ABSTRACT
Background: Experience of childhood maltreatment significantly increases the risk for the development of psychopathology and is associated with impairments in socio-cognitive skills including theory-of-mind (ToM). In turn, neural alterations in ToM processing might then influence future interpersonal interaction and social-emotional understanding.
Objective: To assess resting-state activity in the theory-of-mind network in traumatized and non-traumatized persons.
Methods: Thirty-five women with a history of childhood maltreatment and 31 unaffected women completed a resting-state scan and a ToM localizer task. The peak coordinates from the localizer were used as the seed regions for the resting-state functional connectivity (RSFC) analyses (temporo-parietal junction, dorsomedial prefrontal cortex, middle temporal gyrus and precuneus).
Results: Child abuse was associated with increased RSFC between various ToM regions including the precuneus and the brainstem suggesting altered hierarchical processing in ToM regions. Number of types of abuse was driving the effect for the temporo-parietal junction and the brainstem, while the severity of abuse was linked to increased RSFC between the middle temporal gyrus and the frontal cortex. Post-hoc analyses of brainstem regions indicated the involvement of the serotonergic system (dorsal raphe).
Conclusions: The data indicate a lasting impact of childhood maltreatment on the neural networks involved in social information processing that are integral to understanding others’ emotional states. Indeed, such altered neural networks may account for some of the interpersonal difficulties victims of childhood maltreatment experience.
KEYWORDS: Childhood abuse, functional connectivity, maltreatment, resting state, social cognition, theory of mind (ToM)
HIGHLIGHTS
• Early psychological trauma influences socio-cognitive skills.• Evidence regarding altered resting-state activity in theory of mind network lacking.• Resting-state functional connectivity was increased between Theory of Mind regions and brainstem in people with early maltreatment experience.• Particularly number of types of abuse appeared to be driving the effect for left temporo-parietal junction (TPJ).• The findings support altered resting-state activity between higher cognitive and more basic brain processes after early maltreatment experience.
Abstract
Antecedentes: La experiencia de maltrato infantil aumenta significativamente el riesgo del desarrollo de psicopatología y se encuentra asociado con deficiencia en las habilidades sociocognitivas, incluyendo la teoría-de-la-mente (ToM en su sigla en inglés). A su vez, las alteraciones neuronales en el procesamiento de la ToM podrían así influenciar las interacciones interpersonales futuras y el entendimiento socioemocional.
Objetivo: Evaluar la actividad del estado de reposo en la red de la teoría-de-la-mente en personas traumatizadas y no traumatizadas.
Métodos: Treinta y cinco mujeres con una historia de maltrato infantil y 31 mujeres no afectadas completaron un escáner en estado de reposo y una tarea localizadora de la ToM. Las coordenadas más altas del localizador fueron usadas como las regiones de origen para los análisis de la conectividad funcional del estado de descanso (RSFC en su sigla en inglés; incluyendo la unión temporoparietal, corteza prefrontal dorsomedial, giros cerebrales temporales medios, y precúneo).
Resultados: El abuso infantil fue asociado con un incremento en la RSFC entre varias regiones de la ToM, incluyendo el precúneo y el tronco encefálico, sugiriendo una alteración en el procesamiento jerárquico en las regiones de la ToM. El número de los tipos de abuso estuvo dirigiendo el efecto de la unión temporoparietal y el tronco encefálico, mientras que la severidad del abuso se relacionó a una aumentada RSFC entre los giros cerebrales temporales medios y la corteza frontal. Los análisis post hoc de las regiones del tronco encefálico indicaron el rol del sistema serotoninérgico (rafe dorsal).
Conclusiones: Los datos indican un impacto de largo plazo del maltrato infantil en las redes neuronales involucradas en el procesamiento de la información social que son fundamentales para el entendimiento de los estados emocionales de otros. De hecho, tales redes neuronales alteradas podrían ser responsables de algunas de las dificultades interpersonales que las víctimas de maltrato infantil experimentan.
PALABRAS CLAVES: Abuso infantil, conectividad funcional, maltrato, estado de reposo, cognición social, teoría de la mente (ToM)
Abstract
背景:童年虐待的经历显著增加了精神病理发展的风险,并与社会认知功能损伤相关,其中包括心理理论(ToM)。反之,ToM加工中的神经改变可能会影响未来的人际交往和社会情感理解。
目的:评估受创伤暴露者和未暴露者的心理理论网络静息态活动。
方法:35名有儿童期虐待史的女性和31名未暴露的女性完成了静息态扫描和ToM定位任务。来自定位任务的峰值坐标用作静息态功能连接(RSFC)分析的种子区域(颞顶连接,背内侧前额叶皮层,中颞回和楔前叶)。
结果:童年虐待与各个ToM区域(包括楔前叶和脑干)之间的RSFC增加有关,表明ToM区域的分层加工发生了改变。虐待类型的数量决定颞顶叶连接和脑干的效应,而虐待的严重程度与颞中回和额叶皮质之间的RSFC增加有关。脑干区域的事后分析表明这涉及5-羟色胺能系统(中缝背核)。
结论:数据表明儿童期虐待对社会信息加工中涉及的神经网络有长期影响,而该网络是理解他人情绪状态不可或缺的因素。确实,这种改变的神经网络可能解释了童年虐待受害者中出现的一些人际关系困难。
关键词: 童年虐待, 功能连接, 虐待静息态, 社会认知心理理论(ToM)
1. Introduction
Violence against women is a major public health issue and constitutes a violation of human rights (European Union Agency for Fundamental Rights, 2014). According to official estimates, around 35% of adult women in the European Union have experienced physical, psychological, or sexual violence before the age of 15 (European Union Agency for Fundamental Rights, 2014). Early exposure to such violence including sexual, physical, and emotional abuse can lead to the development of behaviour problems and affect neural structure and plasticity (Teicher & Samson, 2016). The changes in neural structure and plasticity may result in a predisposition towards the development of mental disorders in adulthood, including mood and anxiety disorders, post-traumatic stress disorder (PTSD), substance abuse, and personality disorders (Green et al., 2010; Taillieu, Brownridge, Sareen, & Afifi, 2016). One important and widely accepted consequence of any type of childhood maltreatment is an impairment in socio-emotional development, leading to poorer performance in critical functions such as emotion recognition and understanding, and theory-of-mind abilities (Luke & Banerjee, 2013).
Theory of mind (ToM), or social understanding, is the ability to interpret the thoughts and feelings of other people. Specifically, ToM is the core human socio-cognitive ability to explain and predict the behaviour of others by attributing to them independent mental states, such as beliefs, emotions, intentions, hopes and desires that may be different from our own (Gallagher & Frith, 2003). Because of the inherent violation of personal integrity and autonomy, maltreated individuals may have a different perception of interpersonal interactions and exhibit changes in social abilities, emotions and other mental states associated with such interactions. Indeed, available evidence documents reductions in social abilities in maltreated children (cf. Luke & Banerjee, 2013), whereas work investigating long-term effects of early stressful life events on social skills in adults has only just begun (e.g. Lanius, Bluhm, & Frewen, 2011; Nazarov et al., 2014; Vai et al., 2018). For example, a behavioural study observed that previously maltreated women had more difficulty identifying familial relationship and were less sensitive to emotionally valenced situations (Nazarov et al., 2014). However, to date, little is known how maltreatment affects brain circuitry underlying ToM.
Indeed, complex ToM functions likely recruit a diverse array of brain areas that (presumably) synchronize with one another to integrate different pieces of information to represent ToM at the neural level (e.g., areas involved in self-other distinction, self-referential processing, episodic memory, attention, language) (Schurz, Radua, Aichhorn, Richlan, & Perner, 2014). According to recent reviews and meta-analyses (Schurz et al., 2014; van Veluw & Chance, 2014) neural nodes belonging to this ToM network consist of: (1) the temporo-parietal junction (TPJ) (Brodmann Area 39), (2) the precuneus (mesial extent of Brodmann’s Area 7), (3) the dorsomedial prefrontal cortex (dmPFC), and (4) the middle temporal gyrus (MTG, BA 21). Within this context, a neuroimaging study in maltreated individuals has documented structural network changes in areas involved in social processing (Sun, Haswell, Morey, & De Bellis, 2019). In a small recent functional imaging study, recruitment of brain areas belonging to the mirror neuron system, such as the precentral gyrus and the inferior frontal gyrus, was associated with reduced activation in individuals with more adverse childhood events (Vai et al., 2018). Yet, evidence of basic neural oscillatory changes in ToM networks in maltreated cohorts is scarce.
Characterization of basic resting-state brain networks in persons with mental illness or at-risk may help to identify perturbations of underlying neural oscillations and changed brain dynamics. Within trauma research, only a few studies to date have examined intrinsic functional connectivity (FC) but with much heterogeneity in findings (Olive et al., 2018; Philip et al., 2013; Steuwe et al., 2015; Thomason et al., 2015; van der Werff et al., 2013). Decreased FC between the amygdala and other brain areas such as the putamen, insula, or subgenual anterior cingulate cortex has been documented in maltreated adults (van der Werff et al., 2013) and children (Thomason et al., 2015). Directly contrasting these findings, a task-based study reported increased (rather than decreased) FC for traumatized participants relative to comparisons specifically between midbrain structures (e.g., locus coeruleus) and subcortical brain areas including the amygdala or the thalamus (Steuwe et al., 2015). In their (Steuwe et al., 2015) study, participants viewed animated video sequences of faces with varying emotional expressions and direct or averted gaze. The authors argued that this increased FC may reflect preferential recruitment of a fast subcortical processing route indicative of an innate threat system that is quickly (and probably standardly) active in traumatized persons. Indeed, a hallmark symptom of PTSD is a constant level of alert (hypervigilance), an issue that clinicians aim to moderate before commencing other therapy (Van der Kolk, 2015). However, the findings by (Steuwe et al., 2015) have to be considered tentative as they were 1) task-based and consisted of a re-analysis of a prior fMRI study (Steuwe et al., 2014), and 2) were comprised of a relatively small sample (N = 16 per group).
Given these discrepancies and the interesting hypothesis that an innate state of fear after maltreatment experience may influence social processing, this study assessed the critical question to what extent individuals with and without abuse history show altered resting-state functional connectivity (RSFC) in a ToM network including structures relevant to processing of social signals. Because (1) emerging evidence suggests biological sex differences in neural activation during cognitive (Adenzato et al., 2017) and affective (Derntl et al., 2010) ToM and (2) males are more likely to underreport abuse experience (Finkelhor, Hotaling, Lewis, & Smith, 1990) thus leading to smaller study sizes for comparison, the present sample explicitly focused on an all-female sample. To this end, well-known ToM regions were identified in the present sample by virtue of an independent localizer task. If Steuwe et al. are correct and low-level fast processing interferes with higher-order socio-affective function, then we would also expect to see increased FC between the ToM regions (and possibly the midbrain). Such evidence would then demonstrate long-term neural oscillatory changes after childhood maltreatment experience in a social processing network. Importantly, the identification of impaired social processing nodes would aid in the continued quest for neuroimaging biomarkers of stress-related disorders (Schmidt et al., 2015) and document the long-term impact that violence against women has on interpersonal processes.
2. Methods
2.1. Participants
Thirty-five women with a history of childhood sexual, physical, or emotional abuse (CA) and 31 comparison women without such history or history of other, non-childhood abuse-related traumas in either childhood or adulthood (UC) participated (Table 1). Participants did not differ in age, education, or medication usage but CA participants, relative to UC, had significantly higher levels of depression (t(49.48) = 5.03, p < 0.001, d = 1.21), dissociative experiences (t(45.043) = 3.535, p = 0.001, d = 0.96), current psychopathology (χ2(European Union Agency for Fundamental Rights, 2014) = 26.615, p < 0.001, d = 1.64), trait anxiety (t(63) = 5.63, p < 0.001, d = 1.40) and state anxiety (t(64) = 4.55, p < .001, d = 1.12). However, UC had higher resilience than CA (t(64) = −3.092, p = 0.003, d = 0.76). In addition, CA participants also had significantly higher levels of self-reported empathy (t(64) = 3.05, p = 0.003, d = 0.75).
Table 1.
Demographic and clinical characteristics of the child abuse (CA) and the unaffected comparisons (UC).
| Child abuse group (CA) N = 35 |
Unaffected comparisons (UC) N = 31 |
p | Cohen’s d | |
|---|---|---|---|---|
| Age (years), mean (SD) | 36.16 (12.19) | 36.42 (11.46) | .929 | 0.02 |
| Highest education level (n) | ||||
| High school | 10 | 6 | .397 | |
| Bachelor | 16 | 13 | .174 | |
| Master/PhD/Postdoc | 4 | 7 | .455 | |
| Psychiatric medication (n) | 7 | 2 | .109 | |
| Depression (BDI) score (SD) | 15.68 (10.61) | 5.35 (5.30) | <.001** | 1.21 |
| State anxiety (STAI) score (SD) | 39.17 (8.22) | 30.45 (7.22) | <.001** | 1.12 |
| Trait anxiety (STAI) score (SD) | 47.81 (8.81) | 35.19 (9.24) | <.001** | 1.40 |
| Resilience (RS) score (SD) | 126.86 (17.10) | 138.61 (13.25) | .003* | 0.76 |
| Dissociative experience (DES) score (SD) | 20.50 (12.99) | 10.12 (7.73) | .001* | 0.96 |
| Self-reported empathy (IRI) score (SD) | 72.80 (12.43) | 64.48 (9.29) | .003* | 0.75 |
| IRI – perspective taking (SD) | 18.89 (4.81) | 18.03 (3.99) | .439 | 0.19 |
| IRI – fantasy (SD) | 17.89 (5.51) | 16.13 (5.64) | .206 | 0.32 |
| IRI – empathic concern (SD) | 21.14 (4.39) | 20.10 (4.22) | .329 | 0.24 |
| IRI – personal distress (SD) | 14.86 (4.17) | 10.23 (4.46) | <.001** | 1.08 |
| Current psychopathology (at least one) | 27 | 6 | <.001** | – |
| Mood disorders (n) | 14 | 2 | .002* | – |
| Anxiety disorders (n) | 19 | 2 | <.001** | – |
| PTSD (n) | 7 | 0 | .008* | – |
| Alcohol dependence and abuse (n) | 0 | 2 | .127 | – |
| Substance dependence and abuse (n) | 0 | 2 | .127 | – |
| Psychotic disorders (n) | 1 | 0 | .351 | – |
| Eating disorders (n) | 2 | 1 | .528 | – |
| Personality disorders (n) | 13 | 0 | <.001** | – |
| Borderline personality disorder | 12 | 0 | ||
| Antisocial personality disorder | 1 | 0 | ||
| Suicidality | 10 | 0 | .001* | – |
| Type of abuse | ||||
| Sexual abuse (n) | 29 | 0 | – | |
| Rape (n) | 18 | 0 | – | |
| Sexual assault (n) | 21 | 0 | – | |
| Physical abuse (n) | 26 | 0 | – | |
| Emotional abuse (n) | 21 | 0 | – | |
* p < .01 ** p < .001.
Participants were recruited via self-help groups (CA only), flyers, and social media. Participants were matched for age, sex, handedness, and level of education. The study was approved by the ethical committee of Ghent University Hospital and all participants provided written informed consent prior to the study and received 30 EUR compensation.
2.2. Tom localizer
A Dutch translation of a previously validated ToM localizer (~10 min) was used (Dodell-Feder, Koster-Hale, Bedny, & Saxe, 2011) to identify brain regions involved in ToM, which were then used for seed-based functional connectivity (FC) analysis. Participants read 20 short stories presented in fixed order pertaining either to: characters and their false beliefs about the world (‘false belief’ condition), or to inanimate objects such as maps or photographs, which displayed false information (‘false photograph’ condition). Half of the stories (Schurz et al., 2014) belonged to the ‘false belief’ condition whereas the other half (Schurz et al., 2014) belonged to the ‘false photograph’ condition. Each story was presented for 10,000 ms. Participants then read a statement, presented for 10,000 ms, on that story and responded using an MRI compatible response box (Cedrus) whether they thought the statement was true or false (index finger = true statement, middle finger = false statement). First-level models contained separate regressors for each condition (‘false belief’ and ‘false photograph’) as well as subject movement parameters. To identify regions involved in ToM, a one-sample t-test using the whole group was performed on the false belief > false photograph contrast at the whole-brain level (conflating all false belief statements vs. conflating all false photograph statements), with age, depression scores and trait anxiety scores as regressors of no interest. The coordinates of peak activation (corrected for multiple comparisons (family-wise error, FWE) and thresholded at p < .05 and cluster size k > 10) were then used in the seed-based resting-state FC analyses. There were no significant group differences in the localizer. However, three women (UC = 1, CA = 2) did not complete the localizer task due to practical difficulties (problems with (1) MRI glasses, (2) reading through the helmet, (3) claustrophobia)).
2.3. Questionnaires
2.3.1. SLESQ
The Stressful Life Events Screening Questionnaire (Goodman, Corcoran, Turner, Yuan, & Green, 1998) was used to assess previous traumatic exposure. The SLESQ consists of 13 yes/no items, with sub-items probing age at time of experience and frequency as well as duration of the trauma. Participants in the present study were classified as CA (and included) if they positively answered items 5 (rape), 6 (sexual assault), which together were considered as sexual abuse, 7 (childhood physical abuse), and/or 9 (emotional abuse), and if their age at onset of the abuse was below 17 years. The cut-off of 17 years was chosen to keep similarity with other questionnaires assessing childhood abuse, such as the Childhood Trauma Questionnaire (Bernstein & Fink, 1998). Participants were excluded if they had not experienced CA yet had experienced other serious childhood trauma (e.g. serious accident/illness) or adult interpersonal trauma (e.g. intimate partner violence, physical/sexual assault, etc.). Cronbach’s alpha present sample was 0.754 and omega (ω) = 0.763. To calculate the severity of abuse, a score was associated to the frequency/duration of the trauma as follows: never happened: 0, only once: 1, <6 months: 2, 7 months – 2 years: 3, 2 years – 5 years: 4, >5 years: 5. The scores for the different types of abuse suffered by the participants were summed to obtain the total severity score of each participant.
2.3.2. IRI
The 28-item Interpersonal Reactivity Index (De Corte et al., 2007) measures empathic responsiveness (α = 0.792, ω = 0.806) on four 5-point Likert scales: two cognitive (perspective-taking, α = 0.735, ω = 0.751; fantasy, α = 0.797, ω = 0.820) and two affective (empathic concern, α = 0.752, ω = 0.764; personal distress, α = 0.730, ω = 0.745). The IRI is scored 0–112 (0–28 per scale).
2.3.3. BDI-II
The 21-item Beck Depression Inventory-II (Beck, Steer, & Brown, 1996) measures depressive symptoms (scored 0–63) in adults according to DSM-IV criteria covering cognitive, affective, and somatic aspects of depression (α = 0.934, ω = 0.938).
2.3.4. STAI
The State-Trait Anxiety Inventory (Van der Ploeg, Defares, & Spielberger, 1980) was used to measure state (20 items) and trait anxiety (20 items) on 4-point Likert scales (scored 20–80 for each subscale) (state: α = 0.914, ω = 0.915; trait: α = 0.930, ω = 0.932).
2.3.5. DES
The 28-item visual analogue scale Dissociative Experiences Scale (Ensink & van Otterloo, 1989) measures the extent to which respondents experience dissociative symptoms such as depersonalization, derealization, and disturbances in memory and identity in their daily life (α = 0.897, ω = 0.920).
2.3.6. RS
The Resilience Scale (Wagnild & Young, 1993) measures mental resilience using 26 statements about the self that are judged from ‘strongly disagree’ to ‘strongly agree’ on a 7-point Likert scale (scored 26–182)(α = 0.850, ω = 0.864).
2.3.7. MINI + BPD screening
The Mini-International Neuropsychiatric Interview (Overbeek, Schruers, & Griez, 1999) was administered by two clinical psychology masters students and two PhD students, the latter of whom had been trained by the same licensed clinical psychologist and then subsequently trained the master students. The MINI is a structured interview, assessing current and lifetime histories of Axis I disorders plus one Axis II disorder, antisocial personality disorder, based on DSM-IV criteria. Since previous research underlines a link between experience of childhood abuse and borderline personality disorder (BPD)(Taillieu et al., 2016), an additional MINI-style subsection assessing BPD symptomatology was included. Items for this subsection were designed based on DSM-IV criteria, translated into Dutch, and back-translated.
2.4. Imaging data acquisition
Images were acquired as part of a larger study at Ghent University Hospital using a 3T Magnetom Siemens TrioTim MRI scanner. Firstly, a T1-weighted high-resolution anatomical scan was acquired (repetition time [TR] = 2250 ms, echo time [TE] = 4.18 ms, image matrix = 256 × 256, field of view [FOV] = 256 mm, flip angle = 9°, slice thickness = 1 mm, voxel size = 1.0 × 1.0 × 1.0 mm, number of slices = 176). Then, the ToM localizer scan was acquired using a T2*-weighted Echo Planar Images (EPI) sequence (TR = 2000 ms, TE = 28 ms, image matrix = 64 × 64, FOV = 224 mm, flip angle = 80°, slice thickness = 3.0 mm, voxel size = 3.5 × 3.5 × 3.0 mm, number of slices = 34, 17% gap). Finally, resting-state fMRI data were acquired also using a T2*-weighted Echo Planar Images (EPI) sequence (TR = 2000 ms, TE = 27 ms, image matrix = 64 × 64, FOV = 192 mm, flip angle = 90°, slice thickness = 3.0 mm, voxel size = 3.0 × 3.0 × 3.0 mm, number of slices = 34, 10% gap). The scanning time for resting-state data was 6:06 m. The scanning session also included the acquisition of a functional theory of mind task, the results of which will be reported elsewhere.
2.5. Image preprocessing
Resting-state fMRI data were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) on Matlab. The first 10 volumes were removed for each subject to account for signal saturation effects and the data images were preprocessed by applying slice timing, realignment to adjust for head movement during data acquisition, normalization with the standard EPI template from the Montreal Neurological Institute (MNI) (resampling voxel size = 3 × 3 × 3 mm3) and spatial smoothing to reduce the effects of the bad normalization by using an 8-mm full-width at half-maximum (FWHM) Gaussian kernel. The images were visually inspected to verify the validity of the normalization step for all the data.
The images were segmented (modulated) into non-brain and brain tissue (grey matter, GM; white matter, WM; cerebrospinal fluid, CSF). The functional data were then detrended and filtered with a standard low-pass filter (0.01–0.08 Hz), because only frequencies below 0.1 Hz contribute to regionally specific BOLD correlations, while faster frequencies are related to cardiac or respiratory factors (Cordes et al., 2001).
Due to the fact that spontaneous activity is contaminated by artefacts such as scanner instability or non-neuronal physiological fluctuations, a regression of nuisance step was performed by regressing motion (using a brain mask), white matter (WM mask) and cerebral spinal fluid (CSF mask). All masks were created using SPM8 and visually inspected afterwards.
The last preprocessing step took care of motion using framewise displacement (FWD), a method developed by Power and colleagues to adjust for small head movements that may add spurious noisy variance. Their (Power et al., 2014) method measures how much the head changes position from one frame to the next and is calculated as the sum of the absolute values of the differentiated realignment estimates at every timepoint. The analysis was conducted using their BRAMILA script for framewise displacement with a recommended threshold of <0.5 mm. Since individual subject value means were all well below 0.5, no participant was excluded (mean total FWD = 0.078 mm, FWD range = 0.033–0.217 mm). There were no significant group differences in movement parameters (FWD values).
2.6. Seed-based resting-state functional connectivity analysis
After preprocessing, the data were analysed using seed-based FC taking the following peak coordinates from the ToM localizer as seeds: Right TPJ (MNI coordinates xyz: 46 − 58 20), left TPJ (−48 − 56 24), Precuneus (Adenzato et al., 2017; Beck et al., 1996; Bernstein & Fink, 1998; Cordes et al., 2001; De Corte et al., 2007; Derntl et al., 2010; Dodell-Feder et al., 2011; Ensink & van Otterloo, 1989; Finkelhor et al., 1990; Gallagher & Frith, 2003; Goodman et al., 1998; Green et al., 2010; Lanius et al., 2011; Luke & Banerjee, 2013; Nazarov et al., 2014; Olive et al., 2018; Overbeek et al., 1999; Philip et al., 2013; Power et al., 2014; Schmidt et al., 2015; Schurz et al., 2014; Steuwe et al., 2014, 2015; Sun et al., 2019; Taillieu et al., 2016; Teicher & Samson, 2016; Thomason et al., 2015; Vai et al., 2018; Van der Kolk, 2015; Van der Ploeg et al., 1980; van der Werff et al., 2013; van Veluw & Chance, 2014; Wagnild & Young, 1993), dmPFC (−10 54 28), left MTG (−56 − 2 − 18) and right MTG (52 -Olive et al., 2018; Vai et al., 2018).
The seed-based FC analysis was conducted using REST toolbox, using a user-defined mask (the brain mask) and by creating a 6-mm radius sphere around the single voxel seed. The mean activation of every seed region was calculated and then correlated to all the voxels of the brain, to look at the correlations between each time-series. Correlations were normalized using Fisher transformation, to ensure that all subjects were in the same normalized space.
Second level analysis was performed using SPM8, by entering participants’ connectivity maps with age of participants, mean FWD values and depression scores as covariates, to evaluate possible differences between CA and UC. To compare FC between CA and UC, a two-sample t-test was performed. Given FC analyses of the seed, region were examined across the whole brain, we used the updated AFNI’s 3dClustSim for correction for multiple comparisons (https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) with a voxelwise threshold at p< .001 and the clusterwise threshold at p< .01. Simulations resulted in a cluster size of at least 37 contiguous voxels.
To control for the influence of depressive symptoms, anxiety, number and severity of abuse, empathy, behavioural performance during the ToM task (accuracy) or symptoms of dissociation on the main results, a second-level analysis or correlations of scores with ROIs betas was conducted separately for controls and trauma groups. Connectivity maps of the participants of each group and the respective scores of one group at a time as a covariate were entered using the same threshold as above. Additionally, to assess the influence of a PTSD diagnosis, analyses were rerun excluding all women (n = 7) who would qualify for such a diagnosis. Neither depressive symptoms, anxiety, empathy, dissociative experiences, or borderline personality symptoms influenced the results and will not be discussed further. Importantly, diagnosis of PTSD did also not influence the results (cf. supplementary Tables, Supplementary Figure 1).
Lastly, to be confident that group differences in FC were based on paths that were existing in both groups, the spmT maps of the two groups were overlapped, revealing no significant difference (Supplementary Figure 2).
3. Results
3.1. Seed-based functional connectivity
Maltreatment experience was associated with increased FC between the left TPJ and the midbrain as well as the Precuneus and the midbrain (Table 2, Figure 1). In addition, FC was larger in previously maltreated women (vs. comparison) between the dmPFC seed and the anterior/posterior lobes of right cerebellum, the left pons, the right fusiform gyrus and the uncus. Similarly, FC between the right MTG seed region and the left pons was also increased for traumatized women relative to comparisons. In fact, there was no region where FC was increased for UC relative to CA.
Table 2.
MNI coordinates and anatomical locations of the identified clusters of seed-based functional connectivity analysis. Child abuse > Unaffected comparisons.
| MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| Anatomical location | Cluster size K | x | y | z | Peak t | |
| Left TPJ | Left midbrain | 46 | −9 | −30 | −21 | 4.21 |
| Precuneus | Left pons, midbrain | 94 | −15 | −18 | −30 | 4.13 |
| –9 | –30 | –21 | 4.04 | |||
| -18 | -27 | -39 | 3.88 | |||
| dmPFC | Right cerebellum post. lobe | 90 | 24 | −42 | −45 | 4.61 |
| Post. lobe | 33 | –39 | –45 | 4.60 | ||
| Ant. lobe | 27 | -54 | -36 | 4.57 | ||
| Left pons | 71 | –15 | –18 | –30 | 4.78 | |
| -15 | -24 | -42 | 3.60 | |||
| –18 | –33 | –42 | 3.53 | |||
| Right fusiform gyrus | 62 | 51 | -6 | -30 | 4.14 | |
| 42 | –12 | –30 | 3.97 | |||
| Right limbic lobe, uncus | 33 | -6 | -33 | 3.59 | ||
| Right MTG | Left pons | 41 | −15 | −30 | −30 | 4.09 |
Figure 1.
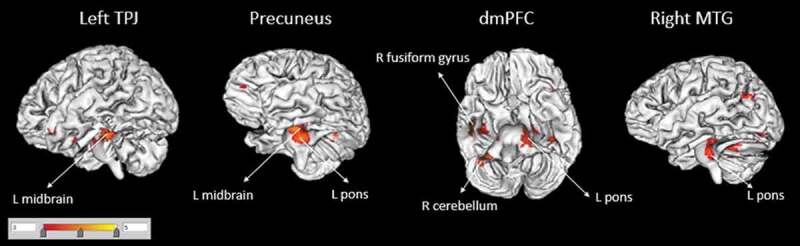
Functional connectivity of seed regions (in larger font, corrected for multiple comparisons) with other brain areas (smaller font) for the Child abuse > Unaffected comparisons contrast. There were no areas for which Unaffected comparisons > Child abuse. TPJ = temporo-parietal junction; dmPFC = dorsomedial prefrontal cortex; MTG = middle temporal gyrus; R, right; L, left.
Within the regions of interest, strong positive correlations were present among dmPFC, lTPJ, and Precuneus for both the CA and UC groups (Figure 2). However, when directly comparing groups, none of the correlation coefficients differed significantly from one another (Fisher r-to-z transform).
Figure 2.
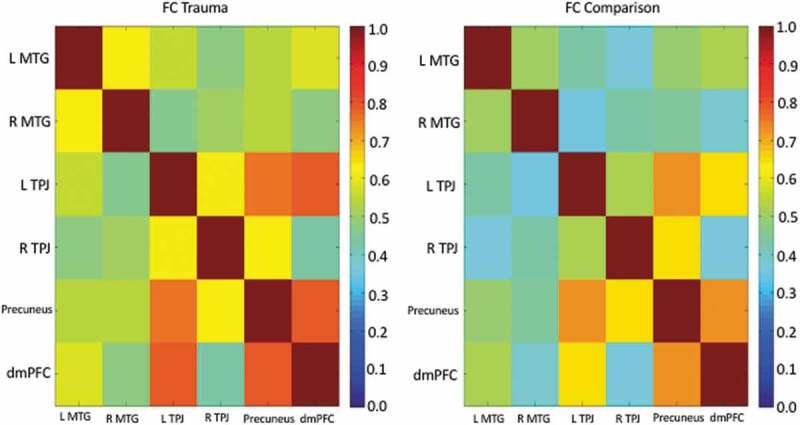
Correlation matrix (correlation coefficient, r) for the ToM to ToM region-of interest correlations for the trauma group (CA, left panel) and unaffected comparison group (UC, right panel) separately.
Interestingly, when the types of abuse or the severity of abuse were taken into consideration, the number of types of experienced abuse revealed a significant positive FC between the left TPJ FC and the midbrain (Table 3, Figure 3). In other words, the more types of abuse a person had experienced, the stronger the FC was between the left TPJ and the midbrain. By comparison, the analysis of FC for the severity of abuse revealed both positive and negative effects (Table 3) most notably a positive effect on the left MTG FC with right frontal regions (orbital gyrus and middle frontal gyrus) and a negative effect on the left TPJ FC with the left superior frontal gyrus and the left insula.
Table 3.
MNI coordinates and anatomical locations of the identified clusters of seed-based functional connectivity analysis for the main effect of number of abuse types and severity on the CA group.
| MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| Seed region | Anatomical location | Cluster size K | x | y | z | Peak t |
| Number of abuse types | ||||||
| Positive | ||||||
| Right TPJ | Left/right cingulate gyrus | 86 | −18 | −36 | 36 | 4.13 |
| 18 | –30 | 33 | 3.73 | |||
| 6 | 36 | 27 | 3.72 | |||
| Left TPJ | Left insula | 118 | −42 | −9 | 0 | 4.21 |
| –39 | –24 | –9 | 3.74 | |||
| Left superior temporal gyrus | -45 | -18 | -3 | 4.04 | ||
| Right thalamus | 75 | 21 | –21 | –3 | 4.45 | |
| Right/Left midbrain | 9 | -24 | -12 | 3.67 | ||
| –3 | –27 | –12 | 3.58 | |||
| Right cingulate gyrus (BA23) | 41 | 3 | -30 | 27 | 3.52 | |
| Precuneus | Right cerebellum post. lobe | 42 | 24 | −36 | −39 | 4.50 |
| dmPFC | Left cingulate gyrus | 63 | −24 | −51 | 24 | 4.42 |
| Severity | ||||||
| Positive | ||||||
| Left MTG | Right orbital gyrus (BA11) | 73 | 3 | 42 | −24 | 4.06 |
| R middle frontal gyrus | 9 | 30 | –15 | 3.39 | ||
| Negative | ||||||
| Right TPJ | Right cingulate gyrus | 41 | 6 | −36 | 27 | 4.01 |
| Right para-hippocampal gyrus | 40 | 30 | –33 | –6 | 3.41 | |
| Left TPJ | Left superior temporal gyrus | 285 | −45 | −18 | 0 | 4.35 |
| Left insula (BA13) | 147 | 42 | –15 | –6 | 4.21 | |
| Right posterior cingulate | 40 | 3 | -30 | 24 | 3.86 | |
| Precuneus | Right hippocampus | 53 | 42 | −18 | −15 | 3.40 |
| Right inferior frontal gyrus | 38 | –51 | 12 | 15 | 4.00 | |
| Left MTG | Right insula | 44 | 45 | 12 | 0 | 3.79 |
Figure 3.
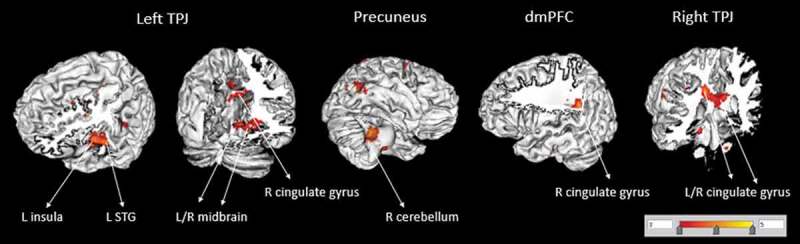
Functional connectivity of seed regions (in larger font, corrected for multiple comparisons) with other brain areas (smaller font) for the main effect of number of abuse on the CA group. TPJ = temporo-parietal junction; dmPFC = dorsomedial prefrontal cortex; STG = superior temporal gyrus; R, right; L, left.
Although there was no main effect of group on the localizer task, an analysis of accuracy on the ToM localizer task revealed both, a positive effect on the CA group FC and a negative effect on both groups. However, these regions were different than those relevant to the main analysis, thus not influencing the present results (cf. Supplementary Table 2).
3.2. Post-hoc analyses of brainstem areas
As brainstem areas consistently emerged in the FC analyses, we sought to investigate, post-hoc, which neurotransmitter system may most likely be involved. We therefore used the Harvard AAN (ascending arousal network) atlas (Edlow et al., 2012) to examine which of these midbrain/brainstem regions, the dorsal raphe (DR), representative for serotonin involvement, the ventral tegmental area (VTA) representative for dopamine involvement or the locus coeruleus (LC) representative for noradrenergic involvement, was more likely to connect with our ToM regions. The results indicated strong positive FC for previously maltreated women relative to comparison women of the DR with the precuneus ROI and the dmPFC ROI. By contrast, while the LC showed strong positive FC with the putamen and thalamus (CA > UC), there were no significant findings for the VTA (Supplementary Table 3, voxelwise p < .001, clusterwise p < .01 as in the main analyses, whole-brain corrected). There was no negative FC.
4. Discussion
Lanius and colleagues (Olive et al., 2018) hypothesize that traumatic experience alters the innate resting-state of the human brain, biasing it toward heightened sensitivity to threat, consistent with the hypervigilance frequently reported by traumatized individuals (cf., DSM-5, PTSD and Acute Stress Disorder diagnoses). To date, evidence for this hypothesis has been limited as support comes from studies with small samples (Steuwe et al., 2015) or studies where FC for midbrain connections differed between a PTSD group without relative to those with dissociative experience but neither from controls (Olive et al., 2018). Improving on earlier work, the present study focused on resting-state ToM seed regions derived from an independent localizer task, yet the brainstem emerged consistently at the whole-brain level after stringent correction for multiple comparisons, but was not one of those seed regions. FC between these brainstem/midbrain areas and several important ToM regions including the TPJ, the Precuneus, the right MTG and the dmPFC was increased relative to comparisons. Consistent with the hypothesis, our data therefore support the role of brainstem regions in biasing the resting-state network after trauma. Specifically, the fact that this positive FC between the TPJ and the midbrain increased with the number of abuse types participants had experienced further cements this hypothesis. In addition, the severity of abuse was associated with FC between the left MTG and frontal areas (middle frontal and orbital gyri). A recent meta-analysis by(Meng et al., 2016), documented that grey matter volume of the MTG (BA 21) is reduced after prolonged trauma, but not after single-incident trauma, thus being consistent with our data and study population. This would further suggest that prolonged trauma does not only alter the structural properties of this region but also changes basic oscillatory functioning. However, the precise (temporal and neuromolecular) mechanisms mediating this effect are presently poorly understood. Specifically, while we made efforts to distinguish between the types of abuse and the severity of experienced abuse, future study should increase efforts to determine the differential impact of these two highly related but separable concepts.
Although changes in grey matter volume (Teicher & Samson, 2016) and structural network connectivity (Sun et al., 2019; Teicher, Anderson, & Polcari, 2014) have been documented after maltreatment experience, how these structural changes may be temporally linked to oscillatory changes and changes of underlying brain chemistry is presently unknown. One possible explanation for the strong midbrain finding is that the midbrain is the starting point of the various neurotransmitter systems of the brain including the dopamine (central tegmental area, VTA), serotonin (dorsal raphe nuclei, DR), and noradrenergic (locus coeruleus, LC) pathways with innervations to the dmPFC and the TPJ (Venkatraman, Edlow, & Immordino-Yang, 2017). Yet the various neurotransmitter systems may implicate different functions. For example, while a hypothesis of a permanent state of threat would more likely imply the locus coeruleus (LC) and noradrenaline (Venkatraman et al., 2017), Abu-Akel (Abu-Akel, 2003) on the other hand suggests that the integrity of the dopaminergic and serotoninergic systems is the neurochemical basis that sustains ToM ability and that abnormalities in this system can account for ToM impairments. Using the DR, LC, and VTA as seeds during exploratory post-hoc analyses to narrow down these competing neurochemical hypotheses, we found that particularly the dorsal raphe showed increased FC with the dmPFC and precuneus for traumatized women relative to comparisons. By contrast, LC showed increased FC with the putamen and thalamus (CA >UC), while the VTA showed no effects. Although these findings may indicate a serotonergic influence on altered ToM ability in maltreated women, it is worth noting that noradrenergic activity (Pudovkina, Cremers, & Westerink, 2002) and presence of noradrenergic transporters (Ordway, Stockmeier, Cason, & Klimek, 1997) have also been observed in the dorsal raphe thus also making an interaction between the serotonin system and the noradrenaline system probable. Therefore, at this stage, it is unclear whether a state of threat driven by noradrenaline may influence (long-term) transmitter systems involved in social information processing (serotonin) or whether these are separable components. While these findings are interesting, they should be considered tentative given their exploratory nature and future (longitudinal) study should aim to optimize their designs for replication efforts.
Non-molecular explanations can also be found on a higher, socio-cognitive level. Recently, a greater network centrality of the precuneus in maltreated persons has been documented (Teicher et al., 2014). Given its role in self-referential (1) thinking and (2) mental imagery (Cavanna & Trimble, 2006), Teicher et al. suggested that the increased precuneus centrality in maltreated individuals may lead to ‘a heightened experience of internal emotions and cravings along with a greater tendency to think about oneself and to engage in self-centred mental imagery (p. 255)’ (Teicher & Samson, 2016). Consistent with this idea, in the present data CA women relative to UC women showed increased precuneus-brainstem (DR) coupling. This suggests that women with an abuse history have an increased focus on internal emotional feelings and harmful self-centred thinking that may be influenced and/or maintained by the brainstem.
As common in maltreatment studies, findings have to be taken with caution due to a small and heterogeneous sample. However, one strength of the study was that it focused exclusively on women thus excluding potential biological sex confounds that may have driven prior divergent findings (Herringa et al., 2013; Lu et al., 2017; Philip et al., 2013; Thomason et al., 2015), especially in light of emerging neural evidence for sex differences in cognitive/affective ToM (Adenzato et al., 2017; Derntl et al., 2010). Yet, this of course limits generalizability and replication in an all-male or sufficiently powered male/female sample would be desirable. A second limitation was that CM was measured retrospectively using a self-report questionnaire and is therefore sensitive to subjectivity and recall biases. Moreover, because the aim was to capture a community sample (rather than rely on a more restricted PTSD diagnosis) no clinician-administered PTSD scales (such as the CAPS) were given. Likewise, the MINI was only scored to gain a broader picture of the variety of psychopathology present in the current sample but analyses of comorbidities focused on the dimensional scales. However, in contrast to prior work, another major strength of the current study was that we relied on an independent localizer task to ensure the adequacy of the probed ToM regions and also used state of the art motion correction to account for motion artefacts thus increasing sensitivity. Finally, while the analyses of the brainstem regions may provide important new leads for further study, it is important to note that these analyses were (1) post-hoc and (2) that our imaging acquisition parameters were not optimized for the study of small brainstem structures and should, therefore, be considered exploratory.
In summary, this study documents the lasting impact that experience of early child abuse has on women and their social information processing neural networks that may impact the future quality of life and attainment of life’s goals (European Union Agency for Fundamental Rights, 2014). Particularly, increased FC consistently connected ToM regions (TPJ, dmPFC, precuneus, middle temporal gyrus) with areas of the brainstem/midbrain. The data thus strongly support prior suggestions that higher-order socio-affective function may be biased already at the brainstem level in resting neural oscillations. Future work could examine whether such resting-state normalizes after therapeutic intervention. Moreover, the neurochemical role of this effect begs inquiry. Lastly, since large-scale studies have shown disrupted cognitive empathy in children of incarcerated women and community women (Thomson, Kuay, Baron-Cohen, & Towl, 2018), future research should also explore the impact of other types of adverse child experiences beyond child abuse.
Supplementary Material
Acknowledgments
The authors would like to warmly thank all women who participated in our research, and all organizations and groups who helped with recruitment. The authors would also like to thank the two anonymous reviewers for their constructive comments on an earlier draft.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary materials
Supplemental data for this article can be accessed here.
References
- Abu-Akel A. (2003). The neurochemical hypothesis of ‘theory of mind’. Medical Hypotheses, 60(3), 382–11. [DOI] [PubMed] [Google Scholar]
- Adenzato M., Brambilla M., Manenti R., De Lucia L., Trojano L., Garofalo S., et al (2017). Gender differences in cognitive theory of mind revealed by transcranial direct current stimulation on medial prefrontal cortex. Scientific Reports, 7, 41219 Epub 2017/ 01/25. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. T., Steer R. A., & Brown G. K. (1996). Manual for the beck depression inventory-II (Second ed.). San Antonio; TX: Psychological Corporation. [Google Scholar]
- Bernstein D. P., & Fink L. (1998). Childhood trauma questionnaire: A retrospective self-report manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Cavanna A. E., & Trimble M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain: a Journal of Neurology, 129(Pt 3), 564–583. [DOI] [PubMed] [Google Scholar]
- Cordes D., Haughton V. M., Arfanakis K., Carew J. D., Turski P. A., Moritz C. H., … & Meyerand M. E. (2001). Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR. American Journal of Neuroradiology, 22(7), 1326–1333. [PMC free article] [PubMed] [Google Scholar]
- De Corte K., Buysse A., Verhofstadt L. L., Roeyers H., Ponnet K., & Davis M. H. (2007). Measuring empathic tendencies. Reliability and Validity of the Dutch Version of the Interpersonal Reactivity Index Psychologica Belgica, 47(4), 235–260. [Google Scholar]
- Derntl B., Finkelmeyer A., Eickhoff S., Kellermann T., Falkenberg D. I., Schneider F., & Habel U. (2010). Multidimensional assessment of empathic abilities: Neural correlates and gender differences. Psychoneuroendocrinology, 35(1), 67–82. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D., Koster-Hale J., Bedny M., & Saxe R. (2011). fMRI item analysis in a theory of mind task. NeuroImage, 55(2), 705–712. [DOI] [PubMed] [Google Scholar]
- Edlow B. L., Takahashi E., Wu O., Benner T., Dai G., Bu L., et al (2012). Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. Journal of Neuropathology and Experimental Neurology, 71(6), 531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensink B. J., & van Otterloo D. (1989). A validation of the dissociative experiences scale in the Netherlands. Dissociation, 2(4), 221–223. [Google Scholar]
- European Union Agency for Fundamental Rights Violence against women: An EU-wide survey. Main results report. Luxembourg: 2014. [Google Scholar]
- Finkelhor D., Hotaling G., Lewis I. A., & Smith C. (1990). Sexual abuse in a national survey of adult men and women: Prevalence, characteristics, and risk factors. Child Abuse & Neglect, 14(1), 19–28. [DOI] [PubMed] [Google Scholar]
- Gallagher H. L., & Frith C. D. (2003). Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences, 7(2), 77–83. [DOI] [PubMed] [Google Scholar]
- Goodman L. A., Corcoran C., Turner K., Yuan N., & Green B. L. (1998). Assessing traumatic event exposure: General issues and preliminary findings for the stressful life events screening questionnaire. Journal of Traumatic Stress, 11(3), 521–542. [DOI] [PubMed] [Google Scholar]
- Green J. G., McLaughlin K. A., Berglund P. A., Gruber M. J., Sampson N. A., Zaslavsky A. M., & Kessler R. C. (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Archives General Psychiatry, 67(2), 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R. J., Birn R. M., Ruttle P. L., Burghy C. A., Stodola D. E., Davidson R. J., & Essex M. J. (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences of the USA, 110(47), 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R. A., Bluhm R. L., & Frewen P. A. (2011). How understanding the neurobiology of complex post-traumatic stress disorder can inform clinical practice: A social cognitive and affective neuroscience approach. Acta Psychiatrica Scandinavica, 124(5), 331–348. [DOI] [PubMed] [Google Scholar]
- Lu S., Gao W., Wei Z., Wang D., Hu S., Huang M., & Li L. (2017). Intrinsic brain abnormalities in young healthy adults with childhood trauma: A resting-state functional magnetic resonance imaging study of regional homogeneity and functional connectivity. The Australian and New Zealand Journal of Psychiatry, 51(6), 614–623. [DOI] [PubMed] [Google Scholar]
- Luke N., & Banerjee R. (2013). Differentiated associations between childhood maltreatment experiences and social understanding: A meta-analysis and systematic review. Developmental Review, 33(1), 1–28. [Google Scholar]
- Meng L., Jiang J., Jin C., Liu J., Zhao Y., Wang W., et al (2016). Trauma-specific grey matter alterations in PTSD. Scientific Reports, 6, 33748 Epub 2016/ 09/22. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarov A., Frewen P., Parlar M., Oremus C., MacQueen G., McKinnon M., & Lanius R. (2014). Theory of mind performance in women with posttraumatic stress disorder related to childhood abuse. Acta Psychiatrica Scandinavica, 129(3), 193–201. [DOI] [PubMed] [Google Scholar]
- Olive I., Densmore M., Harricharan S., Theberge J., McKinnon M. C., & Lanius R. (2018). Superior colliculus resting state networks in post-traumatic stress disorder and its dissociative subtype. Human Brain Mapping, 39(1), 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway G. A., Stockmeier C. A., Cason G. W., & Klimek V. (1997). Pharmacology and distribution of norepinephrine transporters in the human locus coeruleus and raphe nuclei. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 17(5), 1710–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek T., Schruers K., & Griez E. (1999). Mini international neuropsychiatric interview: Nederlandse versie 5.0.0. Maastricht, NE: Universiteit van Maastricht. [Google Scholar]
- Philip N. S., Sweet L. H., Tyrka A. R., Price L. H., Bloom R. F., & Carpenter L. L. (2013). Decreased default network connectivity is associated with early life stress in medication-free healthy adults. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 23(1), 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J. D., Mitra A., Laumann T. O., Snyder A. Z., Schlaggar B. L., & Petersen S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudovkina O. L., Cremers T. I., & Westerink B. H. (2002). The interaction between the locus coeruleus and dorsal raphe nucleus studied with dual-probe microdialysis. European Journal of Pharmacology, 445(1–2), 37–42. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Willmund G. D., Holsboer F., Wotjak C. T., Gallinat J., Kowalski J. T., & Zimmermann P. (2015). Searching for non-genetic molecular and imaging PTSD risk and resilience markers: Systematic review of literature and design of the German armed forces PTSD biomarker study. Psychoneuroendocrinology, 51, 444–458. [DOI] [PubMed] [Google Scholar]
- Schurz M., Radua J., Aichhorn M., Richlan F., & Perner J. (2014). Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci Biobehav R, 42, 9–34. [DOI] [PubMed] [Google Scholar]
- Steuwe C., Daniels J. K., Frewen P. A., Densmore M., Pannasch S., Beblo T., … & Lanius R. A. (2014). Effect of direct eye contact in PTSD related to interpersonal trauma: An fMRI study of activation of an innate alarm system. Social Cognitive and Affective Neuroscience, Epub 2012/ 09/15, 9(1), 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe C., Daniels J. K., Frewen P. A., Densmore M., Theberge J., & Lanius R. A. (2015). Effect of direct eye contact in women with PTSD related to interpersonal trauma: Psychophysiological interaction analysis of connectivity of an innate alarm system. Psychiatry Research, 232(2), 162–167. [DOI] [PubMed] [Google Scholar]
- Sun D., Haswell C. C., Morey R. A., & De Bellis M. D. (2019). Brain structural covariance network centrality in maltreated youth with PTSD and in maltreated youth resilient to PTSD Development and psychopathology, 31(2), 557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillieu T. L., Brownridge D. A., Sareen J., & Afifi T. O. (2016). Childhood emotional maltreatment and mental disorders: Results from a nationally representative adult sample from the USA. Child Abuse & Neglect, 59, 1–12. [DOI] [PubMed] [Google Scholar]
- Teicher M. H., Anderson C. M., & Polcari A. (2014). Childhood maltreatment: Altered network centrality of cingulate, precuneus, temporal pole and insula. Biological Psychiatry, 76, 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M. H., & Samson J. A. (2016). Annual research review: Enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 57(3), 241–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M. E., Marusak H. A., Tocco M. A., Vila A. M., McGarragle O., & Rosenberg D. R. (2015). Altered amygdala connectivity in urban youth exposed to trauma. Social Cognitive and Affective Neuroscience, 10(11), 1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson N. D., Kuay H. S., Baron-Cohen S., & Towl G. J. (2018). The impact of maternal incarceration on their daughter’s empathy. International Journal of Law and Psychiatry, 56, 10–16. [DOI] [PubMed] [Google Scholar]
- Vai B., Riberto M., Ghiglino D., Bollettini I., Falini A., Benedetti F., Poletti S (2018). Mild adverse childhood experiences increase neural efficacy during affective theory of mind. Stress (Amsterdam, Netherlands), 21(1), 84–89. [DOI] [PubMed] [Google Scholar]
- Van der Kolk B. A. (2015). The body keeps the score. New York: Penguin Books. [Google Scholar]
- Van der Ploeg H. M., Defares P. B., & Spielberger C. D. (1980). Handleiding bij de Zelf-Beoordelings Vragenlijst ZBV. Een nederlandstalige bewerking van de Spielberger State-Trait Anxity Inventory[Manual of the Dutch State-Trait Anxiety Inventory]. Lisse, Netherlands: Swets & Zeitlinger. [Google Scholar]
- van der Werff S. J., Pannekoek J. N., Veer I. M., van Tol M. J., Aleman A., Veltman D. J., Zitman F. G., Rombouts S. A., Elzinga B. M. & van der Wee N.J (2013). Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychological Medicine, 43(9), 1825–1836. [DOI] [PubMed] [Google Scholar]
- van Veluw S. J., & Chance S. A. (2014). Differentiating between self and others: An ALE meta-analysis of fMRI studies of self-recognition and theory of mind. Brain Imaging and Behavior, 8(1), 24–38. [DOI] [PubMed] [Google Scholar]
- Venkatraman A., Edlow B. L., & Immordino-Yang M. H. (2017). The brainstem in emotion: A review. Frontiers in Neuroanatomy, 11, 15 Epub 2017/ 03/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagnild G. M., & Young H. M. (1993). Development and psychometric evaluation of the resilience scale. Journal of Nursing Measurement, 1(2), 165–178. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


