Abstract
Background
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by loss of dopaminergic neurons in the substantia nigra. The purpose of this study was to examine neuroprotective effects of Hepad S1, an herbal medicine used for the treatment of PD, in in vitro and in vivo models of PD.
Methods
Differentiated neuronal PC12 cells underwent a cytotoxicity assay and oxidative stress analysis including DCF-DA staining, glutathione, and malondialdehyde, after exposure to 1-methyl-4-phenylpyridium (MPP+). Male Sprague–Dawley rats were used as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD models. After 4-week oral administration of Hepad S1 (200, 300, 400, and 500 mg/kg/day), the levels of complex enzyme I activity and dopamine, and dopaminergic neuronal cell number in substantia nigra were measured by enzyme linked immune-sorbent assay (ELISA) and microscopic observation, respectively. Circulating serotonin and orexin A were also examined by ELISA.
Results
Hepad S1 pretreatment prevented the ability of MPP+ challenge to decrease glutathione and increase lipid peroxidation in cells, indicating antioxidant activity. Hepad S1 recovered MPTP-induced decreases in complex I enzyme activity and enhanced dopamine availability in substantia nigra. Serum levels of serotonin and orexin A were increased by Hepad S1 treatment in model animals. Hepad S1 treatment was associated with the preservation of tyrosine hydroxylase-positive cells in the substantia nigra of MPTP-treated rats.
Conclusions
Hepad S1 exerts antioxidant and neuroprotective effects on neurons of the substantia nigra in a rodent model of PD.
Keywords: Hepad, Korean medicine, Parkinson’s disease, reactive oxygen species, dopamine
1. Introduction
Parkinson’s disease (PD) is a chronic neurodegenerative disorder that produces dopaminergic neuronal loss in the substantia nigrapc).1 Neuronal loss impairs dopamine production and thus motor input to the basal ganglia, leading to the onset of PD symptoms. In the initial stage of disease, symptoms include tremor, slow movement, stiffness, unbalanced posture, and gait; these symptoms later progress to dysphagia, constipation, body temperature disturbance, and other motor symptoms.2 In the patient with PD, they also have lowered plasma level of neurotransmitter including serotonin.3 Especially, the decreased serotonin level in blood reflects the mood disorder such as depression.
Given that radiological examinations do not usually demonstrate clear findings in PD, clinical observations are the most informative for diagnosis. The National Institute of Clinical Excellence diagnostic criteria for PD include responsiveness to levodopa treatment and at least 2 relevant symptoms such as tremor, stiffness, and slow movement.4 A definitive diagnosis of PD is only attained through pathohistological examination of brain tissue via postmortem biopsy.
Although dopamine therapy is the gold standard for PD treatment, use of dopamine medications for 4–5 years is associated with side effects including dyskinesia in 40% of patients, and this percentage increases to 90% of patients after 9–15 years of use.5 Moreover, current pharmacological treatments for PD do not alter disease progression. Therefore, there is a need for novel approaches to protect and preserve neurons in the substantia nigra without producing intolerable side effects in patients with PD.
Hepad S1 is an herbal medicine based on Korean medicinal theory that is comprised of 7 medicinal herbs. Hepad S1 improves PD motor and non-motor symptoms such as sleep disturbance, fatigue, dysphagia, and pain.6 In a previous study, we showed that Hepad protected SH-SY5Y neuronal cell lines against MPTP-induced oxidative stress without producing toxicity.7 And Hepad could modulate adverse cellular responses relating to Parkinson’s disease such as inflammation, apoptosis, and oxidation in neuronal cell.8
The aim of this study is to assess neuro-protective effect of Hepad S1 by regulating oxidative stress in cell culture system. And the efficacies of Hepad S1 were investigated in the level of biological markers relating to PD in the animal model. In this study, we evaluated the therapeutic effects of Hepad S1 in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced model of PD in rats. We measured the therapeutic effects of Hepad S1 on dopamine and complex I enzyme activity as well as cell preservation in the substantial nigra and serum levels of serotonin and orexin A. The Hepad S1, an herbal mixture, would have multi-targeted therapeutic effects in a model of PD.
2. Methods
2.1. Hepad S1 preparation and HPLC analysis
Hepad S1 is an herbal extract prepared by boiling water. The formulation was originally developed based on the theory of Korean medicine. Hepad S1 was prepared according to previous study with minor modification.7 Briefly, Hepad S1 was extracted from a mixture of the herbs Atractylodis Rhizoma, Cnidii Rhizoma, Paeonia Japonica, Poria cocos Wolf, Zizyphi Semen and Glycyrrhizae Radix et Rhizoma in equal weight proportions. In case of Uncariae Ramulus Et Uncus, we added three times larger quantity than other herbs. We named this extract Hepad S1. The extract was prepared from dried herbs (70 g) in 1 L of water boiled at 97 °C for 3 h and condensed by rotary vacuum evaporator (Buchi, Switzerland). The dried powder (11.8 g) was obtained by freeze drying (Ilshinbiobase, Korea) and stored at −80 °C until use. The drug-to-extract ratio was 5.9 (yield percentage, 16.9%). To determine the chemical profile of Hepad S1, 100 mg was dissolved in 10 mL of distilled water and subjected to membrane filtration (0.45 μm). A high performance liquid chromatography (HPLC) analysis was performed using a Shimadzu LC-20A series instrument (Japan). UV detection was performed at 230 nm for paeoniflorin and 250 nm for glycyrrhizin. The column temperature was maintained at 40 °C. Separation was performed on an ACE 5 C18 column (250 mm × 4.6 mm; particle size, 5 μm). The mobile phase was 0.1% acetic acid-water (v/v, solvent A) and acetonitrile (solvent B). The flow rate was 1 mL/min and the gradient was as follows: 0–5 min, 2% B; 5–45 min, 2–100% B; 45–50 min, 100% B; 50–60 min, and 100-2% B. The re-equilibration time was set at 20 min. Standards included paeoniflorin (P0038, Sigma–Aldrich, USA) and glycyrrhizin (glycyrrhizic acid ammonium salt, G2137, Sigma–Aldrich, USA).
2.2. Cell culture
PC12 cells were purchased from the Korean Collection for Type Cultures (Seoul, Korea). Cells were cultured in RPMI medium supplemented with penicillin (100 unit/mL), streptomycin (100 μg/mL), 5% fetal bovine serum, and 10% horse serum at 37 °C in a humidified atmosphere containing 5% CO2. The cells were differentiated into neurons by nerve growth factor (NGF, 50 ng/mL) treatment for 3 days.
2.3. Cytotoxicity assay
PC12 cells were seeded onto 96-well plates at a density of 1.5 × 105 cells/mL and allowed to adhere for 24 h prior to culturing in fresh cell culture medium. Cells were incubated with or without Hepad S1 for 24 h followed by cytotoxicity assay with the Cell Counting Kit-8 assay kit (Dojindo, Japan) as per the manufacturer’s protocol. Absorbance was measured at 450 nm with a microplate reader (Molecular Device, Sunnyvale, CA, USA).
2.4. Oxidative stress analysis
PC12 cells were seeded onto 96-well plates at a density of 2 × 106 cells/mL and allowed to adhere for 24 h prior to culturing in fresh cell culture medium. Cells were pre-incubated with or without Hepad S1 for 2 h followed by 1-methyl-4-phenylpyridium (MPP+, 4 mM) treatment for 24 h. Cells were collected and washed with PBS before reactive oxygen species (ROS) measurement with 2’,7’-dichlorofluorescein diacetate (DCF-DA, 10 μM) staining performed for 15 min in the CO2 incubator. DCF-DA-stained cells were collected into PBS and analysed by flow cytometry (Becton Dickinson, USA). The amounts of total glutathione and malondialdehyde (MDA) were measured using a glutathione assay kit (Sigma, USA) and lipid peroxidation colorimetric/fluorometric assay kit (BioVision, USA) as per manufacturer protocols.
2.5. Animals
Six-week-old male Sprague–Dawley (SD) rats (170–200 g) were purchased from Samtako (Kyunggi, South Korea). Rats were acclimated for 1 week and maintained in a room with constant temperature (22 ± 2 °C) and humidity (55 ± 15%) on a 12-h light-dark cycle with ad libitum access to Harlan 2018S rodent diet (Envigo, USA) and sterilized tap water. The University Animal Care and Use Committee at Daejeon University approved all animal experimental protocols (DJUARB2016-036, Nov. 18, 2016). The PD model was induced by intraperitoneal injection (i.p.) of MPTP (20 mg/kg) once per day for 5 consecutive days. Hepad S1 was administered orally once per day at the same time (i.e., 14:00) for 4 weeks. L-DOPA (10 mg/kg, i.p.) was used as a positive control treatment.
2.6. Serum serotonin and orexin A
Blood was collected from the tail vein and coagulated at room temperature for 30 min. Samples were centrifuged at 1,800g for 15 min and serum was obtained by collecting the supernatant. Concentrations of serotonin and orexin A were measured using rat serotonin and orexin A enzyme linked immunosorbent assay (ELISA) kits (MyBioSource, USA) as per manufacturer protocols.
2.7. Complex I enzyme activity and dopamine in the substantia nigra
After completing treatment, rats were anesthetized by inhalation of 5% ethyl ether (Sigma–Aldrich, USA) for 3 min and sacrificed and the substantia nigra was dissected from the brain. Tissue (500 mg) was homogenized in PBS by sonication at 70% pulse 10 times for 15 s with 50-s intervals using a sonicator (Qsonica, USA). Tissue lysates (supernatants) were obtained by centrifugation at 1,500g for 15 min. Complex I enzyme activity and dopamine were measured using a complex I enzyme activity microplate assay kit (Abcam, United Kingdom) as per manufacturer protocols.
2.8. Histology
Animals were perfused with 0.9% saline solution followed by 4% paraformaldehyde at 20 mL/mL through the left ventricle. The brain was then isolated and post-fixed in 4% paraformaldehyde for 48 hr. Brain sections (25 μm) were prepared by cryomicrotome (Leica CM1850, Germany), incubated with anti-tyrosine hydroxylase (TH) antibody (1:2000) for 24 h at 4 °C, incubated with secondary biotin-conjugated antibody for 2 h at room temperature, and developed with streptavidin peroxidase and avidin-biotin peroxidase complex (Abcam, United Kingdom). Images of identical fields of view were acquired using a microscope (ZEISS AXioskop2, Germany) equipped with a microscope camera (ZEISS AXiocam ICc1, Germany). The TH-positive cells were counted in brains sections from 4 different animals in each group by blinded 2 independent researchers. All the stained cells were count in the area of substantia nigra under bright field microscope.
2.9. Statistical analysis
Data are expressed as the mean ± standard deviation. Data were analysed with unpaired student’s t-tests for 2-group comparisons and one-way analyses of variance (ANOVA) followed by turkey’s test for comparisons of more than 2 groups. All analyses were performed using the SPSS statistical software package, version 11.0 (SPSS Inc., Chicago, IL, USA). A P value of <0.05 was considered statistically significant.
3. Results
3.1. Standardization of Hepad S1
Hepad S1 was standardized by component analysis with HPLC. Paeoniflorin and glycyrrhizin, which are commercially available standard chemicals, were selected as surrogate markers for Paeonia japonica and Glycyrrhizae Radix et Rhizoma, respectively. The HPLC analysis confirmed the presence of these markers in our Hepad S1 preparation (Supplemental Fig. S1).
Fig. 1.
Effects of Hepad S1 on oxidative stress in cultured neurons. Hepad S1 cytotoxicity was measured by wst-8 in NGF-induced neuronal PC12 cells. PC12 cells were treated with Hepad S1 at the indicated concentrations for 2 h (A) without or (B) with 4 mM MPP + for 24 h. (C) Cells were incubated with PBS containing 10 μM DCF-DA for 10 min and then analysed by flow cytometry. (D) Total glutathione and (E) MDA were measured using commercially available assay kits. Data are expressed as the mean ± standard deviation. ###p < 0.001 vs. control; *p < 0.05 and **p < 0.01 vs. vehicle. Con, control.
3.2. Hepad S1 exerts antioxidant effects in PC12 cells
We first examined the cytotoxicity of Hepad S1 in PC12 cells. There was no detectable cytotoxicity up to 100 μg/mL Hepad S1 (Fig. 1A). We then assessed the neuroprotective effects of Hepad S1 in PC12 cells exposed to MPP+, which has been previously reported to increase the production of ROS in PC12 cells.9 Hepad S1 treatment prevented cell death in a dose-dependent manner (Fig. 1B). Moreover, Hepad S1 treatment significantly decreased numbers of DCF-DA positive PC12 cells (Fig. 1C). Glutathione is a major cellular antioxidant that is diminished in substantia nigra of patients with PD.10 Hepad S1 (100 μg/mL) preserved total glutathione in PC12 cells after MPP + challenge (Fig. 1D) and additionally decreased the level of lipid peroxidation in a dose-dependent manner (Fig. 1E). Taken together, these data indicated the neuroprotective and antioxidant effects of Hepad S1 in PC12 cells.
3.3. Hepad S1 up-regulates complex I enzyme activity in the substantia nigra
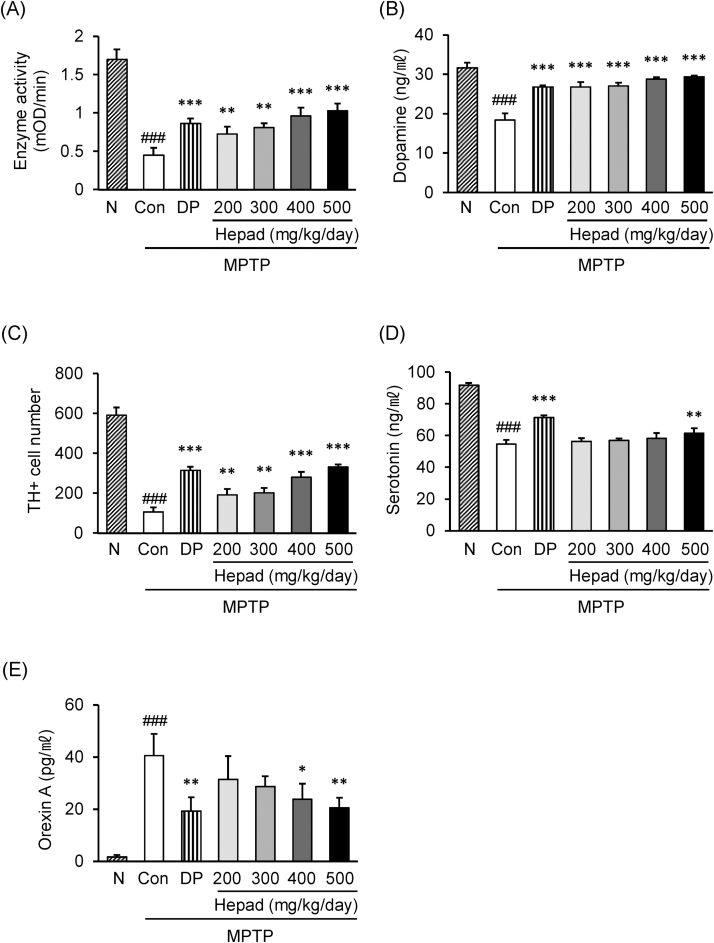
Next, we assessed the antioxidant effects of Hepad S1 on substantia nigra neurons in vivo using a rat MPTP model of PD. The experimental procedure was performed as Supplemental Fig. S2. For in vivo study, we determined the minimum dose based on the clinical application. The dried Hepad S1 (11.8 g) is a standard daily dose for a patient. This dose is equivalent to approximately 200 mg/kg, which is a standard dose for a day in animal experiment. The higher doses were determined by adding 100 mg/kg in each. MPTP injection significantly reduced complex I enzymatic activity in the substantia nigra. This effect was rescued by Hepad S1 treatment; 2-fold increase in 500 mg/kg compared to MPTP control. This result suggested that Hepad S1 regulates the cellular redox system in the substantia nigra (Fig. 2A).
Fig. 2.
Effects of Hepad S1 on MPTP-injected rats. MPTP-injected rats were treated with Hepad S1 at the indicated concentrations for 4 weeks. Substantia nigra in brain were obtained and (A) complex I enzyme activity and (B) dopamine content were measured with a commercially available assay kit. (C) Numbers of TH-positive cells in the substantia nigra. (D) Serum serotonin and (E) serum orexin A were measured using commercially available enzyme-linked immunosorbent assay kits. Data are expressed as the mean ± standard deviation. ###p < 0.001 vs. naïve group; *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control group. N, Naïve group, Con, control group; DP, L-DOPA-treated group.
3.4. Hepad S1 preserves dopamine function in the substantia nigra
The effect of Hepad S1 on dopamine production in the substantia nigra was estimated by neuropeptide quantification with an ELISA. MPTP treatment decreased tissue dopamine levels whereas Hepad S1 treatment increased dopamine concentration in a manner similar to L-DOPA treatment. Treatment with 400 and 500 mg/kg Hepad S1 was more efficacious than L-DOPA for increasing dopamine content in the substantia nigra (Fig. 2B). To confirm these results, we next stained for TH-positive dopaminergic neurons in the substantia nigra.11 Whereas MPTP treatment decreased numbers of TH-positive cells in the substantia nigra and ventral tegmental area (Supplemental Fig. S3), Hepad S1 treatment rescued numbers of TH-positive cells in substantia nigra (Fig. 2C). These results indicated that Hepad S1 protected dopaminergic cells in the substantia nigra against MPTP-induced damage.
3.5. Hepad S1 regulates serum neuropeptide levels
Finally, we investigated the effects of Hepad S1 on serum levels of serotonin and orexin A in MPTP model rats. MPTP treatment decreased the serum level of serotonin to approximately 41% of that in the naive group. In contrast, 500 mg/kg Hepad S1 increased serum serotonin by 11% compared to the control group (Fig. 2D). High levels of orexin A disturb sleep and maintain the waking state, consistent with sleep disturbances in PD. Indeed, MPTP treatment increased the serum level of orexin A compared to the naïve group (Fig. 2E). Hepad S1 treatment significantly decreased the serum neuropeptide level to 58% and 50% of control at doses 400 and 500 mg/kg, respectively. These results revealed that Hepad S1 regulates neuropeptide production after MPTP treatment.
4. Discussion
PD is a motor disorder involving bradykinesia, tremor, posture instability, and stiffness related to a decrease in the number of dopaminergic neurons in the substantia nigra. Although levodopa is the only proved drug for PD, it does not delay or diminish the progressive loss of dopaminergic neurons. In this study, we showed that Hepad S1 treatment increased the number of TH-positive dopaminergic cells in the substantia nigra, consistent with our previous study demonstrating that Hepad increased the number of dopaminergic neurons in rats treated with 6-OHDA.7 We also found that Hepad S1 treatment increased dopamine levels in the substantia nigra and in serum, suggesting that Hepad S1 may ameliorate PD symptoms by restoring dopaminergic function.
ROS are posited to mediate neuronal death in PD.12 Mitochondria produce ROS as an inevitable by-product of energy metabolism; however, overproduction or improper ROS scavenging can lead to disrupted cellular function and cell death.13 Accordingly, cell death attributed to mitochondrial ROS is one suggested trigger of PD pathogenesis.14 We found that Hepad S1 treatment reduced ROS accumulation, preserved glutathione, and decreased lipid peroxidation in neurons differentiated from PC12 cells. Moreover, Hepad S1 elevated complex I enzyme activity in vivo, which is significantly reduced in patients with PD.15 Therefore, our data suggest that Hepad S1 has powerful antioxidant activity that may prevent the loss of dopaminergic neuron cell in substantia nigra.
Motor symptoms are the major complaint in PD; however, ongoing studies increasingly emphasize the importance of non-motor symptoms,16 particularly as way to improve quality of life in patients with PD.17 Sleep disorder is a major non-motor complaint in PD.18 Although patients with PD are generally able to fall sleep, sleep duration and the sleep-wake are typically shorter in patients with PD than in healthy subjects.19 Moreover, patients with PD exhibit diminished rapid eye movement (REM) sleep.20 Accordingly, sleep disturbance impairs quality of life in patients with PD.21 In the present study, we showed that Hepad S1 decreased serum levels of orexin A in MPTP-treated rats. Orexin A is a neuropeptide that regulates the sleep-wake cycle,22 and increased orexin A in the brain disturbs the REM sleep and prolongs the arousal stage.23 Additionally, orexin A is thought to regulate depression and dementia as well as sleep disturbance, which are other non-motor complaints in PD.24, 25 Therefore, orexin A may influence several non-motor syndromes of PD.26, 27 Given the observed effects of Hepad S1 in rodents, future studies should evaluate the effects of Hepad S1 on orexin neuron activation and sleep-wake regulation.
Serotonin (5-HT) is an amine neurotransmitter involving in stress, depression, anxiety, and peristatic movement.28 Serotonergic dysfunction is associated with motor and non-motor symptoms. The patient with PD showed reduced serotonin level in brain.29 The blood level of serotonin is also associated with non-motor syndromes in PD. In our study, the level of serotonin was upregulated by Hepad S1 (Fig. 5A). This result implicated that Hepad S1 might modulate the non-motor symptoms of PD such as depression and anxiety, which are the major non-symptoms of PD.16
All the ingredients of Hepad S1 might be effective on PD. Atractylodis Rhizoma is a traditional herb medicine treating ischemic stroke30 and having anti-oxidant activity.31 In our study, Hepad S1 showed neuroprotective effect, which had been suggested that Atractylodis Rhizoma has similar effects; Atractylodis Rhizoma reduced excitotoxicity‐induced neuronal apoptosis in primary cultured cerebral cortical neurons.32 Cnidii Rhizoma may protect neuronal toxicity in PD based on the finding that ferulic acid from the herb showed neuro-protective activity in ER stress-induced apoptotic cells.33 Paeonia Japonica is known to improve movement activity by increasing the production of serotonin.34 Dai et. al., suggested that the combination of Poria cocos, Radix Glycyrrhizae, and Rhizoma Atractylodis was effective on depression which is a symptom usually accompanied with PD.35 Zizyphi Semen may have an activity controlling central nerve system. Sanjoinine A, a chemical component of Zizyphi Semen extended the sleeping time and latency through activation of GABAA receptor36 and supressed onset of NMDA-induced seizure via inhibiting intracellular calcium influx.37 Uncariae Ramulus is also shown to have strong neuro-protective effect against oxidative stress in mouse cerebral neurons.38 Glycyrrhizae Radix et Rhizoma is well known to protect hippocampal neuronal cell death.39 Liquiritigenin is an active compound of Glycyrrhizae Radix et Rhizoma and suggested that protects dopaminergic neuronal cell death by inhibiting poly ADP-ribose (PAR) accumulation.40 The neuroprotective effect of Hepad S1 might be the combination of pharmacological activity of each herb, which resulting in increased the number of TH-positive cells in substantia nigra.
The combination of herbs is an established technique in Korean medicine for achieving various medicinal effects, such as therapeutic synergy or the neutralization of toxicity.41 In Korean medicine theory, drugs were categorized into Major, Complementary, Neutralizing, and Delivery/Retaining. Hepad S1 consists of 7 herbal medicines containing various chemical compounds. Uncariae Ramulus was a Major in Hepad S1 due to the anti-tremor effect; tremor is one of the main complaints of patients with PD. Complementary, which boosts the efficacy of Major, was Cnidii Rhizoma, Paeonia Japonica, Poria cocos, and Zizyphi Semen. These herbs are known to have spasmolytic and relaxing effects. Atractylodis Rhizoma was used as Delivery/Retaining. Finally, Glycyrrhizae Radix et Rhizoma was used for Neutralizing. This herbal combination of Hepad S1 is thought to enhance therapeutic efficacies more strongly than single plant medicine treatment. The single herb has limited number of active compounds than combination. The multiple compounds with multiple targets in herbal combination interact each other and lead to enhance the medical efficacy and neutralize unexpected side effects. Therefore, herbal combination of Korean medicine has advantages than the single herb treatment. Never the less, the main active compounds of Hepad S1 and the interaction among them must be determined to elucidate the mechanisms of action.
The present study demonstrates that Hepad S1, a Korean medicinal herbal combination, exerted therapeutic effects in a rodent model of PD through neuroprotection, antioxidant activity, and the preservation/restoration of dopamine function. However, behavioural test needs to be validated the efficacy of Hepad S1 on PD. Drug safety test is also needed to be elucidated to warrant clinical application. Despite the limitations, this preclinical data warrants future investigation to inform the utility of Hepad S1 in clinical patients with PD.
Conflict of interest
The authors declare no conflict of interest.
Funding
This work was supported by the RIC program of MOTIE (Ministry of Trade, Industry, and Energy) in Daejeon University, Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (317034-03-2-HD030) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Education) (2017R1D1A1B03034895).
Data availability
Data and materials are available from the authors upon request.
Authors’ contribution
DHK performed the experiments. BJP designed the study and wrote the paper. JJC analyzed the data and wrote the paper. All authors read and approved the final manuscript.
Footnotes
Supplementary figures related to this article can be found in the online version, at doi:https://doi.org/10.1016/j.imr.2019.07.005.
Contributor Information
Jeong June Choi, Email: c27022@dju.ac.kr.
Byung-Jun Park, Email: bjp120@hanmail.net.
Supplementary material
The following are Supplementary data to this article:
High performance liquid chromatography analysis of Hepad S1. (A) Paeoniflorin detected at 230 nm. (B) Glycyrrhizin detected at 250 nm.
Schematic diagram of animal experiment.
Effects of Hepad S1 on tyrosine hydroxylase (TH)-positive neurons in the substantia nigra of MPTP-treated rats. MTPT-injected rats were treated with Hepad at the indicated concentrations for 4 weeks. Brain sections with TH-immunostaining, magnification x400. Arrow indicates substantia nigra.
References
- 1.Alves G., Forsaa E.B., Pedersen K.F., Dreetz Gjerstad M., Larsen J.P. Epidemiology of parkinson’s disease. J Neurol. 2008;255:18–32. doi: 10.1007/s00415-008-5004-3. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 3.Tong Q., Zhang L., Yuan Y., Jiang S., Zhang R., Xu Q. Reduced plasma serotonin and 5-hydroxyindoleacetic acid levels in parkinson’s disease are associated with nonmotor symptoms. Parkinsonism Relat Disord. 2015;21:882–887. doi: 10.1016/j.parkreldis.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Stewart D.A. NICE guideline for parkinson’s disease. Age Ageing. 2007;36:240–242. doi: 10.1093/ageing/afm040. [DOI] [PubMed] [Google Scholar]
- 5.Ahlskog J.E., Muenter M.D. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- 6.Park B. A research on 7 cases of the treatment process for patients with idiopathic parkinson’s disease or parkinsonism. J Orient Neurophsychiatry. 2009;20:283–295. [Google Scholar]
- 7.Baek S.Y., Lee N.R., Kim D.H., Gu A., Kim S.Y., Song D. Protective effect of a novel herbmedicine, hepad, on apoptosis of SH-SY5Y cells and a rat model of parkinson’s disease. Mol and Cell Toxicol. 2015;11:223–230. [Google Scholar]
- 8.Song D.H., Kim G.J., Lee K.J., Shin J.S., Kim D.H., Park B.J. Mitigation effects of a novel herbal medicine, hepad, on neuroinflammation, neuroapoptosis, and neuro-oxidation. Molecules. 2018;23 doi: 10.3390/molecules23112920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langston J.W., Irwin I., Langston E.B., Forno L.S. 1-methyl-4-phenylpyridinium ion (MPP+): identification of a metabolite of MPTP, a toxin selective to the substantia nigra. Neurosci Lett. 1984;48:87–92. doi: 10.1016/0304-3940(84)90293-3. [DOI] [PubMed] [Google Scholar]
- 10.Sofic E., Lange K.W., Jellinger K., Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with parkinson’s disease. Neurosci Lett. 1992;142:128–130. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- 11.White R.B., Thomas M.G. Moving beyond tyrosine hydroxylase to define dopaminergic neurons for use in cell replacement therapies for parkinson’s disease. CNS Neurol Disord Drug Targets. 2012;11:340–349. doi: 10.2174/187152712800792758. [DOI] [PubMed] [Google Scholar]
- 12.Jenner P. Oxidative stress in parkinson’s disease. Ann Neurol. 2003;53 doi: 10.1002/ana.10483. S26,36; discussion S36-8. [DOI] [PubMed] [Google Scholar]
- 13.Parker W.D., Jr., Boyson S.J., Parks J.K. Abnormalities of the electron transport chain in idiopathic parkinson’s disease. Ann Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 14.Bosco D.A., Fowler D.M., Zhang Q., Nieva J., Powers E.T., Wentworth P., Jr. Elevated levels of oxidized cholesterol metabolites in lewy body disease brains accelerate alpha-synuclein fibrilization. Nat Chem Biol. 2006;2:249–253. doi: 10.1038/nchembio782. [DOI] [PubMed] [Google Scholar]
- 15.Schapira A.H., Cooper J.M., Dexter D., Clark J.B., Jenner P., Marsden C.D. Mitochondrial complex I deficiency in parkinson’s disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 16.Poewe W. Non-motor symptoms in parkinson’s disease. Eur J Neurol. 2008;15:14–20. doi: 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 17.Nicoletti A., Mostile G., Stocchi F., Abbruzzese G., Ceravolo R., Cortelli P. Factors influencing psychological well-being in patients with parkinson’s disease. PLoS One. 2017;12 doi: 10.1371/journal.pone.0189682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnulf I., Leu S., Oudiette D. Abnormal sleep and sleepiness in parkinson’s disease. Curr Opin Neurol. 2008;21:472–477. doi: 10.1097/WCO.0b013e328305044d. [DOI] [PubMed] [Google Scholar]
- 19.Boeve B.F., Silber M.H., Ferman T.J., Lucas J.A., Parisi J.E. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16:622–630. doi: 10.1002/mds.1120. [DOI] [PubMed] [Google Scholar]
- 20.Gagnon J.F., Bedard M.A., Fantini M.L., Petit D., Panisset M., Rompre S. REM sleep behavior disorder and REM sleep without atonia in parkinson’s disease. Neurology. 2002;59:585–589. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 21.Scaravilli T., Gasparoli E., Rinaldi F., Polesello G., Bracco F. Health-related quality of life and sleep disorders in parkinson’s disease. Neurol Sci. 2003;24:209–210. doi: 10.1007/s10072-003-0134-y. [DOI] [PubMed] [Google Scholar]
- 22.Piper D.C., Upton N., Smith M.I., Hunter A.J. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur J Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 23.Bourgin P., Huitron-Resendiz S., Spier A.D., Fabre V., Morte B., Criado J.R. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park K.B., Weon H. Orexin receptors mediate long-term depression of excitatory synaptic transmission in the spinal cord dorsal horn. Neurosci Lett. 2017;660:12–16. doi: 10.1016/j.neulet.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 25.Chieffi S., Carotenuto M., Monda V., Valenzano A., Villano I., Precenzano F. Orexin system: the key for a healthy life. Front Physiol. 2017;8:357. doi: 10.3389/fphys.2017.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fronczek R., Overeem S., Lee S.Y., Hegeman I.M., van Pelt J., van Duinen S.G. Hypocretin (orexin) loss in parkinson’s disease. Brain. 2007;130:1577–1585. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- 27.Katsuki H., Michinaga S. Anti-parkinson drugs and orexin neurons. Vitam Horm. 2012;89:279–290. doi: 10.1016/B978-0-12-394623-2.00015-9. [DOI] [PubMed] [Google Scholar]
- 28.Dorszewska J., Prendecki M., Oczkowska A., Rozycka A., Lianeri M., Kozubski W. Polymorphism of the COMT, MAO, DAT, NET and 5-HTT genes, and biogenic amines in parkinson’s disease. Curr Genomics. 2013;14:518–533. doi: 10.2174/1389202914666131210210241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Politis M., Niccolini F. Serotonin in parkinson’s disease. Behav Brain Res. 2015;277:136–145. doi: 10.1016/j.bbr.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 30.Hu W.X., Xiang Q., Wen Z., He D., Wu X.M., Hu G.Z. Neuroprotective effect of atractylodes macrocephalaon polysaccharides in vitro on neuronal apoptosis induced by hypoxia. Mol Med Rep. 2014;9:2573–2581. doi: 10.3892/mmr.2014.2105. [DOI] [PubMed] [Google Scholar]
- 31.Li C.Q., He L.C., Dong H.Y., Jin J.Q. Screening for the anti-inflammatory activity of fractions and compounds from atractylodes macrocephala koidz. J Ethnopharmacol. 2007;114:212–217. doi: 10.1016/j.jep.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Gao Q., Ji Z.H., Yang Y., Cheng R., Yu X.Y. Neuroprotective effect of rhizoma atractylodis macrocephalae against excitotoxicity-induced apoptosis in cultured cerebral cortical neurons. Phytother Res. 2012;26:557–561. doi: 10.1002/ptr.3595. [DOI] [PubMed] [Google Scholar]
- 33.Hiratsuka T., Matsuzaki S., Miyata S., Kinoshita M., Kakehi K., Nishida S. Yokukansan inhibits neuronal death during ER stress by regulating the unfolded protein response. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong J.A., Chung S.H., Lee J.S., Kim S.S., Shin H.D., Kim H. Effects of paeonia radix on 5-hydroxytryptamine synthesis and tryptophan hydroxylase expression in the dorsal raphe of exercised rats. Biol Pharm Bull. 2003;26:166–169. doi: 10.1248/bpb.26.166. [DOI] [PubMed] [Google Scholar]
- 35.Dai Y., Li Z., Xue L., Dou C., Zhou Y., Zhang L. Metabolomics study on the anti-depression effect of xiaoyaosan on rat model of chronic unpredictable mild stress. J Ethnopharmacol. 2010;128:482–489. doi: 10.1016/j.jep.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Ma Y., Han H., Eun J.S., Kim H.C., Hong J.T., Oh K.W. Sanjoinine A isolated from zizyphi spinosi semen augments pentobarbital-induced sleeping behaviors through the modification of GABA-ergic systems. Biol Pharm Bull. 2007;30:1748–1753. doi: 10.1248/bpb.30.1748. [DOI] [PubMed] [Google Scholar]
- 37.Ma Y., Yun S.R., Nam S.Y., Kim Y.B., Hong J.T., Kim Y. Protective effects of sanjoinine A against N-methyl-D-aspartate-induced seizure. Biol Pharm Bull. 2008;31:1749–1754. doi: 10.1248/bpb.31.1749. [DOI] [PubMed] [Google Scholar]
- 38.Lee B., Lee J., Lee K., Jeong J., Lee G., Shin M. Effect of ramulus et uncus uncariae on oxidative stress in cultured mouse cerebral neurons. Korea J Herbol. 2003;18:27. [Google Scholar]
- 39.Yang E.J., Park G.H., Song K.S. Neuroprotective effects of liquiritigenin isolated from licorice roots on glutamate-induced apoptosis in hippocampal neuronal cells. Neurotoxicology. 2013;39:114–123. doi: 10.1016/j.neuro.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Kim H., Park J., Leem H., Cho M., Yoon J.H., Maeng H.J. Rhododendrin-induced RNF146 expression via estrogen receptor beta activation is cytoprotective against 6-OHDA-induced oxidative stress. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20071772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim H.U., Ryu J.Y., Lee J.O., Lee S.Y. A systems approach to traditional oriental medicine. Nat Biotechnol. 2015;33:264–268. doi: 10.1038/nbt.3167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High performance liquid chromatography analysis of Hepad S1. (A) Paeoniflorin detected at 230 nm. (B) Glycyrrhizin detected at 250 nm.
Schematic diagram of animal experiment.
Effects of Hepad S1 on tyrosine hydroxylase (TH)-positive neurons in the substantia nigra of MPTP-treated rats. MTPT-injected rats were treated with Hepad at the indicated concentrations for 4 weeks. Brain sections with TH-immunostaining, magnification x400. Arrow indicates substantia nigra.
Data Availability Statement
Data and materials are available from the authors upon request.