Abstract
Studies of humans with focal brain damage and non-human animals with experimentally induced brain lesions have provided pivotal insights into the neural basis of behavior. As the repertoire of neural manipulation and recording techniques expands, the utility of studying permanent brain lesions bears re-examination. Studies on the effects of permanent lesions provide vital data about brain function that are distinct from those of reversible manipulations. Focusing on work carried out in humans and nonhuman primates, we address the inferential strengths and limitations of lesion studies, recent methodological developments, the integration of this approach with other methods, and the clinical and ecological relevance of this research. We argue that lesion studies are essential to the rigorous assessment of neuroscience theories.
Keywords: neuropsychology, brain damage, humans, nonhuman primates, methods, lesion-behavior mapping, lesion-symptom mapping
Lesion studies: A mainstay of neuroscience
Studying the effects of brain lesions on behavior and cognition is one of the most established and influential methods in neuroscience. In the 19th century, case studies of patients with focal brain damage provided the first evidence that complex cognitive processes, such as those underlying language, have dissociable components that depend on different regions of the brain [1, 2]. Brain lesion studies constituted the foundation of cognitive neuroscience that emerged in the mid to late 20th century. This included seminal work such as Brenda Milner’s demonstration that memory, like language, involves distinct component processes with their own neural substrates [3, 4], as well as the work of Mortimer Mishkin and Leslie Ungerleider on the dissociable contributions of the dorsal and ventral visual pathways in nonhuman primates (NHPs; see Glossary) [5, 6]. These investigations helped to inspire decades of influential new ideas: cognitive theories, intrepid studies of neural activity, and new models of brain function (e.g., [7, 8, 9, 10]). Studies of subjects with focal lesions have since continued to provide fundamental insights in the fields of learning [11, 12], cognitive control [13, 14, 15, 16], social behavior [17, 18], memory [19, 20, 21, 22], and more.
The advent of new neural manipulation methods, both invasive (e.g., optogenetics, chemogenetics) and noninvasive (e.g., transcranial magnetic stimulation, TMS; transcranial focal ultrasound), and the explosion of increasingly sophisticated functional neuroimaging methods, such as positron emission tomography (PET), functional magnetic resonance imaging (fMRI), and electrophysiological measures of neural activity, calls for a critical re-examination of the strengths and limitations of chronic, focal lesion studies. The purpose of this article is to evaluate the role of lesion methods as they relate to other causal and correlative methods in the toolkit of contemporary cognitive and systems neuroscience. Specifically, we discuss four topics: (i) inferences that can be made from lesion studies compared to other methods, (ii) combining lesion studies with correlative methods, (iii) recent advances in lesion methods, and (iv) lesion studies outside the laboratory. Although many of the points raised here may apply to other animal models, we focus on evidence from humans and NHP lesion studies given the similarities between the neuroanatomy of NHPs and humans in terms of cortical expansion [23, 24] and topographical connections (e.g., corticocortical [24, 25] and corticobasal ganglia pathways [26]). Our goal in focusing on humans and NHPs is to highlight the similarities of lesion findings in these models and to emphasize how invasive lesion studies in NHPs have been essential in filling inferential gaps in this work. We conclude that (i) chronic lesion studies provide unique, vital insights into brain function that cannot be achieved via temporary inactivation methods or correlational studies of brain activity, and (ii) integrating insights gleaned from lesion studies with results from other methods is crucial for advancing neuroscience.
What inferences can lesion studies support?
Studies of brain activity, either through electrophysiology, fMRI, PET, or other methods, test whether cognitive processes are associated with activity in neurons, brain regions, or networks. These techniques comprise a powerful set of investigative tools but cannot differentiate the regions that are involved during some cognitive process from those that are necessary for that process. This limitation cannot be addressed by further methodological refinement [27]. In contrast, lesion studies can demonstrate the necessity of a region for a particular cognitive process, not just its mere association with that process. In well-designed studies, null results can be equally informative for constraining neuroscientific theories, particularly when data from other methods (e.g., correlations between a behavioral task condition and neural activity) suggest that a lesion in a specific region should impair an associated behavior. Recent examples of informative null findings include demonstrations that excitotoxic lesions of orbitofrontal cortex (OFC) do not affect stimulus reversal and probabilistic reinforcement learning in NHPs [28, 29], that dorsal anterior cingulate cortex (ACC) damage in humans does not affect behavioral indices of response conflict [13, 30, 31], that humans with ventromedial prefrontal cortex (vmPFC) damage can make choices consistent with their subjective preferences [32, 33], and that non-navigational spatial memory is unaffected by hippocampal damage in NHPs [34]. These findings challenge current models of brain–behavior relationships and push the field towards more refined hypotheses and better behavioral indicators of the processes in question. In the following section we describe key considerations in making inferences from lesion studies, the strengths and limitations of this approach, and how this method differs from others.
Methodological considerations for studies of focal lesions
In human subjects, lesions to a circumscribed brain area can occur following disease, injury, or neurosurgical treatment. Although the lesions are not under experimental control and can vary with regard to etiology, size, laterality, and age of onset, the selection of participants is. Anatomical specificity is determined by the inclusion and exclusion criteria for a given study, a balance between the anatomical region of interest (ROI), the natural patterns of common lesion causes, and pragmatic considerations of sample size and study duration. In NHPs, a focal lesion can be induced via surgical intervention by targeting a region within a predefined anatomical boundary, which affords greater experimental control. The accuracy of the lesion depends on the method used to create it and on experimenter skill. We elaborate on some of the key design considerations for lesion studies in Box 1.
Box 1: How to judge the inferential strength of a lesion study.
A full description of best practices and available methods for designing a lesion study is beyond the scope of the present manuscript. Such resources can be found in the following references: [176–179]). Here, we briefly describe some of the key features to be considered:
Premorbid functioning
In humans, brain lesions are usually caused by a neurological event, such that data are rarely available about premorbid cognitive function (although see [180, 181]). Clinical interviews, level of education, crystallized IQ, and questionnaires on pre- and postmorbid function can help to fill these gaps [182, 183]. In NHPs, subjects with lesions may be compared to subjects with sham surgery, subjects with surgery in another location, or to their own presurgical performance when within-subject comparisons are possible. This latter form of control may also be possible in some rare cases in humans, such as when lesions are made as a planned course of treatment in psychiatric populations (e.g., [13] or in epilepsy). However, the patients undergoing these procedures are not neurologically healthy, which complicates inferences about ‘premorbid’ function.
Control groups
Comparing subjects with lesions to matched controls is critical for establishing the effects of these lesions. This control group should be demographically matched to the lesion group and recruited from the same population. However, there are other factors that accompany brain damage (e.g., use of psychoactive medication) that are not controlled for in these comparisons. Inclusion of a control group comprised of subjects with brain damage sparing the ROI can help account for these factors. In human subjects, lesions are often not constrained to the ROI, and a control group that shares damage outside the ROI can provide greater assurance that behavioral changes in the ROI group are anatomically specific.
Etiology
Lesions in human patients are frequently confounded by features related to the source of damage. For example, ischemic stroke patients are more likely to have cerebrovascular disease and small, “silent” infarcts that may be associated with deficits unrelated to the lesion under study [184], while treatment for brain tumors may have additional neurological consequences (e.g. radiation therapy). The etiology of a lesion is also usually associated with the pattern of damage (e.g., strokes follow vascular branches). Including patients with diverse etiologies can mitigate these problems by reducing lesion covariance and decoupling deficits from a specific neurological disease.
Neuropsychological screening
Experimental tasks are never truly process-pure. For example, a “simple” reinforcement-learning task may depend on working memory, sustained attention, and reading comprehension. Neuropsychological screening tests that tap a wide range of functions can control for potential explanations and provide insight into the latent factors that underlie deficits.
Statistical power
As with many neuroscience methods, sample sizes tend to be low in neuropsychological studies of patients [185]. In studies of brain lesions in human patients, sample size is generally constrained by the difficulty of recruiting, screening and testing a special population of subjects. Dedicated registries of such patients can be one solution, but this requires on-going investment to recruit, assess and maintain such listings of potential research participants, much as centers must invest in supporting other methodological platforms (imaging, etc.) so that individual experiments can be carried out in a timely fashion [186]. Sample sizes are also small in studies of NHPs, mostly due to the costs of studying these animals. Given these constraints, statistical power in studies of human subjects can be most readily improved by testing a large sample of healthy control subjects, who are easier to recruit.
In both cases, destruction of brain tissue causes permanent loss of neurons and, therefore, termination of function in the affected region. Evidence that a lesion reliably alters a behavior (e.g., performance on a task where items must be retained in memory over a delay) can then be used to test a causal link between the brain region and the cognitive process underlying the behavior (e.g., memory). Demonstrating a link between a lesion and a behavioral process, however, is not trivial. A cognitive or behavioral change associated with a lesion must be disentangled from nonspecific symptoms that may accompany brain damage, and other potential sources of between-subject variance. Drawing conclusions from lesion studies depends on evaluating the functional and anatomical selectivity of behavioral changes (i.e., Does a lesion affect related behaviors? Are the effects specific to a certain region?). Dissociation logic, which we discuss in Box 2, has been vital in this effort, not only in lesion studies, but in neuroscience more broadly.
Box 2. Dissociation logic in lesion studies.
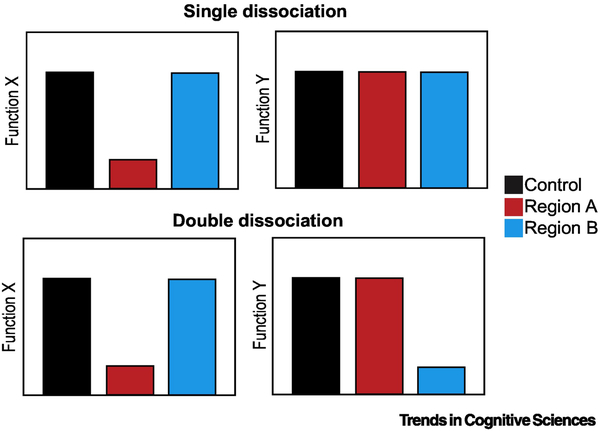
In neuroscience, dissociation logic has played a central role in testing the specificity of region-function relationships. Testing whether a brain region is involved in function X but not function Y is helpful in that it provides stronger evidence for functional specificity than the demonstration of an association (i.e., showing that region A is involved in function X, without testing other functions). However, a single dissociation is still a relatively weak form of inference when these functions can be accounted for by a single process (e.g., if function X is simply a more demanding form of function Y). A crossover double dissociation can be used to make a stronger claim by demonstrating that region A is involved in function X but not Y, whereas region B is involved in Y but not X (Figure I) [187]. Even this case is not impervious to potential alternative single-process explanations [188].
In practice, true dissociations can be challenging to test and require careful consideration of control conditions to ensure that two processes are really independent. One criticism of this approach is that each task might engage the brain in a different way and hence potentially rely on a different set of regions, yielding increasingly granular task dissociations – and ultimately less meaningful distinctions [189]. This problem is not unique to lesion studies but speaks to a broader issue in neuroscience regarding whether theory should lump or split functions. Similar issues were raised regarding fMRI research that appeared to uncover an unending number of functional associations (i.e., Is there a specific brain network for playing board games? What about a network specific for playing checkers?). There is no easy solution to this problem. In any model-fitting exercise, adding parameters may explain more variance but will eventually hamper the generalizability of the model to new data. Similarly, there is an important trade-off in the number of functional dissociations a neuroscientific theory might make and its generalizability outside the experimental settings within which it was developed. That is to say, some dissociations may reveal more significant information about the specialization of brain regions and may also be more likely to stand the test of time than others. The choice of experimental question remains the most vital factor in determining whether an experiment is likely to uncover a crucial new functional dissociation with broader significance, or simply explain noise.
Figure I (Box 2): Schematic demonstrating single and double dissociation logic.
Y-axes indicate to performance in tasks measuring performance in Functions X and Y. Bars represent performance of control group and groups with lesions in regions A and B.
Compensatory processes in lesion studies
Brain damage causes immediate cognitive and behavioral changes, followed by a dynamic period of functional reorganization before subjects reach a chronic state where changes stabilize. In the hyperacute period immediately following injury, both humans and NHPs briefly suffer from the effects of brain edema and inflammation, which cause transient, diffuse brain dysfunction. In the subsequent weeks and months, during the acute phase of a lesion, functional recovery takes place through cellular- and systems-level processes that reorganize circuits at the site of the lesion [35], recruit redundant or alternative pathways, and modify an individual’s behavioral repertoire. While these compensatory processes are sometimes considered uninteresting as they may ‘mask’ deficits observed in the acute phase, they can also provide valuable information about the capacities and limitations of intact neural systems. For example, lesions to striate cortex cause major visual field impairments, but research over several decades has revealed that some basic visual processing is preserved (i.e., “blind-sight”) due to intact processing in separate pathways [36]. A lesion is considered chronic months or years after injury, when the effects of damage on brain function have stabilized. Critically, patients and NHPs tested years, or even decades, from the time of injury will show persistent behavioral deficits in specific tasks, such as the profound lifelong memory impairments caused by bilateral medial temporal lobectomy in patient H.M. [37–41]. Measuring behavior and neural activity at multiple time points in the course of recovery can help distinguish between behavioral and neural changes caused by acute chronic effects of a lesion [42–47].
The etiology of a lesion may also affect how the brain adapts to damage. For example, functional changes following brain damage as a result of stroke are most severe immediately after the event, with some recovery of function occurring rapidly, then reaching a plateau within about 6 months [48, 49]. In contrast, brain tumors cause damage over a long period of time, through compression, distortion, or infiltration of tissue, as well as through edema, microcirculatory effects, and local electrolyte abnormalities [50], followed by the effects of surgical resection of the tumor. Whether the cognitive effects of brain tumors are comparable to those of stroke, particularly when the tumors are slow-growing and allow more time for plasticity to occur, has been a subject of debate [51, 52, 53, 54], and comparison of single- and multistage lesions in animal models suggests that the rate of damage may affect outcome [55]. A recent study compared a large sample of frontal lobe-damaged patients with different lesion etiologies (stroke, fast and slow-growing tumors) in several executive function tests. Etiology was not a strong predictor of patients’ deficits in this sample [56]. Behavioral effects in the chronic phase of focal lesions thus may relate more closely to the region of damage than to etiology in human patients.
The rate at which brain development occurs varies across different brain regions or networks [57]. Cross-sectional and longitudinal developmental lesion studies provide essential information about how lesioning brain regions affects behavior during the course of brain maturation. For example, whereas amygdala damage acquired in infancy in NHPs permanently disrupts normative affective responses to stimuli with either positive and negative valence [58], it only mildly disrupts social cognition in the long term [59]. These results suggest that intact amygdala function across development is necessary for normal emotional, but not social, cognition throughout the lifespan. Adults who incurred vmPFC damage as infants show more exaggerated personality changes (e.g., increased impulsivity, insensitivity to punishment) compared to patients with adult-onset lesions to the same region, who show relatively milder and more subtle personality changes [60]. This work reveals the critical role of these regions for normal developmental trajectories of these complex traits and may provide insight into the processes that occur in developmental psychopathologies such as autism or oppositional defiant disorder.
Comparing chronic lesion methods and temporary manipulations of neural activity
Like lesion studies, manipulation methods (e.g., TMS, pharmacological agents, optogenetics, and chemogenetics) allow researchers to draw causal links between behavior and brain function. However, unlike lesion studies, manipulations of neural activity are temporary and reversible, providing temporal specificity and amenability to within-subject experimental designs. Manipulation methods can also be used to up- and down-regulate activity within a brain region to study how changing neural dynamics affects behavior. Advances in genetic tools have allowed neural manipulations of brain circuits to reach unprecedented levels of detail in rodents (e.g., optogenetics, chemogenetics). Application of genetic targeting technologies in NHPs are comparatively in their infancy but are advancing rapidly [61, 62]. Applications of optogenetics in NHPs have so far been shown to produce transient or weak effects on behavior following perturbation of neuronal targets, findings that are in conflict with lesion or micro-stimulation results within the same regions [63, 64]. Although the source of these differences remains unclear, these techniques hold great promise for advancing our understanding of the functional relevance of separable neural circuits.
Temporary manipulation methods are often assumed to recapitulate what is observed following chronic lesions. However, in practice these methods have fundamentally different effects on brain function that should be considered when drawing interpretations about causal brain-behavior relationships. A recent study in zebra finches elegantly demonstrated that these methods can potentially reach fundamentally different conclusions about the necessity of a region (Figure 1) [65]. Temporary inactivation of a region of the zebra finch brain caused dramatic degradation of birdsong. However, birdsong recovered within days of a permanent lesion to the same region, with major impairments only observed briefly in the hours following damage. Behavioral recovery was associated with the rapid return of spontaneous activity in a region downstream of the lesion, which prior lesion work had established as necessary for normal behavior [66]. These data argue that temporary inactivation may mimic behavioral effects in the acute phase of a lesion, with potentially wide-ranging effects on activity and neural dynamics in distant regions.
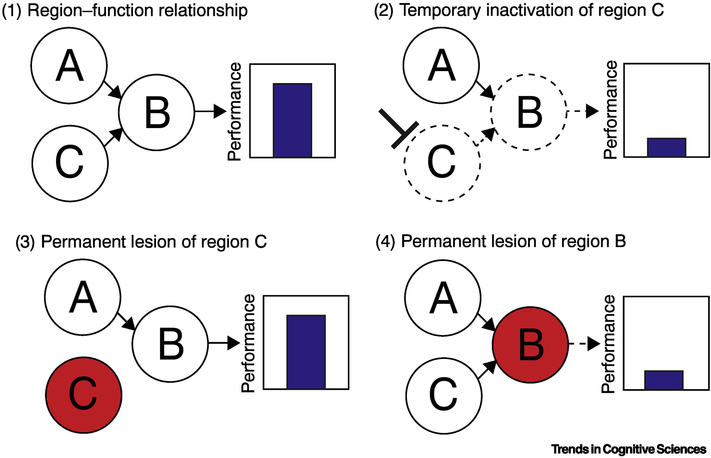
Figure 1. Illustration of Differential Effects of Temporary Inactivation and Permanent Lesions.
This schematic is based on the results of [65]. (1) In this hypothetical example, performance in a task is directly related to the function of region B, which processes crucial information from inputs coming from region A. Region B also receives inputs from region C that are not crucial to the process region B completes for task performance, but exert a separate, secondary influence on the homeostatic activity of this region. (2) Temporary inactivation of region C causes a perturbation of region B, which leads to a disruption of performance. (3) By contrast, permanently lesioning region C does not affect performance because of compensation in region B for the loss of this input. (4) However, a permanent lesion of region B causes a performance deficit that cannot be compensated for.
This example illustrates a key distinction between reversible manipulation and lesion methods that has become increasingly clear in recent years. Chronic lesions can reveal the necessary contributions of damaged brain regions that are not recovered by reorganization and plasticity [67]. Although temporary manipulations also reveal necessary functions of a region in the moment of a perturbance, these “off-target” effects disrupt homeostatic activity within the network of the affected region [68–71], and even coupling across multiple brain systems [72], placing the brain in a previously unencountered physiological state [73]. This is not to say that permanent lesions do not affect activity in regions distant from the site of damage, but instead, that these regions have had time to compensate to the effects of a lesion and reach a new homeostatic state of function. These approaches therefore provide complementary information about the necessity of a region for a given behavior, making them non-interchangeable, mutually informative tools in the neuroscience toolkit.
Converging evidence: Combining lesion studies with other methods
Since different methods provide unique information about the brain, combining methodological approaches yield critical new insights. In the following section, we discuss how combining lesion studies with recordings of neural activity uncovers the necessary contributions of the damaged region to function in distant parts of the brain, and how findings from lesion studies can be used to test hypotheses with other methods.
Combining lesion studies with measurements of brain activity
Methods for studying brain activity (e.g., fMRI, electrophysiology, and PET imaging) have grown tremendously in the past few decades and have benefitted from application of more sophisticated analytic approaches (e.g., multivariate pattern analysis, functional connectivity, model-based analyses) [74, 75, 76, 77]. These studies have provided insight into the information represented by brain activity in different regions, and the time-course over which these representations interact and are maintained. These methods also demonstrate how this information is represented – that is, the code used by neurons for aspects of cognitive processing (e.g., the grid-like coding of space in the entorhinal cortex [78]). However, these methods cannot test the necessity or sufficiency of this activity for the cognitive processes under investigation. For example, the activity of neurons that discriminate expectancy of reward versus punishment does not necessarily indicate that these neurons have any direct bearing on making predictions about outcome value. Such an observation could, in principle, be made with high reliability over many studies, but these data would still not provide evidence that this pattern of activity is causally involved in value prediction per se. Methods that measure correlation between brain activity and behavior provide important information but, alone, they have unavoidable inferential limits.
Studying the effects of lesions on brain activity provides causal evidence for the contribution of a damaged brain region to functional processes measured in connected regions. For example, fMRI studies comparing vmPFC lesion patients to healthy controls demonstrated that vmPFC lesions result in decreased ventral striatum activity during monetary reward anticipation [79] and increased amygdala activity in response to aversive images [80]. A recent EEG investigation of patients with lateral PFC damage showed that the impairments of these subjects in switching between internally and externally directed attention was related to altered theta power during these attentional states [81]. In NHPs, functional recording techniques such as fMRI [82, 83, 84], PET imaging [85], and electrophysiology [86, 87, 88] have been used in conjunction with lesions to characterize causal functional interactions between brain regions. This direction of research promises to provide a better understanding of the causal roles that different brain regions play in the neural dynamics underlying cognitive processes.
Applications of lesion findings in other methodological approaches
Lesion evidence are fertile ground for new hypotheses about neural function that may be tested with other methods. Lesion studies in NHPs that demonstrated a double dissociation for the functions of mid- and posterior dorsolateral prefrontal cortex generated testable predictions about the function of homologous regions. Subsequent human functional imaging experiments confirmed the association of these regions with monitoring information in working memory and conditional selection between competing responses, respectively [89–92]. Similarly, studies of the contributions of perirhinal cortex to memory in macaques [93, 94] led to a series of neuropsychological and fMRI studies in humans that continue to elucidate perirhinal cortex function today [95, 96].
Building converging evidence depends on objective reference points that translate between methods and models. For example, lesion studies have long been used to direct neurophysiological recording studies, when a focal recording site must be chosen prior to chamber and electrode implantation. They can serve a similar role in functional imaging studies by motivating localization of function through ROI analyses [97], especially where statistical thresholding in a whole-brain analyses might obscure effects in smaller regions such as the amygdala or striatum. Coordinates for maximum density of lesion overlap, or results from voxel-based lesion-behavior mapping (described further in the following section), can also be used to define ROIs for functional imaging studies or targets for temporary manipulation studies. Unfortunately, these data are currently dispersed and hard to compare across studies. Collating these data in a meta-analytic platform similar to NeuroSynth (http://neurosynth.org/), and hosting lesion masks and behavioral data in online repositories could help construct a bridge between lesion studies and these other methods. We discuss further applications for data sharing and open science in lesion studies in Box 3.
Box 3: The critical role of lesion studies in open science.
As the scientific community embraces open science applications to make data and analytical tools accessible, researchers working with lesioned subjects should consider ways to collaborate and make provisions for data sharing. Here we outline the advantages of, and practical guidelines for, pursuing collaborative and open scientific methods in lesion work.
Given the rarity of subjects with brain lesions in both human and NHP studies, respectively, the long-term advantages of aggregate or cumulative data in a large database are three-fold: (1) Collaboration and open science can help overcome the oft-cited limitation of small, heterogeneous samples in lesion studies. (2) Repeated measures from the same subject over time can unearth insights on longitudinal changes in behavior from the time of damage onset and resolve unanswered questions regarding plasticity. (3) A larger sample of subjects will make it possible to clarify the effects of individual differences in lesion etiology, psychoactive medication, and other factors that can vary from study to study.
The primary goal is to create an online repository which only vetted researchers can access, to be able to pool data across multiple research sites. Practical guidelines for meeting this goal can be borrowed from the neuroimaging community. There are many databases for fMRI data, such as NeuroVault, OpenfMRI.org, LORIS, COINS, XNAT, NITRC, SciTran, PRIME-DE (exclusively for NHPs), and others, that accept and export their datasets organized according to a data organization standard called ‘brain imaging data structure’ (BIDS; http://bids.neuroimaging.io) [190, 191, 192, 193, 194, 195, 196]. The BIDS standard is designed specifically for fMRI, but a similar standard could be created to organize essential data and metadata for lesion datasets, including lesion masks, VLBM coordinates, self-report measures, neuropsychological data from standard testing batteries, and experimental test results.
In practice, this will take concerted effort and collaboration from laboratories with access to lesion patients and NHPs with focal brain damage. There are several special ethical considerations in sharing these sensitive data from clinical populations, the main ones being the higher likelihood of subject identification given the rare nature of these cases, as well as subjects consenting to having their data being included in a larger database. Access control of such data, similar to that implemented for sensitive genomic information, could also limit risks to patient confidentiality. A resource of this nature, however, could accelerate the rate of discoveries that have yet to be made with existing datasets from lesion samples and provide powerful tools that the broader neuroscience community can use to focus their analyses.
Recent advances in lesion methods
Historically, lesion studies, particularly in humans, have been most closely associated with localizationist approaches to understanding behavioral and cognitive processes. However, no process can occur in a single region without relevant inputs and outputs. A complementary perspective emphasizes how these processes arise from interactions within networks of connected brain regions [98]. Methodological developments are allowing investigators to interrogate information from lesion studies in new ways — particularly in testing both network and regional hypotheses for brain functions. In the following section, we will describe some of the methodological advances that are opening up these lines of inquiry in humans, as well as complementary approaches that can be used in NHPs.
Lesion behavior mapping approaches in humans
Conventionally, chronic lesions are studied to test the role a region plays in a particular function, usually by comparing the effects of damage to one region against a control group of healthy subjects or subjects with damage to a different region (Figure 2a). In investigations of humans, variability in lesion size and extent is unavoidable, and subjects are typically classified into groups based on some a priori anatomical criteria, limiting the spatial resolution of these studies. Voxel-based lesion-behavior mapping (VLBM), or lesion-symptom mapping, takes advantage of the variability of lesions in human patients to make associations between lesions and behavioral performance without the constraint of an ROI ([99–102]; Figure 2b). In its most common application, VLBM uses massive univariate statistics to compare the behavior of patients with damage in each voxel against all other patients in a dataset. This method can thus reveal where damage was most strongly associated with a change in behavior at a more granular level than is possible with group comparisons.
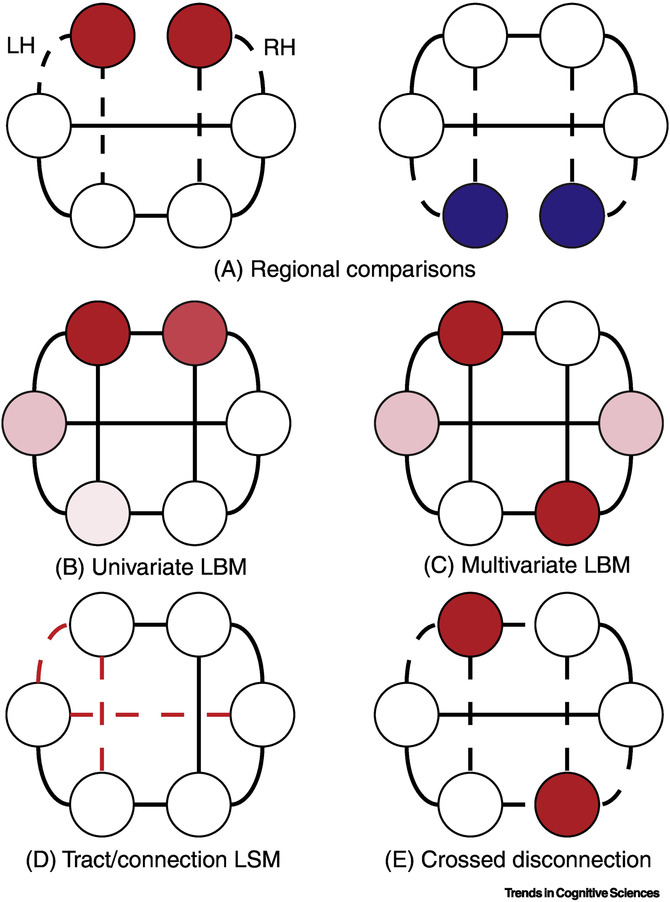
Figure 2: Schematic of different lesion methodologies in a schematic brain network.
In this network, each circular node represents, solid lines represent connections between these regions and dashed lines indicate broken connections between regions caused by a lesion. Nodes are mirrored on the left and right side, representing homologous regions in the right and left ‘hemispheres’ (LH/RH). a. Comparisons between groups with damage to different regions (red and blue nodes) can be used to infer the necessity of these regions for cognitive functions. b. Rather than rely on a priori groups, univariate lesion-behavior mapping (LBM) tests the association of damage with a function across a set of regions, yielding a continuous statistical map for the effect of damage at each location (i.e., the graded colored nodes) c. Multivariate LBM tests the association between a pattern of damage in multiple regions and an observed deficit rather than just any particular region. This method is thus more sensitive to distributed regions that may contribute to some function (i.e., multiple dark red nodes). d. Tract, or connection-based LBM instead tests how damage to specific connections within this network (i.e., red dashed lines) affect function. e. In NHPs, crossed unilateral lesions may be used to ensure that specific regions cannot interact within a hemisphere, allowing inferences about the necessity of this interaction for function.
Recently, several groups have developed multivariate VLBM methods that take a different approach to assessing the localization of function. Although the specific approaches differ, these methods generally test whether there is a consistent relationship between damage in some set of voxels, which may be close together or far apart, and a specific behavior (Figure 2c [99, 103, 104]). Thus, while univariate VLBM tests for the strongest associations between a behavioral change and damage, multivariate approaches test which patterns of damage cause similar changes in behavior. Simulations have shown that this multivariate approach may be particularly useful in cases where damage in multiple regions causes behavior to change in a similar direction (e.g, if damage to the amygdala and OFC caused similar learning deficits). However, more work is needed to directly test how the distinct assumptions of these approaches impact the conclusions they may draw about brain-behavior relationships.
Lesion methods for testing network hypotheses
Many lesion studies aim to test whether damage to a specific gray matter region disrupts a particular behavior. In such cases, white matter damage is frequently treated as a nuisance variable that needs to be controlled for, or a limitation on the interpretation of the data. In contrast, other work has explicitly studied disconnection syndromes—that is, how damage to connections between two or more brain regions affects behavior [98]. In some cases, lesions may primarily affect white matter bundles, disrupting networks while leaving cortical and subcortical gray matter mostly intact. Although VLBM can test associations between white matter damage and behavior in principle, this method does not explicitly test the relationship between disruption of specific tracts, or region-to-region connections and behavior. Recently developed approaches have taken advantage of variability in white matter damage across human patients, and the development of diffusion-tensor imaging tools, to test specific causal relationships between disrupted connections and behavior specifically, using analyses akin to VLBM (Figure 2d). Tract-based lesion-behavior mapping uses white matter atlases in a standard brain space to test if behavioral changes are associated with lesions that interrupt major white matter bundles [105]. Connectome-based lesion-behavior mapping offers a somewhat different approach, using diffusion imaging from subjects with lesions to examine how deficits correlate with metrics of connectivity (e.g., probabilistic tractography) between pre-defined cortical ROIs [106]. However, these methods rely on certain assumptions in reconstructing white matter tracts (e.g., anisotropy of water diffusion, accurate registration of lesions to white matter atlases based on healthy controls), which impose limitations. Diffusion imaging methods can only provide best guesses for the structural connections between regions, and are affected by abnormalities and distortions of brain tissue, potentially affecting interpretation of these data in subjects with brain lesions [106, 107]. Comparison of these estimates against post-mortem histological data may identify long-distance pathways that are difficult to identify with in vivo tractography alone and pathways where these tools are prone to errors [108, 109].
Other work has focused on relating changes in functional connectivity after lesions to changes in behavior. These studies have taken two general approaches: either (1) examining how lesions affect functional connectivity in patients and relating these changes to behavior [e.g. 110], or (2) using information about functional connectivity in healthy subjects to predict the remote effects of brain lesions and testing the relationship between these predicted remote effects and behavior [111, 112]. These tools yield additional insights into the role that network dynamics may play in behavior, beyond testing the contributions of any particular brain region. However, these functional connectivity measures have major limitations. As these measures are correlative, the direction of activity in both healthy and damaged brains is difficult to interpret. For example, changes in connectivity between regions A and B after a lesion to area C could result from either loss of common input from C to both A and B, loss of an input from C to just A or B, or loss of an input from C to an intermediary area D that connects with both A and B. Careful control measures of potential confounds and testing model assumptions also need to be taken into account in interpreting these data – for example, hemodynamic signal in fMRI studies might also be affected cerebrovascular disease [113, 114].
Overall, these new tools are allowing investigators to advance the types of questions that can be asked about the relationships between lesions, brain networks, and behavior. However, parsing whether the behavioral effects of a lesion in a patient are due to loss of function within a damaged region, or depend on critical lines of communication between multiple regions in a network, remains a central challenge in this research. Unfortunately, available analysis tools do not allow these potential sources of variance to compete with each other in the same statistical model to explain the observed behavioral changes in lesion patients. For now, the focus on relating behavior to either network or regional effects of damage depends on the hypothesis tested in individual studies. Lesion-behavior mapping methods also require relatively large samples for sufficient regional coverage and statistical power, which can act as a practical limitation in their use given the challenges of recruiting participants with focal lesions.
Complementary approaches in NHPs for testing regional and network roles
Complementary studies in NHPs can rule out separate contributions of white matter and grey matter to cognitive functions [115]. In recent decades this work has been crucial for refining our understanding of the functions of several brain regions. Two major surgical techniques have been used in NHP work to create focal lesions: aspiration and excitotoxic approaches. Aspiration lesions employ subpial suction and cauterization of tissue, typically under visual guidance with the aid of an operating microscope. In some cases, aspiration lesions affect not only cortex (grey matter) but also axons coursing nearby or through that region (fibers of passage). The extent of this injury depends on the location of the aspirated region and geometry of the underlying white matter tracts. By comparison, excitotoxic lesions induced by local injection of neurotoxins are more selective than aspiration lesions, destroying cell bodies while sparing the underlying fibers of passage. These distinctions, that are practically testable only in animal models, are important for providing evidence about the causal roles of cortical regions in the observed deficits (via excitotoxic damage) versus their connections and interactions (via aspiration damage).
Investigations in NHPs also yield information about regional specializations at a higher resolution than is possible in studies of human patients. Fine-grained targeting of smaller brain regions in NHPs is especially useful for investigating functions of subregions within larger cortical structures (e.g., the orbital and ventromedial regions of prefrontal cortex) that tend to be damaged together in human lesion studies and are known to differ in cytoarchitecture and connectivity to other regions [116]. For example, studies of working memory in patients with frontal lobe damage encompassing several cytoarchitectonic areas revealed impairments in both monitoring within working memory and making conditional selections between competing stimuli [117, 118]. However, experiments with more selective lesions in NHPs were able to show that these functional contributions depended on distinct subregions within the dorsolateral prefrontal cortex [89, 90]. Researchers have similarly used lesions in NHPs to dissociate contributions of sub-regions of PFC in value-based decision-making [42, 119, 120], regulating defensive responses to threatening stimuli [121, 122], and social cognition [123, 124]. These studies in NHPs are highly valuable for constraining search spaces in whole-brain neural recording methods or suggesting target sites for clinical treatments (e.g., as in [125]).
In animal models, the necessity of interactions between different regions can be tested directly through surgically severing specific white matter tracts in some cases (e.g., the corpus callosum or the fornix) or through crossed unilateral lesions in the two hemispheres. Crossed-surgical disconnection lesions leave a single brain region in both hemispheres intact but prevent these regions from interacting within the same hemisphere (e.g., [43]). This technique can answer questions about functional interactions between two, or more [126], brain regions (Figure 2e). Investigations employing crossed-surgical disconnection methods have been informative in supporting circuit interaction models for processes such as value-based decision-making [43, 126–128], discrimination learning [129–131], and memory [132].
Lesion studies outside the laboratory
Applying neuroscientific research findings for diagnosis and treatment of many neurological and psychiatric disorders is complicated by the radical complexity of behavior outside of the laboratory and the challenge of relating these behaviors to operationalizable cognitive processes. In the following section, we describe how lesion studies can connect basic and clinical neuroscience and provide important insights into real-world behaviors.
The role of lesion studies in the clinic
Lesion studies form a unique bridge between basic and clinical neuroscience. Although we have primarily focused on lesion studies in basic science, these findings may also translate into improvements in patient care. Lesion studies directly relate brain dysfunction — in the form of a lesion — to behavioral deficits. Similarly, manipulations of brain activity in NHPs or humans can be used to test causal predictions about relationships between brain activity and behavioral and cognitive symptoms in psychiatric populations [133, 134]. Most immediately, these studies inform neuropsychologists and physicians about likely cognitive impairments in neurological disease, guiding prognosis and treatment [135]. Information gleaned from lesion studies in NHPs can identify potential loci for neurosurgical interventions (e.g., deep brain stimulation), noninvasive manipulations (e.g., TMS) to treat neurological and psychiatric diseases [136, 137], and follow-up studies to reveal the pharmacological, physiological and molecular mechanisms underlying a behavior.
Lesion studies also provide a vital bridge in the other direction: linking behavioral deficits to neurobiology. Importantly, brain lesions are not a model of ‘induced’ psychiatric diseases. Although neurological lesion patients and psychiatric patients may have overlapping symptomatology, the correspondence of symptoms is not complete, the etiologies are very different, and there is far less regional specificity underlying dysfunction in psychiatric disorders [138–140]. However, principled identification of common and distinct deficits in psychiatric and neurological patients could be a promising route for better understanding the neurobiological origins of psychiatric symptoms. Uncovering common deficits in focal brain lesions and psychiatric disorders is in keeping with the goal of symptomatic and neurobiological classifications for psychiatric disease [141]. For example, parallels between the specific social deficits in humans and NHPs with amygdala lesions and people with autism spectrum disorders have provided clues about the neurobiological basis of the latter [142–144]. Finding common cognitive tests or computational principles that link behavioral deficits in lesion patients and psychiatric populations may establish vital connections between neurobiology, cognition, and behavior [145, 146].
Studying real and realistic behavior in lesion studies
The ecological validity of experimental tasks designed to investigate specific constructs in neuroscience is sometimes called into question (e.g., [147]). Lesion studies can uniquely shed light on how specific brain regions support complex cognitive constructs such as personality, morality, aesthetic valuation, and insight, by examining the behavior of subjects with lesions in naturalistic and semi-naturalistic contexts. Verbal, written, and pictorial responses by subjects with brain damage have a long history in neuropsychological research and have revealed the effects of damage on memory [148], perception [149], problem solving [150], and scene construction [151, 152]. Such data shed light on cognitive impairments that simpler behavioral readouts (e.g. binary choices, reaction times) cannot easily access. Although the topic of ecologically valid methods in human neuropsychology has been discussed in depth [153], here we highlight work that involves individual case studies and semi-naturalistic experiments to expand on information gathered from empirically-driven studies of these processes.
Simulating real-world semi-naturalistic contexts allows the effects of lesions to be studied closely in a setting that is perhaps more ecologically meaningful than structured laboratory tasks. For example, the “Multiple Errands Test” (MET) was designed to mimic various “real world” demands on planning, such as shopping for various objects (e.g., a cookie, a candle) under specific efficiency constraints (e.g., spending as little money and time as possible) — behaviors which were thought to be affected by frontal lobe damage [154]. Patients with vmPFC damage showed more errors on the MET and fewer overall completions of the task compared to healthy controls [155]. Likewise, Patient S.M., who had exclusive, bilateral damage to her amygdalae as a result of a congenital condition, had shown impairments on several laboratory tests of fear expression [156]. Based on this evidence, S.M. was taken through several real-world settings known to induce fear in normal subjects, such as a haunted house and a pet shop to handle live snakes and spiders [157]. As predicted, though S.M. expressed feelings of arousal, she did not display the typical ‘fear’ or defensive responses characteristic of normal individuals. These experiments allow a greater appreciation of the function of brain regions in the real world that cannot be easily captured in laboratory settings, and help refine an understanding of what parameters are essential to include in structured experimental tasks.
Tests using naturalistic stimuli or settings have also been developed to characterize deficits in higher-order processes following lesions in NHPs. While a manual test apparatus found no deficit in spatial memory in macaques with hippocampal lesions [158], an analagous foraging task involving navigation revealed this region was essential for memory of spatial [159]. Similar to the test of S.M., unconditioned behavioral responses to common predators such snakes and spiders have been tested in NHPs reared in the laboratory [160]. Remarkably, amygdalectomized NHPs show a fascination with snake stimuli that is reminiscent of the behaviors observed in patient S.M. [161]. In an yet more dramatic demonstration of the essential role of the amydala for survival, an early study released six NHPs with amygdala lesions that into the wild. Four of these animals failed to show appropriate social behaviors, were rejected from social groups, and eventually died as a result [162]. Less constrained tests in such cases may be more sensitive in detecting behavioral deficits and uncovering brain-behavior relationships than more structured tasks.
New and emerging technologies hold promise for advancing naturalistic experiments and generating rich behavioral data both inside and outside the laboratory. Virtual reality (VR) systems can be used to parametrize semi-naturalistic experiments by simulating real-world settings [163, 164]. For example, the MET task described above was recently adapted for a VR environment [165] and has been used to test stroke patients [166], patients with Parkinson’s Disease [167], and patients with obsessive-compulsive disorder [168] and could therefore be used to replicate and build on the findings previously described in vmPFC lesion patients and other lesion groups. Researchers have already started employing VR tasks in studies of NHPs to study spatial memory and navigation, specifically as it relates to foraging behaviors [169]. In addition to VR, smartphones and wearable biosensors (and implantable biosensors, in the case of NHPs) are providing new means of studying behavior, either through experience sampling [170] or measuring movement and physiology [171–173]. These technologies have been used minimally in NHPs and have not yet been applied to test human patients [133, 137] but may be especially useful for studies of mind-wandering, mood, sleep, memory, and decision-making.
Concluding Remarks
In this review, we have described the inferential strengths and weaknesses of the lesion method and contextualized this approach in the larger methodological toolkit available to neuroscience researchers. As with all neuroscientific methods, lesion studies have certain limitations and opportunities for improvement (see “Outstanding Questions” box). As neuroscientists, our primary goal remains building and testing useful models of function that can account for what we can learn through all available methods. We believe that this goal is better served by triangulating evidence from termination, manipulation, and correlational methods to provide convergent, complementary, or divergent evidence for theories of brain function (Figure 3) [174]. Models that can accommodate data from diverse methods should be preferred, as they are robust to different forms of evidence and supported by multiple lines of inquiry [175]. Any model that is based on data solely from one method, even across multiple experiments, is less likely to capture the contributions of a region or network given the complicated spatiotemporal dynamics of brain function and the inferential limitations of each approach. The future challenges of neuroscience will demand a “divide and conquer” solution where models are tested with multiple approaches simultaneously. Successfully implementing this goal will depend on researchers who are focused on different methods and model systems working together towards convergent hypothesis testing. We anticipate that this review will facilitate that exchange by providing a contemporary guide on what lesion studies contribute to neuroscience research.
Outstanding questions for future research.
What is the extent of neuronal plasticity after damage? Is plasticity equivalent between brain areas or are some fundamentally more ‘plastic’ than others?
Genetic techniques have predominantly been used to create tools that can manipulate neuronal function. How might these tools also be used to test the effects of termination of function within specific cell types or selectively lesion circuits with greater specificity than currently available methods?
How might we use emerging models of brain networks to generate hypotheses for lesion results and test cognitive neuroscience models?
Lesions in human subjects typically affect both gray and white matter; however, the effects of this damage are usually examined separately depending on the study question. How can we best test the effects of a lesion on behavior within a single statistical model that appropriately attributes variance to damage within regions or their connections?
How can we better compare experiments across laboratories? How can we achieve a stronger track record of replicating work across multiple research groups working with different sets of subjects?
What kind of meta-analytic tools can we use to synthesize published data across lesion studies in humans and NHP? For example, could comparative anatomy be used to place these data into a common framework that would allow more direct comparison of findings?
In most cases, the judgment of a single rater remains the gold standard in registering lesions to a common brain space. Automated tools for lesion analysis have been showing more promise but remain impractical in many cases (e.g., when research-grade MRI scans or images without major distortions or artifacts are not available). Therefore, can we develop better tools for segmenting and registering the extent of lesion damage in humans and NHP?
How can we better study the rich behavior of lesion subjects outside of constrained laboratory settings? How might these data be used to inform development of neuropsychological tests or be used in conjunction with these tests in clinical assessments?
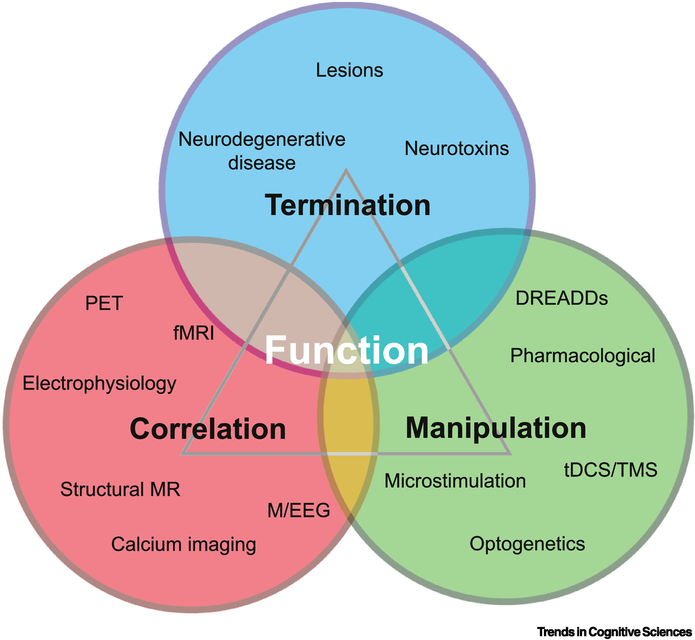
Figure 3: Triangulation of neuroscientific approaches for studying brain function.
Popular neuroscientific methods are sorted by the type of information they provide about brain function. Termination methods involve irreversible changes in brain function through the destruction of some brain region or pathway (via a lesion), neurons with particular characteristics (via a neurotoxin such as MPTP), or vulnerable systems (in neurodegenerative diseases such as Huntington’s disease). Manipulation methods include perturbation techniques that reversibly change brain activity (e.g., optogenetics). Correlation methods include techniques for measuring brain activity and testing the association between this activity and different behaviors and task performance (e.g., task-related fMRI). Triangulating evidence from each of these methods can build a better understanding of brain functions. Abbreviations: DREADD, designer receptor exclusively activated by designer drugs; M/EEG, magnetoencephalography/electroencephalography; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; (f)MRI, (functional) magnetic resonance imaging; PET, positron emission tomography; tDCS, transcranial direct current stimulation; TMS, transcranial magnetic stimulation.
Highlights/Trends Box.
Lesion studies have been fundamental to many core theories in cognitive and behavioral neuroscience.
Lesion work in human and nonhuman primate lesion studies has unique inferential strengths that are distinct from temporary manipulations or correlative measures of neural activity.
New methodological developments are underway that are expanding the range of questions that can be tested in studies of subjects with brain lesions.
Lesion studies form a critical bridge between basic science and behavior in the clinic and real-world settings.
Testing theories with multiple lines of evidence using different approaches, including lesion studies, manipulations of neural activity, and correlations with neural activity, will be essential to the future of neuroscience.
Acknowledgements
We thank Dr Sébastien Tremblay for providing the original impetus for this review and for helpful insights and comments. We also thank Drs Vincent Costa and Linda Yu for constructive comments and suggestions on drafts of this manuscript. This work was supported by funding from the National Institute for Mental Health (NIMH, grants ZIA MH00288712 and F32 MH116592-01A1), the Canadian Institutes of Health Research (CIHR, grants FDN-143212 and PJT 159554), the Natural Sciences and Engineering Research Council of Canada (NSERC, RGPIN 06-066, RGPIN-2019-05176), and the Canada First Research Excellence Fund (Healthy Brains for Healthy Lives).
Glossary
- Aspiration lesion
A lesion made through use of a vacuum pressure (suction) or cautery to physically remove brain tissue within a pre-specified ROI, affecting both gray and underlying white matter
- Cross-disconnection
A technique used to disconnect two or more brain regions in animal models. Unilateral lesions to two target regions are made in opposite hemispheres. As a consequence, these regions can no longer communicate with each other in the same hemisphere, leaving unilaterally intact functioning homologues; see Figure 3
3
- Excitotoxic lesion
A lesion made through the injection of excitotoxic pharmacological agents (e.g., ibotenic acid, quinolinic acid), selectively affecting cell bodies within a pre-specified ROI while sparing the underlying white matter fibers of passage
- Fibers of passage
White matter tracts that do not originate or terminate in a particular brain region but that pass nearby
- Focal lesion
Brain damage that is constrained to a particular region. The focal aspect of such a lesion does not necessarily reflect its volume or restriction to a particular anatomical or functional definition, rather it distinguishes this type of damage from those caused by processes with less precise localization (e.g. non-penetrating traumatic brain injury)
- Lesion-behavior mapping or lesion-symptom mapping
A class of techniques that formally test the association between a lesion and behavioral performance in some task. These include methods for testing the association between behavioral changes and damage within voxels (voxel-based lesion behavior mapping), tracts (tract or connectome-based lesion behavior mapping), functional connections or multi-voxel patterns (multivariate voxel-based lesion behavior mapping); see Figure 2
2
- NHPs
Nonhuman primates. In most contemporary neuroscience experiments, rhesus macaques (Macaca mulatta) or the common marmoset (Callithrix jacchus)
- Redundancy
A function that depends on multiple regions or networks with similar or partially overlapping roles
- Triangulation
Testing the predictions of a hypothesis using two or more different methods. Can provide confirmatory, complementary or divergent evidence that may be used to support, refine or reject the underlying theory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Broca P (1861) Remarques sur le siège de la faculté du langage articulé, suivies d’une observation d’aphémie (perte de la parole). Bulletin et Memoires de la Societe anatomique de Paris 6, 330–357. [Google Scholar]
- 2.Wernicke C (1874) Der aphasische Symptomencomplex: eine psychologische Studie auf anatomischer Basis., M. Crohn und Weigert. [Google Scholar]
- 3.Scoville WB and Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20 (1), 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milner B (1962) Les troubles de la memoire accompagnant des lesions hippocampiques bilaterales In Physiologie de l’hippocampe, pp. 257–272, Centre National de la Recherche Scientifique. [Google Scholar]
- 5.Mishkin M and Ungerleider LG (1982) Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res 6 (1), 57–77. [DOI] [PubMed] [Google Scholar]
- 6.Ungerleider LG and Mishkin M (1982) Two cortical visual systems In Analysis of visual behavior (Ingle DJ et al. eds), pp. 549–586, MIT Press. [Google Scholar]
- 7.McClelland JL et al. (1995) Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102 (3), 419–457. [DOI] [PubMed] [Google Scholar]
- 8.Desimone R and Duncan J (1995) Neural mechanisms of selective visual attention. Annu Rev Neurosci 18, 193–222. [DOI] [PubMed] [Google Scholar]
- 9.Eichenbaum H et al. (1999) The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron 23 (2), 209–26. [DOI] [PubMed] [Google Scholar]
- 10.Cisek P (2007) Cortical mechanisms of action selection: the affordance competition hypothesis. Philos Trans R Soc Lond B Biol Sci 362 (1485), 1585–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foerde K et al. (2013) A role for the medial temporal lobe in feedback-driven learning: evidence from amnesia. J Neurosci 33 (13), 5698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walton ME et al. (2010) Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron 65 (6), 927–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheth SA et al. (2012) Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 488 (7410), 218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansouri FA et al. (2015) Behavioral consequences of selective damage to frontal pole and posterior cingulate cortices. Proc Natl Acad Sci U S A 112 (29), E3940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badre D et al. (2009) Hierarchical cognitive control deficits following damage to the human frontal lobe. Nat Neurosci 12 (4), 515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrides M (2008) Selection between competing responses based on conditional rules In Neuroscience of Rule-Governed Behavior (Bunge SA and Wallis JD eds), pp. 3–22, Oxford University Press. [Google Scholar]
- 17.Shamay-Tsoory SG et al. (2009) Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 132 (Pt 3), 617–27. [DOI] [PubMed] [Google Scholar]
- 18.Dal Monte O et al. (2015) Amygdala lesions in rhesus macaques decrease attention to threat. Nat Commun 6, 10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullally SL et al. (2012) Attenuated boundary extension produces a paradoxical memory advantage in amnesic patients. Curr Biol 22 (4), 261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hebscher M et al. (2016) Memory, Decision-Making, and the Ventromedial Prefrontal Cortex (vmPFC): The Roles of Subcallosal and Posterior Orbitofrontal Cortices in Monitoring and Control Processes. Cereb Cortex 26 (12), 4590–4601. [DOI] [PubMed] [Google Scholar]
- 21.Petrides M (2013) The mid-dorsolateral prefronto-parietal network and the epoptic process In Principles of Frontal Lobe Function (2nd edn) (Stuss DT and Knight RT eds), pp. 79–89, Oxford University Press. [Google Scholar]
- 22.Meunier M et al. (1993) Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci 13 (12), 5418–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wise SP (2008) Forward frontal fields: phylogeny and fundamental function. Trends Neurosci 31 (12), 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandya D et al. (2015) Cerebral cortex: architecture, connections, and the dual origin concept, Oxford University Press. [Google Scholar]
- 25.Pandya DN and Kuypers HG (1969) Cortico-cortical connections in the rhesus monkey. Brain Res 13 (1), 13–36. [DOI] [PubMed] [Google Scholar]
- 26.Haber SN and Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35 (1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logothetis NK (2008) What we can do and what we cannot do with fMRI. Nature 453 (7197), 869–78. [DOI] [PubMed] [Google Scholar]
- 28.Rudebeck PH et al. (2013) Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci 16 (8), 1140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudebeck PH et al. (2017) Specialized Representations of Value in the Orbital and Ventrolateral Prefrontal Cortex: Desirability versus Availability of Outcomes. Neuron 95 (5), 1208–1220 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fellows LK and Farah MJ (2005) Is anterior cingulate cortex necessary for cognitive control? Brain 128 (Pt 4), 788–96. [DOI] [PubMed] [Google Scholar]
- 31.Modirrousta M and Fellows LK (2008) Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J Neurosci 28 (51), 14000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reber J et al. (2017) Selective impairment of goal-directed decision-making following lesions to the human ventromedial prefrontal cortex. Brain 140 (6), 1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaidya AR and Fellows LK (2015) Testing necessary regional frontal contributions to value assessment and fixation-based updating. Nature Communications 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basile BM and Hampton RR (2019) Nonnavigational spatial memory performance is unaffected by hippocampal damage in monkeys. Hippocampus 29 (2), 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wieloch T and Nikolich K (2006) Mechanisms of neural plasticity following brain injury. Curr Opin Neurobiol 16 (3), 258–64. [DOI] [PubMed] [Google Scholar]
- 36.Cowey A (2010) The blindsight saga. Exp Brain Res 200 (1), 3–24. [DOI] [PubMed] [Google Scholar]
- 37.Petrides M (1995) Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. J Neurosci 15 (1 Pt 1), 359–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milner B et al. (1968) Further Analysis of Hippocampal Amnesic Syndrome - 14-Year Follow-up Study of Hm. Neuropsychologia 6 (3), 215–&. [Google Scholar]
- 39.Kennedy DP and Adolphs R (2010) Impaired fixation to eyes following amygdala damage arises from abnormal bottom-up attention. Neuropsychologia 48 (12), 3392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider B and Koenigs M (2017) Human lesion studies of ventromedial prefrontal cortex. Neuropsychologia 107, 84–93. [DOI] [PubMed] [Google Scholar]
- 41.Corkin S (2013) Permanent Present Tense, Basic Books. [Google Scholar]
- 42.Rudebeck PH and Murray EA (2011) Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J Neurosci 31 (29), 10569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baxter MG et al. (2000) Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci 20 (11), 4311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izquierdo A and Murray EA (2004) Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J Neurophysiol 91 (5), 2023–39. [DOI] [PubMed] [Google Scholar]
- 45.Leonard BW et al. (1995) Transient memory impairment in monkeys with bilateral lesions of the entorhinal cortex. J Neurosci 15 (8), 5637–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki WA et al. (1993) Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. J Neurosci 13 (6), 2430–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corbetta M et al. (2005) Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci 8 (11), 1603–10. [DOI] [PubMed] [Google Scholar]
- 48.Jorgensen HS et al. (1995) Outcome and time course of recovery in stroke. Part I: Outcome. The Copenhagen Stroke Study. Arch Phys Med Rehabil 76 (5), 399–405. [DOI] [PubMed] [Google Scholar]
- 49.Verheyden G et al. (2008) Time course of trunk, arm, leg, and functional recovery after ischemic stroke. Neurorehabil Neural Repair 22 (2), 173–9. [DOI] [PubMed] [Google Scholar]
- 50.Esquenazi Y et al. (2017) Critical Care Management of Cerebral Edema in Brain Tumors. J Intensive Care Med 32 (1), 15–24. [DOI] [PubMed] [Google Scholar]
- 51.Desmurget M et al. (2007) Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain 130 (Pt 4), 898–914. [DOI] [PubMed] [Google Scholar]
- 52.Karnath HO and Steinbach JP (2011) Do brain tumours allow valid conclusions on the localisation of human brain functions?--Objections. Cortex 47 (8), 1004–6. [DOI] [PubMed] [Google Scholar]
- 53.Duffau H (2011) Do brain tumours allow valid conclusions on the localisation of human brain functions? Cortex 47 (8), 1016–7. [DOI] [PubMed] [Google Scholar]
- 54.Shallice T et al. (2010) Right posterior cortical functions in a tumour patient series. Cortex 46 (9), 1178–88. [DOI] [PubMed] [Google Scholar]
- 55.Finger S et al. (1973) Brain damage and behavioral recovery: serial lesion phenomena. Brain Res 63, 1–18. [DOI] [PubMed] [Google Scholar]
- 56.Cipolotti L et al. (2015) The impact of different aetiologies on the cognitive performance of frontal patients. Neuropsychologia 68, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raznahan A et al. (2011) Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron 72 (5), 873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bliss-Moreau E et al. (2011) Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behav Neurosci 125 (6), 848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bliss-Moreau E et al. (2017) The effects of neonatal amygdala or hippocampus lesions on adult social behavior. Behav Brain Res 322 (Pt A), 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson SW et al. (1999) Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci 2 (11), 1032–7. [DOI] [PubMed] [Google Scholar]
- 61.Galvan A et al. (2017) Nonhuman Primate Optogenetics: Recent Advances and Future Directions. J Neurosci 37 (45), 10894–10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raper J et al. (2017) Metabolism and Distribution of Clozapine-N-oxide: Implications for Nonhuman Primate Chemogenetics. ACS Chem Neurosci 8 (7), 1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gerits A and Vanduffel W (2013) Optogenetics in primates: a shining future? Trends Genet 29 (7), 403–11. [DOI] [PubMed] [Google Scholar]
- 64.Fetsch CR et al. (2018) Focal optogenetic suppression in macaque area MT biases direction discrimination and decision confidence, but only transiently. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otchy TM et al. (2015) Acute off-target effects of neural circuit manipulations. Nature 528 (7582), 358–63. [DOI] [PubMed] [Google Scholar]
- 66.Aronov D et al. (2008) A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science 320 (5876), 630–4. [DOI] [PubMed] [Google Scholar]
- 67.Hillis AE (2014) Inability to empathize: brain lesions that disrupt sharing and understanding another’s emotions. Brain 137 (Pt 4), 981–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruff CC et al. (2009) Combining TMS and fMRI: from ‘virtual lesions’ to functional-network accounts of cognition. Cortex 45 (9), 1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ilmoniemi RJ et al. (1997) Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport 8 (16), 3537–40. [DOI] [PubMed] [Google Scholar]
- 70.Paus T et al. (1997) Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci 17 (9), 3178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tolias AS et al. (2005) Mapping cortical activity elicited with electrical microstimulation using FMRI in the macaque. Neuron 48 (6), 901–11. [DOI] [PubMed] [Google Scholar]
- 72.Grayson DS et al. (2016) The Rhesus Monkey Connectome Predicts Disrupted Functional Networks Resulting from Pharmacogenetic Inactivation of the Amygdala. Neuron 91 (2), 453–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jazayeri M and Afraz A (2017) Navigating the Neural Space in Search of the Neural Code. Neuron 93 (5), 1003–1014. [DOI] [PubMed] [Google Scholar]
- 74.Haxby JV et al. (2001) Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293 (5539), 2425–30. [DOI] [PubMed] [Google Scholar]
- 75.Biswal B et al. (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34 (4), 537–41. [DOI] [PubMed] [Google Scholar]
- 76.O’Doherty JP et al. (2007) Model-based fMRI and its application to reward learning and decision making. Ann N Y Acad Sci 1104, 35–53. [DOI] [PubMed] [Google Scholar]
- 77.Kriegeskorte N et al. (2006) Information-based functional brain mapping. Proc Natl Acad Sci U S A 103 (10), 3863–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moser EI et al. (2008) Place cells, grid cells, and the brain’s spatial representation system. Annu Rev Neurosci 31, 69–89. [DOI] [PubMed] [Google Scholar]
- 79.Pujara MS et al. (2016) Ventromedial Prefrontal Cortex Damage Is Associated with Decreased Ventral Striatum Volume and Response to Reward. J Neurosci 36 (18), 5047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Motzkin JC et al. (2015) Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry 77 (3), 276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kam JWY et al. (2018) Lateral prefrontal cortex lesion impairs regulation of internally and externally directed attention. Neuroimage 175, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hadj-Bouziane F et al. (2012) Amygdala lesions disrupt modulation of functional MRI activity evoked by facial expression in the monkey inferior temporal cortex. Proc Natl Acad Sci U S A 109 (52), E3640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmid MC et al. (2009) Visually driven activation in macaque areas V2 and V3 without input from the primary visual cortex. PLoS One 4 (5), e5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pinsk MA et al. (2005) Methods for functional magnetic resonance imaging in normal and lesioned behaving monkeys. J Neurosci Methods 143 (2), 179–95. [DOI] [PubMed] [Google Scholar]
- 85.Fox AS et al. (2010) Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci 30 (20), 7023–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rudebeck PH et al. (2013) Effects of amygdala lesions on reward-value coding in orbital and medial prefrontal cortex. Neuron 80 (6), 1519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rudebeck PH et al. (2017) Amygdala Contributions to Stimulus-Reward Encoding in the Macaque Medial and Orbital Frontal Cortex during Learning. J Neurosci 37 (8), 2186–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Padberg J et al. (2010) Lesions in posterior parietal area 5 in monkeys result in rapid behavioral and cortical plasticity. J Neurosci 30 (39), 12918–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petrides M (1985) Deficits in non-spatial conditional associative learning after periarcuate lesions in the monkey. Behav Brain Res 16 (2–3), 95–101. [DOI] [PubMed] [Google Scholar]
- 90.Petrides M (1991) Functional specialization within the dorsolateral frontal cortex for serial order memory. Proc Biol Sci 246 (1317), 299–306. [DOI] [PubMed] [Google Scholar]
- 91.Petrides M et al. (1993) Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci U S A 90 (3), 873–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amiez C et al. (2012) Response selection versus feedback analysis in conditional visuomotor learning. Neuroimage 59 (4), 3723–35. [DOI] [PubMed] [Google Scholar]
- 93.Bussey TJ et al. (2003) Impairments in visual discrimination after perirhinal cortex lesions: testing ‘declarative’ vs. ‘perceptual-mnemonic’ views of perirhinal cortex function. Eur J Neurosci 17 (3), 649–60. [DOI] [PubMed] [Google Scholar]
- 94.Bussey TJ et al. (2002) Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur J Neurosci 15 (2), 365–74. [DOI] [PubMed] [Google Scholar]
- 95.Martin CB et al. (2018) Integrative and distinctive coding of visual and conceptual object features in the ventral visual stream. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davies RR et al. (2004) The human perirhinal cortex and semantic memory. Eur J Neurosci 20 (9), 2441–6. [DOI] [PubMed] [Google Scholar]
- 97.Sutterer MJ and Tranel D (2017) Neuropsychology and cognitive neuroscience in the fMRI era: A recapitulation of localizationist and connectionist views. Neuropsychology 31 (8), 972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Geschwind N (1965) Disconnexion syndromes in animals and man. I. Brain 88 (2), 237–94. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y et al. (2014) Multivariate lesion-symptom mapping using support vector regression. Hum Brain Mapp 35 (12), 5861–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bates E et al. (2003) Voxel-based lesion-symptom mapping. Nat Neurosci 6 (5), 448–50. [DOI] [PubMed] [Google Scholar]
- 101.Kimberg DY et al. (2007) Power in Voxel-based lesion-symptom mapping. J Cogn Neurosci 19 (7), 1067–80. [DOI] [PubMed] [Google Scholar]
- 102.Rorden C et al. (2007) Improving lesion-symptom mapping. J Cogn Neurosci 19 (7), 1081–8. [DOI] [PubMed] [Google Scholar]
- 103.Pustina D et al. (2018) Improved accuracy of lesion to symptom mapping with multivariate sparse canonical correlations. Neuropsychologia 115, 154–166. [DOI] [PubMed] [Google Scholar]
- 104.Smith DV et al. (2013) Decoding the anatomical network of spatial attention. Proc Natl Acad Sci U S A 110 (4), 1518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thiebaut de Schotten M et al. (2014) Damage to white matter pathways in subacute and chronic spatial neglect: a group study and 2 single-case studies with complete virtual “in vivo” tractography dissection. Cereb Cortex 24 (3), 691–706. [DOI] [PubMed] [Google Scholar]
- 106.Gleichgerrcht E et al. (2017) Connectome-based lesion-symptom mapping (CLSM): A novel approach to map neurological function. Neuroimage Clin 16, 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Assaf Y and Pasternak O (2008) Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 34 (1), 51–61. [DOI] [PubMed] [Google Scholar]
- 108.Dauguet J et al. (2007) Comparison of fiber tracts derived from in-vivo DTI tractography with 3D histological neural tract tracer reconstruction on a macaque brain. Neuroimage 37 (2), 530–8. [DOI] [PubMed] [Google Scholar]
- 109.Thiebaut de Schotten M et al. (2011) Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage 54 (1), 49–59. [DOI] [PubMed] [Google Scholar]
- 110.Carter AR et al. (2010) Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol 67 (3), 365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boes AD et al. (2015) Network localization of neurological symptoms from focal brain lesions. Brain 138 (Pt 10), 3061–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fox MD (2018) Mapping Symptoms to Brain Networks with the Human Connectome. N Engl J Med 379 (23), 2237–2245. [DOI] [PubMed] [Google Scholar]
- 113.Rossini PM et al. (2004) Does cerebrovascular disease affect the coupling between neuronal activity and local haemodynamics? Brain 127 (Pt 1), 99–110. [DOI] [PubMed] [Google Scholar]
- 114.Motzkin JC et al. (2014) Ventromedial prefrontal cortex lesions alter neural and physiological correlates of anticipation. J Neurosci 34 (31), 10430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murray EA and Rudebeck PH (2018) Specializations for reward-guided decision-making in the primate ventral prefrontal cortex. Nat Rev Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rudebeck PH and Murray EA (2011) Balkanizing the primate orbitofrontal cortex: distinct subregions for comparing and contrasting values. Ann N Y Acad Sci 1239, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Petrides M and Milner B (1982) Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia 20 (3), 249–62. [DOI] [PubMed] [Google Scholar]
- 118.Petrides M (1985) Deficits on conditional associative-learning tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia 23 (5), 601–14. [DOI] [PubMed] [Google Scholar]
- 119.Noonan MP et al. (2010) Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci U S A 107 (47), 20547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kazama A and Bachevalier J (2009) Selective aspiration or neurotoxic lesions of orbital frontal areas 11 and 13 spared monkeys’ performance on the object discrimination reversal task. J Neurosci 29 (9), 2794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shiba Y et al. (2014) Lesions of either anterior orbitofrontal cortex or ventrolateral prefrontal cortex in marmoset monkeys heighten innate fear and attenuate active coping behaviors to predator threat. Front Syst Neurosci 8, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pujara MS et al. (2019) Heightened defensive responses following subtotal lesions of macaque orbitofrontal cortex. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rudebeck PH et al. (2006) A role for the macaque anterior cingulate gyrus in social valuation. Science 313 (5791), 1310–2. [DOI] [PubMed] [Google Scholar]
- 124.Noonan MP et al. (2010) Does the medial orbitofrontal cortex have a role in social valuation? Eur J Neurosci 31 (12), 2341–51. [DOI] [PubMed] [Google Scholar]
- 125.Rao VR et al. (2018) Direct Electrical Stimulation of Lateral Orbitofrontal Cortex Acutely Improves Mood in Individuals with Symptoms of Depression. Curr Biol 28 (24), 3893–3902 e4. [DOI] [PubMed] [Google Scholar]
- 126.Izquierdo A and Murray EA (2010) Functional interaction of medial mediodorsal thalamic nucleus but not nucleus accumbens with amygdala and orbital prefrontal cortex is essential for adaptive response selection after reinforcer devaluation. J Neurosci 30 (2), 661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fiuzat EC et al. (2017) The Role of Orbitofrontal-Amygdala Interactions in Updating Action-Outcome Valuations in Macaques. J Neurosci 37 (9), 2463–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Clark AM et al. (2013) Interaction between orbital prefrontal and rhinal cortex is required for normal estimates of expected value. J Neurosci 33 (5), 1833–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Browning PG et al. (2015) Evidence for Mediodorsal Thalamus and Prefrontal Cortex Interactions during Cognition in Macaques. Cereb Cortex 25 (11), 4519–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Browning PG et al. (2007) Frontal-temporal disconnection abolishes object discrimination learning set in macaque monkeys. Cereb Cortex 17 (4), 859–64. [DOI] [PubMed] [Google Scholar]
- 131.Bussey TJ et al. (2002) Interaction of ventral and orbital prefrontal cortex with inferotemporal cortex in conditional visuomotor learning. Behav Neurosci 116 (4), 703–15. [PubMed] [Google Scholar]
- 132.Wilson CR et al. (2008) Addition of fornix transection to frontal-temporal disconnection increases the impairment in object-in-place memory in macaque monkeys. Eur J Neurosci 27 (7), 1814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wallis CU et al. (2017) Opposing roles of primate areas 25 and 32 and their putative rodent homologs in the regulation of negative emotion. Proc Natl Acad Sci U S A 114 (20), E4075–E4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kalin NH et al. (2007) Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry 62 (10), 1134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lezak M et al. (2012) Neuropsychological Assessment, Oxford University Press. [Google Scholar]
- 136.Kringelbach ML et al. (2007) Translational principles of deep brain stimulation. Nat Rev Neurosci 8 (8), 623–35. [DOI] [PubMed] [Google Scholar]
- 137.Alexander L et al. (2018) Fractionating Blunted Reward Processing Characteristic of Anhedonia by Over-Activating Primate Subgenual Anterior Cingulate Cortex. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rubinov M and Bullmore E (2013) Fledgling pathoconnectomics of psychiatric disorders. Trends Cogn Sci 17 (12), 641–7. [DOI] [PubMed] [Google Scholar]
- 139.Buckholtz JW and Meyer-Lindenberg A (2012) Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron 74 (6), 990–1004. [DOI] [PubMed] [Google Scholar]
- 140.Barch DM (2017) Resting-State Functional Connectivity in the Human Connectome Project: Current Status and Relevance to Understanding Psychopathology. Harv Rev Psychiatry 25 (5), 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Insel T et al. (2010) Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 167 (7), 748–51. [DOI] [PubMed] [Google Scholar]
- 142.Taubert J et al. (2018) Amygdala lesions eliminate viewing preferences for faces in rhesus monkeys. Proc Natl Acad Sci U S A 115 (31), 8043–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hennessey T et al. (2018) RDoC-based categorization of amygdala functions and its implications in autism. Neurosci Biobehav Rev 90, 115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Losh M et al. (2009) Neuropsychological profile of autism and the broad autism phenotype. Arch Gen Psychiatry 66 (5), 518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huys QJ et al. (2016) Computational psychiatry as a bridge from neuroscience to clinical applications. Nat Neurosci 19 (3), 404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Montague PR et al. (2012) Computational psychiatry. Trends Cogn Sci 16 (1), 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schonberg T et al. (2011) Mind the gap: bridging economic and naturalistic risk-taking with cognitive neuroscience. Trends Cogn Sci 15 (1), 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moscovitch M and Melo B (1997) Strategic retrieval and the frontal lobes: evidence from confabulation and amnesia. Neuropsychologia 35 (7), 1017–34. [DOI] [PubMed] [Google Scholar]
- 149.Chatterjee A (2004) The neuropsychology of visual artistic production. Neuropsychologia 42 (11), 1568–83. [DOI] [PubMed] [Google Scholar]
- 150.Peters SL et al. (2017) The Ventromedial Frontal Lobe Contributes to Forming Effective Solutions to Real-world Problems. J Cogn Neurosci 29 (6), 991–1001. [DOI] [PubMed] [Google Scholar]
- 151.Hassabis D et al. (2007) Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci U S A 104 (5), 1726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.De Luca F et al. (2018) Boundary extension is attenuated in patients with ventromedial prefrontal cortex damage. Cortex 108, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Burgess PW et al. (2006) The case for the development and use of “ecologically valid” measures of executive function in experimental and clinical neuropsychology. J Int Neuropsychol Soc 12 (2), 194–209. [DOI] [PubMed] [Google Scholar]
- 154.Shallice T and Burgess PW (1991) Deficits in strategy application following frontal lobe damage in man. Brain 114 ( Pt 2), 727–41. [DOI] [PubMed] [Google Scholar]
- 155.Tranel D et al. (2007) Impaired behavior on real-world tasks following damage to the ventromedial prefrontal cortex. J Clin Exp Neuropsychol 29 (3), 319–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Adolphs R et al. (1995) Fear and the human amygdala. J Neurosci 15 (9), 5879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Feinstein JS et al. (2011) The human amygdala and the induction and experience of fear. Curr Biol 21 (1), 34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Murray EA and Mishkin M (1998) Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci 18 (16), 6568–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hampton RR et al. (2004) Method for making selective lesions of the hippocampus in macaque monkeys using NMDA and a longitudinal surgical approach. Hippocampus 14 (1), 9–18. [DOI] [PubMed] [Google Scholar]
- 160.Nelson EE et al. (2003) Individual differences in the responses of naive rhesus monkeys to snakes. Emotion 3 (1), 3–11. [DOI] [PubMed] [Google Scholar]
- 161.Chudasama Y et al. (2009) Distinct contributions of the amygdala and hippocampus to fear expression. Eur J Neurosci 30 (12), 2327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dicks D et al. (1969) Uncus and amiygdala lesions: effects on social behavior in the free-ranging rhesus monkey. Science 165 (3888), 69–71. [DOI] [PubMed] [Google Scholar]
- 163.Krakauer JW et al. (2017) Neuroscience Needs Behavior: Correcting a Reductionist Bias. Neuron 93 (3), 480–490. [DOI] [PubMed] [Google Scholar]
- 164.Dombeck DA and Reiser MB (2012) Real neuroscience in virtual worlds. Curr Opin Neurobiol 22 (1), 3–10. [DOI] [PubMed] [Google Scholar]
- 165.Rand D et al. (2009) Training multitasking in a virtual supermarket: a novel intervention after stroke. Am J Occup Ther 63 (5), 535–42. [DOI] [PubMed] [Google Scholar]
- 166.Raspelli S et al. (2011) Validation of a Neuro Virtual Reality-based version of the Multiple Errands Test for the assessment of executive functions. Stud Health Technol Inform 167, 92–7. [PubMed] [Google Scholar]
- 167.Cipresso P et al. (2014) Virtual multiple errands test (VMET): a virtual reality-based tool to detect early executive functions deficit in Parkinson’s disease. Front Behav Neurosci 8, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.La Paglia F et al. (2012) Assessment of executive functions in patients with obsessive compulsive disorder by NeuroVR. Stud Health Technol Inform 181, 98–102. [PubMed] [Google Scholar]
- 169.Dolins FL et al. (2017) Technology advancing the study of animal cognition: using virtual reality to present virtually simulated environments to investigate nonhuman primate spatial cognition. Curr Zool 63 (1), 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nielson DM et al. (2015) Human hippocampus represents space and time during retrieval of real-world memories. Proc Natl Acad Sci U S A 112 (35), 11078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wright SP et al. (2017) How consumer physical activity monitors could transform human physiology research. Am J Physiol Regul Integr Comp Physiol 312 (3), R358–R367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Powell MP et al. (2017) An engineered home environment for untethered data telemetry from nonhuman primates. J Neurosci Methods 288, 72–81. [DOI] [PubMed] [Google Scholar]
- 173.Mitz AR et al. (2017) Using pupil size and heart rate to infer affective states during behavioral neurophysiology and neuropsychology experiments. J Neurosci Methods 279, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.O’Cathain A et al. (2010) Three techniques for integrating data in mixed methods studies. BMJ 341, c4587. [DOI] [PubMed] [Google Scholar]
- 175.Munafo MR and Davey Smith G (2018) Robust research needs many lines of evidence. Nature 553 (7689), 399–401. [DOI] [PubMed] [Google Scholar]
- 176.Fellows LK (2012) Group studies in experimental neuropsychology In APA handbook of research methods in psychology, Vol 2: Research designs: Quantitative, qualitative, neuropsychological, and biological (Cooper H et al. eds), pp. 647–659, American Psychological Association. [Google Scholar]
- 177.Murray EA and Baxter MG (2006) Cognitive Neuroscience and Nonhuman Primates: Lesion Studies In Methods in mind (Senior C et al. eds), pp. 43–69, MIT Press. [Google Scholar]
- 178.Bell AH and Bultitude JH (2017) Methods matter: A primer on permanent and reversible interference techniques in animals for investigators of human neuropsychology. Neuropsychologia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.de Haan B and Karnath HO (2018) A hitchhiker’s guide to lesion-behaviour mapping. Neuropsychologia 115, 5–16. [DOI] [PubMed] [Google Scholar]
- 180.Raymont V et al. (2011) “Studying injured minds” - the Vietnam head injury study and 40 years of brain injury research. Front Neurol 2, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li W et al. (2018) Different patterns of white matter changes after successful surgery of mesial temporal lobe epilepsy. Neuroimage Clin, 101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Grober E and Sliwinski M (1991) Development and Validation of a Model for Estimating Premorbid Verbal Intelligence in the Elderly. Journal of Clinical and Experimental Neuropsychology 13 (6), 933–949. [DOI] [PubMed] [Google Scholar]
- 183.Stout JC et al. (2003) Factor analysis of the frontal systems behavior scale (FrSBe). Assessment 10 (1), 79–85. [DOI] [PubMed] [Google Scholar]
- 184.Vermeer SE et al. (2007) Silent brain infarcts: a systematic review. Lancet Neurol 6 (7), 611–9. [DOI] [PubMed] [Google Scholar]
- 185.Bezeau S and Graves R (2001) Statistical power and effect sizes of clinical neuropsychology research. J Clin Exp Neuropsychol 23 (3), 399–406. [DOI] [PubMed] [Google Scholar]
- 186.Fellows LK et al. (2008) Patient registries in cognitive neuroscience research: advantages, challenges, and practical advice. J Cogn Neurosci 20 (6), 1107–13. [DOI] [PubMed] [Google Scholar]
- 187.Teuber HL (1955) Physiological psychology. Annu Rev Psychol 6, 267–96. [DOI] [PubMed] [Google Scholar]
- 188.Chatham CH and Badre D (2019) How to test cognitive theory with fMRI In New Methods in Cognitive Psychology (Spieler D and Schumacher E eds), Routledge. [Google Scholar]
- 189.Dunn JC (2003) The elusive dissociation. Cortex 39 (1), 177–9. [DOI] [PubMed] [Google Scholar]
- 190.Gorgolewski KJ et al. (2016) The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci Data 3, 160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gorgolewski KJ et al. (2016) NeuroVault.org: A repository for sharing unthresholded statistical maps, parcellations, and atlases of the human brain. Neuroimage 124 (Pt B), 1242–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Milham MP et al. (2018) An Open Resource for Non-human Primate Imaging. Neuron 100 (1), 61–74 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Das S et al. (2011) LORIS: a web-based data management system for multi-center studies. Front Neuroinform 5, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Poldrack RA et al. (2013) Toward open sharing of task-based fMRI data: the OpenfMRI project. Front Neuroinform 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Scott A et al. (2011) COINS: An Innovative Informatics and Neuroimaging Tool Suite Built for Large Heterogeneous Datasets. Front Neuroinform 5, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kennedy DN et al. (2016) The NITRC image repository. Neuroimage 124 (Pt B), 1069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]