Abstract
Background
Hepatocellular carcinoma (HCC) is one of the most common malignancies around the world. It has been verified that the expression of SOX18 is correlated to poor clinical prognosis in patients with ovarian cancer, non-small cell lung cancer, or breast invasive ductal carcinoma. However, the expression pattern and biological function of SOX18 in HCC tissues remains unclear. In this study, we set out to investigate the associated biological function and potential molecular mechanism of the SOX18 gene in HCC cells.
Material/Methods
The mRNA and protein expression levels of experimental related genes were detected by real-time polymerase chain reaction and western blotting assay, respectively. The MTT method was used to assess cell viability, and cell apoptosis analysis was performed by means of FACScan flow cytometry. Wound-healing assay and Transwell analysis were performed to evaluate the ability of cell migration and invasiveness, respectively.
Results
SOX18 was highly expressed in various HCC cell lines. In addition, SOX18 promoted cell viability, migration, and invasion and simultaneously induce cell apoptosis. SOX18 promoted epithelial-to-mesenchymal transition (EMT) progression, and SOX18 downregulation activated the autophagy signaling pathway AMPK/mTOR in HCC cells.
Conclusions
SOX18 downregulation in HCC cells suppressed cell viability and metastasis, induced cell apoptosis and hindered the occurrence and progression of tumor cells by participating in the EMT process and regulating the autophagy signaling pathway AMPK/mTOR.
MeSH Keywords: Apoptosis; Carcinoma, Hepatocellular; Cell Survival; Neoplasm Metastasis; SOXF Transcription Factors
Background
Hepatocellular carcinoma (HCC) is one of the most common malignancies around the world, with high mortality, poor prognosis, and high propensity to infiltration and metastasis, which seriously threatens the life of HCC patients [1,2]. In recent years, abundant progress has been made in basic and clinical research on HCC [3,4]. However, the overall survival rate of patients with HCC is fairly low, primarily because most patients are in the advanced stage of disease at diagnosis and thus lose the opportunity for radical surgical treatment [5]. In addition, the prognosis of patients suitable for surgical therapy is still poor, due to the high potential for blood metastasis, distant metastasis, and recurrence of HCC cells [2]. Therefore, it is essential to investigate the regulatory mechanism of oncogenes and probe oncogenic signal transduction pathways.
The SOX gene family is composed of a class of transcription factors with highly conserved HMG DNA binding domain [6,7]. Sex determining region Y box F (SOXF) 18, SOX18 for short, is a member of the SOX transcription factor F (SOXF) subfamily, which refers to a type of developmental gene that encodes transcription factors [8]. It has been found that there are some associations between SOX transcription factors and human cancers [9]. For example, it has been reported that SOX2 is a potential oncogene in breast and lung cancer tissues [10,11]. Furthermore, recent studies have confirmed that SOX18 is abnormally expressed in various tumor tissues and cell lines, and the aberrant expression of SOX18 is also involved in the occurrence, development, and metastasis of tumors [12]. Research has also demonstrated that SOX18 knockdown could inhibit the growth and invasion of breast cancer cells [13]. Knockdown of SOX18 inhibits the proliferation, migration, and invasion of HCC cells [14]. In addition, it has been verified that SOX18 is associated with poor clinical prognosis in patients with ovarian cancer, non-small cell lung cancer, or breast invasive ductal carcinoma [15].
However, in spite of these advances, the expression pattern and biological function of SOX18 in HCC tissues remains unknown. Here, we set out to investigate the biological function and potential molecular mechanism of SOX18 in HCC cells by constructing models of overexpression and silencing of SOX18. Ultimately, we found that SOX18 was highly expressed in HCC cells and was involved in various biological functions of tumor cells, including tumor cell proliferation, apoptosis, metastasis and epithelial-to-mesenchymal transition (EMT) progression. Overall, SOX18 may serve as a potential target for cancer treatment, providing a new perspective for revealing the molecular mechanism of tumor occurrence and development, and expanding its underlying value in clinical application.
Material and Methods
Cell lines and tissue culture
Eight kinds of human hepatoma cell lines, including Hep3B, Huh-7, MHCC-97H, MHCC-97L, MHCC-LM6, MHCC-LM3, YY-8103, and SK-hep-1, as well as the normal human hepatic cell line MIHA were used in this experiment. All the cell lines aforementioned were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) and were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA, USA), 100 mg/mL penicillin G and 50 μg/mL streptomycin (Life Technologies, Carlsbad, CA, USA) within a humidified incubator containing 5% CO2 at 37°C.
Cell transfections and RNA interference
The MHCC-97H cell line was selected for subsequent experiments and cells were maintained in 6-well plates with a density of 4×105 cells per well. Cells were transiently transfected with: SOX18 siRNA, SOX18 overexpression RNA, si-NC (si-small interfering negative control) and NC with an ultima concentration of 60 nM by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), in accordance with the manufacturer’s instructions. Assays were performed 48 hours after transfection.
RNA extraction and real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s protocol. cDNA was synthesized using the Prime Script RT reagent kit (Takara, Dalian, China) in accordance with the manufacturer’s protocol. RT-PCR was conducted by using the Thermo Power SYBR Green RT-PCR Reagents Kit, and the assay samples were processed using the ABI 7500 Thermocycler System (Applied Biosystems, CA, USA). The RT-PCR cycle was as follows: first preconditioning for 2 minutes at 94°C, followed by 35 cycles for 30 seconds at 94°C, 30 seconds at 63°C, and 1 minute at 72°C, with a final 7 minutes at 72°C; then specimens were held at 4°C. GAPDH served as an internal control, and each specimen was tested in triplicate. The expression level was calculated by 2−ΔΔCT method. The sequences of RT-PCR primers (Invitrogen, Carlsbad, CA, USA) are listed in Table 1.
Table 1.
Sequences of RT-PCR primers.
| Primer | Sequence |
|---|---|
| SOX18 | F: 5′-CGCGTGTATGTTTGGTTC-3′ |
| R: 5′-GTAACCCTGGCAACTC-3′ | |
| E-cadherin | F: 5′-CCGCCGGCGTCTGTAGGAA-3′ |
| R: 5′-AGGGCTCTTTGACCACCGCTCTC-3′ | |
| Vimentin | F: 5′-GGACCAGCTAACCAACGACA-3′ |
| R: 5′-AAGGTCAAGACGTGCCAGAG-3′ | |
| α-SMA | F: 5′-TGCCGCGACCTCAAGATGTG-3′ |
| R: 5′-CACAAGGGTGCTGTAGGTGA-3′ | |
| GAPDH | F: 5′-CACCCACTCCTCCACCTTTG-3′ |
| R: 5′-CCACCACCCTGTTGCTGTAG-3′ |
Protein extraction and western blotting assay
The extraction of total protein was performed by lysing the cultured cells using radioimmunoprecipitation assay lysis buffer (RIPA; Beyotime, Shanghai, China) supplemented with phenylmethylsulfonylfluoride (PMSF; Beyotime, Shanghai, China). The concentrations of proteins were tested by bicinchoninic acid protein assay kit (BCA; Beyotime, Shanghai, China). Equivalent proteins were isolated by means of 12% SDS-PAGE (Beyotime, Shanghai; P0012A) and then were transferred onto a polyvinylidene fluoride (PVDF; Beyotime, Shanghai; FFP28) membrane. The immunoassay of proteins was performed via specific antibodies. The chemiluminescence detection was conducted by means of ECL (Amersham Pharmacia, Piscataway, NJ, USA) system, and density analysis was performed by ImageJ software. Primary antibodies were as follows: SOX18 (ab23342, 1: 500, Abcam, USA), E-cadherin (ab15148, 1: 1000, Abcam, USA), vimentin (ab137321, 1: 500, Abcam, USA), AMPK (ab32047, 1: 1000, Abcam, USA), p-AMPK (ab92701, 1: 1000, Abcam, USA), mTOR (ab2732, 1: 2000, Abcam, USA), p-mTOR (ab109268, 1: 1000, Abcam, USA), and α-SMA (GTX100034, 1: 1000, GeneTex, USA). The antibodies against GAPDH served as an internal reference. HRP-conjugated goat anti-rabbit IgG (ab6741, 1: 5000, Abcam, USA) were used as secondary antibodies.
MTT method
The cell viability was assessed by using MTT Cell Proliferation and Cytotoxicity Assay Kit (Gefan Biotechnology, Shanghai, China). Cells in the overexpression group, the silencing group, and the control group were collected in logarithmic growth stage, and were hydrolyzed by trypsin. After treatment, 100 μL cell suspension of the concentration, of which had been adjusted to 2×103/mL, was added to each well in the 96-well plate.
Cells were cultured in a humidified atmosphere with 5% CO2 at 37°C, and cell viability was measured at 12 hours, 24 hours, and 48 hours after incubation. Then 10 μL MTT solution with a concentration of 5 mg/mL was added to each well and incubated for 4 hours until bluish violet precipitates appeared. Then 100 μL formazan solution was added to each well and cells were incubated for another 4 hours until complete dissolution of the formazan. The absorbance of each well solution was measured at 570 nm by means of a microplate reader, and then the logarithmic phase growth curve was generated according to the absorbance value. All experiments were performed at least 3 times.
Wound-healing assay
A marker pen was used to mark the back of the 6-well cell culture plates and then corresponding cells were seeded into each well such that inoculation fusion rate could reach 100% as a monolayer overnight. After the bottoms of the culture plates were covered by the cells, a 200 μL pipette tip placed perpendicular to the culture plates was used to make several scratches of consistent width. Afterwards, the cell culture solution was poured off and then cells were washed 3 times by PBS buffer to remove cell fragments generated by the scratch. Serum-free medium was added to each well and the culture plates were maintained in an incubator with 5% CO2 at 37°C. Subsequently, pictures and notes were taken at 0 hours and 24 hours, respectively. ImageJ software was used to analyze and calculate the healing rate of the scratch in accordance with the collected images. All experiments were repeated at least 3 times and we used the average migration distance to represented relative cell migration ability.
Transwell invasion assay
In the invasion assay, cell suspensions in which the corresponding cells had been serum-starved for 12 hours, were placed to the upper chamber insert which was coated with Matrigel (BD Biosciences, USA). Next, culture media supplemented with 20% FBS (Life Technologies, Carlsbad, CA, USA) was added into the chamber. Then the cells were maintained in an incubator for 24 hours. A cotton swab was used to remove the upper cells that had not invaded through the membrane. The chamber was air-dried properly, and then the cells that had invaded to the back of the membrane were fixed with 4% methanol for 15 minutes and stained with 0.1% crystal violet for 20 minutes. Lastly, the number of the stained cells within 5 randomly selected fields was counted by means of a light microscope. The results represented the averages of 3 independent experiments.
Cell apoptosis assay
Cell apoptosis was measured by means of Annexin V-FITC/PI Apoptosis Detection Kit (YEASEN, Shanghai, China). Adherent cells as well as suspension cells were harvested and then resuspended in phosphate-buffered saline (PBS). Afterwards, the cell suspension was incubated together with Annexin V-FITC for 15 minutes and PI for 10 minutes. The analysis of apoptosis was performed by means of FACScan flow cytometry.
Statistical analysis
SPSS 22.0 software (Chicago, IL, USA) was used for statistical analyses. All data derived from 3 independent experiments were expressed in the form of mean ± standard deviation. The differences of measurement data among multiple groups were evaluated by one-way analysis of variance (ANOVA). A P value <0.05 was of statistical significance.
Results
SOX18 was highly expressed in various HCC cell lines
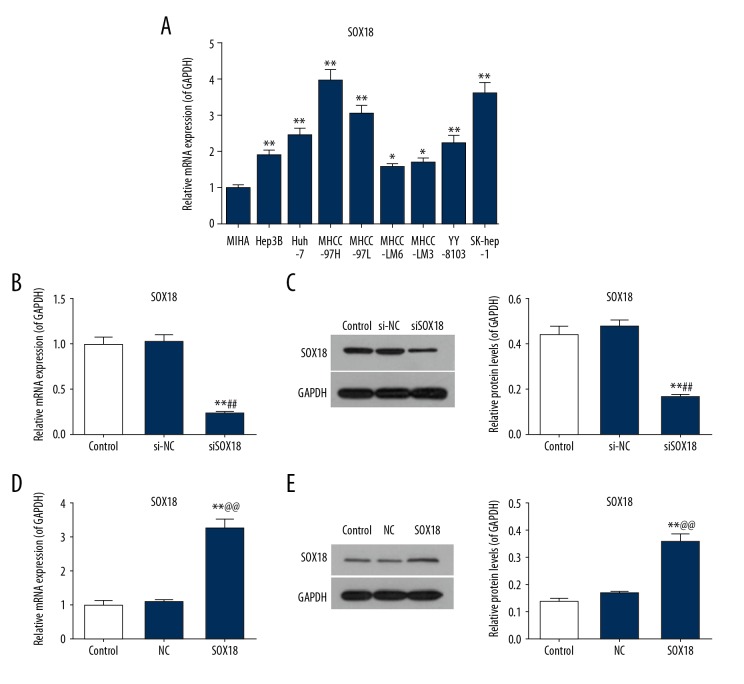
For the purpose of exploring the mechanism of action of SOX18 on the biological function of HCC cells, the mRNA expression levels of SOX18 were evaluated in 8 different HCC cell lines (Hep3B, Huh-7, MHCC-97H, MHCC-97L, MHCC-LM6, MHCC-LM3, YY-8103, and SK-hep-1) and 1 normal immortalized hepatocytes line (MIHA) using real-time PCR. The HCC cell lines, especially the MHCC-97H cells, showed a significantly higher level of SOX18 expression than the normal immortalized hepatocytes (Figure 1A, P<0.05 or P<0.01). MHCC-97H cells were selected for the subsequent experiments. MHCC-97H cells transfected with siSOX18 showed a lower level of SOX18 expression compared to the control group and the si-NC group (Figure 1B, 1C, P<0.01). Nevertheless, the expression of SOX18 in MHCC-97H cells transfected with overexpressing SOX18 was significantly enhanced compared to the control and NC cells (Figure 1D, 1E, P<0.01). These findings suggested that SOX18 might play key roles in occurrence and development of HCC.
Figure 1.
SOX18 was highly expressed in hepatocellular carcinoma (HCC) cells. (A) The mRNA expression level of SOX18 was detected by real-time PCR in 8 hepatoma cell lines (Hep3B, Huh-7, MHCC-97H, MHCC-97L, MHCC-LM6, MHCC-LM3, YY-8103, and SK-hep-1) as well as 1 normal hepatocyte (MIHA) cell line. Due to the significantly highest expression level of SOX18, MHCC-97H cells were selected for the subsequent experiments. (* P<0.05 and ** P<0.01 versus MIHA). The transfection efficiencies of silencing SOX18 (B, C) and overexpressing SOX18 (D, E) were detected by real-time PCR and western blotting assay in MHCC-97H cells. GAPDH served as an internal control. Data were derived from at least 3 independent experiments and were presented as mean ± standard deviation (** P<0.01 versus control, ## P<0.01 versus si-NC, and @@ P<0.01 versus NC). NC – negative control; si-NC – small interfering negative control; siSOX18 – small interfering SOX18.
SOX18 could regulate cell viability and apoptosis in HCC cells
In order to further probe the influences of S0X18 on HCC cells, the behaviors of HCC cells were observed. MTT assay was conducted to determine the effects of SOX18 on the viability of HCC cells. Cell viability in the silencing SOX18 group was significantly decreased in comparison with that in the si-NC group and the control group (Figure 2A, P<0.01). In contrast, cell viability in the overexpressing SOX18 group was significantly increased compared to the NC group and the control group (Figure 2B, P<0.05 or P<0.01). Afterwards, cell apoptosis analysis was performed in HCC cells for the purpose of investigating impacts of SOX18 on the apoptosis of HCC cells. Obviously, cell apoptosis rates in the silencing SOX18 group were significantly increased in comparison with the si-NC group and the control group (Figure 2C, P<0.01). Nevertheless, the cell apoptosis rate in the overexpressing SOX18 group was significantly reduced compared with the NC group and the control group (Figure 2D, P<0.01). The outcomes revealed that SOX18 knockdown could inhibit cell viability and induce cell apoptosis simultaneously in HCC cells.
Figure 2.
Impacts of the expression level of SOX18 on cell viability and apoptosis of hepatocellular carcinoma cells. (A, B) Cell viability was detected by MTT assay in control, si-NC,siSOX18, NC, and SOX18 cells. (C, D) Cell apoptosis analysis was performed by means of FACScan flow cytometry. Data were derived from at least 3 independent experiments and were presented as mean ± standard deviation (* P<0.05 and ** P<0.01 versus control, ## P<0.01 versus si-NC and @ P<0.05 and @@ P<0.01 versus NC). NC – negative control; si-NC – small interfering negative control; siSOX18 – small interfering SOX18.
SOX18 was closely related to cell migration and invasiveness in MHCC-97H cells
Wound-healing assay was implemented to probe the corresponding function of SOX18 on the mobility of MHCC-97H cells. We observed a significant different in cell migration between siSOX18 and SOX18 cells. Cell migration in the SOX18 knockdown group was notably damaged compared with that in the si-NC group and the control group (Figure 3A, P<0.01). By contrast, cell migration in the overexpressing SOX18 group was notably superior to that in the NC group and the control group (Figure 3B, P<0.01). Furthermore, to explore the connection of SOX18 with cell invasion in MHCC-97H cells, Transwell chamber assay was carried out accordingly (Figure 3C, 3D). Apparently, SOX18 was positively correlated with cell invasiveness in MHCC-97H cells. These findings indicated that SOX18 knockdown was able to depress cell migration and invasion in MHCC-97H cells.
Figure 3.
Effects of the expression level of SOX18 on cell migration and invasion in MHCC-97H cells. (A, B) Cell migration was tested by wound-healing assay in MHCC-97H cells. (C, D) Cell invasion assay was performed in Matrigel-coated Transwell chambers in MHCC-97H cells. Data were derived from at least 3 independent experiments and were presented as mean ± standard deviation (** P<0.01 versus control, ## P<0.01 versus si-NC and @@ P<0.01 versus NC). NC – negative control; si-NC – small interfering negative control; siSOX18 – small interfering SOX18.
SOX18 was involved in EMT progression by regulating related genes in HCC cells
To explore the potential molecular mechanism of SOX18 in HCC cells, the mRNA and protein expression levels of EMT related genes for E-cadherin, vimentin, and α-SMA were determined by RT-PCR and western blotting, respectively. Clearly, the mRNA and protein expression levels of E-cadherin in the siSOX18 group were significantly increased in comparison with the NC group and the control group. Nevertheless, the mRNA and protein expression levels of E-cadherin in the SOX18 group were notably decreased compared to the NC group and the control group (Figure 4A, 4D, 4E, P<0.01). Besides, the mRNA and protein expression levels of both vimentin and α-SMA in the siSOX18 group were significantly inferior to that in the NC group and the control group. Apparently, the mRNA and protein expression levels of both vimentin and α-SMA in the SOX18 group showed contrary results (Figure 4B–4E, P<0.01). The outcome showed that SOX18 knockdown was able to promote the expression of E-cadherin, but inhibited the expression of vimentin and α-SMA.
Figure 4.
The expression level of SOX18 could regulate the expression of epithelial to mesenchymal transition (EMT) related proteins E-cadherin, vimentin and α-SMA in hepatocellular carcinoma cells. The mRNA (A–C) and protein (D, E) expression levels of EMT related proteins E-cadherin, vimentin and α-SMA were detected by real-time PCR and western blotting, respectively. GAPDH served as an internal control. Data were derived from at least 3 independent experiments and were presented as mean ± standard deviation (** P<0.01 versus control and @@ P<0.01 versus NC). NC – negative control; siSOX18 – small interfering SOX18; α-SMA – α-smooth muscle actin.
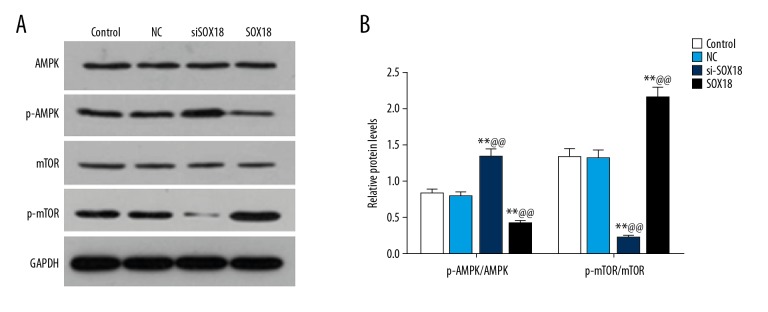
SOX18 could make an impact on the phosphorylation levels of AMPK/mTOR signaling pathway in HCC cells
To investigate the corresponding signaling pathway of SOX18 in HCC cells, western blotting analysis of the AMPK/mTOR signaling pathway was performed in HCC cells. A negative correlation was observed between the relative protein levels of p-AMPK/AMPK and the expression level of SOX18. Contrarily, a positive association was observed between the relative protein levels of p-mTOR/mTOR and the expression level of SOX18 (Figure 5A, 5B, P<0.01). The findings suggested that SOX18 knockdown could increase the phosphorylation levels of AMPK, but decrease the phosphorylation levels of mTOR in HCC cells. In other words, SOX18 knockdown might be capable of activating the AMPK/mTOR signaling pathway to suppress the development of tumor.
Figure 5.
Western blotting analysis of AMPK/mTOR signaling pathway in hepatocellular carcinoma cells. (A) Western blotting assay of phosphorylation levels of AMPK and mTOR in control, NC, siSOX18 and SOX18 cells. (B) The relative protein expression levels of p-AMPK/AMPK and p-mTOR/mTOR were assessed by ImageJ software. GAPDH served as an internal control. Data were derived from at least 3 independent experiments and were presented as mean ± standard deviation (** P<0.01 versus control and @@ P<0.01 versus NC). NC – negative control; siSOX18 – small interfering SOX18; AMPK – adenosine monophosphate activated protein kinase; mTOR – mammalian target of rapamycin.
Either the activator of the AMPK signaling pathway or the specific inhibitor of mTOR signaling pathway could inhibit the viability of MHCC-97H cells in which SOX18 was overexpressed.
Cell viabilities of MHCC-97H cells in which SOX18 was overexpressed were significantly impaired after being disposed by the activator of AMPK signaling pathway AICAR (Figure 6A, P<0.05 or P<0.01). Similarly, cell viabilities of MHCC-97H cells in which SOX18 was overexpressed were also significantly weakened after being treated by the specific inhibitor of mTOR signaling pathway rapamycin (Figure 6B, P<0.05 or P<0.01). The results implied that the corresponding activator or inhibitor of the AMPK/mTOR signaling pathway could be considered to be a novel approach to deactivation of HCC cells.
Figure 6.
Influences of the activator of AMPK signaling pathway AICAR and the specific inhibitor of mTOR signaling pathway rapamycin on the viabilities of MHCC-97H cells in which SOX18 was overexpressed. (A) MHCC-97H cells in which SOX18 was overexpressed were treated by the activator of AMPK signaling pathway AICAR (1 mM) for 12 hours, 24 hours, and 48 hours, respectively. Subsequently, the viabilities of the treated cells above were calculated by MTT assay. (B) MHCC-97H cells in which SOX18 was overexpressed were treated by the specific inhibitor of mTOR signaling pathway rapamycin (10 nM) for 12 hours, 24 hours, and 48 hours, respectively. Next, the viabilities of the treated cells were calculated by MTT assay. Data were derived from at least 3 independent experiments and were presented as mean ± standard deviation (* P<0.05 and ** P<0.01 versus control, @ P<0.05 and @@ P<0.01 versus NC, & P<0.05 and && P<0.01 versus SOX). NC – negative control; AICAR – 5-aminoimidazole-4-carboxamide1-β-D-ribofuranoside.
Discussion
SOX18 plays a major part in the development of blood vessels and lymphatic vessels and its gene mutation or abnormal expression is closely related to the occurrence and development of tumors [16]. Here, in the current study, we assessed the expression level of SOX18 among 8 HCC cell lines (Hep3B, Huh-7, MHCC-97H, MHCC-97L, MHCC-LM6, MHCC-LM3, YY-8103, and SK-hep-1) and 1 normal immortalized hepatocytes (MIHA) cell line. Similar to previous research, we found that SOX18 was highly expressed in various HCC cell lines compared to normal immortalized hepatocytes. Moreover, our results showed that SOX18 knockdown could impair the endogenic expression of SOX18 in HCC cells.
Previous studies have indicated that SOX18 can boost cell proliferation in vascular smooth muscle cells as well as MCF-7 breast cancer cells [17,18]. Consistent with these findings, knocking down the expression of SOX18 could notably restrain cell viability in MHCC-97H cells. Furthermore, our study outcomes suggest that SOX18 knockdown was in a position to induce cell apoptosis in HCC cells. Broadly, SOX18 might boost the growth of HCC cells through the aforementioned approaches, which coincides with the results reported previously [14].
Moreover, in the previous reports, it was found that SOX18 knockdown could generate the inhibition of tumor lymph angiogenesis and mouse melanoma metastasis [19]. Congruously, in this study, we provided evidence that the ability of cell migration in the SOX18 knockdown group was notably weakened compared to that in the si-NC group and the control group. Also, the expression level of SOX18 was found to be positively correlated with the invasive ability of MHCC-97H cells, implying that metastasis of liver cancer cells may be impeded by the downregulation of SOX18.
Epithelial-to-mesenchymal transition (EMT), refers to the process of the transformation of epithelial cells into mesenchymal phenotype cells, which plays a critical role in embryonic development, tumor metastasis, chronic inflammation, tissue reconstruction, and certain fibrotic diseases [20]. EMT is characterized by the disappearance of epithelial cell markers such as E-cadherin and so on, which are replaced by mesenchymal cell markers such as α-SMA muscle actin (α-SMA) and vimentin [21–23]. EMT transcription factors play a vital role in the EMT activation process. They are able to directly or indirectly affect the expression of cell surface proteins (E-cadherin) and cytoskeletal molecules (α-SMA and vimentin) to participate in the regulation of EMT procedure, which impacts on the occurrence and development of tumors [24]. Crucially, E-cadherin expression inhibition is considered to be a hallmark event in the activation of EMT procedure [25,26]. To make further investigation concerning the molecular mechanism of SOX18, the expression levels of several EMT related proteins such as E-cadherin, vimentin, and α-SMA, were assessed. Consistent with the aforementioned reports, our findings showed that mRNA and protein expression levels of E-cadherin in the siSOX18 group were significantly increased, nevertheless, the mRNA and protein expression levels of both vimentin and α-SMA in the siSOX18 group were significantly decreased, indicating that SOX18 knockdown might suppress the development of tumors through the approaches to regulating the correlative proteins in EMT procedure.
Tumors are caused by the disorder of signal transmission which leads to the infinite proliferation of cells. Currently, in-depth studies of abnormal transmission pathways to find effective targets has been a research hotspot in tumor therapy. Autophagy is a conservative self-degradation system which is a process of degrading and recycling long lived proteins, protein aggregates, and damaged organelles in the cytoplasm, and as such plays a vital role in maintaining tissue or organ stability and anti-tumor activities [27]. Adenosine monophosphate activated protein kinase (AMPK) and mammalian rapamycin target protein (mTOR) are pivotal molecules in the regulation of bioenergy metabolism and biosynthesis, and the negative regulatory relationship between them can lead to apoptosis [28]. AMPK can sensitively detect the changes of AMP/ATP ratio caused by rapid and massive cell proliferation, and then be activated [29]. AMPK, activated in different ways, can directly or indirectly inhibit the activity of mTORC1, and cause the phosphorylation process of its related downstream molecules to be blocked, which hinders the syntheses of corresponding proteins and ultimately inhibits the growth and proliferation of tumor cells [30]. In line with the aforementioned findings, in the present study, we observed a negative correlation between the relative protein levels of p-AMPK/AMPK and the expression level of SOX18; and to the contrary, a positive association was observed between the relative protein levels of p-mTOR/mTOR and the expression level of SOX18, implying that SOX18 knockdown might depress the progression of tumors by means of activating the AMPK/mTOR signaling pathway to induce the apoptosis of HCC cells.
Previous studies have shown that AMPK agonist AICAR could inhibit mTOR activity directly or indirectly, impede cell cycle progression, and induce apoptosis in the treatment of renal cell carcinoma [31]. mTOR is a negative regulator of autophagy, and rapamycin is an inhibitor of mTOR, which can induce the occurrence of autophagy and is regarded as an inducer of autophagy [32]. In this study, we found that the cell viability of MHCC-97H cells in which SOX18 was overexpressed were significantly impaired by AICAR or rapamycin, which suggests that the corresponding activator of the AMPK signaling pathway or inhibitor of mTOR signaling pathway might be considered to be a novel approach for deactivation of tumors.
Conclusions
In this study, we demonstrated that SOX18 knockdown in HCC cells suppressed tumor cell proliferation and metastasis, induced tumor cell apoptosis, and hindered the occurrence and development of tumor cells by participating in the EMT process and regulating the autophagy signaling pathway AMPK/mTOR. As a transcription factor, SOX18 regulates a variety of downstream genes, which can affect the biological function of tumor cells by regulating other molecules. Therefore, research on the target of SOX18 might provide references for targeted therapy and drug research, and better understanding of the development of tumors. However, there were some limitations to our study. Future work should include clarifying the relationship between SOX18 and tumors, exploring in great depth the mechanism of signal transduction mediated by SOX18, the value of targeted therapy, and its impact on tumor therapy.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Harley VR, Jackson DI, Hextall PJ, et al. DNA binding activity of recombinant SRY from normal males and XY females. Science. 1992;255(5043):453–56. doi: 10.1126/science.1734522. [DOI] [PubMed] [Google Scholar]
- 2.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of HCC. Ann Surg. 2000;232(1):10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J, Gores GJ, Mazzaferro V. HCC: Clinical frontiers and perspectives. Gut. 2014;63(5):844–55. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruix J, Han KH, Gores G, et al. Liver cancer: Approaching a personalized care. J Hepatol. 2015;62(1 Suppl):S144–56. doi: 10.1016/j.jhep.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang RW, Poon RT. From molecular biology to targeted therapies for HCC: The future is now. Oncology. 2007;72(Suppl 1):30–44. doi: 10.1159/000111705. [DOI] [PubMed] [Google Scholar]
- 6.Wilson M, Koopman P. Matching SOX: Partner proteins and co-factors of the SOX family of transcriptional regulators. Curr Opin Genet Dev. 2002;12(4):441–46. doi: 10.1016/s0959-437x(02)00323-4. [DOI] [PubMed] [Google Scholar]
- 7.Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227(2):239–55. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- 8.Schepers GE, Teasdale RD, Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell. 2002;3(2):167–70. doi: 10.1016/s1534-5807(02)00223-x. [DOI] [PubMed] [Google Scholar]
- 9.Li B, Ge Z, Song S, et al. Decreased expression of SOX7 is correlated with poor prognosis in lung adenocarcinoma patients. Pathol Oncol Res. 2012;18(4):1039–45. doi: 10.1007/s12253-012-9542-8. [DOI] [PubMed] [Google Scholar]
- 10.Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet. 2012;44(10):1111–16. doi: 10.1038/ng.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eom BW, Jo MJ, Kook MC, et al. The lymphangiogenic factor SOX 18: A key indicator to stage gastric tumor progression. Int J Cancer. 2012;131(1):41–48. doi: 10.1002/ijc.26325. [DOI] [PubMed] [Google Scholar]
- 12.Yin H, Sheng Z, Zhang X, et al. Overexpression of SOX18 promotes prostate cancer progression via the regulation of TCF1, c-Myc, cyclin D1 and MMP-7. Oncol Rep. 2017;37(2):1045–51. doi: 10.3892/or.2016.5288. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Ma Y, Wang S, et al. Suppression of SOX18 by siRNA inhibits cell growth and invasion of breast cancer cells. Oncol Rep. 2016;35(6):3721–27. doi: 10.3892/or.2016.4746. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, Wei Z, Jia H, et al. Knockdown of SOX18 inhibits the proliferation, migration and invasion of HCC cells. Oncol Rep. 2015;34(3):1121–28. doi: 10.3892/or.2015.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pula B, Kobierzycki C, Solinski D, et al. SOX18 expression predicts response to platinum-based chemotherapy in ovarian cancer. Anticancer Res. 2014;34(8):4029–37. [PubMed] [Google Scholar]
- 16.Jethon A, Pula B, Olbromski M, et al. Prognostic significance of SOX18 expression in non-small cell lung cancer. Int J Oncol. 2015;46(1):123–32. doi: 10.3892/ijo.2014.2698. [DOI] [PubMed] [Google Scholar]
- 17.Angulo B, Suarez-Gauthier A, Lopez-Rios F, et al. Expression signatures in lung cancer reveal a profile for EGFR-mutant tumours and identify selective PIK3CA overexpression by gene amplification. J Pathol. 2008;214(3):347–56. doi: 10.1002/path.2267. [DOI] [PubMed] [Google Scholar]
- 18.Beroukhim R, Mermel CH, Porter D, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duong T, Proulx ST, Luciani P, et al. Genetic ablation of SOX18 function suppresses tumor lymphangiogenesis and metastasis of melanoma in mice. Cancer Res. 2012;72(12):3105–14. doi: 10.1158/0008-5472.CAN-11-4026. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7(344):re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolos V, Peinado H, Perez-Moreno MA, et al. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: A comparison with Snail and E47 repressors. J Cell Sci. 2003;116(Pt 3):499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- 22.Borok Z. Role for alpha3 integrin in EMT and pulmonary fibrosis. J Clin Invest. 2009;119(1):7–10. doi: 10.1172/JCI38084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson EW, Paik S, Brunner N, et al. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992;150(3):534–44. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- 24.Kantak SS, Kramer RH. E-cadherin regulates anchorage-independent growth and survival in oral squamous cell carcinoma cells. J Biol Chem. 1998;273(27):16953–61. doi: 10.1074/jbc.273.27.16953. [DOI] [PubMed] [Google Scholar]
- 25.Kang HG, Jenabi JM, Zhang J, et al. E-cadherin cell-cell adhesion in ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res. 2007;67(7):3094–105. doi: 10.1158/0008-5472.CAN-06-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen X, Kramer RH. Adhesion-mediated squamous cell carcinoma survival through ligand-independent activation of epidermal growth factor receptor. Am J Pathol. 2004;165(4):1315–29. doi: 10.1016/S0002-9440(10)63390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagca BG, Ozalp O, Kurt CC, et al. Ruxolitinib induces autophagy in chronic myeloid leukemia cells. Tumour Biol. 2016;37(2):1573–79. doi: 10.1007/s13277-015-3947-4. [DOI] [PubMed] [Google Scholar]
- 28.Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. 2012;52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 29.Rajamohan F, Reyes AR, Frisbie RK, et al. Probing the enzyme kinetics, allosteric modulation and activation of alpha1- and alpha2-subunit-containing AMP-activated protein kinase (AMPK) heterotrimeric complexes by pharmacological and physiological activators. Biochem J. 2016;473(5):581–92. doi: 10.1042/BJ20151051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao B, Azmi AS, Ali S, et al. Metformin may function as anti-cancer agent via targeting cancer stem cells: The potential biological significance of tumor-associated miRNAs in breast and pancreatic cancers. Ann Transl Med. 2014;2(6):59. doi: 10.3978/j.issn.2305-5839.2014.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen MB, Wei MX, Han JY, et al. MicroRNA-451 regulates AMPK/mTORC1 signaling and fascin1 expression in HT-29 colorectal cancer. Cell Signal. 2014;26(1):102–9. doi: 10.1016/j.cellsig.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Xia T, Wang J, Wang Y, et al. Inhibition of autophagy potentiates anticancer property of 20(S)-ginsenoside Rh2 by promoting mitochondria-dependent apoptosis in human acute lymphoblastic leukaemia cells. Oncotarget. 2016;7(19):27336–49. doi: 10.18632/oncotarget.8285. [DOI] [PMC free article] [PubMed] [Google Scholar]