Abstract
BACKGROUND:
Chronic kidney disease is a multisystem disorder characterized by a pro-inflammatory state that corresponds with disease morbidity and mortality.
Endogenous danger-associated molecular patterns, including nucleosomes, may contribute to this persistent inflammation. The aim of this study was to profile and evaluate the clinical significance of circulating nucleosomes in patients with Stage 5 chronic kidney disease (CKD5) on hemodialysis (HD).
METHODS:
Under institutional review board approval, plasma samples were collected from 90 CKD5-HD patients (45 male and 45 female) prior to hemodialysis. Normal human plasma samples (25 male and 25 female) were used as a control group. Commercial enzyme-linked immunosorbent and colorimetric assays were used to profile nucleosomes and biochemical markers of kidney injury, inflammation, thrombosis, and renal function in CKD5-HD and control groups. Clinical laboratory parameters were documented from the electronical medical record and correlated to nucleosome levels in the CKD5-HD cohort.
RESULTS:
In comparison to healthy volunteers, the plasma from CKD5-HD patients exhibited markedly elevated nucleosomes (P < 0.0001). Furthermore, nucleosome levels correlated with WBC count (P = 0.025, R = 0.243) and CRP (P = 0.019, R = 0.266) levels. No correlation was found between nucleosomes and the other parameters studied.
CONCLUSIONS:
Our findings indicate extracellular nucleosomes are significantly elevated in CKD5, suggesting increased cell death and/or inflammation. The observed correlation between nucleosomes and parameters of inflammation hints toward a complex, systemic inflammatory process underlying renal deterioration, consistent with the literature. Thus, nucleosomes may play a role in the pathogenesis and progression of chronic kidney disease.
Keywords: kidney failure, chronic - renal insufficiency, chronic - nucleosomes - inflammation
Introduction
Chronic kidney disease (CKD) is a multisystem disorder characterized by a progressive, harmful, and pro-inflammatory state that corresponds with disease morbidity and mortality. This chronic inflammation is thought to result from processes involving the vasculature and immune system that cause a sustained accumulation of pro-inflammatory mediators in renal tissue, leading to progressive renal deterioration, vascular calcification, and endothelial dysfunction. 1 A significant challenge in CKD is the identification of the source of inflammation underlying this disease process. Previous studies have identified pro-inflammatory cytokines including IL-1, IL-6, IL-8, and TNF-α as potential pathophysiological mediators.2 These factors may contribute to fibrosis through the promotion of T cell adhesion and interstitial migration.1 Advanced glycation end products (AGE), which interact with vascular endothelial cells and contribute to inflammation and atherosclerosis, also contribute to this process.3 The elevation of pro-inflammatory mediators facilitates the inappropriate recruitment of immune cells and enhancement of coagulation, leading to renal parenchymal damage and endothelial injury.1
Endogenous danger-associated molecular patterns (DAMPs), including nucleosomes, may contribute to the persistent inflammation seen in CKD. Nucleosomes are histone-DNA complexes normally found within the cell nucleus but are detectable in the blood in certain pathophysiological conditions. Nucleosomes may originate from apoptotic or necrotic cells.4 During apoptosis, nucleosome separation and release occurs via DNA fragmentation mediated by caspase-activated DNAse and DNA fragmentation factor.5 Furthermore, nucleosomes may be released as a component of neutrophil extracellular traps (NETs). NETs are networks of extracellular fibers composed of genomic DNA and core histones expelled by neutrophils to capture and degrade invading microorganisms.4 Nucleosomes may be released from NETs by circulating DNAses.6
Elevated levels of circulating nucleosomes and histones have been linked to the pathogenesis of diseases of different organ systems, including stroke, sepsis, acute lung injury, COPD, toxic liver injury, and pancreatitis.4 Elevated levels of histone H4 were demonstrated in sepsis and associated with loss of endogenous anticoagulant potential in a cohort of septic patients.7 Furthermore, histone stimulation induced release of pro-inflammatory cytokines and decreased cell integrity.7 Histones are also direct mediators of tissue damage; histone accumulation in the infarcted myocardium of mice with permanent coronary artery ligation resulted in increased cardiomyocyte toxicity and cytological changes consistent with cell death.8 Nucleosomes have also been implicated in thrombosis. Extracellular histones promote coagulation and thrombin generation by inducing Toll Like Receptor (TLR)2 and TLR-4 dependent platelet activation.9 Extracellular histones can also induce tissue factor expression in vascular endothelial cells and macrophages via TLR2 and TLR4.10 In humans, extracellular nucleosomes may be associated with the development of deep vein thrombosis.11
Studies of nucleosomes and histones in renal pathology have largely been limited to acute kidney injury (AKI). In AKI, histones are released into extracellular space by necrotic tubular epithelial cells.12 These histones induce toxicity to renal endothelial cells and tubular epithelial cells, and promote leukocyte recruitment, increased vascular permeability, and renal inflammation.12 This process is thought to be mediated by TLR2 and TLR4, leading to the activation of the pro-inflammatory MyD88-NFκB and MAPK pathways.12,13
The purpose of this study was to evaluate the clinical significance of nucleosomes in Stage 5 chronic kidney disease (CKD5) and to characterize the relationship of nucleosomes with kidney injury, inflammation, thrombosis, and renal function. CKD5 is the most severe manifestation of CKD and is characterized by a glomerular filtration rate (GFR) of ≤15 mL/min per 1.73 m2.14 In these patients, maintenance hemodialysis (HD) or kidney transplant is required to replace the function of the kidneys. We hypothesized that the level of circulating nucleosomes would be elevated in patients with CKD5 on hemodialysis (CKD5-HD) due to the setting of increased cell death and impaired clearance of waste products.
Inflammatory and kidney injury markers of interest in CKD include white blood cell (WBC) count, kidney injury molecule 1 (KIM-1), neutrophil gelatinase–associated lipocalin (NGAL), interleukin-18 (IL-18), endocan, C-reactive protein (CRP), and uric acid. Several of these markers were previously identified in our laboratory as elevated in CKD5-HD.15 KIM-1 is released by epithelial cells in the proximal tubules of damaged kidneys during acute kidney injury and promotes epithelial regeneration and tubule cell apoptosis.16,17 NGAL originates from the distal tubule and collecting duct, serving as a marker of tubular damage.16,17 IL-18 is a proinflammatory cytokine.16 Endocan is released by endothelial cells in response to inflammatory stimuli.18 CRP is an acute phase reactant that may bind histones, thus impairing histone clearance.19 Uric acid is a DAMP elevated in the setting of cell death and is a product of nucleotide breakdown.20 Analysis of the association of nucleosomes with these factors may provide insight into the inflammatory pathways by which nucleosomes potentially contribute to the pathophysiology of CKD.
Platelet count, microparticle-tissue factor (MP-TF), platelet-derived growth factor (PDGF), and platelet factor 4 (PF4) were measured to evaluate the relationship of nucleosomes with the hypercoagulability documented in CKD5-HD patients.21 Circulating microparticles are elevated in patients with CKD5-HD and correlate with endothelial dysfunction.22,23 MP-TF is used in this study as a representation of the procoagulant properties of these particles. PDGF and PF4 originate from platelet α granules. PDGF plays a role in cell proliferation and recruitment of mesenchymal cells in the kidney.24 PDGF may contribute to thrombus formation by inducing tissue factor expression in vascular smooth muscle cells.25 PF4 binds with high affinity to heparin and heparin-like molecules, decreasing antithrombin activity and promoting coagulation.26
The association of nucleosomes with renal function was assessed through correlations with estimated glomerular filtration rate (eGFR), creatinine, blood urea nitrogen (BUN), serum potassium (K+), serum chloride (Cl−), and serum calcium (Ca2+). Increased or decreased levels of these parameters are well documented in patients with CKD and are indicative of failure of different aspects of renal function. Fibroblast growth factor 23 (FGF23), a hormone increased in CKD due to phosphate dysregulation, was investigated because studies have shown the elevation of FGF23 is associated with inflammatory mediators.27
Although nucleosomes have demonstrated inflammatory and cytotoxic properties, these observations have largely been limited to in vitro systems. Profiling nucleosomes in CKD5-HD patients will help elucidate the physiological effects these molecules potentially have in humans. Moreover, assessment of the relationship between nucleosomes and parameters of kidney injury, inflammation, thrombosis, and renal function will further our understanding of the role of nucleosomes as a potentially novel pathophysiological mediator in CKD.
Materials and methods
Study Patients
Patients 18 years or older with CKD5 who underwent maintenance outpatient hemodialysis three times a week at Loyola University Medical Center (LUMC), Maywood, Illinois, were eligible for enrollment in the study. Ninety patients (45 males and 45 females) were enrolled in the study. The patients ranged in age from 40 to 87 years, with a median age of 63.8 years. Following consent, plasma samples were collected prior to the dialysis session. Table 1 describes the demographics of the CKD5-HD patients at LUMC outpatient dialysis center enrolled in this study.
Table 1.
CKD5-HD Patient Demographics at LUMC Outpatient Dialysis Center.
| Parameter | # of Patients (% of Total) |
|---|---|
| Total CKD5-HD patients | 90 |
| Male CKD5-HD patients | 45 |
| Female CKD5-HD patients | 45 |
| Age, range (mean) | 40–87 years (63.8 years) |
| β-Blockers | 53 (58.9%) |
| ACE inhibitors | 18 (20%) |
| Aspirin | 42 (46.7%) |
| Insulin | 36 (40%) |
| Antiplatelets | 12 (13.3%) |
| Statins | 52 (57.8%) |
| Anticoagulants | 22 (24.4%) |
| Atrial fibrillation | 19 (21.1%) |
| Heart failure | 33 (36.7%) |
| Left ventricular hypertrophy | 47 (52.2%) |
| Diabetes mellitus | 56 (62.2%) |
Abbreviations: ACE, angiotensin-converting enzyme; CKD5-HD, stage 5 chronic kidney disease hemodialysis; LUMC, Loyola University Medical Center.
Methods
Under institutional review board approval, blood samples from 90 CKD5-HD patients had been collected during December 2014 and stored at −70 °C until analysis. Venous blood samples were collected in 3.2% (0.109 mol/L) sodium citrate tubes immediately before each patient’s dialysis session. The samples were centrifuged at 3000 rpm for 15 minutes, within 2 hours of blood draw, and the resultant plasma was divided into ten 100-mL aliquots and were frozen for storage at −70 °C. Twenty-five male and 25 female plasma samples from healthy individuals were purchased from a biobank as control (George King Biomedical, Overland Park, Kansas). The control plasma samples were collected from nonsmoking, drug-free volunteers (aged 19–54 years, mean 33).
Determination of Biomarker Profile
Commercial enzyme-linked immunosorbent assays were performed using CKD5-HD and control plasma samples to profile extracellular nucleosomes (Roche Diagnostics, Mannheim, Germany), FGF23 (RayBiotech, Norcross, Georgia), CRP (Hyphen Biomed, Neuville-sur-Oise, France), KIM-1 (R&D Systems, Minneapolis, Minnesota), NGAL (R&D Systems, Minneapolis, Minnesota), IL-18 (Medical & Biological Laboratories Co, Ltd, Nagoya, Japan), PDGF (R&D Systems, Minneapolis, Minnesota), PF4 (Hyphen Biomed, Neuville-sur-Oise, France), endocan (Lunginnov, Lille, France), and MP-TF (Hyphen Biomed, Neuville-sur-Oise, France). Uric acid was profiled using a colorimetric assay (Abcam, Cambridge, Massachusetts). All reagents and standard solutions were prepared as directed by the assay manufactures. Patient and control plasmas were thawed and diluted according to the assay instructions.
Electronic Medical Record Chart Review
The patients’ age, sex, diagnoses, comorbidities, medications, and dialysis laboratory test results were collected from their medical records using the Epic electronic medical record. Relevant International Classification of Diseases diagnosis codes that were carried through a patient’s record was documented, which included heart failure, vascular disease, stroke, atrial fibrillation, and diabetes. Medication usage including statins, angiotensin-converting enzyme (ACE) inhibitors, β-blockers, antiplatelet, aspirin, and insulin was recorded. Echocardiograms were reviewed to document whether there was any degree of left ventricular hypertrophy and the ejection fraction. Same day, predialysis blood samples were drawn as part of a monthly dialysis assessment and clinical laboratory data (eGFR, serum potassium, chloride, calcium, CO2, creatinine, blood pH, total protein, albumin, hemoglobin, BUN, platelet count, iron, ferritin, glycated hemoglobin [HbA1c], transferrin, iron saturation, WBC count, glucose, and calcium phosphate) were made available in Epic charts.
Statistical Analysis
Biomarker and chart data were collected in Microsoft Excel and analyzed using GraphPad Prism v7. The results were expressed as mean ± Standard Error of Mean (SEM). Comparison between groups were evaluated using the nonparametric Mann-Whitney U test used for non-normally distributed quantitative data. Correlation analysis was performed using the non-parametric Spearman test to correct for deviations from normality assumption. A P value less than .05 was considered statistically significant. The R values were generated to assess the strength of correlations.
Results
Comparison of extracellular nucleosomes in control vs. CKD5-HD patients
As seen in Figure 1, the circulating plasma levels of extracellular nucleosomes were significantly elevated in the CKD5-HD group compared to that of the control group, demonstrating a percent change of 129.4% (P < 0.0001). The mean nucleosome level in CKD5-HD patients was 15.5 ± 1.48 Arbitrary Units (AU). As shown in Figure 2, the frequency of individuals with circulating nucleosome levels ≥ 10 AU was greater within the CKD5-HD group.
Figure 1.

Bar graph indicating nucleosome mean ± SEM in CKD5-HD patients and control group.
Plasma levels of nucleosomes were significantly elevated CKD5-HD patients (15.5 ± 1.48 AU) compared to that of the control group (6.74 ± 2.09 AU; p < 0.0001)
Figure 2.
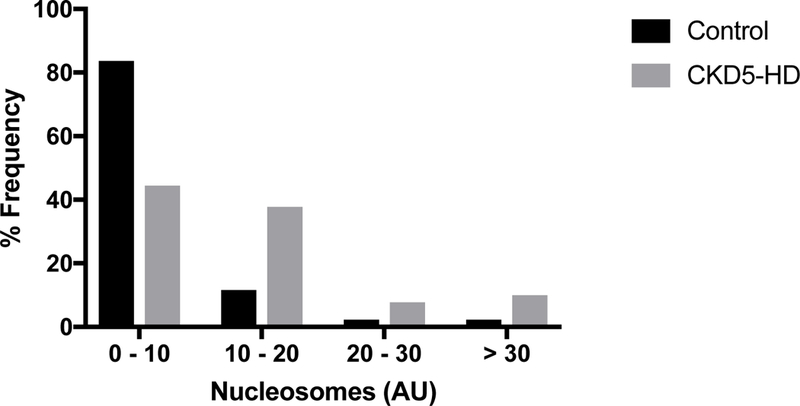
Frequency distribution of nucleosomes in CKD5-HD patients and healthy volunteers.
The nucleosome distribution for both the CKD5-HD and control groups demonstrated a left skew trend.
Comparison of extracellular nucleosomes within the CKD5-HD cohort based on gender, comorbidities, and medications
The difference in nucleosome levels in males compared to females within the CKD5-HD group was not statistically significant (P = 0.626). Furthermore, nucleosome levels were not significantly different when the CKD5-HD group was stratified by concomitant illnesses such as diabetes mellitus (P = 0.679), atrial fibrillation (P = 0.740), left ventricular hypertrophy (P = 0.094), and heart failure (P = 0.654). A significant difference in nucleosome levels was not observed between CKD5-HD patients who used ACE inhibitors (P = 0.544) or beta blockers (P = 0.321 ) versus those that did not take these medications. However, nucleosome levels in CKD5-HD patients who used aspirin (13.1 ± 1.92 AU) were markedly decreased compared to CKD5-HD patients who did not use aspirin (15.9 ± 2.00 AU; P = 0.0438).
Relationship between elevated extracellular nucleosomes and parameters of inflammation and kidney injury in CKD5-HD patients
As seen in Table 2, a positive correlation was observed between extracellular nucleosomes and WBC count in CKD5-HD patients. Extracellular nucleosomes also demonstrated a positive correlation with CRP. No correlation was observed between nucleosomes and uric acid, KIM-1, NGAL, IL-18, and endocan. CRP, KIM-1, NGAL, IL-18, and endocan were markedly increased in the plasma of the CKD5-HD cohort compared to that of the control group. Uric acid was not significantly elevated in the CKD5-HD cohort compared to that of the control group.
Table 2.
Correlation of nucleosomes to parameters of kidney injury and inflammation in CKD5-HD patients.
| Plasma Concentration (mean ± SEM) |
Spearman Correlation with Nucleosomes |
||||
|---|---|---|---|---|---|
| Parameter | CKD5-HD | Controls | P-value | P-value | R |
| WBC count (K/μL) | 6.45 ± 0.437 | - | - | 0.025 | 0.243 |
| CRP (μg/mL) | 13.1 ± 2.01 | 1.26 ± 0.393 | < 0.0001 | 0.019 | 0.266 |
| Uric Acid (mg/dL) | 6.03 ± 0.200 | 6.10 ± 0.209 | 0.513 | 0.574 | −0.060 |
| KIM-1 (ng/mL) | 0.723 ± 0.130 | 0.0591 ± 0.00847 | < 0.0001 | 0.317 | −0.109 |
| NGAL (ng/mL) | 448 ± 9.97 | 54.7 ± 1.79 | < 0.0001 | 0.460 | −0.081 |
| IL-18 (pg/mL) | 493 ± 34.6 | 259 ± 15.5 | < 0.0001 | 0.868 | −0.018 |
| Endocan (pg/mL) | 2.22 ± 0.502 | 1.81 ± 0.0556 | 0.017 | 0.623 | −0.058 |
Correlation of extracellular nucleosomes to parameters of thrombosis in CKD5-HD patients
As seen in Table 3, nucleosomes in CKD5-HD patients did not correlate with MP-TF, platelet count, PDGF, and PF4. MP-TF and PF4 were markedly elevated in the plasma of the CKD5-HD cohort compared to that of the control group. PDGF was not significantly elevated in the CKD5-HD group.
Table 3.
Correlation of nucleosomes to parameters of platelet function in CKD5-HD patients.
| Plasma Concentration (mean ± SEM) |
Spearman Correlation with Nucleosomes |
||||
|---|---|---|---|---|---|
| Parameter | CKD5-HD | Controls | P-value | P-value | R |
| Platelet Count (K/μL) | 179 (7.15) | - | - | 0.545 | 0.066 |
| MP-TF (pg/mL) | 2.97 (0.165) | 0.370 ± 0.036 | < 0.0001 | 0.170 | −0.161 |
| PDGF (pg/mL) | 115 (18.5) | 82.7 ± 16 | 0.405 | 0.314 | 0.110 |
| PF4 (ng/mL) | 94.7 (3.86) | 27.4 ± 2.79 | < 0.0001 | 0.524 | −0.070 |
Correlation coefficients are shown for the total patient population. No changes in significance emerge when patients are separated on the basis of anti-platelet therapy.
Correlation of extracellular nucleosomes to parameters of renal function in CKD5-HD patients
As seen in Table 4, nucleosomes in CKD5-HD patients did not correlate with eGFR, creatinine, BUN, K +, Cl−, Ca2+, and FGF23. FGF23 (1.66 ± 0.264 ng/mL) was statistically increased in the plasma of the CKD5-HD group compared to that of the control group (1.31 ± 0.376 ng/mL; P < 0.0001).
Table 4.
Correlation of nucleosomes to parameters of renal function in CKD5-HD patients.
| Parameter | P-value | R |
|---|---|---|
| eGFR (mL/min) | 0.697 | −0.043 |
| Creatinine (mg/dL) | 0.661 | 0.048 |
| BUN (mg/dL) | 0.542 | 0.067 |
| K+ (mEq/L) | 0.716 | 0.040 |
| Cl− (mmol/L) | 0.990 | 0.001 |
| Ca2+ (mg/dL) | 0.085 | 0.187 |
| FGF23 (ng/mL) | 0.060 | 0.200 |
Discussion
Our results demonstrate a statistically significant increase in circulating nucleosomes in CKD5-HD patients compared to that of healthy volunteers. This finding is consistent with the observation that nucleosomes are elevated in other diseases.28–32 While our study does not distinguish the origin of nucleosomes, it’s plausible that nucleosomes are released from stressed or necrotic tubular epithelial cells. Furthermore, nucleosomes may arise from damaged cells of non-renal origin due to a comorbidity such as cardiovascular disease. Another possibility is that nucleosomes are generated by the inflammatory response of immune cells due to the chronic inflammatory state in CKD. In addition to cell death and inflammation, the increase in circulating nucleosomes may be a product of the uremic environment. Direct renal elimination has been identified as a source of nucleosome clearance; decreased renal function could result in accumulation of nucleosomes in circulation.33
A positive correlation was observed between nucleosomes and WBC count. Although the mean WBC count was within normal limits in our patient population, it is possible that neutrophils have increased activation and immunological activity in CKD, leading to the generation of NETs and nucleosomes. Enhanced released of NETs, termed “NETosis”, plays a role in the pathophysiology of several autoimmune diseases. One study demonstrated NETosis is profoundly increased in rheumatoid arthritis and primarily contributes to elevated nucleosomes detected in serum.34 In systemic lupus erythematosus, enhanced NETosis has been linked to elevated levels of circulating chromatin-containing apoptotic material.35,36 This observation suggest nucleosomes derived from cell death can stimulate neutrophils, leading to increased NETosis and further elevated levels of circulating nucleosomes. In addition, enhanced NETosis has been reported in ANCA-associated vasculitis.37 Collectively, these findings suggest increased neutrophil activity in disease states may lead to increased circulating nucleosomes. Further studies are needed to fully elucidate the relationship between the activity of immune cells and circulating nucleosomes in CKD.
The elevated levels of CRP in our patient population is consistent with previous reports of elevated CRP in CKD.38 We observed a positive correlation between nucleosomes and CRP. Additionally, a correlation between nucleosomes and CRP has been noted in congestive heart failure.28 Prior research indicates CRP has a site capable of interacting with chromatin and can impair the removal of circulating histones.19 The physiological role of CRP in disease has been a topic of discussion. It has been hypothesized that CRP mediates the clearance of damaging products generated by cell death through formation of a CRP complex that either enhances phagocytosis or mediates sequestration to the liver or spleen for degradation.39 A more recent study suggests CRP has a unique role in protecting cells from the toxicity of circulating histones by inhibiting histone integration into cell membranes, preventing cytotoxic calcium influx.40 Furthermore, CRP inhibits histone-induced platelet aggregation and coagulation activation.40 The relationship between nucleosomes and CRP is complex. While the correlation we found suggests nucleosomes play a role in systemic inflammation, it is possible CRP is responsible for mitigating the inflammatory and thrombotic effects of nucleosomes. In addition, elevated CRP might contribute to decreased nucleosome clearance from circulation.
This study did not identify an association between nucleosomes and uric acid, KIM-1, NGAL, IL-18 or endocan. A correlation between nucleosomes and uric acid was predicted because both are thought to be products of cell death. Uric acid levels were not elevated in CKD5-HD patients. Although plasma samples were collected pre-dialysis, the high frequency of hemodialysis undergone by our patient population likely prevented accumulation of uric acid. The lack of correlation between nucleosomes and KIM-1 argues against the notion that nucleosomes originate from proximal tubular epithelial cells. However, KIM-1 is a marker of AKI and therefore KIM-1 levels might not be an accurate proxy for nucleosome release in CKD. On the other hand, NGAL is thought to be a marker of both acute and chronic kidney disease.16 The absence of a relationship between nucleosomes and NGAL in this study suggests nucleosomes do not originate from distal tubular cells. Moreover, our findings propose the elevation of nucleosomes is independent of the signaling pathways involving IL-18 and endocan. Studies have shown that extracellular histones mediate inflammation through activation of the NLRP3 inflammasome in a TLR9-dependent manner in animal models.41 To further our understanding of nucleosome mediated inflammation, future studies should consider investigating the relationship between nucleosomes and the NLRP3 inflammasome in CKD.
Despite previous studies linking nucleosomes to platelet activation, no correlations between nucleosome levels and platelet count or activity were seen in this patient population. Previous studies that have demonstrated a relationship between platelet activation and histones have largely done so in purified in vitro systems in the absence of other potentially complicating factors found in the blood.9,10 Furthermore, we did not observe a correlation between nucleosomes and MP-TF, a procoagulant.
Interestingly, nucleosomes did not correlate with any of the parameters of renal function studied. This observation could be explained by the inherent difference in processes leading to the accumulation of nucleosomes versus metabolites and waste products. In the course of CKD, the nephrotoxic inflammation and remodeling leads to both decreased eGFR and systemic complications. While circulating levels of creatinine, BUN, K+, and Cl− are expected to rise directly as a consequence of decreased eGFR, the concentration of nucleosomes increases for multiple reasons. Nucleosomes may be increased, in part, due to decreased glomerular filtration. In addition, nucleosomes may originate from the systemic complications that involve inflammation and cell death. Thus, we propose the abnormal generation of circulating nucleosomes in CKD should be attributed to the global underlying pathophysiology as opposed to solely a decline in glomerular function, a regional process. It would be interesting to investigate the degree to which the progressive decline in eGFR affects nucleosome concentrations by profiling nucleosomes in each stage of CKD and comparing levels to that of a healthy population.
Limitations
A limitation of this study was the lack of age-matched controls. The age range for the controls was 19 to 54 years, with a mean age of 33 years. The age range for the CKD5-HD patients was 40 to 87 years, with a mean age of 64 years. Therefore, it was not possible to control for age-related changes in the biomarkers and parameters studied. Future studies should consider comparison of biomarkers and parameters using age-matched controls to accurately determine physiological changes in the CKD population.
Conclusion
Circulating nucleosomes were elevated in CKD5-HD patients, indicating an increased release of nucleosomes, suggesting increased cell death and/or inflammation. The observed correlation between nucleosomes and both WBC count and CRP supports our hypothesis that nucleosomes are linked to the chronic inflammatory state present in CKD5-HD patients. The finding that nucleosomes did not correlate with KIM-1, NGAL, IL-18, and endocan implies an absence of interplay between nucleosomes and these markers of kidney injury and inflammation. Furthermore, the lack of correlation between nucleosomes and platelet count, MP-TF, PDGF, and PF4 suggests nucleosomes mediate thrombosis through a biological pathway independent of these factors. Lastly, a lack of correlation between nucleosomes and eGFR, creatinine, BUN, K+, Cl−, Ca2+, and FGF23 implies the changes in these parameters of renal function are independent of the pathophysiologic processes responsible for the abnormal formation of extracellular nucleosomes in CKD5. Overall, the observed correlation between nucleosomes and parameters of inflammation hints toward a complex, systemic inflammatory process underlying renal deterioration, consistent with the literature. Thus, nucleosomes may play a role in the pathogenesis and progression of chronic kidney disease.
Acknowledgments
The authors gratefully acknowledge the assistance of the dialysis center staff at Loyola University Medical Center for the facilitation of the collection of the blood samples from patients with CKD5-HD. We are also thankful to the staff of the hemostasis and thrombosis research laboratories for their technical support. This research was completed as part of the Students Training in Approaches to Research (STAR) program for medical students of the Stritch School of Medicine. We are grateful to Dr. Gail Hecht, assistant dean for medical student research, for her support, encouragement, and fostering of medical research. We also express our gratitude to Mr. Jonas Kingo for generously providing some of the assays used in this study.
Funding
This work was funded by internal research funds from the Department of Pathology of the Loyola University Chicago Health Sciences Division.
Congresses
The content of this paper was presented at the 2016 and 2017 American Society of Nephrology Kidney Week Conferences.
Footnotes
Conflicts of interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.Silverstein DM. Inflammation in chronic kidney disease: Role in the progression of renal and cardiovascular disease. Pediatric Nephrology. 2009;24(8):1445–52. [DOI] [PubMed] [Google Scholar]
- 2.Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, Alleyne S, et al. Immunologic function and survival in hemodialysis patients. Kidney International. 1998;54(1):236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou F, Ren H, Owen W Jr, Guo Z, Chen P, Schmidt A, et al. Enhanced Expression of Receptor for Advanced Glycation End Products in Chronic Kidney Disease. Journal of the American Society of Nephrology. 2004;15(7):1889–96. [DOI] [PubMed] [Google Scholar]
- 4.Chen R, Kang R, Fan XG, Tang D. Release and activity of histone in diseases. Cell Death and Disease. 2014;5(8):e1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu D, Ingram A, Lahti JH, Mazza B, Grenet J, Kapoor A, et al. Apoptotic release of histones from nucleosomes. Journal of Biological Chemistry. 2002;277(14):12001–8. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan MJ, Radic M. Neutrophil Extracellular Traps: Double-Edged Swords of Innate Immunity. The Journal of Immunology. 2012;189(6):2689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekaney M, Otto G, Sossdorf M, Sponholz C, Boehringer M, Loesche W, et al. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Critical Care. 2014;18(5):543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel B, Shinagawa H, Hofmann U, Ertl G, Frantz S. Acute DNase1 treatment improves left ventricular remodeling after myocardial infarction by disruption of free chromatin. Basic Research in Cardiology. 2015;110(2):1–15. [DOI] [PubMed] [Google Scholar]
- 9.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: Involvement of platelet TLR2 and TLR4. Blood. 2011;118(7):1952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Li L, Liu J, Lv B, Chen F. Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-κB and AP-1. Thrombosis Research. 2016;137:211–8. [DOI] [PubMed] [Google Scholar]
- 11.Van Montfoort ML, Stephan F, Lauw MN, Hutten BA, Van Mierlo GJ, Solati S, et al. Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(1):147–51. [DOI] [PubMed] [Google Scholar]
- 12.Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hagele H, Lichtnekert J, et al. Histones from Dying Renal Cells Aggravate Kidney Injury via TLR2 and TLR4. Journal of the American Society of Nephrology. 2012;23(8):1375–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silk E, Zhao H, Weng H, Ma D. The role of extracellular histone in organ injury. Cell Death and Disease. 2017;8(5):e2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Primary care. 2008;35(2):329–344, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMillan R, Skiadopoulos L, Hoppensteadt D, Guler N, Bansal V, Parasuraman R, et al. Biomarkers of Endothelial, Renal, and Platelet Dysfunction in Stage 5 Chronic Kidney Disease Hemodialysis Patients With Heart Failure. Clinical and Applied Thrombosis/Hemostasis. 2018;24(2):235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nickolas TL, Barasch J, Devarajan P. Biomarkers in acute and chronic kidney disease. Current opinion in nephrology and hypertension. 2008;17(2):127–32. [DOI] [PubMed] [Google Scholar]
- 17.Devarajan P The use of targeted biomarkers for chronic kidney disease. Advances in chronic kidney disease. 2010;17(6):469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox LAE, Van Eijk LT, Ramakers BPC, Dorresteijn MJ, Gerretsen J, Kox M, et al. Inflammation-induced increases in plasma endocan levels are associated with endothelial dysfunction in humans in vivo. Shock. 2015;43(4):322–6. [DOI] [PubMed] [Google Scholar]
- 19.Burlingame RW, Volzer MA, Harris J, Du Clos TW. The effect of acute phase proteins on clearance of chromatin from the circulation of normal mice. Journal of immunology. 1996;156(12):4783–8. [PubMed] [Google Scholar]
- 20.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. [DOI] [PubMed] [Google Scholar]
- 21.Nelson K, Thethi I, Cunanan J, Hoppensteadt D, Bajwa R, Fareed J, et al. Upregulation of surrogate markers of inflammation and thrombogenesis in patients with ESRD: Pathophysiologic and therapeutic implications. Clinical and Applied Thrombosis/Hemostasis. 2011;17(3):302–4. [DOI] [PubMed] [Google Scholar]
- 22.Amabile N Circulating Endothelial Microparticles Are Associated with Vascular Dysfunction in Patients with End-Stage Renal Failure. Journal of the American Society of Nephrology. 2005;16(11):3381–8. [DOI] [PubMed] [Google Scholar]
- 23.Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y, et al. Elevation of circulating endothelial microparticles in patients with chronic renal failure. Journal of Thrombosis and Haemostasis. 2006;4(3):566–73. [DOI] [PubMed] [Google Scholar]
- 24.Kok HM, Falke LL, Goldschmeding R, Nguyen TQ. Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease. Nature Reviews Nephrology. 2014;10(12):700–11. [DOI] [PubMed] [Google Scholar]
- 25.Kamimura M, Bea F, Akizawa T, Katus HA, Kreuzer J, Viedt C. Platelet-derived growth factor induces tissue factor expression in vascular smooth muscle cells via activation of Egr-1. Hypertension. 2004;44(6):944–51. [DOI] [PubMed] [Google Scholar]
- 26.Newman PM, Chong BH. Heparin-indiced thrombocytopenia:new evidence for the dynamic binding of purified anti-PF4-haparin antibodies to platelets and the resultant platelet activation. Blood. 2000;96:1(1):182–7. [PubMed] [Google Scholar]
- 27.Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, et al. Fibroblast Growth Factor 23 and Inflammation in CKD. Clinical Journal of the American Society of Nephrology. 2012;7(7):1155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nymo SH, Ueland T, Askevold E, Dahl CP, Gullestad L, Aukrust P, et al. Circulating nucleosomes in chronic heart failure. International Journal of Cardiology. 2016;203:742–3. [DOI] [PubMed] [Google Scholar]
- 29.Zeerleder S, Zwart B, Wuillemin WA, Aarden LA, Groeneveld ABJ, Caliezi C, et al. Elevated nucleosome levels in systemic inflammation and sepsis. Critical Care Medicine. 2003;31(7):1947–51. [DOI] [PubMed] [Google Scholar]
- 30.Holdenrieder S, Stieber P, Bodenmüller H, Busch M, Fertig G, Fürst H, et al. Nucleosomes in serum of patients with benign and malignant diseases. International journal of cancer Journal international du cancer. 2001;95(2):114–20. [DOI] [PubMed] [Google Scholar]
- 31.Amoura Z, Piette JC, Chabre H, Cacoub P, Papo T, Wechsler B, et al. Circulating plasma levels of nucleosomes in patients with systemic lupus erythematosus: correlation with serum antinucleosome antibody titers and absence of clear association with disease activity. Arthritis and rheumatism. 1997;40(12):2217–25. [DOI] [PubMed] [Google Scholar]
- 32.Atan R, May C, Bailey SR, Tanudji M, Visvanathan K, Skinner N, et al. Nucleosome levels and toll-like receptor expression during high cut-off haemofiltration: A pilot assessment. Critical Care and Resuscitation. 2015. December 1;17(4):239–43. [PubMed] [Google Scholar]
- 33.Lichtenstein A V, Melkonyan HS, Tomei LD, Umansky SR. Circulating Nucleic Acids and Apoptosis. Annals of the New York Academy of Sciences. 2006;945(1):239–49. [DOI] [PubMed] [Google Scholar]
- 34.Sur Chowdhury C, Giaglis S, Walker UA, Buser A, Hahn S, Hasler P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: Analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Research and Therapy. 2014;16(3):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dieker J, Tel J, Pieterse E, Thielen A, Rother N, Bakker M, et al. Circulating Apoptotic Microparticles in Systemic Lupus Erythematosus Patients Drive the Activation of Dendritic Cell Subsets and Prime Neutrophils for NETosis. Arthritis and Rheumatology. 2016;68(2):462–72. [DOI] [PubMed] [Google Scholar]
- 36.Decker P TLR9 independent interferon α production by neutrophils on NETosis in response to circulating chromatin, a key lupus autoantigen. Annals of the rheumatic diseases. 2014;73(12):2199–207. [DOI] [PubMed] [Google Scholar]
- 37.Söderberg D, Kurz T, Motamedi A, Hellmark T, Eriksson P, Segelmark M. Increased levels of neutrophil extracellular trap remnants in the circulation of patients with small vessel vasculitis, but an inverse correlation to anti-neutrophil cytoplasmic antibodies during remission. Rheumatology (United Kingdom). 2015;54(11):2085–94. [DOI] [PubMed] [Google Scholar]
- 38.Sharain K, Hoppensteadt D, Bansal V, Singh A, Fareed J. Progressive Increase of Inflammatory Biomarkers in Chronic Kidney Disease and End-Stage Renal Disease. Clinical and Applied Thrombosis/Hemostasis. 2013;19(3):303–8. [DOI] [PubMed] [Google Scholar]
- 39.Robey F, Jones K, Tanaka T, Liu T. Binding of C-reactive protein to chromatin and nucleosome core particles. J biol Chem. 1984;259(11):7311. [PubMed] [Google Scholar]
- 40.Abrams ST, Zhang N, Dart C, Wang SS, Thachil J, Guan Y, et al. Human CRP Defends against the Toxicity of Circulating Histones. The Journal of Immunology. 2013;191(5):2495–502. [DOI] [PubMed] [Google Scholar]
- 41.Huang H, Chen H-W, Evankovich J, Yan W, Rosborough BR, Nace GW, et al. Histones Activate the NLRP3 Inflammasome in Kupffer Cells during Sterile Inflammatory Liver Injury. The Journal of Immunology. 2013;191(5):2665–79. [DOI] [PMC free article] [PubMed] [Google Scholar]


