Abstract
Introduction:
Though cervical cytology, HPV DNA testing, and pre-invasive disease management has significantly reduced the number new diagnoses of cervical cancer, women with persistent oncogenic HPV variants are at significant risk for developing invasive cervical cancer. Early stage and locally advanced disease can be cured, but women with advanced or recurrent disease have a very poor prognosis. This underscores the need for different treatment paradigms for advanced cervical cancer, the most promising of which are novel therapeutics that target the ability of HPV to overcome host immune tolerance.
Areas Covered:
This review includes new therapies being investigated for the treatment of recurrent, metastatic or refractory cervical cancer, separated into broad categories of cellular and non-cellular based strategies.
Expert opinion:
Advanced and recurrent cervical cancer has a poor prognosis with poor response rates to chemotherapy secondary to immune tolerance to HPV, prompting investigations into the development of strategies that will disrupt the immune tolerability for treatment of a once untreatable disease. It is unknown whether it will be these strategies alone or a combination of treatment modalities that will ultimately provide the best outcomes; nevertheless, the new data is promising.
1. Introduction:
The American Cancer Society predicts that in 2017, there will be approximately 12,820 new diagnoses of cervical cancer and approximately 4,210 women will die from cervical cancer. 1 Though promisingly, the death rate from cervical cancer has significantly decreased in the last four decades secondary to effective pap smear screening and will likely decrease further as the currently FDA approved prophylactic vaccines, Gardasil, Gardasil-9, and Cervarix, are more widely utilized and accepted. For those with early stage or locally advanced cervical cancer, chemoradiation is largely curative. Nevertheless, for those patients who present with advanced, metastatic, or recurrent disease, their response rates and progression free survival curves to the current standard chemotherapy still remain dismal, despite the fact that its early stage disease counterpart is typically curable. In addition, despite this disease being now preventable in the developed world, it is still the leading cause of death in the developing nations with limited access to care. This disparity of mortality between the developed and developing nations, as well as the disparity of response between the different stages indicates the need for new strategies to overcome the persistence of human papilloma virus or HPV. Recently one of those new strategies that was awarded FDA approval in 2014, was utilizing the anti-angiogenic agent, bevacizumab, a monoclonal antibody that inhibits vascular endothelial growth factor (VEGF), added to the standard chemotherapy doublet, increasing the response rate to those who have advanced or recurrent cervical cancer to 50%, with a survival advantage of 3.7 months.2 In addition, no previous study had shown a 12-month overall survival of 30% until the addition of bevacizumab with GOG240. The success of GOG240 has prompted further investigations into the development of different targeted strategies to treat cervical cancer, and one such strategy that has been developed is to disrupt the immune tolerance that is inherent to HPV. This review highlights the new immunogenic approaches being developed, in which the immune response is based on tumor-based cells or outside agents, and the rationale behind the development of these cellular and non-cellular based strategies for the treatment of cervical cancer.
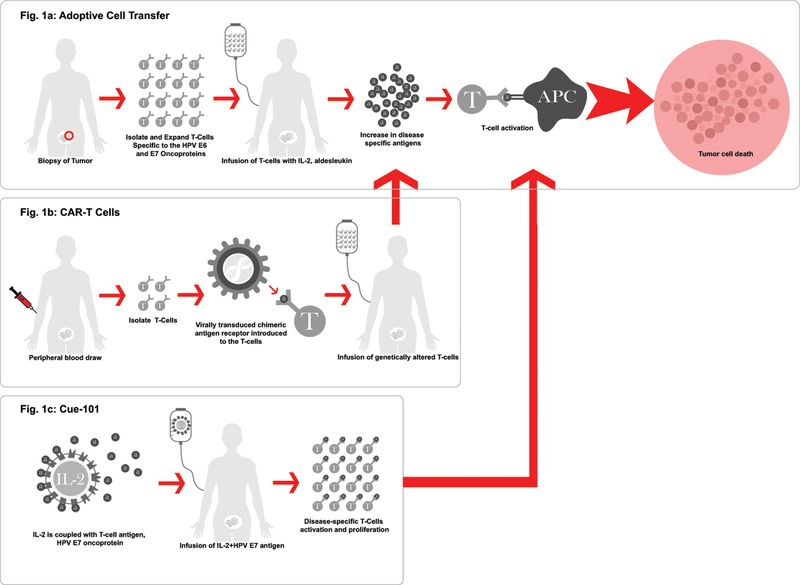
2. Cellular Based Strategies (Figure 1):
Figure 1.
Cellular-based therapies.
2.1. Adoptive T-Cell Transfer (ACT) (Figure 1a):
Adoptive immunotherapy or adoptive T-cell transfer (ACT), involving the autologous T-cell infusion, has shown a complete and durable response in metastatic melanoma and is now being investigated in those with cervical cancer. In other disease sites, preclinical vaccine studies have shown that although the vaccines have shown potential in their ability to provoke a t-cell response, the overall immune and tumor mediated response is minimal since it elicits a cascading immunogenic response of which is uncontrolled in its scale of response. ACT allows for control over the magnitude of response with the ability to select specific T-cells, expand them and then precisely target the tumor at the potential of as high as a 10-fold increase of antigen-specific T-cells.3 Therefore, though not yet proven in an epithelial cancer, the idea of tumor specific T-cell therapy for HPV-related cancers was appealing because of the ability to isolate, expand in-vitro, and administer T-cells specific to the HPV E6 and E7 oncoproteins. These oncoproteins are important targets because it is universally known that although there are approximately 100–150 different genotypes of HPV, greater than 95% of cervical cancers are associated with HPV 16, 18, 31, 33, 37, and 3; with HPV 16 and 18 alone causing upwards of 70% of the cervical cancers.4,5 Additionally, it is known that HPV E6 and E7 intracellular oncoproteins are expressed in HPV-associated cancers harboring those high-risk HPV oncogenes. E6 binds the p53 tumor suppressor protein and E7 binds the retinoblastoma leading to cellular alteration and potentially carcinoma transformation, as they are the players that give HPV to ability to evade the immunologic response and enable access to cell replication.6,7
Therefore, the ability to develop T-cells that can specifically target the carcinogenic E6 and E7 oncoproteins within the high risk HPV oncogenic subtypes should prove effective. In order to prove this theory Stevanovic et al performed a proof-of-concept, prospective trial enrolling patients with locally advanced refractory, metastatic or recurrent squamous or non-squamous cervical cancer who had received prior platinum chemotherapy or chemoradiation. The patients had a surgical excision of their tumor that was at least 1cm3, the tumor cells were expanded with interleukin-2 (IL-2) and tumor-infiltrating T-cells (TILs) were preferentially selected based on their HPV 16 E6 and E7 reactivity (HPV-TILs). Patients were first given IV cyclophosphamide 60mg/kg for the first two days followed by five days of fludarabine 25mg/m2 for the purpose of lymphocytic exhaustion. Patients then received a single dose IV infusion of HPV-TILs, as well as an IV infusion of aldesleukin, at 720,000 IU/kg/dose every eight hours to tolerance (15 doses maximum). Aldesleukin is an IL-2, which is known to have anti-tumor effect through immunostimulation. Out of the nine patients treated, three showed a response, one with a partial response for 3 months and two of which had a complete, durable responses ongoing 15 and 22 months after treatment with 1 dose of the HPV-TILs. Additionally, this therapy was well tolerated with the grade 3 and 4 events being related to the lymphocyte depletion chemotherapy doublet.8 The negative aspect of this trial is the complex nature of obtaining and expanding the HPV-TILs.9
Cellular based strategies of infusing autologous T-cells need to be further explored and expanded, but the results of the HPV-TILs trials thus far suggesting a clinical response in a disease known for its dismal response rates is promising as it clearly demonstrates the importance of the interruption of the immunogenic response in HPV-related cancers. One such trial is a phase II trial launched by Iovance () utilizing LN-145 a proprietary, patient-derived autologous TIL therapy. As a phase II trial, it is investigating the tolerability, safety and efficacy of the TILs in conjunction with IL-2 for expansion in those patients with advanced or recurrent cervical cancer. Additionally, studies are being performed on various combinations of treatment to find an effective treatment regimen for those previously thought to be untreatable, as well as for the development of predictive biomarkers to aid in detection of those patients who will respond to these novel treatments.
2.2. Chimeric antigen receptor T- cells (CAR-Ts) (Figure 1b):
As with ACT or adoptive T-cell transfer, the isolation and expansion of the patients own cellular tumor-infiltrating T-cells (TILs) can often be a complex and time intensive process and only proves to be successful in a small population of patients since it is patient specific therapy.10 This has prompted development of genetically modified T-cells, known as chimeric antigen receptors, or CAR-T cells, a gene-editing method that was just approved for the treatment of recurrent acute lymphoblastic leukemia or ALL based on the Novartis study Eliana () after it showed an astonishing 83% complete response (CR) rate (CI 95%: 71–91%) with a durable response showing negative free marrow at the 3-month interval.11 A form of ACT, but less invasive than removing tumor tissue as in the HPV-TILs process, for CAR-T cell development, the patients T-cells are isolated from the patients’ blood, the chimeric antigen receptor is then added as a tumor targeting agent, and the altered T-cells are infused back into the patient to target the tumor tissue. CARs are synthetic, small molecules that are made up of antibody that binds to specific tumor antigen domains to stimulate a T-cell response.12 CAR-T cells have been developed against a wide variety of tumor antigens across different disease sites, so it is a natural transition to engineer the T-cells retrieved from the blood to specifically target those HPV-related cancers since HPV associated cancers have the specific E6 and E7 oncoproteins to target.13
2.3. Cue-101 (Figure 1c):
Cue-101 is a therapy recently developed that is made up of a IL-2 costimulatory factor coupled with a T-cell that specifically targets the E6 oncoprotein of HPV-18. The design, not unlike adoptive t-cell transfer, is created to specifically target the HPV-specific cancers by its design, but instead of engineering and reinfusing the T-cells back into the patient, this therapy will elicit the exacted T-cell response itself with the IL-2 to maximize its efficacy. Since it only activates HPV specific T-cells it hopefully will be less toxic than traditional IL-2 therapies have been shown to be in the past. Though not in clinical trials yet, it is in the application process for the investigational new drug status by the FDA in order to initiate testing in clinical studies. Preclinical studies have shown Cue-101 to have powerful anti-tumor activity as well as durable response as a monotherapy, as well as in combination with other immunotherapies. For this reason, it is thought that this therapy will likely be effective in combination with other immunotherapies, such as the checkpoint inhibitors.14,15
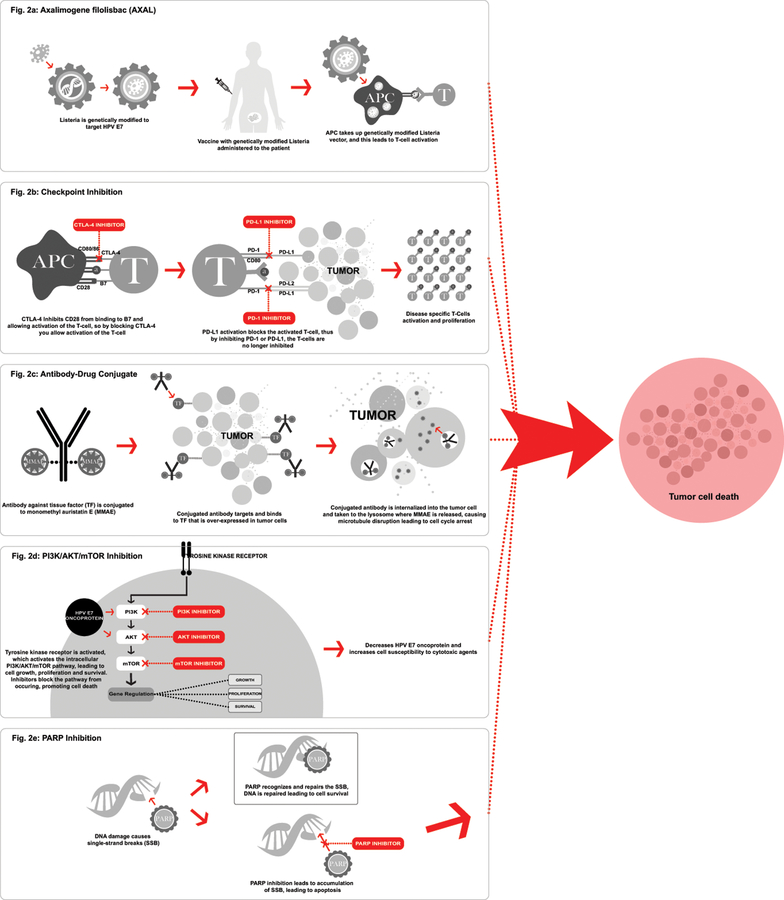
3. Non-Cellular Based Strategies (Figure 2):
Figure 2.
Non-cellular-based therapies.
3.1. Axalimogene filolisbac (AXAL) (Figure 2a):
The HPV specific oncogenes involving the E6 and E7 oncoproteins utilized in the cellular-based strategies discussed above, are correspondingly attractive intracellular targets for therapeutic vaccines as well. Axalimogene filolisbac (AXAL) is the therapeutic vaccine that utilizes a live-attenuated bacterium, Listeria monocytogenes (Lm), to directly target the HPV 16 E7 oncoprotein by delivering antigen directly into the host cell and permitting replication within the cell. Lm is a gram-positive bacterium that has the capability to escape the cellular phagosome, and thus it is able to transfect directly into the host cell, eliciting the macrophages and neutrophils for the innate immune response and the CD 4+ and CD8+ T-Cells for the adaptive immune response; an immunogenic cascade activated to overcome the immune tolerance to HPV. AXAL has shown activity against multiple high-risk HPV variants.7,16,17
In 2009, Maciag et al., exhibited the safety and tolerability of AXAL in a phase I trial, with none of the 15 patients with grade 4 toxicity, several patients with dose-limiting hypotension, and all patients experiencing a flu-like illness caused by a cytokine release that was managed symptomatically. The dose of AXAL determined from this dose escalation study was 1 ×109 CFU.18 At the 2014 American Society of Clinical Oncology (ASCO) annual meeting, Basu et al. presented data from a phase II trial performed in India that enrolled patients with previously treated recurrent or refractory cervical cancer. Patients were to either receive the AXAL vaccine for 3 doses alone or to be followed by five weeks of cisplatin chemotherapy (40mg/m2) with a primary endpoint of overall survival (OS). There was no significant difference between the two treatment arms with an OS of 8.4 months in the AXAL alone group and 8.77 months in the AXAL plus cisplatin group showing that the addition of a platinum therapy is unnecessary. Though there was no difference between the treatment arms, there was found to be a 11% response rate (RR) and 36% 12-month survival in a therapy that was found to be well tolerated indicating the promising nature of the AXAL vaccine.19 With tolerability and efficacy proven, this then prompted the development of GOG/NRG 0265, a phase II trial for those with persistent, recurrent or refractory squamous or non-squamous cervical cancer in those patients who had received at least one prior line of chemotherapy now with evidence of progression. Primary objectives were safety, tolerability, and efficacy as determined by a 12-month survival, as well as secondary objectives of progression-free survival (PFS) and overall survival (OS). Patients were to receive a total of three doses of AXAL at 1×109 CFU every 28 days. To combat the flu-like illness seen in the phase 1 trial by Maciag et al., patients were given prophylactic non-steroidal anti-inflammatory agent and each dose was followed by a 7-day course of ampicillin. Again, AXAL was shown to be well tolerated with, all patients experiencing at lease 1 adverse event (AE) with 91% of the AEs being grade 1 or 2 with the flu-like illness or hypotension and one grade 4 event of pneumonia/sepsis. Promisingly, in preliminary data, AXAL showed a 38.5% 12-month survival, 28% 18-month survival and a median progression-free survival (PFS) of 3.1 months and median overall survival (OS) of 7.7 months.16,20,21
Of note, on October 1, 2015, the FDA suspended the AXAL trials after a former participant tested positive for Listeria three years after the completion of her participation in the trial. It had later been determined that the likely source was from a colonized orthopedic implant that she received during treatment after she had been in a motor vehicle accident, not an active infection. The FDA then subsequently lifted the suspension in December of 2015.22 Additionally, there was a male patient enrolled in a clinical trial investigating use of AXAL for the treatment of a HPV-related oropharyngeal cancer, who developed a flu-like illness that developed into a pneumonia after administration of the axalimogene filolisbac infusion. Blood cultures tested positive for Listeria, with identical antibiotic resistance to AXAL, therefore the trial was terminated. All subsequent tests were negative and the patient never had a recurrence of symptoms. 23
On the heels of the successes of the phase II trials in the recurrent and metastatic cervical cancer population, AIM2CERV (), a randomized phase III trial is currently enrolling patients investigating the use of the AXAL therapy as frontline treatment. This trial will investigate AXAL as an adjuvant treatment in those patients with high-risk locally advanced cervical cancer after they have completed chemoradiation with a primary endpoint of PFS and secondary endpoint of OS. The high-risk eligibility for the patients in this trial are to capture those patients at the highest risk to have refractory or recurrent disease and they define those patients as FIGO stage I-II disease with positive pelvic nodes, FIGO stage III or IVA, or any FIGO stage with para-aortic nodes.24 (Figure 3)
Figure 3.
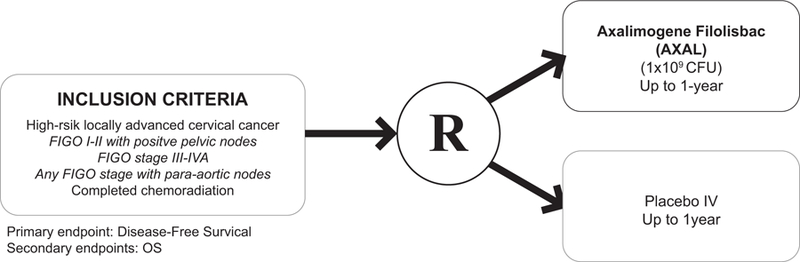
AIM2CERV.
3.2. Checkpoint Inhibition: Anit-CTLA-4 (2b):
Non-cellular strategies investigating the regulation of immunogenic response and T-cell activation have been concurrently examined. This includes distinguishing the co-stimulatory and co-inhibitory receptors that generate the immune-modulatory pathways of the immune checkpoint blockade responsible for T-cell activation, known as checkpoint inhibition. Checkpoint inhibition targets and impedes the inherent checkpoints, allowing for host T-cell activation and ensuing immune response to eliminate the tumor cells; essentially inhibiting the inhibition of the T-cells allowing for an appropriate host immune response to identify the tumor cells. These checkpoints not only determine that the host subject is able to identify non-self-entities and thus invoking a sufficient immune response, but in addition, maintain self-tolerance by modulating the peripheral immune responses, with the intention of minimizing collateral damage from excess reactions. These immunotherapy components that have been developed to target the immune checkpoint receptors are anti-cytotoxic T-lymphocyte-associated antigen 4 (anti-CTLA-4), anti-programmed cell-death 1 (anti-PD-1), and anti-programmed cell-death ligand 1 (anti-PD-L1).
Activation of a T-cell is achieved by a co-stimulatory signal created by the binding of the receptor CD28 to the ligand B7. Found of the surface of activated T-cells, CTLA-4 is an inhibitory receptor that helps to regulate the T-cell immune response by preferentially binding to the B7 ligand on the antigen presenting cell, blocking the binding of CD28 to B7, subsequently inhibiting activation of the T-cell. Thus, tumor cells utilize CTLA-4 to suppress the activation and propagation of hosts’ T-cells against the tumor cells. Consequently, inhibition of CTLA-4 allows for the stimulation of those T-cells to trigger a response in opposition to the tumor cells.25,26 CTLA-4 was the first checkpoint target to be studied in the clinical setting and proved to be an effective therapy prompting approval of ipilimumab, an anti CTLA-4 therapy, by the FDA in 2011 for the treatment of advanced stage melanoma after is showed an OS advantage of 3.6 months.27
At the 2015 ASCO meeting, Lheureux et al. presented data from a Phase I/II study exploring ipilimumab in metastatic or recurrent cervical cancer with primary objectives of safety and response rate. This trial included 42 patients, all who had previously received a platinum therapy. The patients received ipilimumab at 10mg/kg every three weeks for four cycles and then four cycles of maintenance therapy at the same dose ever twelve weeks. Of the 34 evaluable patients, 2 had a partial response and 9 had stable disease, median PFS was 2.5 months (95%CI: 2.3–3.2) and the therapy was overall well tolerated with the grade 3 AEs reported related to diarrhea and colitis. Though the therapy was well tolerated, no complete response was seen in this trial.28 Another study that is investigating the use of ipilimumab for the treatment of cervical cancer is an ongoing phase I trial, GOG 9929 (), enrolling patients with lymph node positive stage IB2 –IVA cervical cancer. After completion of chemoradiation, patients are given sequential ipilimumab at 2 different dose levels, 3mg/kg and 10mg/kg with the primary endpoints of discovering the maximum tolerated dose (MTD) and dose limiting toxicities (DLT) of ipilimumab in this setting. Secondary endpoint is the 1 year disease-free survival (DFS). Most AEs were grade 1–2 and included gastrointestinal distress or endocrinopathies with and self-limiting grade 3 AEs that included elevated lipase, neutropenia and a rash; there were no late toxicities reported. The DFS at the end of the year was 74% indicating that overall the adjuvant immunotherapy was well tolerated and effective.29 This trial is still ongoing. (Table1)
Table 1:
Checkpoint Inhibitor Clinical Trials for Cervical Cancer
| Author | Trial and Eligibility | Agent | ORR | DFS/PFS/OS | AEs |
|---|---|---|---|---|---|
|
Lheureux ASCO 2015 Abstract 3061 |
KEYNOTE-028 () Single arm, Phase I/II; Recurrent and metastatic cervical cancer |
Ipilimumab Anti-CTLA-4 |
Not reported | mPFS 2.5 mos | Diarrhea, colitis |
|
Mayadev Ongoing Trial |
GOG 9929 () Single arm, Phase I; Stage IB2-IVA cervical cancer |
Ipilimumab Anti-CTLA-4 |
Not reported | PFS not reported 1-yr DFS 74% |
GI distress, endocrinopathies, elevated lipase, neutropenia, rash |
|
Frenel ASCO 2016 Abstract 5515 |
KEYNOTE-028 () Single arm, Phase IB; Recurrent and metastatic cervical SCCA |
Pembrolizumab Anti-PD-1 |
12.5%; mDOR 19.3 wks | 6-mos PFS 13% 6-mos OS 66.7 |
Pyrexia, rash, colitis, Guillain-Barre |
|
Schellens ASCO 2017 Abstract 5514 |
KEYNOTE-158 () Single arm, Multi-cohort Phase II; Recurrent and metastatic cervical SCCA |
Pembrolizumab Anti-PD-1 |
17%;ORR > 27 wks: 27% | Not reported | Arthralgia, nausea, pruritis, rash, diarrhea |
|
Hollebecque ASCO2017 Abstract 5504 |
CheckMate-358 () Single arm, Multi-cohort Phase I/II; Recurrent and metastatic HPV-associated cancers |
Nivolumab Anti-PD-1 |
26.3% (31 wks) DCR 71% Note: all responses in cervix patients |
mPFS 5.5 mos | Enterocolitis, hepatitis, dermatitis; neuropathy, endocrinopathy |
|
Santin Ongoing Trial |
NRG GY002 () Single arm, Phase II; Recurrent and metastatic cervical cancer |
Nivolumab Anti-PD-1 |
Not reported | Not reported | Not reported |
|
Papadopoulos ASCO 2016 Abstract 3024 Ongoing Trial |
REGN 2810 () Multi-cohort Phase I; Advanced solid tumors |
Cemiplimab (REGN 2810) Anti-PD-1 |
ORR not reported DCR 63% (27/43) Note: 2 of the responses were SCCA and 1 was anal SCA |
Not reported | Fatigue, nausea, flu-like illness, rash, transaminase elevation, anemia |
|
Tewari Ongoing Trial |
EMPOWER-Cervical 1 () Randomized Phase III; Recurrent and metastatic cervical cancer |
Cemiplimab (REGN 2810) Anti-PD-1 |
Not reported | Not reported | Not reported |
3.3. Checkpoint Inhibition: Anti-PD-1, Anti- PD-L1 (Figure 2b):
PD-1 and its counterpart, PD-L1 are additionally found in the T-cell centered immunotherapy pathway and act similarly to the CTLA-4 inhibitory sequence, but while CTLA-4 acts on inactive T-cells, PD-1 acts on activated T-cells. PD-1, a member of the CD28 family, is typically expressed on activated T-cells (CD4 and CD8), B cells, NK cells and monocytes, while PD-L1 are found on a variety of tissue types such as endothelial, epithelial, and hematopoetic cells (T-cells, B-cells, dendritic cells and macrophages). In normal tissues, the PD-1 serves to orchestrate the immune response, as well as to prevent development of an autoimmune response by creating a self-tolerance to normal tissue through preventing the further activation of T-cells. This dynamic begins with the activation of the T-cell mounted by a response to a cellular abnormality, at which time the PD-1 becomes detectable on the cell surface within 24 hours. The binding of the PD-1 to the T-cell, promotes the binding of PD-1 to its ligand, PD-L1, disseminating an inhibition signal that leads to decreased cytokine production and ultimately decreased proliferation of the cytotoxic T-cells; ultimately protecting the surrounding normal cells from the initial response. In tumors, PD-L1 expression is upregulated and acts to evade the host response by overexpression, thus dampening the immune response to the tumor cells by blocking the T-cell activation and preventing the release of the cytotoxic T-cells. Consequently, the tumor cells exploit the PD-1/PD-L1 system to protect itself from the cytotoxic T-cells, by deactivating the cytotoxic T-cells and preventing the activation of new T-cells, suppressing the immune response. 26,30,31
In head and neck squamous cell carcinoma there are two types of squamous cell carcinomas – carcinogen-induced disease and viral-induced disease. The carcinogen-induced disease involves the down regulation of p16 and p53 mutants, similar to vulvar squamous cancers, and with the viral-induced disease, it is associated with the HPV E6 and E7 oncoproteins and a subsequent upregulation of p16, similar to cervical and anal cancers. In the viral-induced disease, it is thought that with abrasions, HPV is then able to infect the basal epithelial cells, and viral maturation occurs relative to the amount of epithelial differentiation that is occurring. Therefore, there is controversy as to if carcinogen exposure is synergistic or additive to the viral induction of the disease.32 Additionally, in all head and neck squamous cell carcinomas there has been shown to be the presence of TILs and PD-L1 overexpression in approximately 40% of the tumors.33 Correspondingly, multiple studies involving the integration of PD-1 inhibitors have been investigated. Secondary to the similarities of head and neck squamous cell cancers to cervical cancer, it is important to examine the immunotherapy trials from head and neck and cervical cancers.
KEYNOTE-012, a multi-center phase Ib trial enrolling patients with recurrent or metastatic head and neck squamous cell carcinoma and evidence of PD-L1 expression. With primary endpoints of safety and overall response (ORR) as assessed by Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), patients enrolled received PD-1 inhibitor, pembrolizumab, 10mg/kg every 2 weeks. Per RECIST, 18% ORR in all patients (95% CI: 8–32) and was 25% in the HPV-positive group. Moreover, pembrolizumab was well tolerated with the most common AEs of elevated liver enzymes and hyponatremia.34 (Table 2)
Table 2:
Checkpoint Inhibitor Clinical Trials for Other Disease Sites with Significance to Cervical Cancer
| Author | Trial and Eligibility | Agent | ORR | PFS/OS | AEs | Relevance to Cervical SCC |
|---|---|---|---|---|---|---|
|
Papadopoulos ASCO 2017 Abstract 9503 |
() Phase I; Locally advanced and metastatic CSCC |
Cemiplimab (REGN 2810) Anti-PD-1 |
52% DCR 70% Note: all responses in cervix patients |
Not reported | Fatigue, elevated liver enzymes, arthralgia, rash | CSCC was found to have the highest mutational burden with a significant DCR to anti-PD-1 |
|
Sweiwert Lancet 2016 Ongoing Trial |
KEYNOTE-012 () Single arm, Phase Ib; Recurrent and metastatic HNSCC |
Pembrolizumab Anti-PD-1 |
18% Note: 25% ORR in HPV-positive |
mPFS 2 mos Note: 4 mos mPFS in HPV-positive |
Elevated liver enzymes, hyponatremia, fatigue, rash, atrial fibrillation, CHF, diarrhea, neutropenia, pain, and abscess | The virally-induced HNSCC had a significantly better response to immunotherapy |
|
Gandara ASCO 2017 Abstract 9001 Ongoing Trial |
OAK TRIAL () Randomized Phase III, Recurrent or Advanced NSCLC, Treatment Beyond Progression (TBP) |
Atezolizumab Anti-PD-1 |
52% | TBP: atezo: mOS 12.7 mos other: mOS 8.8 mos |
Fatigue, diarrhea, nausea, anemia, pain, pneumonitis, hepatitis, colitis | Illustrates the importance of treating beyond progression and the survival benefit that can be potentially obtained |
Checkmate-141, with a primary endpoint of OS was a phase III trial that enrolled patients with recurrent head and neck squamous cell carcinoma who had received prior platinum therapy. Immunohistochemical staining was used to test the tumors for PD-L1 expression and p16 for HPV status (p16 was only tested in the oropharyngeal tumors). Patients enrolled received either PD-1 inhibitor, nivolumab (3mg/kg), every two weeks or standard single agent chemotherapy, which included methotrexate, docetaxol or cetuximab. OS was 7.5 months in the nivolumab group (95% CI: 5.5–9.1) versus 5.1 months in the standard chemotherapy group (95% CI: 4.0–6.0). Additionally, the success of nivolumab in comparison to single agent chemotherapy was regardless of p16 expression, although p16 expression did seem to relate to a significantly longer median OS. For those patients with a p16-positive tumor, the median OS was 9.1 mos versus 4.4 mos in the nivolumab versus standard-therapy group, respectively (hazard ratio for death, 0.56; 95% CI, 0.32–0.99); and for patients with p16-negative tumors, the median overall survival was 7.5 versus 5.8 mos (hazard ratio, 0.73; 95% CI: 0.42–1.25).35,36
In the head and neck squamous cell carcinoma arena, there were also several studies developed utilizing durvalumab (MEDI4376), a monoclonal antibody that is targeted against PD-L1. The trials included two phase II studies, Hawk () and Condor (), and a phase III trial, Eagle (), that investigated durvalumab or tremelimumab as monotherapy or in combination in patients that had recurrent or metastatic head and neck squamous cell carcinoma after they had failed platinum therapy. The differences in the studies was the PD-L1 expression status of the tumors, Hawk investigated PD-L1 positive, Condor investigated PD-L1 negative, and Eagle, as a phase III trial investigated the monotherapy or combination on both PD-L1 positive and PD-L1 negative. Primary endpoint in the phase II trials was ORR and OS for the phase III trial. At the European Society of Medical Oncology 2017 meeting, Zandberg et al reported an ORR of 16.2% (95%CI:9.9–24.4) in the Hawk trial, the phase II trial that enrolled patients with a high PD-L1 expression. In addition, those with HPV positive tumors demonstrated an ORR of 29.4% versus an ORR of 10.8% in those that were HPV negative highlighting the benefits of immunotherapy in an HPV associated cancer that is associated with a high PD-L1 expression, much like cervical cancer.37 Phase II Condor and Phase III Eagle trials are still ongoing.38,39
Comparably, recent research has shown that the overexpression of PD-L1 is found of approximately 95% of cervical intraepithelial neoplasia (CIN) and 80 % of squamous cell cervical cancers, rendering the PD-1/PD-L1 interaction as a principal immunosuppressive agent in the development of cervical dysplasia or tumorigenesis for cervical cancer, designating this interaction as a key element to target for tumor destruction.40,41 One such study was the phase IB trial designated Keynote-028, presented by Frenel et al. at the 2016 ASCO meeting. This study enrolled patients with recurrent or metastatic cervical cancer that had received prior systemic chemotherapy and the tumor had evidence of PD-L1 expression. The agent used in this trial was anti-PD-1, pembrolizumab, which was given at a dose of 10 mg/kg every 14 days for up to 2 years or until confirmed progression or toxicity. The primary endpoint was ORR, which was reported as 12.5% (3/24; 95% CI: 2.7%−32.4%) with a median duration of response of 19.3 weeks (range, 17.7–52.0 weeks).42 (Table 1)
Furthermore, there are two ongoing studies, Checkmate-358 () and Keynote-158 (), both examining the use of anti-PD-1 therapies for the treatment of HPV-related cancer. CheckMate-358 is a phase I/II multi-cohort, single arm trial in which patients with recurrent or metastatic HPV-associated cancers were treated with nivolumab monotherapy until progression or toxicity. Patients could not have previously received more than 2 lines of systemic chemotherapy. Efficacy was to be determined by the primary endpoint of ORR and secondary endpoints of DOR, PFS, and OS. The cohort included 24 patients with cervical, vaginal and vulvar cancers, with 19 of those patients with cervical cancer and the only cohort to demonstrate not only a response, but a durable response for at least 6 months. Five of the 19 patients had a complete or partial response with an ORR of 26.3% (95%CI: 9.1–51.2) Median PFS was 5.5months (95% CI: 93.5 to not reached) and median OS data is not yet mature. Though HPV and PD-L1 status was not evaluated prior to enrollment, it was investigated post-hoc and interestingly it was found that the disease control rate seems to be similar across cohorts regardless of PD-L1 expression, although the significance is undetermined at this time though likely secondary to the small sample size. In addition, nivolumab was associated with minimal toxicity signifying it as a well-tolerated and effective therapy.43 (Table 1)
Single-arm, multi-cohort phase II trial, KEYNOTE-158, enrolled patients with advanced cervical squamous cell cancer with noted progression or intolerance to standard therapy, utilizing pembrolizumab monotherapy (200mg every 3 weeks) for the treatment of cervical cancer for 2 years or until progression or toxicity. The safety, efficacy and anti-tumor activity of pembrolizumab was determined by endpoints of ORR and DOR. Initial ORR of 17% (95% CI, 8%−31%) with an increase in ORR at the greater than 27-week follow-up of 27% (95% CI 8%−55%). Again, PD-L1 status was not assessed at time of enrollment but was analyzed post-hoc indicating that 87% of the tumors were PD-L1 positive, but the ORR was again irrespective of PD-L1 positivity. 44 An additional phase II study, NRG GY002 (), is currently underway that enrolled patients with recurrent or metastatic cervical cancer with measurable disease who have received one prior systemic treatment regimen. Patients will receive nivolumab every 14 days for a maximum of 46 doses over 92 weeks in the absence of disease progression or intolerable toxicity. Since there have been no prior studies investigating nivolumab as a monotherapy in this patient population, NRG GY002 was designed as a two-stage trial in order to minimize the number of patients exposed to an inactive agent, so if the first stage demonstrates evidence of tumor response, the second stage can be initiated and additional patients can be enrolled. This trial has reached total enrollment with the accrual of 26 patients over both stages.45 Although both CHECKMATE-358 and KEYNOTE-158 trials, and eventually NRG GY002, have small sample sizes, the promising results encouraged the further development into larger phase III trials investigating anti-PD1 immunotherapies as first-line and/or second line treatment for advanced, recurrent or metastatic treatment. (Table 1)
Similar to cervical cancer, in cutaneous squamous cell carcinoma (CSCC) immunosuppression is a well described risk factor and notably CSCC was found to have the highest mutational burden than any other tumor in the Cancer Genome Atlas.46 This high mutational burden is important because cervical cancer also has a high mutational burden and this increase in mutational burden has been shown to be related to a better response from the immunotherapies, thought to be a result of a higher number of neoantigens produced which are then taken up and processed by the antigens presenting cells leading to the activation of the innate immune system.47 Secondary to the untreatable nature of this disease for those with metastatic or locally advanced CSCC, at this time there is not a standard of care treatment for this patient population. At the annual ASCO meeting this year, Papadopoulos et al., presented the phase I open-label study of REGN2810 in expansion cohorts of patients with locally advanced and metastatic CSCC. REGN2810 is a high-affinity, hinge-stabilized IgG4 human anti-PD-1 antibody, obstructing the interaction between PD-1 and PD-L1. Patients on the trial presented by Papadopoulos, were treated with REGN2810 at a dose of 3 mg/kg dose every 14 days up to 48 weeks. 26 patients were enrolled, and remarkably, for both metastatic and locally advanced CSCC, the ORR was 46%, 2 complete responses (7.7%) and 10 partial responses (38.5%). Moreover, disease control rate, defined by the ORR plus the stable disease, was 69.2% (95% CI: 48.2–85.7). Importantly, the responses proved to be not only rapid but also durable when looking at the patients with metastatic disease. 48 This response seen in a disease process once thought to be untreatable gives promise for those with advanced cervical cancer.
Further research has shown that squamous cell carcinomas of the cervix have higher PD-L1 expression than adenocarcinomas, as well as squamous cell carcinomas of the cervix also have more PD-L1 positive tumor-associated macrophages.49 Although this may suggest that checkpoint inhibition may not be a viable therapeutic option in cervical adenocarcinomas, it should be noted that in other solid tumors where checkpoint inhibition has been effective, PD-L1 expression by the tumors has not been shown to be a predictive biomarker. (Table 3) Another exciting observation which suggests that checkpoint inhibition is a viable pathway in cervical cancer, is that when looking at comparing immune signatures of CD8/PD-1/PD-L1 expression within The Cancer Genome Atlas, squamous cell carcinomas of the cervix cluster with tumors in which anti-PD-1 therapies have shown to improve survival in phase III trials - this includes squamous cell cancers of the head and neck, squamous and adenocarcinomas of the lung, cutaneous melanoma, and clear cell kidney carcinoma. Lastly, it has been discovered that there is an abscopal effect of radiation, where in a study of advanced oropharyngeal cancer, PD-1 expression on CD4+ T cells increased 2.5-fold during radiotherapy.50 Notably, the abscopal effect on target lesions beyond the radiation field is one that will require close monitoring, particularly in clinical trials in which patients receiving checkpoint inhibitor therapy may also be receiving localized radiotherapy for palliation of oligometastases. Armed with the successes found in the CSCC trials and with our knowledge of the high overexpression of PD-L1 found in cervical cancer, it is easy to see why there are numerous cervical cancer trials investigating checkpoint immunotherapy.
Table 3:
PD-L1 Expression in Squamous Cell Carcinomas Versus Adenocarcinomas
| SCCA | AdenoCa | P-value | ||
|---|---|---|---|---|
| N (%) | N (%) | |||
| Tumor Cells | PD-L1+ >5% | 83 (54) | 7 (14) | <0.001 |
| PD-L1 neg | 71 (46) | 42 (86) | ||
| Diffuse PD-L1 | 71 (87) | 4 (80) | ||
| Marginal PD-L1 | 11 (13) | 1 (20) | 0.533 | |
| PDL1+ TAM | Yes | 79 (53) | 6 (12) | <0.001 |
| No | 70 (47) | 43 (88) | ||
|
Stromal PD-L1+ cells |
High numbers | 122 (78) | 31 (65) | 0.057 |
| Low numbers | 34 (22) | 17 (35) |
Adapted from Hereen AM, et al. Modern Pathol 2016; 29: 753–63.
At the ASCO 2016 meeting, REGN2810 made an appearance in a phase I dose escalation study. Also presented by the aforementioned Papadopoulos and colleagues, this study investigated the anti-PD-1 antibody as monotherapy and in combination with other anti-tumor treatments in patients with advanced solid tumors. Tumor response and tolerability were studied at escalating doses with no disease limiting toxicities found and a disease control rate of 63%, and interesting to note is that of the responses discovered, two were squamous cell cervical cancers and one was a squamous cell anal cancer. 51 (Table 2) This clearly elucidated the need to further pursue these studies in the cervical cancer patient population.
The first phase III randomized trial of checkpoint inhibition in advanced cervical cancer activated in August 2017 and is known as EMPOWER-Cervical 1 (). This trial is studying the activity, efficacy, and tolerability of the anti-PD-1 molecule cemiplimab, formerly called REGN2810, versus physicians’ choice chemotherapy.52 This trial will enroll patients with recurrent, persistent or metastatic squamous or adenocarcinoma of the cervix with evidence of tumor progression. (Figure 4) It is a second line trial for women that either failed chemotherapy plus bevacizumab or failed chemotherapy alone. Patients will be randomized to receive physicians’ choice chemotherapy until progression including pemetrexed, topotecan versus irinotecan, vinorelbine; or patients will receive REGN2180 IV 350mg every 3 weeks for 2 years or until progression. In terms of the physicians’ choice chemotherapy, the drugs selected have all shown varying degrees of activity in the second-line setting ranging from 7% to 21% overall response (Table 4), but they were chosen based on geographical preferences as this will be an international trial and, in some countries, only specific neoplastic agents are available. Primary endpoint will be OS and secondary endpoints will include, PFS, ORR, AEs and quality of life data. Adverse events are likely to include some of those reported in other trials using checkpoint inhibitors, specifically immune-mediated conditions including pulmonitis, colitis, thyroiditis, and even myocarditis. The management of such AEs will be to withhold the therapy.53 (Table 1)
Figure 4.
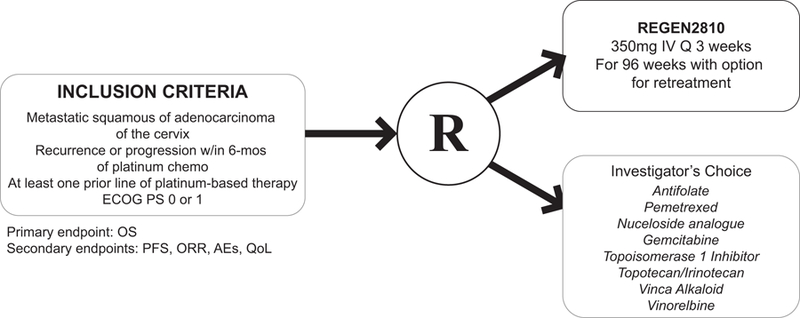
GOG 3016/ENGOT-cx9/EMPOWER Cervical-1.
Table 4:
Phase II Trials in the Second-Line Setting for Recurrent Cervical Cancer
| Agent | N | Histology | Dose | ORR | mOS | Reference |
|---|---|---|---|---|---|---|
| IRINOTECAN | 42 | SCCA | 125 mg/m2 wkly x 4 q 6wks | 21% | 6.4 mos | Verschraegen J Clin Oncol 1997 |
| PEMETREXED | 43 | SCCA & non-SCCA | 500 mg/m2 q21d | 13.90% | 35 wks | Lorusso Ann Oncol 2010 |
| PEMETREXED | 27 | SCCA & non-SCCA | 900 mg/m2 q21d | 15% | 7.4 mos | Miller Gynecol Oncol 2008 |
| TOPOTECAN | 41 | SCCA | 1.5 mg/m2 daily x 5 q21d | 12.50% | 7 mos | Bookman Gynecol Oncol 2000 |
| VINORELBINE | 28 | SCCA | 30 mg/m2 d1 & d8 q21d | 13.70% | Not reported | Muggia Gynecol Oncol 2004 and 2005 |
| 44 | Non-SCCA | 7% | ||||
| GEMCITABINE | 24 | SCCA | 800 mg/m2 wkly x 3,one week off | 8% | 4.9 mos | Schilder Gynecol Oncol 2000 and 2005 |
| 19 | Non-SCCA | 4.50% | 6.5 mos |
In addition to breaking immunologic tolerance, monitoring for abscopal effects when palliative radiotherapy is used for oligometastases, and monitoring for the emergence of immune-mediated adverse events, investigators will be encouraged to treat patients beyond traditional RECIST-designated progression provided the patient is clinically stable and agrees to continue, and no new metastatic lesions have manifested on imaging. The phenomenon of pseudoprogression gained clinical relevance with presentation of the OAK trial at ASCO 2017. The OAK trial is the largest phase III randomized trial studying a PD-L1 inhibitor, atezolizumab, that has been reported. In this trial, patients with advanced non-small cell lung cancer who had previously received two or more lines of chemotherapy were randomized to atezolizumab versus chemotherapy. For the first time in a clinical trial, patients were allowed to continue on therapy beyond progression by RECIST criteria secondary to the belief that there is not only a delayed response with immunotherapy, but also there can also be enlargement of existing lesions secondary to tumor immune infiltration. 51% of the 332 patients identified with disease progression based on RECIST criteria that had received atezolizumab agreed to treatment beyond progression. Of those, 49% (83/168) had stable target lesions and the patients that continued checkpoint inhibition therapy post-progression significantly increased the median OS, with a median OS of 12.7 months (95% CI:9.3–14.9) versus the patients with disease progression who were in the other anti-cancer treatment group with a median OS of 8.8 mos (95%CI: 6.0–12.1).54 (Table 2)
3.4. Antibody-Drug Conjugate (Figure 2c):
Ongoing trial GEN701/GEN702 (), is a trial investigating tistotumab vedotin is an antibody-drug conjugate used as an antibody to target tissue factor (TF) and conjugated to cytotoxic agent, monomethyl auristatin E (MMAE). TF is a factor known to be involved with tumor cell signaling and angiogenesis and thus an attractive target in those cancers that have a high expression of TF, such as cervical cancer. This trial is a phase I/II trial including seven solid tumor types: cervical, ovarian, endometrium, bladder, prostate, esophageal, and lung. Phase I included a dose escalation to determine the recommended phase II dose, which was determined to be 2.0mg/kg of tistotumab vedotin every 3 weeks. The drug thus far has been well tolerated, with AEs consistent with known effects of cytotoxic MMAE of peripheral neuropathy and neutropenia, and conjunctivitis specific to tistotumab vedotin. In the preliminary data released, 34 patients with cervical cancer received the treatment and out of those, 11 achieved a response. Median time of treatment was 4.9 months, with 7 patients still in treatment or in follow-up for progression.55 With the encouraging results of the preliminary data, this trial planning to move forward in the investigation of the antibody-drug conjugate for the treatment of cervical cancer.
3.5. PI3K/AKT/mTOR Inhibition (Figure 2d):
PI3K/AKT/mTOR pathway is an intracellular pathway that regulates the cell cycles and it is known that treatment resistance to standard chemoradiation has been associated with alterations in this pathway and thus inhibitors to PI3K/AKT/mTOR have been developed in the hopes that it will improve the response to treatment.56 Pre-clinical studies supported this theory by exhibiting that AKT inhibitors did in fact block mTOR pathways and decreased cell viability, and additionally mTOR inhibitors have been shown to decrease the levels of the E7 oncoprotein associated with HPV-malignancies.57,58 There was a phase I trial by Hou et al, that was developed that included patients with metastatic or recurrent squamous cell cancer or adenocarcinoma of the cervix who had completed at least one prior treatment regimen. Patients were evaluated for a PIK3CA and/or PTEN loss/mutation prior to enrollment and therapy was matched to their mutation. Findings from this study found that PI3K mutations were more common in patients with squamous cell cervical cancer, 48% versus the 14% found in those with adenocarcinoma. Median OS for both groups was 9.1 months (95% CI: 7.1–11.1) but those with adenocarcinoma had a more significant OS of 14.2 months (95% CI: 7.8 – 20.6) versus the squamous cell population that had an OS of 7.2 months (95% CI: 5.3 – 9.1). In addition, those with adenocarcinoma and a PI3K mutation had an OS of 19.4 months (95% CI: 0 – 43.6). For those who received the matched therapy, there was an increased median PFS of 6.0 months versus PFS of 1.5 months for the unmatched therapy. Additionally, there was a 53% stable disease rate for over 6 months for those with complete and partial response.59 A phase II trial by Tinker et al, utilized mTOR inhibitor, temsirolimus, in patients will recurrent or metastatic cervical cancer, where patients received temsirolimus 25mg IV weekly for 4 week cycles. Of the 38 patients, one patient experienced a partial response, and nineteen patients had stable disease (57.6%) showing that although the response rates were low the percentage of stable disease is promising.60 Again, encouraging results that will prompt the development of phase II and III trials and will likely show promise with conjunction with the other immunotherapeutic regimens.
3.6. PARP inhibition (Figure 2e):
Poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitors (PARPi) are agents that were initially created for cancers that harbor a deleterious BRCA mutation that are unable to repair double-strand breaks through homologous recombination. Thus, PARPi have been studied primarily in BRCA-mutated cancers, and subsequently there have been three PARPi that have been approved for ovarian cancer, olaparib, rucaparib and niraparib. Nonetheless, the recent FDA approval of the PARPi in patients with advanced ovarian cancer was not only limited to those patients who have a BRCA-mutation or a homologous recombination deficiency, but to all patients with recurrent ovarian cancer, as they have shown to also be effective in those patients without such a mutation. Investigations into PARPi for cervical cancer began after preclinical studies showed that a cisplatin-resistant form of cervical cell lines that were created, expressed high levels of PARP, and PARPi exposure to those cells led to cell death.61,62 Additionally, it is widely known that radiation causes DNA damage, thus PARPi would likely have effect in the previously radiated cervical cancer patients. This prompted clinical investigations of PARPi for the treatment of cervical cancer. Initially, there was a phase I/II trial that utilized PARPi, veliparib, in combination with topotecan for patients with recurrent or persistent cervical cancer. This study only showed minimal clinical activity with only 2 partial responses noted out of the 27 patients enrolled (7%, 95% CI: 1–22%), but it did prompt further development of PARPi in combination with other chemotherapy regimens. 63 A phase I/II trial was then created, that enrolled patients with recurrent or metastatic cervical cancer to receive PARPi, veliparib in combination with cisplatin and paclitaxel. As a dose escalation trial, 34 patients were enrolled and the dose limiting toxicities were found to be a grade 4 dyspnea, a persistent grade 3 neutropenia, and febrile neutropenia. Results demonstrated a 34% ORR (95% CI: 20%−53%), median PFS of 6.2 mos (95% CI: 2.0–10.1) and OS of 14.5 mos (95% CI: 8.2–19.4), illustrating the great potential of utilizing PARPi as a combination therapy for the treatment of cervical cancer.64
4. Conclusion:
Even though there are over 100–150 different genotypic variants of HPV, the hope is that one day that at least the few oncogenic variants will be eradicated by prophylactic vaccines, and with improvements in screening, the number of patients with new diagnoses of cervical cancer as well as those with advanced and recurrent cervical cancer will be no longer, but until this end, more effective treatments are needed to treat this devastating disease. As evidenced by the fact that there is not standard therapy for patients once they have a recurrence or persistence of their cervical cancer, these patients do not respond to traditional chemotherapy and there is a significant need for new novel therapeutics for the treatment of those with cervical cancer that have the poorest prognosis. Bevacizumab, as an antiangiogenic agent was the first new targeted strategy for the treatment of advanced or persistent cervical cancer, and now with the evolving development of the cellular and non-cellular based therapies, the armamentarium of new novel therapeutics will quickly develop as the new standard for which we treat a disease that once carried such a poor prognosis.
5. Expert Opinion
The largest risk factor for the development of cancer in those with a HPV-infection is carrying the high-risk HPV-oncogenes and persistence as the HPV-infection evades the host immune response. Thus, it is evident that by targeting the breakdown of the host immune tolerance to the HPV specific oncoproteins, such as HPV E6 and E7, this will aid in the treatment of not only cervical cancer, but additionally the other HPV-related malignancies such as oropharyngeal, anal, vulvar and vaginal cancers. This highlights an important time in oncology where there is a shift from categorizing oncology based on disease site to understanding carcinogenesis based on tumor biology and host response. In fact, in May of 2017, there was the first ever FDA approval of a tissue-agnostic therapy, pembrolizumab, for the treatment of solid tumors with mismatch repair deficiencies or that are microsatellite instability high, focusing on biomarkers expressed rather than tumor type.65 Accordingly, taking a cue from other diseases that much like the HPV-malignancies also harbor specific molecular signatures that can be targeted, this has prompted development of specific targets to angiogenesis, tumor-antigens, signal transduction pathways and tissue factors, all of which in the pre-clinical and early clinical trials have shown great promise. Now there have been cases of patients who had obtained not only a complete response, but also a durable response for a disease that would have only afforded that patient a handful of months with second-line chemotherapy. This clearly demonstrates the ability of immunotherapy and targeted therapy to provide durable therapeutic response in chemo-refractory disease.
Even though promising, as new therapies, most are still in their early phase of development and will need to be further expanded into larger phase II-III studies to confirm their efficacy and safety. In addition, high costs associated to the development of new novel therapies often limits not only the development of new therapies, but also the implementation of those therapies broadening the gap between moderate increases in clinical benefit and the high cost of new therapeutics disparaging the promotion of drug discovery. Furthermore, there needs to be research into establishing predictive biomarkers for cervical cancer, as well as further research into determining the efficacy of combination strategies of these targeted strategies. Nevertheless, if the trend continues to expose immunotherapies and targeted therapies as effective as they have been thus far, we will be upon a new frontier of treatment for advanced, metastatic and recurrent cervical cancer. We are in an era where likely these combinations of novel therapeutics will serve an essential role in overcoming acquired drug resistance and immune tolerance, our critical obstacles of effective treatment of advanced cancers, no matter the tumor type. While promoting early prevention through continued encouragement of the prophylactic vaccine and implementation of appropriate screening strategies will still be at the forefront for cervical cancer prevention, until cervical cancer is completely eradicated, we will need to develop an armamentarium of effective treatments for our patients with recurrent, metastatic, or refractory cervical cancer, those patients who need it the most.
Article Highlights:
Advanced or recurrent metastatic cervical cancer has a poor prognosis with dismal PFS and RR to current therapeutic options secondary to the immune tolerance of HPV
Bevacizumab was the first targeted therapy approved by the FDA to show survival benefit in a chemo-refractory disease prompting more research into the development of targeted agents
Recently there has been development of cellular and non-cellular based targeted strategies for the treatment of recurrent, metastatic or persistent cervical cancer
Cellular-based strategies highlighted here include the adoptive cell transfer of HPV-TILs, CAR-T-cells, and Cue-101
Non-cellular- based strategies highlighted here include the AXAL-therapeutic vaccine, checkpoint inhibitors, anti-CTLA-4, anti-PD-1, and anti-PD-L1; as well as antibody-drug conjugate, PI3K/AKT/mTor inhibitors and PARP inhibitors.
Thus far, the immunotherapies and targeted therapeutics studied in pre-clinical and phase I and II trials have been promising, prompting further development of targeted therapy as a mainstay for the treatment of advanced, recurrent or metastatic cervical cancer.
Acknowledgments
Funding:
This manuscript has not been funded.
K Tewari is a consultant for Genentech/Roche, Clovis and Merck. He also has research grants from Genentech, Clovis, AstraZeneca, Merck, Pfizer and Tesaro and is also on the speaker’s bureau for Roche, Merck, Clovis and Tesaro.
Footnotes
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References:
- 1.What Are the Key Statistics About Cervical Cancer? https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed August 15, 2017.
- 2.Tewari KS, Sill MW, Long HJ, et al. Improved Survival with Bevacizumab in Advanced Cervical Cancer. N Engl J Med 2014;370(8):734–743. doi: 10.1056/NEJMoa1309748.**Landmark trial showing survival benefit and increased response rates for patients with advnaced cervical cancer
- 3.Zsiros E, Tsuji T, Odunsi K. Adoptive T-Cell Therapy Is a Promising Salvage Approach for Advanced or Recurrent Metastatic Cervical Cancer. J Clin Oncol 2015;33(14):1521–1522. doi: 10.1200/JCO.2014.60.6566. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N Engl J Med 2003;348(6):518–527. doi: 10.1056/NEJMoa021641.*Important retrospective review that helped lead to the identification of the oncogenic HPV subtypes
- 5.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8.*Largest study of HPV genotypes
- 6.zur Hausen H Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002;2(5):342–350. 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 7.Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11–001. Gynecol Oncol Res Pract 2017;4(1):10. doi: 10.1186/s40661-017-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevanović S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol 2015;33. doi: 10.1200/JCO.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zsiros E, Tsuji T, Odunsi K. Adoptive T-Cell Therapy Is a Promising Salvage Approach for Advanced or Recurrent Metastatic Cervical Cancer. J Clin Oncol 2015;33(14):1521–1522. doi: 10.1200/JCO.2014.60.6566. [DOI] [PubMed] [Google Scholar]
- 10.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of Tumor-Infiltrating Lymphocyte Cultures for Use in Adoptive Transfer Therapy for Melanoma Patients. J Immunother 2003;26(4):332–342. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2305721/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buechner J, Grupp SA, Maude SL, et al. Global Registration Trial of Efficacy and Safety of CTL019 in Pediatric and Young Adult Patients with Relapsed/Refractory (R/R) Acute Lymphoblastic Leukemia (ALL): Update to the Interim Analysis. Clin Lymphoma Myeloma Leuk 2017;17:S263–S264. doi: 10.1016/j.clml.2017.07.030. [DOI] [Google Scholar]
- 12.Hinrichs CS. Molecular Pathways: Breaking the Epithelial Cancer Barrier for Chimeric Antigen Receptor and T-cell Receptor Gene Therapy. Clin Cancer Res 2016;22(7):1559–1564. doi: 10.1158/1078-0432.CCR-15-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinrichs CS, Stevanovic S, Draper L, et al. Adoptive transfer of tumor infiltrating lymphocytes for metastatic cervical cancer. J Immunother Cancer 2013;1(1):P15. doi: 10.1186/2051-1426-1-S1-P15. [DOI] [Google Scholar]
- 14.Cue Biopharma Announces Selection of Lead Candidate Cue-101 Targeting https://www.cuebiopharma.com/2017mar22-cue-biopharma-to. Accessed September 4, 2017.
- 15.First Cervical Cancer Immunotherapy from Cue Biopharma Ready for Testing https://cervicalcancernews.com/2017/04/03/cue-biopharma-develops-first-immunotherapy-for-cervical-cancer/. Accessed October 17, 2017.
- 16.Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11–001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract 2017;4:9. doi: 10.1186/s40661-017-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourla AB, Zamarin D. Immunotherapy: New Strategies for the Treatment of Gynecologic Malignancies. Oncology (Williston Park) 2016;30(1):59–66, 69. http://www.ncbi.nlm.nih.gov/pubmed/26791846. Accessed August 30, 2017.**Detailed review of immunotherapy treatment strategies for gynecologic cancer
- 18.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine 2009;27. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 19.Basu P, Mehta AO, Jain MM, Gupta S, Nagarkar RV, Kumar V, et al. ADXS11–001 immunotherapy targeting HPV-E7: Final results from a phase 2 study in Indian women with recurrent cervical cancer. J Clin Oncol 2014;32(Suppl): abstract 5610. [Google Scholar]
- 20.Huh WK, Brady WE, Moore KN, Lankes HA, Monk BJ, Aghajanian C, et al. A phase 2 study of live-attenuated Listeria monocytogenes cancer immunotherapy (ADXS11–001) in the treatment of persistent or recurrent cancer of the cervix (GOG-0265). J Clin Oncol 201. [Google Scholar]
- 21.Huh W, Brady WE, Dizon DS, et al. <strong>A prospective phase II trial of the listeria-based human papillomavirus immunotherpay axalimogene filolisbac in second- and third-line metastatic cervical cancer: A NRG oncology group trial</strong>. Gynecol Oncol 2017;145:220. doi: 10.1016/j.ygyno.2017.03.506. [DOI] [Google Scholar]
- 22.Furlow B AXAL Immunotherapy Well-Tolerated in Recurrent Metastatic Cervical Cancer: Page 2 of 2 | Cancer Network | The Oncology Journal https://vpn.nacs.uci.edu/+CSCO+1h756767633A2F2F6A6A6A2E706E617072656172676A6265782E70627A++/asco-2016-gynecologic-cancers/axal-immunotherapy-well-tolerated-recurrent-metastatic-cervical-cancer/page/0/1. Accessed October 15, 2017.
- 23.Sacco JJ, Evans M, Harrington KJ, et al. Systemic listeriosis following vaccination with the attenuated Listeria monocytogenes therapeutic vaccine, ADXS11–001. Hum Vaccin Immunother 2016;12(4):1085–1086. doi: 10.1080/21645515.2015.1121338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herzog T, Backes FJ, Copeland L, Estevez Diz MD, Hare TW, Huh W, et al. AIM2CERV: a randomized phase 3 study of adjuvant AXAL immunotherapy following chemoradiation in patients who have high-risk locally advanced cervical cancer (HRLACC). J Immunother Can
- 25.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12(4):252–264. 10.1038/nrc3239.*Article highlighting the immune checkpoint pathway in cancer therapy
- 26.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015;33(17):1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodi FS, O’Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lheureux S, Butler MO, Clarke B, et al. A phase I/II study of ipilimumab in women with metastatic or recurrent cervical carcinoma: A study of the Princess Margaret and Chicago N01 Consortia. J Clin Oncol 2015;33(15_suppl):3061. doi: 10.1200/jco.2015.33.15_suppl.3061. [DOI] [Google Scholar]
- 29.Mayadev J, Brady WE, Lin YG, et al. A phase I study of sequential ipilimumab in the definitive treatment of node positive cervical cancer: GOG 9929. J Clin Oncol 2017;35(15_suppl):5526. doi: 10.1200/JCO.2017.35.15_suppl.5526. [DOI] [Google Scholar]
- 30.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 2007;19(7):813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 31.Shen C-R, Chen Y-S. Immune checkpoint blockade therapy: The 2014 Tang prize in biopharmaceutical science. Biomed J 2015;38(1):5. doi: 10.4103/2319-4170.151150. [DOI] [PubMed] [Google Scholar]
- 32.Mannarini L, Kratochvil V, Calabrese L, et al. Human Papilloma Virus (HPV) in head and neck region: review of literature. Acta Otorhinolaryngol Ital 2009;29(3):119–126. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2815356/. [PMC free article] [PubMed] [Google Scholar]
- 33.Keck MK, Zuo Z, Khattri A, et al. Integrative Analysis of Head and Neck Cancer Identifies Two Biologically Distinct HPV and Three Non-HPV Subtypes. Clin Cancer Res 2015;21(4):870 LP-881. http://clincancerres.aacrjournals.org/content/21/4/870.abstract. [DOI] [PubMed] [Google Scholar]
- 34.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2017;17(7):956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 35.Gillison ML, Blumenschein G, Fayette J, et al. Abstract CT099: Nivolumab (nivo) vs investigator's choice (IC) for recurrent or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): CheckMate-141. Cancer Res 2016;76(14 Supplement):CT099 LP-CT099. http://cancerres.aacrjournals.org/content/76/14_Supplement/CT099.abstract. [Google Scholar]
- 36.Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zandberg D, Algazi A, Jimeno A, et al. 1042ODurvalumab for recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): Preliminary results from a single-arm, phase 2 study. Ann Oncol 2017;28(suppl_5). doi: 10.1093/annonc/mdx374. [DOI] [Google Scholar]
- 38.Gilbert J, Le Tourneau C, Mehanna H, et al. Phase II, randomized, open-label study of durvalumab (MEDI4736) or tremelimumab monotherapy, or durvalumab + tremelimumab, in patients with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): CONDOR. J Immunother Cancer 2015;3(Suppl 2):P152–P152. doi: 10.1186/2051-1426-3-S2-P152. [DOI] [Google Scholar]
- 39.Ferris RL, Even C, Haddad R, et al. Phase III, randomized, open-label study of durvalumab (MEDI4736) monotherapy, or durvalumab + tremelimumab, versus standard of care (SoC), in recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): eagle. J Immunother Cancer 2015;3(Suppl 2):P150–P150. doi: 10.1186/2051-1426-3-S2-P150. [DOI] [Google Scholar]
- 40.Mezache L, Paniccia B, Nyinawabera A, Nuovo GJ. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol 2015;28(12):1594–1602. 10.1038/modpathol.2015.108. [DOI] [PubMed] [Google Scholar]
- 41.Heong V, Ngoi N, Tan DSP. Update on immune checkpoint inhibitors in gynecological cancers. J Gynecol Oncol 2017;28(2):e20. doi: 10.3802/jgo.2017.28.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frenel J-S, Le Tourneau C, O’Neil BH, et al. Pembrolizumab in patients with advanced cervical squamous cell cancer: Preliminary results from the phase Ib KEYNOTE-028 study. J Clin Oncol 2016;34(15_suppl):5515. doi: 10.1200/JCO.2016.34.15_suppl.5515. [DOI] [Google Scholar]
- 43.Hollebecque A, Meyer T, Moore KN, et al. An open-label, multicohort, phase I/II study of nivolumab in patients with virus-associated tumors (CheckMate 358): Efficacy and safety in recurrent or metastatic (R/M) cervical, vaginal, and vulvar cancers. J Clin Oncol 2017;35(15_suppl):5504. doi: 10.1200/JCO.2017.35.15_suppl.5504. [DOI] [Google Scholar]
- 44.Schellens JHM, Marabelle A, Zeigenfuss S, Ding J, Pruitt SK, Chung HC. Pembrolizumab for previously treated advanced cervical squamous cell cancer: Preliminary results from the phase 2 KEYNOTE-158 study. J Clin Oncol 2017;35(15_suppl):5514. doi: 10.1200/JCO.2017.35.15_suppl.5514. [DOI] [Google Scholar]
- 45.Nivolumab in Treating Patients With Persistent, Recurrent, or Metastatic Cervical Cancer - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02257528. Accessed October 17, 2017.
- 46.Pickering CR, Zhou JH, Lee JJ, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res 2014;20(24):6582–6592. doi: 10.1158/1078-0432.CCR-14-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9(1):34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papadopoulos KP, Owonikoko TK, Johnson ML, et al. REGN2810: A fully human anti-PD-1 monoclonal antibody, for patients with unresectable locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC)—Initial safety and efficacy from expansion cohorts (ECs) of phase I study. J Clin Oncol 2017;35(15_suppl):9503. doi: 10.1200/JCO.2017.35.15_suppl.9503. [DOI] [Google Scholar]
- 49.Heeren AM, Punt S, Bleeker MCG, et al. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol 2016;29(7):753–763. doi: 10.1038/modpathol.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parikh F, Duluc D, Imai N, et al. Chemoradiotherapy-Induced Upregulation of PD-1 Antagonizes Immunity to HPV-Related Oropharyngeal Cancer. Cancer Res 2014;74(24):7205 LP-7216. http://cancerres.aacrjournals.org/content/74/24/7205.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papadopoulos KP, Crittenden MR, Johnson ML, et al. A first-in-human study of REGN2810, a monoclonal, fully human antibody to programmed death-1 (PD-1), in combination with immunomodulators including hypofractionated radiotherapy (hfRT). J Clin Oncol 2016;34(15_suppl):3024. doi: 10.1200/JCO.2016.34.15_suppl.3024. [DOI] [Google Scholar]
- 52.Burova E, Hermann A, Waite J, et al. Characterization of the Anti–PD-1 Antibody REGN2810 and Its Antitumor Activity in Human <em>PD-1</em> Knock-In Mice. Mol Cancer Ther 2017;16(5):861 LP-870. http://mct.aacrjournals.org/content/16/5/861.abstract. [DOI] [PubMed] [Google Scholar]
- 53.Study of REGN2810 in Adults With Cervical Cancer - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03257267. Accessed October 22, 2017.
- 54.Gandara DR, Von Pawel J, Sullivan RN, et al. Impact of atezolizumab (atezo) treatment beyond disease progression (TBP) in advanced NSCLC: Results from the randomized phase III OAK study. J Clin Oncol 2017;35(15_suppl):9001. doi: 10.1200/JCO.2017.35.15_suppl.9001.**First trial to give clinical relevance to the concept of pseudoprogression for patients on immunotherapy
- 55.Genmab Announces Preliminary Cervical Cancer Data from Tisotumab Vedotin Phase I/II Study http://files.shareholder.com/downloads/AMDA-KPIBN/5123554297x0x946853/AD8E3C8F-3062-4550-827E-207F236C826D/22CA_Tisotumabvedotindata_160617.pdf. Accessed September 4, 2017.
- 56.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 2009;9(8):550–562. 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 57.Rashmi R, DeSelm C, Helms C, et al. AKT Inhibitors Promote Cell Death in Cervical Cancer through Disruption of mTOR Signaling and Glucose Uptake. Cheng JQ, ed. PLoS One 2014;9(4):e92948. doi: 10.1371/journal.pone.0092948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh K-J, Kalinina A, Park N-H, Bagchi S. Deregulation of eIF4E: 4E-BP1 in Differentiated Human Papillomavirus-Containing Cells Leads to High Levels of Expression of the E7 Oncoprotein. J Virol 2006;80(14):7079–7088. doi: 10.1128/JVI.02380-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hou M-M, Liu X, Wheler J, et al. Targeted PI3K/AKT/mTOR therapy for metastatic carcinomas of the cervix: A phase I clinical experience. Oncotarget 2014;5(22):11168–11179. doi: 10.18632/oncotarget.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tinker AV, Ellard S, Welch S et al. Phase II study of temsirolimus (CCI-779) in women with recurrent, unresectable, locally advanced or metastatic carcinoma of the cervix. A trial of the NCIC Clinical Trials Group (NCIC CTG IND 199). Gynecol Oncol 2013;130(2):269–274. doi: 10.1016/J.YGYNO.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Michels J, Vitale I, Galluzzi L, et al. Cisplatin Resistance Associated with PARP Hyperactivation. Cancer Res 2013;73(7):2271 LP-2280. http://cancerres.aacrjournals.org/content/73/7/2271.abstract. [DOI] [PubMed] [Google Scholar]
- 62.Reinbolt RE, Hays JL. The Role of PARP Inhibitors in the Treatment of Gynecologic Malignancies. Front Oncol 2013;3:237. doi: 10.3389/fonc.2013.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kunos C, Deng W, Dawson D, et al. A phase I–II evaluation of veliparib (NSC#737664), topotecan, and filgrastim or pegfilgrastim in the treatment of persistent or recurrent carcinoma of the uterine cervix: An NRG Oncology/Gynecologic Oncology Group study. Int J Gynecol Cancer 2015;25(3):484–492. doi: 10.1097/IGC.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thaker PH, Salani R, Brady WE, et al. A phase I trial of paclitaxel, cisplatin, and veliparib in the treatment of persistent or recurrent carcinoma of the cervix: an NRG Oncology Study (NCT#01281852). Ann Oncol 2017;28(3):505–511. 10.1093/annonc/mdw635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lemery S, Keegan P, Pazdur R. First FDA Approval Agnostic of Cancer Site — When a Biomarker Defines the Indication. N Engl J Med 2017;377(15):1409–1412. doi: 10.1056/NEJMp1709968.**First FDA approval of a tumor-agnostic therapy