Short abstract
Erythropoietin (EPO) is a cytokine mainly induced in hypoxia conditions. Its major production site is the kidney. EPO primarily acts on the erythroid progenitor cells in the bone marrow. More and more studies are highlighting its secondary functions, with a crucial focus on its role in the central nervous system. Here, EPO may interact with up to four distinct isoforms of its receptor (erythropoietin receptor [EPOR]), activating different signaling cascades with roles in neuroprotection and neurogenesis. Indeed, the EPO/EPOR axis has been widely studied in the neurodegenerative diseases field. Its potential therapeutic effects have been evaluated in multiple disorders, such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, spinal cord injury, as well as brain ischemia, hypoxia, and hyperoxia. EPO is showing great promise by counteracting secondary neuroinflammatory processes, reactive oxygen species imbalance, and cell death in these diseases. Multiple studies have been performed both in vitro and in vivo, characterizing the mechanisms through which EPO exerts its neurotrophic action. In some cases, clinical trials involving EPO have been performed, highlighting its therapeutic potential. Together, all these works indicate the potential beneficial effects of EPO.
Keywords: erythropoietin, neuroinflammation, neuroprotection, neurodegeneration
Introduction
Erythropoietin (EPO) is a 30-kDa molecular weight glycoprotein of 165 amino acids, coded by the EPO gene, located on Chromosome 7 (Law et al., 1986). EPO presents one small O-linked glycan and three tetra-antennary N-linked polysaccharide units, fundamental for its secretion, molecular stability, and binding to its receptor, erythropoietin receptor (EPOR; Tsuda et al., 1988). EPO is a type I cytokine essential for erythroid development and maturation (Brines and Cerami, 2005). Its expression is canonically induced by both hypoxia-inducible factor-1 (HIF-1) and HIF-2 (Keswani et al., 2011; Maiese et al., 2012), transduction factors sensitive to hypoxia and anemia. EPO expression is thus activated during the hematopoiesis process, where it coordinates the hormonal modulation on the hematopoietic system (Jelkmann, 2001). EPO’s main site of production changes throughout human life. During fetal development, blood cells are produced in the liver, and EPO is produced locally in this organ. After birth, the major EPO’s production site becomes the kidney peritubular cells (Jelkmann, 2001). Secondary EPO production sites have been described, and EPO’s expression in organs other than the kidney accounts for about 15% to 20% of the total production throughout the organism (Ponce et al., 2013). Although the kidney peritubular interstitial cells remain the main site of EPO production and secretion, a more pleiotropic role for EPO has been demonstrated in studies showing its expression in the endothelium and in the central nervous system (CNS; Anagnostou et al., 1990; Digicaylioglu et al., 1995; Marti et al., 1996). Within the CNS, EPO’s production and secretion has been demonstrated in the hippocampus, cortex, and midbrain. Furthermore, in the development of the brain and vascular system, EPO has been demonstrated to have a relevant function in promoting and stimulating neurogenesis (Shingo et al., 2001; Lombardero et al., 2011). Moreover, EPO limits cell injury and blocks the production of reactive oxygen species (ROS; Dang et al., 2010). Its neuroprotective effect and its role in counteracting oxidative stress indicate a potential beneficial effect for the molecule in the treatment of neurodegenerative diseases. EPO is currently the most sold biopharmaceutical product worldwide according to the Food and Drug Administration, and in particular, recombinant human EPO (rhEPO) is indicated in the treatment of anemic patients and diseases associated with low concentrations of EPO in plasma (European Medicines Agency; U.S. Food and Drug Administration; Ng et al., 2003). Remarkably, these patients show significant cognitive improvements, leading to the conjecture that EPO could be used for the treatment of neurodegenerative diseases (Sargin et al., 2011).
EPOR: Different Localizations for a Pleiotropic Function
EPOR presents a 225-amino acids extracellular domain, a single transmembrane section of 23 amino acids, and a cytoplasmic domain of 235 amino acids (Constantinescu et al., 1999; Marcuzzi et al., 2016). The extracellular component presents two domains, D1 and D2, both necessary for EPO’s binding (de Vos et al., 1992; Matthews et al., 1996). In the hematopoietic system, EPO’s binding to its receptor leads to homodimerization, with the activation of the associated Janus tyrosine kinase 2 (JAK2) and secondary signaling molecules, such as the signal transducer and activator of transcription 5 (STAT5), phosphatidylinositol 3-kinase (PI3K), and mitogen-activated protein kinase (MAPK; Darnell et al., 1994; Constantinescu et al., 1999). This signaling leads to differentiation and lineage commitment of erythroid colony-forming unit and burst-forming unit progenitors, indicating that EPO’s signaling is necessary for red cell maturation (Constantinescu et al., 1999; Brines and Cerami, 2005; Richmond et al., 2005).
In the past 20 years, many studies have reported various nonhematopoietic functions of EPO and EPOR in endothelial cells, myoblasts differentiation, colon, and in the CNS (Anagnostou et al., 1990; Ogilvie et al., 2000; Arcasoy et al., 2003; Brines and Cerami, 2005; Maiese et al., 2012). Interestingly, it has been demonstrated that biologically active EPO is synthesized in the brain with important roles in neurodevelopment and in the modulation of neurotransmission (Masuda et al., 1994; Digicaylioglu et al., 1995). As EPOR is detected at the embryonic state in neurons and astrocytes of the brain and spinal cord, EPO seems to have an important function in the brain already in the fetal stage (Juul et al., 1999). Linked to this finding, it has been demonstrated that EPOR is present in rats’ oligodendrocytes and astrocytes in culture and that rhEPO administration enhances their maturation and proliferation, suggesting that the EPO/EPOR interaction is important in tissue repair after damage (Sugawa et al., 2002).
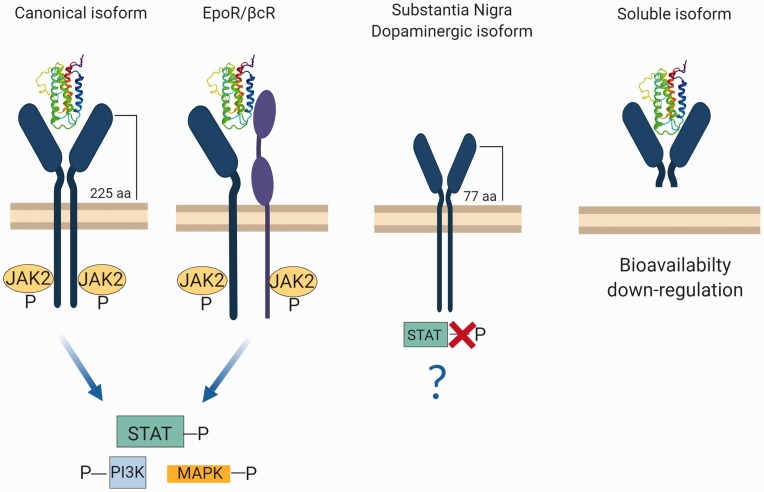
The different functions exerted in the CNS with respect to the hematopoietic system could be explained by the fact that EPOR occurs in at least four different isoforms, expressed specifically in different tissues (Ostrowski and Heinrich, 2018; Figure 1).
Figure 1.
Different isoforms of EPOR in the CNS. In its canonical isoform, EPO’s binding leads to the homodimerization of the receptor and phosphorylation of JAK2 molecules. This activates specific intracellular pathways (STAT-PI3K-MAPK). In the second case, the EPOR monomer interacts with the beta common receptor βcR activating the JAK2 pathway. A third isoform of the receptor occurs in dopaminergic neurons of the substantia nigra, where EPOR results altered in the extra regional domain. In this case, the isoform is shorter than the full form, leading to the lack of STAT phosphorylation. Last, a soluble version of the receptor has been found in the murine brain. This isoform interacts with EPO, with no activation of any downstream pathway. This leads to a reduced availability of EPO, thereby reducing its interaction with other isoforms of EPOR. Made in ©BioRender—biorender.com
aa = amino acids; EPOR = erythropoietin receptor; JAK2 = Janus tyrosine kinase 2; MAPK = mitogen-activated protein kinase; PI3K = phosphatidylinositol 3-kinase; STAT = signal transducer and activator of transcription.
The canonical isoform, which is mainly present in the hematopoietic system, is present in the brain (Constantinescu et al., 1999). EPO’s binding to EPOR leads to the formation of a homodimer, which phosphorylates JAK2 and activates the subsequent intracellular pathway that enables erythroid differentiation and maturation (Constantinescu et al., 1999). In the brain, the canonical isoform activation by EPO mediates EPO’s action in neuroinflammation and hypoxia (Lombardero et al., 2011).
The second isoform of EPOR is involved in neural tissue protection (Brines et al., 2004). In this case, the EPOR monomer interacts with the beta common receptor βcR (CD131), a typical receptor subunit of interleukin 3, interleukin 5, and granulocyte-macrophage colony stimulating factor (Brines and Cerami, 2005). The most accredited hypothesis is that the dimer formation results in a specific tissue—protective receptor. The activation of this receptor seems to activate the same JAK2 signaling pathway as the canonical homodimer (Brines et al., 2004; Brines and Cerami, 2012).
The third isoform of the receptor has been found in dopaminergic neurons of the substantia nigra, where EPOR results altered in the extra regional domain, being shorter than the full form (Marcuzzi et al., 2016). EPOR truncated isoform does not implicate STAT phosphorylation, suggesting the presence of another mechanism of action not yet characterized (Marcuzzi et al., 2016). This could be useful for the identification of possible drugs targeting specifically this isoform.
Last, an extracellular soluble version of the receptor has been shown to occur in the murine brain. This isoform interacts with EPO, with no activation of any downstream mediator. This leads to a reduced bioavailability of EPO, thereby limiting its interaction with other isoforms of EPOR. The expression of this isoform results downregulated during hypoxia, which triggers a mechanism that opposes full-length EPOR (Soliz et al., 2007).
Even if the presence of all these different isoforms paves the way for new interesting mechanisms through which EPO could exert its function in the brain, many questions remain unanswered. Indeed, it is necessary to investigate how EPO’s binding to each isoform activates a specific intracellular pathway, modulating the expression of target genes. Once these mechanisms have been elucidated, it will be possible to develop new isoform-selective drugs, resulting in a more specific therapy.
EPO Is Involved in Canonical Cellular Pathways of Neurodegeneration
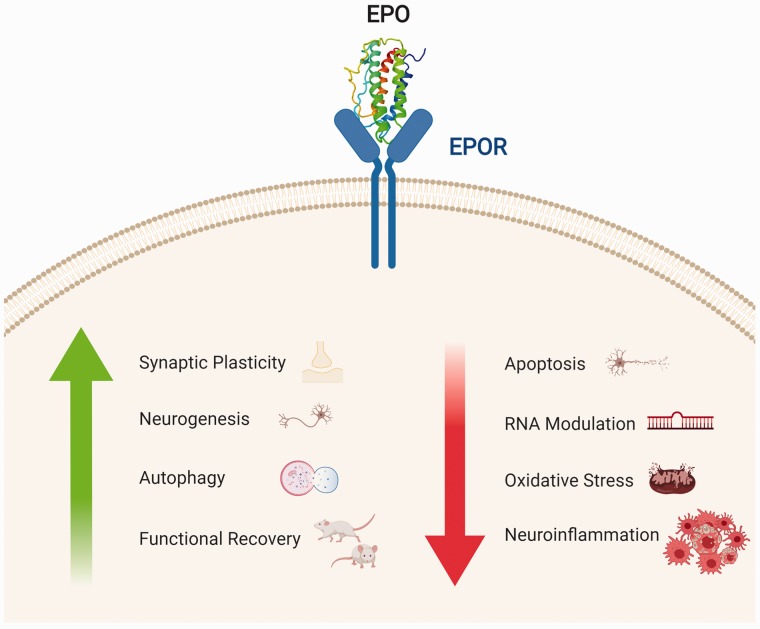
EPO’s production and function in the brain are now widely investigated. Multiple studies in vitro and in preclinical in vivo models have highlighted the beneficial effects of the cytokine. EPO exerts a neurotrophic action on multiple pathways involved in neurodegeneration (Figure 2; Bartesaghi et al., 2005; Maiese et al., 2012; Chong et al., 2013; Bond and Rex, 2014; Jelkmann, 2016; Hernández et al., 2017).
Figure 2.
Neurotrophic role of EPO’s binding with EPOR. In the CNS, EPO’s binding to its receptor leads to an increase in neuroprotective actions (synaptic plasticity, neurogenesis, autophagy) with an overall functional recovery in animal models. Conversely, the binding leads to a decrease in apoptosis, expression of miRNAs regulating the apoptotic process, oxidative stress, and neuroinflammation. Made in ©BioRender—biorender.com
EPO = erythropoietin; EPOR = erythropoietin receptor.
Regulation of Cellular Proliferation: Effects on Neurogenesis and Apoptosis
Considering the fact that EPO’s primary function is the regulation of the development and maturation of erythroid cells, it is worth investigating whether a similar function could be exerted by targeting neuronal cells. EPO’s effects on neurogenesis are now widely studied, representing a key pathway through which EPO could exert its neuroprotective roles in neurodegenerative diseases (Chong et al., 2013). Furthermore, the ablation of the canonical isoform of EPOR in knockout murine models results in massive apoptosis, associated with a reduction of neural progenitor cells and death at Embryonic Day 13.5 (Yu et al., 2002). As EPO’s effects on neurogenesis are pleiotropic, they may concern neural precursors cells (NPCs) of different origin as well and may include increased proliferation of progenitors with accelerated differentiation, a process described in hypoxic mesencephalic precursors cells (Studer et al., 2000). Likewise, similar effects of EPO in neural stem cells isolated from both murine E14 ganglionic eminence and adult subventricular zone have been characterized (Shingo et al., 2001). Furthermore, EPO seems to play a pivotal role in the enhancement of hippocampal neurogenesis (Osredkar et al., 2010; Hassouna et al., 2016). EPO-led neurogenesis was also observed in spinal cord-derived neural stem cells (Zhang et al., 2018). EPO can also indirectly promote neurogenesis through the activation of brain-derived neurotrophic factor (BDNF; Wang et al., 2004). Besides increasing neurogenesis, EPO can inhibit the apoptosis process (Merelli et al., 2015). The activation of EPOR downstream molecules JAK2 and PI3K, together with the regulation of the apoptotic proteins Bad and Bcl-xL, leads to a decrease in the apoptosis process (Bartesaghi et al., 2005; Shang et al., 2012; Hernández et al., 2017).
Modulation by Oxygen: Implications of Hypoxia and ROS Formation
EPO is perhaps the first ever protein whose expression was reported to be oxygen-dependent (Jelkmann, 2007). The discovery of the HIF-1α was indeed made possible by targeting EPO itself as downstream protein regulated by hypoxia (Semenza et al., 1991). Today, however, HIF-2α, also known as EPAS-1 (endothelial PAS domain protein 1), seems to be more critical than HIF-1 in overexpressing EPO during hypoxia (Chavez et al., 2006; Haase, 2013). Although the classical hypoxia-dependent EPO/EPOR system was long believed to target the kidney-erythroid axis, today, the brain axis is highlighted as an important protective mechanism, based on the observation that EPO and EPOR are expressed in those regions of the brain that are most sensitive to hypoxia, that is, the hippocampus and the telencephalon (Digicaylioglu et al., 1995). Thus, it is tentative to speculate that EPO’s physiological role in the brain is to act as a protective agent against hypoxia (Rabie and Marti, 2008) and, possibly, ischemia. To this end, the role of EPO in brain ischemia has been widely studied and will be discussed in depth in a subsequent section. Even so, it is worth noting that ischemia-preconditioned neurons protect neighboring astrocytes through an EPO-mediated activation of antioxidant proteins and enzymes (Wu et al., 2017). ROS also have a role in perpetrating tissue damage and neuronal death in numerous other neurodegenerative disorders (Kim et al., 2015; Liu et al., 2017b). More specifically, EPO antioxidant mechanisms reduce beta-amyloid-induced cell death (Li et al., 2008) and increases tyrosine hydroxylase (TH)-positive neurons in a rat model of Parkinson’s disease (PD; Erbaş et al., 2015).
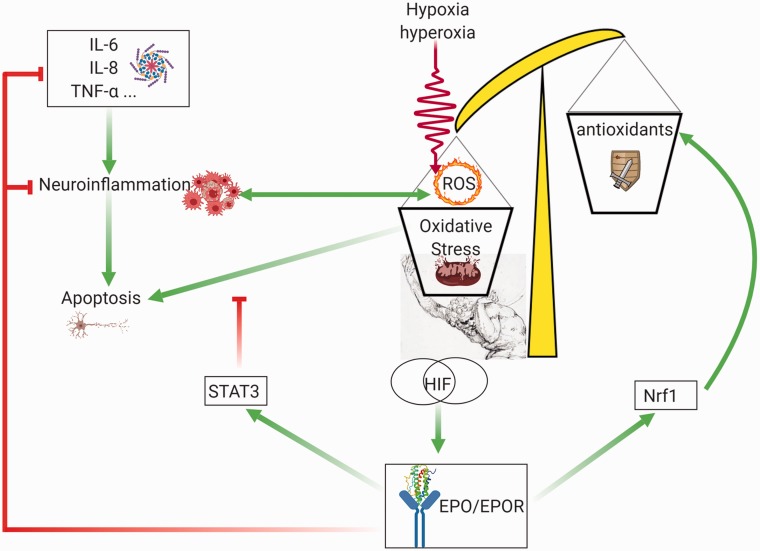
If EPO and HIF-1 respond to hypoxia, it is tempting to believe that excess oxygen, or hyperoxia, has opposite outcomes. Despite being a straightforward paradigm, literature is, however, very controversial. Although a few early studies support this view (Fletcher et al., 1973; Kokot et al., 1994a, 1994b), more recent studies testing the effects of transient hyperoxia on EPO blood levels show increased, not decreased, circulating EPO (Balestra et al., 2006, 2004; Donati et al., 2017; Kiboub et al., 2018). This has also been confirmed in a clinical trial (Hyperoxia, Erythropoiesis and Microcirculation in Critically Ill Patient, NCT02481843). Still another study shows a significant increase of EPO production after a short-term hyperoxic stimulus, only when preceded by antioxidants administration (Momeni et al., 2011; Rocco et al., 2014). This apparently paradoxical response has a strong mechanistic background. Indeed, despite their name, HIFs are stabilized not only by hypoxia but also by hyperoxia. This feature was observed in rats’ brains exposed to 50% oxygen for 3 weeks (Benderro et al., 2012) as well as in mice exposed to 30% oxygen (Terraneo et al., 2017). These key findings are indirectly confirmed in growing prostate cancer (Terraneo et al., 2014), myocardium (Wikenheiser et al., 2009; Zara et al., 2012; Gyongyosi et al., 2018) as well as newborn rats hepatocytes and liver hemopoietic cells (Marconi et al., 2014). It has been pointed out that the main reason for the high sensitivity of HIF’s to hyperoxia may not be related to HIF mRNA but rather to a decreased expression of the prolyl hydroxylases that degrade and address HIF’s toward ubiquitination (Terraneo and Samaja, 2017). The generation of oxidative stress in both hypoxia and hyperoxia may play an additional pivotal role in HIF stabilization and hence EPO production. Although ROS blockade is clearly associated with increased prolyl hydroxylation and therefore decreased HIF activity (Chandel et al., 2000; Brunelle et al., 2005; Guzy et al., 2005; Kaelin, 2005), it is not clear in which direction lack or excess oxygen modulates the oxidative stress in the brain. For example, long-term 30% oxygen cause less oxidative stress than 10% oxygen (Terraneo et al., 2017). Nevertheless, the recent finding of a molecular mechanisms for HIF-1α activation alternative of hydroxylation, which depends on the cAMP-PKA pathway, could provide an explanation for this apparently contradictory behavior (Bullen et al., 2016). The mechanism through which EPO/EPOR interaction modulates oxidative stress has been summarized in Figure 3.
Figure 3.
Role of the EPO/EPOR pathway in opposing the oxidative stress induced by hypoxia or hyperoxia. Both hypoxia and hyperoxia are sources of oxidative stress and activate the HIF pathway, which leads to increased EPO expression and EPO/EPOR interaction, which activates the antiapoptotic STAT3 pathway, and increases the antioxidant enzymes expression through the Nrf1 pathway. Green arrows: activation. Red lines: inhibition. Made in ©BioRender—biorender.com
EPO = erythropoietin; EPOR = erythropoietin receptor; HIF-1 = hypoxia-inducible factor 1; IL-6 = interleukin-6; IL-8 = interleukin-8; Nrf1 = nuclear respiratory factor 1; TNF-α = tumor necrosis factor alpha; ROS = reactive oxygen species; STAT3 = signal transducer and activator of transcription 3.
EPO’s Function in Suppressing Neuroinflammation
EPO has emerged as a multifunctional tissue-protective cytokine, and this is partially due to its anti-inflammatory effects (Wang et al., 2011; Sanchis-Gomar et al., 2014). Indeed, when rhEPO is administered in the setting of focal brain ischemia in rats, it crosses the blood–brain barrier and reduces injury severity by 50% to 75% (Brines et al., 2000). rhEPO appears effective in preclinical models of other types of injury in the nervous system whose gravity is exacerbated by inflammation (Sugawa et al., 2002). In a preclinical model of multiple sclerosis (consisting in experimental autoimmune encephalitis), rhEPO reduced the production and release of proinflammatory cytokines and chemokines, and the influx of inflammatory cells into the injured tissue (Agnello et al., 2002). EPO’s neuroprotective effect is mediated by EPOR; this is particularly relevant during hypoxia, where it has been proposed to contribute to endogenous neuroprotection during carotid endarterectomy (Fantacci et al., 2006; Carelli et al., 2016a; Terraneo et al., 2017; Terraneo and Samaja, 2017). The mechanism whereby EPO exerts its neuroprotective action might involve activation of the antiapoptotic STAT3 pathway (Ponce et al., 2013; Jia et al., 2014), the increase of antioxidant enzymes expression, and the reduction of ROS production (Bartesaghi et al., 2005; Chavez et al., 2006).
Novel Potential Targets: Modulation of RNA Expression
In recent years, the role of the noncoding epigenome in disease mechanisms is gaining more and more importance. Among the multiple mechanisms implicated in neurodegeneration, a new attention has been posed on the alteration of RNA processing and function in neurodegenerative diseases (Liu et al., 2017a). New studies have implicated more and more different classes of noncoding RNAs (ranging from microRNAs to long noncoding RNAs) in neurodegeneration (Salta and De Strooper, 2017). An epigenetic activity for EPO has been reported for erythroid cells proliferation (Perreault et al., 2017), but a similar regulation in the brain is yet to be fully characterized. Interestingly, EPO downregulates two microRNA molecules (miR-451 and miR-855-5p) in the SH-SY5Y neuroblastoma cell line (Alural et al., 2014). This downregulation seems to be responsible for the neurotrophic, neuroprotective, antioxidant, and antiapoptotic effects observed after EPO’s administration in SH-SY5Y (Alural et al., 2014). EPO’s downregulation of miR-451 also has a possible implication in the progression of glioblastoma (Alural et al., 2017). Further studies by transcriptomic approach investigated the deregulated RNAs which could be associated with EPOR’s signaling (Sollinger et al., 2017). Indeed, EPOR deletion in rodent fetal NPCs is associated with altered expression of key transcriptional and epigenetic regulators in the developing CNS. Multiple transcriptomic analysis revealed different factors involved, such as REST and NRF1. REST is an epigenetic modifier involved in neural differentiation and plasticity (Gao et al., 2011). NRF1 is a key regulator of antioxidant response and mitochondrial biogenesis (Lee et al., 2011). These two factors could act mediating EPO’s downstream pathway in the developing CNS (Sollinger et al., 2017).
EPO’s Implications in Neurodegenerative Diseases
To this day, there is no curative therapy for the treatment of neurodegenerative diseases. Along with the canonical first line therapies used for each specific neurodegenerative disease, new therapies are now being investigated. Epigenetic therapy, gene therapy, stem cells therapy, and even 3-D organoids are gaining more and more relevance in this context (Sakthiswary and Raymond, 2012; Coppedè, 2014; Piguet et al., 2017; Bordoni et al., 2018). Even so, the potentiality of a single-drug therapy is not to be underestimated, and EPO’s role as a therapeutic agent in neurodegenerative diseases is clearly worth investigating (Brines and Cerami, 2005).
Parkinson’s Disease
PD is the second most common neurodegenerative disorder. Its symptoms can be both motor (bradykinesia, rigidity, and tremor) and nonmotor (these can precede the manifestation of the disease; Tysnes and Storstein, 2017). The two main pathological hallmarks are the death of dopaminergic neurons in the substantia nigra pars compacta (Wirdefeldt et al., 2011) and the presence of alpha synuclein aggregates, noted as primary components of Lewy bodies (Benskey et al., 2016). These aggregates lead to cellular toxicity with an increase in oxidative stress and neuroinflammation (Benskey et al., 2016). Dopaminergic agonists (such as L-3,4-dihydroxyphenylalanine, L-DOPA) are the first line of therapy for PD treatment, but they are only a symptomatic remedy as they do not provide permanent or disease-altering effects (Nutt and Wooten, 2005). Various studies have reported EPO’s ability to counteract numerous processes altered in PD, such as oxidative stress, inflammation, and mitochondrial dysfunction (Jang et al., 2016). Neuroprotective effects have been demonstrated in numerous in vitro and in vivo PD models. Examples include improvements in the vitality and an alteration of the Bax/Bcl-2 ratio in PC12 cells intoxicated with 1-methyl-4-phenylpyridinium (Wu et al., 2007). EPO treatment induces the autophagy process in rotenone-treated SH-SY5Y cells, as observed with a specific analysis of autophagy markers, such as Beclin-1, AMPK, and ULK-1 (Jang et al., 2016). Another possible pathway in which EPO could be involved is the activation of PI3K/Akt/FoxO3a signaling, known to be able to prevent apoptosis (Maiese et al., 2012). It has been shown that PC12 cells pretreated with 6-hydroxydopamine show a major vitality after EPO’s administration (Jia et al., 2014).
EPO is involved in neuroinflammation modulation with different neurotrophic actions on astrocytes, microglia, and neurons (Bond and Rex, 2014). Indeed, EPO is able to preserve the blood–brain barrier through the inhibition of microvascular endothelial cells’ apoptosis and astrocytes’ activation (Maiese et al., 2012). Specifically, astrocytes respond to EPO’s presence through the activation of JNK and p38-MAPK, a decrease of glial swelling, and a reduction of blood–brain barrier permeability (Gunnarson et al., 2009; Tang et al., 2013). It has also been shown that the upregulation of the stress-induced intermediate filament protein is associated with glial hypertrophy, and it is decreased by EPO, independently of the MAPK pathway (Gonzalez et al., 2013). Moreover, EPO may directly influence the reactive microglia, which has the ability to engulf dying neurons through the increased expression of phosphatidylserine receptor, which specifically targets apoptotic neurons (De et al., 2002; Bond and Rex, 2014). EPO decreases the in vitro levels of the phosphatidylserine receptor on the microglial plasma membrane, suggesting its ability to redirect microglia and prevent its proliferation (Chong et al., 2003). Interestingly, EPO’s specific action on neurons is still widely unknown, but the presence of EPOR’s receptor indicates a specific function for the molecule in this context (Constantinescu et al., 1999; Brines and Cerami, 2005, 2012). Moreover, EPO has been found to block apoptosis in retinal neurons (Grimm et al., 2002; Weishaupt et al., 2004; Bond and Rex, 2014).
Different signaling effects of EPO as a neuroprotective molecule have been tested in vivo, specifically in rodent models of PD. It has been reported that EPO restores the levels of TH in rats in which parkinsonism has been induced with rotenone or 6-hydroxydopamine and decreases the levels of tumor necrosis factor alpha, indicating that EPO could exert its mechanisms through the modulation of neuroinflammation (Qi et al., 2014; Erbaş et al., 2015). The importance of EPO’s paracrine release has been investigated, with particular reference to the neuroprotective effects of a class of NPCs able to release erythropoietin (Er-NPCs) in a murine model of PD (Marfia et al., 2011; Carelli et al., 2016b, 2017b). The injection of Er-NPCs in mice, pretreated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, promotes functional recovery and enhances survival of nigrostriatal neurons associated to the restored levels of TH. These effects are likely due to a modulation of the neuroinflammatory process, as Er-NPCs are able to reduce the expression of early proinflammatory and microglia markers (Carelli et al., 2018). To this day, only two clinical trials have studied the role of rhEPO in PD patients (Pedroso et al., 2012; Jang et al., 2014). Pedroso et al. (2012) demonstrated the safety of ior-EPOCIM, a recombinant human EPO produced in Cuba and approved for use in humans, in 10 PD patients (Pedroso et al., 2012). They evaluated its possible neuroprotective effects and demonstrated improvements in motor functions, cognitive status, and mood. To investigate any possible side effect linked to the hematopoietic action of EPO, haematological parameters and blood pressure were evaluated, noting no adverse reactions (Pedroso et al., 2012). The second clinical trial showed that rhEPO has positive effects on nonmotor symptoms in PD patients, such as pain, apathy, and sexual difficulty. Again, no side effects due to hypertension or iron deficiency were observed, and this was true for the whole 12 months observational period (Jang et al., 2014).
Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common form of dementia, clinically characterized by a progressive loss of memory and a decline in cognitive functions (Karch et al., 2014). The first evidences indicating that EPO could have a role in improving cognitive functions arose from observations relating to nonneurological diseases, where patients in hemodialysis treated with EPO demonstrated an improvement in cognitive functions (Grimm et al., 1990; Temple et al., 1995). These early observations led to more in-depth studies aiming to characterize the pleiotropic effects of the molecule and its potential benefits in diseases where cognitive function was impaired, such as AD (Rabie and Marti, 2008; Hernández et al., 2017). At a cellular level, the hallmarks of the disease are the presence of extracellular neuritic plaques (due to Aβ aggregation) and intracellular neurofibrillary tangles (characterized by a hyperphosphorylation of the tau protein; Blennow et al., 2006). So far, no curative treatment has been found for AD, and classic pharmacological therapy includes acetylcholinesterase inhibitors, N-methyl-D-aspartate antagonists, and their possible combination (Madav et al., 2019). For this reason, it is necessary to evaluate the therapeutic potentials of new approaches and molecules, such as EPO. Indeed, multiple studies have already been performed, showing promising results (Sun et al., 2019). The therapeutic effects of EPO have been first studied at a cellular level, using both stable cell lines, such as PC12 (Li et al., 2008), and primary hippocampal neurons (Viviani et al., 2005). The molecule seems to exert its effect through the blockade of the apoptotic pathway and the protection from Aβ toxicity (Ma et al., 2009; Esmaeili Tazangi et al., 2015). Moreover, it is possible to observe a taming of the neuroinflammatory response (Shang et al., 2011, 2012) and an increase of antioxidant mechanisms (Li et al., 2008). Interestingly, EPO also shows a neurotrophic function in primary hippocampal neurons through the expression of BDNF (Viviani et al., 2005).
Most of these neuroprotective effects observed in vitro have been evaluated in vivo in preclinical models of the disease. First of all, EPO injections reduced the levels of Aβ in transgenic and Aβ treated mice and rats (Lee et al., 2012; Shang et al., 2012; Armand-Ugón et al., 2015; Samy et al., 2016; Rodríguez Cruz et al., 2017), demonstrating the molecule’s ability in tackling the first pathological hallmark of the disease. Furthermore, EPO seemed to decrease tau hyperphosphorylation (Li et al., 2015), reduce neuroinflammation and oxidative stress (Shang et al., 2012; Maurice et al., 2013), and increase neurogenesis (Arabpoor et al., 2012). Preclinical murine models have become crucial for the assessment of behavioral functions. Indeed, EPO treatment improved cognitive results in AD mice models, when learning and spatial memory were evaluated. Both in transgenic and Aβ treated mice models, EPO’s administration led to improvements in fear-conditioning and step-down avoidance tests, where the treated animals showed a recovery of associative learning memory (Lee et al., 2012; Li et al., 2015). AD animals performed better in tests aimed at evaluating spatial memory (Morris Water Maze and Y-maze) when treated with EPO, indicating effects for the molecule in this type of learning (Maurice et al., 2013; Rodríguez Cruz et al., 2017). Furthermore, the molecule seems to have a recovery effect on synaptic plasticity defects (Adamcio et al., 2008; Esmaeili Tazangi et al., 2015). This, together with the fact that EPOR is expressed in the hippocampus (Lee et al., 2012), suggests the possibility for elective effects of EPO in this disease (Hernández et al., 2017). Although no clinical trials involving EPO have been performed so far in AD, one study investigated the effect of rhEPO on the amyloidogenic pathway proteins in patients affected by chronic kidney disease associated with cognitive dysfunction. Even if the patients enrolled in this study were young (age range 20–39), the platelets level of AD-associated proteins was altered: The amyloid precursor protein and α secretase decreased, and BACE1, presenilin 1, and Aβ increased. Western blot analysis revealed that these protein abnormalities could be reverted by rhEPO treatment, along with improved neuropsychological tests scoring (Vinothkumar et al., 2018), thereby suggesting promise for EPO in AD treatment. Furthermore, in a clinical report of an AD patient with trochanteric fracture of the left femur and chronic renal failure associated with severe anemia, rhEPO treatment increased the speech level after 2 days and the food intake after 8 days, which substantially improved the patient’s quality of life (Kumagai et al., 2018). These authors state that the daily living activities could improve due to the EPO antidepressant effects that preceded the correction of anemia (Kumagai et al., 2018).
Amyotrophic Lateral Sclerosis
Out of all the motor neuron diseases, amyotrophic lateral sclerosis (ALS) is the most prevalent. It is characterized by the progressive loss of upper and lower motor neurons, leading to muscle atrophy, paralysis, and death 2 to 5 years after the first diagnosis (Wijesekera and Leigh, 2009). Perhaps the most tragic effect of ALS, which eventually leads to patients’ death, is the respiratory muscle weakness, which causes ventilator insufficiency and tissue hypoxia (Carilho et al., 2011). This observation led to the investigation of the role of EPO in ALS. Initial studies focused on the expression levels of EPO in cerebrospinal fluid, serum, and plasma of ALS patients. Studies demonstrated a decrease of EPO levels in ALS patients’ cerebrospinal fluid but not in patients’ serum (Brettschneider et al., 2007; Widl et al., 2007). Further studies indicated that EPO levels were decreased in the plasma of only a subset of ALS patients, which are those that presented with respiratory failure (Carilho et al., 2011). In vitro study aimed to elucidate how EPO could exert its protective actions in ALS patients and demonstrated that EPO prevented apoptotic neuronal changes and could even decrease the levels of the superoxide dismutase (SOD1) aggregates (Nagańska et al., 2010; Cho et al., 2011). The preclinical investigation of the beneficial effects of EPO was performed in SOD1-G93A mice, a model of familial ALS (Grignaschi et al., 2007). The results obtained in these studies, all performed in SOD1-G93A mice, are controversial. Indeed, two of them indicated that EPO delays only symptoms onset with not beneficial effects on mice survival (Grignaschi et al., 2007; Grunfeld et al., 2007). On the contrary, other studies indicated that EPO treatment significantly delayed symptom onset, prolonged life span, preserved motor neurons, and modulated the immune-inflammatory response (Koh et al., 2007; Noh et al., 2014). Even so, two different clinical trials aiming at studying rhEPO’s effects in ALS patients were started. Both studies demonstrated that treatment was safe and well tolerated, with no increase in the levels of haematocrit and haemoglobin (Lauria et al., 2009; Kim et al., 2014). The Phase III of the study performed by Lauria et al. (2009) indicated that no differences were observed in the rate of death or secondary outcomes in patients treated with EPO, suggesting that different doses of the molecule or a more target specific administration could be necessary (Lauria et al., 2015).
Spinal Cord Injury
Spinal cord injury (SCI) is a debilitating condition caused by high-energy trauma. It is characterized by severe neurological dysfunctions ranging from sensation loss to partial or complete limb paralysis (Profyris et al., 2004). The trauma leads to the release of toxic substances such as free radicals, lipid peroxidase, eicosanoids, and glutamate, causing neuronal and glial apoptosis (Hock, 1998; Profyris et al., 2004; Mofidi et al., 2011). The main strategy to promote recovery following SCI is the reduction of secondary degeneration occurring after the initial injury. There is now no curative therapy, and the current main therapeutic strategy is the reduction of secondary degeneration through the administration of high-dose corticosteroids, surgical stabilization, and decompression (King et al., 2007; Furlan et al., 2011). Various pharmacological agents are currently being evaluated in humans and animals, aimed at reducing SCI consequences, and EPO is resulting to be a very promising candidate (Celik et al., 2002; Leist et al., 2004; Gorio et al., 2005; Cetin et al., 2006; Carelli et al., 2014, 2015, 2017a). The beneficial neuroprotective and neurotrophic effects have been demonstrated in vitro and in vivo, where EPO’s administration led to a partial recovery of motor function (Rees et al., 2010; Mofidi et al., 2011; Cerri et al., 2012). EPO seemed to exert its beneficial effects through the prevention of secondary injury and neurodegeneration, probably through the combination of anti-inflammatory and neuroprotective actions (Gorio et al., 2002, 2005; Simon et al., 2011; Grégoire et al., 2015). Indeed, EPO led to antiapoptotic and anti-inflammatory effects, along with edema reduction (Simon et al., 2011, 2016; Nekoui and Blaise, 2017; Zhang et al., 2018).
Stem cells and stem cell-derived molecules have shown beneficial effects to a variable extent in the treatment of SCI (Martino and Pluchino, 2006; Romanko et al., 2007). Er-NPCs also show a beneficial effect when applied to SCI, leading to a promotion of axonal regeneration and an increase in TH-positive fibers in the ventral portion of the lumbosacral cord of the treated mice (Carelli et al., 2014, 2015, 2017a). Furthermore, Er-NPCs modify the microenvironment reducing the production of proinflammatory cytokines, such as tumor necrosis factor alpha and IL-6, and the infiltration of inflammatory cells (Carelli et al., 2014, 2015, 2017a). EPO also induces neurogenesis increasing BDNF’s activity (Mofidi et al., 2011; Carelli et al., 2015). To this day, only one clinical trial has been designed to study the role of rhEPO in SCI (Costa et al., 2015) in which it has been shown that the administration of rhEPO in acute SCI is safe. Unfortunately, the limited number of patients recruited and the short observational period were not sufficient to demonstrate EPO’s efficacy (Costa et al., 2015).
Brain Ischemia
Ischemic stroke is due to transient or permanent reduction in cerebral blood flow, generally caused by the occlusion of a cerebral artery, an embolus, or local thrombosis, which cause acute ischemia stroke (AIS; Dirnagl et al., 1999). The most common consequence of AIS is hypoxia, or the imbalance between oxygen supply and consumption (Dirnagl et al., 1999). Activation of HIF’s upregulates downstream factors, such as the vascular endothelial growth factor and EPO, which exert a neuroprotective action against ischemic damage (Dirnagl et al., 1999). Further consequences of ischemic strokes are the increase in ROS, as well as the inflammatory response by means of induction of proinflammatory genes, as tumor necrosis factor alpha and interleukin 1 beta (Ruscher et al., 1998). In in vitro models of hypoxia, EPO’s expression is upregulated in both murine astrocytes and neurons (Suzuki et al., 2001). It seems that EPO acts on forebrain neural stem cells, suggesting its involvement in neurogenesis (Dirnagl et al., 1999). In primary hippocampal and cortical neurons subjected to cerebral ischemia, cell viability improves following EPO treatment, suggesting its involvement in apoptosis and cell recovery (Dirnagl et al., 1999; Marti, 2004). Another study confirms EPO’s role in apoptosis: EPO pretreatment in cerebral cortical cells indeed activates antiapoptotic genes, such as XIAP and c-IAP2 (Ruscher et al., 1998; Suzuki et al., 2001).
Several experimental works demonstrated the involvement of the EPO/EPOR system in neuroprotection (Rothwell and Hopkins, 1995). rhEPO administration prevented ischemia-induced learning disability by rescuing hippocampal CA1 neurons from lethal ischemic damage (Lewczuk et al., 2000), while another study showed that the endogenous EPO/EPOR system helps protecting hypoxic astrocytes and oligodendrocyte precursor cells, showing that the suppression of endogenous EPO in astrocytes implies decreased protection of oligodendrocyte precursor cells and cell death (Bernaudin et al., 2000). This hypothesis is further supported by the fact that in the mammalian brain exposed to ischemic infarct or hypoxic damage, the EPO/EPOR system results activated and EPOR is expressed in neuronal fibers (Shingo et al., 2001). Other studies demonstrate that even though EPOR transcription is not directly influenced by hypoxia in vivo, anemic stress and ischemic conditions enhance its expression (Lewczuk et al., 2000; Digicaylioglu and Lipton, 2001). Another work on in vivo rabbits demonstrate that rhEPO is neuroprotective during cerebral AIS, showing a significant reduction of necrotic cortical neurons (Sanchez et al., 2009). Furthermore, rhEPO reduces the activation of astrocytes and the recruitment of leukocytes and of proinflammatory cytokines as IL-6 and tumor necrosis factor, suggesting that EPO can help reducing ischemia-induced inflammation (Sakanaka et al., 1998).
A few clinical trials tested the potential effects of EPO in AIS patients. The safety of rhEPO was demonstrated, showing that rhEPO, when infused intravenously 3 days after stroke, can pass the blood–brain barrier reducing the infarct size (Ehrenreich et al., 2002). Another study tested EPO’s effects on long-term neurological outcomes in AIS patients and evaluated EPO’s safety and benefits (Tsai et al., 2015). By contrast, a meta-analysis supporting the view that the efficacy evidence is inconsistent indicates that EPO’s administration in AIS patients is not recommended (Yao et al., 2017). It is thus evident that more studies are needed to assess rhEPO efficacy in AIS patients.
Brain Hypoxia and Hyperoxia
The discovery that HIF-1α binds to the EPO enhancer sequence in response to hypoxia (Semenza and Wang, 1992) univocally addresses the key role of EPO/EPOR to protect the neurological functions in brain tissue challenged not only by hypoxia (Sanchez et al., 2009; Terraneo et al., 2017), but also by ischemia (Sakanaka et al., 1998), carotid clamping (Carelli et al., 2016a), and hypoxia/reoxygenation (Kato et al., 2011). The most relevant mechanisms underlying neuroprotection involve either the prevention of neuronal apoptosis through upregulation of Akt phosphorylation (Chong et al., 2002; Caretti et al., 2008) or the increase of vascular NO availability through activation of eNOS (Beleslin-Cokic et al., 2004). In support of these mechanisms, treatment with rhEPO may provide clues for EPO-based therapy in pediatric chronic hypoxia by rescuing hypoxia-induced depression in hippocampal neurogenesis and oligodendrocyte progenitor populations (Chung et al., 2015), as well as in improving neurologic outcomes in newborns affected with hypoxic-ischemic encephalopathy (Zhu et al., 2009). To prevent erythropoiesis, carbamylated EPO, a derivative devoid of erythropoietic activity, represents a solid pharmacological tool to protect brain in severely hypoxic mice (Fantacci et al., 2006).
As stated earlier, hyperoxia may represent a valuable bench to test the hypothesis linked to the endogenous hypoxia-induced, EPO-mediated neuroprotection. The effect of experimental hyperoxia on brain EPO overexpression was examined in two studies with controversial outcomes. While breathing 50% oxygen for 3 weeks upregulates HIF-2α and EPO expressions in mouse brain (Benderro et al., 2012), breathing 30% oxygen for 4 week increases EPOR, but not EPO expression (Terraneo et al., 2017). As the EPO/EPOR system of neuroprotection requires the availability of both EPO and EPOR, it remains to be understood whether the recruitment of this system depends only on oxygen availability or other components are to be ruled in.
Future Challenges
A critical attention needs to be put to avoid the risk of adverse reactions, which would involve the hematopoietic system when EPO is chronically administered. To this end, EPO analogues are currently being investigated (Sun et al., 2019). These can present reduced erythropoietic activity or be posttranscriptionally modified to yield a molecule with enhanced clearance or selectively target the EPOR receptor (King et al., 2007; Armand-Ugón et al., 2015; Collino et al., 2015; Culver et al., 2017; Yoo et al., 2017). The development of novel EPO analogues with a more specific action and fewer side effects necessarily requires deeper investigation to decipher the relationship between the different isoforms of the receptor and the different functions of EPO.
Conclusions
Considering all the advances made in the recent years, EPO has shown great promise in the treatment of neurodegenerative diseases. It is able to target multiple pathways, which are deregulated in neurodegeneration, leading to an overall neuroprotective effect (Table 1).
Table 1.
Summary of the Studies Describing EPO’s Neurotrophic Effect in Neurodegenerative Diseases.
| Pathology | Description | References |
|---|---|---|
| Parkinson’s disease | ||
| In vitro | EPO increases cell viability altering the Bax/Bcl-2 ratio in PC12 cells intoxicated with MPP+, modulates the autophagy process in rotenone-treated SH-SY5Y cells, activates the PI3K/Akt/FoxO3a pathway, and modulates neuroinflammation. | Wu et al., 2007; Maiese et al., 2012; Bond and Rex, 2014; Jia et al., 2014; Jang et al., 2016 |
| In vivo | EPO restores TH levels and reduces the expression of early proinflammatory cytokines and microglia markers. | Qi et al., 2014; Erbas et al., 2015; Carelli et al., 2016a, 2017b, 2018 |
| Clinical trials | EPO has positive effects on nonmotor symptoms, such as pain, apathy, and sexual difficulty. | Pedroso et al., 2012; Jang et al., 2014 |
| Alzheimer’s disease | ||
| In vitro | EPO is used to block the apoptotic pathway and protects from Aβ toxicity, increases antioxidant mechanisms, and has a neurotrophic function in primary hippocampal neuron through BDNF’s expression. | Viviani et al., 2005; Li et al., 2008; Ma et al., 2009; Shang et al., 2011, 2012; Esmaeili Tazangi et al., 2015 |
| In vivo | EPO decreases tau hyper phosphorylation, reduces neuroinflammation and oxidative stress, increases neurogenesis, improves cognitive results in AD mice models, and improves synaptic plasticity but no effect on anxiety and spontaneous activity. | Lee et al., 2012; Armand-Ugón et al., 2015; Li et al., 2015; Samy et al., 2016; Hernández et al., 2017; Rodríguez Cruz et al., 2017 |
| Clinical trials | EPO improves neuropsychological test scoring in chronic kidney disease and ameliorates AD patient’s daily living. | Kumagai et al., 2018; Vinothkumar et al., 2018 |
| Amyotrophic lateral sclerosis | ||
| In vitro | EPO prevents apoptotic neuronal changes and decreases the levels of the SOD1 aggregates. | Nagańska et al., 2010; Cho et al., 2011 |
| In vivo | EPO administration delays symptoms, preserves motor symptoms, and modulates the neuroinflammatory response, but results are controversial with regard to the life span. | Grignaschi et al., 2007; Grunfeld et al., 2007; Koh et al., 2007; Noh et al., 2014 |
| Clinical trials | EPO administration has no adverse effects | Lauria et al., 2009; Kim et al., 2014 |
| Spinal cord injury | ||
| In vivo | EPO administration leads to a partial recovery of motor function, prevents the secondary injury through anti-inflammatory and neuroprotective actions, increases TH-positive fibers of lumbosacral cord of treated mice, reduces the production of proinflammatory cytokines, and induces neurogenesis increasing BDNF activity. | Gorio et al., 2002, 2005; Rees et al., 2010; Mofidi et al., 2011; Simon et al., 2011; Cerri et al., 2012; Carelli et al., 2014, 2015, 2017b |
| Clinical trials | EPO’s effect was not efficiently evaluated. | Costa et al., 2015 |
| Brain ischemia | ||
| In vitro | EPO acts on neurogenesis, is involved in apoptotic and cellular recovery, and activates antiapoptotic genes. | Ruscher et al., 1998; Dirnagl et al., 1999; Suzuki et al., 2001; Marti, 2004 |
| In vivo | EPO administration is involved in neuroprotection. | Shingo et al., 2001; Sanchez et al., 2009 |
| Clinical trials | EPO reduces infarct size. | Tsai et al., 2015; Yao et al., 2017 |
| Brain hypoxia and hyperoxia | ||
| In vivo | EPO ameliorates the metabolic stress and induces neuroprotection in chronic hypoxia. | Chung et al., 2015; Fantacci et al., 2006 |
| Clinical trials | EPO treatment in newborns with hypoxic-ischemic encephalopathy improves neurological outcomes. | Zhu et al., 2009 |
Note. EPO = erythropoietin; MPP+ = 1-methyl-4-phenylpyridinium; PI3K = phosphatidylinositol 3-kinase; TH = tyrosine hydroxylase; BDNF = brain-derived neurotrophic factor; AD = Alzheimer’s disease; SOD1 = superoxide dismutase.
Summary
EPO is a cytokine with a broad action on the CNS, where it is able to target multiple pathways that are deregulated in neurodegenerative diseases, leading to an overall neuroprotective effect.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge the economic support of the “Neurogel-en-Marche” Foundation (France), AUS Niguarda Onlus (Italy) to S.C., and Fondazione “Romeo and Enrica Invernizzi” to A. M. D. G.
References
- Adamcio B., Sargin D., Stradomska A., Medrihan L., Gertler C., Theis F., Zhang M., Müller M., Hassouna I., Hannke K., Sperling S., Radyushkin K., El-Kordi A., Schulze L., Ronnenberg A., Wolf F., Brose N., Rhee J. S., Zhang W., Ehrenreich H. (2008). Erythropoietin enhances hippocampal long-term potentiation and memory. BMC Biol, 6, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnello D., Bigini P., Villa P., Mennini T., Cerami A., Brines M. L., Ghezzi P. (2002). Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res 952, 128–134. [DOI] [PubMed] [Google Scholar]
- Alural B., Ayyildiz Z. O., Tufekci K. U., Genc S., Genc K. (2017). Erythropoietin promotes glioblastoma via miR-451 suppression. Vitam Horm, 105, 249–271. [DOI] [PubMed] [Google Scholar]
- Alural B., Duran G. A., Tufekci K. U., Allmer J., Onkal Z., Tunali D., Genc K., Genc S. (2014). EPO mediates neurotrophic, neuroprotective, anti-oxidant, and anti-apoptotic effects via downregulation of miR-451 and miR-885-5p in SH-SY5Y neuron-like cells. Front Immunol, 5, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou A., Lee E. S., Kessimian N., Levinson R., Steiner M. (1990). Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc Natl Acad Sci U S A, 87, 5978–5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabpoor Z., Hamidi G., Rashidi B., Shabrang M., Alaei H., Sharifi M. R., Salami M., Dolatabadi H. R., Reisi P. (2012). Erythropoietin improves neuronal proliferation in dentate gyrus of hippocampal formation in an animal model of Alzheimer’s disease. Adv Biomed Res, 1, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcasoy M. O., Jiang X., Haroon Z. A. (2003). Expression of erythropoietin receptor splice variants in human cancer. Biochem Biophys Res Commun, 307, 999–1007. [DOI] [PubMed] [Google Scholar]
- Armand-Ugón M., Aso E., Moreno J., Riera-Codina M., Sánchez A., Vegas E., Ferrer I. (2015). Memory improvement in the AβPP/PS1 mouse model of familial Alzheimer’s disease induced by carbamylated-erythropoietin is accompanied by modulation of synaptic genes. J Alzheimers Dis, 45, 407–421. [DOI] [PubMed] [Google Scholar]
- Balestra C., Germonpre P., Poortmans J. R., Marroni A. (2006). Serum erythropoietin levels in healthy humans after a short period of normobaric and hyperbaric oxygen breathing: The “normobaric oxygen paradox”. J Appl Physiol, 100, 512–518. [DOI] [PubMed] [Google Scholar]
- Balestra C., Germonpre P., Poortmans J., Marroni A., Schiettecatte J., Collard J. F., Snoeck T. (2004). Erythropoietin production can be enhanced by normobaric oxygen breathing in healthy humans. Undersea Hyperb Med, 31, 53–57. [PubMed] [Google Scholar]
- Bartesaghi S., Marinovich M., Corsini E., Galli C. L., Viviani B. (2005). Erythropoietin: A novel neuroprotective cytokine. Neurotoxicology, 26, 923–928. [DOI] [PubMed] [Google Scholar]
- Beleslin-Cokic B. B., Cokic V. P., Yu X., Weksler B. B., Schechter A. N., Noguchi C. T. (2004). Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood, 104, 2073–2080. [DOI] [PubMed] [Google Scholar]
- Benderro G. F., Sun X., Kuang Y., Lamanna J. C. (2012). Decreased VEGF expression and microvascular density, but increased HIF-1 and 2alpha accumulation and EPO expression in chronic moderate hyperoxia in the mouse brain. Brain Res, 1471, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskey M. J., Perez R. G., Manfredsson F. P. (2016). The contribution of alpha synuclein to neuronal survival and function – Implications for Parkinson’s disease. J Neurochem, 137, 331–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernaudin M., Bellail A., Marti H. H., Yvon A., Vivien D., Duchatelle I., Mackenzie E. T., Petit E. (2000). Neurons and astrocytes express EPO mRNA: Oxygen-sensing mechanisms that involve the redox-state of the brain. Glia, 30, 271–278. [PubMed] [Google Scholar]
- Blennow K., de Leon M. J., Zetterberg H. (2006). Alzheimer’s disease. Lancet, 368, 387–403. [DOI] [PubMed] [Google Scholar]
- Bond W. S., Rex T. S. (2014). Evidence that erythropoietin modulates neuroinflammation through differential action on neurons, astrocytes, and microglia. Front Immunol, 5, 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoni M., Rey F., Fantini V., Pansarasa O., Di Giulio A. M., Carelli S., Cereda C. (2018). From neuronal differentiation of iPSCs to 3D neuro-organoids: Modelling and therapy of neurodegenerative diseases. Int J Mol Sci, 19, 3972–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J., Widl K., Schattauer D., Ludolph A. C., Tumani H. (2007). Cerebrospinal fluid erythropoietin (EPO) in amyotrophic lateral sclerosis. Neurosci Lett, 416, 257–260. [DOI] [PubMed] [Google Scholar]
- Brines M., Cerami A. (2005). Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci, 6, 484–494. [DOI] [PubMed] [Google Scholar]
- Brines M., Cerami A. (2012). The receptor that tames the innate immune response. Mol Med, 18, 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M., Grasso G., Fiordaliso F., Sfacteria A., Ghezzi P., Fratelli M., Latini R., Xie Q. W., Smart J., Su-Rick C. J., Pobre E., Diaz D., Gomez D., Hand C., Coleman T., Cerami A. (2004). Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A, 101, 14907–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M. L., Ghezzi P., Keenan S., Agnello D., de Lanerolle N. C., Cerami C., Itri L. M., Cerami A. (2000). Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A, 97, 10526–10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle J. K., Bell E. L., Quesada N. M., Vercauteren K., Tiranti V., Zeviani M., Scarpulla R. C., Chandel N. S. (2005). Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metabol, 1, 409–414. [DOI] [PubMed] [Google Scholar]
- Bullen J. W., Tchernyshyov I., Holewinski R. J., DeVine L., Wu F., Venkatraman V., Kass D. L., Cole R. N., Van Eyk J., Semenza G. L. (2016). Protein kinase A-dependent phosphorylation stimulates the transcriptional activity of hypoxia-inducible factor 1. Sci Signal, 9, ra56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli S., Ghilardi G., Bianciardi P., Latorre E., Rubino F., Bissi M., Di Giulio A. M., Samaja M., Gorio A. (2016. a). Enhanced brain release of erythropoietin, cytokines and NO during carotid clamping. Neurol Sci, 37, 243–252. [DOI] [PubMed] [Google Scholar]
- Carelli S., Giallongo T., Gerace C., De Angelis A., Basso M. D., Di Giulio A. M., Gorio A. (2014). Neural stem cell transplantation in experimental contusive model of spinal cord injury. J Vis Exp, e52141. [DOI] [PMC free article] [PubMed]
- Carelli S., Giallongo T., Gombalova Z., Merli D., Di Giulio A. M., Gorio A. (2017. a). EPO-releasing neural precursor cells promote axonal regeneration and recovery of function in spinal cord traumatic injury. Restor Neurol Neurosci, 35, 583–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli S., Giallongo T., Gombalova Z., Rey F., Gorio M. C. F., Mazza M., Di Giulio A. M. (2018). Counteracting neuroinflammation in experimental Parkinson’s disease favors recovery of function: Effects of Er-NPCs administration. J Neuroinflammation, 15, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli S., Giallongo T., Marfia G., Merli D., Ottobrini L., Degrassi A., Basso M. D., Di Giulio A. M., Gorio A. (2015). Exogenous adult postmortem neural precursors attenuate secondary degeneration and promote myelin sparing and functional recovery following experimental spinal cord injury. Cell Transplant, 24, 703–719. [DOI] [PubMed] [Google Scholar]
- Carelli S., Giallongo T., Viaggi C., Gombalova Z., Latorre E., Mazza M., Vaglini F., Di Giulio A. M., Gorio A. (2016. b). Grafted neural precursors integrate into mouse striatum, differentiate and promote recovery of function through release of erythropoietin in MPTP-treated mice. ASN Neuro, 8, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli S., Giallongo T., Viaggi C., Latorre E., Gombalova Z., Raspa A., Mazza M., Vaglini F., Di Giulio A. M., Gorio A. (2017. b). Recovery from experimental parkinsonism by intrastriatal application of erythropoietin or EPO-releasing neural precursors. Neuropharmacology, 119, 76–90. [DOI] [PubMed] [Google Scholar]
- Caretti A., Bianciardi P., Ronchi R., Fantacci M., Guazzi M., Samaja M. (2008). Phosphodiesterase-5 inhibition abolishes neuron apoptosis induced by chronic hypoxia independently of hypoxia-inducible factor-1 alpha signaling. Exp Biol Med, 233, 1222–1230. [DOI] [PubMed] [Google Scholar]
- Carilho R., de Carvalho M., Kuehl U., Pinto S., Pinto A., Kromminga A., Costa J. (2011). Erythropoietin and amyotrophic lateral sclerosis: Plasma level determination. Amyotroph Lateral Scler 12, 439–443. [DOI] [PubMed] [Google Scholar]
- Celik M., Gökmen N., Erbayraktar S., Akhisaroglu M., Konakc S., Ulukus C., Genc S., Genc K., Sagiroglu E., Cerami A., Brines M. (2002). Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A, 99, 2258–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri G., Montagna M., Madaschi L., Merli D., Borroni P., Baldissera F., Gorio A. (2012). Erythropoietin effect on sensorimotor recovery after contusive spinal cord injury: An electrophysiological study in rats. Neuroscience, 219, 290–301. [DOI] [PubMed] [Google Scholar]
- Cetin A., Nas K., Büyükbayram H., Ceviz A., Olmez G. (2006). The effects of systemically administered methylprednisolone and recombinant human erythropoietin after acute spinal cord compressive injury in rats. Eur Spine J, 15, 1539–1544. [DOI] [PubMed] [Google Scholar]
- Chandel N. S., McClintock D. S., Feliciano C. E., Wood T. M., Melendez J. A., Rodriguez A. M., Schumacker P. T. (2000). Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem, 275, 25130–25138. [DOI] [PubMed] [Google Scholar]
- Chavez J. C., Baranova O., Lin J., Pichiule P. (2006). The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci, 26, 9471–9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho G. W., Kim G. Y., Baek S., Kim H., Kim T., Kim H. J., Kim S. H. (2011). Recombinant human erythropoietin reduces aggregation of mutant Cu/Zn-binding superoxide dismutase (SOD1) in NSC-34 cells. Neurosci Lett, 504, 107–111. [DOI] [PubMed] [Google Scholar]
- Chong Z. Z., Kang J. Q., Maiese K. (2002). Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation, 106, 2973–2979. [DOI] [PubMed] [Google Scholar]
- Chong Z. Z., Kang J. Q., Maiese K. (2003). Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol, 138, 1107–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Z. Z., Shang Y. C., Mu Y., Cui S., Yao Q., Maiese K. (2013). Targeting erythropoietin for chronic neurodegenerative diseases. Expert Opin Ther Targets, 17, 707–720. [DOI] [PubMed] [Google Scholar]
- Chung E., Kong X., Goldberg M. P., Stowe A. M., Raman L. (2015). Erythropoietin-mediated neuroprotection in a pediatric mouse model of chronic hypoxia. Neurosci Lett, 597, 54–59. [DOI] [PubMed] [Google Scholar]
- Collino M., Thiemermann C., Cerami A., Brines M. (2015). Flipping the molecular switch for innate protection and repair of tissues: Long-lasting effects of a non-erythropoietic small peptide engineered from erythropoietin. Pharmacol Ther, 151, 32–40. [DOI] [PubMed] [Google Scholar]
- Constantinescu S. N., Ghaffari S., Lodish H. F. (1999). The erythropoietin receptor: Structure, activation and intracellular signal transduction. Trends Endocrinol Metab, 10, 18–23. [DOI] [PubMed] [Google Scholar]
- Coppedè F. (2014). The potential of epigenetic therapies in neurodegenerative diseases. Front Genet, 5, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa D. D., Beghi E., Carignano P., Pagliacci C., Faccioli F., Pupillo E., Messina P., Gorio A., Redaelli T. (2015). Tolerability and efficacy of erythropoietin (EPO) treatment in traumatic spinal cord injury: A preliminary randomized comparative trial vs. methylprednisolone (MP). Neurol Sci, 36, 1567–1574. [DOI] [PubMed] [Google Scholar]
- Culver D. A., Dahan A., Bajorunas D., Jeziorska M., van Velzen M., Aarts L. P. H. J., Tavee J., Tannemaat M. R., Dunne A. N., Kirk R. I., Petropoulos I. N., Cerami A., Malik R. A., Brines M. (2017). Cibinetide improves corneal nerve fiber abundance in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain. Invest Ophthalmol Vis Sci, 58, BIO52–BIO60. [DOI] [PubMed] [Google Scholar]
- Dang J., Jia R., Tu Y., Xiao S., Ding G. (2010). Erythropoietin prevents reactive oxygen species generation and renal tubular cell apoptosis at high glucose level. Biomed Pharmacother, 64, 681–685. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Kerr I. M., Stark G. R. (1994). Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science, 264, 1415–1421. [DOI] [PubMed] [Google Scholar]
- De S. R., Ajmone-Cat M. A., Nicolini A., Minghetti L. (2002). Expression of phosphatidylserine receptor and down-regulation of pro-inflammatory molecule production by its natural ligand in rat microglial cultures. J Neuropathol Exp Neurol, 61, 237–244. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. (1992). Human growth hormone and extracellular domain of its receptor: Crystal structure of the complex. Science, 255, 306–312. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M., Bichet S., Marti H. H., Wenger R. H., Rivas L. A., Bauer C., Gassmann M. (1995). Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci U S A, 92, 3717–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digicaylioglu M., Lipton S. A. (2001). Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature, 412, 641–647. [DOI] [PubMed] [Google Scholar]
- Dirnagl U., Iadecola C., Moskowitz M. A. (1999). Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci, 22, 391–397. [DOI] [PubMed] [Google Scholar]
- Donati A., Damiani E., Zuccari S., Domizi R., Scorcella C., Girardis M., Giulietti A., Vignini A., Adrario E., Romano R., Mazzanti L., Pelaia P., Singer M. (2017). Effects of short-term hyperoxia on erythropoietin levels and microcirculation in critically Ill patients: A prospective observational pilot study. BMC Anesthesiol, 17, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H.Hasselblatt, M., Dembowski, C., Cepek, L., Lewczuk, P., Stiefel, M., Bohn, M. (2002). Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med, 8, 495–505. [PMC free article] [PubMed] [Google Scholar]
- Erbaş O., Çınar B. P., Solmaz V., Çavuşoğlu T., Ateş U. (2015). The neuroprotective effect of erythropoietin on experimental Parkinson model in rats. Neuropeptides, 49, 1–5. [DOI] [PubMed] [Google Scholar]
- Esmaeili Tazangi P., Moosavi S. M., Shabani M., Haghani M. (2015). Erythropoietin improves synaptic plasticity and memory deficits by decrease of the neurotransmitter release probability in the rat model of Alzheimer’s disease. Pharmacol Biochem Behav, 130, 15–21. [DOI] [PubMed] [Google Scholar]
- Fantacci M., Bianciardi P., Caretti A., Coleman T. R., Cerami A., Brines M., Samaja M. (2006). Carbamylated erythropoietin ameliorates the metabolic stress induced in vivo by severe chronic hypoxia. Proc Natl Acad Sci U S A, 103, 17531–17536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. W., Gallagher N. I., Warnecke M. A., Donati R. M. (1973). Erythropoiesis and hyperoxia. Proc Soc Exp Biol Med, 144, 569–574. [DOI] [PubMed] [Google Scholar]
- Furlan J. C., Noonan V., Cadotte D. W., Fehlings M. G. (2011). Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: An evidence-based examination of pre-clinical and clinical studies. J Neurotrauma, 28, 1371–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Ure K., Ding P., Nashaat M., Yuan L., Ma J., Hammer R. E., Hsieh J. (2011). The master negative regulator REST/NRSF controls adult neurogenesis by restraining the neurogenic program in quiescent stem cells. J Neurosci, 31, 9772–9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. F., Larpthaveesarp A., McQuillen P., Derugin N., Wendland M., Spadafora R., Ferriero D. M. (2013). Erythropoietin increases neurogenesis and oligodendrogliosis of subventricular zone precursor cells after neonatal stroke. Stroke, 44, 753–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A., Gokmen N., Erbayraktar S., Yilmaz O., Madaschi L., Cichetti C., Di Giulio A. M., Vardar E., Cerami A., Brines M. (2002). Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci U S A, 99, 9450–9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A., Madaschi L., Di Stefano B., Carelli S., Di Giulio A. M., De Biasi S., Coleman T., Cerami A., Brines M. (2005). Methylprednisolone neutralizes the beneficial effects of erythropoietin in experimental spinal cord injury. Proc Natl Acad Sci U S A, 102, 16379–16384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignaschi G., Zennaro E., Tortarolo M., Calvaresi N., Bendotti C. (2007). Erythropoietin does not preserve motor neurons in a mouse model of familial ALS. Amyotroph Lateral Scler, 8, 31–35. [DOI] [PubMed] [Google Scholar]
- Grimm C., Wenzel A., Groszer M., Mayser H., Seeliger M., Samardzija M., Bauer C., Gassmann M., Remé C. E. (2002). HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med, 8, 718–724. [DOI] [PubMed] [Google Scholar]
- Grimm G., Stockenhuber F., Schneeweiss B., Madl C., Zeitlhofer J., Schneider B. (1990). Improvement of brain function in hemodialysis patients treated with erythropoietin. Kidney Int, 38, 480–486. [DOI] [PubMed] [Google Scholar]
- Grégoire C. A., Goldenstein B. L., Floriddia E. M., Barnabé-Heider F., Fernandes K. J. (2015). Endogenous neural stem cell responses to stroke and spinal cord injury. Glia, 63, 1469–1482. [DOI] [PubMed] [Google Scholar]
- Grunfeld J. F., Barhum Y., Blondheim N., Rabey J. M., Melamed E., Offen D. (2007). Erythropoietin delays disease onset in an amyotrophic lateral sclerosis model. Exp Neurol, 204, 260–263. [DOI] [PubMed] [Google Scholar]
- Gunnarson E., Song Y., Kowalewski J. M., Brismar H., Brines M., Cerami A., Andersson U., Zelenina M., Aperia A. (2009). Erythropoietin modulation of astrocyte water permeability as a component of neuroprotection. Proc Natl Acad Sci U S A, 106, 1602–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzy R. D., Hoyos B., Robin E., Chen H., Liu L., Mansfield K. D., Simon M. C., Hammerling U., Schumacker P. T. (2005). Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab, 1, 401–408. [DOI] [PubMed] [Google Scholar]
- Gyongyosi A., Terraneo L., Bianciardi P., Tosaki A., Lekli I., Samaja M. (2018). The impact of moderate chronic hypoxia and hyperoxia on the level of apoptotic and autophagic proteins in myocardial tissue. Oxid Med Cell Longev, 2018, 5786742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase V. H. (2013). Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev, 27, 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassouna I, et al. (2016). Revisiting adult neurogenesis and the role of erythropoietin for neuronal and oligodendroglial differentiation in the hippocampus. Mol Psychiatry, 21, 1752–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández C. C., Burgos C. F., Gajardo A. H., Silva-Grecchi T., Gavilan J., Toledo J. R., Fuentealba J. (2017). Neuroprotective effects of erythropoietin on neurodegenerative and ischemic brain diseases: The role of erythropoietin receptor. Neural Regen Res, 12, 1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock N. H. (1998). Neuroprotective agents in acute ischemic stroke. J Cardiovasc Nurs, 13, 17–25. [DOI] [PubMed] [Google Scholar]
- Jang W., Kim H. J., Li H., Jo K. D., Lee M. K., Yang H. O. (2016). The neuroprotective effect of erythropoietin on rotenone-induced neurotoxicity in SH-SY5Y cells through the induction of autophagy. Mol Neurobiol, 53, 3812–3821. [DOI] [PubMed] [Google Scholar]
- Jang W., Park J., Shin K. J., Kim J. S., Youn J., Cho J. W., Oh E., Ahn J. Y., Oh K. W., Kim H. T. (2014). Safety and efficacy of recombinant human erythropoietin treatment of non-motor symptoms in Parkinson’s disease. J Neurol Sci, 337, 47–54. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. (2001). The role of the liver in the production of thrombopoietin compared with erythropoietin. Eur J Gastroenterol Hepatol, 13, 791–801. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. (2007). Erythropoietin after a century of research: Younger than ever. Eur J Haematol, 78, 183–205. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. (2016). Erythropoietin. Front Horm Res, 47, 115–127. [DOI] [PubMed] [Google Scholar]
- Jia Y., Mo S. J., Feng Q. Q., Zhan M. L., OuYang L. S., Chen J. C., Ma Y. X., Wu J. J., Lei W. L. (2014). EPO-dependent activation of PI3K/Akt/FoxO3a signalling mediates neuroprotection in in vitro and in vivo models of Parkinson’s disease. J Mol Neurosci, 53, 117–124. [DOI] [PubMed] [Google Scholar]
- Juul S. E., Yachnis A. T., Rojiani A. M., Christensen R. D. (1999). Immunohistochemical localization of erythropoietin and its receptor in the developing human brain. Pediatr Dev Pathol, 2, 148–158. [DOI] [PubMed] [Google Scholar]
- Kaelin W. G., Jr.(2005). ROS: Really involved in oxygen sensing. Cell Metabol, 1, 357–358. [DOI] [PubMed] [Google Scholar]
- Karch C. M., Cruchaga C., Goate A. M. (2014). Alzheimer’s disease genetics: From the bench to the clinic. Neuron, 83, 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Aoyama M., Kakita H., Hida H., Kato I., Ito T., Goto T., Hussein M. H., Sawamoto K., Togari H., Asai K. (2011). Endogenous erythropoietin from astrocyte protects the oligodendrocyte precursor cell against hypoxic and reoxygenation injury. J Neurosci Res, 89, 1566–1574. [DOI] [PubMed] [Google Scholar]
- Keswani S. C., Bosch-Marcé M., Reed N., Fischer A., Semenza G. L., Höke A. (2011). Nitric oxide prevents axonal degeneration by inducing HIF-1-dependent expression of erythropoietin. Proc Natl Acad Sci U S A, 108, 4986–4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiboub F. Z., Balestra C., Loennechen O., Eftedal I. (2018). Hemoglobin and erythropoietin after commercial saturation diving. Front Physiol, 9, 1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G. H., Kim J. E., Rhie S. J., Yoon S. (2015). The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol, 24, 325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Y., Moon C., Kim K. S., Oh K. W., Oh S. I., Kim J., Kim S. H. (2014). Recombinant human erythropoietin in amyotrophic lateral sclerosis: A pilot study of safety and feasibility. J Clin Neurol, 10, 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King V. R., Averill S. A., Hewazy D., Priestley J. V., Torup L., Michael-Titus A. T. (2007). Erythropoietin and carbamylated erythropoietin are neuroprotective following spinal cord hemisection in the rat. Eur J Neurosci, 26, 90–100. [DOI] [PubMed] [Google Scholar]
- Koh S. H., Kim Y., Kim H. Y., Cho G. W., Kim K. S., Kim S. H. (2007). Recombinant human erythropoietin suppresses symptom onset and progression of G93A-SOD1 mouse model of ALS by preventing motor neuron death and inflammation. Eur J Neurosci, 25, 1923–1930. [DOI] [PubMed] [Google Scholar]
- Kokot F., Franek E., Kokot M., Wiecek A. (1994. a). Erythropoietin secretion in patients with chronic renal failure after pure oxygen breathing. Nephron, 67, 436–440. [DOI] [PubMed] [Google Scholar]
- Kokot M., Kokot F., Franek E., Wiecek A., Nowicki M., Dulawa J. (1994. b). Effect of isobaric hyperoxemia on erythropoietin secretion in hypertensive patients. Hypertension, 24, 486–490. [DOI] [PubMed] [Google Scholar]
- Kumagai R., Koike M., Iwase Y., Ichimiya Y. (2018). Erythropoietin preparation drastically improved activities of daily living in a patient with severe dementia. Psychiatry Clin Neurosci, 72, 849. [DOI] [PubMed] [Google Scholar]
- Lauria G., Campanella A., Filippini G., Martini A., Penza P., Maggi L., Antozzi C., Ciano C., Beretta P., Caldiroli D., Ghelma F., Ferrara G., Ghezzi P., Mantegazza R. (2009). Erythropoietin in amyotrophic lateral sclerosis: A pilot, randomized, double-blind, placebo-controlled study of safety and tolerability. Amyotroph Lateral Scler, 10, 410–415. [DOI] [PubMed] [Google Scholar]
- Lauria G., et al. (2015). Erythropoietin in amyotrophic lateral sclerosis: A multicentre, randomised, double blind, placebo controlled, phase III study. J Neurol Neurosurg Psychiatry, 86, 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. L., Cai G. Y., Lin F. K., Wei Q., Huang S. Z., Hartz J. H., Morse H., Lin C. H., Jones C., Kao F. T. (1986). Chromosomal assignment of the human erythropoietin gene and its DNA polymorphism. Proc Natl Acad Sci U S A, 83, 6920–6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. S., Lee C., Hu T., Nguyen J. M., Zhang J., Martin M. V., Vawter M. P., Huang E. J., Chan J. Y. (2011). Loss of nuclear factor E2-related factor 1 in the brain leads to dysregulation of proteasome gene expression and neurodegeneration. Proc Natl Acad Sci U S A, 108, 8408–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. T., Chu K., Park J. E., Jung K. H., Jeon D., Lim J. Y., Lee S. K., Kim M., Roh J. K. (2012). Erythropoietin improves memory function with reducing endothelial dysfunction and amyloid-beta burden in Alzheimer’s disease models. J Neurochem, 120, 115–124. [DOI] [PubMed] [Google Scholar]
- Leist M., et al. (2004). Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science, 305, 239–242. [DOI] [PubMed] [Google Scholar]
- Lewczuk P., Hasselblatt M., Kamrowski-Kruck H., Heyer A., Unzicker C., Siren A. L., Ehrenreich H. (2000). Survival of hippocampal neurons in culture upon hypoxia: Effect of erythropoietin. Neuroreport, 11, 3485–3488. [DOI] [PubMed] [Google Scholar]
- Li G., Ma R., Huang C., Tang Q., Fu Q., Liu H., Hu B., Xiang J. (2008). Protective effect of erythropoietin on beta-amyloid-induced PC12 cell death through antioxidant mechanisms. Neurosci Lett, 442, 143–147. [DOI] [PubMed] [Google Scholar]
- Li Y. P., Yang G. J., Jin L., Yang H. M., Chen J., Chai G. S., Wang L. (2015). Erythropoietin attenuates Alzheimer-like memory impairments and pathological changes induced by amyloid β42 in mice. Brain Res, 1618, 159–167. [DOI] [PubMed] [Google Scholar]
- Liu E. Y., Cali C. P., Lee E. B. (2017. a). RNA metabolism in neurodegenerative disease. Dis Model Mech, 10, 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Zhou T., Ziegler A. C., Dimitrion P., Zuo L. (2017. b). Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid Med Cell Longev, 2017, 2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardero M., Kovacs K., Scheithauer B. W. (2011). Erythropoietin: A hormone with multiple functions. Pathobiology, 78, 41–53. [DOI] [PubMed] [Google Scholar]
- Ma R., Xiong N., Huang C., Tang Q., Hu B., Xiang J., Li G. (2009). Erythropoietin protects PC12 cells from beta-amyloid(25-35)-induced apoptosis via PI3K/Akt signaling pathway. Neuropharmacology, 56, 1027–1034. [DOI] [PubMed] [Google Scholar]
- Madav Y., Wairkar S., Prabhakar B. (2019). Recent therapeutic strategies targeting beta amyloid and tauopathies in Alzheimer’s disease. Brain Res Bull, 146, 171–184. [DOI] [PubMed] [Google Scholar]
- Maiese K., Chong Z. Z., Shang Y. C., Wang S. (2012). Erythropoietin: New directions for the nervous system. Int J Mol Sci, 13, 11102–11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi G. D., Zara S., De Colli M., Di Valerio V., Rapino M., Zaramella P., Dedja A., Macchi V., De Caro R., Porzionato A. (2014). Postnatal hyperoxia exposure differentially affects hepatocytes and liver haemopoietic cells in newborn rats. PLoS One, 9, e105005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcuzzi F., Zucchelli S., Bertuzzi M., Santoro C., Tell G., Carninci P., Gustincich S. (2016). Isoforms of the erythropoietin receptor in dopaminergic neurons of the substantia nigra. J Neurochem, 139, 596–609. [DOI] [PubMed] [Google Scholar]
- Marfia G., Madaschi L., Marra F., Menarini M., Bottai D., Formenti A., Bellardita C., Di Giulio A. M., Carelli S., Gorio A. (2011). Adult neural precursors isolated from post mortem brain yield mostly neurons: An erythropoietin-dependent process. Neurobiol Dis, 43, 86–98. [DOI] [PubMed] [Google Scholar]
- Marti H. H. (2004). Erythropoietin and the hypoxic brain. J Exp Biol, 207, 3233–3242. [DOI] [PubMed] [Google Scholar]
- Marti H. H., Wenger R. H., Rivas L. A., Straumann U., Digicaylioglu M., Henn V., Yonekawa Y., Bauer C., Gassmann M. (1996). Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci, 8, 666–676. [DOI] [PubMed] [Google Scholar]
- Martino G., Pluchino S. (2006). The therapeutic potential of neural stem cells. Nat Rev Neurosci, 7, 395–406. [DOI] [PubMed] [Google Scholar]
- Masuda S., Okano M., Yamagishi K., Nagao M., Ueda M., Sasaki R. (1994). A novel site of erythropoietin production. Oxygen-dependent production in cultured rat astrocytes. J Biol Chem, 269, 19488–19493. [PubMed] [Google Scholar]
- Matthews D. J., Topping R. S., Cass R. T., Giebel L. B. (1996). A sequential dimerization mechanism for erythropoietin receptor activation. Proc Natl Acad Sci U S A, 93, 9471–9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T., Mustafa M. H., Desrumaux C., Keller E., Naert G., de la C., García-Barceló M., Rodríguez Cruz Y., Garcia Rodríguez J. C. (2013). Intranasal formulation of erythropoietin (EPO) showed potent protective activity against amyloid toxicity in the Aβ25-35 non-transgenic mouse model of Alzheimer’s disease. J Psychopharmacol, 27, 1044–1057. [DOI] [PubMed] [Google Scholar]
- Merelli A., Czornyj L., Lazarowski A. (2015). Erythropoietin as a new therapeutic opportunity in brain inflammation and neurodegenerative diseases. Int J Neurosci, 125, 793–797. [DOI] [PubMed] [Google Scholar]
- Mofidi A., Bader A., Pavlica S. (2011). The use of erythropoietin and its derivatives to treat spinal cord injury. Mini Rev Med Chem, 11, 763–770. [DOI] [PubMed] [Google Scholar]
- Momeni M., De Kock M., Devuyst O., Liistro G. (2011). Effect of N-acetyl-cysteine and hyperoxia on erythropoietin production. Eur J Appl Physiol, 111, 2681–2686. [DOI] [PubMed] [Google Scholar]
- Nagańska E., Taraszewska A., Matyja E., Grieb P., Rafałowska J. (2010). Neuroprotective effect of erythropoietin in amyotrophic lateral sclerosis (ALS) model in vitro. Ultrastructural study. Folia Neuropathol, 48, 35–44. [PubMed] [Google Scholar]
- Nekoui A., Blaise G. (2017). Erythropoietin and nonhematopoietic effects. Am J Med Sci, 353, 76–81. [DOI] [PubMed] [Google Scholar]
- Ng T., Marx G., Littlewood T., Macdougall I. (2003). Recombinant erythropoietin in clinical practice. Postgrad Med J, 79, 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh M. Y., Cho K. A., Kim H., Kim S. M., Kim S. H. (2014). Erythropoietin modulates the immune-inflammatory response of a SOD1(G93A) transgenic mouse model of amyotrophic lateral sclerosis (ALS). Neurosci Lett, 574, 53–58. [DOI] [PubMed] [Google Scholar]
- Nutt J. G., Wooten G. F. (2005). Clinical practice. Diagnosis and initial management of Parkinson’s disease. N Engl J Med, 353, 1021–1027. [DOI] [PubMed] [Google Scholar]
- Ogilvie M., Yu X., Nicolas-Metral V., Pulido S. M., Liu C., Ruegg U. T., Noguchi C. T. (2000). Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J Biol Chem, 275, 39754–39761. [DOI] [PubMed] [Google Scholar]
- Osredkar D., Sall J. W., Bickler P. E., Ferriero D. M. (2010). Erythropoietin promotes hippocampal neurogenesis in in vitro models of neonatal stroke. Neurobiol Dis, 38, 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski D., Heinrich R. (2018). Alternative erythropoietin receptors in the nervous system. J Clin Med, 7, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso I., Bringas M. L., Aguiar A., Morales L., Alvarez M., Valdés P. A., Alvarez L. (2012). Use of Cuban recombinant human erythropoietin in Parkinson’s disease treatment. MEDICC Rev, 14, 11–17. [DOI] [PubMed] [Google Scholar]
- Perreault A. A., Benton M. L., Koury M. J., Brandt S. J., Venters B. J. (2017). Epo reprograms the epigenome of erythroid cells. Exp Hematol, 51, 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet F., Alves S., Cartier N. (2017). Clinical gene therapy for neurodegenerative diseases: Past, present, and future. Hum Gene Ther, 28, 988–1003. [DOI] [PubMed] [Google Scholar]
- Ponce L. L., Navarro J. C., Ahmed O., Robertson C. S. (2013). Erythropoietin neuroprotection with traumatic brain injury. Pathophysiology, 20, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profyris C., Cheema S. S., Zang D., Azari M. F., Boyle K., Petratos S. (2004). Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis, 15, 415–436. [DOI] [PubMed] [Google Scholar]
- Qi C., Xu M., Gan J., Yang X., Wu N., Song L., Yuan W., Liu Z. (2014). Erythropoietin improves neurobehavior by reducing dopaminergic neuron loss in a 6-hydroxydopamine-induced rat model. Int J Mol Med, 34, 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabie T., Marti H. H. (2008). Brain protection by erythropoietin: A manifold task. Physiology (Bethesda), 23, 263–274. [DOI] [PubMed] [Google Scholar]
- Rees S., Hale N., De Matteo R., Cardamone L., Tolcos M., Loeliger M., Mackintosh A., Shields A., Probyn M., Greenwood D., Harding R. (2010). Erythropoietin is neuroprotective in a preterm ovine model of endotoxin-induced brain injury. J Neuropathol Exp Neurol, 69, 306–319. [DOI] [PubMed] [Google Scholar]
- Richmond T. D., Chohan M., Barber D. L. (2005). Turning cells red: Signal transduction mediated by erythropoietin. Trends Cell Biol, 15, 146–155. [DOI] [PubMed] [Google Scholar]
- Rocco M., D'Itri L., De Bels D., Corazza F., Balestra C. (2014). The “normobaric oxygen paradox”: A new tool for the anesthetist? Minerva Anestesiol, 80, 366–372. [PubMed] [Google Scholar]
- Rodríguez Cruz Y., Strehaiano M., Rodríguez Obaya T., García Rodríguez J. C., Maurice T. (2017). An intranasal formulation of erythropoietin (Neuro-EPO) prevents memory deficits and amyloid toxicity in the APPSwe transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis, 55, 231–248. [DOI] [PubMed] [Google Scholar]
- Romanko M. J., Zhu C., Bahr B. A., Blomgren K., Levison S. W. (2007). Death effector activation in the subventricular zone subsequent to perinatal hypoxia/ischemia. J Neurochem, 103, 1121–1131. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Hopkins S. J. (1995). Cytokines and the nervous system II: Actions and mechanisms of action. Trends Neurosci, 18, 130–136. [DOI] [PubMed] [Google Scholar]
- Ruscher K., Isaev N., Trendelenburg G., Weih M., Iurato L., Meisel A., Dirnagl U. (1998). Induction of hypoxia inducible factor 1 by oxygen glucose deprivation is attenuated by hypoxic preconditioning in rat cultured neurons. Neurosci Lett, 254, 117–120. [DOI] [PubMed] [Google Scholar]
- Sakanaka M., Wen T. C., Matsuda S., Masuda S., Morishita E., Nagao M., Sasaki R. (1998). In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A, 95, 4635–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakthiswary R., Raymond A. A. (2012). Stem cell therapy in neurodegenerative diseases: From principles to practice. Neural Regen Res, 7, 1822–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salta E., De Strooper B. (2017). Noncoding RNAs in neurodegeneration. Nat Rev Neurosci, 18, 627–640. [DOI] [PubMed] [Google Scholar]
- Samy D. M., Ismail C. A., Nassra R. A., Zeitoun T. M., Nomair A. M. (2016). Downstream modulation of extrinsic apoptotic pathway in streptozotocin-induced Alzheimer’s dementia in rats: Erythropoietin versus curcumin. Eur J Pharmacol, 770, 52–60. [DOI] [PubMed] [Google Scholar]
- Sanchez P. E., Fares R. P., Risso J. J., Bonnet C., Bouvard S., Le-Cavorsin M., Georges B., Moulin C., Belmeguenai A., Bodennec J., Morales A., Pequignot J. M., Baulieu E. E., Levine R. A., Bezin L. (2009). Optimal neuroprotection by erythropoietin requires elevated expression of its receptor in neurons. Proc Natl Acad Sci U S A, 106, 9848–9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Gomar F., Garcia-Gimenez J. L., Pareja-Galeano H., Romagnoli M., Perez-Quilis C., Lippi G. (2014). Erythropoietin and the heart: Physiological effects and the therapeutic perspective. Int J Cardiol, 171, 116–125. [DOI] [PubMed] [Google Scholar]
- Sargin D., El-Kordi A., Agarwal A., Müller M., Wojcik S. M., Hassouna I., Sperling S., Nave K. A., Ehrenreich H. (2011). Expression of constitutively active erythropoietin receptor in pyramidal neurons of cortex and hippocampus boosts higher cognitive functions in mice. BMC Biol, 9, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L., Nejfelt M. K., Chi S. M., Antonarakis S. E. (1991). Hypoxia-inducible nuclear factors bind to an enhancer element located 3' to the human erythropoietin gene. Proc Natl Acad Sci U S A, 88, 5680–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L., Wang G. L. (1992). A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol, 12, 5447–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y. C., Chong Z. Z., Wang S., Maiese K. (2011). Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Curr Neurovasc Res, 8, 270–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y. C., Chong Z. Z., Wang S., Maiese K. (2012). Prevention of β-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY), 4, 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo T., Sorokan S. T., Shimazaki T., Weiss S. (2001). Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci, 21, 9733–9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon F., Scheuerle A., Gröger M., Vcelar B., McCook O., Möller P., Georgieff M., Calzia E., Radermacher P., Schelzig H. (2011). Comparison of carbamylated erythropoietin-FC fusion protein and recombinant human erythropoietin during porcine aortic balloon occlusion-induced spinal cord ischemia/reperfusion injury. Intensive Care Med, 37, 1525–1533. [DOI] [PubMed] [Google Scholar]
- Simon F. H., Erhart P., Vcelar B., Scheuerle A., Schelzig H., Oberhuber A. (2016). Erythropoietin preconditioning improves clinical and histologic outcome in an acute spinal cord ischemia and reperfusion rabbit model. J Vasc Surg, 64, 1797–1804. [DOI] [PubMed] [Google Scholar]
- Soliz J., Gassmann M., Joseph V. (2007). Soluble erythropoietin receptor is present in the mouse brain and is required for the ventilatory acclimatization to hypoxia. J Physiol, 583, 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollinger C., Lillis J., Malik J., Getman M., Proschel C., Steiner L. (2017). Erythropoietin signaling regulates key epigenetic and transcription networks in fetal neural progenitor cells. Sci Rep, 7, 14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer L., Csete M., Lee S. H., Kabbani N., Walikonis J., Wold B., McKay R. (2000). Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci, 20, 7377–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawa M., Sakurai Y., Ishikawa-Ieda Y., Suzuki H., Asou H. (2002). Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci Res, 44, 391–403. [DOI] [PubMed] [Google Scholar]
- Sun J., Martin J. M., Vanderpoel V., Sumbria R. K. (2019). The promises and challenges of erythropoietin for treatment of Alzheimer’s disease. Neuromolecular Med, 21, 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Tanaka K., Nogawa S., Dembo T., Kosakai A., Fukuuchi Y. (2001). Phosphorylation of signal transducer and activator of transcription-3 (Stat3) after focal cerebral ischemia in rats. Exp Neurol, 170, 63–71. [DOI] [PubMed] [Google Scholar]
- Tang Z., Sun X., Huo G., Xie Y., Shi Q., Chen S., Wang X., Liao Z. (2013). Protective effects of erythropoietin on astrocytic swelling after oxygen-glucose deprivation and reoxygenation: Mediation through AQP4 expression and MAPK pathway. Neuropharmacology, 67, 8–15. [DOI] [PubMed] [Google Scholar]
- Temple R. M., Deary I. J., Winney R. J. (1995). Recombinant erythropoietin improves cognitive function in patients maintained on chronic ambulatory peritoneal dialysis. Nephrol Dial Transplant, 10, 1733–1738. [PubMed] [Google Scholar]
- Terraneo L., Paroni R., Bianciardi P., Giallongo T., Carelli S., Gorio A., Samaja M. (2017). Brain adaptation to hypoxia and hyperoxia in mice. Redox Biol, 11, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terraneo L., Samaja M. (2017). Comparative response of brain to chronic hypoxia and hyperoxia. Int J Mol Sci, 18, 1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terraneo L., Virgili E., Caretti A., Bianciardi P., Samaja M. (2014). In vivo hyperoxia induces hypoxia-inducible factor-1alpha overexpression in LNCaP tumors without affecting the tumor growth rate. Int J Biochem Cell Biol, 51C, 65–74. [DOI] [PubMed] [Google Scholar]
- Tsai T. H., Lu C. H., Wallace C. G., Chang W. N., Chen S. F., Huang C. R., Tsai N. W., Lan M. Y., Sung P. H., Liu C. F., Yip H. K. (2015). Erythropoietin improves long-term neurological outcome in acute ischemic stroke patients: A randomized, prospective, placebo-controlled clinical trial. Crit Care, 19, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda E., Goto M., Murakami A., Akai K., Ueda M., Kawanishi G., Takahashi N., Sasaki R., Chiba H., Ishihara H. (1988). Comparative structural study of N-linked oligosaccharides of urinary and recombinant erythropoietins. Biochemistry, 27, 5646–5654. [DOI] [PubMed] [Google Scholar]
- Tysnes O. B., Storstein A. (2017). Epidemiology of Parkinson’s disease. J Neural Transm (Vienna), 124, 901–905. [DOI] [PubMed] [Google Scholar]
- Vinothkumar G., Krishnakumar S., Sureshkumar, Shivashekar G., Sreedhar S., Preethikrishnan, Dinesh S., Sundaram A., Balakrishnan D., Riya, Venkataraman P. (2018) Therapeutic impact of rHuEPO on abnormal platelet APP, BACE 1, presenilin 1, ADAM 10 and Aβ expressions in chronic kidney disease patients with cognitive dysfunction like Alzheimer’s disease: A pilot study. Biomed Pharmacother, 104, 211–222. [DOI] [PubMed] [Google Scholar]
- Viviani B., Bartesaghi S., Corsini E., Villa P., Ghezzi P., Garau A., Galli C. L., Marinovich M. (2005). Erythropoietin protects primary hippocampal neurons increasing the expression of brain-derived neurotrophic factor. J Neurochem, 93, 412–421. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang Z., Wang Y., Zhang R., Chopp M. (2004). Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke, 35, 1732–1737. [DOI] [PubMed] [Google Scholar]
- Wang W., Kagaya Y., Asaumi Y., Fukui S., Takeda M., Shimokawa H. (2011). Protective effects of recombinant human erythropoietin against pressure overload-induced left ventricular remodeling and premature death in mice. Tohoku J Exp Med, 225, 131–143. [DOI] [PubMed] [Google Scholar]
- Weishaupt J. H., Rohde G., Pölking E., Siren A. L., Ehrenreich H., Bähr M. (2004). Effect of erythropoietin axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci, 45, 1514–1522. [DOI] [PubMed] [Google Scholar]
- Widl K., Brettschneider J., Schattauer D., Süssmuth S., Huber R., Ludolph A. C., Tumani H. (2007). Erythropoietin in cerebrospinal fluid: Age-related reference values and relevance in neurological disease. Neurochem Res, 32, 1163–1168. [DOI] [PubMed] [Google Scholar]
- Wijesekera L. C., Leigh P. N. (2009). Amyotrophic lateral sclerosis. Orphanet J Rare Dis, 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser J., Wolfram J. A., Gargesha M., Yang K., Karunamuni G., Wilson D. L., Semenza G. L., Agani F., Fisher S. A., Ward N., Watanabe M. (2009). Altered hypoxia-inducible factor-1 alpha expression levels correlate with coronary vessel anomalies. Dev Dyn, 238, 2688–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirdefeldt K., Adami H. O., Cole P., Trichopoulos D., Mandel J. (2011). Epidemiology and etiology of Parkinson's disease: A review of the evidence. Eur J Epidemiol, 26(Suppl 1), S1–S58. [DOI] [PubMed] [Google Scholar]
- Wu X. M., Qian C., Zhou Y. F., Yan Y. C., Luo Q. Q., Yung W. H., Zhang F. L., Jiang L. R., Qian Z. M., Ke Y. (2017). Bi-directionally protective communication between neurons and astrocytes under ischemia. Redox Biol, 13, 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Shang Y., Sun S. G., Liu R. G., Yang W. Q. (2007). Protective effect of erythropoietin against 1-methyl-4-phenylpyridinium-induced neurodegenaration in PC12 cells. Neurosci Bull, 23, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Wang D., Li H., Shen H., Shu Z., Chen G. (2017). Erythropoietin treatment in patients with acute ischemic stroke: A systematic review and meta-analysis of randomized controlled trials. Curr Drug Deliv, 14, 853–860. [DOI] [PubMed] [Google Scholar]
- Yoo S. J., Cho B., Lee D., Son G., Lee Y. B., Soo Han H., Kim E., Moon C. (2017). The erythropoietin-derived peptide MK-X and erythropoietin have neuroprotective effects against ischemic brain damage. Cell Death Dis, 8, e3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Shacka J. J., Eells J. B., Suarez-Quian C., Przygodzki R. M., Beleslin-Cokic B., Lin C. S., Nikodem V. M., Hempstead B., Flanders K. C., Costantini F., Noguchi C. T. (2002). Erythropoietin receptor signalling is required for normal brain development. Development, 129, 505–516. [DOI] [PubMed] [Google Scholar]
- Zara S., Macchi V., De Caro R., Rapino M., Cataldi A., Porzionato A. (2012). pPKCalpha mediated-HIF-1alpha activation related to the morphological modifications occurring in neonatal myocardial tissue in response to severe and mild hyperoxia. Eur J Histochem, 56, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Fang X., Huang D., Luo Q., Zheng M., Wang K., Cao L., Yin Z. (2018). Erythropoietin signaling increases neurogenesis and oligodendrogenesis of endogenous neural stem cells following spinal cord injury both in vivo and in vitro. Mol Med Rep, 17, 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Kang W., Xu F., Cheng X., Zhang Z., Jia L., Ji L., Guo X., Xiong H., Simbruner G., Blomgren K., Wang X. (2009). Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics, 124, e218–226. [DOI] [PubMed] [Google Scholar]