Abstract
Background:
The large-scale National Lung Cancer Screening Trial demonstrated an increased detection of early-stage lung cancers using low-dose computed tomography scan in the screening population. It also demonstrated a 20% reduction of lung cancer-related deaths in these patients.
Aims:
Although both solid and subsolid lung nodules are evaluated in studies, subsolid and partially calcified lung nodules are often overlooked.
Materials and Methods:
We reviewed transthoracic fine-needle aspiration (FNA) cases from lung nodule patients in our clinics and correlated cytological diagnoses with radiologic characteristics of lesions. A computer search of the pathology archive was performed over a period of 12 months for transthoracic FNAs, including both CT- and ultrasound-guided biopsies.
Results:
A total of 111 lung nodule cases were identified. Lesions were divided into three categories: solid, subsolid, and partially calcified nodules according to radiographic findings. Of 111 cases, the average sizes of the solid (84 cases), subsolid (22 cases), and calcified (5 cases) lesions were 1.952 ± 2.225, 1.333 ± 1.827, and 1.152 ± 1.984 cm, respectively. The cytological diagnoses of three groups were compared. A diagnosis of malignancy was made in 64.28% (54 cases) in solid, 22.72% (5 cases) in subsolid, and 20% (1 case) in partially calcified nodules. Among benign lesions, eight granulomatous inflammations were identified, including one case of solid, five cases of subsolid, and two cases of calcified nodules.
Conclusions:
Our study indicates that solid nodules have the highest risk of malignancy. Furthermore, the cytological evaluation of subsolid and partially calcified nodules is crucial for the accurate diagnosis and appropriate clinical management of lung nodule patients.
Keywords: Cytological diagnosis, non-small cell lung carcinoma, solid, subsolid lung nodules, transthoracic fine-needle aspiration
INTRODUCTION
Lung cancer is the leading cause of cancer-related death throughout the world.[1,2] In 2018, the estimated number of new cases of lung cancer is approximately 234,030 patients annually, and the estimated number of deaths from lung cancer is approximately 154,050 patients, representing one-quarter of all cancer deaths in the United States.[1] Although knowledge in the prevention and development of targeted therapies has progressed rapidly, the overall mortality of lung cancer is still high.[3,4,5,6,7,8,9,10,11]
Over the past decade, several clinical trials using highly sensitive imaging technology, such as low-dose computed tomography (LDCT), have been implemented for the early detection of lung cancer and improvement of lung cancer patients' survival.[12,13] Focusing on individuals at high risk for the development of lung cancer, these screening trials (especially the recent National Lung Cancer Screening Trial [NLST] in the United States) have demonstrated an increased detection proportion of Stage I lung cancers, and early detection was associated with a 20% reduction of lung cancer-related deaths in the screening population.[14,15] While high-resolution imaging has evidently improved our ability to detect small lung cancers at a curable stage, lung cancer screening trials in both the US and Europe have also shown high false-positive rates.[12,13,14,15,16] In the European NELSON trial, 1451 nodules (19.2%) diagnosed as indeterminate and 119 nodules (1.6%) diagnosed as positive eventually required further assessment after the initial screening, but only 70 cases (0.9%) were confirmed to be lung cancer after further presurgical workup.[16] In the NLST from the United States, 780 (51%) cases required further imaging assessment, and some of them even required up to five repeats, but only 31 (3.97%) cases were eventually diagnosed as lung cancer.[14,15] Molecular markers in the sputum, bronchial epithelium, blood, and tumor tissues are being evaluated,[10,11,17] and although studies show promise of biomarkers in improvement of the specificity of diagnostic or screening tests, these biomarkers are still not being used clinically. Therefore, an accurate diagnosis of lung nodules plays a critical role in appropriate management of patients.[18,19,20]
Clinically, the diagnosis of lung nodules involves a combination of radiologic surveillance and cytological examination of lesional cells. Several approaches have been routinely used to obtain cytological material, such as minimally invasive transthoracic[21,22,23,24] and endoscopic transbronchial fine-needle aspiration (FNA).[25,26,27] Among these procedures, CT- and ultrasound (US)-guided transthoracic lung biopsies are particularly useful for diagnosing peripheral lung nodules, and these procedures also have lower costs and shorter hospital stay for patients.[21,22,23,24] Furthermore, although solid lung nodules are routinely biopsied, subsolid and partially calcified lung nodules are often not biopsied.[28,29]
In this study, we reviewed lung nodule patients who underwent transthoracic FNA of their lesion and correlated cytological diagnosis of these lung nodules with their radiologic characteristics.
MATERIALS AND METHODS
Case collection
Data were collected from a computer search of the archives of the department of pathology over a 12-month period from January 2013 to December 2013. The dataset was comprised transthoracic CT- and/or US-guided lung biopsies. Based on radiographic features, cases were reviewed and divided into three categories: solid, subsolid, and partially calcified nodules/lesions. The World Health Organization and the International Association for the Study of Lung Cancer/American Thoracic Society classification criteria were used for the determination of histological subtypes of lung cancer.[2]
Patients and public involvement
In this study, we evaluated the histological diagnoses of CT- and/or US-guided transthoracic FNA, retrospectively. All data were retrieved from a pathology database. Patients and the public were not directly involved.
Preparation of cytological smears
FNA smears were prepared using both air-dried and wet-fixed methods. The air-dried smears were stained with Diff-Quik (DQ) method (DQ stain) and used for the immediate on-site evaluation. Additional smears were wet-fixed with 95% alcohol and stained by Papanicolaou (Pap) method (Pap stain) in the cytopathology laboratory.
Cellblock and core biopsy material preparations
For cellblock preparation, the aspiration needle was rinsed with 10–20 cc of Hanks' balanced salt solution (Sigma, St. Louis, MO, USA) into a 50-cc centrifuge tube. The centrifuge tubes were spun down using a microcentrifuge at 1870 rpm for 10 min (Hettich, Beverly, MA, USA). Cellular material was recovered in 1–2 cc of 10% neutral buffered formalin and fixed overnight. After fixation in formalin, the pellet was embedded with paraffin and processed in the histology laboratory. The section was cut at 5-micron and stained with the hematoxylin and eosin (H and E). The core biopsy materials were fixed in 10% neutral buffered formalin and processed in the histology laboratory, with subsequent H and E staining.
Evaluation and statistical analysis of data
In our study, the cytologic diagnostic criteria of “malignancy” were defined by the identification of malignant cells in the biopsy material; the criteria of “benign” were defined by the absence of malignant cells; the criteria of “suspicious for malignancy” were defined by the presence of markedly atypical cells with the amount of atypical cells insufficient for the diagnosis of malignancy; and the criteria of “nondiagnostic” were defined by the lack of sufficient lesional cells.
Solid lung nodules were defined as completely solid soft-tissue density lesions on CT images, subsolid nodules included nodules with pure ground-glass appearance and part-solid ground-glass nodules, while partially calcified nodules referred to nodules containing calcifications on thin-section CT images.[29]
The Student's t, Fisher's exact, and/or Chi-square tests were used for statistical analyses. Different types of lung nodules were correlated with their demographic characteristics. Differences were considered statistically significant when P ≤ 0.05 (P < 0.05). All P value statistical tests were two-sided.
RESULTS
Clinical information
Our study group consisted of 49 females and 62 males, with a male:female ratio of 1.27:1. The median age of patients was 63.29 years, ranging from 23 to 87 years (with 86.5% of patients older than 50 years). Of 111 cases, 84 cases were solid, 22 cases were subsolid, and 5 cases were partially calcified lung nodules [Figure 1]. The median size of the solid, subsolid, and calcified lesions was 1.952 ± 2.225, 1.333 ± 1.827, and 1.152 ± 1.984 cm, respectively. There was no significant difference among these three groups (P > 0.05). The patients' clinical information with age and gender distribution is summarized in Table 1.
Figure 1.
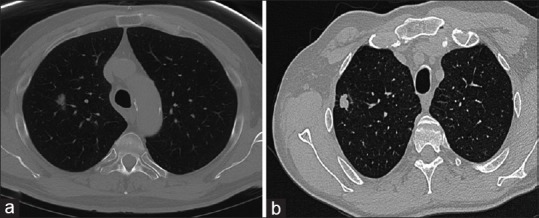
Representative image of solid and subsolid lung nodules. (a) A subsolid nodule defined as focal nodular area of increased lung attenuation through which lung parenchymal structures, such as the pulmonary vessels or airway structures, can be observed, with or without a small solid component. (b) A solid nodule defined as complete soft-tissue density on computed tomography
Table 1.
Clinical characteristics of study cases
| Characteristics | Solid (n=84) | Subsolid (n=22) | Calcified (n=5) |
|---|---|---|---|
| Gender case(%) | |||
| Male | 46(54.76) | 15(68.18) | 1(20) |
| Female | 38(45.24) | 7(31.82) | 4(80) |
| Age(years) | |||
| Median | 62.24 | 59.23 | 68.4 |
| Range | 31-86 | 23-85 | 53-87 |
| Lesion size(cm) | |||
| Median | 1.952±2.225 | 1.333±1.827 | 1.152±1.984 |
| Range | 0.7-9 | 0.8-7.4 | 2.5-6.5 |
Cytological diagnosis of lung nodules
Overall, 60 cases (54.06%) were diagnosed as malignant, 32 cases (28.83%) were diagnosed as benign lesions, 10 cases (9.00%) were diagnosed as suspicious for malignancy, and 9 cases (8.11%) were nondiagnostic [Table 2]. Representative cytological features of lung nodules are shown in Figure 2.
Table 2.
Cytological diagnoses of lung nodules
| Lung nodules | Malignant, n (%) | Benign, n (%) | Suspicious, n (%) | Nondiagnostic, n (%) |
|---|---|---|---|---|
| Solid (n=84) | 54 (48.65) | 15 (13.52) | 7 (6.30) | 8 (7.21) |
| Subsolid (n=22) | 5 (4.51) | 14 (12.61) | 2 (1.80) | 1 (0.90) |
| Calcified (n=5) | 1 (0.90) | 3 (2.70) | 1 (0.90) | N/A |
| Total (n=111) | 60 (54.06) | 32 (28.83) | 10 (9.00) | 9 (8.11) |
N/A: Not applicable
Figure 2.
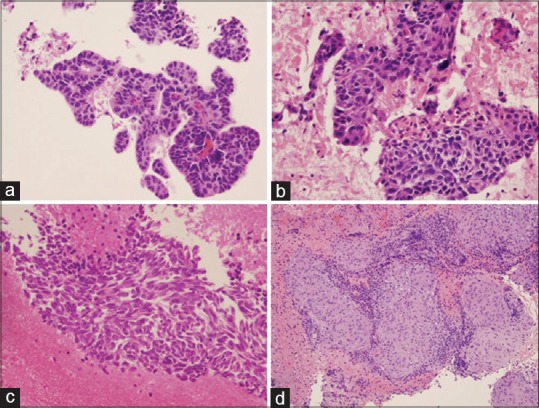
Cytological diagnosis of lung nodules. (a) Primary lung adenocarcinoma. Tumor cells show acinar and glandular arrangement with hyperchromatic nuclei. (b) Primary lung squamous cell carcinoma. Tumor cells demonstrate pleomorphic nuclei with few dyskeratotic cells. (c) Primary lung small cell carcinoma. Tumor cells reveal fine chromatin pattern, nuclear crowding and molding, and tumor necrosis. (d) Granulomatous inflammation. The section reveals clusters of epithelioid histiocytes, scattered multinucleated giant cells, and inflammatory cells. All photos are taken from cellblock H and E preparations at ×20
In solid nodules (n = 84), 54 lesions (64.29%) were diagnosed as malignant, 15 lesions (17.86%) were diagnosed as benign, 7 cases (8.33%) were diagnosed as suspicious for malignancy, and 8 lesions (9.52%) were considered nondiagnostic. The malignant cases comprised 25.93% (n = 14) adenocarcinoma (ADC), followed by 24.10% (n = 13) squamous cell carcinoma (SqCC), 18.53% (n = 10) small cell lung carcinoma (SCLC), 11.11% (n = 6) poorly differentiated non-small cell carcinoma (PDCA), 5.56% (n = 3) lymphoma, and 1.85% (n = 1) carcinoid. In the benign group, we found 1 case (6.67%) of granulomatous inflammation and 14 cases with reactive bronchial epithelium and/or inflammatory lesions.
In subsolid nodules (n = 22), 5 cases (22.73%) were diagnosed as malignant, 14 cases (63.64%) were diagnosed as benign lesions, 2 cases (9.01%) were diagnosed as suspicious for malignancy, and 1 case (4.55%) was considered nondiagnostic. Of the malignant lesions, 3 cases (60%) were diagnosed as ADC and 2 cases (40%) were diagnosed as SCC. In the benign group, we found 5 cases (35.71%) of granulomatous inflammations and 9 cases of reactive bronchial epithelium and/or inflammatory lesions.
In partially calcified nodules (n = 5), 1 case (20.00%) was diagnosed as malignant (i.e., ADC), 3 cases (60.00%) were diagnosed as benign lesions, and 1 case (20.00%) was diagnosed as suspicious for malignancy. In the benign group, we found 2 cases (66.67%) of granulomatous inflammation and 1 case of reactive bronchial epithelium and/or inflammatory lesions.
In cases of granulomatous inflammation, we also did multiple special stains including periodic acid–Schiff, Grocott's methenamine silver, and acid-fast bacteria stains. All these stains were negative for microorganisms in all cases.
Taken together, the solid nodules revealed the highest malignant rate and subsolid and partially calcified nodules had a higher incidence of granulomatous inflammation [Tables 2 and 3]. We also compared malignant lesions in both solid and subsolid nodules. The different subtypes of tumors are summarized in Figure 3.
Table 3.
Cytological diagnoses of malignant nodules
| Carcinomas | Solid, n(%) | Subsolid, n(%) | Calcified, n(%) |
|---|---|---|---|
| ADC | 14(25.93) | 3(60.00) | 1(100) |
| SqCC | 13(24.1) | 2(40.00) | N/A |
| SCLC | 10(18.53) | N/A | N/A |
| PDCA | 6(11.11) | N/A | N/A |
| Lymphoma | 3(5.56) | N/A | N/A |
| Carcinoid | 1(1.85) | N/A | N/A |
| Metastasis | 7(12.92) | N/A | N/A |
| Total | 54(100) | 5(100) | 1(100) |
ADC: Adenocarcinoma, SqCC: Squamous cell carcinoma, SCLC: Small cell lung carcinoma, PDCA: Poorly differentiated non-small cell carcinoma, N/A: Not applicable
Figure 3.
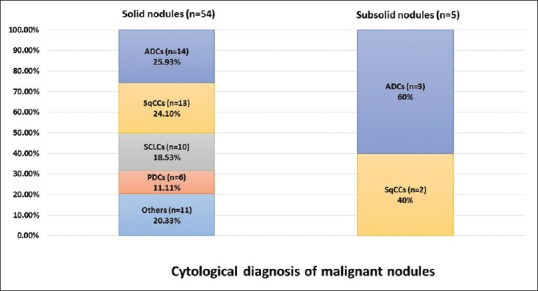
Comparison of the cytological diagnosis of solid and subsolid nodules
DISCUSSION
The diagnosis of lung nodules involves a combination of radiologic surveillance and the morphological examination of the lesion. The NLST trial in the United States using low-dose CT has demonstrated an increased detection rate of Stage I lung cancers.[13,14,15] This approach has been associated with a 20% reduction of lung cancer-related deaths in the screening population. The study clearly demonstrates a beneficial effect for individuals who are at high risk for the development of lung cancers, such as smokers aged from 55 to 80 years with at least a 30 pack-year smoking history and/or smokers who have quit <15 years.[19,28,29,30,31] However, the limitations of current screening approaches have also been reported, such as lack of cost-effectiveness, risk of radiation, and high false-positive results in the screening population.[18,31,32] How to best implement a screening strategy for the early detection of lung cancer is still a clinical challenge.
In the United States, 94 million individuals who are current or former smokers are at high risk of developing lung cancer, and the majority of smokers harbor small lung nodules.[12,14,15,31] The current clinical guideline for the management of lung nodules is mainly based on studies of noncalcified solid pulmonary nodules (SPN). Although several studies have addressed the issue of the management of subsolid and/or partially calcified lung nodules, evidence-based guidelines are still necessary. A study of 629 patients with SPN ranging in size from 4 mm to 30 mm found that 65% of the nodules were benign, 23% malignant, and 12% indeterminate.[16] While nodule size has traditionally been used to predict whether a lesion is benign or malignant,[12,13,14,15,28,29,30,31] high-resolution CT screening should detect an increasing proportion of smaller nodules that cannot be readily dismissed as benign due to their morphological changes. Thus, it is becoming increasingly important to distinguish small benign nodules from cancer using minimally invasive tissue sampling procedures and reducing the use of more invasive procedures to classify lung nodules.
Transthoracic FNA, with CT or US guidance, is a minimally invasive and effective procedure used to diagnose and subclassify pulmonary nodules >5 mm in size.[20,22,32,33] In addition, the resection rate of nonmalignant nodules after CT-guided FNA is significantly lower.[34] The procedure also has relatively fewer complications such as pneumothorax and hemorrhage.[21,24,30] The incidences of these complications range from 0% to 61% for pneumothorax and 11% for hemorrhage, respectively.[21] Other complications, such as air embolism and tumor seeding, may occur but are extremely rare.[21,24,29] Transthoracic FNA is considered a safe and accurate method for diagnosis and staging of pulmonary lesions. Furthermore, transthoracic FNA can also provide material for molecular testing of the tumor.[23,25,27] Recent advances in targeted and immune therapy have made the subclassification of non-SCLC (NSCLC) important for appropriate clinical management. For example, many targeted therapeutic agents are now available to treat lung cancers with specific molecular alterations, such as epidermal growth factor receptor mutations, anaplastic lymphoma kinase, and c-ros oncogene 1 gene rearrangements.[5,6,7,10,11] More recently, checkpoint blockade immunotherapy has been demonstrated to be an effective therapy for treating lung cancers in selected patients.[35] Therefore, accurate morphological diagnosis and molecular characterization of lung cancers are critical for targeted and personalized therapies.
In this retrospective study, we evaluated 111 cytological cases, including 84 solid, 22 subsolid, and 5 calcified nodules. Based on cyto-histopathological feature, we categorized all nodules in four groups; malignant, benign, suspicious, and nondiagnostic. Of all cases, 60 cases (54.06%) were malignant tumors, 32 cases (28.83%) were benign lesions, 10 cases (9%) were suspicious, and 9 cases (8.11%) were nondiagnostic cases. In our study, 48.65% of solid, 4.51% of subsolid, and 0.90% of partially calcified nodules were diagnosed as malignant. Of all malignant cases, 83.33% were diagnosed as NSCLCs, whereas 16.67% were diagnosed as SCLCs. ADC predominated among the malignant cases (30%), followed by SqCC (25%), and SCLC (16.67%), poorly differentiated non-small cell carcinoma (10%), and the rest comprised metastatic deposit, lymphoma, and carcinoid (18.33%). Among benign lesions, eight granulomatous inflammations were identified, including one case of solid, five cases of subsolid, and two cases of calcified nodules. In our study, we only found five cases in which the lesion was diagnosed as benign on transthoracic FNA, but the follow-up resection of the lesion proved to be malignancy. These cases include four cases of solid and one case of subsolid nodules. False-negative diagnosis has also been reported and associated with the location and the size of lesions, as well as in subsolid lesions.[18,32,33] Therefore, clinically suspicious cancer cases with benign cytological diagnosis still need to be further evaluated using a multimodality approach.
CONCLUSIONS
In summary, currently, screening guidelines have been developed to improve the detection of early cancer in high-risk patients. Clinical signs and symptoms of lung cancer patients, such as persistent cough, shortness of breath, and bloody sputum, often do not appear until there is advanced stage disease, making early detection difficult. Only 15% of patients diagnosed with localized disease have those clinical presentations. Thus, early detection of the tumor in a high-risk population is challenging and plays an important role in improvement of the clinical survival rate of lung cancer patients. We correlated the cytomorphological diagnoses of lung nodules with radiologic characteristics in high-risk patients. In our study, we found that solid nodules revealed the highest risk of malignancy, but subsolid and partially calcified nodule might also be associated with malignant lesions. Our study also indicates that cytological evaluation of subsolid and partially calcified nodules is crucial for the accurate diagnosis and the appropriate clinical management of lung nodule patients.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare no conflicts of interest.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author. YYZ, GG, HYW, ZAH, PL, and RH carried out data collection. YYZ, GG, HYW, and ZAH analyzed and interpreted the results and performed the statistical analysis. QKL carried out reviewing the slides and drafting the manuscript. YYZ, GG, HYW, ZAH, FA, and EG carried out reviewing and drafting the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
The study was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions.
LIST OF ABBREVIATIONS (In alphabetic order)
ADC – Adenocarcinoma
ALK – Anaplastic lymphoma kinase
CT – Computed tomography
DQ – Diff-Quik
EGFR – Epidermal growth factor receptor
FNA – Fine-needle aspiration
H and E – Hematoxylin and eosin
LDCT – Low-dose computed tomography
N/A – Not applicable
NLST – National Lung Cancer Screening Trial
NSCLC – Non-small cell lung carcinoma
Pap – Papanicolaou
PDCA – Poorly differentiated non-small cell carcinoma
ROS1 – c-ros oncogene 1
SCLC – Small cell lung carcinoma
SPN – Solid pulmonary nodule
SqCC – Squamous cell carcinoma
US – Ultrasound
WHO – The World Health Organization
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Contributor Information
Yangying Zhou, Email: yzhou122@jhu.edu.
Gary Gong, Email: ggong1@jhmi.edu.
Haiyan Wang, Email: hwang171@jhmi.edu.
Zahra Alikhassy Habibabady, Email: zaryalikhassy@gmail.com.
Peggy Lang, Email: mlang5@jhmi.edu.
Russell Hales, Email: rhales1@jhmi.edu.
Frederic Askin, Email: faskin@jhmi.edu.
Ed Gabrielson, Email: egabriel@jhmi.edu.
Qing Kay Li, Email: qli23@jhmi.edu.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10:1240–2. doi: 10.1097/JTO.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 3.New M, Keith R. Early detection and chemoprevention of lung cancer. F1000Res. 2018;7:61. doi: 10.12688/f1000research.12433.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouvinhas C, De Mello RA, Oliveira D, Castro-Lopes JM, Castelo-Branco P, Dos Santos RS, et al. Lung cancer: A brief review of epidemiology and screening. Future Oncol. 2018;14:567–75. doi: 10.2217/fon-2017-0486. [DOI] [PubMed] [Google Scholar]
- 5.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 6.Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, et al. Erlotinib in lung cancer – Molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–44. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 7.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–80. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 8.Munfus-McCray D, Cui M, Zhang Z, Gabrielson E, Askin F, Li QK. Comparison of EGFR and KRAS mutations in primary and unpaired metastatic lung adenocarcinoma with potential chemotherapy effect. Hum Pathol. 2013;44:1286–92. doi: 10.1016/j.humpath.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Li QK, Singh A, Biswal S, Askin F, Gabrielson E. KEAP1 gene mutations and NRF2 activation are common in pulmonary papillary adenocarcinoma. J Hum Genet. 2011;56:230–4. doi: 10.1038/jhg.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–25. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–50. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Mandrekar SJ, Hillman SL, et al. CT screening for lung cancer: Five-year prospective experience. Radiology. 2005;235:259–65. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- 13.van Klaveren RJ, Oudkerk M, Prokop M, Scholten ET, Nackaerts K, Vernhout R, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361:2221–9. doi: 10.1056/NEJMoa0906085. [DOI] [PubMed] [Google Scholar]
- 14.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, et al. National Lung Screening Trial Research Team. The national lung screening trial: Overview and study design. Radiology. 2011;258:243–53. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diederich S, Thomas M, Semik M, Lenzen H, Roos N, Weber A, et al. Screening for early lung cancer with low-dose spiral computed tomography: Results of annual follow-up examinations in asymptomatic smokers. Eur Radiol. 2004;14:691–702. doi: 10.1007/s00330-003-2200-5. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Beer DG. Molecular predictors of prognosis in lung cancer. Ann Surg Oncol. 2012;19:669–76. doi: 10.1245/s10434-011-1967-x. [DOI] [PubMed] [Google Scholar]
- 18.Gelbman BD, Cham MD, Kim W, Libby DM, Smith JP, Port JL, et al. Radiographic and clinical characterization of false negative results from CT-guided needle biopsies of lung nodules. J Thorac Oncol. 2012;7:815–20. doi: 10.1097/JTO.0b013e31824abd9c. [DOI] [PubMed] [Google Scholar]
- 19.Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med. 2012;185:363–72. doi: 10.1164/rccm.201104-0679CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callister ME, Baldwin DR. How should pulmonary nodules be optimally investigated and managed? Lung Cancer. 2016;91:48–55. doi: 10.1016/j.lungcan.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Koegelenberg CF, Bolliger CT, Irusen EM, Wright CA, Louw M, Schubert PT, et al. The diagnostic yield and safety of ultrasound-assisted transthoracic fine-needle aspiration of drowned lung. Respiration. 2011;81:26–31. doi: 10.1159/000319576. [DOI] [PubMed] [Google Scholar]
- 22.Minot DM, Jaben E, Aubry MC, Voss JS, Vine RL, Lee PU, et al. Evolution of transthoracic fine needle aspiration and core needle biopsy practice: A comparison of two time periods, 1996-1998 and 2003-2005. Diagn Cytopathol. 2012;40:876–81. doi: 10.1002/dc.21666. [DOI] [PubMed] [Google Scholar]
- 23.Ferretti GR, Busser B, de Fraipont F, Reymond E, McLeer-Florin A, Mescam-Mancini L, et al. Adequacy of CT-guided biopsies with histomolecular subtyping of pulmonary adenocarcinomas: Influence of ATS/ERS/IASLC guidelines. Lung Cancer. 2013;82:69–75. doi: 10.1016/j.lungcan.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Heerink WJ, de Bock GH, de Jonge GJ, Groen HJ, Vliegenthart R, Oudkerk M. Complication rates of CT-guided transthoracic lung biopsy: Meta-analysis. Eur Radiol. 2017;27:138–48. doi: 10.1007/s00330-016-4357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feller-Kopman D, Yung RC, Burroughs F, Li QK. Cytology of endobronchial ultrasound-guided transbronchial needle aspiration: A retrospective study with histology correlation. Cancer. 2009;117:482–90. doi: 10.1002/cncy.20049. [DOI] [PubMed] [Google Scholar]
- 26.Stoll LM, Yung RC, Clark DP, Li QK. Cytology of endobronchial ultrasound-guided transbronchial needle aspiration versus conventional transbronchial needle aspiration. Cancer Cytopathol. 2010;118:278–86. doi: 10.1002/cncy.20103. [DOI] [PubMed] [Google Scholar]
- 27.Yung RC, Otell S, Illei P, Clark DP, Feller-Kopman D, Yarmus L, et al. Improvement of cellularity on cell block preparations using the so-called tissue coagulum clot method during endobronchial ultrasound-guided transbronchial fine-needle aspiration. Cancer Cytopathol. 2012;120:185–95. doi: 10.1002/cncy.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Filippo M, Saba L, Concari G, Nizzoli R, Ferrari L, Tiseo M, et al. Predictive factors of diagnostic accuracy of CT-guided transthoracic fine-needle aspiration for solid noncalcified, subsolid and mixed pulmonary nodules. Radiol Med. 2013;118:1071–81. doi: 10.1007/s11547-013-0965-4. [DOI] [PubMed] [Google Scholar]
- 29.Chung K, Jacobs C, Scholten ET, Goo JM, Prosch H, Sverzellati N, et al. Lung-RADS category 4X: Does it improve prediction of malignancy in subsolid nodules? Radiology. 2017;284:264–71. doi: 10.1148/radiol.2017161624. [DOI] [PubMed] [Google Scholar]
- 30.Wiener RS, Gould MK, Arenberg DA, Au DH, Fennig K, Lamb CR, et al. An official American Thoracic Society/American college of chest physicians policy statement: Implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med. 2015;192:881–91. doi: 10.1164/rccm.201508-1671ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Black WC, Gareen IF, Soneji SS, Sicks JD, Keeler EB, Aberle DR, et al. Cost-effectiveness of CT screening in the national lung screening trial. N Engl J Med. 2014;371:1793–802. doi: 10.1056/NEJMoa1312547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piplani S, Mannan R, Lalit M, Manjari M, Bhasin TS, Bawa J, et al. Cytologic-radiologic correlation using transthoracic CT-guided FNA for lung and mediastinal masses: Our experience. Anal Cell Pathol (Amst) 2014;2014:343461. doi: 10.1155/2014/343461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sangha BS, Hague CJ, Jessup J, O'Connor R, Mayo JR. Transthoracic computed tomography-guided lung nodule biopsy: Comparison of core needle and fine needle aspiration techniques. Can Assoc Radiol J. 2016;67:284–9. doi: 10.1016/j.carj.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Barta JA, Henschke CI, Flores RM, Yip R, Yankelevitz DF, Powell CA, et al. Lung cancer diagnosis by fine needle aspiration is associated with reduction in resection of nonmalignant lung nodules. Ann Thorac Surg. 2017;103:1795–801. doi: 10.1016/j.athoracsur.2016.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]


