Abstract
Background
To explore the association between biologically effective dose (BED) and survival rates in Child-Pugh A classification (CP-A) small hepatocellular carcinoma (HCC) patients treated with stereotactic body radiation therapy (SBRT).
Methods
This retrospective study included 108 small HCC patients who were treated with SBRT between 2011 and 2014. The prescribed dose delivered to the tumor were 48Gy/8f, 49Gy/7f, 50Gy/5f and 54Gy/6f. The median biologically effective dose (BED10) of the total prescribed dose was 100Gy (76.8–102.6Gy). Factors associated with the survival rate were examined using the Cox proportion hazards model, and the factors associated with radiation-induced liver injury (RILD) were examined by logistic regression analysis.
Results
For these patients, the median follow-up time was 42 months (6–77 months), and the 1-, 2- and 3-year overall survival (OS) rates were 96.3, 89.8 and 80.6%, respectively. The 1-, 2- and 3-year progression-free survival (PFS) rates were 85.2, 70.1 and 60.6%, respectively. The 1-, 2- and 3-year local control (LC) rates were 98.1, 96.2 and 95.1%, respectively. The 1-, 2- and 3-year distant metastasis- free survival (DMFS) rates were 86.1, 72.8 and 61.2%. The OS, PFS and DMFS were significantly higher in the BED10 ≥ 100Gy group than in the BED10 < 100Gy group (OS: p = 0.020; PFS: p = 0.017; DMFS: p = 0.012). The PLT count was a predictive factor of RILD.
Conclusions
SBRT is a safe and effective option for CP-A HCC patients. A BED10 value greater than 100Gy and lower CP score are associated with improved OS and PFS. Additionally, the peripheral PLT count are predictive factors of RILD.
Electronic supplementary material
The online version of this article (10.1186/s12885-019-6063-9) contains supplementary material, which is available to authorized users.
Keywords: Hepatocellular carcinoma, Stereotactic body radiation therapy, Radiation-induced injury, Biologically effective dose, Platelet
Background
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third most common cause of cancer mortality worldwide, and only approximately 20–30% of patients with HCC are eligible for surgical treatment, including liver resection and liver transplantation [1, 2]. Accumulating data have shown that stereotactic body radiation therapy (SBRT) is a safe and effective treatment for HCC, especially in patients with inoperable or recurrent HCC [3–5]. Furthermore, Su et al. [6] compared the efficacy of stereotactic ablative radiation therapy (SABR) versus liver resection for treating small HCC (< 5 cm) patients with Child-Pugh class A (CP-A) cirrhosis and concluded that SABR has local effects that are similar to those of liver resection. Wahl et al. [7] reported that SBRT and radiofrequency ablation (RFA) were equally effective for treating small HCCs. We conducted this retrospective study to evaluate the efficacy of SBRT and identify prognostic factors related to the efficacy of SBRT in patients with HCC.
In other cancers, such as lung and cervical cancers, with the delivery of increasing biologically effective doses (BEDs) to lesions, the OS of patients increased [8–10]. However, due to the scarcity of data on HCC, the relationship between the BEDs and the efficacy of SBRT in HCC patients was included in our study.
Radiation-induced liver injury (RILD) is often a fatal complication of SBRT and should be avoided in patients with HCC; furthermore, the CP classification is an important predictive factor of RILD [11–13]. Therefore, we investigated the incidence of RILD in the included patients and searched for predictive factors among their clinical data, including biochemical parameters and the peripheral platelet (PLT) and white blood cell (WBC) counts.
Methods
Enrolled patients’ characteristics and SBRT parameters
We conducted a retrospective observation of CP-A HCC patients. The eligibility criteria were the following: (a) primary HCC diagnosed by a surgeon and/or radiologist and oncologist according to the international guidelines for the management of HCC or by pathology [14]; (b) single lesion and longest tumor diameter < 5.0 cm; (c) CP-A classification; (d) Eastern Cooperative Oncology Group (ECOG) score 0–1; (e) distances between tumor and normal organs (esophagus, stomach, duodenum, bowel) were more than 5 mm; (f) unsuitable for other therapies, such as patients with heart disease, uncontrolled diabetes, uncontrolled hypertension, etc. (g) rejecting other therapies such as resection, liver transplantation, etc. (h) platelet count≥50 × 109/L, white blood count≥1.5 × 109/L and (i) patients infected with hepatitis B virus who were treated with adefovir or entecavir; patients infected with hepatitis C virus whose HCV DNA were negative. The exclusion criteria were the following: (a) tumor thrombus; (b) lymph node involvement; and (c) extrahepatic metastasis. Patients were treated with SBRT at The Fifth Medical Center of PLA General Hospital between 2011 and 2014. They did not receive any other treatments, such as RFA or transcatheter arterial chemoembolization (TACE) before enrollment into our study. All patients were managed in multidisciplinary setting with all legitimate treatment options available and provided with written informed consent before treatment.
The baseline data of the 108 patients in our study are listed in Table 1.ALBI parameter in this study was calculated from baseline blood work according to Johnson et al. [15]. ALBI = [log10bilirubin × 0.66] + [albumin× (− 0.085)], where bilirubin is in μmol/L and albumin is in g/L; the cutoffs were used to assign each patient to one of three prognostic groups indicating the ALBI grade (range, 1 to 3). The cutoffs were as follows: xb ≤ − 2.60 (ALBI grade 1), > − 2.60 to ≤1.39 (ALBI grade 2), and > − 1.39 (ALBI grade 3).
Table 1.
Clinical, biochemical characteristics and radiation planning parameters of patients enrolled in this study
| Variables | n |
|---|---|
| Clinical and biochemical characteristics | |
| Sex | |
| Male | 80 (74.07%) |
| Female | 28 (25.93%) |
| Age (years) | |
| Median | 54 |
| Range | 37–77 |
| Underlying liver disease | |
| Hepatitis B | 97 (89.81%) |
| Hepatitis C | 7 (6.48%) |
| Alcoholic hepatitis | 3 (2.78%) |
| None | 1 (0.93%) |
| Maximum tumor diameter (cm) | |
| Median | 2.3 |
| Range | 0.7–4.9 |
| AFP (ng/ml) a | |
| Median | 33.13 |
| Range | 0.8–7896 |
| Child-Pugh score | |
| 5 | 97 (89.81%) |
| 6 | 11 (10.19%) |
| ALBI grade | |
| Grade 1 | 34 (31.48%) |
| Grade 2 | 74 (68.52%) |
| WBC count (×109/L) | |
| Median | 4.86 |
| Range | 1.53–9.4 |
| PLT count (×109/L) | |
| Median | 118 |
| Range | 50–283 |
| Radiation planning parameters | |
| Isodose line of maximum dose | |
| Median (range, %) | 72 (60–86) |
| Residual normal liver volume | |
| Median (range, cm3) | 1324 (834–2493) |
| Mean dose of whole liver volume minus GTV | |
| Median (range, Gy) | 9.07 (4.04–12.05) |
| Dose received by volume of liver | |
| D700c | 4.99 (1.40–13.50) |
| BED10b of Plan target volume (PTV) | |
| Median (range, Gy) | 100 (76.8–102.6) |
Radiation treatment technique
All enrolled patients underwent the implantation of 4 to 6 fiducials one week prior to SBRT (CyberKnife, Accuray, USA). All plans were designed using G4 CyberKnife MultiPlan (version 4.0.2) and were applied with dynamic respiration tracking combined with fiducial tracking.
An oncologist contoured the gross tumor volume (GTV) and organs at risk (normal liver, kidneys, esophagus, stomach, duodenum, bowel and spinal cord). The planning target volume (PTV) was expanded 3–5 mm around the GTV, which contoured 100% of GTV. The prescribed dose delivered to the tumor were 48Gy/8f, 49Gy/7f, 50Gy/5f and 54Gy/6f. The selection of dose depended on the relation between lesion and bile duct. If the distance between tumor and bile duct was less than 3 mm, the prescribed dose delivered to the tumor were 48Gy/8f or 49Gy/7f. The normal tissue dose was within the normal radiotherapy tolerance dose (TG-101) [16]. In this study, both the single dose and the total dose varied, so the BED was chosen as the parameter of reaction dose fractionated schemes.
The BED was calculated according to the value of α/β (10Gy, BED10) using the formula BED = D (1 + d/[α/β]) [17, 18], where D is the total dose delivered, and d is the dose per fraction. The median BED10 of the total prescribed dose was 100Gy (76.8–102.6Gy) in our research.
Follow-up study
All patients underwent a liver function assessment and routine blood examinations before treatment. After SBRT, the patients were followed every 3 months for 1 year and every 6 months thereafter until June 2018.
Toxicity evaluation
Radiation-related toxicity was measured based on Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC) [19].
The liver toxicity reaction evaluation was based on the definition of RILD, of which there are two types: classic RILD and non-classic RILD.
Classic RILD usually manifests as symptoms of fatigue, hepatomegaly, and anicteric ascites, etc., 1–3 months after radiotherapy. Moreover, the serum alkaline phosphatase (ALP) level in these patients increases to more than twice the normal level, while the serum transaminase and bilirubin levels in remain normal [20, 21].
Non-classic RILD occurs in patients with underlying chronic hepatic diseases, who suffer from jaundice and/or remarkably elevated serum transaminase levels (increased by more than fivefold compared to normal levels) [22, 23].
Meanwhile, CP score progression (increasing by 2 or more scores) was also a clinical metric for non-classic RILD [24] .
Tumor recurrence and treatment
When the recurrence/metastasis was confirmed, second-line treatments were individualized according to the number and location of the recurrent tumors and the liver function status, while considering the patient’s preferences. Therapeutic options included repeated SBRT, targeted therapy and conservative treatment.
Statistical analysis
OS was calculated starting from the date of SBRT to the date of the final follow-up or demise of the patients. PFS was estimated starting from the date of SBRT to the date of disease progression or patient death. LC was defined starting from the date of SBRT to the date of treated-lesion progression or patient death. DMFS was defined starting from the date of SBRT to the date of distant metastasis occurrence (out-field relapse). OS, PFS, LC and DMFS were estimated using the Kaplan-Meier method. OS-, PFS-, LC- and DMFS- related group analyses of BED10 were performed using the log rank test. Univariate and multivariable hazard ratios were calculated using the Cox proportion hazard model. A binary logistic regression method was employed to investigate each prognostic factor of RILD. Variables with p-values less than 0.2 in the univariate analysis were included in the multivariate analysis with forward selection. For comparisons between the baseline variables, the χ2 test and Fisher’s exact test were performed.
All statistical analyses were performed using SPSS 22.0 software (IBM) and STATA 15.0 software. P values < 0.05 were considered statistically significant.
Results
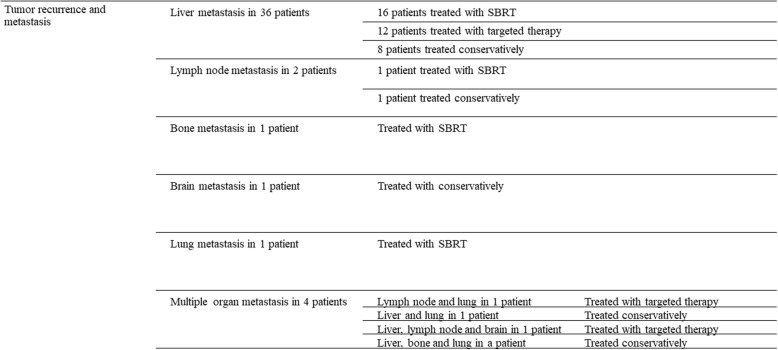
The median follow-up period was 42 months (range, 6–77 months). By June 2018, 45 patients had experienced relapse or metastasis; 36 patients experienced liver metastasis, 2 experienced lymph node metastasis, 1 experienced bone metastasis, 1 experienced brain metastasis, 1 experienced lung metastasis and 4 experienced multiple organ metastases. The treatment was ultimately selected by the patient (Fig. 1).
Fig. 1.
The treatment was selected by the patient
Survival outcomes
After 6 months of SBRT, there were 65 patients with CR (60.19%), 30 patients with PR (27.78%), 4 patients with SD (3.70%) and 9 patients with PD (8.33%). The response rate was (CR + PR)/108 × 100% = 87.96%, and the disease control rate was (CR + PR + SD)/ 108 × 100% = 91.67%.
By June 2018, 27 patients died: 6 patients died of hepatic failure; 6 died of upper gastrointestinal bleeding; 1 died of hepatorenal syndrome; 1 died of infectious shock; 1 died of pulmonary or brain metastasis complications; and 12 died of unknown causes.
The 1-, 2- and 3-year OS rates were 96.3, 89.8 and 80.6%, respectively (Fig. 2a). The 1-, 2- and 3-year PFS rates were 85.2, 70.1 and 60.6%, respectively (Fig. 2b). The 1-, 2- and 3-year LC rates were 98.1, 96.2 and 95.1%, respectively (Fig. 2c).
Fig. 2.
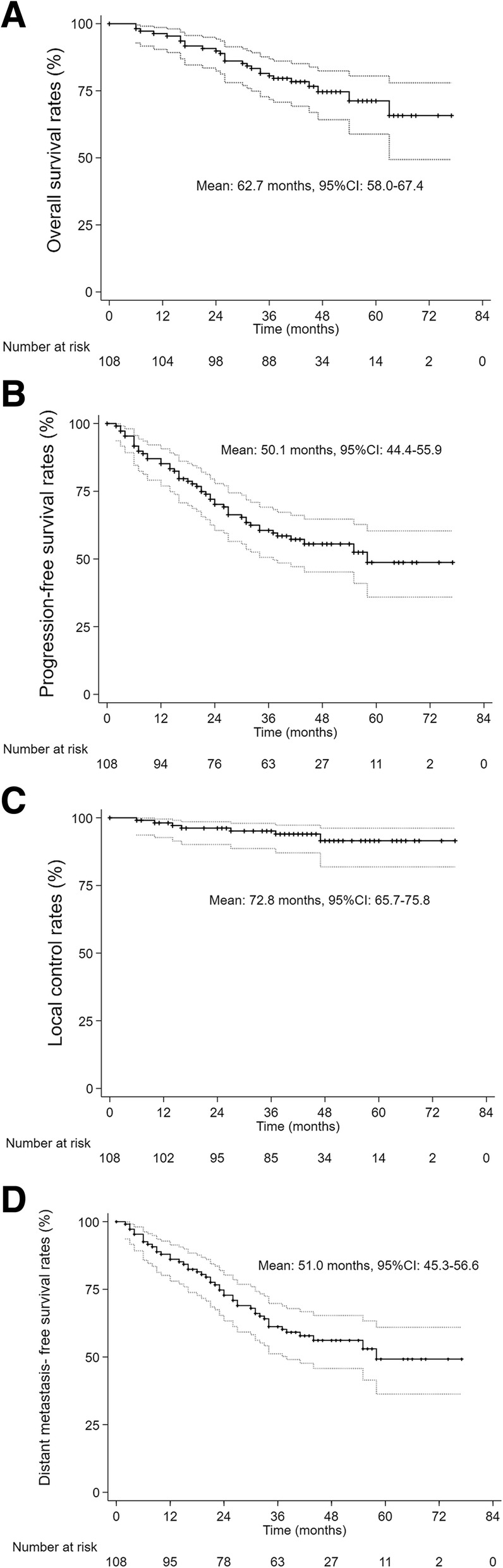
Kaplan-Meier curve of overall survival (a), progression-free survival (b), local control (c) and distant metastasis-free survival (d)
The 1-, 2- and 3-year DMFS rates were 86.1, 72.8 and 61.2%, respectively (Fig. 2d). The 5-year cumulative OS, PFS, LC and DMFS were 71.2,48.7,91.5 and 49.2%, respectively.
In the Cox proportional hazard model, the Child-Pugh score and BED10 were independent prognostic factors of OS (Table 2), PFS (Table 3) and DMFS (Table 4) on multivariate analysis.
Table 2.
Univariate and multivariate Cox regression analysis of OS
| Univariate Cox regression | Multivariate Cox regression | |||
|---|---|---|---|---|
| Patient details | p value | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) |
| Sex (male/female) | 0.214 | 1.856 (0.700–4.922) | ||
| Age | 0.983 | 1.000 (0.960–1.043) | ||
| Hepatitis type (B/C/alcoholic/others) | 0.645 | 1.167 (0.606–2.247) | ||
| Maximum tumor diameter | 0.368 | 1.222 (0.790–1.889) | ||
| ECOG PS | 0.432 | 1.621 (0.486–5.402) | ||
| AFP | 0.968 | 1.000 (1.000–1.000) | ||
| Child-Pugh score (5/6) | 0.095 | 2.300 (0.866–6.108) | 0.048 | 2.740 (1.011–7.425) |
| ALBI grade | 0.708 | 1.156 (0.542–2.465) | ||
| WBC count | 0.181 | 0.822 (0.618–1.095) | ||
| PLT count | 0.134 | 0.993 (0.984–1.002) | ||
| Residual normal liver volume | 0.375 | 0.999 (0.997–1.001) | ||
| Mean dose of whole liver volume minus GTV | 0.810 | 0.997 (0.972–1.022) | ||
| D700 | 0.252 | 1.151 (0.905–1.463) | ||
| BED10 of PTV | 0.030 | 0.960 (0.925–0.996) | 0.017 | 0.955 (0.919–0.992) |
Table 3.
Univariate and multivariate Cox regression analysis of PFS
| Univariate Cox regression | Multivariate Cox regression | |||
|---|---|---|---|---|
| Patient details | p value | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) |
| Sex (male/female) | 0.094 | 1.864 (0.900–3.858) | ||
| Age | 0.673 | 0.993 (0.962–1.025) | ||
| Hepatitis type (B/C/alcoholic/others) | 0.043 | 1.546 (1.013–2.359) | ||
| Maximum tumor diameter | 0.848 | 1.026 (0.789–1.334) | ||
| ECOG PS | 0.388 | 1.505 (0.595–3.805) | ||
| AFP | 0.440 | 1.000 (1.000–1.000) | ||
| Child-Pugh (5/6) | 0.071 | 2.100 (0.938–4.071) | 0.029 | 2.500 (1.096–5.705) |
| ALBI grade | 0.156 | 0.656 (0.367–1.174) | ||
| WBC count | 0.051 | 0.850 (0.723–1.001) | ||
| PLT count | 0.997 | 0.993 (0.992–1.003) | ||
| Residual normal liver volume | 0.375 | 0.999 (0.997–1.001) | ||
| Mean dose of whole liver volume minus GTV | 0.524 | 0.959 (0.845–1.090) | ||
| D700 | 0.839 | 1.018 (0.857–1.210) | ||
| BED10 of PTV | 0.020 | 2.063 (1.120–3.803) | 0.020 | 2.063 (1.120–3.803) |
Table 4.
Univariate and multivariate Cox regression analysis of DMFS
| Univariate Cox regression | Multivariate Cox regression | |||
|---|---|---|---|---|
| Patient details | p value | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) |
| Sex (male/female) | 0.114 | 1.801 (0.868–3.736) | ||
| Age | 0.777 | 0.995 (0.964–1.028) | ||
| Hepatitis type (B/C/alcoholic/others) | 0.080 | 1.668 (0.940–1.028) | ||
| Maximum tumor diameter | 0.901 | 1.017 (0.780–1.326) | ||
| ECOG | 0.220 | 1.799 (0.704–4.594) | ||
| AFP | 0.471 | 1.000 (1.000–1.000) | ||
| Child-Pugh score (5/6) | 0.054 | 2.214 (0.996–4.965) | 0.021 | 2.653 (1.160–6.067) |
| ALBI grade | 0.487 | 0.714 (0.277–1.843) | ||
| WBC count | 0.061 | 0.854 (0.724–1.007) | ||
| PLT count | 0.320 | 0.997 (0.992–1.003) | ||
| Residual normal liver volume | 0.982 | 1.000 (0.998–1.002) | ||
| Mean dose of whole liver volume minus GTV | 0.898 | 0.993 (0.886–1.112) | ||
| D700 | 0.496 | 1.064 (0.889–1.274) | ||
| BED10 of PTV | 0.013 | 0.964 (0.937–0.992) | 0.006 | 0.959 (0.932–0.988) |
Comparison between BED10 ≥ 100Gy and BED10 < 100Gy
To further examine the BED10, we divided the patients into two groups with a BED10 of 100Gy as the cutoff, i.e., the BED10 ≥ 100Gy and BED10 < 100Gy groups. There were no differences in the detail of the patients between the two groups (Table 5). There were 84 patients in the BED10 ≥ 100Gy group and 24 patients in the BED10 < 100Gy group. The OS, PFS and DMFS rates were significantly higher in the BED10 ≥ 100Gy group than in the BED10 < 100Gy group (OS: p = 0.020, Fig. 3a; PFS: p = 0.017, Fig. 3b; DMFS: p = 0.012, Fig. 3d). However, there were no significant differences in LC between the two groups (p = 0.409, Fig. 3c).
Table 5.
Details of the patients in the two groups
| BED10 ≥ 100 Gy | BED10 < 100 Gy | ||
|---|---|---|---|
| Details | p value | ||
| Sex (male/female) | 62/22 | 18/6 | 0.907 |
| Age | 54.33 ± 8.82 | 54.60 ± 11.59 | 0.885 |
| Maximum tumor diameter | 2.50 ± 1.02 | 2.74 ± 1.10 | 0.406 |
| ECOG PS (0/1) | 78/6 | 22/2 | 0.844 |
| Child-Pugh score (5/6) | 75/9 | 22/2 | 0.734 |
| ALBI grade (1/2) | 25/59 | 9/15 | 0.472 |
| WBC count | 5.00 ± 1.99 | 4.68 ± 1.51 | 0.528 |
| PLT count | 127.06 ± 59.06 | 112.04 ± 39.47 | 0.244 |
| Residual normal liver volume | 1345.33 ± 286.81 | 1390.36 ± 329.11 | 0.614 |
| D700 | 5.50 ± 2.27 | 4.41 ± 3.17 | 0.087 |
| Percent of PTV volume enclosed by isodose line (%) | 92.09 ± 1.69 | 91.53 ± 1.77 | 0.280 |
Fig. 3.
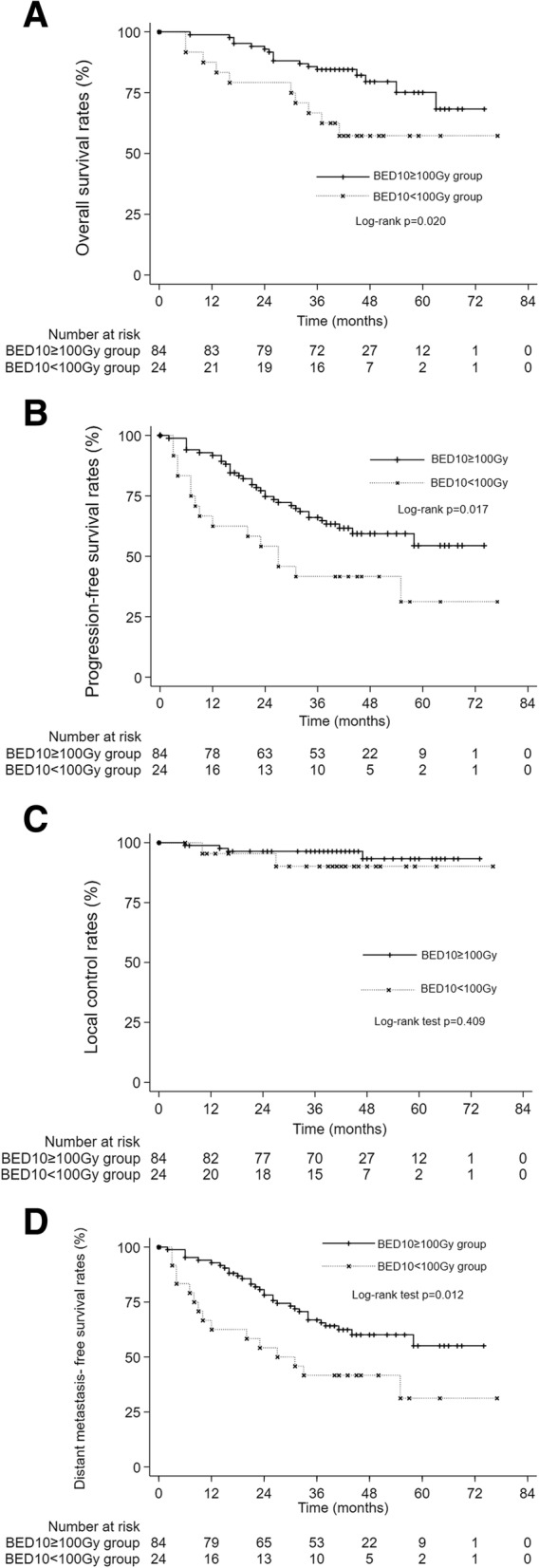
A OS in the BED10 ≥ 100 Gy and BED10 < 100 Gy groups. The 1-, 2- and 3-year OS rates were 98.8, 92.9 and 84.5% in the BED10 ≥ 100 Gy group and 87.5, 79.2 and 66.7% in the BED10 < 100 Gy group, respectively (p = 0.020). B PFS in the BED10 ≥ 100 Gy and BED10 < 100 Gy groups. The 1-, 2- and 3-year PFS rates were 91.7, 74.7 and 66.0% in the BED10 ≥ 100 Gy group and 62.5, 54.2 and 41.7% in the BED10 < 100 Gy group, respectively (p = 0. 017). C LC in the BED10 ≥ 100 Gy and BED10 < 100 Gy groups. The 1-, 2- and 3-year LC rates were 98.8, 96.4 and 96.4% in the BED10 ≥ 100 Gy group and 95.5, 95.5 and 90.2% in the BED10 < 100 Gy group, respectively (p = 0.409). D DMFS in the BED10 ≥ 100 Gy and BED10 < 100 Gy groups. The 1-, 2- and 3-year DMFS rates were 92.9, 80.7 and 66.9% in the BED10 ≥ 100 Gy group and 62.5, 54.2 and 41.7% in the BED10 < 100 Gy group, respectively (p = 0.012)
Toxicity outcomes
All 108 patients finished the SBRT treatments. Grade 1–2 acute toxicity reactions occurred in 32 patients, including abdominal pain (3 patients in BED10 ≥ 100Gy group, 1 patients in BED10 < 100Gy group, p = 0.89), fatigue (13 patients in BED10 ≥ 100Gy group, 5 patients in BED10 < 100Gy group, p = 0.53), vomiting and anorexia (22 patients were in BED10 ≥ 100Gy group, 6 patients were in BED10 < 100Gy group, p = 0.92), which could be relieved gradually by symptomatic treatment.
Liver toxicity
The most serious complication of RILD is the liver failure-resulted death. We reviewed the causes of death of four patients who died within a year after receiving SBRT, among which only one died from liver failure. The patient’ tumor progressed three months after treatment, but his liver function remained normal. The patient only received conservative treatment afterwards. Therefore, we contributed the death of this patient to tumor progression instead of RILD.
In our research, eight patients were diagnosed with RILD through laboratory tests; 4 patients showed classic RILD, and 4 patients showed non-classic RILD (The parameters of RILD patients were shown in Table 6). Among these patients, 1 patient (1/24) was in BED10<100Gy group while 7 patients (7/84) were in BED10 ≥ 100Gy group (p = 0.49). The condition of all patients who were diagnosed with RILD was relieved after symptomatic treatment, and none of these patients died. The analysis of the factors influencing RILD is shown in Table 7. A lower PLT count was associated with an increased risk of RILD on multivariate analysis.
Table 6.
The parameters of RILD patients before and after SBRT
| ALP | Bilirubin | ALT | AST | ALB | Ascites | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre- | Post- | Pre- | Post- | Pre- | Post- | Pre- | Post- | Pre- | Post- | Pre- | Post- | |
| Classic RILD | 131 | 341 | 9.8 | 9.5 | 28 | 39 | 32 | 32 | 43 | 36 | – | – |
| 67 | 80 | 12.8 | 9.9 | 43 | 34 | 43 | 29 | 46 | 35 | – | ++ | |
| 59 | 327 | 14.5 | 8.4 | 13 | 18 | 16 | 29 | 44 | 41 | – | + | |
| 113 | 362 | 7.8 | 8.2 | 16 | 31 | 46 | 38 | 42 | 44 | – | – | |
| Non-Classic RILD | 67 | 136 | 22.7 | 51.6 | 30 | 608 | 28 | 363 | 36 | 40 | – | – |
| 79 | 81 | 18.5 | 35.6 | 28 | 17 | 37 | 42 | 39 | 34 | – | – | |
| 79 | 116 | 12 | 16 | 22 | 214 | 27 | 223 | 34 | 33 | – | – | |
| 73 | 147 | 26.1 | 51.4 | 34 | 749 | 42 | 678 | 36 | 32 | – | + | |
Table 7.
Univariate and multivariate logistic regression analysis of RILD
| Univariate logistic regression | Multivariate logistic regression | |||
|---|---|---|---|---|
| Patient details | p value | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) |
| Sex (male/female) | 0.116 | 0.304 (0.069–1.345) | ||
| Age | 0.793 | 0.542 (0.890–1.058) | ||
| Hepatitis type (B/C/alcoholic/others) | 0.096 | 0.019 (0.309–3.269) | ||
| Maximum tumor diameter | 0.015 | 0.096 (0.531–2.092) | ||
| Child-Pugh score (5/6) | 0.999 | 0.000 | ||
| ALBI grade | 0.398 | 0.523 (0.116–2.352) | ||
| WBC count | 0.009 | 0.422 (0.220–0.808) | ||
| PLT count | 0.006 | 0.966 (0.942–0.990) | 0.034 | 0.974 (0.950–0.998) |
| Residual normal liver volume | 0.973 | 1.001 (0.999–1.004) | ||
| Mean dose of whole liver volume minus GTV | 0.821 | 0.997 (0.972–1.024) | ||
| D700 | 0.002 | 0.029 (0.901–1.695) | ||
| BED10 of PTV | 0.696 | 1.018 (0.930–1.116) | ||
Discussion
In this study, we retrospectively reviewed a large group of CP-A HCC patients treated with SBRT over a three-year span as an extended analysis of our previous observations [25]. Compared to prior studies, the OS, PFS, LC and DMFS rates were all satisfactory in this study. Su et al. [4] reported 3- and 5-year OS rates of 73.5 and 64.3% and 3- and 5-year PFS rates of 58.3 and 36.4%, respectively, with doses of 42-46Gy administered in 3–5 fractions and single-fraction doses of 28–30 Gy. Among 77 HCC patients treated with SBRT, Andres et al. [3] found 1- and 2-year OS rates of 81.8 and 56.6%, respectively, and 1- and 2-year LC rates of 99%. The total dose of 45 Gy in 3 fractions was prescribed to the 80% isodose line. Their OS rates were lower than ours, which have been caused by the inclusion of CP-B patients and/or patients with previous treatments. This finding was confirmed by their conclusion that the CP-B classification was associated with a poor prognosis. Moreover, previous treatments may also be an affect factor. Ueno et al. [26] reviewed 296 patients with single nodular HCC ≤ 5 cm with Child-Pugh A between 2001 and 2011 who underwent surgical resection (SR, n = 136) and radiofrequency thermal ablation (RFA, n = 160), and they found that 5-year OS rates of SR and RFA among all patients were 70.1 and 69.8%, respectively (P = 0.14). The 5-year OS was similar to the 5-year cumulative OS in our result.
CP score was an influential factor in OS and PFS in our study, which may be related to the extent to which CP reflects cirrhosis, which is associated with occurrence of complication and tumorigenesis. Moreover, we found that the OS, PFS and DMFS rates also increased significantly with BED10 ≥ 100Gy in treating HCC with SBRT, but with no significant difference in the LC rates. The correlation between LC and BEDs in our study was consistent with Ohri’ findings [27], but he didn’t explore the relation between BEDs and survival rates. In addition to initial radiotherapy, the efficacy of subsequent treatment after recurrence was also a factor affecting OS. The relation between PFS and BEDs of our study, we speculate that SBRT could change immune microenvironment, which can be proven by DMFS. This assumption is also supported by some other tumor-related articles. Lee et al. [28] reported that ablative radiotherapy dramatically increased T-cell priming in draining lymphoid tissues, leading to reduction/eradication of the primary tumor or distant metastases. Meanwhile, antitumor immunity significantly contributes to the superior response induced by one dose of 20Gy compared with that induced by 4 doses of 5Gy. Schaue et al. [29] studied the tumor-specific immune response in mice with murine melanoma irradiated with 15Gy administered in different fractionated dose schemes, and the results showed that a single dose of 7.5Gy or higher, but not lower than 5Gy, was immune-stimulatory, as mediated by tumor-reactive T cells. They all showed that under the condition that the total dose remained unchanged, the superior anti-tumor immune response was related with the higher single dose. And it is the same with BEDs, which increase with higher single dose when the total dose remained the same. Therefore, with higher BEDs causing a better anti-tumor immune microenvironment, the recurrence rates of tumor decreased. However, it’s only our hypothesis. In our other studies, we found that in these patients different BED10 values lead to different changes of immune system, such as NK cell functions, and further experiments are in progress (data not shown).
The influential factor of RILD is the pretreatment PLT count, as demonstrated by Velec et al. [11]. Some studies shown that the PLT count indirectly reflects the degree of liver cirrhosis by indicating the degree of portal hypertension and hypersplenism [30, 31]. Nozaki et al. [32] performed in vitro and in vivo studies, and they have proven that thrombopoietin promotes liver regeneration and improves liver cirrhosis by increasing the PLT level, indirectly implying that PLT decrease would worsen liver cirrhosis. We think it is the relation between the PLT count and liver cirrhosis, an influential factor of RILD that makes PLT count an influential factor of RILD [33]. In clinical work, we found Child-Pugh score and PLT were not compatible. Degree of liver cirrhosis could affect liver nutrition supply by affecting liver hemodynamics, which may affect liver function. However, liver cirrhosis degree is not the only influence factor of liver function, which may be affected by many other factors, including effective liver volume, hepatic cell function and compensate ability of liver, etc. Moreover, PLT is not a parameter affecting Child-Pugh score. Our result reminds us that besides Child-Pugh score, PLT is also a non-negligible factor.
Though the limited dose of bile duct was not shown in TG-101, previous studies showed that higher dose radiation of bile duct was related with higher risk of stenosis. Barney et al. [34] reported one case of grade 3 biliary stenosis after SBRT where the patient was treated with a dose of 50Gy in five fractions. Takahisa et al. [35] showed that the true threshold for biliary stenosis would be between 40Gy in 5 fractions and 80Gy in 5 fractions, and a special caution was necessary when treating patients with more than 40Gy in 5 fractions. Before this study, we had 2 cases with obstructive jaundice after 50Gy/5f and 54Gy/6f, and their PET-CT showed bile duct stenosis but no active lesion. Since then, we adopted the dose fraction of 49Gy/7f and 48Gy/8f in SBRT of small HCC when the tumor is near to bile duct to reduce this risk. And until now, no patient has had bile duct stenosis by the two doses and fraction sizes above, so we consider they were safe fractionated regimens. Considering both the relation of BEDs and survival rates in our study, we think the trade-off between the efficacy and risk of SBRT in tumors near the bile duct deserves further study, and the optimal dose fractionated regimens needs large-sized samples to explore.
This study has several limitations. First, this was a retrospective study. Second, the number of patients in the two groups is much different, which may cause bias. A relevant prospective clinical trial is in progress (Clinicaltrails NCT 03295500). We hope to get objective and accurate results.
Conclusion
SBRT is a safe and effective option for CP-A HCC patients, and an increased BED10 and lower CP score are associated with improved OS and PFS. The results indicate that a strategy escalating radiation doses over a limited time frame are worth exploring in a prospective clinical trial. Meanwhile, PLT count should be considered, which is a predictive factor of RILD in SBRT of HCC.
Additional file
The information of the 108 patients involved in the study. (XLSX 15 kb)
Acknowledgements
We appreciate Ye Lin (University of Chinese Academy of Sciences) for her valuable suggestions and editing support.
Abbreviations
- AFP
Alpha fetoprotein
- ALP
alkaline phosphatase
- BED
Biologically effective dose
- CIs
Confidence intervals
- CP
Child-Pugh
- CR
Complete response
- CT
computed tomography
- DMFS
distant metastasis-free survival
- ECOG
Eastern cooperative oncology group
- GTV
Gross target volume
- HCC
Hepatocellular carcinoma
- HRs
Hazard ratios
- LC
Local control
- MRI
Magnetic resonance imaging
- OS
Overall survival
- P
P-value
- PD
Progressive disease
- PET-CT
Positron emission tomography-computed tomography
- PR
Partial response
- PTV
Planning target volume
- RFA
Radiofrequency ablation
- RILD
Radiation-induced liver disease
- SBRT
Stereotactic body radiation therapy
- SD
Stable disease
Authors’ contributions
JS and TZ involved in drafting the manuscript and revising it critically for important intellectual content, and JS, WH and JD made substantial contributions to analysis and interpretation of data; JW, AZ and DL made substantial contributions to acquisition of data; WL and DZ were responsible for follow up content. XD was responsible for the final approval of the version to be published. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This study was supported by grants from Beijing Municipal Science and Technology Commission Fund (Z171100001017181) whose responsible person was Wengang Li, who was responsible for follow up content.
Availability of data and materials
The information of the 108 patients involved in this study is presented in the Additional file 1.
Date sharing is not applicable to this article as no datasets were generated or analyzed during the study.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of 302 Hospital of PLA (People’s Liberation Army) and was conducted in accordance with the Declaration of Helsinki and internationally accepted ethical guidelines. During their admission for SBRT treatment, the patients enrolled in this study provided written informed consent for their information to be stored in hospital databases and used for research.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Sun and Tao Zhang contributed equally to this work.
Contributor Information
Jing Sun, Email: 519299998@qq.com.
Tao Zhang, Email: 13641304338@126.com.
Jia Wang, Email: 675601310@qq.com.
Wengang Li, Email: doctor302@163.com.
Aimin Zhang, Email: zhangaimin761213@163.com.
Weiping He, Email: hewpbj302@163.com.
Dan Zhang, Email: 1090409588@qq.com.
Dong Li, Email: ld_1768@163.com.
Junqiang Ding, Email: 799435134@qq.com.
Xuezhang Duan, Email: duanxuezhang2006@163.com.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. Ca A Cancer J Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Huertas A, Baumann AS, Saunier-Kubs F, Salleron J, Oldrini G, Croisã-Laurent V, Barraud H, Ayav A, Bronowicki JP, Peiffert D. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol. 2015;115(2):211–216. doi: 10.1016/j.radonc.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Su TS, Liang P, Lu HZ, Liang J, Gao YC, Zhou Y, Huang Y, Tang MY, Liang JN. Stereotactic body radiation therapy for small primary or recurrent hepatocellular carcinoma in 132 Chinese patients. J Surg Oncol. 2016;113(2):181–187. doi: 10.1002/jso.24128. [DOI] [PubMed] [Google Scholar]
- 5.Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, Johnstone PA, Cardenes HR. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81(4):e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Su TS, Liang P, Liang J, Lu HZ, Jiang HY, Cheng T, Huang Y, Tang Y, Deng X. Long-term survival analysis of stereotactic ablative radiotherapy versus liver resection for small hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2017;98(3):639. doi: 10.1016/j.ijrobp.2017.02.095. [DOI] [PubMed] [Google Scholar]
- 7.Wahl D, Stenmark M, Tao Y, Pollom E, Caoili E, Lawrence T, Schipper M, Feng M. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34(5):452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stahl JM, Ross R, Harder EM, Mancini BR, Soulos PR, Finkelstein SE, Shafman TD, Dosoretz AP, Evans SB, Husain ZA. The association between biologically effective dose (BED) and radiation treatment schedule on overall survival in stage I non-small cell lung cancer (NSCLC) patients treated with stereotactic body radiation therapy (SBRT) Int J Radiat Oncol Biol Phys. 2016;96(5):1011–1020. doi: 10.1016/j.ijrobp.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 9.Zhu L, Zhang S, Xu X, Wang B, Wu K, Deng Q, Xia B, Ma S. Increased biological effective dose of radiation correlates with prolonged survival of patients with limited-stage small cell lung Cancer: a systematic review. PLoS One. 2016;11(5):e0156494. doi: 10.1371/journal.pone.0156494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beskow C, Ågrencronqvist AK, Lewensohn R, Tomadasu I. Biological effective dose evaluation and assessment of rectal and bladder complications for cervical cancer treated with radiotherapy and surgery. J Contemp Brachytherapy. 2012;4(4):205–212. doi: 10.5114/jcb.2012.32554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velec M, Haddad CR, Craig T, Wang L, Lindsay P, Brierley J, Brade A, Ringash J, Wong R, Kim J. Predictors of liver toxicity following stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2017;97(5):939. doi: 10.1016/j.ijrobp.2017.01.221. [DOI] [PubMed] [Google Scholar]
- 12.Jung J, Yoon SM, Kim SY, Cho B, Park JH, Kim SS, Song SY, Lee SW, Ahn SD, Choi EK. Radiation-induced liver disease after stereotactic body radiotherapy for small hepatocellular carcinoma: clinical and dose-volumetric parameters. Radiat Oncol. 2013;8(1):1–7. doi: 10.1186/1748-717X-8-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray LJ, Dawson LA. Advances in stereotactic body radiation therapy for hepatocellular carcinoma. Semin Radiat Oncol. 2017;27(3):247. doi: 10.1016/j.semradonc.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 14.European oF, R, Treatment Of C, Llovet J, Ducreux M, Lencioni R, Bisceglie AD, Galle P, Dufou J, Easleortc C: European association for the study of the liver, European organisation for research and treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma 2012. [DOI] [PubMed]
- 15.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach—the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L. Stereotactic body radiation therapy: the report of AAPM task group 101. Med Phys. 2010;37(8):4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 17.Jones B, Dale RG, Deehan C, Hopkins KI, Morgan DA. The role of biologically effective dose (BED) in clinical oncology. Clin Oncol. 2001;13(2):71–81. doi: 10.1053/clon.2001.9221. [DOI] [PubMed] [Google Scholar]
- 18.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62(740):679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 19.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC) Int J Radiat Oncol Biol Phys. 2015;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31(5):1237. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 21.Liang SX, Huang XB, Zhu XD, Zhang WD, Cai L, Huang HZ, Li YF, Chen L, Liu MZ. Dosimetric predictor identification for radiation-induced liver disease after hypofractionated conformal radiotherapy for primary liver carcinoma patients with child–Pugh grade a cirrhosis. Radiother Oncol. 2011;98(2):265–269. doi: 10.1016/j.radonc.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Cheng JC, Wu JK, Lee PC, Liu HS, Jian JJ, Lin YM, Sung JL, Jan GJ. Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2004;60(5):1502–1509. doi: 10.1016/j.ijrobp.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 23.Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, Ten Haken RK. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76(3):S94–S100. doi: 10.1016/j.ijrobp.2009.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapman Tobias R., Bowen Stephen R., Schaub Stephanie K., Yeung Rosanna H., Kwan Sharon W., Park James O., Yu Lei, Harris William P., Johnson Guy E., Liou Iris W., Nyflot Matthew J., Apisarnthanarax Smith. Toward consensus reporting of radiation-induced liver toxicity in the treatment of primary liver malignancies: Defining clinically relevant endpoints. Practical Radiation Oncology. 2018;8(3):157–166. doi: 10.1016/j.prro.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Zhang T, Sun J, He W, Li H, Piao J, Xu H, Duan X. Stereotactic body radiation therapy as an effective and safe treatment for small hepatocellular carcinoma. BMC Cancer. 2018;18(1):451. doi: 10.1186/s12885-018-4359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueno M, Hayami S, Shigekawa Y, Kawai M, Hirono S, Okada KI, Tamai H, Shingaki N, Mori Y, Ichinose M. Prognostic impact of surgery and radiofrequency ablation on single nodular HCC ⩽5 cm: cohort study based on serum HCC markers. J Hepatol. 2015;63(6):1352–1359. doi: 10.1016/j.jhep.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Ohri N, Tomé WA, Méndez Romero A, Miften M, ten Haken RK, Dawson LA, Grimm J, Yorke E, Jackson A. Local control after stereotactic body radiation therapy for liver tumors. Int J Radiat Oncol Biol Phys. 2018. [DOI] [PMC free article] [PubMed]
- 28.Lee Y, Auh S, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaue D, Ratikan JA, Iwamoto KS, Mcbride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83(4):1306–1310. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Feng Y, Ma X, Wang G, Wu H, Xie X, Zhang C, Zhu Q. Diagnostic efficacy of noninvasive liver fibrosis indexes in predicting portal hypertension in patients with cirrhosis. PLoS One. 2017;12(8):e0182969. doi: 10.1371/journal.pone.0182969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim MY, Jeong WK, Baik SK. Invasive and non-invasive diagnosis of cirrhosis and portal hypertension. World J Gastroenterol. 2014;20(15):4300–4315. doi: 10.3748/wjg.v20.i15.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nozaki R, Murata S, Nowatari T, Maruyama T, Ikeda N, Kawasaki T, Fukunaga K, Ohkohchi N. Effects of thrombopoietin on growth of hepatocellular carcinoma: is thrombopoietin therapy for liver disease safe or not? Hepatol Res. 2013;43(6):610–620. doi: 10.1111/hepr.12006. [DOI] [PubMed] [Google Scholar]
- 33.Liang SX, Zhu XD, Xu ZY, Zhu J, Zhao JD, Lu HJ, Yang YL, Chen L, Wang AY, Fu XL. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys. 2006;65(2):426–434. doi: 10.1016/j.ijrobp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 34.Barney BM, Haddock MG, Miller RC, Olivier KR. Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol. 2012;7(1):67. doi: 10.1186/1748-717X-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahisa E, Atsuya T, Naoko S, Yohei O, Yousuke A, Naoyuki S, Etsuo K. Acceptable toxicity after stereotactic body radiation therapy for liver tumors adjacent to the central biliary system. Int J Radiat Oncol Biol Phys. 2013;85(4):1006–1011. doi: 10.1016/j.ijrobp.2012.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The information of the 108 patients involved in the study. (XLSX 15 kb)
Data Availability Statement
The information of the 108 patients involved in this study is presented in the Additional file 1.
Date sharing is not applicable to this article as no datasets were generated or analyzed during the study.