ABSTRACT
Plasmid-mediated antimicrobial resistance has emerged as one of the principal global issues, posing significant threats to public health. Herein, we reported a mobile tigecycline resistance mechanism Tet(X4) on both plasmid and chromosome in Escherichia coli strains from migratory birds in China. Besides tigecycline, these tet(X4)-positive strains also exhibited elevated MICs to the FDA newly approved tetracycline antibiotics, eravacycline (4 µg/ml) and omadacycline (8 µg/ml). Worrisomely, the tet(X4)-carrying plasmids and chromosome also shared high homology with the plasmids from human. Taken together, Tet(X4) represents another emerging antimicrobial threat and collective efforts from different sectors are needed to control its further spread.
KEYWORDS: Tigecycline resistance, migratory birds, tet(X4), eravacycline, omadacycline
Recent studies have showed that wild birds, especially migratory birds, may play an important role in the emergence and transmission of antimicrobial resistance and infectious diseases [1]. Meanwhile, the tigecycline resistance has also sporadically occurred (although not in migratory birds), primarily due to overexpression of efflux pumps and the newly identified tigecycline-inactivating mechanisms Tet(X3) and Tet(X4) [2–4]. Here we reported the tet(X4) gene in Escherichia coli strains from migratory birds in China, on both plasmid and chromosome, which raised a serious public health concern.
In 2018, three tigecycline-resistant E. coli strains, namely 2FT39, 2FT38-2, and 2ZN37-2, were isolated from stool samples of the little egrets (Egretta garzetta) from Guangdong, China, during a wild bird antimicrobial resistance surveillance study (Table S1). Susceptibility testing results showed that they were all resistant to tigecycline, tetracycline, florfenicol, and sulfamethoxazole-trimethoprim (Table S2). In addition, E. coli 2FT39 was also resistant to ciprofloxacin and E. coli 2FT38-2 was resistant to gentamicin. Interestingly, the three E. coli strains also exhibited significantly higher minimal inhibitory concentrations (MICs) to the FDA newly approved tetracycline antibiotics, eravacycline (4 µg/ml) and omadacycline (8 µg/ml), in comparison to the results from previous surveillance studies [5,6].
Further conjugation experiments by filter mating showed that the tigecycline resistance could be successfully transferred from E. coli 2FT38-2 into the recipient E. coli C600, Salmonella Typhimurium ATCC 14028 and clinical KPC-2-producing Klebsiella pneumoniae 1332, with the transfer efficiencies of (4.3 ± 0.7)×10−2, (1.2 ± 0.4)×10−6 and (1.6 ± 0.5)×10−4, respectively. Meanwhile, the resistance to tetracycline, florfenicol, sulfamethoxazole-trimethoprim and gentamicin were co-transferred (Table S2). The transconjugants also exhibited at least 8-fold increases of MICs against eravacycline and omadacycline, in comparison to the recipients. By contrast, the tigecycline resistance in E. coli 2FT39 failed to transfer via conjugation, but was successfully transferred into E. coli JM109 by electroporation, along with tetracycline and florfenicol resistance (Table S2). Similarly, the 2FT39 transformant had 512- and 32-fold increases of MICs against eravacycline (4 µg/ml) and omadacycline (8 µg/ml), respectively. However, the tigecycline resistance in E. coli 2ZN37-2 failed to transfer either by conjugation or electroporation.
Genomic DNA of them was then completely sequenced by combination of Nanopore GridION and Illumina HiSeq platforms (Nextomics, Wuhan, China), followed by assembling with Unicycler and annotating with the RAST server [7,8]. Subsequently, the bioinformatics analyses of them were conducted via the CGE server (https://cge.cbs.dtu.dk/services/), with ResFinder for detection of antimicrobial resistance genes, Multi-Locus Sequence Typing (MLST) for sequence types (STs), and PlasmidFinder for plasmid replicon types [9–11]. E. coli 2FT39 (Accession number: SSWK00000000), 2FT38-2 (SSWJ00000000), and 2ZN37-2 (SSWI00000000) all harbored a gene [namely tet(X4)] homologous to tet(X) with 94.5% amino acid sequence identity, which has been confirmed to share the similar degradation mechanism for tetracyclines (including tigecycline) by hydroxylation at C11a [4]. In this study, further gene cloning of tet(X4) from 2ZN37-2 into a pBAD24 vector also showed 64-, 512-, and 32-fold increases of MICs for tigecycline (16 µg/ml), eravacycline (4 µg/ml) and omadacycline (8 µg/ml), respectively.
The MLST analysis showed that the E. coli 2FT39, 2FT38-2, and 2ZN37-2 strains were non-clonal and belonged to three distinct STs: ST1196, ST6833, and ST641 (Table S1). Especially, the tet(X4) gene was identified on two different plasmids (namely p2FT39-3 and p2FT38-2-1) in strain 2FT39 and 2FT38-2, and on the chromosome of strain 2ZN37-2 (c2ZN37-2), respectively. These were further confirmed by S1-PFGE, I-CeuI PFGE, and Southern blot hybridization (Figure S1 and S2).
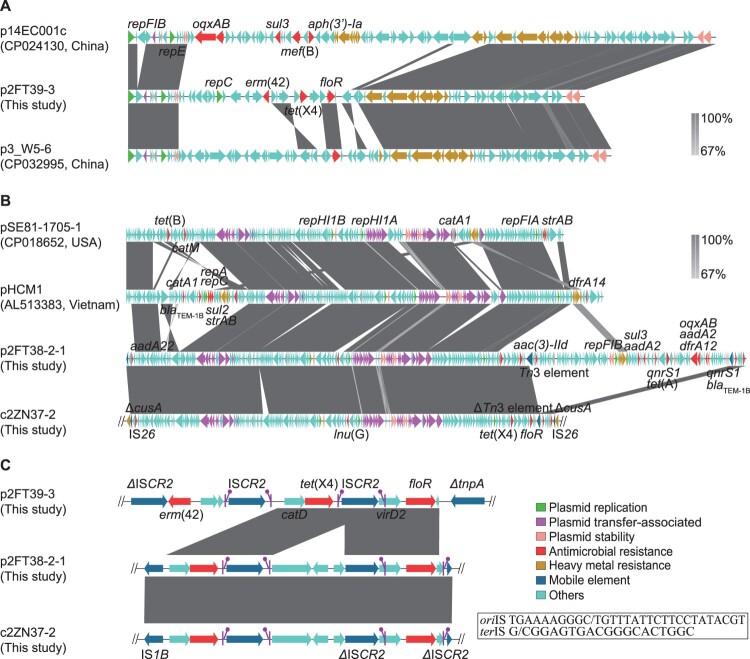
Among them, p2FT39-3 was 68.714 kb in size and harbored 75 open reading frames (ORFs), including tet(X4), erm(42) and floR (Figure 1(A)). Plasmid structural analysis showed that it belonged to the F-:A18:B- plasmid group but lacked the entire conjugative transfer region, which could explain its failure in the conjugation experiment. Blast analysis showed that p2FT39-3 shared similar plasmid backbone (70% query coverage and 99% average sequence identity) against some other plasmids deposited in GenBank, such as p14EC001c (CP024130) from a clinical E. coli strain and p3_W5-6 (CP032995) from an E. coli strain isolated from a contaminated waterway of wild birds.
Figure 1.
Comparative analysis of tet(X4)-harboring plasmids and chromosome. (A) Structures of p14EC001c (CP024130), p3_W5-6 (CP032995) and the tet(X4)-harboring plasmid p2FT39-3. (B) Structures of pSE81-1705-1 (CP018652), pHCM1 (AL513383), the tet(X4)-harboring plasmid p2FT38-2-1 and tet(X4)-harboring chromosome region in E. coli 2ZN37-2. (C) Genetic environments of tet(X4). Results of nucleotide sequence alignment are generated with Easyfig [15]. The arrows represent the positions and transcriptional directions of the ORFs. Regions of homology are marked by shading. The putative oriIS (left-facing sticks in purple) and terIS (right-facing sticks in purple) of the ISCR2 element are also illustrated (5′ to 3′). Δ symbol indicates that the gene is truncated.
p2FT38-2-1 was 283.255 kb in length and contained 349 ORFs, including the resistance genes aadA2, aac(3)-Iid, aadA22, blaTEM−1B, qnrS1, oqxAB, lnu(G), floR, sul3, tet(A), dfrA12 and tet(X4) (Figure 1(B)). Plasmid structural analysis showed that it belonged to IncHI1 incompatibility group, harboring an intact conjugation transfer region, which was in concordance with its conjugability described above. Interestingly, p2FT38-2-1 also harbored partial skeleton of the F-:A8:B- plasmid, including plasmid replication, transfer and stability regions, and was similar to the plasmids pSE81-1705-1 (CP018652) and pHCM1 (AL513383) in Salmonella spp. from human samples.
The tet(X4) gene in E. coli 2ZN37-2 was identified in a 194.2 kb genomic island on its chromosome (4931.759 kb), along with some other antimicrobial resistance genes, including aadA22, blaTEM−1B, qnrS1, lnu(G), and floR (Figure 1(B)). This genomic island was flanked by two copies of IS26 and inserted into a heavy metal resistance gene cusA, with high homology (99%) to the sequence of p2FT38-2-1, likely as a result of IS26-mediated genetic element mobilization. Similar insertion sequence-mediated chromosome integrations have also been described in other resistance genes, including aphA1, tet(D) and blaSHV [12].
We then examined the tet(X4)-neighboring genetic elements among the three strains, and found that tet(X4) was located upstream of ISCR2 and downstream of a hydrolase gene catD (Figure 1(C)). Unlike other insertion sequences, a single copy of ISCR2 could utilize a rolling-circle transposition process to transpose adjacent DNA sequences [13]. It has been found to be associated with the acquisition of diverse antimicrobial resistance genes, including blaVEB−1 [14]. Interestingly, the catD-tet(X4) element in p2FT39-3 was found be flanked by two copies of ISCR2, which strongly suggested that ISCR2 was associated with the mobilization of tet(X4). It was likely that an intact ISCR2 copy originally mobilized catD-tet(X4) by a rolling-circle transposition process and that a secondary process of homologous recombination between two ISCR2 copies led to the deletion of one copy of ISCR2. In combination with the conjugability observed in p2FT38-2-1, our results also suggested that the tet(X4) gene might be able to transfer into other plasmid vectors and spread into other bacterial strains.
In summary, we reported the emergence of tet(X4)-encoding tigecycline resistance mechanism in E. coli strains from migratory birds, further highlighting that migratory birds may serve as a reservoir for the dissemination of emerging antimicrobial resistance. Worrisomely, the emergence of tet(X4) challenges the effectiveness of the entire family of tetracycline antibiotics, including the FDA newly approved eravacycline and omadacycline. Further spread of tet(X4) into clinical multidrug-resistant pathogens may create extensively drug-resistant or pandrug-resistant strains, and result in untreatable infections. A continuous surveillance of tet(X4) in humans, animals, and their environments should be considered for understanding and tackling the dissemination of tigecycline resistance.
Geolocation information
These tet(X4)-positive E. coli strains were isolated from migratory birds in Shenzhen (E. coli 2FT39; E. coli 2FT38-2) and Huizhou (E. coli 2ZN37-2), China, respectively.
Acknowledgements
JS, LC, Y-HL, and X-PL designed the study. CC, C-YC, YZ, QH, X-TW, and GL collected the samples and conducted the experiments. JS, LC, and CC analyzed and interpreted the data. JS, LC, BNK, and CC drafted the manuscript. All authors reviewed, revised, and approved the final report.
Supplementary Material
Funding Statement
This work was supported by National Institutes of Health [grant numbers R01AI090155, R21AI117338, R21AI135250]; the Program for Innovative Research Team in the University of Ministry of Education of China [grant number IRT_17R39, granted to Prof. Ya-Hong Liu by Ministry of Education of the People’s Republic of China in Jun 2017]; the National Key Research and Development Program of China [grant number 2016YFD0501300, granted to Prof. Ya-Hong Liu by Ministry of Science and Technology of the People’s Republic of China in Jun 2016]; the Foundation for Innovation and Strengthening School Project of Guangdong, China [grant number 2016KCXTD010, granted to Prof. Ya-Hong Liu by Department of Education of Guangdong Province, China, in Mar 2017].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Allen HK, Donato J, Wang HH, et al. . Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol. 2010;8(4):251–259. doi: 10.1038/nrmicro2312 [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Hu D, Zhang Q, et al. . Efflux pump overexpression contributes to tigecycline heteroresistance in Salmonella enterica serovar Typhimurium. Front Cell Infect Microbiol. 2017;7, article number 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun J, Chen C, Cui C-Y, et al. . Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat Microbiol. 2019. DOI: 10.1038/s41564-019-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He T, Wang R, Liu D, et al. . Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat Microbiol. 2019. DOI: 10.1038/s41564-019-0445-2. [DOI] [PubMed] [Google Scholar]
- 5.Abdallah M, Olafisoye O, Cortes C, et al. . Activity of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including multidrug-resistant isolates, from New York City. Antimicrob Agents Chemother. 2015;59(3):1802–1805. doi: 10.1128/AAC.04809-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfaller MA, Huband MD, Shortridge D, et al. . Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe as part of the 2016 SENTRY antimicrobial surveillance Program. Antimicrob Agents Chemother. 2018;62(4):e02327–17. doi: 10.1128/AAC.02327-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wick RR, Judd LM, Gorrie CL, et al. . Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13(6):e1005595. doi: 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aziz RK, Bartels D, Best AA, et al. . The RAST server: rapid annotations using subsystems technology. BMC Genomics. 2008;9, article number 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zankari E, Hasman H, Cosentino S, et al. . Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67(11):2640–2644. doi: 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen MV, Cosentino S, Rasmussen S, et al. . Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol. 2012;50(4):1355–1361. doi: 10.1128/JCM.06094-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carattoli A, Zankari E, Garcia-Fernandez A, et al. . In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58(7):3895–3903. doi: 10.1128/AAC.02412-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Partridge SR, Kwong SM, Firth N, et al. . Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev. 2018;31(4):e00088–17. doi: 10.1128/CMR.00088-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toleman MA, Bennett PM, Walsh TR.. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol Mol Biol Rev. 2006;70(2):296–316. doi: 10.1128/MMBR.00048-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poirel L, Mugnier PD, Toleman MA, et al. . ISCR2, another vehicle for blaVEB gene acquisition. Antimicrob Agents Chemother. 2009;53(11):4940–4943. doi: 10.1128/AAC.00414-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan MJ, Petty NK, Beatson SA.. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27(7):1009–1010. doi: 10.1093/bioinformatics/btr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.