Histiocytic disorders are uncommon and often affect multiple organ systems. They pose diagnostic challenges because of their rarity and the fact that the nosology of these lesions is still being decided. ALK-positive histiocytosis is one of the newest subtypes and was originally described about ten years ago, wherein there was a predilection for neonates and infants with multi-organ involvement [1]. Since then, ten additional cases have been reported, with only one having exclusive intracranial disease, along with involvement of the cavernous sinus [2, 6]. Here, we report two additional cases with exclusive involvement of the central nervous system.
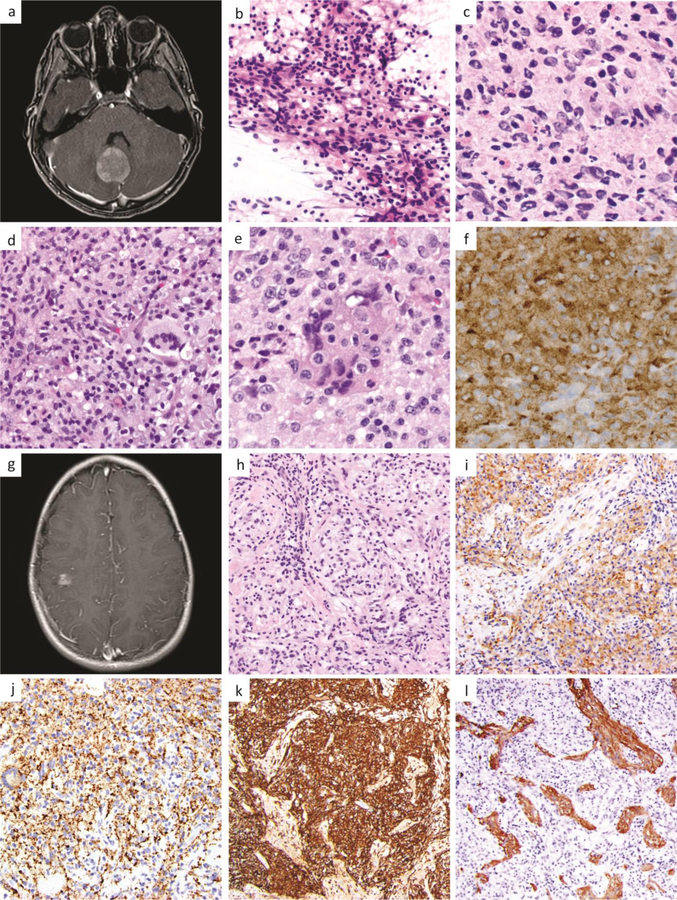
Case 1 is a 7-year-old girl who presented with a one-month history of headaches and vomiting. Magnetic resonance imaging (MRI) showed an infiltrating 3 cm mass in the cerebellar vermis. The mass was associated with diffusion restriction and was radiologically suspicious for medulloblastoma (Fig. 1a). She underwent gross total resection followed by observation with MRI every three months. Postoperative whole-body PET-CT scan showed no evidence of systemic disease. At one-year post-operative follow-up, there is no evidence of recurrence on neuroimaging. Her only neurologic deficit is a minimal slurring of speech and difficulty with phonation.
Fig 1.
Case 1, 7-year-old girl with a 3 cm enhancing cerebellar mass seen on MRI (a). Intraoperative smear showed multinucleation, pleomorphism and tapering eosinophilic cytoplasm which was initially confused with a glial neoplasm (b). Frozen section (c) and permanent sections (d-f) showed additional histologic features including Touton-like giant cells (d), nuclear grooves and emperipolesis (e), and ALK-positivity (f). Case 2, 10-year-old girl with an enhancing 1.4 cm right-sided cortical mass seen on MRI (g). Histopathologic workup revealed a histiocytic lesion (h) with ALK-positivity (i), C68-positivity (j), CD163-positivity (k), and entrapped GFAP-positive gliotic brain (l).
Case 2 is a 10-year-old girl who presented with medically refractory seizures and was found to have a homogenously enhancing 1.4 cm mass in the right pericentral cortical region on head MRI (Fig. 1g). She underwent focal corticectomy followed by observation. Postoperatively, she has been doing well and has only had one reported seizure. She has not had a recurrent seizure while on antiepileptic therapy. At six-month post-operative follow-up, there is no evidence of recurrence on neuroimaging. She has no remarkable findings on physical and neurological exam.
Consistent with prior reported cases, microscopic examination in both cases showed sheet-like aggregates of large epithelioid cells with irregularly folded nuclei and fine chromatin, foamy cells, Touton-like giant cells, and focal emperipolesis (Fig. 1b–e, h). Immunohistochemical workup in both cases showed ALK expression (Fig. 1f, i), Factor XIIIa, CD68 (Fig. 1j), and CD163 (Fig. 1k) positivity, patchy staining for S-100 protein, and lack of CD1a, BRAF V600E, or GFAP reactivity, although the latter highlighted adjacent and entrapped brain parenchyma with reactive astrocytosis (Fig. 1l). The histopathology observed in these cases of ALK-positive histiocytosis show overlapping features with those of Erdheim-Chester disease (ECD), juvenile xanthogranuloma (JXG), Rosai-Dorfman disease (RDD), and Langerhans cell histiocytosis (LCH). In particular, foamy cells, Touton-like giant cells, variable S-100 staining, and the presence of Factor XIIIa expression suggests the possibility of JXG or ECD [7]. Emperipolesis can be seen in RDD and folded or grooved nuclei are present in LCH. A CD1a immunostain can be used to further rule out LCH. Rarely, ALK-positive histiocytosis can also be confused with astrocytic lesions, particularly at intraoperative consultation where the presence of pleomorphic nuclei, multinucleation and tapering eosinophilic cytoplasm can resemble the histologic appearance of a pleomorphic astrocytoma (Fig. 1b).
Targeted next-generation sequencing identified in-frame KIF5B-ALK gene fusions in both cases. Both cases harbored fusions linking exons 1–24 of KIF5B to exons 20–29 of ALK (Supplementary Table 1 and Supplementary Figure 1 [Online Resource 1]), identical to the five systemic cases of ALK-positive histiocytosis described by Chang et al [2]. In case 1, fusion testing was performed using the Archer FusionPlex Solid Tumor next-generation RNA sequencing assay targeting 53 genes. In case 2, targeted capture-based next-generation DNA sequencing was performed using the UCSF500 Cancer Panel as previously described [3]. No additional pathogenic mutations, amplifications, deletions, or fusions were identified involving any other targeted genes, including BRAF, MAP2K1, KRAS, NRAS, and PIK3CA.
Some histiocytic disorders can show specific genetic signatures. BRAF V600E mutation is most common in both Langerhans cell histiocytosis and Erdheim-Chester disease and this is detectable using immunohistochemistry. Other MAPK pathway alterations are often found in those that are negative for BRAF V600E mutant protein. ALK gene fusions have been identified in a subset of histiocytic neoplasms [4–5] including KIF5B-ALK in a case of ALK-positive histiocytosis. In the recently published series by Chang et al, five samples showed KIF5B-ALK gene fusion, with identical fusion breakpoints to the two localized central nervous system cases reported here [2]. Of note, ALK gene fusions are known to be present in a spectrum of other human tumors including lung adenocarcinomas and inflammatory myofibroblastic tumors and often correlate with sensitivity to small molecule kinase inhibitors such as crizotinib [8]. The prior report of an ALK-positive histiocytosis case responding to crizotinib [2] illustrates the utility of targeted therapy in the treatment of this disease entity. So far, in our two cases, this has not been necessary because both underwent gross total resection and have not shown any evidence of recurrence to date.
These cases, along with the recently published series by Chang et al, 2018 extend the clinical and histomorphologic spectrum of ALK-positive histiocytosis to include localized central nervous system involvement and a broader age range than previously appreciated [1]. These cases also underscore the importance of an integrated histologic and genetic approach for the diagnosis and treatment of challenging histiocytic central nervous system lesions.
Supplementary Material
Acknowledgments:
D.A.S is supported by NIH Director’s Early Independence Award (DP5 OD021403).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References:
- 1.Chan JK, Lamant L, Algar E, et al. (2008) ALK+ histiocytosis: a novel type of systemic histiocytic proliferative disorder of early infancy. Blood 112(7):2965–8. https://10.1182/blood-2008-03-147017 [DOI] [PubMed] [Google Scholar]
- 2.Chang KTE, Tay AZE, Kuick CH, et al. (2018) ALK-positive histiocytosis: an expanded clinicopathologic spectrum and frequent presence of KIF5B-ALK fusion. Mod Pathol 32(5):598–608. https://10.1038/s41379-018-0168-6 [DOI] [PubMed] [Google Scholar]
- 3.Kline CN, Joseph NM, Grenert JP et al. (2017) Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro-oncology 19(5):699–709. https://10.1093/neuonc/now254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond EL, Durham BH, Haroche J, et al. (2016) Diverse and Targetable Kinase Alterations Drive Histiocytic Neoplasms. Cancer Discov 6(2):154–65. https://10.1158/2159-8290.CD-15-0913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estrada-veras JI, O’brien KJ, Boyd LC, et al. (2017) The clinical spectrum of Erdheim-Chester disease: an observational cohort study. Blood Adv 1(6):357–366. https://10.1182/bloodadvances.2016001784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang H, Gheorghe G, North PE, Suchi M (2018) Expanding the Phenotype of ALK-positive Histiocytosis: A Report of 2 Cases. Pediatr Dev Pathol 21(5):449–455. https://10.1177/1093526617740784 [DOI] [PubMed] [Google Scholar]
- 7.Louis DN, Ohgaki H, Wiestler OD, et al. (2016) WHO classification of tumours of the central nervous system (Revised 4th edition). Lyon, France. [Google Scholar]
- 8.Yoshihara K, Wang Q, Torres-garcia W, et al. (2015) The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene 34(37):4845–54. https://10.1038/onc.2014.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.