Abstract
Opioid use can induce immunosuppression; however, it is unclear whether opioid use increases infections in patients with advanced cancers. This study assessed the association between opioid use in the week before death and mortality among patients with advanced lung cancer having sepsis. Data on opioid usage in the week before death, general information, and clinical information of the patients were collected retrospectively. The primary outcome was the association between opioid use in the week before death and mortality after sepsis. The study included 980 patients who died of advanced lung cancer between January 2003 and June 2017 (sepsis related: 413, unrelated to sepsis: 567). The average morphine equivalent daily dose in the final week was higher in the sepsis group (313.5 ± 510.5 mg) than in the nonsepsis group (125.2 ± 246.9 mg, P < .001). A significant association was found between the average morphine equivalent daily dose in the final week and mortality due to sepsis (odds ratio: 1.02, 95% confidence interval: 1.01-1.02, P < .001). This was especially evident when the dose was increased by 10 mg in the final week. Furthermore, older age, male sex, and a lower body mass index were associated with an increased risk of mortality after developing sepsis. Opioid use in the week before death may be associated with mortality for patients with advanced lung cancer having sepsis.
Keywords: cancer, cancer-related pain, epidemiology, NSCLC, lung cancer
Introduction
Lung cancer is a major global health issue and one of the deadliest types of cancer.1 Lung cancers are often diagnosed at an advanced stage and therefore require appropriate palliative care.2,3 The key purpose of palliative care for patients with advanced cancer is to provide appropriate pain control and, consequently, an improved quality of life.4 Opioids are often used for this purpose5 and are prescribed by clinicians in accordance with World Health Organization guidelines.6
However, recent reports about the effects of opioids on the immune system suggest that they may have negative impacts on the long-term outcomes of patients with cancer.7 Specifically, the immune suppression caused by opioid use may increase infection rates,8,9 especially for patients with advanced cancer who are often already immunocompromised due to chemotherapy or malnutrition.10 A previous study suggested a possible association between the excessive use of opioids and shortened survival times for patients with advanced non-small cell lung cancer (NSCLC).11 A more recent study reported that the increased use of opioids results in increased infection rates in patients with advanced cancer.12 Because patients with advanced lung cancer are often exposed to infections and consequent sepsis,13 further studies on this topic are needed.
To our knowledge, no studies have assessed the association between the occurrence of sepsis in patients with advanced lung cancer and the amount of opioid use; thus, our study aimed to assess this association. We hypothesized that patients with terminal stage advanced lung cancer who died due to sepsis were associated with higher quantities of opioid consumption compared to patients who died of other causes.
Materials and Methods
This retrospective observational study was approved by the institutional review board (approval number: B-1707/408-104). Because this was a retrospective review of patient medical records, the requirement for informed consent was waived. The study used the medical records of all adult patients (≥19 years of age) who were diagnosed with advanced lung cancer and died while hospitalized at the single tertiary academic hospital between January 2007 and June 2017. Advanced lung cancer was defined as a primary lung cancer that was found to be inoperable at the time of diagnosis. Small-cell lung cancer (SCLC) was defined as an extensive stage cancer at the time of diagnosis, whereas NSCLC was defined as a stage IIIB or IV cancer. Lung cancer staging was conducted according to the 7th edition of the American Joint Committee on Cancer guidelines.14 Exclusion criteria for patient selection were as follows: inaccurate information on opioid usage in the final week before death, palliative surgery performed in the week before death, or the occurrence of other primary cancers between the time of diagnosis and death. The study center was a 1360-bed tertiary care hospital as of July 2017. All medical records have been managed and stored electronically since 2003.
Definition of Sepsis in Patients With Advanced Lung Cancer
To define sepsis at the time of death for patients with lung cancer, we used the standards obtained from 2 previously conducted studies12,15: (1) a positive blood culture test in the week before death; (2) an abnormally high white blood cell count, neutrophil/granulocyte ratio, C-reactive protein level, and procalcitonin level in the week before death; and (3) a Sepsis-related Organ Failure Assessment (SOFA) score of ≥2. Patients who exhibited either the first or second criterion concurrently with the third criterion were considered to have sepsis. The selection of patients with sepsis was based on this definition and was verified by the 2 main investigators of this study, who are certified intensivists.
Measurements
We collected data on each patient’s sex, age, body mass index (BMI, kg/m2), histology of cancer (SCLC or NSCLC), year of death, presence of distant metastasis, history of chemotherapy/radiotherapy, history of smoking, cause of death, and the average morphine equivalent daily dose (MEDD, mg) in the final week before death. Distant metastasis only included metastases of the bones or major organs. Cause of death was categorized as sepsis, respiratory failure without sepsis, cardiologic, neurologic, or others (eg, unclear, gastrointestinal bleeding, acute kidney injury, endocrinologic). The infection source was further categorized as respiratory, digestive, genitourinary, unknown, or others (eg, skin, wound, cardiac, brain, spinal cord, brain). To calculate the average MEDD in the final week, the total opioid usage in the 7 days preceding and including the date of death was accurately measured. Using a standard conversion ratio, the sum of these values was divided by 7 to calculate the average MEDD for the final week16 (Appendix A).
Outcomes
The primary outcome of this study was the potential association between sepsis as a cause of death in patients with terminal stage advanced lung cancer and opioid usage (based on the average MEDD for the week before death). The secondary outcome was the clinical factors associated with the occurrence of sepsis in patients with terminal stage advanced lung cancer.
Statistical Methods
The patients were grouped into quartiles based on opioid usage. The χ2 test was used to estimate possible demographic differences in the categorical data for each group. Analysis of variance was also used to find differences in the demographic data between the groups of continuous data, along with the Scheffe test. Similarly, a χ2 test and t test were used to compare the groups with sepsis at the time of death and those without sepsis.
Finally, univariable and multivariable logistic regression analysis was performed to determine whether opioid usage was independently associated with sepsis at the time of death. All covariates (age, BMI, histology of cancer, distant metastasis, receipt of chemotherapy or radiotherapy, history of smoking, and year of death) were included in the multivariable model for adjustment. Our main independent variable, the average MEDD in the last week, was included in 2 different multivariable models as a continuous variable and as a categorical variable in quartile to avoid multicollinearity in the models. Hosmer-Lemeshow test was used to confirm goodness of fit of the 2 multivariable models. The results of the multivariable model were presented as odds ratio (OR) with 95% confidence interval (CI). Additionally, the restricted cubic spline was used to present the log odds of development of sepsis according to average MEDD for the final week. R software (version 3.6.0; R Foundation, Vienna, Austria) was used for all analyses, and P < .05 was considered to be statistically significant.
Results
A total of 1085 Asian adult patients with advanced lung cancer died while hospitalized at the institution between January 2007 and June 2017. The following patients were excluded from the study: 24 patients who had an additional primary cancer at the time of death, 12 patients who died within a week after a palliative operation, and 69 patients who had inaccurate records on opioid usage in the final week before death. In all, 980 patients were included in our analysis. Of these patients, 413 died of sepsis and 567 died of other causes.
Baseline Characteristics by Average MEDD in the Final Week
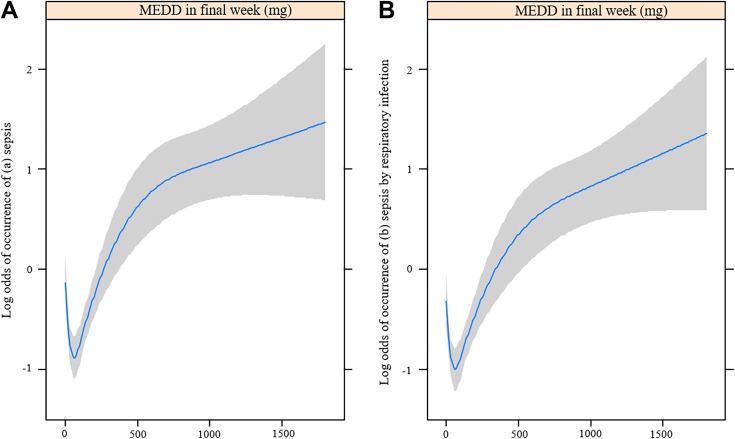
Table 1 shows the baseline patient characteristics by average MEDD in the final week of life by quartiles. The proportions of deaths due to sepsis were significantly different between the quartiles of opioid usage: quartile 1 (<30 mg): 44.0% (102/232); quartile 2 (30-60 mg): 30.7% (62/202); quartile 3 (60-165 mg): 33.7% (98/291); and quartile 4 (>165 mg): 58.8% (150/255; P < .001). In addition, age (P < .001), presence of distant metastasis (P = .007), history of chemotherapy (P < .001), history of radiotherapy (P = .006), and the cause of death (P < .001) were significantly different between the quartiles. Figure 1A and B are restricted cubic splines that show the relationship between average MEDD in the final week and the log odds of death caused by sepsis and death caused by sepsis due to respiratory infection, respectively. The log odds of mortality from sepsis and from sepsis caused by respiratory infection tended to increase with increased average MEDDs in the final week of life.
Table 1.
Baseline Characteristics of Patients With Advanced Lung Cancer for Average MEDD in Last Week by Quartiles.a
| Variable | Average MEDD in Last Week | P Value | |||
|---|---|---|---|---|---|
| Q1 < 30, n = 232 | 30 ≤ Q2 < 60, n = 202 | 60 ≤ Q3 < 165, n = 291 | 165 < Q4, n = 255 | ||
| Sex: male | 170 (73.3) | 148 (73.3) | 210 (72.2) | 194 (76.1) | .768 |
| Age, years | 70.1 (10.9) | 68.1 (11.5) | 65.5 (10.8) | 66.5 (10.8) | <.001 |
| Body mass index, kg/m2 | 21.3 (3.6) | 21.3 (3.8) | 21.7 (3.4) | 21.4 (3.6) | .294 |
| Histology of cancer | |||||
| NSCLC | 186 (80.2) | 154 (76.2) | 230 (79.0) | 199 (78.0) | |
| SCLC | 46 (19.8) | 48 (23.8) | 61 (21.0) | 56 (22.0) | |
| Distant metastasis (bone or major organ) | 43 (18.5) | 62 (30.7) | 89 (30.6) | 64 (25.1) | .007 |
| Chemotherapy | 19 (8.2) | 43 (21.3) | 66 (22.7) | 58 (22.7) | <.001 |
| Radiotherapy | 94 (40.5) | 87 (43.1) | 151 (51.9) | 137 (53.7) | .006 |
| History of smoking | 205 (88.4) | 174 (86.1) | 251 (86.3) | 228 (89.4) | .622 |
| Cause of death | <.001 | ||||
| Sepsis | 102 (44.0) | 62 (30.7) | 98 (33.7) | 150 (58.8) | |
| Respiratory failure without sepsis | 103 (44.4) | 122 (60.4) | 168 (57.7) | 94 (36.9) | |
| Cardiovascular disease | 5 (2.2) | 3 (1.5) | 6 (2.1) | 3 (1.2) | |
| Neurologic disease | 13 (5.6) | 7 (3.5) | 5 (1.7) | 3 (1.2) | |
| Othersb | 9 (3.9) | 8 (4.0) | 14 (4.8) | 5 (2.0) | |
| Year of death | .011 | ||||
| 2007-2010 | 90 (38.8) | 52 (25.7) | 89 (30.6) | 70 (27.5) | |
| 2011-2014 | 81 (34.9) | 73 (36.1) | 108 (37.1) | 113 (44.3) | |
| 2015-2017 | 61 (26.3) | 77 (38.1) | 94 (32.3) | 72 (28.2) | |
Abbreviations: MEDD, morphine equivalent daily dose; NSCLC, non-small cell lung cancer; SCLC, small-cell lung cancer.
a Presented as mean with standard deviation or number with percentage.
b Others: Unclear, gastrointestinal bleeding, acute kidney injury, endocrinologic disease.
Figure 1.
Restricted cubic spline showing opioid dosages for the occurrence of (A) sepsis and (B) sepsis by respiratory infection in patients with lung cancer. MEDD indicates morphine equivalent daily dose (mg).
Baseline Characteristics by Sepsis Diagnosis
Table 2 shows the differences between patients who died of sepsis and patients who died of other causes. Patients who died of sepsis had significantly higher average MEDDs in the final week of life compared with patients who died of other causes (sepsis: 313.5 ± 510.5 mg vs nonsepsis: 125.2 ± 246.9; P < .001). In addition, the proportion of males (sepsis: 78.7% vs nonsepsis: 70.0%; P = .003) and age (sepsis: 69.7 ± 9.9 years vs nonsepsis: 65.7 ± 11.7 years; P < .001) were also significantly higher among patients who died of sepsis. Body mass index was significantly lower in the sepsis group (sepsis: 21.0 ± 3.6 kg/m2 vs nonsepsis: 21.7 ± 3.5 kg/m2; P = .003), as was the proportion of patients with distant metastases (sepsis: 22.8% vs nonsepsis: 28.9%; P = .031). Respiratory infection was the most common infection type (91.3%).
Table 2.
Demographics and Baseline Characteristics According to Sepsis in Patients With Advanced Lung Cancer.a
| Variable | Patients With Advanced Lung Cancer (n = 980) | P Value | |
|---|---|---|---|
| Sepsis (n = 413) | Nonsepsis (n = 567) | ||
| Sex: male | 325 (78.7) | 397 (70.0) | .003 |
| Age, years | 69.7 (9.9) | 65.7 (11.6) | <.001 |
| BMI, kg/m2 | 21.0 (3.6) | 21.7 (3.5) | .003 |
| MEDDb (mg) | 313.5 (510.5) | 125.2 (246.9) | <.001 |
| Cancer type | .212 | ||
| NSCLC | 332 (80.4) | 437 (77.1) | |
| SCLC | 81 (19.6) | 130 (22.9) | |
| Distant metastasis (bone or major organ) | 94 (22.8) | 164 (28.9) | .031 |
| Chemotherapy | 61 (14.8) | 125 (22.0) | .004 |
| Radiotherapy | 186 (45.0) | 283 (49.9) | .131 |
| History of smoking | 361 (87.4) | 497 (87.7) | .909 |
| Infection source | <.001 | ||
| Respiratory | 377 (91.3) | 0 (0.0) | |
| Digestive | 13 (3.1) | 7 (1.2) | |
| Genitourinary | 4 (1.0) | 0 (0.0) | |
| Othersc | 6 (1.5) | 0 (0.0) | |
| Unknown | 13 (3.1) | 0 (0.0) | |
Abbreviations: BMI, body mass index; MEDD, morphine equivalent daily dose; NSCLC, non-small cell lung cancer; SCLC, small-cell lung cancer.
a Presented as mean with standard deviation or number with percentage.
b MEDD: Average MEDD in last week.
c Others: Skin, wound, cardiac, brain, spinal cord, bone.
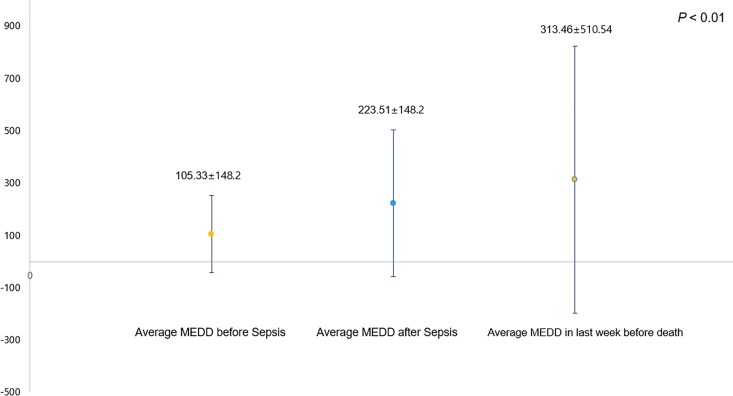
Figure 2 shows the average MEDD before and after the diagnosis of sepsis in patients with advanced lung cancer. The average MEDD before sepsis diagnosis was lower than the average MEDD after the diagnosis of sepsis (MEDD before sepsis: 105.3 ± 148.2 mg vs MEDD after sepsis: 223.5 ± 148.2 mg; P < .01).
Figure 2.
Opioid use before and after sepsis diagnosis in patients with advanced lung cancer. MEDD indicates morphine equivalent daily dose (mg).
Logistic Regression Analysis for the Occurrence of Sepsis
Table 3 shows the results of uni- and multivariable logistic regression analyses for death due to sepsis in patients with advanced lung cancer. A significant association was observed between the 10 mg increase of average MEDD in the final week of life and death from sepsis (OR: 1.02, 95% CI: 1.01-1.02, P < .001; model 1). As a categorical variable, in the Q4 group, the average MEDD in the final week of life was associated with death from sepsis, compared with that in the Q1 group (Q4, OR: 1.82, 95% CI: 1.62-3.49, P < .001; model 2). In addition, age (OR: 1.03, 95% CI: 1.02-1.05, P < .001), male sex (OR: 1.48, 95% CI: 1.07-2.05, P < .001), and BMI (OR: 0.95, 95% CI: 0.91-0.99; P = .013) were also significantly associated with death from sepsis (model 1).
Table 3.
Univariable and Multivariate Logistic Regression Analysis for Development of Sepsis in Patients With Advanced Lung Cancer.a
| Univariable Model | P Value | Multivariable Model | P Value | |
|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | |||
| Age, years | 1.04 (1.02-1.05) | <.001 | 1.03 (1.02-1.05) | <.001 |
| Male sex (vs female) | 1.58 (1.18-2.13) | .002 | 1.48 (1.07-2.05) | <.001 |
| BMI, kg/m2 | 0.95 (0.91-0.98) | .003 | 0.95 (0.91-0.99) | .013 |
| NSCLC (vs SCLC) | 1.22 (0.89-1.67) | .213 | 1.40 (0.99-1.97) | .058 |
| Distant metastasis | 0.72 (0.54-0.97) | .031 | 0.94 (0.68-1.31) | .718 |
| Chemotherapy | 0.61 (0.44-0.86) | .004 | 0.70 (0.48-1.00) | .049 |
| Radiotherapy | 0.82 (0.64-1.06) | .132 | 0.97 (0.72-1.30) | .835 |
| History of smoking | 0.98 (0.67-1.44) | .909 | 0.77 (0.51-1.17) | .219 |
| Year of death | ||||
| 2007-2010 | 1 | 1 | ||
| 2011-2014 | 1.03 (0.76-1.40) | .847 | 0.85 (0.62-1.19) | .854 |
| 2015-2017 | 0.96 (0.69-1.32) | .789 | 0.74 (0.51-1.06) | .101 |
| MEDDb per 10 mg (model 1) | 1.02 (1.01-1.02) | <.001 | 1.02 (1.01-1.02) | <.001 |
| MEDDb in quartile (model 2) | ||||
| Q1 < 30 | 1 | 1 | ||
| 30 ≤ Q2 < 60 | 0.56 (0.38-0.84) | .005 | 0.68 (0.42-1.05) | .058 |
| 60 ≤ Q3 < 165 | 0.66 (0.46-0.94) | .020 | 0.87 (0.60-1.27) | .474 |
| 165 < Q4 | 1.82 (1.27-2.61) | .001 | 2.38 (1.62-3.49) | <.001 |
Abbreviations: BMI, body mass index; CI, confidence interval; MEDD, morphine equivalent daily dose; NSCLC, non-small cell lung cancer; SCLC, small-cell lung cancer.
a Hosmer-Lemeshow test = χ2 = 12.17, df = 8 (P = .144) in model 1, χ2 = 10.78, df = 8 (P = .215) in model 2.
b MEDD: Average MEDD in last week.
Discussion
Our study shows that patients with terminal stage advanced lung cancer who died of sepsis used higher quantities of opioids compared with patients with terminal stage advanced lung cancer who died of other causes; this result was statistically significant in the multivariate logistic analysis. This finding is in agreement with the results of a study in which the daily increased oral morphine equivalent was associated with a risk of infection in patients with advanced cancer.12 A unique characteristic of our study is that it only included deaths caused by sepsis in patients with terminal stage advanced cancer. In addition, our study suggests that the risk of death due to sepsis is positively associated with increased age, male sex, and NSCLC cancer type.
This study shows that excessive opioid use by patients with advanced cancer could contribute to the development of sepsis. Because sepsis is common among hospitalized patients with cancer,17 this finding may have clinical significance. Furthermore, effective pain control that avoids excessive opioid use is now an important issue in the palliative care of patients with advanced cancer.18 Therefore, our findings suggest that opioid dosage may affect mortality from sepsis for patients with advanced lung cancer.
Because of the effects of opioids on the immune system, we hypothesized that the excessive use of these drugs may be associated with death due to sepsis in patients with advanced lung cancer. The suppression of the immune system through opioid usage and the consequent worsening of long-term oncologic outcomes are important issues, although they are still controversial.19 Furthermore, it remains unclear how opioids affect the immune system, and the findings pertaining to induced immunosuppression and immunostimulation are paradoxical.20,21 However, unlike previously conducted studies, our findings show that the excessive use of opioids is associated (although weakly) with infections in patients with advanced cancer.12 In general, a patient with advanced cancer is already in an immunocompromised state, and their condition worsens as the cancer progresses to the terminal stage.10 Based on the results of a previous study, which reported increased rates of opportunistic infections in opioid abusers,8 we suggest that the excessive use of opioids by immunocompromised patients with advanced cancer can increase infection rates.
A few points should be considered in the interpretation of our study’s findings. First, infection and sepsis in the patients may not have been affected by the immune system, but by various other factors. For example, in the case of aspiration pneumonia, mental status and swallowing difficulty are important causative factors.22 In the case of urinary tract infections, the time taken for Foley catheter insertion inevitably has effects, whereas all nosocomial infections are likely to be affected by the length of the hospitalization period.23 Furthermore, chemotherapy history and nutritional status should also be considered. Despite the fact that our study could not consider the type and number of chemotherapy agents/treatments administered and the exact assessment of the nutritional status could not be performed, factors such as the history of chemotherapy and BMI had no effects on death due to sepsis in patients with advanced lung cancer. Therefore, considering the drawbacks arising from the retrospective nature of this study, the excessive use of opioids has a significant association, rather than a clear causal relationship, with death due to sepsis in patients with advanced lung cancer. Moreover, the difference in the ORs between the average MEDD in the final week and death caused by sepsis was 1.002, which is extremely close to the value of 1 and further supports our hypotheses.
Another important issue is the appropriateness of the definition of sepsis, considering the characteristics of patients with advanced lung cancer. Infection and sepsis, severe sepsis, and septic shock cannot be easily distinguished, and their definitions change often.24 We used the standards suggested by Shao et al12 who defined infection in patients with advanced cancer. Thus, the first step in defining the infection was based on the results of a blood culture test. Then, laboratory test results were considered, including white blood cell counts, neutrophil granulocyte ratios, C-reactive protein levels, and procalcitonin levels. Second, to assess organ dysfunction, SOFA scores were used. Lastly, the systemic inflammatory response syndrome (SIRS) sign was used in parallel. Patients with advanced lung cancer can have symptoms similar to those of SIRS due to the unstable hemodynamic status that results from various reasons other than infection.25 Therefore, an SIRS assessment in patients with terminal stage advanced lung cancer would have low specificity and was not performed in this study. However, there are no established tools or systems that can effectively judge and define sepsis in patients with lung cancer; therefore, additional studies must be conducted.
This study had a few limitations, including the retrospective design of the experiment. Second, we only accurately assessed opioid use (amount) in the final week before death; the rate of increase in opioid usage, according to disease progression, could not be considered. Third, the changes and developments that the clinics underwent, along with the changes in the physicians, across the 15 years (2003-2017) of the study could not be taken into consideration. Lastly, to calculate the sums of the different types of opioids, conversions needed to be performed; the accuracy of the conversion method we used is still controversial.26 However, this study’s strength lies in the fact that it is the first study to assess the association between opioid usage and death from sepsis in patients with advanced cancer.
In conclusion, our study showed a significant association between the amount of opioids used in the final week before death and death due to sepsis in patients with advanced lung cancer.
Appendix
Appendix A.
Equianalgesic Opioid Conversion Table.
| Opioid | Administration Route | Dose Equivalent to 10 mg of Oral Morphine (mg) |
|---|---|---|
| Morphine | Oral | 10 |
| Morphine | Intravenous | 3.3 |
| Morphine | Epidural | 0.33 |
| Hydromorphone | Oral | 2 |
| Fentanyl | Intravenous | 0.03 |
| Oxycodone | Oral | 7 |
| Codeine | Oral | 80 |
| Tramadol | Oral | 40 |
Footnotes
Authors’ Note: This retrospective observational study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (SNUBH; approval number: B-1707/408-104). Because this was a retrospective review of patient medical records, the requirement for informed consent was waived.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Tak Kyu Oh, MD  https://orcid.org/0000-0002-4027-4423
https://orcid.org/0000-0002-4027-4423
References
- 1. Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The International Epidemiology of Lung Cancer: latest trends, disparities, and tumor characteristics. J Thorac Oncol. 2016;11(10):1653–1671. [Google Scholar]
- 2. Leppert W, Turska A, Majkowicz M, Dziegielewska S, Pankiewicz P, Mess E. Quality of life in patients with advanced lung cancer treated at home and at a palliative care unit. Am J Hosp Palliat Care. 2012;29(5):379–387. [DOI] [PubMed] [Google Scholar]
- 3. Yoong J, Park ER, Greer JA, et al. Early palliative care in advanced lung cancer: a qualitative study. JAMA Intern Med. 2013;173(4):283–290. [DOI] [PubMed] [Google Scholar]
- 4. Singer PA, Martin DK, Kelner M. Quality end-of-life care: patients’ perspectives. JAMA. 1999;281(2):163–168. [DOI] [PubMed] [Google Scholar]
- 5. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2): e58–68. [DOI] [PubMed] [Google Scholar]
- 6. Azevedo Sao Leao Ferreira K, Kimura M, Jacobsen Teixeira M. The WHO analgesic ladder for cancer pain control, twenty years of use. How much pain relief does one get from using it? Support Care Cancer. 2006;14(11):1086–1093. [DOI] [PubMed] [Google Scholar]
- 7. Ninkovic J, Roy S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids. 2013;45(1):9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roy S, Ninkovic J, Banerjee S, et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol. 2011;6(4):442–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oh TK, Jeon JH, Lee JM, et al. Investigation of opioid use and long-term oncologic outcomes for non-small cell lung cancer patients treated with surgery. PLoS One. 2017;12(7): e0181672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pergolizzi J, Boger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an international expert panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008;8(4):287–313. [DOI] [PubMed] [Google Scholar]
- 11. Zylla D, Kuskowski MA, Gupta K, Gupta P. Association of opioid requirement and cancer pain with survival in advanced non-small cell lung cancer. Br J Anaesth. 2014;113(suppl 1):i109–i116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shao YJ, Liu WS, Guan BQ, et al. Contribution of opiate analgesics to the development of infections in advanced cancer patients. Clin J Pain. 2017;33(4):295–299. [DOI] [PubMed] [Google Scholar]
- 13. Radford JA, Ryder WD, Dodwell D, Anderson H, Thatcher N. Predicting septic complications of chemotherapy: an analysis of 382 patients treated for small cell lung cancer without dose reduction after major sepsis. Eur J Cancer. 1992;29A(1):81–86. [DOI] [PubMed] [Google Scholar]
- 14. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 15. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Management of cancer pain: adults. Cancer Pain Guideline Panel. Agency for Health Care Policy and Research. Am Fam Physician. 1994;49(8):1853–1868. [PubMed] [Google Scholar]
- 17. Williams MD, Braun LA, Cooper LM, et al. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8(5): R291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manchikanti L, Kaye AM, Knezevic NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician. 2017;20(2S): S3–S92. [PubMed] [Google Scholar]
- 19. Kim R. Anesthetic technique and cancer recurrence in oncologic surgery: unraveling the puzzle. Cancer Metastasis Rev. 2017;36(1):159–177. [DOI] [PubMed] [Google Scholar]
- 20. Liang X, Liu R, Chen C, Ji F, Li T. Opioid system modulates the immune function: a review. Transl Perioper Pain Med. 2016;1(1):5–13. [PMC free article] [PubMed] [Google Scholar]
- 21. Plein LM, Rittner HL. Opioids and the immune system—friend or foe. Br J Pharmacol. 2018;175(14):2717–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin BJ, Corlew MM, Wood H, et al. The association of swallowing dysfunction and aspiration pneumonia. Dysphagia. 1994;9(1):1–6. [DOI] [PubMed] [Google Scholar]
- 23. Yu SM, Park KY. Risk factors for nosocomial urinary tract infection in the intensive care unit with a positive urine culture and Foley catheterization [in Korean]. Taehan Kanho Hakhoe Chi. 2007;37(7):1149–1158. [DOI] [PubMed] [Google Scholar]
- 24. Sterling SA, Puskarich MA, Glass AF, Guirgis F, Jones AE. The impact of the sepsis-3 septic shock definition on previously defined septic shock patients. Crit Care Med. 2017;45(9):1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andrejak C, Terzi N, Thielen S, et al. Admission of advanced lung cancer patients to intensive care unit: a retrospective study of 76 patients. BMC Cancer. 2011;11:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’Bryant CL, Linnebur SA, Yamashita TE, Kutner JS. Inconsistencies in opioid equianalgesic ratios: clinical and research implications. J Pain Palliat Care Pharmacother. 2008;22(4):282–290. [DOI] [PubMed] [Google Scholar]