Abstract
Biomechanical changes in the sclera likely underlie the excessive eye elongation of axial myopia. We studied the biomechanical characteristics of myopic sclera at the microscopic level using scanning acoustic microscopy (SAM) with 7-μm in-plane resolution. Guinea pigs underwent form-deprivation (FD) in one eye from 4–12 days of age to induce myopia, and 12-μm-thick scleral cryosections were scanned using a custom-made SAM. Two-dimensional maps of the bulk modulus (K) and mass density (ρ) were derived from the SAM data using a frequency-domain approach. We assessed the effect on K and ρ exerted by: 1) level of induced myopia, 2) region (superior, inferior, nasal or temporal) and 3) eccentricity from the nerve using univariate and multivariate regression analyses. Induced myopia ranged between −3D and −9.3D (Mean intraocular difference of −6.2 +/− 1.7D, N = 11). K decreased by 0.036 GPa for every 1.0 D increase in induced myopia across vertical sections (p < 0.001). Among induced myopia right eyes, K values in the inherently more myopic superior region were 0.088 GPa less than the inferior region (p = 0.002) and K in the proximal nasal region containing the central axis were 0.10 GPa less than temporal K (p = 0.036). K also increased 0.12 GPa for every 1 mm increase in superior vertical distance (p < 0.001), an effect that was blunted after 1 week of FD. Overall, trends for ρ were less apparent than for K. ρ values increased by 20.7 mg/cm3 for every 1.00 D increase in induced myopia across horizontal sections (p < 0.001), and were greatest in the region containing the central posterior pole. ρ values in the inherently more myopic superior region were 13.1 mg/cm3 greater than that found in inferior regions among control eyes (p = 0.002), and increased by 11.2 mg/cm3 for every 1 mm increase in vertical distance (p = 0.001). This peripheral increase in ρ was blunted after 1 week of FD. Scleral material properties vary depending on the location in the sclera and the level of induced myopia. Bulk modulus was most reduced in the most myopic regions (both induced myopia and inherent regional myopia), and suggests that FD causes microscopic local decreases in sclera stiffness, while scleral mass density was most increased in the most myopic regions.
Keywords: Scanning acoustic microscopy, myopia, guinea pig, elastic properties
1. Introduction
Myopia, or near-sightedness, is a common eye disease affecting approximately 2.3 billion people worldwide, and the global prevalence is rising (Holden et al., 2016; Vitale et al., 2008). In certain populations, especially in East and Southeast Asia, it is at epidemic levels (Holden et al. 2016; Lin et al., 1999; Saw et al., 1996). More than 95% of myopia cases result from excessive eye axial length (Curtin, 1985; Zadnik, 1995). While myopia is not often a significant cause for concern when mild, eyes with high myopia (HM, defined as more than −6.0 diopters (D) of near-sightedness) can progress to pathologic myopia (PM) (Hayashi et al., 2010), with up to 70% of patients experiencing sight-threatening pathology (Celorio and Pruett, 1991; Grossniklaus and Green, 1992; Yannuzzi et al., 1993). A key pathological change in HM eyes is scleral thinning (Avetisov et al., 1983; Curtin and Teng, 1957), which predisposes the eye to developing local outpouchings (staphylomas), which are commonly found in PM (Curtin and Karlin, 1970; Hsiang et al., 2008). Alterations in scleral structural elements likely underlie this pathological change, resulting in the viscoelastic sclera in the myopic eye becoming more pliable, and hence more readily deformed under constant pressure (Castren and Pohjola, 1962; Phillips and McBrien, 1995). These scleral structural changes have been shown in mammalian models, which demonstrated increased scleral collagen degradation and decreased scleral collagen synthesis with the development of myopia (McBrien et al., 2000, 2001; Norton and Rada, 1995). Furthermore, both mammalian models and HM patients show reduced collagen cross-linking and changes in collagen-fiber diameter (Avetisov et al., 1983; Harper and Summers, 2015; Phillips et al. 2000; Rada et al. 2000; Phillips and McBrien, 2008). The causes underlying these changes in the local and regional material properties of the sclera, however, have not yet been fully understood.
The guinea pig (GP) is a well-established model of myopia, accomplished through either form-deprivation myopia (FDM) or lens-induced myopia (Howlett and McFadden, 2006; Zeng et al., 2013). In addition to serving as an excellent model of myopic sclera in primates, this animal model also has the practical advantage of yielding rapid myopia development. Recent work showed that the peripapillary zone around the optic nerve rapidly expands during development of myopia. In addition, large regional staphylomas can develop in the neighboring crescent area as early as from 4 weeks of induced myopia (Zeng et al., 2013; Zeng and McFadden, 2013). In a small mammal, like the guinea pig whose eyes are relatively close to the ground, a natural asymmetry in refractive error occurs between the upper and lower visual fields (Zeng et al., 2013). At elevations that view the ground, the eye is myopic, while those viewing the sky are hyperopic. Natural adaptation is the plausible explanation for this, where myopia is adaptively advantageous for focusing on near objects on the ground using the superior retina; and vice versa for focusing on far objects in the sky using the inferior retina. Such a pattern is also observed in the human eye, but on a much smaller scale.
Various methods have been employed in studying the biomechanical properties of ocular tissues ex vivo, but these techniques have specific limitations. Strip-extensiometry (Kling et al., 2012; Richoz et al., 2014) and inflation testing (Coudrillier et al., 2015; Grytz et al., 2014; Lombardo et al., 2014) are two such methods, where evaluation of tissue elasticity is limited to the macroscopic scale. On the other hand, atomic force microscopy is able to produce high-resolution images of tissue surface contours and Young’s modulus, but is limited by slow scanning speeds and a narrow field of view (i.e., a few square μm) (Dias et al., 2015; Lavanya et al., 2016). Use of a scanning acoustic microscope (SAM) overcomes many serious limitations of these methods and is the only approach that has the potential to both provide estimates of material properties at fine resolution and over the entire eye. Studies have shown that SAM at frequencies between 100MHz and 1.5 GHz is viable for the analysis of tissue mechanical properties in diverse soft tissue types (Beshtawi et al., 2013; Hozumi et al., 2004; Rohrbach et al., 2015; Saijo et al., 1997). However, few studies have reported SAM measurements of ocular tissues. Beshtawi et al. (2013) used a 761-MHz SAM to compare speed-of-sound at the μm scale in crosslinked corneas with controls and found good agreement between histology and speed-of-sound in the treated cases. In another study, two human retinas were successfully imaged at a 1-GHz center frequency, and high contrast existed among the acoustic properties of different retinal structures (Marmor et al., 1977).
Our group recently developed a novel SAM device that was successfully tested for application on mouse and human ocular tissues (Rohrbach et al., 2015, 2017a, 2017b, 2018a). We demonstrated that tissue mechanical properties at 7-μm resolution significantly differ among layers of murine retinas (Rohrbach et al., 2015). Furthermore, our system is able to resolve the microanatomy of human ocular tissues and characterize the material properties of different tissue types that are expected to play an important role in eye disorders (Rohrbach et al., 2017a). For instance, epithelia tissue of human cornea was found to have a lower speed-of-sound when compared to the stroma (Rohrbach et al., 2018a), a fact that allowed more accurate estimation of epithelia thickness (using high-frequency ultrasound scanning) to detect and monitor corneal pathologies such as keratoconus (Silverman et al., 2009). Recently, employing the GP model of myopia, we demonstrated the feasibility of assessing scleral material properties through a pilot project that investigated the average posterior pole biomechanical properties, and assessed microscopic scleral changes in a vertical plane in a single highly myopic, unilaterally form deprived GP (D. Rohrbach et al., “Fine-resolution elastic-property maps of myopic sclera by means of acoustic microscopy,” 2015 IEEE International Ultrasonics Symposium, Taipei, 2015, pp. 1–4). These preliminary findings suggested a possible regional difference in scleral biomechanical properties.
Therefore, in the present study, we employed a high resolution SAM system to image and compare untreated and myopic scleras extracted from 11 GPs that underwent unilateral FDM and were selected to encompass a range of different levels of myopia, so that the relationship between refractive changes and scleral biomechanical changes could be fully assessed. Additionally, micro-biomechanical maps of the two-dimensional regional variation were made in both horizontal (in the temporal-nasal plane) and vertical sections (in the dorsal-ventral plane). Because prominent changes in eye shape in myopic guinea pig eyes occur around the optic nerve head (ONH, Bowrey et al. 2017), assessment of eccentricity-dependent changes in elastic properties on either side of the ONH was also made.
2. Materials and methods
2.1. Animals and raising conditions
Guinea pigs (N=11) were form-deprived by raising young animals with a translucent diffuser worn in front of the right eye for 8 days (from 4–12 days of age). During this period, animals were kept with their mothers in white-light LED illumination as previously described (Bowrey et al. 2015). After 7 days, cycloplegia was induced with 1–2 drops of 1% cyclopentolate and refractive error was measured (using a Nidek autorefractor) 1.5–2 hours later, with diffusers replaced after each measurement (McFadden et al., 2004). Within this manuscript, we define “inherent regional myopia” as the inherent, regional difference in which the region superior to the optic nerve head is more myopic than the inferior region, as previously described (Zeng et al. 2013). We define “induced myopia” as the level of induced form deprivation myopia (which includes any inherent regional variations). All procedures were approved under Australian animal ethics legislative requirements and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. Sample preparation
At 12 days of age, animals were sacrificed by first deeply anaesthetizing with isoflurane in oxygen and then injecting the heart with 0.5 ml of pentobarbitone. Freshly-enucleated eyes were either flash-frozen in liquid nitrogen or more slowly frozen using a beaker of isopentane surrounded by liquid nitrogen (to avoid cracks) and stored at −80°C until embedded in medium (Tissue-Tek optimum cutting temperature, O.C.T.). Serial cryosections (12-μm thick) from unfixed frozen tissues were taken in either a vertical (6 animals) or horizontal orientation (5 animals) across the entire posterior pole from FD-treated and contralateral control eyes. Vertical (superior/inferior) sections were cut in one of three planes and mounted onto slides: through the central axis, at the nasal edge of ONH, or through the vertical midline of the ONH. Horizontal (nasal/temporal) sections were cut in one of two planes and mounted onto slides: through either the horizontal midline of the ONH or through the inferior edge of the ONH and the central axis. To ensure complete thawing and rehydration of the specimens, slides were placed in a saline bath for 10 min at room temperature before being imaged with SAM.
2.3. Acoustic microscopy
SAM data were acquired using a custom-designed system as described previously (Rohrbach et al., 2015, 2017a, 2017b, 2018a). Briefly, the SAM system utilizes a 250-MHz transducer (Fraunhofer IBMT, Sulzbach, Germany) with F-number 1.16 and a 160-MHz bandwidth. The transducer was excited using a 300-MHz monocyle pulser (GEOZONDAS, Vilnius, Lithuania). The lateral resolution and depth of field of the transducer were 7 μm and 72 μm, respectively. The microscope slides were mounted in an “upside down” configuration on a three-axis, high-precision, scanning stage (Newport, Irvine, CA, USA). The radio-frequency (RF) echo signals were amplified (MITEQ, Hauppauge, NY, USA) and digitized at 2.5 GHz using a 12-bit HD oscilloscope (HDO6104, Teledyne Lecroy, Chestnut Ridge, NY, USA). The samples were raster scanned in two dimensions with a step size of 2 μm and a drop of degassed and filtered saline was used as coupling fluid. The temperature was measured pre and post scan using thermocouples. Custom LabVIEW (National Instruments, Austin, TX, USA) software was used to control the SAM system.
2.4. Signal and data processing
Two-dimensional (2D) maps of quantitative acoustic properties were formed using a frequency-domain and model-based approach as described in our recent publication (Rohrbach et al., 2017b). Briefly, at each scan location, the RF echo signals were Fourier transformed and normalized (i.e., divided) by the Fourier transform of a reference signal (i.e., a signal from a glass plate). This normalized spectrum was used to directly compute four independent variables: sample thickness (d), speed of sound (c), acoustic attenuation (α), and acoustic impedance (Z) using standard and validated algorithms (Hozumi et al., 2004; Rohrbach et al., 2017b). Briefly, at each scan location the acoustic signals is composed of two reflections corresponding to the water-tissue and tissue-glass interfaces. The time of flights of these reflections are used to compute d and c. The amplitude of the first reflections is directly related to Z. Finally, α is estimated from the amplitude and the spectral content of second reflection. In this study, we decided to report two independent mechanical properties directly estimates from the Z and c: Bulk modulus (K) and mass density (ρ) which were calculated from first principles using ρ =Zc and K= ρc, respectively (Briggs, 1992). K (which is similar and complimentary to Young’s Modulus) measures how resistant a tissue is to compression (i.e., a larger K value signifies a stiffer tissue). In linear isotropic media, bulk and Young’s moduli are directly related through the Poison’s ratio and behave qualitatively similarly. In addition, 2D maps of signal amplitude were also formed by taking the maximum of the envelope of the RF signals at each scan location.
2.5. Image Segmentation
The 2D parameter maps were divided into FD-treated and contralateral control eyes (CON). For each eye, measurements were taken at comparable regions both superior (SUP) and inferior (INF) as well as nasal (NAS) and temporal (TMP) to the optic nerve for vertical and horizontal sections, respectively. Distances were measured relative to either the horizontal or vertical ONH midline for vertical and horizontal distances, respectively. The ONH center was defined as the intersection between the horizontal and vertical ONH midlines.
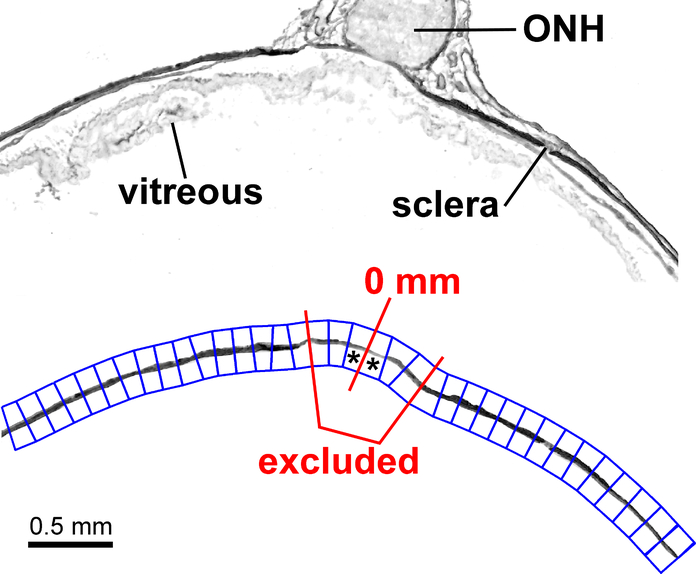
The sclera and optic nerve were manually-segmented to exclude unrelated tissue sections (e.g., the retina, choroid and extraocular tissue), preparation artifacts, and areas that exhibited sectioning artifacts (e.g., tissue folding). All further image processing was performed based on these exclusion masks. Upon selection of the ONH, the image-skeleton of these maps were calculated using a distance transform. This procedure is a common image-processing algorithm and often referred to as binary image skeletonizing (Pratt, 1991). The skeleton of the binary mask represented the midline of the scleral axial thickness and was used to calculate the lateral distance from the ONH, which was further used to divide the sclera into lateral regions of interest (ROIs). Figure 1, top panel, depicts SAM amplitude maps prior to manual segmentation from a vertical section through midpoint of the ONH. Each ROI extended 0.2 mm in length along the axial midline of the sclera, with the distance of 0 mm defined at the center of the ONH (Fig. 1, bottom panel). Each map was then manually-segmented to focus on the sclera and optic nerve (Fig. 1, bottom panel). All ROIs that were observed to contain optic nerve or only extraocular tissue (as opposed to solely scleral tissue) were excluded from the analysis (example shown in Fig. 1- red line outline).
Figure 1.
Region of interest (ROI) selection based on amplitude image of a vertical section through the optic nerve head (ONH) midline before (top panel) and after (bottom panel) manual segmentation to isolate the sclera and ONH. The 0 mm mark is roughly at the horizontal midline of the ONH. * (asterisk) denotes ROI 1 on both sides of the 0 mm mark. ROIs were place semi-automatically (through software detecting the sclera), then adjusted manually. ROIs bounded by the red lines were excluded from analysis due to their position underlying the ONH in the unsegmented top panel.
Within each ROI, the mean and standard deviation of each parameter value were calculated. Acoustic parameters derived from the SAM data obtained from all imaged sclera in both directions (in nasal to temporal and inferior to superior) were averaged over 0.2-mm wide regions of interest automatically defined and originating at the ONH center. For these analyses, data that were within 0.6 mm of the ONH center were ignored, since the optic nerve itself is approximately 1–1.2 mm in diameter. Since only two of the four acoustic parameters are independent (see explanation above), we limited the detailed analysis to K and ρ.
For each parameter, the ROIs in each region (SUP, INF, NAS, TMP) were grouped into distance quintiles (composed of equal number of ROIs within each quintile). Specifically, 5 vertical quintiles were defined in terms of the range of distances from the ONH in mm being: #1) 0.60–1.39, #2) 1.40–1.99, #3) 2.00–2.39, #4) 2.40–3.39 and #5) 3.40–5.20 mm. Horizontals quintiles were defined as: #1) 0.60–1.39, #2) 1.40–1.79, #3) 1.80–2.39, #4) 2.40–3.19 and #5) 3.20–4.6 mm. The combination of the first two quintiles (#1 and #2) were defined as “proximal” vertical and horizontal regions. The combination of the two most eccentric quintiles (#4 and #5) were defined as “distal” vertical and horizontal regions. Vertical sections through the central axis (which did not contain the landmark of the ONH) were omitted from region and eccentricity analyses.
2.6. Statistical Analysis
Univariate and multivariate regression analyses were performed to assess the association between biomechanical parameters (K or ρ) and 1) level of induced myopia, 2) vertical region (i.e., SUP, INF) and horizontal region (i.e., NAS, TMP), 3) vertical and horizontal eccentricity, and the interaction of these three factors (region, eccentricity and level of myopia) using STATA13 software (StataCorp, College Station, TX). Specifically, with the linear regression analysis, a coefficient (slope) and p-value to summarize the magnitude and precision of the relationship between main independent variable and the outcome variable of interest was reported. Using a binned scatterplot approach, the underlying relationship was summarized graphically by plotting the mean outcome variable at each value of the independent variable in order to provide a more visually compelling way to represent the same data as a conventional scatterplot, but allows one to better infer the underlying relationship between outcome and independent variable values. The linear regression line within binned scatterplots provides a coefficient and standard error that quantifies the magnitude and precision of the observed relationship, takes into account the underlying inter-sample variance and reports it as an overall p-value for the regression line across all the data points (versus visualizing error bars at each x value). The spread of the binned scatterpoints around the regression line is informative about the precision of the slope of the relationship. If the spread is tight (vs. dispersed) around the regression line, the p-value of the observed relationship would be small (vs. large). Statistical results were considered significant for p-values less than 0.05.
3. Results
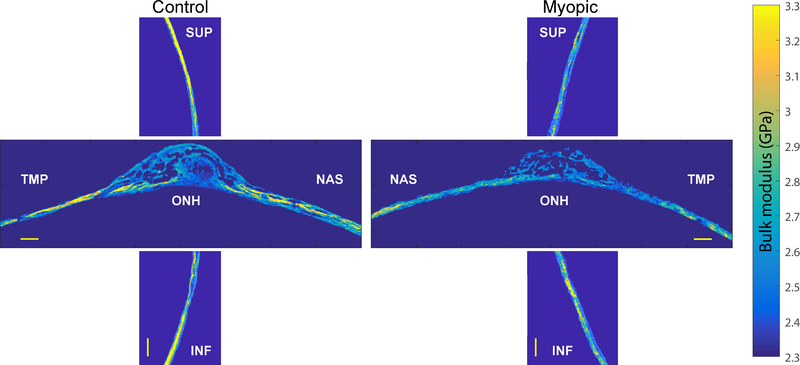
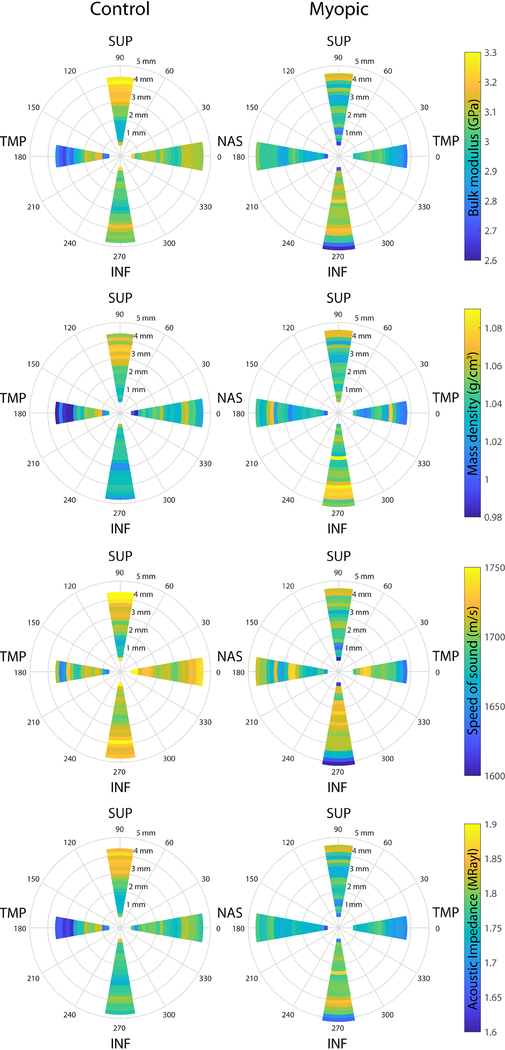
Figure 2 depicts representative 2D K maps of manually-segmented sclera for untreated left eyes (left panel) and FD eyes with high levels of induced myopia (right panel horizontal section −9.3 D and vertical section −7.2 D). Sections are taken near the vertical midpoint of the ONH. Figure 3 shows the quantitative average values for four relevant acoustic parameters (K, ρ, speed of sound and acoustic impedance). Data in Figure 3 were averaged and color-coded at each location for each scanning direction and are shown for both the control and myopic eye. When combining both vertical and horizontal sections, overall, the average K value was 3.047 +/− 0.213 GPa (mean +/− standard deviation) in control eyes and 3.015 +/− 0.217 GPa across all myopic eyes, which was significantly different (p = 0.031). Overall, the average ρ value was 1.042 +/− 0.047 g/cm3 in control eyes and 1.042 +/− 0.045 g/cm3 across all myopic eyes. This difference was not significant (p = 0.832). However, averaging across the entire eye cancels out opposing regional differences. Rather, an advantage of obtaining fine resolution (7 micron) biomechanical data provided by SAM, is that it allows analysis of how K and ρ change with: level of induced myopia, vertical or horizontal region, and distance from the ONH midpoint. This finer resolution data makes differences more readily apparent.
Figure 2.
Color-coded bulk modulus amplitude maps derived from vertical sections (vertical panels) and horizontal sections (horizontal panels) of sclera that were taken from untreated control eyes (left panel) and eyes that underwent form deprivation myopia (right panel) with a resulting relative induced myopia of −7.2 D (horizontal section) and −9.3 D (vertical section). Both horizontal and vertical sections were cut through the optic nerve head midline. Superior sclera (SUP) is located on the top of the image, inferior sclera (INF) at the bottom, nasal sclera (NAS) toward the midline and temporal sclera (TMP) toward the lateral border of the image. Scale bar = 200 microns. ONH = optic nerve head.
Figure 3.
Color-coded amplitude maps representing the quantitative average values for four relevant acoustic parameters (bulk modulus, A; mass density, B; speed of sound, C; acoustic impedance, D) were averaged for control (left column) and myopic eyes (right column). Acoustic parameters derived from the SAM data obtained from all imaged sclera in both directions (in nasal (NAS) to temporal (TMP) and inferior (INF) to superior (SUP)) were averaged over 0.2-mm wide regions of interest automatically defined and originating at the midpoint of the optic nerve head (ONH, 0 mm) and plotted on a polar graph. For these analyses, data within 0.6 mm of the ONH midpoint were omitted.
3.1. Bulk Modulus (K)
3.1.1. Regional Differences in Control Eyes
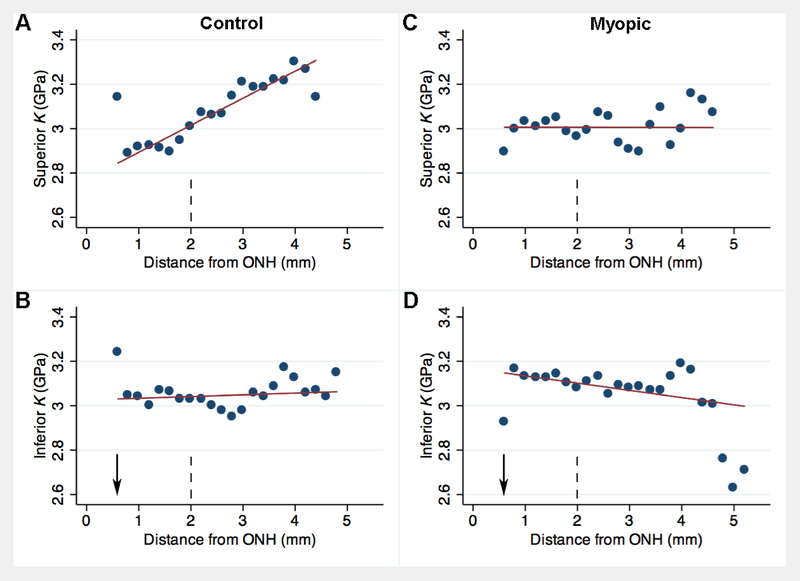
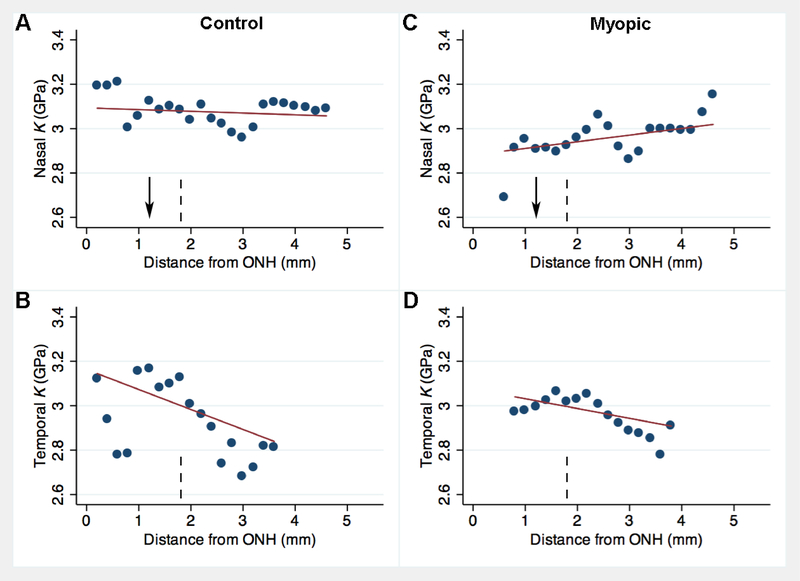
Among control left eyes, in terms of eccentricity-dependent changes within the SUP region, K increased by 0.122 GPa for every 1 mm increase in vertical distance (p < 0.001, Fig. 4A) from the ONH horizontal midline (Fig. 1, “0 mm” mark) with control tissues showing greatest compressibility in the superior peripheral sclera, and being relatively “stiffer” at the ONH. However, this pattern did not occur elsewhere, and in temporal sclera the far periphery had a tendency to have slightly lower K values, opposite to that observed in the SUP region. Statistically, K was not influenced in a significant way by an increase in eccentricity within the INF (coefficient = +7.7, p = 0.542, Fig. 4B), NAS (coeff. = − 0.0078 GPa, p = 0.508, Fig. 5A) or TMP regions (coeff. = −90, p = 0.172, Fig. 5B).
Figure 4.
Binned scatterplot comparing vertical distance (horizontal axis, in mm from horizontal midline of the optic nerve head (ONH)) to bulk modulus (vertical axis, K, in GPa) for control eyes (A,B) and contralateral myopic eyes (C,D) in vertically-sectioned guinea pig eyes from near the ONH to 5 mm away in the periphery. Top panels represent superior distance and bottom panels represent inferior distance from the ONH horizontal midline. Each dot represents the mean from up to 12 ROIs from 6 animals measured at the given distance. Red line is the linear regression trendline for N = 233 – 251 data points. Vertical dashed line notes upper boundary of the “proximal” region. Arrow marks approximate location of the central axis of the eye.
Figure 5.
Binned scatterplot comparing horizontal distance (horizontal axis, in mm from vertical midline of the optic nerve head (ONH)) to bulk modulus (vertical axis, K, in GPa) for control eyes (A,B) and contralateral myopic eyes (C,D) in horizontally-sectioned guinea pig pup eyes. Top panels represent nasal distance and bottom panels represent temporal distance from the ONH vertical midline. Each dot represents the mean from up to 10 ROIs from 5 animals measured at the given distance. Red line is the linear regression trendline for N = 175 – 182 data points. Vertical dashed line notes upper boundary of the “proximal” region. Arrow marks approximate location of the central axis of the eye.
Among control eyes, in terms of region-dependent changes, on average, K did not significantly differ between SUP and INF regions (Fig. 3, top panel, p = 0.852) nor between NAS and TMP regions (p = 0.078). However, in proximal vertical regions, superior K was 0.128 GPa less than inferior K (p < 0.001). In distal vertical regions, superior K was 0.125 GPa greater than inferior K (p = 0.005). In distal horizontal regions, nasal K was 0.245 GPa greater than temporal K (p < 0.001).
3.1.2. Regional Differences in Eyes with Induced Myopia
Among myopic right eyes within the INF region, K decreased by 0.033 GPa for every 1 mm eccentricity from the optic nerve (p = 0.043, Fig. 4D). K was not influenced in a significant way by an increase in distance within the SUP (coeff. = −0.0003, p = 0.992, Fig. 4C), NAS (coeff. = +0.003, p = 0.093, Fig. 5C) or TMP regions (coeff. = −0.044, p = 0.263, Fig. 5D).
Among eyes with induced myopia, the average superior K values were 0.088 GPa less than inferior K (p = 0.002, Fig. 3, top panel), specifically due to differences in the proximal vertical regions, where superior K was 0.103 GPa less than inferior K (p = 0.027). K did not differ significantly between NAS and TMP regions (p = 0.133). However, in proximal horizontal regions, nasal K was 0.10 GPa less than temporal K (p = 0.036).
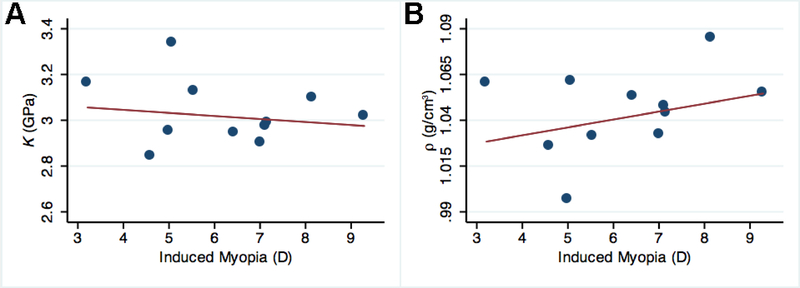
Among induced myopia eyes, in terms of refraction-dependent changes, when averaged across the entire eye (including SUP, INF, NAS and TMP regions), K decreased by 0.013 GPa for every 1.00 D increase in induced myopia (range −3.0 to −9.3 D) (p = 0.024, Fig. 6A). K decreased by 0.036 GPa/ 1.00 D of induced myopia (p < 0.001) when averaged across vertical sections and decreased by 0.051 GPa/ 1.00D of induced myopia in horizontal sections (p < 0.001). Specifically, within the INF region, K decreased by 0.05 GPa for every 1.00 D increase in induced myopia (p < 0.001). Within the NAS region, K increased by 0.075 GPa/1.00 D (p < 0.001). K was not influenced by the level of induced myopia in the SUP (p = 0.121) or TMP regions (p = 0.511).
Figure 6.
Binned scatterplot comparing level of induced myopia (horizontal axis, in D, diopters) to bulk modulus (A, vertical axis, K, in GPa) or mass density (B, vertical axis, ρ, in g/cm3) for myopic eyes averaged across regions superior, inferior, nasal and temporal to the ONH midpoint in vertically- and horizontally-sectioned guinea pig eyes. Red lines are the linear regression trendlines for N = 415 data points.
3.1.3. Differences between Induced Myopia and Control Eyes
Within the SUP region, the average K was 0.122 GPa lower in eyes with induced myopia relative to control eyes (p < 0.001). As eccentricity from the ONH increased, K decreased by 0.012 GPa for every 1.00 D increase in induced myopia among the SUP region (p = 0.003).
Relative induced myopia had no significant influence on the average K within the other retinal regions (INF:coeff. = −0.04 GPa, p = 0.053; NAS: p = 0.070; TMP: p = 0.524; Fig. 3, top panel). However, K significant decreased by 0.007 GPa/ 1.00 D among the INF region of all eyes (p = 0.009). Moreover, K was 0.076 GPa lower in induced myopia eyes relative to control eyes when averaged across all vertical distances examined (p < 0.001), but did not differ among the horizontal sections in either the NAS (p = 0.138), or TMP regions (p = 0.478, example in Fig. 3 right (control) versus left (myopic) panels).
3.2. Mass Density (ρ)
3.2.1. Regional Differences in Control Eyes
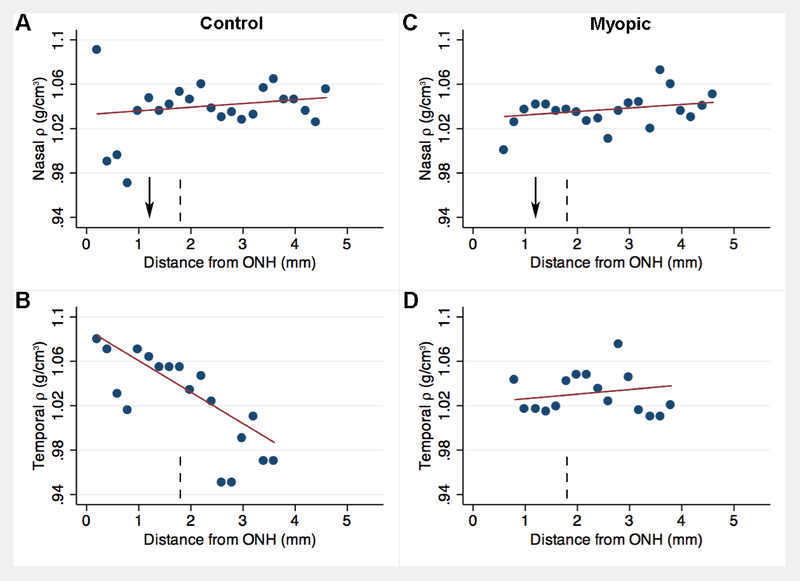
Overall, trends for ρ were less apparent than for K. Within the SUP region of control eyes, ρ increased by 11.2 mg/cm3 for every 1 mm increase in vertical eccentricity away from the ONH (p = 0.001, Fig. 7A). ρ was not significantly influenced by increased eccentricity within the INF region of control (coeff. = −3.1, p = 0.151, Fig. 7B). Eccentricity-dependent changes also occurred in the horizontal meridian. Specifically, ρ decreased by 28.3 mg/cm3 for every 1 mm increase in horizontal eccentricity away from the ONH in the TMP region (p = 0.008, Fig. 8B), but ρ did not significantly vary at different eccentricities within the NAS region (coeff. = +3.3, p = 0.517, Fig. 8A).
Figure 7.
Binned scatterplot comparing vertical distance (horizontal axis, in mm from horizontal midline of the optic nerve head (ONH)) to mass density (vertical axis, ρ, in g/cm3) for control eyes (A,B) and contralateral myopic eyes (C,D) in vertically-sectioned guinea pig eyes from near the ONH to 5 mm away in the periphery. Top panels represent superior distance and bottom panels represent inferior distance from the ONH horizontal midline. Each dot represents the mean from up to 12 ROIs from 6 animals measured at the given distance. Red line is the linear regression trendline for N = 233 – 251 data points. Vertical dashed line notes upper boundary of the “proximal” region. Arrow marks approximate location of the central axis of the eye.
Figure 8.
Binned scatterplot comparing horizontal distance (horizontal axis, in mm from vertical midline of the optic nerve head (ONH)) to mass density (vertical axis, ρ, in g/cm3) for control eyes (A,B) and contralateral myopic eyes (C,D) in horizontally-sectioned guinea pig pup eyes. Top panels represent nasal distance and bottom panels represent temporal distance from the ONH vertical midline. Each dot represents the mean from up to 10 ROIs from 5 animals measured at the given distance. Red line is the linear regression trendline for N = 175 – 182 data points. Vertical dashed line notes upper boundary of the “proximal” region. Arrow marks approximate location of the central axis of the eye.
Among control eyes, SUP ρ was on average 13.1 mg/cm3 greater than INF ρ (p = 0.002, Fig. 3, second row). Specifically, within distal vertical regions, SUP ρ was 31.8 mg/cm3 greater than INF ρ (p < 0.001). ρ did not differ between NAS and TMP regions (p = 0.985). However, within distal horizontal regions, nasal ρ was 50.4 mg/cm greater than temporal ρ (p = 0.022).
3.2.2. Regional Differences in Induced Myopia Eyes
Among induced myopia eyes, within the INF region, ρ increased by 4.9 mg/cm3 for every 1 mm (p = 0.023, Fig. 7D). ρ was not influenced by increased distance within the SUP (coeff. = −3.5, p = 0.209, Fig. 7C), NAS (coeff. = +3.2, p = 0.580, Fig. 8C) or TMP regions (coeff. = +4.2, p = 0.705, Fig. 8D). However, on average, ρ did not significantly differ between SUP and INF regions (p = 0.525, Fig. 3, second row) nor between NAS and TMP regions (p = 0.514).
Among induced myopia eyes, in terms of refraction-dependent changes, when averaged across the entire eye (including SUP, INF, NAS and TMP regions), ρ increased by 4.3 mg/cm3 for every 1.00 D increase in induced myopia (range −3.0 to −9.3 D) (p < 0.001, Fig. 6B). ρ increased by 20.7 mg/cm3/ 1.00 D of induced myopia when averaged across horizontal sections (p < 0.001), but was not influenced by the level of induced myopia when averaged across vertical sections (p = 0.272). This was primarily due to changes within the NAS region, where ρ increased by 25.6 mg/cm3 for every 1.00 D increase in induced myopia (p < 0.001), while ρ was not influenced by the level of induced myopia in the TMP region (p = 0.112). Within the SUP region, ρ increased by 2.3 mg/cm3 for every 1.00 D increase in induced myopia (p = 0.043), whereas INF region ρ decreased by 3.5 mg/cm3 for every 1.00 D increase (p = 0.002).
3.2.3. Differences between Induced Myopia and Control Eyes
Eyes with induced myopia showed a different pattern in regional variation in ρ compared to control eyes. In the superior sclera, the mass density was highest 3 mm from the ONH in control eyes, and lowest at the same eccentricity in induced myopia eyes, while opposite changes occurred closer to the ONH, with increased mass density in induced myopia eyes (Fig 7A,C). Based on linear fits to the data within the SUP region (Fig. 7), the change in ρ with increased eccentricity from the ONH was 14.7 mg/cm3 lower in induced myopia eyes relative to control eyes (p = 0.002) and 8.0 mg/cm3 greater within the INF region (p = 0.009). Interestingly, in temporal sclera, the high mass density near the ONH and much lower mass density in the temporal periphery seen in control eyes was not observed in induced myopia eyes (cf. Fig. 8B, D). Overall, the change in ρ with increased eccentricity from the ONH was 32.5 mg/cm3 greater in induced myopia eyes within the TMP region (p = 0.038), while no significant differences occurred within the NAS region (p = 0.985, Fig. 8A,C).
Of note, the results remain qualitatively similar whether we include fixed effect for each animal (to account for any possible inter-relatedness of repeated measures within a given eye) or if we simply restrict the analysis to a single section from each eye (i.e., without repeated measures within a given eye).
4. Discussion
In this study, by using a SAM system operating at 250-MHz on thin tissue sections, we quantitatively assessed the material properties of sclera from form-deprived and untreated fellow eyes. K and ρ values were reported. K is related to the Young’s modulus together with the Poisson’s ratio or shear modulus. K is the inverse of compressibility, and is akin to stiffness. A lower K suggests that under the same uniform change in pressure, the structure of the sclera is more distensible. It should be noted that our measure of K was not influenced by scleral thickness, because the measures are taken perpendicular to the scleral surface at a microscopic scale and are independent of its depth.
The SAM system has been validated using simulation and experimental studies in numerous organ systems (Lawton et al. 2019; (Rohrbach et al., 2015, 2017a, 2017b, 2018a, 2018b). In this study, the use of cryosections guarantees that the freshly-excised (i.e., ex vivo) properties are maintained and the use of a consistent and optimized sample preparation protocol (i.e., thawing and hydration) also guarantees consistent and repeatable results. Nevertheless, the values may not reflect true in vivo conditions as it is the case with all microscopy imaging modalities.
Along the vertical scleral meridian, K values decreased with increases in FD-induced myopia and K values increased with vertical eccentricity. Interestingly, K values of the guinea pig sclera also varied with inherent regional myopia, in that K values were lower in the relatively more myopic superior region than in the hyperopic inferior region. Our results suggest that the region of the sclera that corresponds to inherent lower field myopia is biomechanically more distensible than the scleral region corresponding to the relatively hyperopic upper visual field (Fig. 3).
K significantly increased with increased superior vertical distance from the optic nerve. This peripheral increase in “stiffness” in the superior sclera was blunted after 1 week of myopia inducement. In the horizontal meridian, the scleral K in the nasal region containing the central axis was mostly less than in the temporal region in induced myopia eyes, and decreased with the level of myopia, suggesting that myopic sclera was more distensible. However, in general, the changes in K in the horizontal meridian were much less than those observed in the vertical.
These findings are consistent with the notion that the greater the myopia (be it the inherent regional myopia found in the superior region as compared to the inferior region, or the greater level of myopia induced by FD), the lower the value of K. Of note, prior reports suggest that higher levels of myopia (in guinea pigs, monkeys and humans) are associated with a macroscopically weaker and softer sclera (Cui et al., 2011; Curtin et al., 1979; Funata and Tokoro, 1990; McBrien et al., 2001). These studies consistently suggested a thinner scleral wall, which may, to some extent, explain a weaker sclera. However, the macroscopic characteristics may be unrelated to microscopic properties. Specifically, it was unclear from previous studies whether the intrinsic scleral-material properties such as stiffness (e.g., K) are different in myopic eyes. Previously, studies found decreased collagen-fibril diameters in myopic eyes when compared to non-myopic eyes (Cui et al., 2011), which would lead to different material properties of scleral tissues at the micrometer scale. Different types of collagen change differently, and the ratio of collagen types is changed in myopic sclera. Moreover, fibril diameters decrease only after a substantial period of time, therefore we would not expect to see this change after the relatively short-term FD (10 days) employed in the present work. Despite this, in the present work we demonstrated that scleral material properties of myopic eyes differ significantly from non-myopic eyes, which might be due to early variations of collagen fibril size and concentration or a different structural alteration. Future studies should investigate the relationship between collagen-fibril diameters, concentration, and orientation with the intrinsic material properties at the micrometer length scale.
The second tissue property we reported was ρ, which measures mass per unit volume and in the sclera is determined by the mass density and concentration of scleral components including collagen (predominantly type I collagen), proteoglycans and other glycoproteins. Changes in mass density can be related to morphological changes due to myopia.
ρ values increased with the level of induced myopia (greatest in the nasal region, which contains the central axis). ρ values in the inherently more myopic superior region were greater than that found in inferior regions only among control eyes (trends were less consistent in induced myopia eyes). Superior ρ values increased with increased vertical distance from the nerve in control eyes. This peripheral increase in ρ was blunted after 1 week of myopia inducement. Along the horizontal meridian, ρ values only differed between nasal and temporal regions at the distal edge (where ρ was greater in the nasal region containing the central axis); and temporal ρ values decreased with increased horizontal distance in control eyes. Nevertheless, the trends for ρ were, in general, less apparent than for K.
For animals that developed myopia from FD, K values decreased and ρ values increased with increased levels of myopia, thereby suggesting that the lack of visual feedback can lead to alteration in these parameters, and potentially other regional scleral biomechanical properties at the microscopic level. This is corroborated by previous research that has shown that FD causes axial lengthening of the eye near the posterior pole and optic nerve, but shortening in the peripheral regions of the eye, which shrink and become relatively hyperopic (Zeng and McFadden, 2013). The spatial resolution afforded by SAM imaging permits assessing the material properties at length scales of a few μm. Therefore, in the myopic sclera, the SAM measurements are likely to be affected by microstructural changes such as collagen fibril mechanical properties, structure and orientation. Elastic properties assessed with macroscopic methods (e.g., strip-extensiometry and inflation testing) cannot be directly compared to SAM measurements because such methods typically depend on the macroscopic properties of the entire sclera (e.g., scleral thickness and bulk scleral mechanical properties) or possess a much coarser spatial resolution.
SAM is sensitive to myopia-related changes of the intrinsic scleral material properties that are directly connected to alterations in the collagen connective-tissue network. For instance, increased collagen degeneration, and decreased scleral collagen synthesis, crosslinking and fiber diameter changes have been reported for high myopia in animal models and myopia patients and should affect SAM measurements (Avetisov et al., 1983; Norton and Rada, 1995; Phillips and McBrien, 1995). Specifically, myopia-related changes are shown to be dependent on which part of the sclera is investigated and the level of induced myopia. We also hypothesize that additional region-dependent effects may also be present in the different layers of the sclera. Such investigations are readily performed with our SAM system with its spatial resolution of better than 10 μm and will be useful to assess the impact of local scleral treatments aimed to correct myopic abnormalities in scleral biomechanical properties.
Supplementary Material
Highlights.
SAM allows the assessment of scleral material properties at a 7-μm resolution
Myopia-related scleral biomechanical changes vary by location and level of myopia
Generally, more myopic regions and eyes are associated with lower bulk modulus
More myopic regions and eyes are associated with greater mass density
Acknowledgements
We thank Daniel Gross for his technical support and assistance with the SAM and we thank Anette Jakob for providing and assisting with the 250 MHz transducer. We also thank Jessica Pan, Ph.D. for her biostatistical assistance, Albert Chung and Kenoh Murakami for their help with SAM measurements and analysis, Quan Wen for cryosectioning and Judith Ow for her help with manuscript preparation. This work was supported in part by the National Institutes of Health (EB016117 (JM) and EY023595 (QVH)), Hunter Medical Research Institute (G1400967 (SAM)), the Riverside Research Fund for Biomedical Engineering Research, a Career Development Award from Research to Prevent Blindness (QVH) and an unrestricted grant from Research to Prevent Blindness to Columbia University. The funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- Avetisov ES, Savitskaya NF, Vinetskaya MI, Iomdina EN. A study of biochemical and biomechanical qualities of normal and myopic eye sclera in humans of different age groups. Metab Pediatr Syst Ophthalmol. 1983;7(4): 183–8 [PubMed] [Google Scholar]
- Beshtawi IM, Akhtar R, Hillarby MC, O’Donnell C, Zhao X, Brahma A, Carley F, Derby B, Radhakrishnan H. Scanning acoustic microscopy for mapping the microelastic properties of human corneal tissue. Curr Eye Res. 2013. April;38(4):437–44. [DOI] [PubMed] [Google Scholar]
- Bowrey HE, Metse AP, Leotta AJ, Zeng G, McFadden SA. The relationship between image degradation and myopia in the mammalian eye. Clin Exp Optom. 2015. November;98(6):555–63. [DOI] [PubMed] [Google Scholar]
- Bowrey HE, Zeng G, Tse DY, Leotta AJ, Wu Y, To CH, Wildsoet CF, McFadden SA. The Effect of Spectacle Lenses Containing Peripheral Defocus on Refractive Error and Horizontal Eye Shape in the Guinea Pig. Invest Ophthalmol Vis Sci. 2017. May 1;58(5):2705–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs A Acoustic microscopy. Oxford : New York: Clarendon Press; Oxford University Press, 1992. [Google Scholar]
- Castren JA, Pohjola S. Myopia and scleral rigidity. Acta Ophthalmol (Copenh). 1962;40:33–6. [DOI] [PubMed] [Google Scholar]
- Celorio JM, Pruett RC. Prevalence of lattice degeneration and its relation to axial length in severe myopia. Am JOphthalmol. 1991. January 15;111(1):20–3. [DOI] [PubMed] [Google Scholar]
- Coudrillier B, Pijanka J, Jefferys J et al. Effects of age and diabetes on scleral stiffness. J Biomech Eng. 2015. July;137(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D, Trier K, Zeng J et al. Effects of 7-methylxanthine on the sclera in form deprivation myopia in guinea pigs. Acta Ophthalmol. 2011. June;89(4):328–34. [DOI] [PubMed] [Google Scholar]
- Curtin BJ. The myopias: basic science and clinical management. Philadelphia: Harper & Row, 1985. [Google Scholar]
- Curtin BJ, Iwamoto T, Renaldo DP. Normal and staphylomatous sclera of high myopia. An electron microscopic study. Arch Ophthalmol. 1979. May;97(5):912–5. [DOI] [PubMed] [Google Scholar]
- Curtin BJ, Karlin DB. Axial length measurements and fundus changes of the myopic eye. I. The posterior fundus. Trans Am Ophthalmol Soc. 1970;68:312–34. [PMC free article] [PubMed] [Google Scholar]
- Curtin BJ, Teng CC. Scleral changes in pathological myopia. Trans Am Acad Ophthalmol Otolaryngol. 1957;62:777–788. [PubMed] [Google Scholar]
- Dias J, Diakonis VF, Lorenzo M et al. Corneal stromal elasticity and viscoelasticity assessed by atomic force microscopy after different cross linking protocols. Exp Eye Res. 2015. September;138:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funata M, Tokoro T. Scleral change in experimentally myopic monkeys. Graefes Arch Clin Exp Ophthalmol. 1990;228(2):174–9. [DOI] [PubMed] [Google Scholar]
- Goh WS, Lam CS. Changes in refractive trends and optical components of Hong Kong Chinese aged 19–39 years. Ophthalmic Physiol Opt. 1994. October;14(4):378–82. [PubMed] [Google Scholar]
- Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina. 1992;12(2):127–33. [DOI] [PubMed] [Google Scholar]
- Grytz R, Fazio MA, Libertiaux V et al. Age- and race-related differences in human scleral material properties. Invest Ophthalmol Vis Sci. 2014. November 11;55(12):8163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AR, Summers JA. The dynamic sclera: extracellular matrix remodeling in normal ocular growth and myopia development. Exp Eye Res. 2015. April; 133:100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Ohno-Matsui K, Shimada N et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010. August;117(8):1595–611, 1611.e1–4. [DOI] [PubMed] [Google Scholar]
- Holden BA, Fricke TR, Wilson DA et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016. May;123(5):1036–42. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Form-deprivation myopia in the guinea pig (Cavia porcellus). Vision Res. 2006. January;46(1–2):267–83 [DOI] [PubMed] [Google Scholar]
- Hozumi N, Yamashita R, Lee CK et al. Time-frequency analysis for pulse driven ultrasonic microscopy for biological tissue characterization. Ultrasonics. 2004. April;42(1–9):717–22. [DOI] [PubMed] [Google Scholar]
- Hsiang HW, Ohno-Matsui K, Shimada N et al. Clinical characteristics of posterior staphyloma in eyes with pathologic myopia. Am J Ophthalmol. 2008. July;146(1):102–110. [DOI] [PubMed] [Google Scholar]
- Kling S, Ginis H, Marcos S. Corneal biomechanical properties from two-dimensional corneal flap extensiometry: application to UV-riboflavin cross-linking. Invest Ophthalmol Vis Sci. 2012. July 27;53(8):5010–5. [DOI] [PubMed] [Google Scholar]
- Lawton AK, Engstrom T, Rohrbach D, Omura M, Turnbull DH, Mamou J, Zhang T, Schwarz JM, Joyner AL. Cerebellar folding is initiated by mechanical constraints on a fluid-like layer without a cellular pre-pattern. Elife. 2019. April 16;8 pii: e45019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavanya Devi AL, Nongthomba U, Bobji MS. Quantitative characterization of adhesion and stiffness of corneal lens of Drosophila melanogaster using atomic force microscopy. J Mech BehavBiomedMater. 2016. January;53:161–173. [DOI] [PubMed] [Google Scholar]
- Lin LL, Shih YF, Tsai CB et al. Epidemiologic study of ocular refraction among schoolchildren in Taiwan in 1995. Optom Vis Sci. 1999. May;76(5):275–81. [DOI] [PubMed] [Google Scholar]
- Lombardo G, Serrao S, Rosati M, Lombardo M. Analysis of the viscoelastic properties of the human cornea using Scheimpflug imaging in inflation experiment of eye globes. PLoS One. 2014. November 14;9(11):e112169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor MF, Wickramasinghe HK, Lemons RA. Acoustic microscopy of the human retina and pigment epithelium. Invest Ophthalmol Vis Sci. 1977. July;16(7):660–6. [PubMed] [Google Scholar]
- McBrien NA, Cornell LM, Gentle A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Invest Ophthalmol Vis Sci. 2001. September;42(10):2179–87. [PubMed] [Google Scholar]
- McBrien NA, Lawlor P, Gentle A. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Invest Ophthalmol Vis Sci. 2000. November;41(12):3713–9. [PubMed] [Google Scholar]
- McFadden SA, Howlett MH, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004. March;44(7):643–53. [DOI] [PubMed] [Google Scholar]
- Norton TT, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995. May;35(9):1271–81. [DOI] [PubMed] [Google Scholar]
- Phillips JR, McBrien NA. Form deprivation myopia: elastic properties of sclera. Ophthalmic Physiol Opt. 1995. September;15(5):357–62. [PubMed] [Google Scholar]
- Phillips JR, Khalaj M, McBrien NA. Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest Ophthalmol Vis Sci. 2000. July;41(8):2028–34. [PubMed] [Google Scholar]
- Pratt WK. Digital Image Processing, John Wiley & Sons, Inc., 1991. [Google Scholar]
- Rada JA, Achen VR, Penugonda S, Schmidt RW, Mount BA. Proteoglycan composition in the human sclera during growth and aging. Invest Ophthalmol Vis Sci. 2000. June;41(7):1639–48. [PubMed] [Google Scholar]
- Richoz O, Kling S, Zandi S, Hammer A, Spoerl E, Hafezi F. A constant-force technique to measure corneal biomechanical changes after collagen cross-linking. PLoS One. 2014. August 27;9(8):e105095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach D, Ito K, Lloyd HO et al. Material Properties of Human Ocular Tissue at 7-μm Resolution. Ultrason Imaging. 2017a. September;39(5):313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach D, Jakob A, Lloyd HO, Tretbar SH, Silverman RH, Mamou J. A Novel Quantitative 500-MHz Acoustic Microscopy System for Ophthalmologic Tissues. IEEE Trans Biomed Eng. 2017b. March;64(3):715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach D, Lloyd HO, Silverman RH, Mamou J. Fine-resolution maps of acoustic properties at 250 MHz of unstained fixed murine retinal layers. J Acoust Soc Am. 2015. May;137(5):EL381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach D, Silverman RH, Chun D, Lloyd HO, Urs R, Mamou J. Improved High-Frequency Ultrasound Corneal Biometric Accuracy by Micrometer-Resolution Acoustic-Property Maps of the Cornea. Transl Vis Sci Technol. 2018. April 14;7(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach D, Mamou J. Autoregressive Signal Processing Applied to High-Frequency Acoustic Microscopy of Soft Tissues. IEEE Trans Ultrason Ferroelectr Freq Control. 2018. November;65(11):2054–2072. [DOI] [PubMed] [Google Scholar]
- Saijo Y, Tanaka M, Okawai H, Sasaki H, Nitta SI, Dunn F. Ultrasonic tissue characterization of infarcted myocardium by scanning acoustic microscopy. Ultrasound Med Biol. 1997;23(1):77–85. [DOI] [PubMed] [Google Scholar]
- Saw SM, Katz J, Schein OD, Chew SJ, Chan TK. Epidemiology of myopia. Epidemiol Rev. 1996;18(2):175–87. [DOI] [PubMed] [Google Scholar]
- Silverman RH, Patel MS, Gal O, et al. Effect of corneal hydration on ultrasound velocity and backscatter. Ultrasound Med Biol. 2009; 35: 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale S, Ellwein L, Cotch MF, Ferris FL 3rd, Sperduto R. Prevalence of refractive error in the United States, 1999–2004. Arch Ophthalmol. 2008. August;126(8):1111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollensak G, Iomdina E. Crosslinking of scleral collagen in the rabbit using glyceraldehyde. J Cataract Refract Surg. 2008. April;34(4):651–6.. [DOI] [PubMed] [Google Scholar]
- Yannuzzi LA, Sorenson JA, Sobel RS, et al. Risk factors for idiopathic rhegmatogenous retinal detachment. Am J Epidemiol. 1993. 137:749–57. [PubMed] [Google Scholar]
- Zadnik K The Glenn A. Fry Award Lecture (1995). Myopia development in childhood. Optom Vis Sci. 1997. August;74(8):603–8. [PubMed] [Google Scholar]
- Zeng G and McFadden SA. Inhibition in peripheral scleral lengthening during the development of myopia in the guinea pig. Invest. Ophthalmol. Vis. Sci. 2013;54(15):5180. [Google Scholar]
- Zeng G, Bowrey HE, Fang J, Qi Y, McFadden SA. The development of eye shape and the origin of lower field myopia in the guinea pig eye. Vision Res. 2013. January 14;76:77–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.