Abstract
Stretching a muscle of the upper limb elicits short (M1) and long-latency (M2) reflexes. When the participant is instructed to actively compensate for a perturbation, M1 is usually unaffected and M2 increases in size and is followed by the voluntary response. It remains unclear if the observed increase in M2 is due to instruction-dependent gain modulation of the contributing reflex mechanism(s) or results from voluntary response superposition. The difficulty in delineating between these alternatives is due to the overlap between the voluntary response and the end of M2. The present study manipulated response accuracy and complexity to delay onset of the voluntary response and observed the corresponding influence on electromyographic activity during the M2 period. In all active conditions, M2 was larger compared with a passive condition where participants did not respond to the perturbation; moreover, these changes in M2 began early in the appearance of the response (∼50 ms), too early to be accounted for by voluntary overlap. Voluntary response latency influenced the latter portion of M2, with the largest activity seen when accuracy of limb position was not specified. However, when participants aimed for targets of different sizes or performed movements of various complexities, reaction time differences did not influence M2 period activity, suggesting voluntary activity was sufficiently delayed. Collectively, our results show that while a perturbation applied to the upper limbs can trigger a voluntary response at short latency (<100 ms), instruction-dependent reflex gain modulation remains an important contributor to EMG changes during the M2 period.
Keywords: long-latency reflex, M2, StartReact effect, superposition, reaction time
fast perturbations applied to the upper limbs can elicit stereotypical, electromyographic (EMG) responses in the stretched muscle. The first response (M1) occurs at short latency (∼25–50 ms) and reflects input from a spinal reflex pathway (Liddell and Sherrington 1924). This is followed by a longer latency (∼50–100 ms) response (M2), which receives input from group II afferents travelling a spinal pathway (Lourenço et al. 2006; Matthews 1984) as well as group I afferents traversing a longer transcortical route (Capaday et al. 1991; Cheney and Fetz 1984; Evarts and Tanji 1976; Lourenço et al. 2006; MacKinnon et al. 2000; Matthews et al. 1990; Omrani et al. 2014; Pruszynski et al. 2011a, 2014). The magnitude of M1 is dependent on peripheral factors such as muscle spindle sensitivity and characteristics of the mechanical perturbation (Matthews 1986; Pruszynski et al. 2009, 2011b). While these factors can also influence M2 (Calancie and Bawa 1985; Pruszynski et al. 2009, 2011b), the size of the long-latency response can be influenced by the task requirements or instructions provided to the participant. When the participant is instructed to “resist” or “compensate” for the perturbation, M2 increases in magnitude and is followed a voluntary response; when the participant is told to “not intervene” or “let go,” M2 is smaller in comparison (e.g., Calancie and Bawa 1985; Colebatch et al. 1979; Hammond 1956; MacKinnon et al. 2000).
The reason for the increase in the size of M2 when instructed to act against the perturbation has been a matter of contention for some time. One view attributes the amplitude modulation to be the result of an increase in the excitability of the reflex pathways (Calancie and Bawa 1985; Colebatch et al. 1979; Hammond 1956; Lee and Tatton 1978; Pruszynski et al. 2008; Selen et al. 2012; Yang et al. 2011). The opposing view suggests that the amplitude modulation is an artifact of the superposition of the voluntary response onto the end of the long-latency response (Crago et al. 1976; Houk 1978; Lewis et al. 2006; Manning et al. 2012; Ravichandran et al. 2013; Rothwell et al. 1980). Part of the disagreement appears to stem from the problem of clearly delineating the M2 response from the voluntary response in EMG recordings. To distinguish between the two potential mechanisms, it is critical that the M2 and voluntary EMG responses can be clearly demarcated from one another.
Studies have previously relied on indirect techniques to determine the onset of the voluntary response [i.e., reaction time (RT)]. For example, Hammond (1956) used a mechanical tap to the wrist (which did not evoke reflexive activity) as a proprioceptive imperative signal and found a mean voluntary RT of about 100 ms for elbow flexion responses. This type of proprioceptive RT was subsequently used as the estimate of voluntary response latency during perturbation conditions, where participants responded by flexing the elbow following an imposed extension. Hammond found that EMG activity changed in size based on instruction (“resist” vs. “let-go”) and this occurred at a latency shorter than the estimated RT. As a result, he concluded that M2 modulation likely resulted from presetting excitability of the contributing stretch reflex mechanism (see also Calancie and Bawa 1985; Colebatch et al. 1979; Lee and Tatton 1978; Pruszynski et al. 2008; Selen et al. 2012; Yang et al. 2011).
Although Hammond (1956) originally assumed that the M2 and voluntary responses did not overlap in time, there is evidence showing that voluntary RTs to a mechanical perturbation can be considerably shorter than 100 ms (e.g., Day et al. 1983; Evarts and Granit 1976; Evarts and Vaughn 1978; Lewis et al. 2006; MacKinnon et al. 2000; Manning et al. 2012; Ravichandran et al. 2013; Shemmell 2009, 2015). As an example, to dissociate M2 from voluntary activity, Evarts and Granit (1976) instructed participants to supinate or pronate the forearm in response to a perturbation (occurring either in the supination or pronation direction). When biceps were stretched (pronation perturbation) and a supination response was required, M1, M2, and voluntary activity all occurred in the same muscle and RT could not be determined precisely. In contrast, when the same supination response was required in reacting to a supination perturbation, M1 and M2 appeared in pronators but only the voluntary response remained in biceps. Using this technique, Evarts and Granit (1976) and others (e.g., Evarts and Vaughn 1978; MacKinnon et al. 2000; Ravichandran et al. 2013) reported voluntary RTs to a proprioceptive stimulus to be as short as 70 ms. Assuming similar RTs when M2 and the voluntary response occurred in the same muscle, any observed M2 modulation could have resulted from overlapping voluntary activity onto the M2 response.
This overlap hypothesis was tested in an experiment by Rothwell et al. (1980). The authors manipulated predictability of the imperative (perturbation) signal, an effect known to produce voluntary RT differences (to visual or auditory stimuli), and compared modulation of M2 between a “resist” and “let-go” instruction. Larger changes to M2 period activity were observed in the conditions with more predictable perturbations (and presumably shorter RTs). It was concluded that rather than changing excitability of the reflex pathways (i.e., instruction/goal-dependent gain modulation), changes to M2 magnitude resulted primarily from the overlap of a “rapid voluntary event.” However, voluntary response magnitudes were also larger for conditions expected to have shorter RTs. Therefore, it is plausible RT differences were not present, instead only the magnitude of the voluntary response differed, generating the appearance of different voluntary latencies.
While substantial evidence exists suggesting that early voluntary responses may contribute to changes in M2 magnitude when participants actively compensate for a stretch perturbation, it remains unclear whether Hammond's (1956) original mechanism of presetting excitability of the contributing reflex pathway(s) also plays a role. The purpose of the present study was to further examine whether the changes in M2 magnitude result from instruction-dependent reflex gain modulation and/or overlapping voluntary activity. To address this question, it was imperative that we delayed the onset of the voluntary response, in turn minimizing its overlap onto M2.
One method that has been shown to increase RT (in a nonperturbation task) is to increase the movement accuracy (e.g., Fitts and Peterson 1964; Lajoie and Franks 1997; Sidaway 1991) or number of movement components (e.g., Henry and Rogers 1960; Ketelaars et al. 1997, 1999; Lajoie and Franks 1997). Previous perturbation studies, particularly those that have provided evidence of short-latency voluntary RTs, have typically used simple untargeted active responses following a perturbation (e.g., Crago et al. 1976; Day et al. 1983; Evarts and Vaughn 1978; Hammond 1956; MacKinnon et al. 2000; Manning et al. 2012; Rothwell et al. 1980). The absence of specified endpoint accuracy and/or complexity yields short RTs, which increases the probability of superposition of the voluntary response into the M2 period.
In this study we delayed voluntary responses during a perturbation paradigm by increasing either the accuracy (experiment 1) or complexity (experiment 2) requirements of the task. In experiment 1, participants reacted to a wrist extension perturbation by flexing the wrist quickly and accurately to a narrow or a wide target. Reflexive and voluntary responses from the targeted conditions were compared with untargeted active as well as passive responses (i.e., similar conditions used by groups arguing against instruction-dependent gain modulation; e.g., Crago et al. 1976; Manning et al. 2012). In experiment 2, we compared low-complexity unidirectional movements to high-complexity reversal movements. Experiment 2 had the additional goal of controlling the magnitude of the voluntary response between active conditions. Reversal movements were chosen because the beginning of the voluntary EMG response (the portion that may overlap onto M2) is similar in magnitude and shape to that of a unidirectional movement, when amplitude and size of the first target are controlled (Gottlieb 1998). We reasoned that controlling the size of the voluntary response may permit observations of voluntary RT changes in which differences in RT are not confounded by differences in response magnitude.
If superposition of the voluntary response is the main source of instruction- or goal-dependent M2 period modulation (as suggested by Crago et al. 1976; Day et al. 1983; Manning et al. 2012; Rothwell et al. 1980; and others), we expected that delaying the voluntary response in both experiments 1 and 2 would result in an M2 response approaching a similar magnitude to the passive condition. Alternatively, if reflex pathway gain modulation is the main factor (Colebatch et al. 1979; Hammond 1956; Lee and Tatton 1978), there should be minimal influence of voluntary response timing and thus we expected the size of the M2 response to change only as a categorical function of whether or not participants actively compensated for the perturbation. Finally, if M2 period activity during active conditions is due to a combination of changes in reflex gain and voluntary superposition, we expected M2 magnitudes to increase during all active conditions, compared with the passive condition, with a further increase observed in the latter half of the M2 period, when the voluntary overlap is greatest.
METHODS
Participants and Apparatus
Twenty right-handed volunteers (9 females, 11 males; 20–46 yr), free of any neuromuscular abnormalities and capable of correctly performing the various conditions, participated in at least one of three experiments lasting ∼90 min. Experimental procedures were approved by the University of British Columbia Ethics Committee, and informed written consent was collected before each testing session.
Participants were positioned in a height-adjustable chair facing an oscilloscope monitor (placed ∼1 m in front) resting on a table. Both elbows were flexed at 100°, and hands were semipronated with the wrist joints aligned with the manipulanda rotational axes. Connected to the right manipulandum was a torque motor (Aeroflex TQ 82W-1C), and a metal handle adjoined to the motor shaft was placed near the right metacarpophalangeal joints. To prevent lateral wrist movement, padded stops were adjusted on either side of the wrist. Custom-molded thermoplastic enveloped the hand and allowed movement to occur about the wrist without the fingers having to grasp the metal handle. Participants were also asked to keep the hands and fingers relaxed and to only move the right wrist joint. Angular position information pertaining to the right wrist was continuously provided on the oscilloscope as feedback. The home position was 10° of wrist flexion and visually defined on the oscilloscope by arrows. The left manipulandum was immovable and positioned at 20° of wrist extension.
All perturbation trials began with a slight extension preload ramped slowly (over 500 ms) to 0.25 Nm. Participants were instructed to resist by lightly activating wrist flexors against the load and to hold their right wrist at the home position. A random foreperiod (2,750–3,750 ms) followed onset of the preload and was terminated by a 1.5-Nm extension perturbation lasting 150 ms.
Experimental Paradigm
Experiment 1: movement accuracy.
At the start of the experiment, each participant (n = 11) was provided with ∼15 familiarization trials for each condition. In addition, five practice trials were given before the start of each condition to ensure correct performance of the required task. Each testing condition consisted of a single block of trials where the participant was instructed to either 1) “not intervene with the perturbation and slowly move back to the home position” [do not intervene/passive condition (DNI)], 2) “flex the right wrist as fast as possible following the perturbation” (active untargeted: ACT), or 3) “flex the right wrist into the target zone as fast and as accurately as possible following the perturbation” (active wide target: ACT-Wi; active narrow target: ACT-Na). A target was only visible on the oscilloscope for the two targeted conditions and was 10° wide for the ACT-Wi condition and 5° wide for ACT-Na. Both target positions were centered around 22.5° of wrist flexion. Feedback for the ACT condition involved continuously reminding participants to “react as fast as possible.” For the ACT-Wi and ACT-Na conditions, participants were reminded to respond “as fast and as accurately as possible.” Moreover, for the two targeted conditions, participants were also verbally informed by the experimenter if they either “hit the target” or “missed short or long.” This accuracy component was determined by the amplitude of the initial peak flexion displacement of the return movement in relation to the target. Collection within a block continued until at least 20 correct trials were performed (i.e., no false starts, failure to respond, or missing the center of the target by >12.5°). Block order was randomized, and a 5-min rest was provided between conditions to prevent fatigue.
Experiment 2: movement complexity.
Similar to experiment 1, participants (n = 10) were provided with ∼15 familiarization trials for each condition and five practice trials were given before the start of each condition in the testing phase. The testing conditions consisted of three blocks of trials where participants were instructed to either 1) “not intervene with the perturbation and slowly move back to the home position” (DNI), 2) “flex the right wrist into the flexion target zone as fast and as accurately as possible following the perturbation” (active narrow target: ACT-Na), and 3) or “flex the right wrist into the flexion target zone and immediately extend into the extension target zone as fast and as accurately as possible following the perturbation” (active reversal: ACT-R). The flexion target was identical to the ACT-Na target used in experiment 1. The extension target position was positioned at 2.5° of extension, and was 5° wide. When performing in the ACT-Na and ACT-R conditions, participants were continuously reminded to respond “as fast and as accurately as possible.” After each trial, participants were also informed if they either “hit the target(s)” or “missed short or long.” Block order was randomized and collection within a block continued until at least 20 correct trials were performed. A 5-min rest was provided between blocks to prevent fatigue.
Visual RT conditions.
To ensure our manipulations of movement accuracy and complexity influenced RT, each participant performed an additional visual RT task after completing the perturbation conditions. The home position was 30° of wrist extension, approximately the position the motor was expected to move the wrist to in the perturbation conditions (determined from pilot testing). The warning signal was represented by a line on the oscilloscope moving up to the home position. Following a variable foreperiod (2,750–3,750 ms), the line jumped into the centre of the flexion target area (22.5° of flexion). The participants in experiment 1 were instructed to either “flex their wrist as fast as possible after the line jump” (visual active untargeted: V-ACT) or to “flex the right wrist into the target zone as fast and as accurately as possible following the line jump” (visual active wide target: V-ACT-Wi; visual active narrow target: V-ACT-Na). The participants in experiment 2 were instructed to either “flex the right wrist into the flexion target zone as fast and as accurately as possible following the line jump” (V-ACT-Na) or “flex the right wrist into the flexion target zone and immediately extend into the extension target zone as fast and as accurately as possible following the line jump” (visual active reversal: V-ACT-R). The targets were placed in the same position as the perturbation conditions and feedback was provided on a trial-by-trial basis. Block order was randomized and participants performed 10 practice trials for one condition, immediately followed by at least 20 testing trials. The testing blocks were separated by a 5-min rest period.
Experiment 3: wrist perturbations and startle-triggered responses.
Recent work has shown that perturbations applied to the elbow (Ravichandran et al. 2013) or ankle (Campbell et al. 2013) can elicit a startle reflex, characterized by activation of sternoicleidomastoid (SCM). Important for the context of the present study, startling stimuli are known to involuntarily trigger prepared movements at short latencies (<100 ms), a phenomenon known as the StartReact effect (for reviews, see Carlsen et al. 2012; Rothwell et al. 2006; Shemmell et al. 2015). If the perturbations used in experiments 1 and 2 elicited a startle reflex and StartReact effect, potential voluntary RT differences (as observed in the Visual RT conditions) could be nullified (e.g., Maslovat et al. 2011a). Thus, to examine whether the wrist perturbations and the experimental protocols used in this study were capable of eliciting a startle reflex, we collected data from three participants (recruited for their known disposition to being startled) and monitored indicators of the startle reflex [EMG data were recorded bilaterally from sternocleidomastoid (SCM) and unilaterally from orbicularis oculi (OOc)]. Participants were positioned in a setup identical to experiments 1 and 2, with the addition of a loudspeaker placed 30 cm behind the head capable of eliciting a startling acoustic stimulus (SAS; see Forgaard et al. 2013; Maslovat et al. 2011a,b for a similar setup).
At the beginning of testing, participants were asked to remain relaxed and fixate on the oscilloscope monitor. After 5–10 s, a 120 dB (1,000 Hz, 50 ms, SAS) was delivered via the loudspeaker. This first trial was used to examine each participant's baseline auditory startle reflex in the absence of any motor preparation. Following the baseline startle trial, participants performed 15 practice trials followed by 25 testing trials in both a DNI block and an ACT (untargeted) block (identical protocols and instructions to experiment 1). To make direct comparisons with the auditory startle reflex, on five random testing trials, a 120 dB SAS was delivered simultaneous with perturbation onset.
Data Collection and Analysis
Surface EMGs were collected from the right wrist flexor (FCR) and extensor (ECR) carpi radialis muscles (as well as left OOc and bilaterally from SCM, experiment 3 only) using bipolar preamplified surface electrodes connected to an external amplifier (experiment 1: model 544, Therapeutics Unlimited, Iowa City, IA; experiments 2 and 3: model DS-80, Delsys, Natick, MA). EMGs were amplified at 2–4 K and band-pass filtered from 30 to 1,000 Hz in experiment 1 and 20 to 1,000 Hz in experiments 2 and 3. Positional data were collected using a potentiometer (model 6637S-1-103, Bourns, Riverside, CA) connected to the right wrist manipulandum. All signals were sampled at 2 kHz using a 1401Plus data acquisition system and a computer running Spike2 (CED, Cambridge, UK) software. Offline data analysis was accomplished using Spike2 and custom-written LabVIEW (National Instruments, Austin, TX) software.
At the beginning of analysis, EMG data were baseline corrected and full-wave rectified. A 700-ms window (200 ms pre- to 500 ms postperturbation) was placed around each mechanical perturbation or visual imperative signal. While error trials were recycled online during data collection, we conducted an additional check of individual trial data from EMG recordings and the displacement profiles to ensure correct performance. Good trials were submitted to an individual condition ensemble average for each participant.
For experiments 1 and 2, the ensemble averages for each perturbation condition (from each participant) were used to determine stretch reflex onset and offset times (using a similar method to MacKinnon et al. 2000; Manning et al. 2012). Mean background EMG and SD were first determined from 100 to 5 ms before delivery of the perturbation. A horizontal cursor was placed at 3SD above mean activity. The first rise in activity (∼20–35 ms postperturbation), greater than 3SD above baseline was marked as M1 onset. The onset of M2 was more difficult to determine because M1 and M2 often overlapped; thus the trough in activity between these two events (occurring around 50 ms) was marked as M2 onset. Due to overlapping voluntary activity in the active conditions, M2 offset could only be determined in the DNI conditions. This point was marked as the first decrease in activity below 3SD above baseline following M2 onset.
For the main analysis, we analyzed the ensemble wrist flexor EMG data in five time periods relative to perturbation onset. The first epoch was used to determine baseline EMG, occurring 25 ms before onset of the perturbation. The second epoch contained the short-latency (M1) response, occurring 25–50 ms postperturbation. While M2, our main response of interest, occurs 50–100 ms following the perturbation, we chose to divide this period further into M2a (50–75 ms) and M2b (75–100 ms). Finally, magnitude of the voluntary response was captured in an epoch (VOL) occurring between 100 and 200 ms postperturbation. Previous studies (e.g., Lee and Tatton 1978; Pruszynski et al. 2008, 2009; Ravichandran et al. 2013) have used a similar temporal segregation to more closely analyze the timecourse of M2 as well as the voluntary response.
Integrated area of each of the predefined epochs (Background, M1, M2a, M2b, VOL) was calculated on a trial-by-trial basis using the rectified EMG data. The means and SD from each condition were then normalized to the largest M2 value obtained from each participants' DNI ensemble average (experiment 1 DNI mean peak: 0.25 mV, SD: 0.11 mV; experiment 2 DNI mean peak: 0.18 mV, SD: 0.07 mV). In experiment 1, mean rectified background EMG was 0.021 mV (±0.009 mV) and for experiment 2 mean background EMG was 0.018 mV (±0.006 mV).
Kinematic profiles following each perturbation were also analyzed. Spike2 software was used to identify Peak Extension Amplitude and the subsequent initial Peak Flexion Amplitude on a trial-by-trial basis. These marker positions were verified visually and exported along with the associated latency values (Peak Extension Latency and Peak Flexion Latency) relative to perturbation onset.
For the visual RT conditions, we were interested in quantifying onset of voluntary activity on a trial-by-trial basis. Onset of activity in FCR (RT) was defined as the point at which rectified EMG began a sustained rise above baseline levels (−100 to −5 ms before the imperative signal). The location of this point was determined by displaying FCR activity on a monitor with a superimposed horizontal cursor indicating 3SD above baseline activity. A vertical cursor was placed at the first point (after the imperative signal) that activity increased above the horizontal cursor and remained above this level for >5 ms.
Identical procedures were used to determine the onset of activity in startle indicator muscles (L-SCM, R-SCM, and OOc) for experiment 3. The startle reflex elicited by either auditory or somatosensory stimuli is characterized by early activity in OOc and symmetric bilateral bursts in the left and right SCM. Thus for a trial to be considered as showing a positive startle reflex, both left and right SCM as well as OOc had to be activated within 120 ms of the SAS or the perturbation (Álvarez-Blanco et al. 2009; Brown et al. 1991; Carlsen et al. 2007, 2011).
Statistical Analysis
Kinematic landmarks (Peak Extension Amplitude, Peak Extension Latency, Peak Flexion Amplitude, Peak Flexion Latency) and integrated normalized EMG data from the first four epochs of interest (Background, M1, M2a, M2b) were analyzed using four-condition (experiment 1: DNI, ACT, ACT-Wi, ACT-Na) and three-condition (experiment 2: DNI, ACT-Na, ACT-R) repeated-measures ANOVA. Because a voluntary response was not present for the DNI conditions, integrated normalized EMG data for the VOL epoch was analyzed using a three-condition (experiment 1: ACT, ACT-Wi, ACT-Na) repeated-measures ANOVA or a paired-samples t-test (experiment 2: ACT-Na, ACT-R). Trend analysis (experiment 1) and a paired-sample t-test (experiment 2) were used to analyze the visual RT data. For the ANOVA results, to adjust any violation to the assumption of sphericity, Greenhouse-Geiser corrected P values were reported, along with the uncorrected degrees of freedom. Partial eta squared (ηp2) was used to convey effect sizes and post hoc analyses were performed using the Dunn-Bonferonni corrected t-test. For all measures, the level of statistical significance was set at P = 0.05.
RESULTS
Experiment 1
Behavioral differences in visual RT.
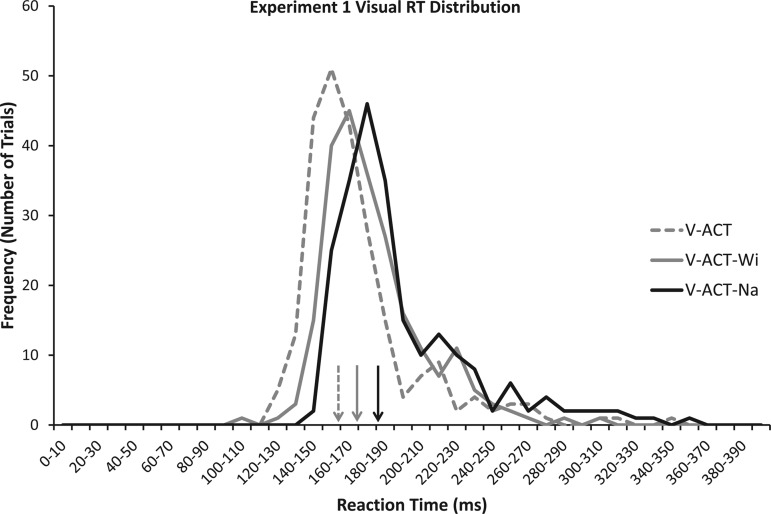
In the visual RT experiment, we found a significant linear trend among the three conditions [F(2,20) = 30.61, P < 0.001, ηp2 = 0.75]. Mean RT was shortest for untargeted condition (V-ACT: 168.9 ms), increased ∼10 ms when participants aimed for the 10° target (V-ACT-Wi: 179.2 ms), and increased another ∼10 ms when aiming for the narrow (5°) target (V-ACT-Na: 190.8 ms). Figure 1 shows a distribution of visual RTs from the three different accuracy conditions.
Fig. 1.
Reaction time frequency distributions of the visual reaction time (RT) conditions in experiment 1. Mean values are displayed by vertical arrows above the x-axis. Note how the distribution (and mean value) for the visual active untargeted (V-ACT; untargeted, dashed grey line/arrow) condition is earliest, followed by visual active wide target (V-ACT-Wi; wide target, solid grey line/arrow). The longest RTs were observed in the visual active narrow target (V-ACT-Na) condition (solid black line/arrow).
Kinematics following a rapid wrist perturbation.
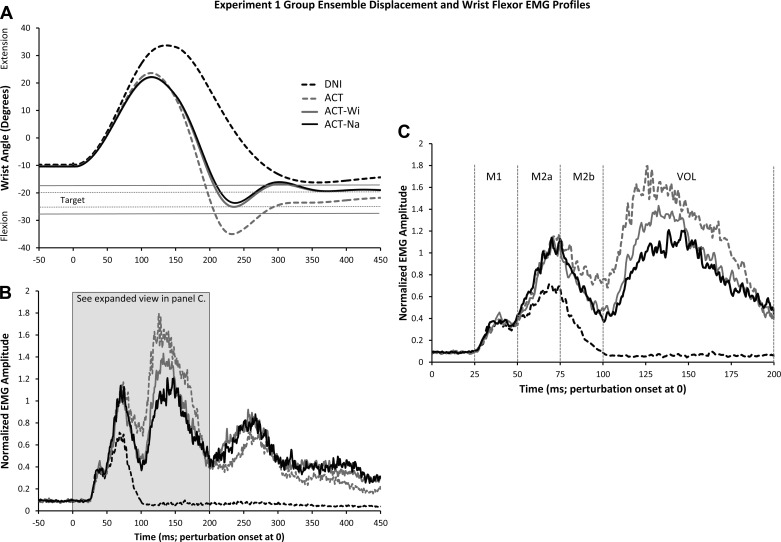
The rapid wrist perturbation induced large changes in the angle of the wrist; however, the intention to respond actively changed the four kinematic measures examined. Figure 2A shows the group ensemble displacement profiles for the various perturbation conditions (also see Table 1 for mean values and statistical results of all comparisons). The DNI condition resulted in greatest (34.9°) Peak Extension Amplitude [F(3,30) = 43.11, P < 0.001, ηp2 = 0.81] at a significantly longer latency [134.2 ms; F(3,30) = 35.17, P < 0.001, ηp2 = 0.78]. No significant differences with regards to Peak Extension were observed among the three active conditions (ACT: 24.3°, 112.1 ms; ACT-Wi: 22.9°, 112.6 ms; ACT-Na: 22.9°, 112.6 ms). A different pattern of results was observed when Peak Flexion Amplitude [F(3,30) = 30.82, P < 0.001, ηp2 = 0.76] was examined. Participants moved furthest into flexion (P values <0.001) when actively responding as fast as possible but not aiming for a target (ACT: −39.8°). Even though the target zones were centered about the same position for the two targeted conditions, participants consistently moved further into flexion when aiming for the wide target (P = 0.045; ACT-Wi: −27.7°; ACT-Na: −25.9°). Despite differences in flexion amplitude among the three active conditions, Peak Flexion Latency did not differ significantly (P values ≥0.88; ACT: 232.0 ms; ACT-Wi: 226.4 ms; ACT-Na: 239.5 ms). The active conditions did, however, differ from DNI, where peak flexion was reached significantly later [381.2 ms; P values <0.002, F(3,30) = 30.46, P < 0.001, ηp2 = 0.75].
Fig. 2.
Group ensemble displacement and wrist flexor EMG data for the perturbation conditions in experiment 1. A: wrist displacement data, normalized by participant (n = 11). Do not intervene (DNI) condition: dashed black line. ACT: dashed grey. ACT-Wi: solid grey. ACT-Na: solid black. Positive values signify wrist extension and negative values wrist flexion. Target positions are represented by the solid black lines (wide target) and dashed black lines (narrow target). B: group ensemble EMG data, normalized by participant. EMG amplitude value of 1 corresponds to peak reflex value from DNI condition from each participant. C: expanded view of the epochs of interest shown in B.
Table 1.
Results from experiment 1
| Means (SD) |
Omnibus ANOVA |
Post Hoc |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | DNI | ACT | ACT-Wi | ACT-Na | df | F | P | ηp2 | DNI | ACT | ACT-Wi | ACT-Na |
| Peak Extension, ° | 34.9 (7.3) | 24.3 (7.3) | 22.9 (8.0) | 22.9 (7.0) | 3, 30 | 43.11 | <0.001 | 0.81 | ||||
| DNI | — | <0.001 | <0.001 | <0.001 | ||||||||
| ACT | — | 0.35 | 0.16 | |||||||||
| ACT-Wi | — | >0.99 | ||||||||||
| ACT-Na | — | |||||||||||
| Peak Extension Latency, ms | 134.2 (12.6) | 112.1 (8.8) | 112.6 (9.7) | 112.6 (8.6) | 3, 30 | 35.17 | <0.001 | 0.78 | ||||
| DNI | — | 0.001 | <0.001 | 0.001 | ||||||||
| ACT | — | >0.99 | >0.99 | |||||||||
| ACT-Wi | — | >0.99 | ||||||||||
| ACT-Na | — | |||||||||||
| Peak Flexion, ° | −18.5 (6.9) | −39.8 (6.0) | −27.7 (1.8) | −25.9 (2.3) | 3, 30 | 30.82 | <0.001 | 0.76 | ||||
| DNI | — | <0.001 | 0.032 | 0.072 | ||||||||
| ACT | — | <0.001 | <0.001 | |||||||||
| ACT-Wi | — | 0.045 | ||||||||||
| ACT-Na | — | |||||||||||
| Peak Flexion Latency, ms | 381.2 (78.8) | 232.0 (16.2) | 226.4 (35.9) | 239.5 (17.0) | 3, 30 | 30.46 | <0.001 | 0.75 | ||||
| DNI | — | 0.001 | 0.001 | 0.002 | ||||||||
| ACT | — | >0.99 | 0.88 | |||||||||
| ACT-Wi | — | >0.99 | ||||||||||
| ACT-Na | — | |||||||||||
| M1 onset, ms | 29.5 (3.0) | 29.5 (3.1) | 29.3 (2.2) | 29.5 (2.5) | 3, 30 | 0.08 | 0.97 | <0.01 | ||||
| DNI | — | — | — | — | ||||||||
| ACT | — | — | — | |||||||||
| ACT-Wi | — | — | ||||||||||
| ACT-Na | — | |||||||||||
| M2 onset, ms | 48.6 (3.3) | 48.4 (3.0) | 49.0 (3.7) | 49.2 (3.1) | 3, 30 | 0.81 | 0.50 | 0.08 | ||||
| DNI | — | — | — | — | ||||||||
| ACT | — | — | — | |||||||||
| ACT-Wi | — | — | ||||||||||
| ACT-Na | — | |||||||||||
| M2 offset, ms | 94.2 (8.4) | — | — | — | — | — | — | — | ||||
| DNI | — | — | — | — | ||||||||
| ACT | — | — | — | |||||||||
| ACT-Wi | — | — | ||||||||||
| ACT-Na | — | |||||||||||
| Baseline EMG Activity, NU·ms | 2.1 (1.3) | 2.2 (1.0) | 2.2 (1.1) | 2.2 (1.0) | 3, 30 | 0.33 | 0.81 | 0.03 | ||||
| DNI | — | — | — | — | ||||||||
| ACT | — | — | — | |||||||||
| ACT-Wi | — | — | ||||||||||
| ACT-Na | — | |||||||||||
| M1 activity, NU·ms | 7.3 (3.6) | 7.3 (3.2) | 7.7 (3.0) | 7.7 (3.0) | 3, 30 | 0.52 | 0.67 | 0.05 | ||||
| DNI | — | — | — | — | ||||||||
| ACT | — | — | — | |||||||||
| ACT-Wi | — | — | ||||||||||
| ACT-Na | — | |||||||||||
| M2a activity, NU·ms | 14.0 (2.4) | 20.1 (3.8) | 19.2 (4.1) | 20.4 (6.0) | 3, 30 | 12.76 | <0.001 | 0.56 | ||||
| DNI | — | 0.001 | 0.003 | 0.009 | ||||||||
| ACT | — | >0.99 | >0.99 | |||||||||
| ACT-Wi | — | >0.99 | ||||||||||
| ACT-Na | — | |||||||||||
| M2b activity, NU·ms | 7.6 (3.4) | 21.8 (7.7) | 17.0 (6.6) | 17.6 (6.7) | 3, 30 | 33.84 | <0.001 | 0.77 | ||||
| DNI | — | <0.001 | 0.001 | <0.001 | ||||||||
| ACT | — | 0.039 | 0.021 | |||||||||
| ACT-Wi | — | >0.99 | ||||||||||
| ACT-Na | — | |||||||||||
| VOL activity, NU·ms | 6.9 (3.2) | 118.7 (41.8) | 93.0 (42.2) | 81.8 (33.8) | 2, 20 | 17.75 | <0.001 | 0.64 | ||||
| DNI | — | — | — | — | ||||||||
| ACT | — | 0.008 | 0.003 | |||||||||
| ACT-Wi | — | 0.035 | ||||||||||
| ACT-Na | — | |||||||||||
Means (SD) and statistical results from experiment 1. NU, normalized unit [value of 1 corresponds to peak reflex EMG value from (do not intervene) DNI condition]; NU·ms, integrated area of normalized EMG data; ACT, active untargeted; ACT-Wi, active wide target; ACT-Na, active narrow target; VOL, voluntary response epoch; M1, short latency; M2, long latency. Following a significant omnibus test, post hoc testing was conducted using the Dunn-Bonferroni corrected t-test.
EMG activity following a rapid wrist perturbation.
Rapidly perturbing the right wrist into extension evoked at least two clear responses in the EMG recording from wrist flexors. Figure 2, B and C, shows group ensemble averages from each condition (also see mean values and a statistical summary in Table 1). The first response, M1, had mean onset latencies of 29.5 (±3.0) ms, 29.5 (±3.1) ms, 29.3 (±2.2) ms, and 29.5 (±2.5) ms for the DNI, ACT, ACT-Wi, and ACT-Na conditions, respectively. Corresponding onset times for the second response, M2, were 48.6 (±3.4) ms, 48.4 (±3.1) ms, 49.0 (±3.7) ms, and 49.2 (±3.2) ms. M2 offset was obtained only for the passive condition and was 94.2 (±8.4) ms. The voluntary response was observed only for the active conditions. As the voluntary response sometimes began earlier than 100 ms and overlapped with the end of M2, we could not determine its exact onset latency nor could we mark the end of M2 for these conditions.
Before each perturbation, a slight extension preload was applied by the motor. To counter the load, and maintain the limb at the home position, participants generated a small contraction in wrist flexors. This preperturbation muscle activity did not differ significantly among DNI, ACT, ACT-Wi, and ACT-Na [F(3,30) = 0.33, P = 0.81, ηp2 = 0.03]. Likewise, EMG activity during the M1 period was not statistically different across the four conditions [F(3,30) = 0.52, P = 0.67, ηp2 = 0.05].
While preparing to respond against the perturbation did not influence background activity or the short-latency response, marked differences were observed between the passive and active conditions for the epochs containing the long-latency response (see Fig. 2, B and C). For the first half of M2 (M2a), a significant main effect was found [F(3,30) = 12.76, P < 0.001, ηp2 = 0.56]. Post hoc tests revealed increased activity for the active conditions [ACT: 20.1 normalized units (NU)·ms; ACT-Wi: 19.2; ACT-Na = 20.4 NU·ms] compared with passive (DNI = 14.0 NU·ms). No significant differences were found among the three active conditions (P values >0.99). Analysis of the latter half of M2 (M2b) also revealed EMG differences [F(3,30) = 33.84, P < 0.001, ηp2 = 0.77], but post hoc tests uncovered a different pattern of results. As expected, the DNI instruction resulted in the lowest level of activity (M = 7.6 NU·ms; P values ≤0.001). Asking the participants to flex the wrist as fast as possible (ACT) produced the highest level of activity (M = 21.8 NU·ms; P values ≤0.04), while having the participants flex the wrist with an accuracy component produced significantly less activity (ACT-Wi: 17.0 NU·ms; ACT-Na: 17.6 NU·ms). As we will argue, the increased activity observed in the M2b period for the ACT condition resulted from a larger and likely shorter latency voluntary response, producing increased superposition onto the end of the M2 response.
While modulation of EMG activity was clearly observed during the epochs containing the M2 response, we also found significant EMG differences among active conditions in the VOL (100–200 ms) epoch [F(2,20) = 17.75, P < 0.001, ηp2 = 0.64]. Post hoc analysis revealed significant differences (P values ≤0.035) among all three active conditions (see Fig. 2, B and C). Activity was highest in the ACT condition (118.7 NU·ms), lowest for ACT-Na (81.8 NU·ms), and intermediary for the ACT-Wi condition (93.0 NU·ms).
Experiment 2
Behavioral differences in visual RT.
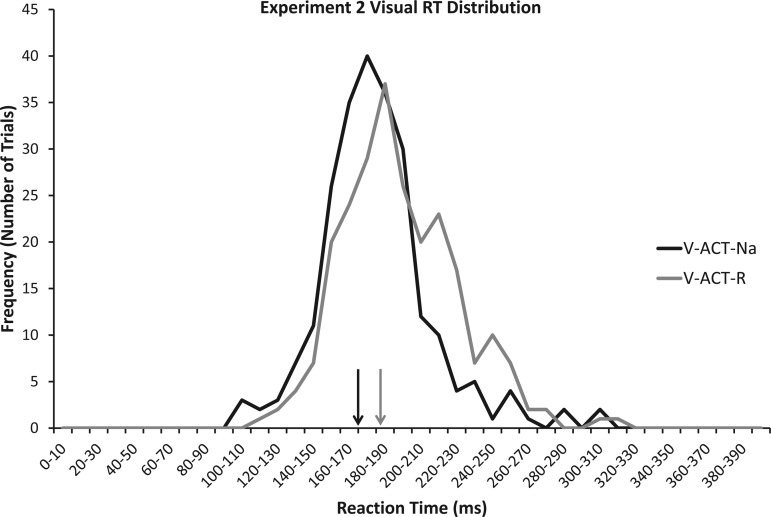
We observed significant RT differences between the unidirectional and reversal conditions [t(9) = 3.2, P = 0.011]. As expected, mean RT was significantly longer for the reversal movement (V-ACT-R: 193.1 ms) compared with the unidirectional movement (V-ACT-Na: 180.7 ms). Figure 3 shows a distribution of visual RTs for the two conditions. Figure 3 also shows that the mean RT for the ACT-Na condition was ∼10 ms earlier than it was for experiment 1.
Fig. 3.
RT frequency distributions of the visual RT conditions in experiment 2. Mean values are displayed by vertical arrows above the x-axis. Note how the distribution (and mean RT value) for the V-ACT-Na (unidirectional movement, black line/arrow) condition is earlier than the V-ACT-R (reversal movement, solid grey line/arrow) condition.
Kinematics following a rapid wrist perturbation.
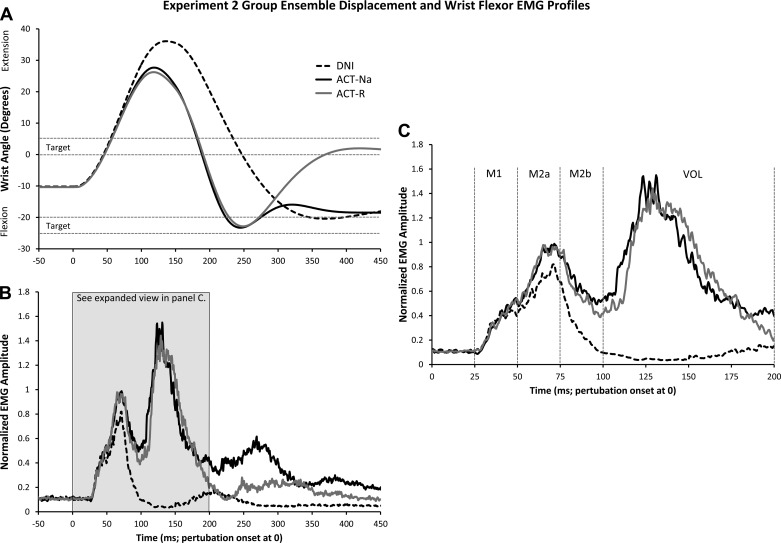
Similar to experiment 1, the perturbation produced large excursions in wrist angle, and the intention to respond influenced all kinematic measures examined (see Fig. 4A and Table 2). Peak Extension Amplitude was significantly larger [F(2,18) = 50.96, P < 0.001, ηp2 = 0.85] and occurred later [F(2,18) = 31.23, P < 0.001, ηp2 = 0.78] for the DNI condition (P values = 0.001; 36.8°, 137.0 ms). There were no differences between the active conditions (ACT-Na: 27.9°, 117.5 ms; ACT-R: 26.5°, 117.0 ms). Analysis of Peak Flexion also revealed differences in the Amplitude [F(2,18) = 13.34, P = 0.002, ηp2 = 0.60] and Latency [F(2,18) = 85.85, P < 0.001, ηp2 = 0.91]. Values obtained for the DNI condition were latest (377.9 ms) and of smallest amplitude (−21.7°). As expected, because participants aimed for the same flexion target in the ACT-Na and ACT-R conditions, Peak Flexion Amplitude did not differ significantly (P = 0.32). Although also not statistically significant (P = 0.10), the latency of Peak Flexion was ∼6 ms later for the ACT-R condition. As will be argued in the discussion, this small latency difference (see Fig. 3A) in reaching peak flexion might provide indirect evidence of a RT difference between ACT-R and ACT-Na. For the ACT-R condition, we also calculated measures associated with the second extension (i.e., reversal) movement. The target was placed from 0 to 5° of extension, and participants reached an average wrist angle of 4.0° of extension, 394.9 ms after perturbation onset.
Fig. 4.
Group ensemble displacement and wrist flexor EMG data for the perturbation conditions in experiment 2. A: wrist displacement data, normalized by participant (n = 10). DNI condition: dashed black line. ACT-Na: solid black. ACT-R: grey. Positive values signify wrist extension and negative values wrist flexion. Target positions are represented by the dashed black lines. B: group ensemble EMG data, normalized by participant. EMG amplitude value of 1 corresponds to peak reflex value from DNI condition from each participant. C: expanded view of the epochs of interest shown in B.
Table 2.
Results from experiment 2
| Means (SD) |
Omnibus ANOVA |
Post Hoc |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | DNI | ACT-Na | ACT-R | df | F | P | ηp2 | DNI | ACT-Na | ACT-R |
| Peak Extension, ° | 36.8 (2.9) | 27.9 (3.1) | 26.5 (3.1) | 2, 18 | 50.96 | <0.001 | 0.85 | |||
| DNI | — | <0.001 | <0.001 | |||||||
| ACT-Na | — | 0.18 | ||||||||
| ACT-R | — | |||||||||
| Peak Extension Latency, ms | 137.0 (8.9) | 117.5 (4.2) | 117.0 (4.4) | 2, 18 | 31.23 | <0.001 | 0.78 | |||
| DNI | — | 0.001 | 0.001 | |||||||
| ACT-Na | — | >0.99 | ||||||||
| ACT-R | — | |||||||||
| Peak Flexion, ° | −21.7 (3.8) | −26.4 (1.7) | −27.5 (1.7) | 2, 18 | 13.34 | 0.002 | 0.60 | |||
| DNI | — | 0.033 | 0.005 | |||||||
| ACT-Na | — | 0.32 | ||||||||
| ACT-R | — | |||||||||
| Peak Flexion Latency, ms | 377.9 (35.2) | 245.1 (16.1) | 251.0 (17.9) | 2, 18 | 85.85 | <0.001 | 0.91 | |||
| DNI | — | <0.001 | <0.001 | |||||||
| ACT-Na | — | 0.105 | ||||||||
| ACT-R | — | |||||||||
| M1 onset, ms | 29.7 (2.0) | 30.3 (1.6) | 30.0 (2.1) | 2, 18 | 1.40 | 0.27 | 0.14 | |||
| DNI | — | — | — | |||||||
| ACT-Na | — | — | ||||||||
| ACT-R | — | |||||||||
| M2 onset, ms | 50.4 (3.9) | 50.9 (4.3) | 50.6 (3.8) | 2, 18 | 0.32 | 0.71 | 0.03 | |||
| DNI | — | — | — | |||||||
| ACT-Na | — | — | ||||||||
| ACT-R | — | |||||||||
| M2 offset, ms | 93.7 (5.9) | — | — | — | — | — | — | |||
| DNI | — | — | — | |||||||
| ACT-Na | — | — | ||||||||
| ACT-R | — | |||||||||
| Baseline EMG activity, NU·ms | 2.8 (1.2) | 2.6 (1.1) | 2.6 (0.9) | 2, 18 | 1.48 | 0.26 | 0.14 | |||
| DNI | — | — | — | |||||||
| ACT-Na | — | — | ||||||||
| ACT-R | — | |||||||||
| M1 activity, NU·ms | 7.8 (2.6) | 7.8 (2.2) | 7.8 (2.1) | 2, 18 | <0.01 | 0.98 | <0.01 | |||
| DNI | — | — | — | |||||||
| ACT-Na | — | — | ||||||||
| ACT-R | — | |||||||||
| M2a activity, NU·ms | 15.5 (2.5) | 19.2 (4.6) | 19.4 (3.1) | 2, 18 | 7.76 | 0.004 | 0.46 | |||
| DNI | — | 0.063 | 0.014 | |||||||
| ACT-Na | — | >0.99 | ||||||||
| ACT-R | — | |||||||||
| M2b activity, NU·ms | 7.2 (1.9) | 15.9 (6.2) | 14.4 (4.7) | 2, 18 | 15.51 | 0.001 | 0.63 | |||
| DNI | — | 0.010 | 0.003 | |||||||
| ACT-Na | — | 0.71 | ||||||||
| ACT-R | — | |||||||||
| VOL activity, NU·ms | 7.5 (4.2) | 80.2 (37.9) | 78.5 (44.8) | t(9) | 0.34 | 0.74 | — | |||
| DNI | — | — | — | |||||||
| ACT-Na | — | — | ||||||||
| ACT-R | — | |||||||||
Means (SD) and statistical results from experiment 2; ACT-R, reversal movement. Following a significant omnibus test, post hoc testing was conducted using the Dunn-Bonferroni corrected t-test.
EMG activity following a rapid wrist perturbation.
Figure 4, B and C, shows group ensemble EMG averages from each condition (also see values and a statistical summary in Table 2). M1 had mean onset latencies of 29.7 (±2.0) ms, 30.3 (±1.6) ms, and 30.0 (±2.1) ms for the DNI, ACT-Na, and ACT-R conditions, respectively. Onset times for M2 were 50.4 (±3.9) ms, 50.9 (±4.3) ms, and 50.6 (±3.8) ms. M2 offset was only obtained for DNI and was 93.7 (±5.9) ms. The third response, representing voluntary activity, was observed during the ACT-Na and ACT-R conditions. As this response was often continuous with the end of M2, we did not mark its onset latency, or the offset of M2 for these conditions. However, because the magnitude of activity during the VOL epoch did not differ, and the voluntary response appeared of similar magnitude and shape (see Fig. 4, B and C), we inferred a small voluntary response latency shift between active conditions (see below).
Analysis of EMG activity during the preperturbation and reflex periods replicated the findings of experiment 1. No significant differences were found for the preperturbation [F(2,18) = 1.48, P = 0.26, ηp2 = 0.14] or M1 epochs [F(2,18) < 0.01, P = 0.98, ηp2 < 0.01]; however, differences were found for the epochs containing the M2 response [M2a: F(2,18) = 7.76, P = 0.004, ηp2 = 0.46; M2b: F(2,18) = 15.51, P = 0.001, ηp2 = 0.63]. Activity during the M2a and M2b epochs was larger for both active conditions compared with DNI, but the two active conditions (ACT-Na, ACT-R) did not differ significantly (P values ≥0.71; see Fig. 4, B and C, and Table 2).
Similar to the M2b period, analysis of EMG activity for the VOL epoch revealed no significant difference between the two active conditions [t(9) = 0.34, P = 0.74]. Furthermore, inspection of the group ensemble EMG profiles (see Fig. 4, B and C) revealed a comparable magnitude voluntary response between ACT-Na and ACT-R. Because voluntary response magnitude was similar, we examined the group EMG ensemble to infer a latency shift (see Fig. 4, B and C). Indeed the voluntary response for the ACT-R (solid grey line) condition appeared delayed relative to the ACT-Na (solid black line) condition. Despite inferred differences in voluntary response onset, M2b activity did not differ significantly between the active conditions (P = 0.71), suggesting minimal voluntary superposition onto the end of M2.
Experiment 3
Wrist perturbations and startle triggered responses.
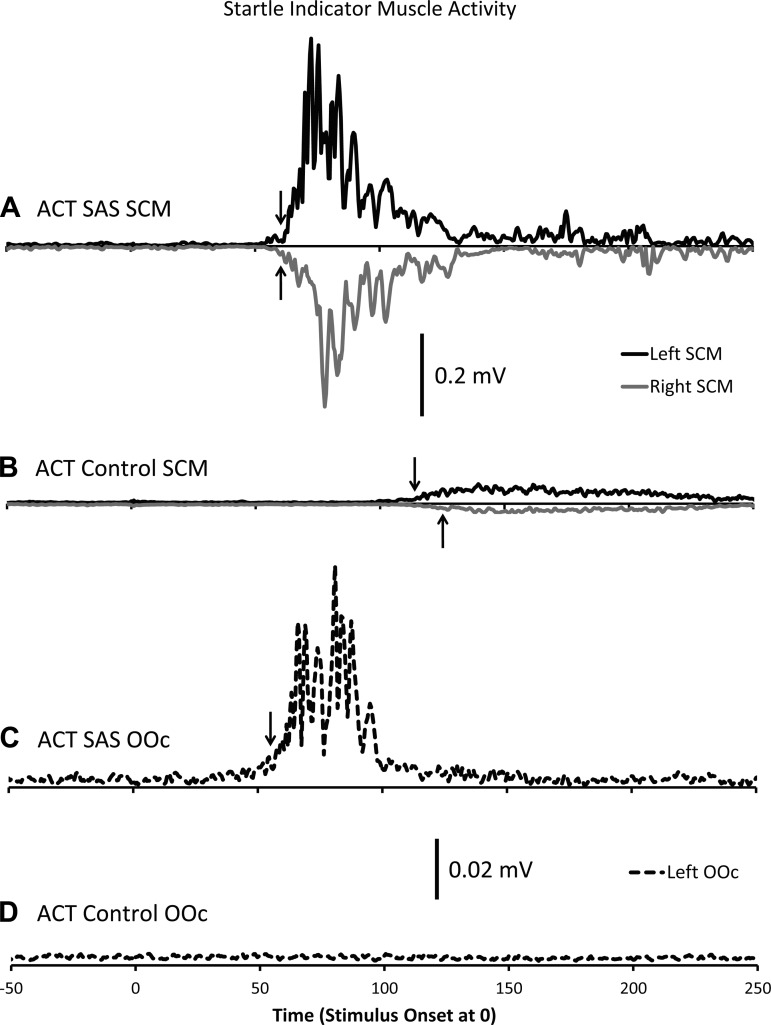
All participants displayed positive indicators of a startle reflex on the control trial. After onset of the SAS, OOc was activated first at a mean latency of 37 ms followed by bursts in left and right SCM at 58 and 60 ms, respectively. For the DNI perturbation-only trials, we found no incidence of activity in either SCM or OOc. However, when a SAS was delivered simultaneous with a perturbation (DNI + SAS), 93.3% of trials displayed a positive startle reflex. OOc was activated at a mean onset of 43 ms (mean intraparticipant SD = 5.1 ms), followed by right SCM at 65 (±2.9) ms and left SCM at 66 (±3.8) ms.
Of primary interest in this experiment was startle reflex incidence during ACT perturbation-only trials. Based on the criteria of bilateral SCM and OOc activation within 120 ms of the stimulus (in this case the wrist perturbation), none of the trials were classified as showing a startle reflex. We did note a small general increase in late SCM activity (often greater than 120 ms); however, left and right SCM were not activated simultaneously, and this activity was never accompanied by OOc activity (see Fig. 5, B and D). This is in stark contrast to the ACT + SAS trials where a SAS was delivered simultaneous with the perturbation, in which 100% of trials showed indicators of a startle reflex, with OOc activated first at a mean latency of 44 (±6.7 ms), followed by left SCM at 56 (±3.6) ms and right SCM at 57 (±3.4) ms (see Fig. 5, A and C). The findings of this control experiment suggested that the perturbations and the predictable presentation protocol we used in experiments 1 and 2 did not evoke a startle reflex. We were therefore confident that the voluntary responses observed in experiments 1 and 2 were not responses triggered involuntarily by the startle reflex (i.e., no StartReact effect).
Fig. 5.
Ensemble EMG data from an exemplar participant in a control experiment conducted to determine whether the perturbations used in experiments 1 and 2 were capable of eliciting a startle reflex. Solid black: left sternocleidomastoid (SCM); solid grey: right SCM; dashed black: left orbicularis oculi (OOc). Black arrows denote mean onset time. A: average SCM data from 5 ACT trials where a startling auditory stimulus (SAS) was unexpectedly paired with the perturbation. B: average SCM data from 25 ACT trials without a SAS (identical to ACT trials in experiment 1). C: average OOc data from the same trials as A. D: average OOc data from same trials as in B. Note how trials with the SAS (A and C) have a burst in left OOc, followed by large bilateral bursts in SCM. This is in contrast to the control ACT trials (B and D) where no OOc activity was observed and activity in left and right SCM were small and not activated symmetrically.
DISCUSSION
An extension perturbation applied to the wrist-evoked short- and long-latency responses in the stretched wrist flexors. When participants acted against the perturbation, the short-latency response was unaffected, while activity during the long-latency response period increased and was followed by a voluntary response. This study was primarily concerned with whether changes in activity during the M2 period result from instruction- or goal-dependent reflex gain modulation, superposition of the voluntary response, or a combination of the two mechanisms. By employing behavioral manipulations that are known to influence voluntary RT, we varied the degree to which the voluntary response overlapped with the epochs containing the long-latency response. We found that activity during the first half of the M2 epoch (M2a) was influenced by the intention to respond but was not sensitive to the latency or magnitude of the voluntary response (see Figs. 2, B and C, and 4, B and C). Similarly, activity during the latter half of the M2 epoch (M2b) was greater when participants compensated for the perturbation. However, unlike M2a activity, under certain conditions (i.e., ACT condition, experiment 1), M2b was also influenced by the voluntary response. Our findings support a hybrid mechanism in which both reflex gain modulation and a superpositioned voluntary response can influence EMG activity during the long-latency response period. Moreover, these findings showcase goal-dependent wrist flexor M2 period modulation in the absence of a startle reflex or StartReact effect.
RT, Triggered Reactions, and Modulation of the Long-Latency Reflex
Researchers examining the long-latency response have long understood the importance of estimating voluntary RT in perturbation paradigms. While the seminal work by Hammond (1956) calculated proprioceptive RT at ∼100 ms, subsequent studies have reported RT values in response to muscle stretch at latencies as short as ∼70 ms (e.g., Evarts and Granit 1976, 1978; MacKinnon et al. 2000; Manning et al. 2012; Ravichandran et al. 2013). While we are not arguing that voluntary superposition cannot play a role in M2 period modulation, we found clear modulation of M2 activity at a latency (50–75 ms) shorter than the earliest reported voluntary response. Moreover, modulation of M2a period activity was similar across all active conditions, which (as argued below) exhibited different amounts of voluntary response superposition during the M2b period. Previous studies have shown sub-70-ms M2 changes between a “let-go” and “compensate” task (Colebatch et al. 1979) or a target analog version of the verbal “do-not intervene” vs. “compensate” (e.g., Pruszynski et al. 2008; Yang et al. 2011) or even between two “do-not intervene” conditions that differed only in the stability of the environment in which they were performed (Shemmell et al. 2009). However, a majority of studies argue against instruction/goal-dependent modulation of the long-latency response compared the verbal “do-not intervene” to a “compensate or resist” instruction (e.g., Crago et al. 1976; Manning et al. 2012; Ravichandran et al. 2013; Shemmell et al. 2009). These studies have often only found modulation during the M2b period, thus attributing the findings to voluntary response superposition. Pruszynski et al. (2008) suggested there may be ambiguity associated with verbal instructions and participants may interpret instructions differently. Even though we made comparisons using the verbal “do-not intervene” instruction with various active conditions, we have provided clear evidence of instruction-dependent M2a period modulation, a finding in line with the recent work using targets (as opposed to verbal instructions) to specify participant behavior (e.g., Pruszynski et al. 2008; Yang et al. 2011).
The manipulations of accuracy requirements (experiment 1) and response complexity (experiment 2) were intended to influence the latencies of the voluntary response. Analysis of EMG data from our visual RT conditions confirmed that these manipulations of response requirements resulted in significant voluntary RT differences ranging from ∼10 to ∼20 ms in response to a visual stimulus (see Figs. 1 and 3). However, analysis of the voluntary responses to the stretch perturbations indicated that the perturbations resulted not only in shorter voluntary response onsets but also may have compressed the magnitude of RT differences between some of the active conditions. To infer voluntary latency differences between perturbation conditions, we examined the interaction between wrist kinematics, voluntary response magnitude, and magnitude of activity in the M2b epoch.
The perturbation we applied moved the wrist into extension; however, the intention to compensate resulted in a smaller (and shorter latency) Peak Extension Amplitude compared with the passive motion in the DNI condition. This finding is in line with work showing the urgency or intention to respond to a perturbation can influence peak joint excursion (Crevecoeur et al. 2013). However, we found no significant differences between active conditions (in both experiments 1 and 2) in terms of the joint angle or latency at which peak extension was reached (see Figs. 2A and 4A), suggesting a similar level of urgency when compensating for the perturbation. There were differences in Peak Flexion Amplitude, depending on the active condition. When there was no accuracy requirement (ACT, experiment 1), participants moved further into flexion compared with when a target was present (ACT-Wi and ACT-Na, experiment 1). Despite differences in Peak Flexion Amplitude among all three active conditions in experiment 1 (ACT > ACT-Wi > ACT-Na), peak flexion positions were reached at similar latencies (P values >0.80). This was likely a result of RT differences and the corresponding change in the magnitude of the voluntary response among conditions (ACT > ACT-Wi > ACT-Na). Certainly both shorter RTs and larger voluntary responses can account for why participants moved further distances in a similar period of time. In experiment 2, when subjects had to move to the same initial target position (ACT-Na vs. ACT-R), we found no significant differences in flexion amplitudes nor in the magnitudes of the voluntary responses. We did, however, note a small increase in Peak Flexion Latency, being ∼6 ms later for the ACT-R condition (see Fig. 4A). Although this difference only approached statistical significance (P = 0.10), 6 ms was similar to the difference observed between onsets of the voluntary responses in the ensemble EMG profiles (see Fig. 4, B and C, solid grey vs. solid black profiles).
If the voluntary responses to the perturbations were not sensitive to our response accuracy/complexity manipulations, but instead were automatically “triggered” by the stretch perturbation (e.g., Crago et al. 1976; Evarts and Granit 1976; Evarts and Vaughn 1978; Houk 1978; Ravichandran et al. 2013; Shemmell 2015), similar RT latencies would be expected among the three active conditions in experiment 1 and the two active conditions from experiment 2. Because the magnitude of EMG activity during the VOL epoch differed between active conditions in experiment 1, the triggered response hypothesis would predict these differences to also appear in the M2b period. However, we only found increased M2b activity for the ACT condition, whereas the values obtained for ACT-Wi vs. ACT-Na did not differ. We can interpret this finding as further evidence of response latency differences between active conditions. Similar to the visual RT data, the ACT condition had what appeared to be the earliest (and largest) voluntary response and thus exhibited the greatest degree of superposition. Adding an accuracy component delayed the voluntary response, thus minimizing superposition onto the end of M2. Furthermore, because magnitude of activity during the VOL epoch differed between targeted conditions (ACT-Wi and ACT-Na), but M2b period activity did not, we reason these two targeting conditions may have had minimal overlap from the voluntary response in the M2b period.
While the visual RT data from experiment 1 demonstrated differences ranging from 10 to 20 ms (V-ACT < V-ACT-Wi < V-ACT-Na; see Fig. 1), closer analysis of the M2b period for the perturbation conditions revealed a selective increase in activity for the ACT condition occurring 20 to 25 ms earlier than ACT-Wi or ACT-Na (see Fig. 2, B and C). Typically when RT is reduced (in this situation from the visual to proprioceptive imperative signal), differences in RT between conditions may also be expected to decrease (e.g., Maslovat et al. 2011a). This raises an intriguing question as to why the temporal separation between ACT and the two targeting conditions may have increased. One possibility is the presence of a “triggered response” in the ACT condition but not for ACT-Wi or ACT-Na. Although the definition of a triggered response varies in the literature, in the context of studies that have examined M2, the triggered response is believed to be the voluntary response elicited at shortened latency (see Lewis et al. 2006; Manning et al. 2012). The ACT condition in our study was similar to that used by studies that have proposed the concept of a triggered response (i.e., untargeted movement with participants instructed to respond as quickly as possible; e.g., Crago et al. 1976; Evarts and Granit 1976; Evarts and Vaughn 1978). However, exactly why we would observe a triggered response for the untargeted condition but not the conditions with imposed accuracy constraints remains unclear. As an alternative mechanism, the magnitude of the long-latency response may have scaled with the intended distance to move against the perturbation. This may be similar to the recent work showing the size of the long-latency response in elbow flexors can increase (or decrease) with the distance moved against (or with) an elbow perturbation (Pruszynski et al. 2008). In experiment 1 of the present study, participants moved the wrist ∼15° further into flexion in the ACT condition, compared with ACT-Wi and ACT-Na. However, despite these large kinematic differences, M2a activity remained similar among the three active conditions suggesting the differences in the M2b period may not have been from scaling of the long-latency response. Future studies may be needed to further distinguish whether modulation of M2 period activity results from overlap of a triggered response or scaling of the long-latency response magnitude with intended movement distance.
Although an exact mechanism for a perturbation “triggered response” and how it differs from a voluntary response remains elusive in the literature (e.g., see Crago et al. 1976; Evarts and Vaughn; Lewis et al. 2006; Manning et al. 2012; Pruszynski et al. 2011), comparisons have been made with the StartReact effect (Lewis et al. 2006; Ravichandran et al. 2013; Shemmell et al. 2009). Ravichandran et al. (2013) showed that unexpectedly delivering an elbow flexion or extension position perturbation when participants were planning an elbow extension movement elicited a startle reflex as well as the intended voluntary response in elbow extensors at short latency (73 ms). Because it is well known that auditory startle stimuli can elicit a startle reflex, which triggers a prepared voluntary response (i.e., StartReact effect), Ravichandran et al. (2013) argued that the StartReact effect is a mechanism responsible for perturbation triggered responses. We conducted a third experiment to investigate whether the perturbations in our protocol were capable of eliciting a startle reflex. While the auditory startle stimulus reliably evoked a startle reflex (see Fig. 5), the wrist perturbations never elicited startle in either the DNI or the ACT condition. Despite the lack of a startle reflex (and therefore StartReact effect), as discussed above, the results of the ACT condition in experiment 1 demonstrated that the voluntary response could be elicited at short latency, such that it superimposed onto the end of M2. Thus a perturbation evoked startle reflex was likely not the mechanism responsible for triggering the voluntary response at short latency.
One of the first studies to suggest that modulation of activity during the M2 period is due to an overlapping voluntary response also implemented a behavioral manipulation in attempt to vary RT. Rothwell et al (1980) changed the predictability of the perturbation and found the largest increase in M2 activity between “let-go” and “resist” conditions when the onset of the perturbation was most predictable. Although this work has been cited extensively as evidence for voluntary response superposition contributing to increased M2 activity (e.g., Lewis et al. 2006; Manning et al. 2012; Shemmell et al. 2009), we believe this interpretation may be tempered by differences in the magnitude of voluntary responses across the “resist” conditions. Comparable to our findings in experiment 1, the magnitude of the voluntary response was largest when RT was expected to be shortest (Rothwell et al. 1980 also could not estimate RT directly in the perturbation conditions). It is plausible that RT differences between active conditions were negligible (for both Rothwell et al. 1980 and experiment 1 of the present study); instead, it was the magnitude of the voluntary response that differed, creating the appearance of latency differences between the voluntary responses. Even though the findings from the two targeting (ACT-Wi and ACT-Na) conditions in experiment 1 provided evidence that this was likely not the case (i.e., different magnitude voluntary responses but no differences in M2b activity), we can turn to the results of our second experiment to further disentangle the interactions among voluntary response magnitude, onset latency, and superposition into the M2 period.
In experiment 2, activity during the M2a and M2b epochs was larger during the active conditions (see Fig. 4). Similar to the targeting conditions in experiment 1, we found no further modulation of M2b activity as a function of the active condition. In contrast to the first experiment, the magnitude of EMG activity in the VOL epoch was similar between active conditions, and furthermore, the size and shape of the ensemble voluntary responses appeared qualitatively comparable (Gottlieb et al. 1998; see Fig. 4, B and C). This allowed us to indirectly infer a RT difference between the unidirectional and reversal movements. As expected (Ketelaars et al. 1997, 1999; Lajoie and Franks 1997), the ensemble EMG profile of the reversal condition (ACT-R; Fig. 4, gray trace) was delayed relative to unidirectional condition (ACT-Na; Fig. 4, solid black trace). Inspection of the M2b epoch revealed that the two active conditions did not significantly differ. A difference in voluntary response onset, but no significant difference in M2b period activity, suggests the voluntary response was sufficiently delayed in both conditions, minimizing superposition onto the end of M2. Taken together with the findings from the targeting conditions in experiment 1, the use of accuracy constraints following a perturbation may delay the voluntary response outside of the long-latency response epochs.
Conclusion
The present study examined sources that may contribute to stretch reflex modulation following expected torque perturbations applied at the wrist. Across all active conditions, we noted a general increase in the size of the long-latency reflex, beginning early in the appearance of the response (∼50 ms). Because this increased activity began at a latency shorter than the earliest reported voluntary responses or triggered reactions, we can attribute the magnitude changes to an increase in reflex circuit excitability (or gain modulation). Activity during the latter half of M2 was also found to be larger for all active conditions (compared to DNI), but we found a further increase in activity for the condition with the earliest and largest voluntary response. However, we also showed that early triggering of the voluntary response was not a result of the StartReact effect. In summary, the findings of this study support a hybrid mechanism by which both changes in reflex gain and a superimposed voluntary response contribute to instruction/goal-dependent modulation of the long-latency reflex period.
GRANTS
This work was supported by Natural Sciences and Engineering Research Council (NSERC) Discovery Grants (to R. Chua and I. M. Franks) as well as an NSERC Doctoral Fellowship (to C. J. Forgaard).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.J.F., I.M.F., D.M., and R.C. conception and design of research; C.J.F. and L.C. performed experiments; C.J.F. and L.C. analyzed data; C.J.F., I.M.F., D.M., L.C., and R.C. interpreted results of experiments; C.J.F. prepared figures; C.J.F. drafted manuscript; C.J.F., I.M.F., D.M., and R.C. edited and revised manuscript; C.J.F., I.M.F., D.M., L.C., and R.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Prof. Parveen Bawa for the mentorship and numerous intellectual discussions. We also acknowledge several anonymous reviewers for suggestions and comments on an earlier version of the manuscript.
REFERENCES
- Alvarez-Blanco S, Leon L, Valls-Solé J. The startle reaction to somatosensory inputs: different response pattern to stimuli of upper and lower limbs. Exp Brain Res 195: 285–292, 2009. [DOI] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain 114: 1891–1902, 1991. [DOI] [PubMed] [Google Scholar]
- Calancie B, Bawa P. Firing patterns of human flexor carpi radialis motor units during the stretch reflex. J Neurophysiol 53: 1179–1193, 1985. [DOI] [PubMed] [Google Scholar]
- Campbell AD, Squair JW, Chua R, Inglis JT, Carpenter MG. First trial and StartReact effects induced by balance perturbations to upright stance. J Neurophysiol 110: 2236–2245, 2013. [DOI] [PubMed] [Google Scholar]
- Capaday C, Forget R, Fraser R, Lamarre Y. Evidence for a contribution of the motor cortex to the long-latency stretch reflex of the human thumb. J Physiol 440: 243–255, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Dakin CJ, Chua R, Franks IM. Startle produces early response latencies that are distinct from stimulus intensity effects. Exp Brain Res 176: 199–205, 2007. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Lam MY, Chua R, Franks IM. Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci Biobehav Rev 35: 366–376, 2011. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Franks IM. Preparation for voluntary movement in healthy and clinical populations: evidence from startle. Clin Neurophysiol 123: 21–33, 2012. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol 349: 249–272, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC, McCloskey DI, Potter EK. Subject instruction and long latency reflex responses to muscle stretch. J Physiol 292: 527–534, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol 39: 925–935, 1976. [DOI] [PubMed] [Google Scholar]
- Crevecoeur F, Kurtzer I, Bourke T, Scott SH. Feedback responses rapidly scale with the urgency to correct for external perturbations. J Neurophysiol 110: 1323–1332, 2013. [DOI] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Marsden CD. Interaction between the long-latency stretch reflex and voluntary electromyographic activity prior to a rapid voluntary motor reaction. Brain Res 270: 55–62, 1983. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Granit R. Relations of reflexes and intended movements. In: Understanding the Stretch Reflex, edited by Homma S. Amsterdam, The Netherlands: Elsevier, 1976, p. 1–14. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Tanji J. Reflex and intended responses in motor cortex pyramidal tract neurons of monkey. J Neurophysiol 39: 1069–1080, 1976. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Vaughn WJ. Intended arm movements in response to externally produced arm displacements in man. In: Cerebral Motor Control in Man: Long-Loop Mechanisms, edited by Desmedt JE. Basel, Switzerland: Karger, 1978, p. 178–192. [Google Scholar]
- Fitts PM, Peterson JR. Information capacity of discrete motor responses. J Exp Psychol 67: 103–112, 1964. [DOI] [PubMed] [Google Scholar]
- Forgaard CJ, Maslovat D, Carlsen AN, Chua R, Franks IM. Startle reveals independent preparation and initiation of triphasic EMG burst components in targeted ballistic movements. J Neurophysiol 110: 2129–2139, 2013. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL. Muscle activation patterns during two types of voluntary single-joint movement. J Neurophysiol 80: 1860–1867, 1998. [DOI] [PubMed] [Google Scholar]
- Hammond PH. The influence of prior instruction to the subject on an apparently involuntary neuro-muscular response. J Physiol 132: 17P–18P, 1956. [PubMed] [Google Scholar]
- Henry FM, Rogers DE. Increased response latency for complicated movements and a “memory drum” theory of neuromotor reaction. Res Q 31: 448–458, 1960. [Google Scholar]
- Houk JC. Participation of reflex mechanisms and reaction-time processes in the compensatory adjustments to mechanical disturbances. In: Cerebral Motor Control in Man: Long-Loop Mechanisms, edited by Desmedt JE. Basel, Switzerland: Karger, 1978, p. 193–213. [Google Scholar]
- Ketelaars MA, Garry MI, Franks IM. On-line programming of simple movement sequences. Hum Mov Sci 16: 461–483, 1997. [Google Scholar]
- Ketelaars MA, Khan MA, Franks IM. Dual-task interference as an indicator of on-line programming in simple movement sequences. J Exp Psychol Hum Percept Perform 25: 1302–1315, 1999. [Google Scholar]
- Lajoie JM, Franks IM. Response programming as a function of accuracy and complexity: Evidence from latency and kinematic measures. Hum Mov Sci 16: 485–505, 1997. [Google Scholar]
- Lee RG, Tatton WG. Long loop reflex in man: clinical applications. In: Cerebral Motor Control in Man: Long-Loop Mechanisms, edited by Desmedt JE. Basel, Switzerland: Karger, 1978, p. 320–333. [Google Scholar]
- Lewis GN, MacKinnon CD, Perreault EJ. The effect of task instruction on the excitability of spinal and supraspinal reflex pathways projecting to the biceps muscle. Exp Brain Res 174: 413–425, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell EG, Sherrington C. Reflexes in response to stretch (myotatic reflexes). Proc R Soc Lond B 96: 212–242, 1924. [Google Scholar]
- Lourenço G, Iglesias C, Cavallari P, Pierrot-Deseilligny E, Marchand-Pauvert V. Mediation of late excitation from human hand muscles via parallel group II spinal and group I transcortical pathways. J Physiol 572: 585–603, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon CD, Verrier MC, Tatton WG. Motor cortical potentials precede long-latency EMG activity evoked by imposed displacements of the human wrist. Exp Brain Res 131: 477–490, 2000. [DOI] [PubMed] [Google Scholar]
- Manning CD, Tolhurst SA, Bawa P. Proprioceptive reaction times and long-latency reflexes in humans. Exp Brain Res 221: 155–166, 2012. [DOI] [PubMed] [Google Scholar]
- Maslovat D, Hodges NJ, Chua R, Franks IM. Motor preparation and the effects of practice: evidence from startle. Behav Neurosci 125: 226, 2011a. [DOI] [PubMed] [Google Scholar]
- Maslovat D, Hodges NJ, Chua R, Franks IM. Motor preparation of spatially and temporally defined movements: evidence from startle. J Neurophysiol 106: 885–894, 2011b. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Evidence from the use of vibration that the human long-latency stretch reflex depends upon spindle secondary afferents. J Physiol 348: 383–415, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol 374: 73–90, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB, Farmer SF, Ingram DA. On the localization of the stretch reflex of intrinsic hand muscles in a patient with mirror movements. J Physiol 428: 561–577, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani M, Pruszynski JA, Murnaghan CD, Scott SH. Perturbation-evoked responses in primary motor cortex are modulated by behavioral context. J Neurophysiol 112: 2985–3000, 2014. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Scott SH. Rapid motor responses are appropriately tuned to the metrics of a visuospatial task. J Neurophysiol 100: 224–238, 2008. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Lillicrap TP, Scott SH. Temporal evolution of “automatic gain-scaling”. J Neurophysiol 102: 992–1003, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature 478: 387–390, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Scott SH. The long-latency reflex is composed of at least two functionally independent processes. J Neurophysiol 106: 449–459, 2011b. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Omrani M, Scott SH. Goal-dependent modulation of fast feedback responses in primary motor cortex. J Neurosci 34: 4608–4617, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran VJ, Honeycutt CF, Shemmell J, Perreault EJ. Instruction-dependent modulation of the long-latency stretch reflex is associated with indicators of startle. Exp Brain Res 230: 59–69, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD. Influence of voluntary intent on the human long-latency stretch reflex. Nature 286: 3, 1980. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. The startle reflex, voluntary movement, and the reticulospinal tract. Suppl Clin Neurophysiol 58: 223–231, 2006. [DOI] [PubMed] [Google Scholar]
- Selen LP, Shadlen MN, Wolpert DM. Deliberation in the motor system: reflex gains track evolving evidence leading to a decision. J Neurosci 32: 2276–2286, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemmell J. Interactions between stretch and startle reflexes produce task-appropriate rapid postural reactions. Front Integr Neurosci 9: 2, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemmell J, An JH, Perreault EJ. The differential role of motor cortex in stretch reflex modulation induced by changes in environmental mechanics and verbal instruction. J Neurosci 29: 13255–13263, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidaway B. Motor programming as a function of constraints on movement initiation. J Mot Behav 23: 120–130, 1991. [DOI] [PubMed] [Google Scholar]
- Yang L, Michaels JA, Pruszynski JA, Scott SH. Rapid motor responses quickly integrate visuospatial task constraints. Exp Brain Res 211: 231–242, 2011. [DOI] [PubMed] [Google Scholar]