Abstract
Background:
Placement of ventriculoperitoneal shunt is a standard treatment for hydrocephalus. The risk of shunt malfunction in the first year is 25%–40% making endoscopic third ventriculostomy (ETV) a feasible option in those patients with shunt failure.
Aim:
The aim of this study was to evaluate ETV as a viable option in patients with shunt malfunction and to correlate the clinical outcome following successful ETV with functional and radiological outcomes.
Materials and Methods:
All patients who underwent ETV as a diversion procedure for hydrocephalus following shunt failure or malfunction over 1 year were studied. Functional outcome was evaluated by Wee function independence measure score carried out preoperatively, postoperatively, and at 6-month follow-up. Similar comparison was carried out for radiological parameters such as effacement of gyri, periventricular lucency, frontal horn diameter (maximum), Evans’ index, and third ventricular diameter.
Results:
Of 15 patients, 61.5% were shunt free after ETV. All the failures were noted in the first month following the procedure. The factors, which showed statistically significant correlation with the outcome of ETV, included age (P = 0.030), preoperative functional score (P = 0.006), and all the three components of the functional scoring, namely self-care score (P = 0.087), motor control score (P = 0.035), and neurocognitive score (P = 0.003). Parameters such as Evans’ index, maximum frontal horn diameter, and third ventricular diameter showed no significant difference between preoperative and postoperative scans. In follow-up imaging, only the frontal horn diameter showed a significant improvement (P = 0.047).
Conclusion:
ETV leads to significant neurocognitive improvement and postoperative functional status making it a viable option in patients who present with shunt malfunction.
Keywords: Endoscopic third ventriculostomy, hydrocephalus, pediatric, ventriculoperitoneal shunt, shunt failure
Introduction
Hydrocephalus is defined as an active distension of the ventricular system resulting from inadequate passage of cerebrospinal fluid (CSF) from its point of production within the ventricles to its point of absorption into the systemic circulation.[1] A recent study revealed that pediatric hydrocephalus results in 38,200–39,900 annual hospital admissions, with total hospital charges of 1.4-2 billion US dollars. Hydrocephalus accounts for 3.1% of all pediatric hospital charges.[2]
Shunt placement has been the standard treatment for these patients. The risk of shunt malfunction is relatively high ranging from 25% to 40% following the first year of shunt placement, followed by a 4%–5% increase every year.[3] Hence, shunt failure is almost inevitable during a patient’s life. In view of this near inevitability of shunt failure, endoscopic third ventriculostomy (ETV) is now considered as an advancement for the patients who present with shunt malfunction.
The aim of this study was to evaluate ETV as a viable option in patients with shunt malfunction and to correlate the clinical outcome following successful ETV with the functional and radiological outcomes.
Materials and Methods
All consecutive patients who underwent ETV as a diversion procedure for hydrocephalus following shunt failure or malfunction from January 2014 to February 2015, were included in the study. Patients with coagulopathies or those who underwent ETV as the primary drainage procedure were excluded from the study.
These patients were evaluated for symptoms and signs of raised intracranial pressure by clinical examination and investigations preoperatively and postoperatively. Signs such as neck stiffness, cerebellar signs, and focal neurological deficits were also elicited. In all patients, the evaluation of the cause of the shunt failure was carried out.
Preoperatively, various radiological parameters such as effacement of gyri, periventricular lucency, frontal horn diameter (maximum), Evans’ index, and third ventricular diameter were studied and were compared with the immediate postoperative and follow-up period imaging. All the patients who were involved in the study were also functionally evaluated by a Wee function independence measure (WeeFIM) score for functional status in preoperative period and in follow-up period after 6 months.[4]
The statistical analysis for continuous variables was carried out using Student’s t-test or analysis of variance. For preoperative and postoperative categorical data, comparison was carried out with McNemar’s test. Functional status, which was measured using the WeeFIM scoring, was compared preoperatively and postoperatively using the Wilcoxon signed-rank test. All tests were two-sided, and P value <0.05 was considered as significant.
Results
A total of 15 patients of hydrocephalus underwent ETV during the 12-month study. All these patients were initially managed with shunt placement and subsequently presented with shunt malfunction. The age of the patients ranged between 11 months and 35 years with a mean age of 10.5 years. Of the seven patients who were less than 15 years of age were considered as pediatric age group and the remaining eight were considered as adult patients. A total of 86.6% patients were male (13/15). Hydrocephalus in these patients was due to various reasons such as congenital hydrocephalus, Dandy–Walker syndrome, posterior fossa space occupying lesions, and post-tuberculosis (TB) meningitis [Figure 1].
Figure 1.
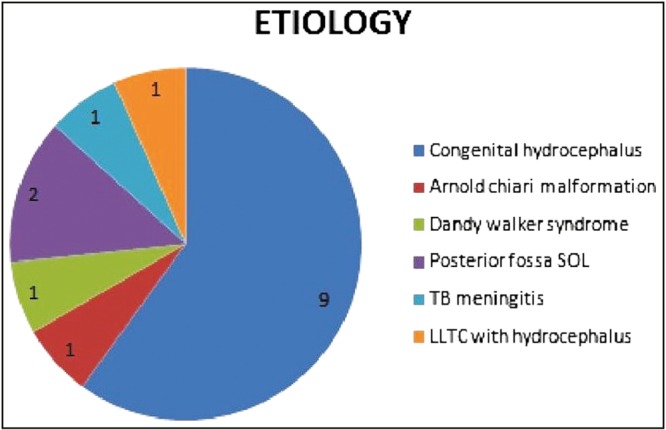
Etiology of hydrocephalus in study group, SOL = Space occupying lesion, LLTC = Low lying tethered cord
Of the 15 patients, 61.5% were shunt free following ETV (8/15). Of the seven patients not considered successful, two were lost to follow-up, and in another two, the procedure had to be abandoned due to severe ependymal inflammation (one patient, 6.6%) and poor anatomical details during neuroendoscopy (one patient, 6.6%). The rest three had failed procedure and needed a repeat shunt placement within a mean of 8.2 days (early failure). No delayed failures in our study group were observed up to a mean follow-up of 10.38 months.
Functional assessment was carried out by comparing preoperative WeeFIM scores to the score in the follow-up period. The mean improvement was 6.23% with a range of 0%–17.8% [Figure 2]. The mean improvement in the self-care and motor control component of the functional score was 4.05% and 1.33%, respectively. The mean improvement in the cognitive component of the functional score was 16.49% [Figure 3].
Figure 2.
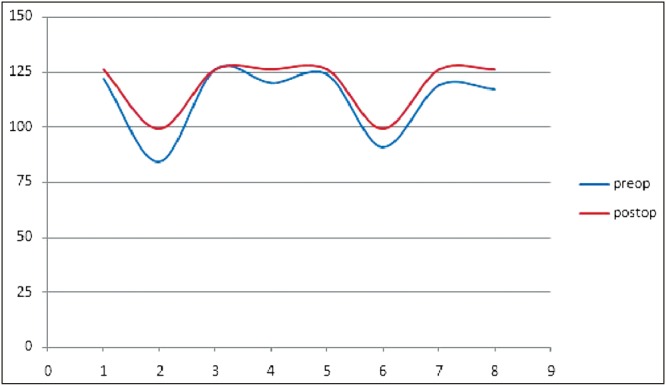
Functional score comparison (max score, 126)
Figure 3.
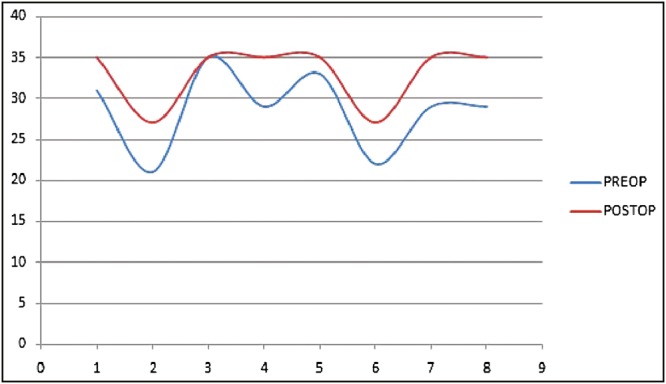
Cognitive score comparison
The factors, which showed statistically significant correlation with the outcome of ETV, included age, preoperative functional score (WeeFIM), and all the three components of the functional scoring, namely self-care score, motor control score, and neurocognitive score [Table 1].
Table 1.
Functional predictors of outcome of ETV in post shunt failure patients
| Parameter | P value |
|---|---|
| Age | 0.030 |
| Wee FIM total score | 0.030 |
| Neuro-cognitive component of Wee FIM | 0.030 |
| Self care component of Wee FIM | 0.011 |
| Motor component of Wee FIM score | 0.030 |
Radiological data were evaluated in all patients with the help of computed tomography/magnetic resonance imaging (CT/MRI) carried out preoperatively and in the immediate postoperative period. In patients who improved following ETV alone, six patients were evaluated with follow-up imaging at 6 months to 1 year after the surgery. Parameters such as Evans’ index, maximum frontal horn diameter, and third ventricular diameter showed no significant difference between preoperative and postoperative scans. In follow-up imaging (at least after 6 months), only the frontal horn diameter showed a significant improvement (P = 0.047). Rest of the factors, which were studied, did not show significant impact on the outcome [Table 2].
Table 2.
Parameters that did not significantly predict outcome after ETV
| Predictors of Outcome | P Value |
|---|---|
| Sex | 0.715 |
| Preoperative Glasgow Coma scale (GCS) | 0.071 |
| Preoperative Radiological Parameters | |
| Evans Index | 0.171 |
| Maximum Frontal Horn Diameter | 0.284 |
| 3rd Ventricular Diameter | 0.127 |
| Effacement of Gyri | 0.123 |
| Periventricular Lucency | 0.212 |
| Intra Operative Complications | 0.928 |
| Post Operative Glasgow coma Scale | 0.151 |
| Period for which patient is in Ventriculo Peritoneal Shunt | 0.502 |
| Number of Shunts done before ETV | 0.198 |
Discussion
ETV has greatly progressed due to refinement of neuroendoscopic techniques and the optics since the time it was first described by Sayers and Kosnik[5] in 1976. Modern endoscopes with their fiber optics and advanced light sources allow for excellent resolution of ventricular anatomy and safe fenestration of the floor of the third ventricle.
Studies have reported an overall ETV success rate of 52%.[6] ETV also has a higher proven efficacy in the management of obstructive hydrocephalus with success rates of 82% and 76.7% in adults and children, respectively, as indicated in the studies by Boschert et al.[7] and Cinalli et al.[8] The need of prompt follow-up in the first year following the ETV is also indicated by various studies because of a high failure rate in the first month following the procedure and increasing number of ETV failures in first year following surgery.[2]
In our study, the success rate was 61.5% (eight successful ETV of 13 secondary ETV preformed and two patients were lost to follow-up and their outcome was not included) and was comparable with the outcome of previous studies.[9,10,11] Of all the 15 patients involved in the study, in two patients (13.3%), the procedure was abandoned in view of poor anatomical landmarks and severe ependymal inflammation. A total of 26% of ETV abandoning rate is described in a study conducted by Brockmeyer et al.[12] over 98 patients for various reasons such as unfavorable anatomy, hemorrhage, and inability to perform cisternostomy, but most of the cases in this series underwent ETV as a primary diversion procedure and no specific data could be found on the rate at which the procedure was abandoned in patients of secondary ETV.
Among multiple factors that were thought to effect the outcome of ETV in various studies, a special consideration was given to age of patient and etiology of underlying pathology. The review of some studies suggested that the clinical response to ETV is different in children compared to that in adult population secondary to the age of onset, CSF dynamics, and changes in viscoelastic properties of the brain parenchyma.[8,13,14] The success rate of ETV in children with less than 2 years of age ranged between 0% and 83% with a mean success rate of 47.8%, which was significantly less than the success rate in adults, which was up to 75% in obstructive etiologies.[15,16] In our data, seven patients belonged to the pediatric age group, of these four had failure of procedure, and hence a success rate of 48.5%. Among these four patients, three were less than 2 years of age, which mirrored the results of previous studies.
The role of primary ETV in children for different types of hydrocephalus has been elaborated in literature. Children with congenital or acquired aqueductal stenosis have a good outcome ranging between 70% and 90% in the long term.[17,18] ETV, in patients with posterior fossa tumors, has a success rate of more than 90% either as a temporary or permanent measure for the treatment of hydrocephalus.[19,20,21] ETV in children with hydrocephalus with postinfectious etiology has a lower outcome than when it is carried out in patients with noninfectious etiology possibly due to adhesions and scarring in basal cisterns.[22]
Outcome for hydrocephalus following TB meningitis is better than the ventriculo-peritoneal shunt placement as the risk of shunt failure is very high.[23] Also the outcome of secondary ETV is much better than when it is carried out as a primary CSF diversion procedure with a success rate of 71% and 27%, respectively.[22,24]
Etiology had shown no statistically significant impact on the outcome in our study, probably, because of a very small sample size, and we support the idea of the need for further study with a larger sample size.
In our study, of the 15 patients who underwent ETV following shunt malfunction, three patients had undergone repeated shunting, and the highest number of shunts in a single patient was three and the mean time for which the patients were on shunt was 3.4 years. The results in our study also showed no statistically significant correlation with the outcome of the ETV.
Most importantly, all the patients who underwent secondary ETV were evaluated for the preoperative and the postoperative functional status and this functional outcome was compared with the clinical improvement in all the patients. This functional assessment was conducted using the WeeFIM scoring system (maximum score of 126). It is a functional score, which is obtained with the sum of three other scores, which include self-care score, motor control score, and neurocognitive score. This is an objective scoring system, which is easy to perform and is effective in evaluating the functional capabilities of the patient and the quality of life based on the final score. No study was found till date, which used WeeFIM functional assessment in the patients who underwent a diversion procedure for hydrocephalus.
Preoperative functional assessment that was performed with WeeFIM scoring showed a significant correlation with the outcome. A similar correlation was also found with the neurocognitive component of the WeeFIM score, which was comparable to the functional improvement in patients who underwent shunt placement following ETV failure. The neurocognitive abilities of children significantly improved following ETV allowing a better development, and hence an enhanced quality of life. These findings should be correlated with the functional outcome after a longer follow-up as the duration of follow-up was only 6–14 months in this study.
All the patients in the study underwent either CT or MRI in the preoperative period, and ventriculomegaly, periventricular lucency, and effacement of gyri were present in almost all of them. Evans’ index (mean, 0.399), maximum frontal horn diameter (mean, 2.13), and maximum third ventricular diameter (mean, 1.633) were calculated in all the patients, which showed no significant correlation (P value being 0.083, 0.081, and 0.718, respectively) with the outcome, but their role in predicting the outcome cannot be ruled out in view of small sample size in our study.
In 75% of the patients who clinically improved following ETV alone, follow-up imaging showed a significant improvement in the maximum frontal horn diameter (P = 0.047), whereas parameters such as Evans’ index (P = 0.518) and third ventricular diameter (P = 0.274) showed insignificant improvement unlike many previous studies.[25] All the patients with clinical improvement concurrently had radiological and functional improvement but their correlations can only be established after a larger study is conducted.
In spite of limitations of small number of patients and a short period of follow-up, our study portrays the experience at our institute with secondary ETV, and along with other studies, it supports ETV as a viable option in patients with shunt malfunction.
Conclusion
ETV when performed following shunt malfunction helped 61.5% of the patients to become shunt independent as observed over a mean follow-up of 10.8 months. All the failures occurred in the first month following the procedure. Age and preoperative functional status were found to be significantly correlating with the outcome. The sex, etiology, duration for which the patient is on shunt, number of shunts undergone earlier, and radiological parameters including Evans’ index, maximum frontal horn diameter, and third ventricular diameter did not influence the outcome to a level of statistical significance. In patients with a good clinical outcome following surgery, there was a significant improvement in the maximum frontal horn diameter. ETV leads to significant neurocognitive improvement and postoperative functional status making it a viable option in patients who present with shunt malfunction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rekate HL. The definition and classification of hydrocephalus: a personal recommendation to stimulate debate. Cerebrospinal Fluid Res. 2008;5:2. doi: 10.1186/1743-8454-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SH, Kong DS, Seol HJ, Shin HJ. Endoscopic third ventriculostomy in patients with shunt malfunction. J Korean Neurosurg Soc. 2011;49:217–21. doi: 10.3340/jkns.2011.49.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon TD, Riva-Cambrin J, Srivastava R, Bratton SL, Dean JM, Kestle JR Hydrocephalus Clinical Research Network. Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr. 2008;1:131–7. doi: 10.3171/PED/2008/1/2/131. [DOI] [PubMed] [Google Scholar]
- 4.Msall ME, DiGaudio K, Rogers BT, LaForest S, Catanzaro NL, Campbell J, et al. The functional independence measure for children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities. Clin Pediatr (Phila) 1994;33:421–30. doi: 10.1177/000992289403300708. [DOI] [PubMed] [Google Scholar]
- 5.Sayers MP, Kosnik EJ. Percutaneous third ventriculostomy: experience and technique. Childs Brain. 1976;2:24–30. doi: 10.1159/000119596. [DOI] [PubMed] [Google Scholar]
- 6.Buxton N, Macarthur D, Robertson I, Punt J. Neuroendoscopic third ventriculostomy for failed shunts. Surg Neurol. 2003;60:201–3. doi: 10.1016/s0090-3019(03)00317-3. discussion 203-4. [DOI] [PubMed] [Google Scholar]
- 7.Boschert J, Hellwig D, Krauss JK. Endoscopic third ventriculostomy for shunt dysfunction in occlusive hydrocephalus: long-term follow up and review. J Neurosurg. 2003;98:1032–9. doi: 10.3171/jns.2003.98.5.1032. [DOI] [PubMed] [Google Scholar]
- 8.Cinalli G, Salazar C, Mallucci C, Yada JZ, Zerah M, Sainte-Rose C. The role of endoscopic third ventriculostomy in the management of shunt malfunction. Neurosurgery. 1998;43:1323–7. doi: 10.1097/00006123-199812000-00030. discussion 1327-9. [DOI] [PubMed] [Google Scholar]
- 9.Marton E, Feletti A, Basaldella L, Longatti P. Endoscopic third ventriculostomy in previously shunted children: a retrospective study. Childs Nerv Syst. 2010;26:937–43. doi: 10.1007/s00381-010-1130-1. [DOI] [PubMed] [Google Scholar]
- 10.Melikian A, Korshunov A. Endoscopic third ventriculostomy in patients with malfunctioning CSF-shunt. World Neurosurg. 2010;74:532–7. doi: 10.1016/j.wneu.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Heshmati B, Habibi Z, Golpayegani M, Salari F, Anbarlouei M, Nejat F. Endoscopic third ventriculostomy in children with failed ventriculoperitoneal shunt. Asian J Neurosurg. 2019;14:399–402. doi: 10.4103/ajns.AJNS_93_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brockmeyer D, Abtin K, Carey L, Walker ML. Endoscopic third ventriculostomy: an outcome analysis. Pediatr Neurosurg. 1998;28:236–40. doi: 10.1159/000028657. [DOI] [PubMed] [Google Scholar]
- 13.Dusick JR, McArthur DL, Bergsneider M. Success and complication rates of endoscopic third ventriculostomy for adult hydrocephalus: a series of 108 patients. Surg Neurol. 2008;69:5–15. doi: 10.1016/j.surneu.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Enchev Y, Oi S. Historical trends of neuroendoscopic surgical techniques in the treatment of hydrocephalus. Neurosurg Rev. 2008;31:249–62. doi: 10.1007/s10143-008-0131-y. [DOI] [PubMed] [Google Scholar]
- 15.Niknejad HR, Depreitere B, De Vleeschouwer S, Van Calenbergh F, van Loon J. Results of endoscopic third ventriculostomy in elderly patients ≥65 years of age. Clin Neurol Neurosurg. 2015;130:48–54. doi: 10.1016/j.clineuro.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Oertel JM, Baldauf J, Schroeder HW, Gaab MR. Endoscopic options in children: experience with 134 procedures. J Neurosurg Pediatr. 2009;3:81–9. doi: 10.3171/2008.11.PEDS0887. [DOI] [PubMed] [Google Scholar]
- 17.Ghritlaharey RK, Budhwani KS, Shrivastava DK, Srivastava J. Ventriculoperitoneal shunt complications needing shunt revision in children: a review of 5 years of experience with 48 revisions. Afr J Paediatr Surg. 2012;9:32–9. doi: 10.4103/0189-6725.93300. [DOI] [PubMed] [Google Scholar]
- 18.Irani F, Elkambergy H, Okoli K, Abou DS. Recurrent symptomatic pleural effusion due to a ventriculopleural shunt. Respir Care. 2009;54:1112–4. [PubMed] [Google Scholar]
- 19.El-Ghandour NM. Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in the treatment of obstructive hydrocephalus due to posterior fossa tumors in children. Childs Nerv Syst. 2011;27:117–26. doi: 10.1007/s00381-010-1263-2. [DOI] [PubMed] [Google Scholar]
- 20.Fritsch MJ, Doerner L, Kienke S, Mehdorn HM. Hydrocephalus in children with posterior fossa tumors: role of endoscopic third ventriculostomy. J Neurosurg. 2005;103:40–2. doi: 10.3171/ped.2005.103.1.0040. [DOI] [PubMed] [Google Scholar]
- 21.Klimo P, Jr, Goumnerova LC. Endoscopic third ventriculocisternostomy for brainstem tumors. J Neurosurg. 2006;105:271–4. doi: 10.3171/ped.2006.105.4.271. [DOI] [PubMed] [Google Scholar]
- 22.Drake JM Canadian Pediatric Neurosurgery Study Group. Endoscopic third ventriculostomy in pediatric patients: the Canadian experience. Neurosurgery. 2007;60:881–6. doi: 10.1227/01.NEU.0000255420.78431.E7. discussion 881-6. [DOI] [PubMed] [Google Scholar]
- 23.Bhagwati S, Mehta N, Shah S. Use of endoscopic third ventriculostomy in hydrocephalus of tubercular origin. Childs Nerv Syst. 2010;26:1675–82. doi: 10.1007/s00381-010-1183-1. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien DF, Javadpour M, Collins DR, Spennato P, Mallucci CL. Endoscopic third ventriculostomy: an outcome analysis of primary cases and procedures performed after ventriculoperitoneal shunt malfunction. J Neurosurg. 2005;103:393–400. doi: 10.3171/ped.2005.103.5.0393. [DOI] [PubMed] [Google Scholar]
- 25.Sarmast A, Khursheed N, Ramzan A, Shaheen F, Wani A, Singh S, et al. Endoscopic third ventriculostomy in noncommunicating hydrocephalus: report on a short series of 53 children. Asian J Neurosurg. 2019;14:35–40. doi: 10.4103/ajns.AJNS_187_16. [DOI] [PMC free article] [PubMed] [Google Scholar]


