Abstract
Context: HemoHIM is a medicinal herbal preparation of Angelica gigas Nakai (Apiaceae), Cnidium officinale Makino (Umbelliferae), and Paeonia japonica Miyabe (Paeoniaceae) developed for immune regulation. HemoHIM has been investigated for its ability to enhance tissue self-renewal and stimulate immune systems. To date, studies on the protective effects of HemoHIM against gastritis and gastric ulcers have not been conducted.
Objective: The protective effects of HemoHIM using models of indomethacin and ethanol/hydrochloric acid (EtOH/HCl)-induced gastric mucosal injury were investigated.
Materials and methods: Rats were divided into five groups (n = 10): control, indomethacin, or EtOH/HCl groups, HemoHIM 250, 500 mg kg−1, and cimetidine 100 mg kg−1, respectively. Indomethacin (80 mg kg−1) and 60% EtOH/150 mM HCl were administered orally 1 h after the administration of samples and rats were anesthetized 3 h after induction. The lesion area (%), inhibition ratio (%), and total acidity were investigated, and tissues were histopathologically analyzed using hematoxylin and-eosin (H&E) staining.
Results: HemoHIM significantly reduced gastric injury in indomethacin-induced model (250 and 500 mg kg−1; 64.30% and 67.75%, p < 0.001) compared to indomethacin group. In the EtOH/HCl-induced model, HemoHIM reduced gastric lesion (250 and 500 mg kg−1; 61.05% and 73.37%, p < 0.001) and gastric acidity (250 and 500 mg kg−1; 37.80 and 45.20 meq L−1, p < 0.001) compared to EtOH/HCl group. H&E staining of the gastric mucosa showed decreased erosion and hemorrhage in HemoHIM group compared to EtOH/HCl group.
Discussion and conclusions: Based on the results, HemoHIM is potential candidate for the treatment of gastritis and gastric ulcers.
Keywords: Inflammation, gastritis, gastric ulcer, Angelica gigas Nakai, Cnidium officinale Makino, Paeonia japonica Miyabe
Introduction
Gastric injury is one of the world’s most common diseases; it and related diseases are very difficult to completely cure. Recently, the prevalence of gastric injury is rising due to environmental factors such as the increased consumption of fatty foods, smoking, stress, alcohol, and Helicobacter pylori infection, nonsteroidal anti-inflammatory drugs (NSAIDs) (Satyanarayana 2006; Kim et al. 2018), resulting in erosion, erythema, hemorrhage, and edema (Kushima et al. 2005; Yeo et al. 2018). This inflammatory disease of the gastrointestinal mucous membrane is called gastritis, which is more likely to develop into chronic gastritis, (gastric ulcer) when the damage is more advanced than mucous plates (Lee et al. 2015; Lee et al. 2016). The secretion of gastric acid is caused by the expression of histamine 2 receptor (H2r) by histamine, activation of cholecystokinin 2 receptor (CCK2r) by gastrin, and activation of muscarinic acetylcholine receptor M3 (M3r) by acetylcholine (Lee et al. 2005). H2r accelerates the secretion of gastric acid into the stomach lumen via the cAMP/protein kinase A/proton pump pathway (Takeuchi et al. 1999). Thus, H2-receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) are considered representative of gastritis and gastric ulcers. Typical gastritis and gastric ulcer drugs include H2RAs such as ranitidine, cimetidine, and famotidine, which inhibit the secretion of gastric acid, and PPIs, such as omeprazole and pantoprazole (Park et al. 2017). Although these drugs showed clinically excellent therapeutic effects, side effects have been reported such as digestive disorders, rashes, and urticaria (Lee et al. 2015). Therefore, development of new drugs to treat gastritis and gastric ulcers using natural plants or herbs which have no toxicity and side effects is promising. Recently, the protective effects of herbal extracts against gastric injuries such as the protective effects of Lycium chinense Miller (Solanaceae) on acute gastric lesion in mice (Lee et al. 2015) and inhibitory effects of cabbage juice on EtOH-HCl induced gastritis in rats (Hong et al. 2013) were reported.
HemoHIM is an herbal preparation consisting of roots of Angelica gigas Nakai (Apiaceae), Cnidium officinale Makino (Umbelliferae), and Paeonia japonica Miyabe (Paeoniaceae) that has antioxidant and immune-modulating activities (Jo et al. 2005; Shin et al. 2006; Park et al. 2012). In addition, HemoHIM can protect gastrointestinal and immune hematopoietic systems against radiation (Park et al. 2005). In several studies, the herbal ingredients of HemoHIM, or compounds containing them, have shown protective effects against gastric injuries. Jiyu-tang containing Angelica Radix inhibits gastric and ileac mucosal ulcers induced by indomethacin (Kang et al. 2007) and polysaccharides from Angelica Radix attenuates gastric injury, promoting normal gastric epithelial cell migration and proliferation (Lam et al. 2010). In addition, Cnidii Rhizoma protects the stomach against ethanol (EtOH)-induced gastric injury (Shin et al. 2011). The nine herbal mixture types containing Cnidii Rhizoma exert antimicrobial and antioxidant activities against Helicobacter pylori (Park and Kim 2006). Paeonia Radix protects the gastric mucosa from oxidative stress caused by acidified EtOH and compound 48/80 (Ohta et al. 2006; Kim et al. 2018). In addition, the antioxidant and inhibitory effects on Helicobacter pylori have been found in beverages containing Angelica Radix, Cnidii Rhizoma and Paeonia Radix (Park 2007). HemoHIM alleviated airway inflammation (Shin et al. 2017) and had the anti-inflammatory effects in colitis (Lee et al. 2007). Thus, HemoHIM may be a good candidate for treatment and prevention of gastritis and gastric ulcers, due to the excellent effects of the herbal ingredients.
The NSAID indomethacin-induced bleeding, ulcers, and erosions in the gastrointestinal tract in both animal and human trials (Djahanguiri 1969). In addition, EtOH/HCl causes severe damage and necrotic lesions to the gastric mucosa (Hong et al. 2013). The animal models of gastric injuries induced by indomethacin and EtOH/HCl have been widely used in studies to evaluate the protective effects of herbal products against gastritis and gastric ulcers. Sreeja et al. (2018) and de Araújo et al. (2018) studied the gastroprotective action of Sphenodesme involucrata var. paniculata (C. B. Clarke) Munir (Lamiaceae), Kalanchoe brasiliensis Cambess (Crassulaceae), and Kalanchoe pinnata (Lamarck) Persoon (Crassulaceae) leaf juices using EtOH, EtOH/HCl-, and indomethacin-induced experimental gastric ulcer models. Therefore, in the present study, the protective effects of HemoHIM against indomethacin- and EtOH/HCl-induced gastric mucosal injury in rat models were investigated.
Materials and methods
Materials
Formalin, indomethacin, cimetidine, sodium carbonate, Tween 80, ethanol (EtOH), carboxymethyl cellulose (CMC), and phosphoric acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). The reference standards, chlorogenic acid was purchased from USP reference standard (Rockville, MD, USA), paeoniflorin and nodakenin were purchased from Wako Pure Chemical Industries (Osaka, Japan). HPLC-grade methanol and water were purchased from Honeywell, Burdick & Jackson (Muskegon, MI, USA). Phosphate buffered saline (PBS) was purchased from Gibco (Grand Island, NY, USA), HCl was purchased from Daejung Chemicals (Gyeonggi-do, Republic of Korea), and sodium hydroxide was purchased from Duksan Pure Chemical (Gyeonggi-do, Republic of Korea).
Preparation of HemoHIM
HemoHIM was prepared according to the method described in detail in our previous report (Jo et al. 2005). The standardized HemoHIM (Batch no: HHH009) containing nodakenin (50-150 mg/100 g), chlorogenic acid (25–60 mg/100 g), and paeoniflorin (200–400 mg/100 g) was manufactured by Kolmar BNH (Sejong-si, Republic of Korea). Briefly, traditional Korean medicinal plants, Angelica gigas, Cnidium officinale, and Paeonia japonica were extracted for 4 h in boiling water to obtain a total extract. Half of the extract was precipitated with 95% EtOH to obtain an EtOH-insoluble polysaccharide fraction. Finally, the HemoHIM was obtained by adding the polysaccharide fraction to the remaining half of the extract, concentrated to a solid content of 30% ± 3%, freeze-dried (MCFD8512, Ilshin Lab, Seoul, Republic of Korea) and stored at 4 °C until use.
HPLC analysis
The analyses were performed using an HPLC system (Prominence HPLC system, Shimadzu, Kyoto, Japan) with Capcell Pak C18 column (4.6 × 250 mm2, 5 μm, Shiseido, Tokyo, Japan). The mobile phase included 0.1% phosphoric acid in water (Solvent A) and 0.1% phosphoric acid in acetonitrile (Solvent B). The flow rate was set at 1.0 mL/min and column temperature was kept at 30 °C. The auto-sampler was conditioned at 4 °C and the injection volume was 10 μL. The UV detector wavelength was set at 230 nm. The reference standards of chlorogenic acid, paeoniflorin, and nodakenin were accurately weighed, dissolved in 80% methanol, and then diluted to appropriate concentration ranges for the establishment of calibration curves. HemoHIM was dissolved in 50 volumes (w/v) of 80% methanol and then filtered through a 0.45 μm PVDF filter membrane (Chromdisc, Daegu, Korea). The filtrate was injected into the HPLC system.
Animals and experimental protocol
Male Sprague Dawley (SD) rats 5- and 6-weeks-old were used for indomethacin-induced (five groups of ten) and EtOH/HCl-induced models (five groups of ten), respectively. Rats were purchased from DooYeol Biotech (Seoul, Republic of Korea) and housed in a controlled environment (25 °C ± 2 °C, 55% ± 5% relative humidity under a 12 h light-dark cycle). Throughout the experiment, the rats were allowed free access to food and tap water. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Korea Kolmar Animal Ethics Committee (18-KBH-I-01).
Indomethacin-induced gastric ulcer model
Rats were divided into five groups: control, indomethacin, HemoHIM 250 mg kg−1, HemoHIM 500 mg kg−1, and cimetidine 100 mg kg−1. HemoHIM (250, 500 mg kg−1) dissolved in 0.5% CMC or cimetidine (100 mg kg−1) dissolved in 20% Tween 80, were administered orally after 24 h of fasting. Indomethacin (80 mg kg−1) dissolved in 5% sodium bicarbonate was administered orally 1 h after the administration of HemoHIM or cimetidine. Three hours after administration of indomethacin, rats were anesthetized and euthanized. The inner and outer layers of the gastric wall were fixed with 4% neutral formalin. After 30 min, the stomachs were dissected and washed with PBS.
EtOH/HCl-induced gastritis model
Rats were divided into five groups: control, EtOH/HCl, HemoHIM 250 mg kg−1, HemoHIM 500 mg kg−1, and cimetidine 100 mg kg−1. HemoHIM (250 or, 500 mg kg−1) dissolved in 0.5% CMC or cimetidine (100 mg kg−1) dissolved in 20% Tween 80, were administered orally after 24 h of fasting. Next, 60% EtOH/150 mM HCl was administered orally 1 h after the administration of HemoHIM or cimetidine. One hour after administration of 60% EtOH/150 mM HCl, rats were dissected. The gastric juice was centrifuged at 3,000 rpm for 20 min and the total acidity was assayed (Nam et al. 2014). Briefly, 0.5% dimethylaminobenzene alcohol solution and 1% phenolphthalein alcohol solution were added to the gastric juice supernatant. The solution was titrated by adding 0.1 N NaOH solution until the red color disappeared. The total acidity of the titration value is expressed as meq/L/100 g using the following formula: Acidity = (Volume of NaOH × Normality of NaOH × 100)/0.1 (meq/L).
Analysis of gastric lesions
For the determination of gastric lesion area, the inner surface of the stomach was photographed and analyzed using Image J software (NIH ImageJ, NIH, Bethesda, MD). Lesion area (%) was determined for the lesion area of the stomach tissue. The inhibition ratio was calculated as follows: Inhibition ratio (%) = {(control lesion area - sample lesion area)/control lesion area}) × 100. For histopathological analysis, the stomachs were fixed in 10% neutral formalin and fixed overnight. Sections of the gastric glands were sliced at a thickness of 5 μm and stained with hematoxylin and eosin (H&E). The stained sections were examined under microscope for any damage or change in morphology of the particular tissue.
Statistical analysis
The results are presented as the mean ± standard error or the mean (S.E.M). One-way analysis of variance (ANOVA) followed by Duncan’s multiple range test used for comparing three or more groups. The GraphPad Prism5.0 (GraphPad Prism Software, San Diego, CA) program was used for statistical analysis. p values < 0.05 were considered statistically significant.
Results
Identification and quantification of chlorogenic acid, paeoniflorin, and nodakenin using HPLC
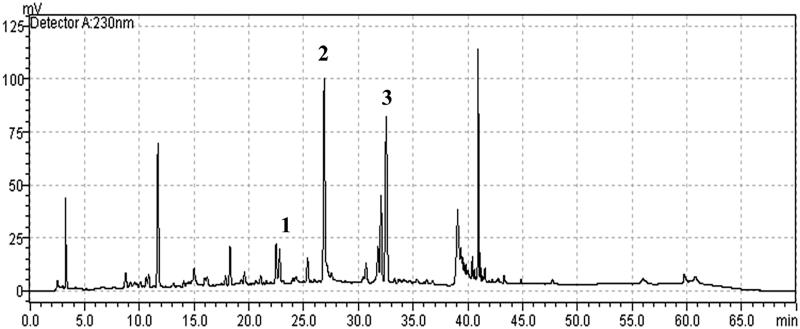
The main compounds in the standardized composition of HemoHIM were investigated using HPLC at 230 nm (Figure 1). The identified compounds in HemoHIM included chlorogenic acid (retention time: 22.488 min), paeoniflorin (retention time: 26.880 min), and nodakenin (retention time: 32.061 min). The quantities of chlorogenic acid, paeoniflorin, and nodakenin were 37, 358, and 106 mg/100 g, respectively. Thus, this batch of HemoHIM was found to contain the compounds within the range of the production management standards of chlorogenic acid (25–60 mg/100 g), paeoniflorin (200–400 mg/100 g), and nodakenin (50–150 mg/100 g).
Figure 1.
HPLC chromatogram of HemoHIM. Peak number indicated: 1: chlorogenic acid, 2: paeoniflorin, 3: nodakenin.
HemoHIM inhibits the reddening and hemorrhage in indomethacin-induced gastric ulcer model
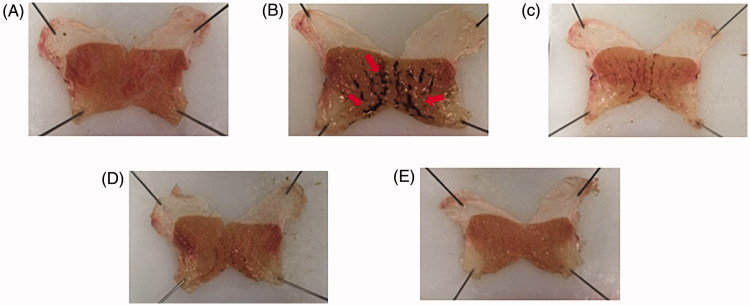
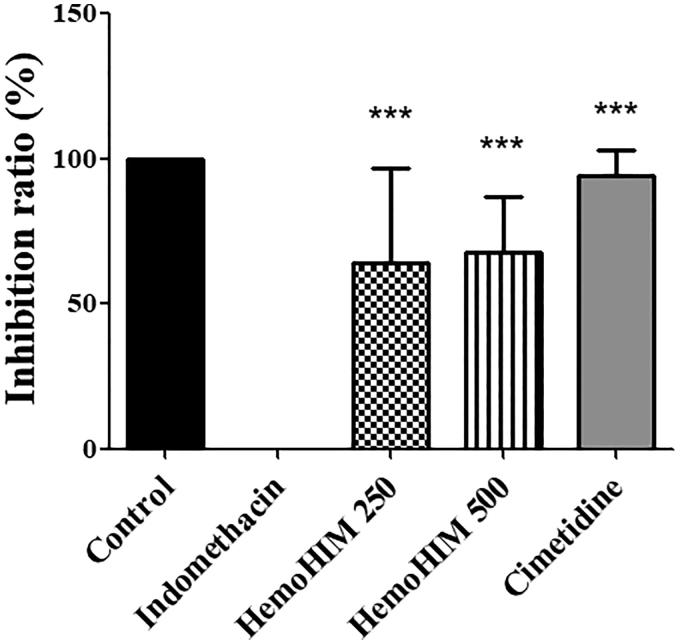
First, the protective role of HemoHIM against indomethacin-induced ulcers was investigated using rats. The body weights were not significantly different between the groups (Table 1). Gross findings of the rats in the indomethacin group showed numerous large lesions in the entire gastric mucosa such as signs of clear reddening and hemorrhage compared with the rats in the control group (arrow heads in Figure 2(B)). However, pretreatment with HemoHIM significantly decreased reddening and hemorrhage (Figures 2(C,D)). HemoHIM showed significant inhibition of gastric injury in indomethacin-induced model (250 mg kg−1; 64.30% ± 8.98% and 500 mg kg−1; 67.75% ± 5.33%, p < 0.001, respectively) compared to indomethacin group. Inhibition ratio in the cimetidine 100 mg kg−1 was 94.07% ± 2.37% (p < 0.001) (Figure 3, Table 2).
Table 1.
Number of animals and body weight on indomethacin and EtOH/HCI-induced gastric mucosal injury models.
| Group | Animals | No. of animals | Body weight (g) |
|---|---|---|---|
| Indomethacin-induced model | |||
| Control | 10 | 153.60 ± 2.88 | |
| Indomethacin | 10 | 152.55 ± 2.50 | |
| HemoHIM 250 | SD-rat, 5w, male | 10 | 152.33 ± 2.96 |
| HemoHIM 500 | 10 | 151.64 ± 2.30 | |
| Cimetidine | 10 | 155.07 ± 4.58 | |
| EtOH/HCI-induced model | |||
| Control | 10 | 226.43 ± 3.12 | |
| Et/OH/HCI | 10 | 224.10 ± 2.56 | |
| HemoHIM 250 | SD-rat, 6w, male | 10 | 221.96 ± 1.92 |
| HemoHIM 500 | 10 | 223.38 ± 2.65 | |
| Cimetidine | 10 | 224.64 ± 3.17 |
Figure 2.
Effect of HemoHIM on stomach appearance in indomethacin-induced rat. Arrow heads indicate reddening and hemorrhage.(A) Control (B) Indomethacin (C) HemoHIM 250 mg kg−1 (D) HemoHIM 500 mg kg−1 (E) Cimetidine 100 mg kg−1.
Figure 3.
Effect of HemoHIM on inhibition ratio (%) in indomethacin-induced rats. Data are expressed as mean ± SEM (n = 10). Comparison was made between indomethacin and each sample treatment group. Significant differences as compared with indomethacin group (***p < 0.001).
Table 2.
Effect of HemoHIM on lesion area (%) in indomethacin and EtOH/HCI-induced rats.
| Group | Lesion area (%) |
|---|---|
| Indomethacin-induced ulcer model | |
| Control | 0 |
| Indomethacin | 10.11 ± 1.57 |
| HemoHIM 250 | 4.03 ± 1.10** |
| HemoHIM 500 | 3.49 ± 0.79*** |
| Cimetidine | 0.27 ± 0.05*** |
| EtOH/HCI-induced gastritis model | |
| Control | 0 |
| Et/OH/HCI | 25.69 ± 2.65 |
| HemoHIM 250 | 10.12 ± 1.74*** |
| HemoHIM 500 | 5.92 ± 1.00*** |
| Cimetidine | 7.64 ± 0.90*** |
Lesion area (%) indicates the damaged area. Thus, HemoHIM significantly reduced damaged area compared to the induction group (indomethacin and EtOH/HCl group). Significant differences as compared with indomethacin group and EtOH/HCl group (**p < 0.01, ***p < 0.001).
HemoHIM suppresses lesions and total gastric acidity in EtOH/HCl-induced gastritis model
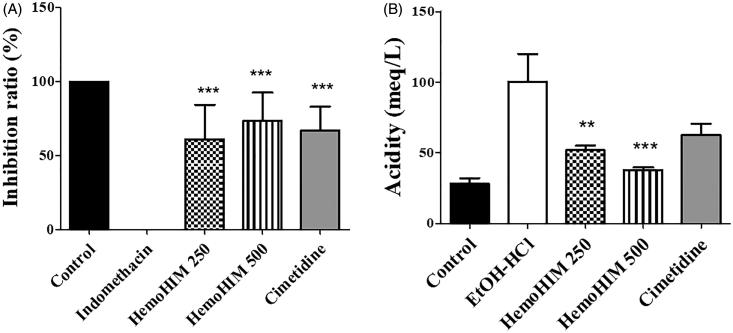
HemoHIM showed significant inhibition of gastric injury in EtOH/HCl-induced model (250 mg kg−1; 61.05% ± 7.22% and 500 mg kg−1; 73.37% ± 7.12%, p < 0.001, respectively) compared to EtOH/HCl group. Inhibition ratio in the cimetidine 100 mg kg−1 was 94.07% ± 2.37% (p < 0.001) (Figure 4, Table 2). Next, whether the protective effects of HemoHIM against EtOH/HCl-induced gastritis resulted from regulating gastric acidity was examined. In the EtOH/HCl group, the gastric acidity increased to 100.40 ± 17.22 meq L−1 compared with the control group (28.20 ± 3.50 meq L−1). However, the HemoHIM treatment groups showed significantly decreased acidity to 37.80 ± 8.79 meq L−1 in the 250 mg kg−1 group (p < 0.01) and to 45.20 ± 7.76 meq L−1 in the 500 mg kg−1 group (p < 0.001) (Figure 4(B)).
Figure 4.
Effect of HemoHIM on stomach appearance and the changes of secretion of gastric acid in EtOH/HCl induced rats. (A) Inhibition ratio (%) (B) Acidity (meq/L). Data are expressed as mean ± SEM (n = 10). Comparison was made between EtOH/HCl and each sample treatment group. Significant difference as compared with EtOH/HCl group (**p < 0.01, ***p < 0.001).
HemoHIM inhibits gastric lesions including erosion and hemorrhage in EtOH/HCl-induced gastritis model based on histopathological analysis
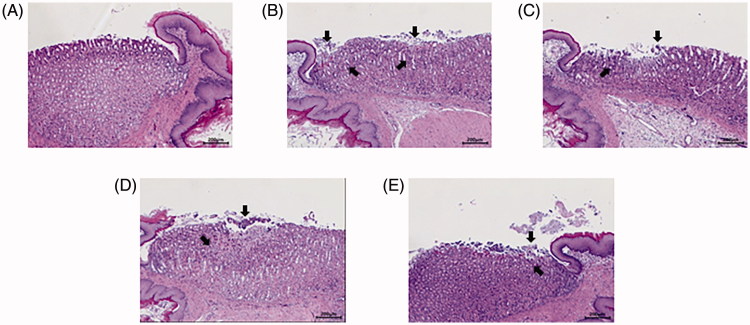
Next, the protective effects of HemoHIM in EtOH/HCl-induced gastritis model were investigated. The body weights were not significantly different between the groups (Table 1). Based on histopathological examination, the rats in the control group showed smooth and clear gastric mucosa. However, in the EtOH/HCl group, the rats showed significant pathological changes such as erosion, hemorrhage and loose cell arrangement (arrow heads in Figure 5). In contrast, the rats in the HemoHIM 250, 500 mg kg−1, and cimetidine group showed less erosion and hemorrhage compared to the EtOH/HCl group, indicating that HemoHIM and cimetidine are effective in inhibiting gastric damage.
Figure 5.
Hematoxylin-eosin (H&E) staining of histological section of the gastric mucosa in the gastric glands in EtOH/HCl-induced rats. Arrow heads indicate erosion and hemorrhage. (A) Control (B) EtOH/HCl (C) HemoHIM 250 mg kg−1 (D) HemoHIM 500 mg kg−1 (E) Cimetidine 100 mg kg−1.
Discussion
Gastritis and gastric ulcer are common diseases worldwide, and the incidence is rising among not only adults but also children and minors (Zhao et al. 2019). Major causes include Helicobacter pylori infection, overuse of NSAIDs, alcohol consumption, stress, and irregular eating habits (Lee et al. 2015). Gastritis is an inflammation disease in all parts of the stomach that causes trimming, nausea, indigestion, and heartburn (Hong et al. 1992). The stimulated gastric acid secretion increases internal pH and gastric mucosal damage (Nam et al. 2014). The gastritis symptoms can progress further to damage of submucosa or muscular layers in the stomach wall, which is called a gastric ulcer. The ulcer does not occur in normal mucosa and is associated with diffuse gastritis (Jee 2010; Lee et al. 2015). Gastric ulcers resulting from NSAID use are caused by gastric mucosal cell damage due to the increase of mucosal permeability of active radicals, decrease of blood flow, and damage of vascular endothelial cells resulting from changes in blood cell functions (Lee et al. 2005). PPIs such as omeprazole, pantoprazole, and rabeprazole as well as H2RAs including cimetidine, ranitidine, famotidine, and misoprostol, have been developed to treat the symptoms caused by NSAIDs, however, these drugs have various side effects such as diarrhea, nausea, stomachache, and skin rash (Miederer 1986; Lim et al. 2012). NSAIDs have been shown to damage the mucosa membrane and weaken the mucosal resistance (Satyanarayana 2006). Indomethacin, a first generation NSAID, damages gastric mucosa by inhibiting of cyclooxygenase (COX), thus, limiting synthesis of prostaglandins (PGs) (Takeuchi et al. 1991; Sagar and Ahamed 1999). Indomethacin suppresses both isoforms of the COX enzyme (COX-1 and COX-2), induces severe damage in gastric tissue accompanied by gastrointestinal bleeding (Peskar et al. 2001; Suleyman et al. 2009). In addition, results from several studies indicated that indomethacin induces lipid peroxidation and has pro-oxidant activity by generating ROS (Takeuchi et al. 1991; Miura et al. 2002). To assess the protective effects of HemoHIM against gastric damage caused by NSAIDs, the indomethacin-induced gastric damage model was used according to previous studies (Babyatsky et al. 1996; Ji and Baek 2011). The reduction of lesion area is the most direct indicator of treatment effectiveness for gastric injury. In the present study, the increased lesion area caused by indomethacin was significantly reduced by administration of HemoHIM, which is a direct result of suppressing gastric damage. Based on the antioxidant effect of HemoHIM's previous study, HemoHIM potentially inhibited gastric injury by inhibiting ROS (Shin et al. 2006).
Gastric injury caused by EtOH/HCl is a widely used gastritis model in which EtOH directly stimulates the gastric mucosa to remove free antioxidants, thereby increasing free radicals, lipid peroxidation, and PG, further exacerbating acute gastritis (Ishii et al. 1976; Anandan et al. 1999; Sreeja et al. 2018). In the EtOH/HCl-induced gastric injury rat model experiments performed in the present study, the area of gastric lesions was reduced in HemoHIM and cimetidine groups compared with the EtOH/HCl group. Histopathological analysis showed hemorrhage and erosion in EtOH/HCl group, however, the incidence of damage was lower in HemoHIM and cimetidine groups than in the EtOH/HCl group. The results obtained with HemoHIM are similar to previous results that showed rats with EtOH/HCl-induced acute gastric lesions recovered normal mucosal structure after being fed soy skim milk fermented by lactic acid bacteria (Liu et al. 2009). Gastric acid secretion is an important factor in gastric damage (Okcu et al. 1992). Gastric fluid in healthy people is controlled by normal secretion which is necessary for food digestion, however, stimulation by chemicals due to mucous membrane damage or blood flow disorder promotes gastric acid secretion (Okabe et al. 1986). In experiments in this study, gastric acidity was increased due to stimulation with EtOH/HCl, but suppressed by the administration of HemoHIM and cimetidine. The H2RA cimetidine is a drug that inhibits the secretion of gastric acid in the stomach via nerve stimulation by directly antagonizing the histamine receptor of cell walls (Hung and Lee 1991). Further studies are needed to determine whether HemoHIM acts as an H2RA inhibiting histamine secretion, or as an H+/K+ ATPase inhibitor. HemoHIM has previously been shown to have antioxidant properties (Shin et al. 2006) and anti-inflammatory effects on epithelium in the colon mucosa of TNBS-treated rats (Lee et al. 2007). Therefore, HemoHIM could effectively suppress the damage by eliminating the free radicals which can directly cause injury.
In the present study, the effects of HemoHIM on gastric mucosal injury induced by indomethacin and EtOH/HCl were investigated. In both models, HemoHIM had significant effects compared with cimetidine, a well-known drug for gastritis and gastric ulcers, in the gastric lesion index and gastric acid secretary parameters. Further studies on the key regulation targets of HemoHIM in these models and the effects of HemoHIM on chronic gastritis in animal models will be required to confirm the effectiveness of HemoHIM as a preventive or treatment drug against gastric injuries.
Conclusions
The results from this study demonstrated that oral administration of HemoHIM protected against indomethacin- and EtOH/HCl-induced gastric mucosal injuries in rat models. HemoHIM inhibited pathological changes including lesion area, acidity, erosion, and hemorrhage induced by indomethacin or EtOH/HCl. These findings strongly suggest that HemoHIM is potential candidate for the treatment of gastritis and gastric ulcers. However, further studies are necessary to analyze proinflammatory cytokines and identify the molecular mechanism underlying the treatment efficacy of HemoHIM in gastritis and gastric ulcers.
Funding Statement
This research was supported by a grant from Kolmar BNH Co., Ltd., Korea.
Author contributions
All authors contributed to this study. H.K. Kim, H.S. Lee, S.K. Jo and U.H. Jung, and H.R. Park conceived and designed the experiments; S.K. Kim, D.A. Kwon, Y.S. Kim, and S.H Baek performed the experiments; S.K. Kim, D.A. Kwon, and Y.S. Kim, and S.H Baek contributed to the interpretation and statistical analysis of the data; and D.A. Kwon, H.S. Lee, wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Anandan R, Rekha RD, Saravanan N, Devaki T. 1999. Protective effects of Picrorrhiza kurroa against HCl/ethanol-induced ulceration in rats. Fitoterapia. 70(5):498–501. [Google Scholar]
- Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. 1996. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 110(2):489–497. [DOI] [PubMed] [Google Scholar]
- de Araújo E, Guerra G, Araújo D, de Araújo A, Fernandes J, de Araújo Júnior R, da Silva V, de Carvalho T, Ferreira L, Zucolotto S. 2018. Gastroprotective and antioxidant activity of Kalanchoe brasiliensis and Kalanchoe pinnata leaf juices against indomethacin and ethanol-induced gastric lesions in rats. Int J Mol Sci. 19:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djahanguiri B. 1969. The production of acute gastric ulceration by indomethacin in the rats. Scand J Gastroenterol. 4(3):265–267. [PubMed] [Google Scholar]
- Hong YJ, Kim SY, Han J, Lim YI, Park KY. 2013. Inhibitory effects of cabbage juice and cabbage-mixed juice on the growth of AGS human gastric cancer cells and on HCl-ethanol induced gastritis in rats. J Food Sci Nutr. 42:682–689. [Google Scholar]
- Hong YC, Park CY, Lee WC, Lee KS. 1992. Case-control study on effects of alcohol intake and smoking to gastritis of Korean adult men. J Prev Med Public Health. 25:238–246. [Google Scholar]
- Hung CR, Lee CH. 1991. Protective effect of cimetidine on tannic acid-induced gastric damage in rats. J Pharm Pharmacol. 43(8):559–563. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Fujii Y, Homma M. 1976. Gastric acid stimulating action of cysteamine in the rat. Eur J Pharmacol. 36(2):331–336. [DOI] [PubMed] [Google Scholar]
- Jee SR. 2010. Differential diagnosis of benign and malignant gastric ulcer. Korean J Gastrointest Endosc. 40:79–82. [Google Scholar]
- Ji HC, Baek TH. 2011. A comparative study of Pyeongwi-san, Ijin-tang and Pyeongjintang extracts on indomethacin-induced gastric mucosal lesions in mice. J Korean Med. 32:102–117. [Google Scholar]
- Jo SK, Park HR, Jung U, Oh H, Kim SH, Yee ST. 2005. Protective effect of a herbal preparation (HemoHIM) on the self-renewal tissues and immune system against γ-irradiation. J Food Sci Nutr. 34:805–813. [Google Scholar]
- Kang A, Choi EY, Kim HJ, Han YS, Lim SW. 2007. The effects of Jiyu-tang against gastric and ileac mucosal ulcer induced by indomethacin in mouse. J Korean Med. 28:224–240. [Google Scholar]
- Kim YS, Park HJ, Song JB, Lee DH, Kim HC. 2018. Anti-ulcer effects of HT074 on HCl/EtOH induced gastric injury. Kor J Herbol. 33:9–18. [Google Scholar]
- Kushima H, Hiruma-Lima CA, Santos MA, Viana E, Coelho-Ferreira M, Brito A. 2005. Gastroprotective activity of Pradosia huberi on experimentally induced gastric lesions in rodents: role of endogenous sulphydryls and nitric oxide. J Ethnopharmacol. 101(1–3):61–67. [DOI] [PubMed] [Google Scholar]
- Lam RYY, Lin ZX, Sviderskaya E, Cheng C. 2010. Application of a combined sulphorhodamine B and melanin assay to the evaluation of Chinese medicines and their constituent compounds for hyperpigmentation treatment. J Ethnopharmacol. 132(1):274–279. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Choi HS, Lee JH, Jung CJ, Lee MY. 2005. The effects of ethyl acetate fraction of Sanguisorba officinalis L. on experimentally-induced acute gastritis and peptic ulcers in rats. J Food Sci Nutr. 34:1545–1552. [Google Scholar]
- Lee HJ, Kim SR, Moon CJ, Kim JC, Bae CS, Kang SS, Jung U, Park HR, Jo SK, Kim SH. 2007. Anti-inflammatory activities of a herbal preparation (HemoHIM) in colitis induced by trinitrobenzene sulfonic acid in rats. Korean J Vet Res. 47:19–24. [Google Scholar]
- Lee JY, Kwon OJ, Noh JS, Roh SS. 2016. Protective effects of red ginseng according to steaming time on HCl/ethanol-induced acute gastritis. Appl Biol Chem. 59(4):365–372. [Google Scholar]
- Lee AR, Lee JY, Kim MY, Shin MR, Shin SH, Seo BI, Kwon OJ, Roh SS. 2015. Protective effects of a Lycium chinense ethanol extract through anti-oxidative stress on acute gastric lesion mice. Kor J Herbol. 30(6):63–68. [Google Scholar]
- Lim SY, Byun JS, Kim DJ, Kwak MA. 2012. Protective effects of BJS-mix001, in indomethacin induced gastric damages in rats. J Physiol Pathol Kor Med. 26:181–188. [Google Scholar]
- Liu CF, Hu CL, Chiang SS, Tseng KC, Yu RC, Pan TM. 2009. Beneficial preventive effects of gastric mucosal lesion for soy-skim milk fermented by lactic acid bacteria. J Agric Food Chem. 57(10):4433–4438. [DOI] [PubMed] [Google Scholar]
- Miederer S. 1986. Will anti-ulcer drugs soon differ only in their side effects? Fortschritte Der Medizin. 104(3):918–920. [PubMed] [Google Scholar]
- Miura T, Muraoka S, Fujimoto Y. 2002. Lipid peroxidation induced by indomethacin with horseradish peroxidase and hydrogen peroxide: involvement of indomethacin radicals. Biochem Pharmacol. 63(11):2069–2074. [DOI] [PubMed] [Google Scholar]
- Nam DE, Kim OK, Shim TJ, Lee JK, Hwang KT. 2014. Inhibitory effects of chios mastic gum on gastric acid secretion by histamine-related pathway in a rat model and primary parietal cells. J Food Sci Nutr. 43:1500–1509. [Google Scholar]
- Ohta Y, Kobayashi T, Hayashi T, Inui K, Yoshino J, Nakazawa S. 2006. Preventive effect of Shigyaku-san on progression of acute gastric mucosal lesions induced by compound 48/80, a mast cell degranulator, in rats. Phytother Res. 20(4):256–262. [DOI] [PubMed] [Google Scholar]
- Okabe S, Jino H, Nishida A. 1986. Effects of 15(R)-15-methyl prostaglandin E2 (arbaprostil) on gastric secretion and various gastric lesions induced in rats. Jpn J Pharmacol. 40(2):329–337. [DOI] [PubMed] [Google Scholar]
- Okcu N, Onuk M, Yilmaz A, Gundogdu M, Baran T. 1992. The effects of omeprazole and ranitidine on the gastric ulcer healing. Doga Trop J Med Sci. 16:657–658. [Google Scholar]
- Park HR, Jo SK, Choi NH, Jung U. 2012. HemoHIM ameliorates the persistent down-regulation of Th1-like immune responses in fractionated γ-irradiated mice by modulating the IL-12p70-STAT4 signaling pathway. Radiation Res. 177(5):676–684. [DOI] [PubMed] [Google Scholar]
- Park YS, Kim YH. 2006. The effect of medicinal herb extract on antimicrobial activity against Helicobacter pylori and antioxidant activity. J East Asian Soc Diet Life. 16:199–206. [Google Scholar]
- Park HR, Kim SH, Yee ST, Byun MW, Jo SK. 2005. Effect of a herb mixture (HIM-I) on the protection of the hematopoietic-immune system and self-renewal tissues against radiation damage. J Food Sci Nutr. 34:605–612. [Google Scholar]
- Park I, Park S, Yun J, Choi G, Kim H, Seo Y, Cho J. 2017. Protective effect of Litsea japonica fruit flesh extract on stress-induced gastritis in rats. J Fd Hyg Safety. 32(6):536–541. [Google Scholar]
- Park YS. 2007. Development of functional beverages using distilled extract of Korean medicinal herb. J East Asian Soc Diet Life. 17:384–392. [Google Scholar]
- Peskar BM, Maricic N, Gretzer B, Schuligoi R, Schmassmann A. 2001. Role of cyclooxygenase-2 in gastric mucosal defense. Life Sci. 69(25/26):2993–3003. [DOI] [PubMed] [Google Scholar]
- Sagar V, Ahamed RN. 1999. Gastric mucosal cellular changes induced by indomethacin (NSAID) in male albino rats. Indian J Exp Biol. 37(4):365–369. [PubMed] [Google Scholar]
- Satyanarayana M. 2006. Capsaicin and gastric ulcers. Crit Rev Food Sci Nutr. 46(4):275–328. [DOI] [PubMed] [Google Scholar]
- Shin SH, Kim DS, Kim MJ, Kim SH, Jo SK, Byun MW, Yee ST. 2006. Protective effects of a herbal composition (HemoHIM) against apoptosis induced by oxidative stress of hydrogen peroxide. J Food Sci Nutr. 35:1127–1132. [Google Scholar]
- Shin NR, Kim SH, Ko JW, Park SH, Lee IC, Ryu JM, Kim JC, Shin IS. 2017. HemoHIM, a herbal preparation, alleviates airway inflammation caused by cigarette smoke and lipopolysaccharide. Lab Anim Res. 33(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin IS, Lee MY, Seo CS, Lim HS, Kim JH, Jeon WY, Shin HK. 2011. Palmul-tang, a traditional herbal formula, protects against ethanol-induced acute gastric injury in rats. J Kor Med. 32:74–84. [Google Scholar]
- Sreeja PS, Arunachalam K, Saikumar S, Kasipandi M, Dhivya S, Murugan R, Parimelazhagan T. 2018. Gastroprotective effect and mode of action of methanol extract of Sphenodesme involucrata var. paniculata (CB Clarke) Munir (Lamiaceae) leaves on experimental gastric ulcer models. Biomed Pharmacother. 97:1109–1118. [DOI] [PubMed] [Google Scholar]
- Suleyman H, Cadirci E, Albayrak A, Polat B, Halici Z, Koc F, Hacimuftuoglu A, Bayir Y. 2009. Comparative study on the gastroprotective potential of some antidepressants in indomethacin-induced ulcer in rats. Chem Biol Interact. 180(2):318–324. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Kajimura M, Kodaira M, Lin S, Hanai H, Kaneko E. 1999. Up-regulation of H2 receptor and adenylate cyclase in rabbit parietal cells during prolonged treatment with H2-receptor antagonists. Dig Dis Sci. 44(8):1703–1709. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Ueshima K, Hironaka Y, Fujioka Y, Matsumoto J, Okabe S. 1991. Oxygen free radicals and lipid peroxidation in the pathogenesis of gastric mucosal lesions induced by indomethacin in rats. Relation to gastric hypermotility. Digestion. 49(3):175–184. [DOI] [PubMed] [Google Scholar]
- Yeo D, Hwang SJ, Kim WJ, Youn HJ, Lee HJ. 2018. The aqueous extract from Artemisia capillaris inhibits acute gastric mucosal injury by inhibition of ROS and NF-kB. Biomed Pharmacother. 99:681–687. [DOI] [PubMed] [Google Scholar]
- Zhao X, Li J, Meng Y, Cao M, Wang J. 2019. Treatment effects of Jinlingzi powder and its extractive components on gastric ulcer induced by acetic acid in rats. J Evid Based Complementary Altern Med. 2019:7365841. [DOI] [PMC free article] [PubMed] [Google Scholar]