Abstract
Glycogen synthase kinase-3β (GSK-3β) is involved in a wide variety of cellular processes, and implicated in a growing list of human diseases. Recent drug inhibition studies have suggested a role for GSK-3β in mitosis in animals. Here, we take an alternative approach to understanding GSK-3β function in mitosis by genetic mutational analysis in Drosophila. GSK-3β function is well conserved between Drosophila (Zw3) and humans, frequently operating similarly in pathways, as diverse as the Wnt signaling and circadian rhythm pathways, and sharing a key role in the development of the neuromuscular junction. Unlike drug inhibitor studies, we find that loss of function mutations of zw3 result in markedly curved, or bent, metaphase spindles that exhibit metaphase delay. These defects do not routinely result in mitotic catastrophe, and argue that Zw3 plays a role in the maintenance of the mitotic spindle, rather than an essential role in spindle morphogenesis. Consistent with a mitotic function, we observe a complex and dynamic localization of Zw3 during cell division. These studies provide genetic data that validate and extend drug inhibition studies on a novel mitotic role for glycogen synthase kinase in the maintenance of the mitotic spindle.
Keywords: GSK-3β, Zw3, sgg, mitosis, Drosophila
Introduction
Studied for over 25 years, glycogen synthase kinase 3β (GSK-3β) participates in signaling via kinase cascades, and has been found to play roles in a wide variety of metabolic and cytoskeletal roles (reviewed in ref. 1). For example, this regulatory enzyme plays an important role in the wingless/hedgehog signaling pathway during animal development, in which it directs the proteolysis of P-catenin and Cubitus interruptus (reviewed in ref. 2). GSK-3β phosphorylates a consensus sequence, X(S/T)XXXSp, which often must be preprimed by phosphorylation at a nearby serine residue by one of a number of upstream kinases.3,4 The numerous cellular roles of this protein have stimulated human drug trials of GSK-3β inhibitors on such diverse diseases as depression, Alzheimer’s, diabetes and other neurodegenerative disorders.5
In light of the implications for human health, a thorough understanding of the biology of GSK-3β is crucial. Additional evidence points to specific roles for this kinase in cell division, including modulating the stability of cyclins D and E and affecting microtubule dynamics by regulating an assortment of MAPs, such as APC, tau and Map1B.6,7 A potential direct role for this kinase in regulating mitotic machinery in animal cells was suggested by drug inhibition experiments utilizing competitive inhibitors to the ATP-binding site of the enzyme.8 These chemical inhibitor studies document a number of mitotic defects in cultured cells, including perturbations of mitotic microtubule dynamics and failure of chromosome congression. As a class, GSK3 inhibitors vary in the type and extent of mitotic defects that they induce. However, the specificity and selectivity of several GSK3 inhibitors has recently been revealed to be lower than previously assumed.9–12 This underscores the risk of relying on a limited panel of test kinases to determine the selectivity of an inhibitor. The cross-reactivity of these inhibitors for other kinases may explain their variable effects upon mitosis that can range from benign to catastrophic.13 These confounding data make it imperative to validate the inhibitor studies with genetic analyses to uncover the bona fide role of GSK3 in mitosis.
A growing body of evidence suggests that the cellular destiny of protein targets phosphorylated by GSK-3β is recognition of the phosphoepitope by a member of the SCF family of E2 ubiquitin ligases and subsequent degradation (reviewed in ref. 14). In Drosophila, several substrates, including Ci, Armadillo (β-catenin), period and timeless, are marked for degradation by SCF complex via phosphorylation by GSK-3β, also known as Zw3, or Shaggy in Drosophila.15 We have previously shown that Slimb/SCF complex is required for the fidelity of centrosome duplication in Drosophila, raising the possibility that GSK-3β may mark important mitotic substrates of SCF complex.16 Furthermore, immunolocalization of GSK-3β in the mitotic spindle in Hela cells,8 as well as GFP-GSK-3β in Drosophila syncytial embryos, supports the idea of a putative GSK-3β function in mitosis.17
Our group recently performed a genetic protein-trap screen for genes whose protein products localize to the mitotic apparatus. This screen yielded a novel GFP-GSK-3β fusion that can fully substitute for the native gene throughout the life cycle of the fly. Herein we describe the effect of loss of function of GSK-3β on mitosis in Drosophila. This analysis complements existing drug inhibition studies and provides the first mutational data supporting a role for this protein kinase in spindle organization.
Results
A GFP-Zw3 kinase chimera fully substitutes for the native gene.
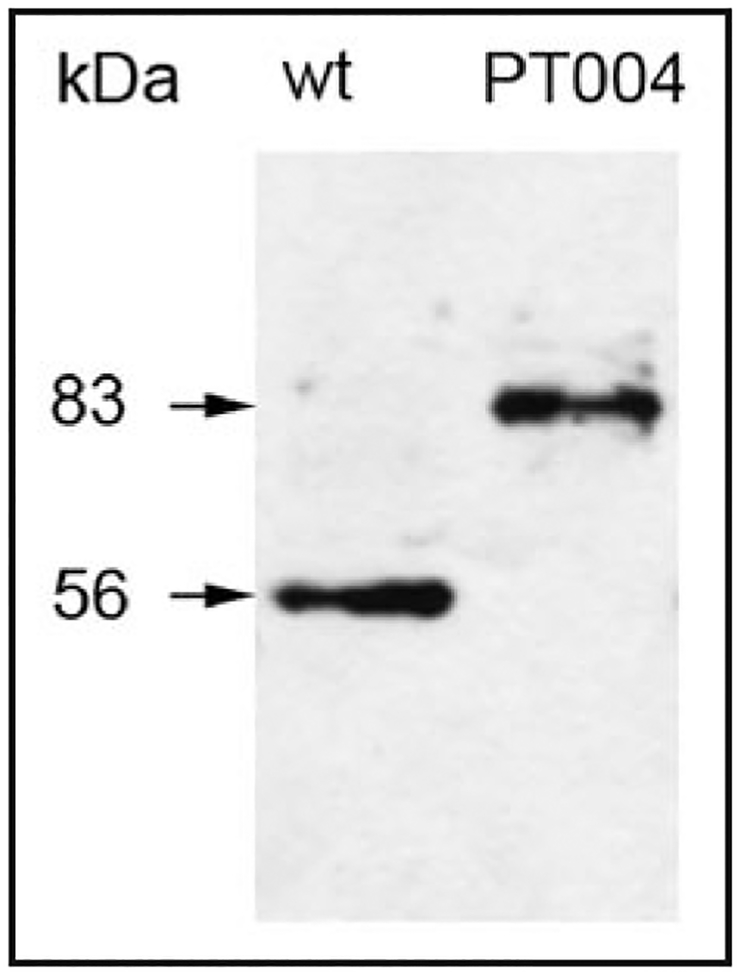
We recovered a GFP-Zw3 chimera in an unbiased protein-trap screen for proteins that localize to the Drosophila mitotic apparatus. The protein-trap transposable element inserted itself within the large second intron of Zw3 kinase, wherein its GFP exon was expressed in-frame after the first 29 amino acids of the protein (Fig. 1). The GFP module is added at the N-terminus of the protein, well outside of the C-terminal kinase domain. Because the protein-trap strategy results in modification of the native locus, such an insertion can be considered analogous to a standard gene replacement. Animal stocks homozygous for the protein-trap insertion are readily maintained in long-term culture with no apparent deleterious effects in females or hemizygous males, arguing for the validity of the observed GFP-Zw3 localization patterns. The insertion line complements, and is fully viable, in combination with a deficiency for the zw3 region of the X chromosome (Df(1)64j4) as well. Western blots of GFP-Zw3 animals reveal a single major band of 83 kDa, corresponding to the appropriate chimeric protein (Fig. 2). No native unmodified Zw3 is detected at 56 kDa in this line, indicating that the artificial GFP exon is rarely bypassed by alternative splicing, as is the case within an independently-derived GFP protein trap allele of zw3.17
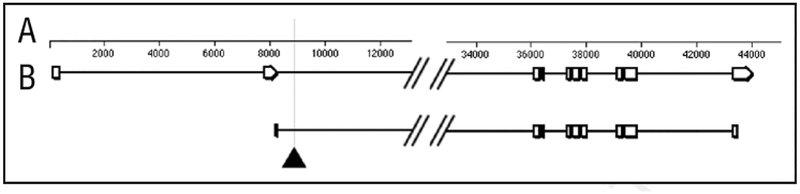
Figure 1.
Map of the zw3 primary transcript(A) and coding sequence (B). The dark triangle marks the site of insertion of the protein trap construct with in the large second intron.34 This site represents a ‘hotspot’ for P element insertion, and is well upstream of a series of alternatively spliced exons near the dista lend of the transcript (not shown).
Figure 2.
Western blot of Drosophila larval protein extracts from wildtype (wt) and GFP-Zw3 (PT004) lines. Anti-GSK-3 recognizes a single band of approximately 56 kDa in wildtype animals and an upshifted band of approximately 83 kDa in the GFP-Zw3 line.
GFP-Zw3 undergoes a dynamic pattern of subcellular localization to cytoskeletal components during syncytial mitosis.
Although GFP-Zw3 is detectable throughout the cytoplasm of syncytial embryos, it displays a complex and dynamic pattern of localization during mitosis. While always detectable on centrosomes throughout syncytial mitosis, GFP-Zw3 transiently accumulates within the prometaphase, and subsequently in the telophase nucleoplasm (Fig. 3). During spindle assembly, GFP-Zw3 enrichment within the nucleus decreases concomitantly with a general increase in abundance throughout the cytoplasm: a behavior that is consistent with release from the nuclear compartment during nuclear envelope breakdown and diffusion throughout the cytoplasm. At metaphase, GFP-Zw3 is distributed with uniformity, some discernable association with the mitotic spindle, and exclusion from condensed chromosomes. Also, enrichment of GFP-Zw3 along the pseudocleavage furrows is observed. This physical and temporal-specific localization pattern in embryos is mostly recapitulated by an independently-derived GFP-fusion of Zw3: some differences may be attributable to the alternative splicing of the GFP cassette within that allele.17
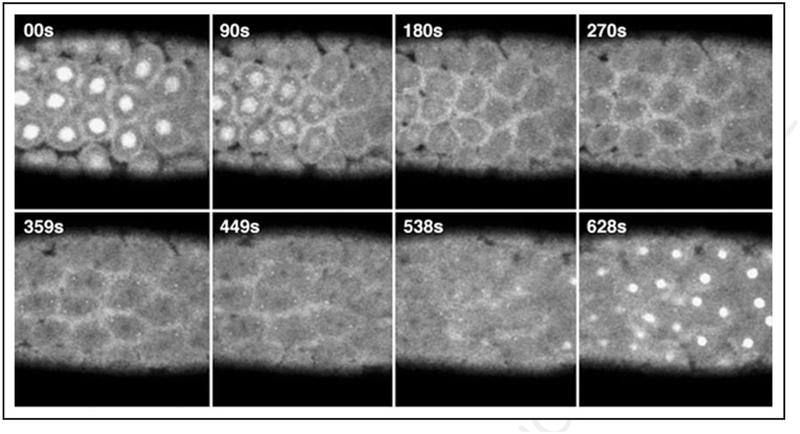
Figure 3.
Panel of time-lapse confocal images of GFP fluorescence taken at the cortical surface of a homozygous GFP-Zw3 syncytial Drosophila embryo. Note that although centrosomes tend to move out of the plane of focus during time-lapse imaging (e.g., panels 359 s to 628 s), we find GFP-Zw3 localized to centrosomes throughout rounds of syncytial mitosis. Shown are selected frames at the times indicated after centrosome separation during prophase († = 00 s) and progress through anaphase (~† = 449 s later). Chromosomes, which do not fluoresce and appear as dark shadows, can be seen in roughly metaphase configuration in panels 270 to 359s. S-phase of the subsequent round of division is shown in the last panel († = 628 s). At room temperature, rounds of division in both wt and GFP-Zw3 embryos occurred approximately every 10.5 min. Time units are in seconds (s) and scale bar is 50 μm.
Zw3 localization during somatic divisions in larval giant neuroblasts differs from syncytial divisions.
Previous studies limited the analysis of GFP-Zw3 to the germline and specialized syncytial divisions of Drosophila.17 To assess whether the spindle defects we observe during syncytial mitosis were a consequence of a critical role for Zw3 that is limited to this specialized form of division, or reflected a broader and essential role for this kinase in regulating cellular mitotic machinery, we analyzed the behavior of Zw3 during somatic mitotic divisions within giant larval neuroblasts.
We examined the subcellular localization of both homozygous GFP-Zw3 in 3rd instar larval Drosophila neuroblasts by short-term culture and time-lapse microscopy. Through a round of mitosis, the subcellular localization of GFP-Zw3 shares certain features with the localization observed in syncytial divisions, and yet differs in others. Parallel to syncytial divisions, GFP-Zw3 is enriched at centrosomes throughout mitosis in larval neuroblasts (Fig. 4, Suppl. Movie S1). During prophase, GFP-Zw3 becomes highly enriched within the nucleus in a transient manner. Confocal imaging reveals enrichment throughout the nucleoplasm, with the exception of condensing chromatin, and no detectable enrichment on the nuclear membrane (e.g., Fig. 4B).
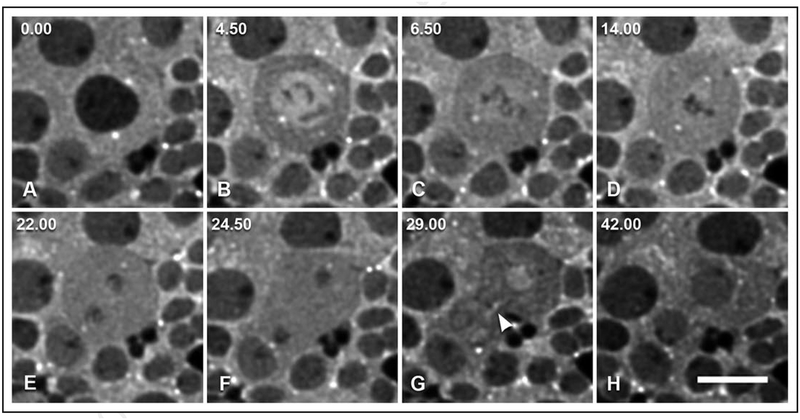
Figure 4.
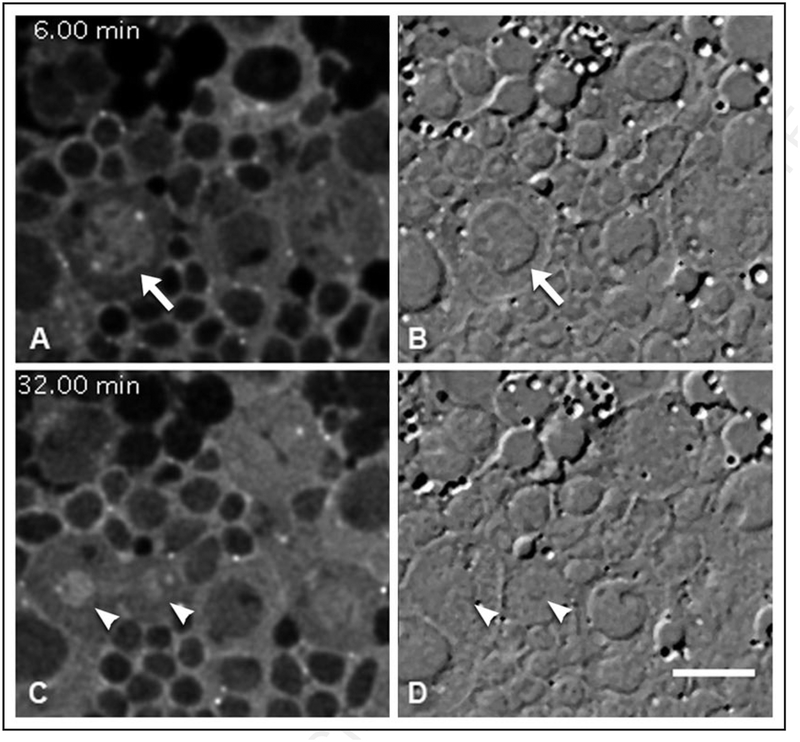
Selected widefield immunofluorescence images from a time-lapse collection of GFP-Zw3 in short-term cultured larval giant neuroblasts (see Suppl. Movie S1). This series is from one round of mitosis. Shown are cytoplasmic and centrosomal localization of GFP-Zw3 during pro-metaphase and metaphase (A, B), influx into the nucleus at the onset of metaphase (C and D), late anaphase and telophase (E and F), concentration in the nucleus in late telophase (G), and post-cytokinesis (H). Also, enrichment along the central spindle becomes apparent during telophase (arrowhead, G). Units of time are denoted in minutes.seconds. Scale bar is 10 μm.
The nucleoplasmic enrichment dissipates concomitantly with nuclear envelope breakdown (Fig. 5, also Suppl. Movie S2). Although the accumulation of GFP-Zw3 in the prophase nucleus is uniform, upon nuclear envelope breakdown GFP-Zw3 spreads into the cytoplasm and a fraction associated with the mitotic spindle is revealed (Fig. 4D, Suppl. Movies S1 and S2). Subsequently, enrichment along the central spindle is observed (Fig. 4G, arrowhead) that persists through late telophase; this pattern of localization is difficult to assess during syncytial divisions due to cytoplasmic background signal.
Figure 5.
Selected timepoints from confocal GFP-Zw3 fluorescence and corresponding widefield DIC time-lapse images (see supplemental Movie S2) of ex vivo larval giant neuroblasts. The field contains several dividing neuroblasts amidst smaller glial cells. The arrow marks a prophase nucleus enriched for GFP-Zw3 (A) and corresponding DIC image of the prominent nuclear envelope (B). Twenty-six minutes later, arrowheads mark the boundary of the GFP-Zw3 enriched telophase nuclei (C) in this asymmetric division, with discernable nuclear envelope [arrowheads in (D)] just visible in the corresponding DIC view. Scale bar is 10 μm.
Strikingly, GFP-Zw3 transiently re-accumulates within telophase nuclei during formation of the nuclear envelope (Fig. 5, Suppl. Movie S2). As the telophase nuclei increase in volume, however, GFP-Zw3 is removed or degraded from the interphase nucleus (Fig. 4H) until little or no GFP-Zw3 is detectable within interphase nuclei. There is no precedent for the complex and dynamic pattern of localization of Zw3 to mitotic apparatus, centrosomes and telophase nucleus during mitosis. This complex localization argues for pleiotropic involvement of Zw3 in multiple, and likely disparate, processes during mitosis in somatic cells.
Zw3 loss of function affects the morphogenesis of the mitotic apparatus.
Explicit and dynamic localization of GFP-Zw3 during mitosis prompted a search for the effects of loss of function on cell division. As hemizygous hypomorphic animals (zw3b12/Y) survive to late larval stages, the central nervous system (CNS) from such 3rd instar larvae was examined for defects. The ratio of metaphase to anaphase cells in larval brain chromosome spreads can detect difficulties in the transition from metaphase to anaphase, often by a marked elevation of this ratio. Orcein-stained chromosome spreads from wildtype siblings were found to have a metaphase-to-anaphase ratio (M/A) of 3.4 to 1, while zw3b12 mutant brains had an elevated M/A of 7.3 to 1 (Table 1). This increase in M/A indicated a modest increase in the duration of the metaphase state of mutant cells.17
Table 1.
Metaphase-to-anaphase ratios and percentage of PSCS for wildtype and hemizygous mutant larval brains
| Genotype | M/A ratio | PSCS (%) |
|---|---|---|
| Oregon R | 3.4 | 1.1 |
| Zw3b12/Y | 7.3 | 11.5 |
For M/A ratios shown, 4 wildtype (Oregon R) brains and 16 mutant (zw3bl2/Y) brains were analyzed. For premature sister chromatid separation (PSCS) analysis, 6 wildtype (Oregon R) brains and 47 mutant (zw3bl2/Y) brains were processed.
We examined the morphology of the metaphase spindle by confocal microscopy to detect any abnormality that could be responsible for the observed delay in the metaphase to anaphase transition in the mutant CNS. Severe mitotic abnormalities, such as polyploid or aneuploid nuclei, or mitotic arrest, are not observed in the mutant neuroblasts (Fig. 6). Rather, we observe moderate anomalies both in the structure of bi-polar spindles, and in relative microtubule density. The perturbations consist of curved or bent spindles (Fig. 6), with the metaphase plate of chromosomes frequently shifted off-axis and near the cortex. The gently elevated mitotic index argues that many, if not all, of the cells with abnormalities are able to nonetheless exit mitosis.
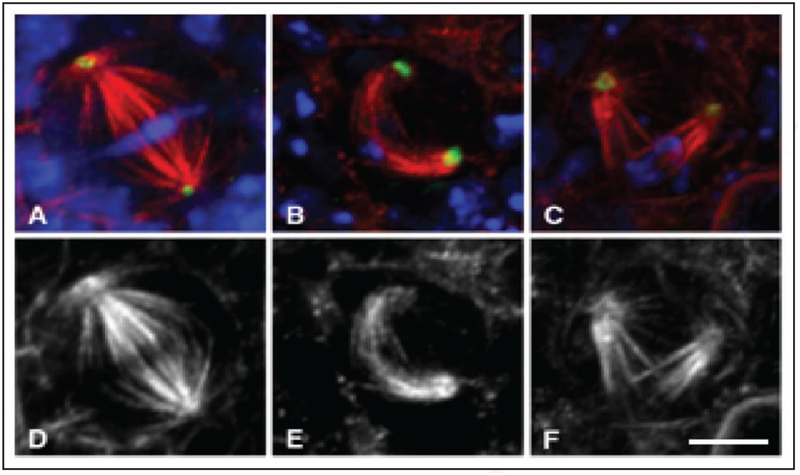
Figure 6.
Confocal images of metaphase 3rd instar from wildtype (A and D) and hemizygous mutant zw3b12 neuroblasts (B, C, E and F) immunostained to show DNA (blue), α-tubulin (red), and γ-tubulin (green), and with α-tubulin shown separately in D–F. Scale bar is 5 μm.
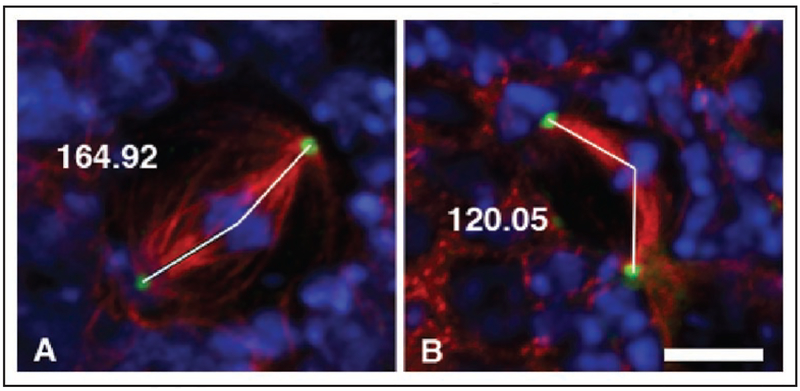
By marking the geometric center of metaphase chromosome mass, we were able to quantitate the extent of curved spindles by measuring the angle formed between the centrosomes and center of chromosome mass in both wildtype and mutant neuroblasts (Fig. 8). The increased incidence of curved spindles in mutant neuroblasts is marked, with considerable variation in the degree of the observed bend. This effect is not observed in wildtype, and can be rescued in mutant cells by heat-shock induced expression of Zw3 kinase (Table 2).
Figure 8.
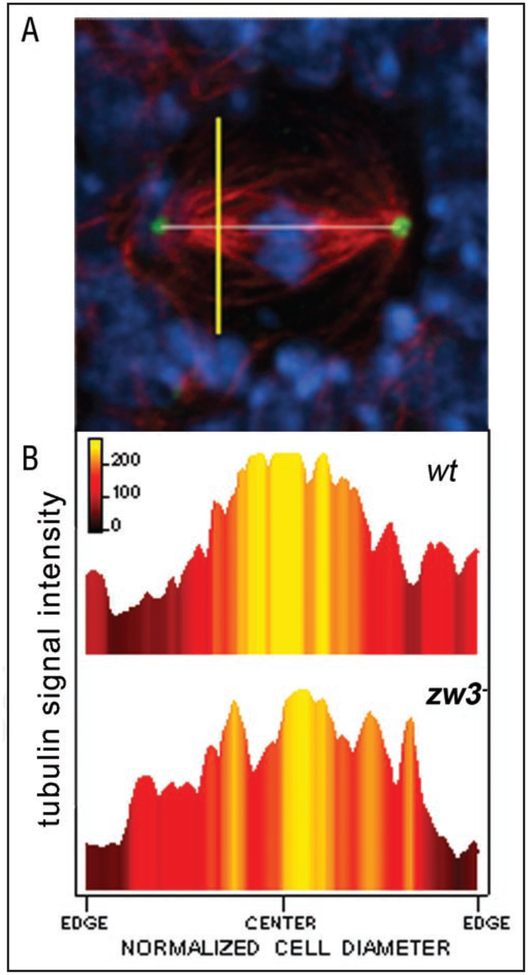
Analysis of metaphase spindle microtubule density and structure in wildtype and zw3b12 mutant neuroblasts. Linescans of tubulin fluorescence intensity through hemispindle microtubules were obtained at the midpoint between centrosomes and chromosomes (A): a line was drawn connecting the centrosomes to set the spindle length (white line), and a linescan measurement was taken perpendicular to the spindle axis and at a distance of 25% of the spindle length from each centrosome (e.g., yellow line). Maximum intensity values from linescans (B) were obtained from both wildtype and mutant spindles (w† and zw3b12, respectively). The color gradient in the graphs correlates to the relative peak signal intensity at a given point across a cell, and can be directly compared between wildtype and mutant neuroblasts spindles. Color scale is shown, in which yellow is the highest relative intensity and dark maroon is the lowest relative intensity.
Table 2.
Quantitation of aberrant spindle angles seen in hypomorphic zw3 larval neuroblasts
| Genotype | Mean spindle angle (degrees) | n |
|---|---|---|
| Oregon R | 167.59 ± 9.49 | 23 spindles |
| zw3b12/Y | 136.26 ± 35.29 | 33 spindles |
| zw3b12/Y; hsZw3A (rescue) | 168.44 ± 9.95 | 27 spindles |
Measurements were taken of the angle between the centrosomes and center of metaphase chromosome mass as illustrated in Figure 8. Data was collected from wildtype (Oregon R), hemizygous mutant (zwSb12), and mutant animals that had been rescued by ectopic expression of wildtype Zw3 (zw3b12/Y; hsZw3A). The data are based on six wildtype Oregon R brains, thirteen zw3 mutant brains, and eight zw3 mutant brains whose phenotype was rescued by ectopic expression of native Zw3.
In order to assess the overall microtubule structure of the mutant spindles, we compared linescans of tubulin fluorescence-intensity across normalized wildtype and mutant spindles under constant confocal conditions (Fig. 8). At the site of measurement, wildtype Drosophila spindles occupy approximately 25% of the cell diameter (Fig. 8B), specified by the yellow shading localized in the center of the cell and associated with microtubule fluorescence. Mutant spindle microtubule arrays were apt to have notable gaps between microtubule bundles, typically found in proximity to the cell membrane (Fig. 8B). Secondly, comparison of the total area of high tubulin intensity (Fig. 8B, yellow area) in mutant and wildtype Drosophila cells yields detection of a lower overall density of microtubules in mutant spindles. Whether kinetochore fibers or overlapping polar microtubules are contributing more to this effect is not clear from our dataset.
The zw3 mitotic phenotype differs between somatic and syncytial divisions.
Zw3 loss-of-function lethality occurs during late embryogenesis in mutant larvae is a result of the perdurance of maternal Zw3 through embryogenesis. Therefore, to observe the effects of loss-of-function during syncytial divisions, germline clones of both available loss of function alleles, zw3M11−1 and zw3sggD127, were generated with the FLP recombinase system as previously described.18 Our data are in good agreement with previous studies that observed some degree of mitotic catastrophe, including nuclei arrested in mitosis, and/or culled from the pool of dividing nuclei by the nuclear fallout checkpoint, during syncytial divisions.18,19 As shown in Fig. S3, both multipolar spindles and nuclear loss were observed in these mutants. These types of defects were never observed in somatic divisions. It has previously been suggested that the zw3 syncytial defects may be due to a misregulation of the actin cytoskeleton, which plays a unique role in preventing dividing syncytial nuclei from interfering with each other in the absence of cell boundaries (Fig. S3).19 Our data showing the subcellular localization of Zw3-GFP during syncytial divisions place it in the right time and place to affect peudocleavage furrow formation (e.g., Fig. 3, 449 s).
The mitotic checkpoint in syncytial divisions is unable to pause mitotic cycling during these rapid mitoses to permit the correction of even minor defects, and instead triggers the subsequent elimination of any nuclei that fail to successfully divide. Therefore, action by the checkpoint resulting in nuclear fallout is not informative of the severity of the causative mitotic defect. We suspect that the severity of the zw3 mitotic defect is relatively mild, because we observe approximately 1 in 5 mutant embryos successfully complete syncytial divisions and cellularize. Although it is tempting to hypothesize that the impact of Zw3 upon syncytial mitosis will largely parallel its impact on somatic mitosis, it is clearly involved in essential processes, such as pseudocleavage furrow formation, that have no parallel in somatic divisions. Therefore, we expect Zw3 function to differ between syncytial and somatic mitosis. How Zw3 is integrated into this highly specialized type of mitosis during early embryogenesis is beyond the scope of our current investigation.
Zw3 loss of function impacts, but does not abrogate, the spindle assembly checkpoint.
Taken as a whole, the elevated M/A ratio may reflect the action of the spindle assembly checkpoint in response to the zw3-induced spindle defects. To verify the integrity of the checkpoint, wildtype and mutant brains were challenged with the microtubule poison, colchicine. An active functional checkpoint is expected to respond to the microtubule poison by delaying the onset of anaphase. If the checkpoint is not functional, then a frequency of premature sister chromatid separation (PSCS) upwards of 40% would be apparent as the cells attempt to enter anaphase.20 We find that wildtype neuroblasts respond to colchicine with a robust metaphase block, and only 1.1% exhibit slippage into PSCS (Table 1). On the other hand, neuroblasts from zw3b12 mutant animals exhibit a modestly impaired checkpoint with up to 11.5 % of the metaphase figures showing PSCS. Although the spindle assembly checkpoint is slightly impaired, it remains robust enough to account for the slow passage of mutant cells through metaphase.
Discussion
Glycogen synthase kinase is a pleiotropic modulator of many different cellular processes. Much attention has recently been focused on the potential use of small molecule inhibitors of GSK-3β to treat a variety of both lethal and non-lethal human diseases.5 Previously, experiments utilizing a variety of ATP-competitive inhibitors of GSK-3β on cultured human cells reported severe mitotic catastrophe after treatment, arguing for a role for GSK-3β in modulating the assembly of the mitotic apparatus.8,13,21 Interpretation of the drug-inhibitor studies is complicated by both the documented and potential cross-reactivity of the ATP-competitive inhibitors with other important mitotic enzymes, which may result in complicated cellular phenotypes due to varying degrees of inhibition and resulting in a mixture of effects.
To help validate the putative functions of GSK-3β in mitosis in animal cells suggested by drug inhibitor studies, we examined the distribution of enzyme and the phenotype of mutations in the lone Drosophila homolog of gsk-3β, zw3 (zeste white-3, known later as shaggy, sgg). Zw3 localization to the cell division machinery within syncytial embryos shows some similarity to the pattern seen in mammalian tissue culture cells.17 However, we augmented the above findings by examination of canonical mitotic cells, neuroblasts, and focused on the dynamics of Zw3 localization within living cells in a model organism.
Two lines of data argue that our Zw3-GFP chimera faithfully reports the behavior of the native protein. First, immunolocalization of native Zw3 is consistent with that observed in living cells expressing Zw3-GFP (data not shown). Second, our Zw3-GFP line behaves identically to a similar independently derived line during embryogenesis.17
During syncytial divisions in Drosophila, GFP-Zw3 is consistently enriched in centrosomes during the entire mitotic cycle. Furthermore, we observe dynamic localization of GFP-Zw3 to the nucleus and vicinity of the spindle. This pattern of localization supports a model for Zw3-mediated regulation of the actin cytoskeleton and its interactions with syncytial mitotic spindles that has been proposed by others.18,19 Not surprisingly, the dramatically different mitotic machinery of syncytial divisions displays different sensitivities and consequences to zw3 loss of function: an analysis of which is beyond the scope of our study of somatic divisions.
On the other hand, in the somatic divisions of the giant neuroblasts, GFP-Zw3 is initially excluded from the nucleoplasm during interphase, but accumulates within the nucleus during prophase prior to nuclear envelope breakdown. That this accumulation is not due to passive diffusion across the nuclear membrane is suggested by the higher concentration of GFP-Zw3 seen within the nucleus during prophase as compared with the levels in the cytoplasm. Subsequently, GFP-Zw3 appears to diffuse to the surrounding cytoplasm upon nuclear envelope breakdown to achieve a near-uniform distribution throughout the mitotic cell. Strikingly, GFP-Zw3 transiently re-accumulates within telophase nuclei during the latter stages of cell division, followed once again by exclusion from the nucleus as the cell enters interphase. GFP-Zw3 enrichment within the prophase and telophase nucleus correlates with periods of chromatin remodeling. However, our morphological analysis of mutant cells does not reveal a chromatin phenotype that might result from the observed nuclear localization. As the extent and modest severity of the observed mitotic defects do not seem to account for the overall lethality we observe, it is possible that other cellular roles for Zw3, perhaps regulated by the observed nuclear localization, are essential for viability.
Although in vitro analysis of human GSK-3β in animal cell lines has not been reported to undergo a similar pattern of transient nuclear accumulation during mitosis, it should be noted that time-lapse analysis of GSK-3β behavior in living mammalian cells has also not been reported. Coupled with its sensitivity to fixation conditions, it is possible for transient accumulations of GSK-3β, such as those we observe in fly cells, to be difficult to discern in fixed animal cells. Indeed, aldehyde fixation abrogates the localization of Zw3 to centrosomes and to the nucleus during mitosis in giant neuroblasts, as visualized by both indirect immunofluorescence with anti-Zw3 and anti-GFP as well as direct detection of GFP-Zw3 after fixation (Fig. S1). It is possible that aldehyde-fixed mammalian cells may show these same experimental idiosyncrasies/peculiarities.
In contrast, during the transition from prometaphase to metaphase in giant neuroblasts, the reduction of GFP-Zw3 concomitant with nuclear envelope breakdown reveals an enrichment of this kinase to the metaphase microtubule spindle, and subsequent central spindle after anaphase. This enrichment is clearly visible in confocal time-lapse, but is suppressed by out-of-focus background and not visible in widefield fluorescence time-lapse (Fig. S2; Movie S3). We suspect that this modest enrichment that is detectable by confocal sectioning may reflect a role for Zw3 in the regulation of microtubule stability, or polymerization, within the spindle. Based on this localization, we looked for a mitotic role for Zw3 in Drosophila by phenotypic analysis of genetic loss-of-function experiments.
Examination of hemizygous zw3b12 mutant larval neuroblasts showed an increased M/A ratio, consistent with the activation of the mitotic checkpoint due to a spindle defect. Indeed, the mitotic checkpoint is able to sense spindle defects and arrest mitosis in zw3b12 mutant neuroblasts: microtubule disruption by colchicine effectively blocks mitosis at metaphase in the mutant neuroblasts, albeit with a minimal level of slippage and concomitant PSCS. Therefore, the metaphase delay seen in untreated mutant neuroblasts is likely to be caused by the checkpoint response to zw3-mediated spindle defects.
Mutant neuroblasts consistently formed a bipolar mitotic spindle without severe assembly defects, such as split poles or monopolar arrays. Notwithstanding correct establishment of productive bipolar mitotic spindles, these arrays frequently display a bent or curved shape that was the only notable defect that could account for the observed checkpoint-mediated metaphase delay. This phenotype may be a direct effect of loss of function of spindle-associated Zw3, and is consistent with a potential role for this kinase in regulating microtubule stability within the spindle. However, it appears that mitotic checkpoint-mediated delay is sufficient to help correct these defects, as very few expected products from a mitotic catastrophe, such as easily detected polyploid and aneuploid cells, are found in the mutants.
Our genetic analysis of eukaryotic cells in living Drosophila tissues is consistent with drug inhibition studies of human cell lines: these data are in agreement with the effects of allosteric inhibitors of GSK3 on cultured cells and in keeping with only a subset of noted effects in the presence of ATP-competitive inhibitors, which may suffer more off-target effects.8,13,21 The Drosophila zw3 phenotype is entirely consistent with the limited effects on mitosis observed in mammalian cells by allosteric inhibitors, such as TDZD-8.13 These allosteric inhibitors bind to a pocket outside of the active site of GSK3 and are uncompetitive with ATP.22,23 Allosteric inhibitors are thought to be more specific than the ATP-competitive class inhibitors, as the putative binding pocket is not a conserved feature among kinase family members and is thought to be unique to GSK3 kinases only. Although they are highly effective in inhibiting GSK3-β activity in cells and animals, they do not cause catastrophic mitotic defects.22,23
The relatively modest mitotic phenotype seen in the zw3 mutant is similar to the effects of non-ATP-competitive allosteric drug inhibition by TDZD- 8 on the mitotic apparatus of both mammalian tissue culture cells and in vitro assembled Xenopus spindles (manuscript in preparation): we observe that the microtubule arrays in the mutant spindles are longer, yet appeared less dense, with more visible gaps. Consistent with this, we see an overall delay in metaphase that is consistent with activation of the spindle checkpoint. It is possible that one or more of the observed defects in microtubule organization is causing checkpoint activation. Comparison between wildtype and mutant spindles reveals a tendency for microtubule bundles to shift off center together with the chromosomes. The tendency for visible gaps between bundles of microtubules can be interpreted as a form of spindle splitting, or a result of the loss of polar microtubules, thus unveiling separable kinetochore fibers. Closer inspection our confocal data reveals lower overall tubulin fluorescence intensity between wildtype and mutant spindles. To the extent that the imaging conditions were held constant between all specimens, this data suggests a primary defect in the regulation of microtubule stability in the mutant background: an interpretation supported by the known interaction between GSK-3β and MAPs.24–27 Nonetheless, potential variation in fixation, antibody penetration and epitope accessibility limits the analysis of this result. To determine whether the observed spindle defects are caused by changes in spindle microtubule dynamics, future efforts will directly probe the effects of GSK-3β inhibition on spindle microtubule dynamics in vivo.
In contrast, the ATP-competitive inhibitors, such as SB415286, resulted in the perturbations observed above, but in addition suffered a host of catastrophic mitotic defects.8,13,21 It is possible that mammalian GSK-3β plays additional essential roles during mitosis from those of the fly homolog, and that these roles are only revealed by the ATP-competitive class of inhibitors. However, the ATP-competitive inhibitor studies rely on a subset of compounds that have demonstrated cross-reactivity with key mitotic kinases, such as CDK1, and that also remain untested against a large cohort of other mitotic kinases and phosphatases. The most commonly utilized inhibitor used in studies examining the role of GSK-3P on mitosis, SB415286,8,13,21 has an IC50 of 0.9 μM for CDK1 kinase in vitro,10 within an order of magnitude of the IC50 constants for GSK3-a (0.077μM) and GSK3-α/β (0.133 μM).10,28 Therefore, the working concentration (30 μM) of SB415286 used in those studies8,13,21 is likely to significantly inhibit CDK1 kinase, which can cause the observed phenotypes by itself (reviewed in ref. 29). Any inhibition of CDK1 kinase will have contributed to their observations, and compromised the conclusions reached.
Similar confounding issues are seen for other ATP-competitive inhibitors of GSK3 used in studies of mitosis. The GSK3 inhibitor AR-A014418 causes severe mitotic defects in cultured cells.13 However, it has not been tested for inhibition against mitotic enzymes outside of CDK2 and CDK5,30 and has recently exhibited a surprising lack of selectivity for GSK3-β in a cell-based assay.12 Furthermore, to distinguish the effects of any CDK1 kinase inhibition by GSK3 inhibitors, including AR-A014418, a compound thought to be a specific inhibitor of CDK1 kinase, RO-31–8220, was utilized as a negative control for GSK3 inhibition.13 Surprisingly, other studies have shown RO-31–8220 to be an equally potent (IC5Q =6.8 nM) inhibitor of GSK3 as AR-A014418.28,31 Therefore, experiments utilizing ATP-competitive inhibitors of GSK should be approached with caution: our genetic approach, that avoids issues of small-molecule specificity, highlights the experimental constraints, as well as the differences in results and conclusions drawn, or using cultured cell systems with chemical inhibitors.
Our data, and those resulting from inhibition studies utilizing allosteric non-ATP-competitive inhibitors, supports a role for GSK3 in affecting microtubule dynamics in the mitotic spindle. In an absence of GSK3 function, the mitotic spindle appears to lose tension and activates the spindle checkpoint. That these defects are not catastrophic, and can be resolved by spindle machinery, is apparent in the observed, very low frequency of aberrant cells that would be expected to arise from severe spindle defects.
The enrichment of GFP-Zw3 along the spindle at metaphase may reflect a role in spindle assembly at that point in time. It is tempting to argue that Zw3 may be required for the spindle to fully come under tension and thus deactivate the checkpoint. However, other perturbations, such as taxol treatment, which are known to relax spindle tension, do not result in such bent spindles and off-axis metaphase plates (reviewed in refs. 32 and 33). Instead Zw3 may modulate the interaction of spindle and astral microtubules with the spindle positioning machinery at the cortex or somehow affect the morphogenesis of the metaphase plate. Identification of additional Zw3 kinase substrates during mitosis may shed some light on this issue in the future.
Human GSK-3β has been shown to regulate the microtubule association of the Adenomatous polyposis coli (APC) protein,27 which binds and tracks the plus-ends of microtubules. Furthermore, the Drosophila APC homologs (APC1, APC2) are known to interact with Zw3 during syncytial divisions.19 Therefore, it will be interesting to determine whether the spindle defects we observe in mutant neuroblasts are linked to misregulation of APC protein. Because human GSK-3β can fully substitute for Drosophila Zw3 during embryogenesis and partially during subsequent life cycle stages,34 it is likely that the mitotic role we observe for fly GSK-3β can be successfully extrapolated to human cells.
Given that phosphorylation by GSK-3β can also mark proteins for degradation, the speculation that this function is important for aspects of its role(s) in mitosis is appealing. Future efforts can now be directed towards identifying novel GSK-3β substrates during mitosis, and determining the functional outcome of such events.
Materials and Methods
Fly stocks.
The Drosophila line expressing Zw3-GFP (PT004) was generated using the protein-trap technique, as described in ref. 35. Briefly, GFP expressing lines were established from larvae that had been screened for fluorescence. The location and nature of each insertion was determined using inverse PCR, following protocols established by the Berkeley Drosophila Genome Project. In the case of line PT004, the GFP module was inserted in-frame after the first 29 amino acids of Zw3. In subsequent genetic analysis, two amorphic embryonic lethal alleles, zw3sggD127 and zw3M11−1, were used interchangeably in this study, and Df(1)64j4 was utilized to test GFP-Zw3 for partial loss of function. We also utilized a strong hypomorphic larval lethal allele, zw3b12, for neuroblast analysis. Whereas germline clones were made with both zw3sggD127 = FRT101/FM7i, [w + mC = ActGFP]JMR3 and zw3M11−1 = FRT101/FM7a lines using standard protocols. Rescue experiments utilized hsZw3A (a gift from Dr. E. Siegfried, Penn St. Univ.). Both homozygous and hemizygous zw3b12 and zw3M11−1 lines could be rescued to adulthood with a series of heat shock treatments driving expression of hsZw3A. Fly stocks were maintained using standard culture methods.
Microscopy.
Time-lapse imaging of GFP-Zw3 embryos was performed on dechorionated embryos under halocarbon oil with a Zeiss LSM510 confocal microscope. Images were collected with bi-directional scans at 10 s intervals with a 40X n.a. 1.3 lens. Time-lapse imaging of ex vivo larval giant neuroblasts was performed on a Zeiss axiovert widefield microscope equipped with an Orca-ER cooled CCD camera, 63X n.a. 1.4 lens, and operated by Metamorph software (Molecular Devices). Neuroblast cultures were prepared according to ref. 36, with the following modifications. After dissection in 0.7% NaCl, a larval brain was teased apart in a 7 μl drop of Schneider’s medium (Gibco) containing 20% FBS (fetal bovine serum, Gibco) on a 25 X 45 #2 coverslip. It was then overlayed with a 170 μm thick sheet of 1.5% agarose/Schneiders/20% FBS. To flatten the cells and inhibit evaporation, an 18 × 18 mm # 1 coverslip was overlaid on the agarose and the edges sealed with halocarbon oil (#700 Halocarbon Products). These preparations behaved well, with normal cell divisions, for several hours. Confocal/DIC imaging of live neuroblasts was performed with preparations as above on a Nikon TE2000 inverted microscope workstation equipped with a Perkin Elmer Spinning disk confocal operated by Metamorph software. Detector noise in the time-lapse fluorescence datasets was suppressed with the aid of the Huygens Essential deconvolution software (Scientific Volume Imaging, Netherlands).
Larval brains were fixed and stained according to ref. 37, and imaged on a Zeiss LSM 510 confocal microscope using a 63X n.a. 1.4 lens. Microtubules were labeled utilizing a rat monoclonal anti-a tubulin (YOL1/34; Accurate Chemical) diluted 1:200 and Alexa 647 conjugated goat anti-rat secondary antibody (Molecular Probes) at a 1:200 dilution. Centrosomes were visualized with a mouse monoclonal anti-y tubulin (GTU-8 8; Sigma) at a dilution of 1:200 and Alexa 488 goat anti-mouse secondary antibody (Molecular Probes). DNA was stained with DAPI (Sigma) at a concentration of 0.5 μg/ml. Actin was visualized with Alexa Fluor 488 phalloidin (1:500, Molecular Probes) and Zw3 visualized with anti-GSK3, clone 4G-1E, Alexa-488 conjugate (1:250, Upstate).
Detection of PSCS was performed on Orcein-stained chromosome spreads, as described.20 Metaphase spindle angle measurements were performed on confocal data from wildtype and zw3 mutant neuroblasts as described in Figure 8. Care was taken to limit measurements to spindles in which the centrosomes were coplanar. Briefly, lines were drawn from each centrosome to the center of the metaphase chromosome mass, and the resulting acute-side angle recorded utilizing both MetaMorph software (Molecular Devices Inc.,) and ImageJ.38
Analysis of the organization of wildtype and mutant metaphase microtubule bundles was based on linescans of pixel intensity taken from the middle of hemispindles. Linescans were measured on a single length marked from one edge of a cell to the other edge, and traversing the middle of the hemispindle (illustrated in Fig. 8A, yellow line) using MetaMorph software. This line was positioned perpendicular to the main spindle axis (Fig. 8A, white line). Because the neuroblasts varied in their diameter at this point, the linescans were normalized to one another to preserve the relative distribution of microtubule density across the cells. To compare wildtype and mutant microtubule distributions, maximum pixel intensities were calculated for each point across the linescan dataset; this permits visualization of all aberrantly offset microtubule bundles across the entire dataset without bias or averaging effects. Data normalization and plotting was accomplished with Igor Pro software (Wavemetrics).
Western blots.
Western blots were performed on extracts from 3rd instar larvae using the commercial antibodies and indicated dilutions: GSK-3β/Zw3, (Rabbit mAb 22C10, 1:2000, Cell Signaling), and actin (1:2000, Sigma A2066). Detection utilized ECL™ reagents and protocols (Amersham Life Science).
Figure 7.
Confocal images of wildtype (A) and zw3b12 hemizygous mutant neuroblasts (B) taken from fixed and immunostained whole-mount 3rd instar brains, and illustrating how spindle angle measurements were made to compile the data in Table 2 (See Materials and Methods). DNA is shown in blue, microtubules in red, and centrosomes in green. Lines were drawn from each centrosome to the center of the chromosome mass, and the angle measured. Scale bar is 5 μm.
Acknowledgements
We thank Dr. Xavier Morin for the protein-trap transposable element construct, and Dr. Esther Siegfried (Penn St. Univ.) for various zw3 allelic fly stocks, Thomas Wall for isolated the GFP-Zw3 protein trap line, and Kevin Crosby and Will Ratzan for their technical assistance. We gratefully recognize members of the Mitchison laboratory (Systems Biology, Harvard Medical School) for thoughtful discussions on the project, and Dr. S. Kim for comments on the manuscript. This work was supported by the NIH grant # GM066328.
Abbreviations:
- APC
adenomatous polyposis coli
- CNS
central nervous system
- FCS
fetal calf serum
- GFP
green fluorescent protein
- GSK
glycogen synthase kinase
- M/A
metaphase to anaphase ratio
- MAP
microtubule associated protein
- PSCS
premature sister chromatid separation
- zw3
zeste-white 3
Footnotes
References
- 1.Jope RS, Johnson GV. The glam our and gloom of glycogen synthase kinase-3. Trends Biochem Sci 2004; 29:95–102. [DOI] [PubMed] [Google Scholar]
- 2.Moon RT. Wnt/beta-catenin pathway. Sci STKE 2005; 2005:1. [DOI] [PubMed] [Google Scholar]
- 3.Fiol CJ, Wang A, Roeske RW, Roach PJ. Ordered multisite protein phosphorylation. Analysis of glycogen synthase kinase 3 action using model peptide substrates. J Biol Chem 1990; 265:6061–5. [PubMed] [Google Scholar]
- 4.Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem 1991; 266:15555–8. [PubMed] [Google Scholar]
- 5.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov 2004; 3:479–87. [DOI] [PubMed] [Google Scholar]
- 6.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 1998; 12:3499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welcker M, Singer J, Loeb KR, Grim J, Bloecher A, Gurien-West M, Clurman BE, Roberts JM. Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol Cell 2003; 12:381–92. [DOI] [PubMed] [Google Scholar]
- 8.Wakefield JG, Stephens DJ, Tavare JM. A role for glycogen synthase kinase-3 in mitotic spindle dynamics and chromosome alignment. J C ell Sci 2003; 116:637–46. [DOI] [PubMed] [Google Scholar]
- 9.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some com monly used protein kinase inhibitors. Biochem J 2000; 351:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci 2004; 25:471–80. [DOI] [PubMed] [Google Scholar]
- 11.Quiroz JA, Gould TD, Manji HK. Molecular effects of lithium. Mol Interv 2004; 4:259–72. [DOI] [PubMed] [Google Scholar]
- 12.Selenica M-L, Jensen HS, Larsen AK, Pedersen ML, Helboe L, Leist M, Lotharius J. Efficacy of small-molecule glycogen synthase kinase-3 inhibitors in the postnatal rat model of tau hyperphosphorylation. Br J Pharmacol 2007; 152:959–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tighe A, Ray-Sinha A, Staples OD, Taylor SS. GSK-3 inhibitors induce chromosome instability. BMC Cell Biol 2007; 8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smelkinson MG, Kalderon D. Processing of the Drosophila hedgehog signaling effector Ci-155 to the repressor Ci-75 is mediated by direct binding to the SCF component Slimb. Curr Biol 2006; 16:110–6. [DOI] [PubMed] [Google Scholar]
- 15.Grima B, Lamouroux A, Chelot E, Papin C, Limbourg-Bouchon B, Rouyer F. The F-box protein slimb controls the levels of clock proteins period and timeless. Nature 2002; 420:178–82. [DOI] [PubMed] [Google Scholar]
- 16.Wojcik EJ, Glover DM, Hays TS. The SCF ubiquitin ligase protein slimb regulates centrosome duplication in Drosophila. Curr Biol 2000; 10:1131–4. [DOI] [PubMed] [Google Scholar]
- 17.Bobinnec YM orin X, Debec A. Shaggy/GSK-3beta kinase localizes to the centrosome and to specialized cytoskeletal structures in Drosophila. Cell Motil Cytoskeleton 2006; 63:313–20. [DOI] [PubMed] [Google Scholar]
- 18.Peifer M, Sweeton D, Casey M, Wieschaus E. wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development 1994; 120:369–80. [DOI] [PubMed] [Google Scholar]
- 19.McCartney BM, McEwen DG, Grevengoed E, Maddox P, Bejsovec A, Peifer M. Drosophila APC2 and Armadillo participate in tethering mitotic spindles to cortical actin. Nat Cell Biol 2001; 3:933–8. [DOI] [PubMed] [Google Scholar]
- 20.William s BC, Goldberg ML. Determinants of Drosophila zw10 protein localization and function. J Cell Sci 1994; 107:785–98. [DOI] [PubMed] [Google Scholar]
- 21.Izumi N, Fumoto K, Izumi S, Kikuchi A. GSK-3beta regulates proper mitotic spindle formation in cooperation with a component of the gamma-tubulin ring complex, GCP5. J Biol Chem 2008; 283:12981–91. [DOI] [PubMed] [Google Scholar]
- 22.Conde S, Perez DI, Martinez A, Perez C, Moreno FJ. Thienyl and phenyl alpha-halomethyl ketones: new inhibitors of glycogen synthase kinase (GSK-3beta) from a library of compound searching. J Med Chem 2003; 46:4631–3. [DOI] [PubMed] [Google Scholar]
- 23.Martinez A, Alonso M, Castro A, Dorronsoro I, Gelpf JL, Luque FJ, Perez C, Moreno FJ. SAR and 3D -QSAR studies on thiadiazolidinone derivatives: exploration of structural requirements for glycogen synthase kinase 3 inhibitors. J Med Chem 2005; 48:7103–12. [DOI] [PubMed] [Google Scholar]
- 24.Goold RG, Gordon-Weeks PR. Microtubule-associated protein 1B phosphorylation by glycogen synthase kinase 3beta is induced during PC12 cell differentiation. J Cell Sci 2001; 114:4273–84. [DOI] [PubMed] [Google Scholar]
- 25.Lovestone S, Hartley CL, Pearce J, Anderton BH. Phosphorylation of tau by glycogen synthase kinase-3 beta in intact mammalian cells: the effects on the organization and stability of microtubules. Neuroscience 1996; 73:1145–57. [DOI] [PubMed] [Google Scholar]
- 26.Sperber BR, Leight S, Goedert M, Lee VM. Glycogen synthase kinase-3 beta phosphorylates tau protein at multiple sites in intact cells. Neurosci Lett 1995; 197:149–53. [DOI] [PubMed] [Google Scholar]
- 27.Zumbrunn J, Kinoshita K, Hyman AA, Nathke IS. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr Biol 2001; 11:44–9. [DOI] [PubMed] [Google Scholar]
- 28.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol 2000; 7:793–803. [DOI] [PubMed] [Google Scholar]
- 29.Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, Heimbrook DC, Chen L. Selective small-molecule inhibitor reveals critical mitotic functions of hum an CDK1. Proc Natl Acad Sci USA 2006; 103:10660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhat R Structural Insights and Biological Effects of Glycogen Synthase Kinase 3-specific Inhibitor AR-A014418. J Biol Chem 2003; 278:45937–45. [DOI] [PubMed] [Google Scholar]
- 31.Hers I, Tavare JM, Denton RM. The protein kinase C inhibitors bisindolylmaleimide I (GF 109203x) and IX (Ro 31–8220) are potent inhibitors of glycogen synthase kinase-3 activity. FEBS Lett 1999; 460:433–6. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman DB, Pearson CG, Yen TJ, Howell BJ, Salmon ED. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol Biol Cell 2001; 12:1995–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waters JC, Chen RH, Murray AW, Gorbsky GJ, Salmon ED, Nicklas RB. Mad2 binding by phosphorylated kinetochores links error detection and checkpoint action in mitosis. Curr Biol 1999; 9:649–52. [DOI] [PubMed] [Google Scholar]
- 34.Siegfried E, Chou TB, Perrimon N. wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell 1992; 71:1167–79. [DOI] [PubMed] [Google Scholar]
- 35.Morin X, Daneman R, Zavortink M, Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc Natl Acad Sci USA 2001; 98:15050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleming SL, Rieder CL. Flattening Drosophila cells for high-resolution light microscopic studies of mitosis in vitro. Cell Motil Cytoskeleton 2003; 56:141–6. [DOI] [PubMed] [Google Scholar]
- 37.Wojcik E, Basto R, Serr M, Scaerou F, Karess R, Hays T Kinetochore dynein: its dynamics and role in the transport of the Rough deal checkpoint protein. Nat Cell Biol 2001; 3:1001–7. [DOI] [PubMed] [Google Scholar]
- 38.Rasband WS. ImageJ. US National Institutes of Health, Bethesda, MD USA. [Google Scholar]