Abstract
Robotic therapy enables mass practice of complex hand movements after stroke, but current devices generally enforce patients to reproduce prescribed kinematic patterns using rigid actuators, without considering individuals’ unique impairment characteristics, thereby reducing their efficacy. In this early-stage study, we tested the feasibility of a novel, theory-based “biomimetic” approach to restoring mechanics of complex hand tasks with subject-specific assistance patterns. Twelve chronic stroke survivors performed two simulated functional tasks, hand open and simulated pinch task (distal pad press). Assistance was provided by non-restraining actuators (exotendons) that counteracted ‘subject-specific’ impairments, identified during unassisted task performance. There was no constraint of movement to predefined patterns. Assistance patterns required to complete tasks were significantly different across subjects, reflecting high variability in impairment and required assistance patterns. For hand open, range of motion and interjoint coordination were significantly improved for severely impaired patients, while movement quality was enhanced (reduction in jerk) for those less impaired. For simulated pinch, subject-specific assistance restored task mechanics before injury, as patients were able to direct fingertip force towards the direction normal to surface; angular deviation reduced from 16.8°±10.4° to 3.7°±2.6°. Notably, electromyography data confirmed that subjects maintained an effort level under assistance comparable to unassisted conditions. The proposed method could lead to a novel paradigm for hand rehabilitation that restores complex task mechanics with a subject-specific assistance reflecting individual impairment characteristics while promoting subjects’ participation.
Keywords: hand, stroke, robotic rehabilitation, subject-specific, targeted muscle assistance, functional task
I. Introduction
Significant degradation of upper extremity (UE) function is common among stroke survivors (Mayo et al., 1999), which greatly diminishes their quality of life (Carod-Artal et al., 2000; Clarke and Black, 2005; Nichols-Larsen et al., 2005). Distal segments of the UE (especially the hand) are thought to be more affected (Twitchell, 1951; Colebatch and Gandevia, 1989; Saladin, 1996), leading to significant impact on overall UE function (Wing and Lederman, 1998; Hermsdörfer et al., 2003). In general, restoration of hand function is critical in UE functional recovery (Heller et al., 1987). Unfortunately, functional recovery of the hand post-stroke is generally slow and limited; in an observational study, up to 40% of patients had limited use of fine hand use after 3 months, and the number even increased to 45% at 18 months (Welmer et al., 2008).
Robotic systems potentially offer substantial advantages to hand rehabilitation because they allow patients to systematically practice complex multi-joint movements (Balasubramanian et al., 2010), an approach difficult to administer in conventional training administered by therapists. However, so far the efficacy of current robotic systems is found limited; while many robotic devices for hand rehabilitation produce statistically-significant functional gains, several controlled studies failed to find UE robotic training superior to conventional therapy (Fischer et al., 2007; Connelly et al., 2010; Lo et al., 2010). Different systematic reviews also concluded that no significant change in activities of daily living (ADL) resulting from robotic rehabilitation (Prange et al., 2006; Mehrholtz et al., 2008; Kwakkel et al., 2008). While more recent review studies found robot-assisted training more effective than conventional therapies in UE functional recovery, such difference was not observed in ADL recovery (Bertani et al., 2017; Veerbeek et al., 2017).
We argue that, due to the complexity of human hand and its function, many existing robotic devices cannot effectively address two important aspects of stroke rehabilitation: restoration of task mechanics before injury, in both kinematic (movement) and kinetic (force generation) aspects, and engagement of participants. While the importance of restoration of task mechanics over compensation has been emphasized (Roby-Brami et al., 2003; Krakauer, 2005; Levin et al., 2009; Nordin et al., 2014), complexity of functional hand tasks and altered motor control pose unique challenges to restoration of pre-stroke task mechanics using existing robotic approaches. For instance, many robotic devices adopt rather simplified actuation strategies, such as cable-driven, endpoint-actuation mechanisms (Fischer et al., 2007; Dovat et al., 2008), which may not precisely control movements of multiple hand joints and may result in non-physiologic movements. Other devices with more complex electromechanical design can reproduce complex multi-joint hand movements (Takahashi et al., 2008; Schabowsky et al., 2010; Susanto et al., 2015). However, soliciting active participation of patients using this type of systems could be a challenging issue since these devices typically use rigid (non-backdrivable) actuators and position-feedback controllers to reproduce complex multi-degrees-of-freedom (DOF) kinematics. Furthermore, kinematics of the tasks to be trained are often pre-defined (Marchal-Crespo and Reinkensmeyer, 2009), thus preempting error-based approaches to motor learning (Kawato and Gomi, 1992; Emken and Reinkensmeyer, 2005).
More importantly, these constrained devices do not reflect ‘subject-specific’ patterns of physiological impairment of patients in their muscle control (e.g., muscle weakness to be assisted, or spasticity to be compensated) because the pattern of assistance is solely determined by the device’s pre-defined joint kinematics (and kinematic error). Most multi-DOF robots provide joint torques required to reduce kinematic errors (joint angles), and do not consider underlying impairment mechanisms (i.e., muscle function impairment). Many robotic systems produce complex multi-DOF movements using mechanical constraints (e.g., gear ratio; Schabowsky et al., 2010), which allow very little variation in the produced movement patterns; the same type of assistance (multi-joint torque) applies regardless of individual impairment characteristics. Thus, these devices may not solicit effective active participation of subjects, because their assistance patterns do not reflect the individual’s unique impairment characteristics.
A desired rehabilitation system for the hand, therefore, should restore mechanics of functional tasks, in both kinematic (movements) and kinetic (force production) aspects. Assistance should be provided by actuators that do not constrain movement to a predefined trajectory, thereby facilitating error-based learning. It should also provide ‘subject-specific’ assistance that reflect each patient’s impairment in their muscle level, which could not only reduce the overall assistance level but also promote subject participation.
In this study, we test the feasibility of a novel robot-assisted training method for hand rehabilitation that could properly emphasize the aforementioned elements of the desired robotic rehabilitation. In the proposed method, first, subject-specific abnormalities of muscle use in individual patients are identified from their altered task performance using an established biomechanical model that was developed and validated in our previous in vivo and in vitro studies (Lee et al., 2008; Lee and Kamper, 2009). Based on the identified impairment, we use a biomimetic device, equipped with exotendons that mimic anatomy of hand musculotendons, to provide targeted assistance to major hand muscles (Lee et al., 2014; Lee et al., 2018) to complete these tasks. We first postulate that the proposed method will result in different assistance patterns across patients, reflecting significant differences in impairment among individual patients. We further hypothesize that, given the subject-specific assistance, the targeted muscle assistance will ‘restore’ normal mechanics of complex hand tasks, including kinematics of finger extension, i.e., interjoint coordination (Cirstea and Levin, 2007) and/or movement smoothness (Rohrer et al., 2002), and reduction in the shear force at fingertip during force exertion that contributes to grip instability post-stroke (Seo et al., 2010). More importantly, we hypothesize that the effort level of patients during assisted task performance, gauged by their electromyography (EMG) data, will be comparable to that of unassisted performance, as the controller was tuned to provide a minimum level of overall assistance required to complete the task.
II. Methods
A. Subjects
Twelve chronic stroke survivors (age: mean±SD = 63±12 yrs; 3 females; minimum 1 year since the onset of stroke) participated in the study. Inclusion criteria were: 1) Fugl-Meyer Assessment of Upper Extremity (FMAUE) score ≥ 30; and 2) residual finger movements of the affected hand. Exclusion criterion was severe spasticity/contracture of the finger flexor muscles (modified Ashworth scale ≥ 3). Subjects were divided into two groups based on their impairment assessed by the FMAUE score; 6 subjects were categorized into a mild/moderate-impairment group (MI; FMAUE ranged from 45 to 62), and 6 into a severe/moderate-impairment group (SI: FMAUE from 30 to 42). The experimental protocol was approved by the institutional review boards at the MedStar Health Research Institute and the Catholic University of America, and written informed consent was obtained from each subject prior to participation.
B. Instrumentation
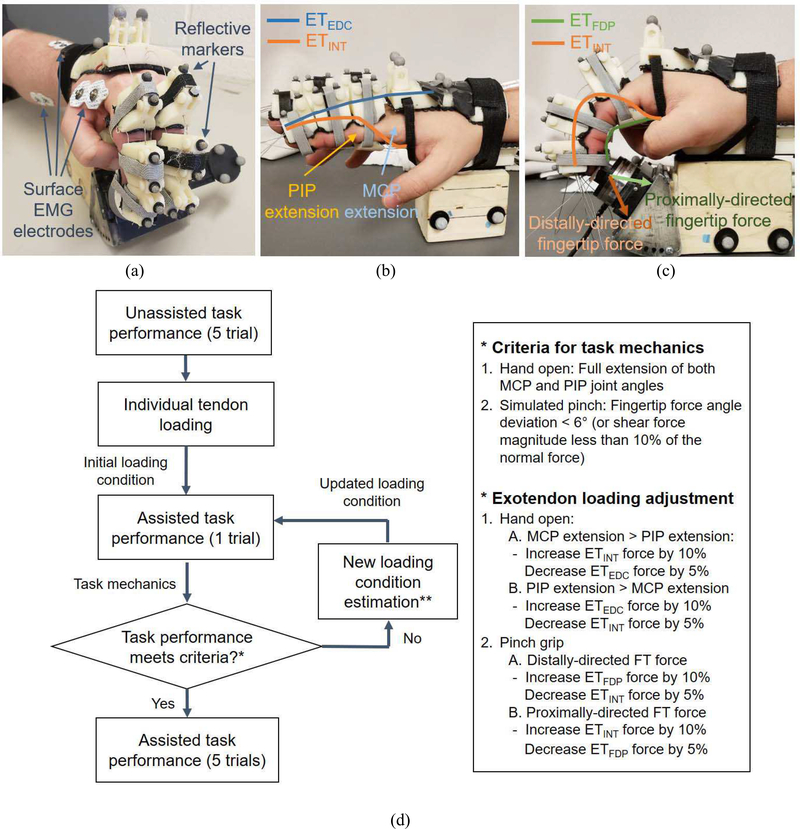
Patients wore an exotendon device, a modified version of our recently developed biomimetic device (BiomHED; Lee et al., 2014; Lee et al., 2018) that provided assistance (Fig. 1). Briefly, for each of the index and middle fingers, four cables were routed through custom thermoplastic components attached to the dorsal and palmar aspects of the finger via Velcro straps. These cables (ExoTendon; ET) replicated the anatomical configuration of four hand musculotendons: ETEDC routed to replicate the anatomical configuration of the extensor digitorum communis (EDC) tendon, ETFDP for the flexor digitorum profundus (FDP) tendon, and ETINT for the dorsal and palmar interosseous tendons. We previously found that tension applied to each exotendon reproduced kinetic effects of the multi-articular tendon that it replicated (i.e., joint torques and spatial coordination of the multi-joint movements; Lee et al., 2014). When the assistance was provided, the exotendon force was linearly increased to the assistance level during the first second (to avoid possible reflex response) and maintained at the level for the duration of the task performance. Assistance was produced by actuators (brushed DC motor with gearheads, A-max 16, GP 16A with reduction ratio 84:1, Maxon Motor AG, Switzerland), which created tension in the corresponding exotendon.
Fig. 1. Experimental setup.
(a) Experimental setup showing electrodes and markers (b,c) Simulated functional tasks: subject performing (b) hand open task; (c) palmar press task with assistance from BiomHED. For hand open, assistance level of ETEDC and ETINT, and for palmar press, assistance level of ETFDP and ETINT were adjusted to provide ‘subject-specific’ assistance based on each patient’s impairment pattern (and/or task performance); (d) Flowchart of the iterative scheme that determined an assistance pattern for individual patient based on the observed task mechanics
Three pairs of disposable, self-adhesive silver/silver chloride bipolar surface electrodes (Noraxon, Scottsdale, AZ, USA) were used for surface EMG recordings. One pair of electrodes were placed on the hand to record the activity of an intrinsic hand muscle [first dorsal interosseous (FDI)], and two pairs on the forearm to record extrinsic hand muscle activities from 1st and 2nd compartments of the flexor digitorum superficialis (FDS) and EDC, respectively. To ensure accurate placement of each electrode, EMG signals from the electrodes were inspected while subjects performed several finger and wrist movements associated with the target muscle and adjacent (wrist) muscles. The EMG signals were sampled at 1000 Hz. After the electrode placement, subjects first created maximum activations, for the normalization purpose, by performing the following tasks: finger extension (EDC), finger flexion with the DIP joint extended (FDS), and index finger abduction (FDI).
Additionally, finger movements were recorded at 60Hz using an 8-camera motion capture system (Osprey Digital RealTime System; Motion Analysis Corp., Santa Rosa, CA, USA). Two small reflective markers were placed on each segment of the fingers, and four markers on the dorsum of the hand (Fig. 1a,b). The fingertip force data during simulated pinch task was recorded by a 6-DOF load cell (Mini40, ATI Industrial Automation, Apex, NC, USA). The EMG and load cell signals were recorded using the Labview (National Instruments, Austin, TX, USA), which was also synchronized with the motion capture system that sent a trigger signal to the Labview interface.
C. Target tasks
Patients performed two types of functional hand tasks:
1). Hand open (finger extension)
Subjects were instructed to perform a timed finger extension task (hand open). A customized graphical user interface (GUI) built in the LabView environment provided visual cues regarding the timing of the movement, i.e., when to start and complete the movement, in the form of sliding bar with tick marks (one for the movement phase, and the other for the hold phase). The movement time was set to 3 seconds, and subjects were asked to complete the movement as the bar reached the first indicator (at the 3-second mark), then to maintain the posture for another 3 seconds until the bar reached the second indicator (at the 6-second mark).
2). Simulated pinch
Subjects performed a ‘simulated’ palmar pinch task with their index and middle fingers (distal pad press; Fig. 1c). The location and orientation of the load cell were adjusted for each subject so that the finger joint angles were similar to those used during typical palmar pinch, i.e., distal interphalangeal (DIP) joint angle run ≈ 0°: proximal interphalangeal (PIP) joint annotgle ≈ 45°; and metacarpophalangeal (MCP) joint angle ≈ 45°. Subjects were asked to press on the sensor with their index and middle fingers as if they performed pinch tasks.
D. Protocol
For each task, patients first performed the task without assistance (5 trials). Then, based on the task dynamics produced by individual tendon force for each subject while they rest (kinematics for hand open; kinetics for simulated pinch task), the initial assistance level for the exotendons was determined, which is set to about 50% to 60% of the force level required to perform the task by the device alone. An iterative process to adjust the assistance pattern (i.e., modulating the level of assistive force for each exotendon), based on biomechanics of three major hand musculotendons (extrinsic extensor, extrinsic flexor, intrinsic muscles) and their effects on the task mechanics (Valero-Cuevas et al., 2000; Lee et al., 2008), was then employed to find a ‘subject-specific’ assistance pattern that restores normal task mechanics for each patient. Note that the initial exotendon loading level was determined based on our initial pilot testing with a smaller number of patients (n =3), in which the iterative tuning processes with several initial loading conditions were implemented and compared. Details of these iterative tuning processes for the two target tasks are described below (also see Fig. 1d):
1). Hand open
For the hand open task, two exotendons mimicking extrinsic extensor (ETEDC) and intrinsic muscles (ETINT) were used to provide assistance, and the assistance pattern for each patient was determined based on the observed movement deficits. Previous studies on hand biomechanics showed that the EDC muscle provides a larger MCP extension moment (compared to distal joints), while the intrinsic muscles (DI/PI) mainly extends distal joints (DIP/PIP) while flexing the MCP joint (An et al., 1983). Therefore, based on the spatial coordination pattern of the PIP and MCP joints observed during hand open, we adjusted the force levels of the two exotendons (ETEDC and ETINT).
First, an initial loading level for each exotendon was determined. While subjects remain relaxed, each of the exotendons was actuated with varying levels of force, and the exotendon force level that fully extends either the distal joints (ETINT) or the proximal joint (ETEDC) was found. Then, the initial assistance level for each exotendon (to be used during assisted task performance) was determined as 50% of the forces used in these individual loading conditions.
Subjects then performed the finger extension with assistance (actuating both exotendons), then the desired assistance level of the individual exotendon force for each subject was adjusted iteratively based on the observed task mechanics (kinematics). Namely, if the resulting MCP joint extension was smaller than the PIP joint extension, ETEDC force level was increased by 10% while ETINT force level was decreased by 5%. Conversely, if the degree of MCP joint extension was greater than that of PIP joint, ETINT force level was increased by 10% while ETEDC force level was decreased by 5%. The process was iteratively performed until a desired between-joint spatial coordination (i.e., both PIP and MCP joints were fully extended) was achieved (Fig. 1d). In addition, the overall assistance level (for both exotendons) was decreased by 10% if patients reached full extension within 1 second (difficulty adjustment). Once the desired assistance pattern was determined, subjects performed the task five times with the fixed assistance pattern.
2). Simulated pinch with two fingers
Pinch tasks were performed in the second session, administered after a 15-minute break period. First, maximum voluntary force was first recorded for each subject, and the target force level for each subject was set to 40% of his/her maximum voluntary force.
Similar to the hand open task, the initial assistance level of each exotendon was first determined. Patients were asked to maintain the posture (without actively engaging in the force production) as the force level for each exotendon (ETFDP or ETINT) that produced approximately 60% of the target force was determined. Then, an iterative process (similar to hand open) was implemented to find the ‘subject-specific’ assistance pattern for each patient. Namely, the assistance pattern was determined based on the observed fingertip force direction. The EDC muscle typically produces a proximally-directed dorsal fingertip force, while the intrinsic muscles produce a dorsal fingertip force pointing towards distal direction (Valero-Cuevas et al., 2000; Lee et al., 2008). Thus, when the fingertip force produced during assisted trials was found pointing towards the distal direction, we increased the level of ETFDP force by 10% and decreased the ETINT force by 5%; if the force was directed towards the proximal direction, the ETINT force level was increased by 10% and the ETFDP level decreased by 5%. This process was repeated until the shear fingertip force was reduced less than approximately 15% of the dorsal fingertip force (Fig. 1d). Similar to hand open, overall assistance level was decreased if patients reached the target force too fast (within 1 second). Once the desired assistance pattern was determined, subjects performed five assisted trials.
E. Data analysis
1). Task kinematics (Hand open)
The marker data was first low-pass filtered (5Hz). The markers on the dorsum of the device were then used to establish the local coordinate system. After the local coordinate system was established, markers on the three segments of each finger were used to fit a two-dimensional plane for the finger, and all markers were projected to the plane. The joint angles were then computed by using a dot product of the two segment vectors. Here, hyperextension was detected by the direction of the cross-product of the vectors.
Then, the following kinematic measures were computed between the two conditions (unassisted vs. assisted). Here, we focused on kinematic analysis of the PIP and MCP joint movements as the movement of the DIP joint is typically coupled with that of the PIP joint. Previous studies also showed that DIP and PIP joint motions are highly correlated (or coupled) during functional movements (Kuo et al., 2006; Leijnse et al., 2010). The PIP-MCP joint coordination has been typically examined when functionality of the hand post-stroke is assessed (e.g., Raghavan et al., 2010).
Range of motion (ROM): The hand ROM of each subjects during hand open was quantified using two finger joint angles (PIP and MCP). From the three-dimensional marker data, the angular profiles of the PIP and MCP joints of the index and middle fingers were first computed. Then, the change in the sum of MCP and PIP extension angles between the initial and final postures during hand open was computed between the two conditions (unassisted vs. assisted) and compared.
Joint coordination (r-value): Spatiotemporal coordination of the two finger joints (PIP vs. MCP) was assessed by the correlation coefficients between the two angular profiles. Note that previous studies showed that natural (unconstrained) movements typically represent linear covariation of joints used in movements (Gottlieb et al., 1996). Additionally, extrinsic finger muscles – due to its multiarticular nature – produce highly-correlated PIP and MCP joint movements (Nimbarte et al., 2008).
Smoothness (Jerk): Smoothness of movements has been used to gauge motor performance (movement quality) in both healthy (Balasubramanian et al., 2010) and patient (Rohrer et al., 2002; Kahn et al., 2006a) populations. We computed the jerk metrics (the third time derivative of joint angle) from the PIP and MCP joint angles and averaged across all four joints (PIP, MCP of the index and middle fingers).
2). Task kinetics (Simulated pinch)
For both conditions (unassisted vs. assisted), the normal and shear fingertip force magnitudes were computed during the force maintenance phase (3s – 5s), and the fingertip force angle (deviation from the normal direction) was computed from the force projected onto the sagittal plane. The force vector in the lateral (radial/ulnar) direction was not included in the analysis.
3). EMG data
For both tasks (Hand open and Simulated pinch task), activation levels of the three muscles (EDC, FDS, FDI) were computed for both conditions (unassisted vs. assisted) during the steady-state phase (from 3-second to 5-second) and averaged. The activation level of each muscle was expressed as the percentage of its maximum activation level.
4). Statistical analysis
For the task variables (ROM, rvalues, jerk, target fingertip force magnitude, fingertip force angle), assistance level (motor torque), and subject participation (EDC activation level), a two-factor mixed analysis of variance (ANOVA) was performed with the assistance as a within-subject factor, and the group as a between-subject factor (SPSS Statistics, Ver. 22; IBM Corp., Armonk, NY, USA).
III. Results
When assisted, all subjects were able to meet the task mechanics criteria for both tasks (details of these criteria is provided in Fig. 1d). Two to eight iterations (mean±SD = 4.9±3.5) were required to find a ‘subject-specific’ assistance pattern for each task.
A. Hand Open Task: Change in task kinematics
1). Between-subject variability in assistance patterns
Significantly different patterns of assistance were required across subjects to complete the task (Table 1). A higher level of assistance (motor torque) was required for patients with severe impairment (SI group) than the moderately-impaired patients (MI group), particularly for the extrinsic extensor (EDC; p = 0.020) (Table 1a), indicating that patients in the SI group had more severe weakness of their extrinsic extensor muscles.
TABLE I.
Subject-specific assistance (motor torque) applied to exotendons during:
| Group | Subject number | (a) Hand open | (b) Palmar press | ||
|---|---|---|---|---|---|
| ETEDC | ETINT | ETFDP | ETINT | ||
| Severely-impaired (SI) | 2 | 506 | 108 | 344 | 235 |
| 6 | 362 | 108 | 398 | 235 | |
| 7 | 289 | 72 | 217 | 163 | |
| 8 | 163 | 181 | 289 | 54 | |
| 9 | 217 | 108 | 217 | 72 | |
| 12 | 127 | 344 | 253 | 90 | |
| Mean (SD) | 277(141) | 154 (100) | 286 (73) | 142 (81) | |
| Moderately-impaired (MI) | 1 | 108 | 181 | 271 | 181 |
| 3 | 90 | 163 | 344 | 434 | |
| 4 | 90 | 72 | 434 | 163 | |
| 5 | 163 | 108 | 524 | 199 | |
| 10 | 90 | 181 | 307 | 72 | |
| 11 | 145 | 108 | 181 | 434 | |
| Mean (SD) | 114(32) | 136 (46) | 344 (121) | 247 (151) | |
Shade indicates the exotendon that received a greater level of assistance
2). Improvement in task kinematics
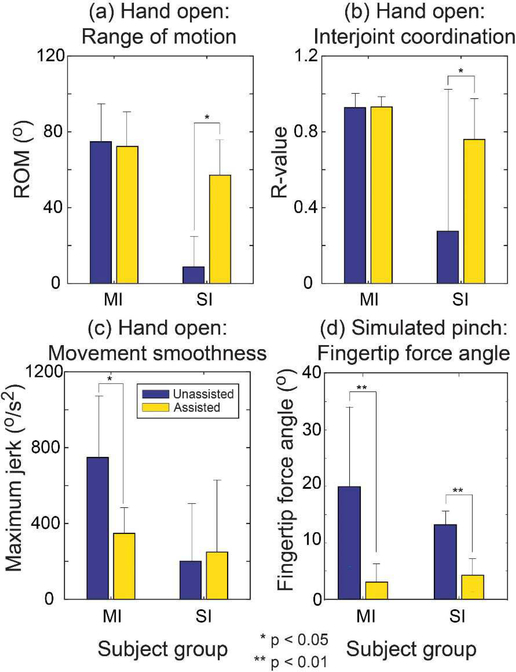
We found significant differences in kinematics across subjects in both the mild/moderate and the severe groups. The assistance significantly improved the ROM values for subjects with severe impairment (p < 0.01; Fig. 2a). Examination of individual joint movements showed that the increase in the ROM was achieved in a subject-specific manner: for instance, for those who received a higher level of assistance to their extrinsic extensor muscles, the increase in the ROM of the MCP joints was greater than that of the PIP joints (e.g., subject 6: ΔMCP ROM = 52.6° vs. ΔPIP ROM = 35.6°; ETEDC force =362N vs. ETINT force = 108N). In contrast, a greater increase in the PIP ROM (than the MCP ROM) was observed for the subjects who received greater assistance to their intrinsic muscles (e.g., subject 12: ΔMCP ROM = 2.1° vs. ΔPIP ROM = 50.8°; ETEDC force =127N vs. ETINT force = 344N). We did not find such differences in the mild/moderate group.
Fig. 2.
Change in the task mechanics: (a) Hand open: ROM; (b) Hand open: interjoint coordination; (c) Hand open: Movement smoothness; and (d) Palmar press (simulated pinch): fingertip force angle (SI: Severely-impaired; MI: Moderately-impaired).
The assistance also improved the spatiotemporal coordination of the finger joints (PIP and MCP) for subjects with severe impairment. For these subjects (SI group), when assisted, the r-value between the MCP and PIP movements increased from 0.13±0.72 to 0.79±0.19 under assistance (p = 0.008), indicating that the two joints moved more in synchrony under assistance (Fig. 2b). However, similar to ROM, there was no significant effect of assistance for the subjects with moderate impairments as they were already able to produce coordinated movements without assistance (mean±SD r-value = 0.93±0.07 [unassisted] vs. 0.95±0.03 [assisted]; p = 0.31).
Conversely, movement smoothness was significantly enhanced (i.e., significant reduction in maximum jerk) for the subjects with moderate impairment (p = 0.01), indicating that they were able to extend their fingers in a more controlled manner (Fig. 2c). However, there was no significant change in smoothness of movements for those with severe impairment (p = 0.25).
Lastly, the assistance also significantly improved the between-trial variability in the movements, particularly for the Mi group. For instance, the between-trial variability in the joint angles at the final posture was significantly reduced under assistance (mean±SD of the variance in the final posture: 4.7°±1.2° [unassisted] vs. 2.4°±1.0° [assisted]; p = 0.008).
3). Subject participation: EMG data
For both patient groups, the level of volitional effort during assisted movements was not significantly different from the level observed during unassisted movements. The activation level of the three muscles were 58.3±27.3% (EDC), 25.8±13.7% (FDS), 30.6±24.1% (FDI) without assistance, and 44.9±30.4% (EDC), 25.6±33.1% (FDS), 24.9±23.5% (FDI) under assistance (all p-values > 0.15).
B. Simulated Pinch Task (Distal Pad Press): Change in task kinetics
1). Between-subject variability in assistance patterns
Similar to the hand open, varying patterns of assistance across subjects were required to complete the simulated pinch task (Table 1b). A larger number of subjects (8 out of 12) produced fingertip force towards distal direction, which required the FDP force (ETFDP) greater than the intrinsic force (ETINT). The assistance level was not significantly different between the two groups (p = 0.341 for ETFDP; p = 0.160 for ETINT), although the target force magnitudes (determined from their voluntary maximum fingertip forces) of the MI group were significantly higher than those of the SI group (p = 0.041) (Table 2).
TABLE II.
Mean (SD) values of the fingertip force magnitude and angle during voluntary (unassisted) and assisted force production: (a) subjects with severe impairment (SI); (b) subjects with moderate impairment (MI)
| Fingertip force magnitude (N) | Fingertip force angle (°)* | ||||
|---|---|---|---|---|---|
| Group | Sub ID | Unassisted | Assisted | Unassisted | Assisted |
| (a) SI | 2 | 7.5 (0.6) | 8.7 (0.5) | 8.9 (2.9) | −0.8 (1.7) |
| 6 | 6.1 (0.5) | 6.8 (0.9) | 25.6 (14.5) | 0.1 (3.5) | |
| 7 | 6.4 (0.1) | 6.2 (0.2) | 15.8 (1.9) | 2.5 (1.3) | |
| 8 | 4.7 (0.1) | 5.5 (0.3) | −8.7 (1.5) | 3.4 (1.6) | |
| 9 | 1.7 (0.2) | 3.6 (0.2) | −46.0 (2.9) | −6.6 (8.6) | |
| 12 | 5.2 (0.6) | 5.6 (0.4) | 14.6 (9.8) | −4.7 (5.7) | |
| (b) MI | 1 | 7.8 (0.3) | 7.9 (0.3) | 18.0(4.2) | −6.4 (4.6) |
| 3 | 12.0 (1.1) | 11.9 (0.3) | 11.1 (3.7) | 4.0 (3.2) | |
| 4 | 10.6 (0.2) | 10.8 (0.1) | −8.9 (1.8) | 0.8 (1.9) | |
| 5 | 10.4 (0.3) | 12.0(1.3) | −12.0 (2.6) | −8.6 (7.1) | |
| 10 | 3.1 (0.5) | 4.0 (0.3) | 15.7(11.7) | −3.8 (2.6) | |
| 11 | 19.2 (4.0) | 17.3 (3.5) | 13.4 (8.4) | −1.8 (4.3) | |
Here, a positive angle denotes the deviation of the force vector toward distal direction, and a negative angle toward proximal direction.
2). Improvement in task kinetics
During distal pad press (simulate pinch), the subject-specific assistance helped subjects decrease the deviation of their fingertip force from the normal direction, as the shear (tangential) component of the fingertip force significantly decreased under assistance (Fig. 3). During unassisted trials, the fingertip force was deviated from the normal (palmar) direction by 20.0°±14.2° for the SI group, and 13.6°±3.5° for the MI group (Table 2), while the shear force direction also varied across subjects (distal shear force for 8 subjects; proximal shear force for 4 subjects). Under assistance, the angular deviation of the fingertip force decreased to 3.2°±2.5° for the SI group (p = 0.025) and 4.2°±2.8° for the MI group (p = 0.001). Additionally, the between-trial variability of the force vector angle (mean±SD) was smaller under assistance (3.8°±2.3°) than that without assistance (5.5°±4.4°), although the difference did not reach statistical significance (p = 0.25).
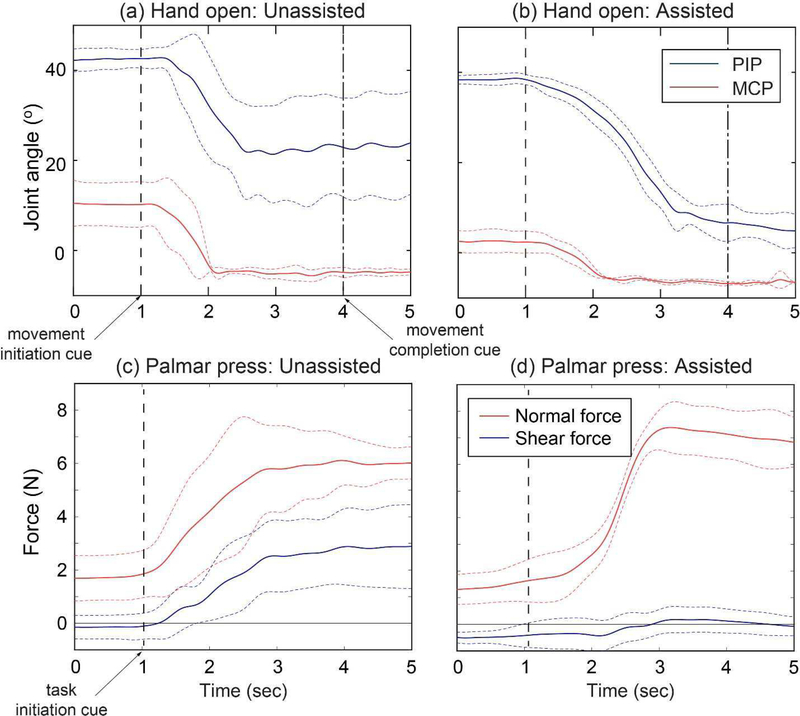
Fig. 3.
Representative cases for the improvement in task mechanics; (a,b) Joint angular profiles (subject 5: MI group) during hand open; (c,d) fingertip force trajectories (subject 6: SI group) during palmar press (mean: solid line; SD: dotted line). Note that the between-trial variability was generally large under unassisted conditions for both tasks, while the task performance became more consistent under assistance. During hand open, not only the range of motion was improved under assistance, but the subject was also able to control the timing (initiation, speed) of the movement (patients produced fast, less-controlled movements under no assistance). For the palmar press, the shear fingertip force was significantly reduced under assistance. For both tasks, a large degree of reduction in the between-trial variability of the task performance was also observed (indicated by the SD lines).
3). Subject participation: EMG data
Similar to the hand open task, there was no significant change in the effort level (measured by EMG) under assistance. The mean activation level of the two agonist/synergist muscles (FDS and FDI) actually increased to a small degree under assistance, while the difference was not statistically significant. The activation level of the three muscles were 27.7±17.8% (EDC), 30.5±17.4% (FDS), 19.8±14.8% (FDI) without assistance, and 31.0±25.0% (EDC), 31.8±28.0% (FDS), 28.0±21.7% (FDI) under assistance (all p-values > 0.11).
IV. Discussion
In this study, we demonstrated feasibility of a novel approach to restore mechanics of functional tasks of the hand by providing an individualized, subject-specific pattern of targeted muscle assistance, determined by the functional deficit of each patient observed during his/her task performance. For each task, an assistance pattern for each subject was computed by using the observed kinematic (hand open) or kinetic (simulated pinch) deficits during voluntary task performance to identify (and reinforce) underlying biomechanical deficiencies of the extrinsic and/or intrinsic hand musculotendons. our approach provides two unique advantages: restoration of normal mechanics (kinematics/dynamics) of functional hand tasks, and effective solicitation of subject participation during assisted movements.
A. Restoring task mechanics in a subject-specific manner
Recent studies suggested that restoration of task mechanics post-stroke, rather than use of compensatory strategies, is more desirable to achieve functional improvement (Michaelsen et al., 2001; Roby-Brami et al., 2003; Levin et al., 2009; Lum et al., 2009; Kitago et al., 2012). Although patients may achieve functional improvement by the use of compensatory strategies (e.g., constraint-induced movement therapy), such functional gains typically do notgeneralize to other type of tasks (Kitago et al., 2012). More importantly, training that emphasizes recovery/restoration of task mechanics could lead to greater functional gain (e.g., reaching; Michaelsen et al., 2006). We showed that our approach could restore important kinematic and/or kinetic aspects of functional tasks for each subject.
Different kinematic aspects of the finger extension were affected for the two subject groups (SI and MI) by the subject-specific assistance. During hand open, for subjects with more severe impairment (SI group), the proposed subject-specific approach mainly improved the spatiotemporal coordination of finger joints (RoM and joint coordination), similar to other robotic devices previously developed (Takahashi et al., 2008; Schabowsky et al., 2010). An increase in ROM during hand open could greatly improve the functionality of patients, as an inability to extend the fingers and the thumb (hand open) has a significant impact on hand function post-stroke (Lang et al., 2009). More importantly, the proposed approach significantly improved the between-joint (PIP-MCP) coordination, indicated by the significant increase in r-values; note that proper coordination of the PIP and MCP joints is crucial in object manipulation (Santello and Soechting, 1998), thus considered critical in improving hand function post-stroke (Raghavan, 2007). On the other hand, for subjects with moderate impairment (MI group), the proposed assistance paradigm mainly enhanced the smoothness of movements, as quantified by the significant reduction in the peak angular jerk values. Without assistance, patients in the MI group tend to produce a fast, rather jerky extension movement despite the instruction (“complete the movement as the timing bar hits the 3-second mark”). Under assistance, in contrast, they were able to produced slow, smooth movements (Fig. 3b). Movement smoothness is typically correlated with the degree of stroke recovery (Rohrer et al., 2002), and proper control of movement speed was identified as one of critical factors in stroke recovery, as shown by a recent imaging (fMRI) study (Buma et al., 2016). Therefore, the proposed training could also help the patients who already recovered the hand ROM (extension), as the training could improve their movement quality (smoothness) that could lead to further improvement.
For the simulated pinch task (distal palmar press), we also showed that the targeted ‘subject-specific’ assistance can improve the kinetics of the functional task, i.e., significant reduction of shear (distal/proximal) force. Previous studies showed that proper control of normal/shear force is important to maintain stable grip (Johansson and Westling 1984; Flanagan et al., 1999). However, coordination of fingertip forces (forces normal/shear to grip surface) is found generally impaired following stroke (Seo et al., 2009), as it would require a substantial level of involvement of both cortical (e.g., posterior parietal cortex; Ehrsson et al., 2003) and subcortical (cerebellum; Kawato et al., 2003) structures. While a previous study (Seo et al., 2011) demonstrated that patients may be able to voluntarily reduce the shear fingertip force when the visual feedback of force direction was provided, the degree of improvement (shear force reduction) observed in their study was relatively small (shear-to-normal force ratio reduced from 58% to 41%). Moreover, patients with a greater level of motor impairment are not likely to be able to voluntarily control the force direction even when the visual feedback of the force direction is provided. The proposed method could provide ‘subject-specific’ assistance (determined from each patient’s task performance) to help patients redirect the fingertip force towards the normal direction. The biomimetic assistance used in the study could also provide somatosensory (coordinated joint moments) and tactile (fingertip force direction sensed at the fingerpad) feedback during task, which would be beneficial in functional recovery. Previous studies demonstrated the importance of sensory input to motor learning, as the movement-related afference provide important information regarding task performance (Krakauer et al., 1999; Cauraugh et al., 2000). Lesion of primary somatosensory cortex was also found to significantly hamper the motor learning process in monkeys (Pavlides et al., 1993). Note that, when assistance is delivered in typical joint-based exoskeletons (via straps), unlike exotendon-based systems, they produce shear/translational internal forces (joint reaction) that are very different from those produced during voluntary movements (Nef et al., 2007). It should be noted that the control of fingertip force (i.e., force direction control) was significantly impaired in both subject groups (MI and SI; Table 2), indicating that this type of fine motor control tends to be impaired regardless of the clinical functionality scores of individual patients. Since proper fingertip force control could be critical in the UE functionality, as demonstrated in the previous study (Seo et al., 2010, 2011), the proposed training for the simulated pinch task would be beneficial to most patients with varying functional impairment levels.
The proposed device can train patients to perform sophisticated (multi-DOF) movements or force production tasks employing proper coordination patterns, which are crucial components of many functional activities. Thus, our device could be employed in a task-oriented training that emphasizes restoration of proper task mechanics that could lead to greater functional gain (Michaelsen et al., 2006).
B. Non-restraining, subject-specific assistance: Soliciting subject participation
Previous studies (Israel et al., 2006; Kahn et al., 2006b; Hornby et al., 2008) showed that, while a robot-assisted training may restore ‘normal’ kinematics of task (by enforcing patients to follow pre-defined kinematic patterns/trajectories), this could also result in “reduced volitional drive necessary for motor memory consolidation” for patients with neurological injury (Hornby et al., 2008). Furthermore, many robotic systems are ‘rigid’ and do not allow kinematic errors to take place during movements, as they are programed to follow ‘desired’ trajectories during training. But kinematic errors produced during movement execution (and their active correction) are found critical in promoting motor adaptation and neuroplasticity (Patton et al., 2006; Emken et al., 2007).
Our proposed method employs a force-based control approach that utilizes non-restraining (non-rigid) actuators (exotendons). Therefore, the device would allow patients to deviate from the target movement patterns in case they use improper muscle coordination patterns. Such deviation, if occurs, could facilitate an error-based learning process (Emken et al., 2007). In addition, since the device provided only the assistance required to complete the task, determined from kinematic/kinetic deficiency of patients’ voluntary task performance, subjects overall maintained their effort level, as confirmed by no-significant difference between the EMG data collected during assisted and unassisted trials.
The proposed control strategy, targeted muscle assistance based on identification of impaired muscle (via biomechanical analyses), is conceptually similar to recent control strategies developed to promote active participation, i.e., ‘assist-as-needed’ strategy (Cai et al., 2006; Pehlivan et al., 2016) or impedance control (Krebs et al., 2003), in that the assistance level is adjusted based on users’ engagement to the task. The main difference would be that the exotendons in our system provide ‘biomimetic’ assistance that resembles kinetic action of human multiarticular musculotendons to the muscles identified to be deficient. Therefore, subjects need to maintain activation of other less-impaired muscles, instead of reducing overall effort level, which would be advantageous to promote their active participation. Additionally, our previous study showed that targeted assistance could also induce significant change in the muscle ‘coordination’ pattern during assisted task performance (Lee et al., 2018).
C. Limitations of the study
This study focused on restoring motor control of fingers; thus, thumb movements were neither assisted nor examined. Since precise control of thumb-tip forces is also important in performing manual tasks (Johanson et al., 2001), future studies could provide similar subject-specific assistance for the thumb-tip force production to restore normal mechanics of manual tasks.
This early stage study had a cross-sectional design. While the capacity of the proposed approach to emphasize important elements of stroke rehabilitation, i.e., restoring task mechanics and soliciting subject participation, was demonstrated in this study, clinical benefits of the proposed method should be demonstrated in a longitudinal training study to determine efficacy.
Lastly, our study was not a ‘controlled’ study. Although our system employs a unique ‘subject-specific’ tuning process to restore task mechanics, its performance was not directly compared with the performance of other types of robotic systems; such direction comparisons in a controlled study would be required to clearly demonstrate the advantage of the proposed system.
V. Conclusion
In this study, we demonstrated the feasibility of a novel robotic approach to restoring mechanics of functional hand tasks by providing an individualized pattern of targeted assistance to impaired muscles using non-restraining (underconstrained), biomimetic actuators. The proposed scheme identifies deficiency in muscle kinetics (that needs to be reinforced) from their voluntary task performance, and only the level of assistance required to complete the task was provided. Thus patients maintained their effort level during assisted trials, while the assistance focused on restoring task mechanics (kinematics/kinetics), providing somatosensory (proprioceptive and/or tactile) feedback that could promote their functional recovery. The proposed technique presents a novel training paradigm to efficiently restore the functionality of the hand post-stroke by emphasizing three important aspects of effective stroke rehabilitation, i.e., restoring task mechanics; error-based learning; and subject participation.
References
- [1].An KN, Ueba Y, Chao EY, Cooney WP, Linscheid RL. 1983. Tendon excursion and moment arm of index finger muscles, J Biomech 16: 419–425. [DOI] [PubMed] [Google Scholar]
- [2].Balasubramanian S, Klein J, Burdet E. 2010. Robot-assisted rehabilitation of hand function. Curr Opin Neurol 23: 661–670. [DOI] [PubMed] [Google Scholar]
- [3].Bertani R, Melegari C, De Cola MC, Bramanti A, Bramanti P, Calabro RS. 2017. Effects of robot-assisted upper limb rehabilitation in stroke patients: a systematic review with meta-analysis, Neurol Sci 38: 1561–1569. [DOI] [PubMed] [Google Scholar]
- [4].Buma FE, van Kordelaar J, Raemaekers M, van Wegen EEH, Ramsey NF, Kwakkel G. 2016. Brain activation is related to smoothness of upper limb movements after stroke, Exp Brain Res 234: 2077–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cai LL, Fong AJ, Otoshi CK, Liang Y, Burdick JW, Roy RR, Edgerton VR. 2006. Implication of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning, J Neurosci 26: 10564–10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Carod-Artal J, Egido JA, Gonzalez JL, de Seijas EV. 2000. Quality of life among stroke survivors evaluated 1 year after stroke: experience of a stroke unit, Stroke 31: 2995–3000. [DOI] [PubMed] [Google Scholar]
- [7].Cirstea CM, Levin MF. 2008. Improvement of arm movement patterns and endpoint control depends on type of feedback during practice in stroke survivors, Neurorehabil Neural Repair 21: 398–411. [DOI] [PubMed] [Google Scholar]
- [8].Clarke P, Black SE. 2005. Quality of life following stroke: negotiating disability, identity, and resources, J Appl Gerontol 24: 319–336. [Google Scholar]
- [9].Colebatch JG, Gandevia SC. 1989. The distribution of muscular weakness in upper motor neuron lesions affecting the arm, Brain 112: 749–763. [DOI] [PubMed] [Google Scholar]
- [10].Collins DF, Refshauge KM, Todd G, Gandevia SC. 2005. Cutaneous Receptors Contribute to Kinesthesia at the Index Finger, Elbow, and Knee, J Neurophysiol 94: 1699–1706. [DOI] [PubMed] [Google Scholar]
- [11].Connelly L, Jia Y, Toro ML, Stoykov ME, Kenyon RV, Kamper DG. 2010. A pneumatic glove and immersive virtual reality environment for hand rehabilitative training after stroke. IEEE Trans Neural Syst Rehabil Eng 18: 551–559. [DOI] [PubMed] [Google Scholar]
- [12].Cordo PJ, Horn JL, Kunster D, Cherry A, Bratt A, Gurfinkel V. 2011. Contribution of skin and muscle afferent input to movement sense in the human hand, J Neurophysiol 105: 1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dorvat L, Lambercy O, Gassert R, Maeder T, Milner T, Leong TC, Burdet E. 2008. HandCARE: A cable-actuated rehabilitation system to train hand function after stroke, IEEE Trans Neural Syst Rehabil Eng 16: 582–591. [DOI] [PubMed] [Google Scholar]
- [14].Ehrsson HE, Fagergren A, Johansson RS, Forssberg H. 2003. Evidence for the involvement of the posterior parietal cortex in coordination of fingertip forces for grasp stability in manipulation, J Neurophysiol 90: 3295–3303. [DOI] [PubMed] [Google Scholar]
- [15].Emken JL, Reinkensmeyer DJ. 2005. Robot-enhanced motor learning: accelerating internal model formation during locomotion by transient dynamic amplification, IEEE Trans Neural Syst Rehabil Eng 13: 33–39. [DOI] [PubMed] [Google Scholar]
- [16].Emken JL, Benitez R, Sideris A, Bobrow JE, Reinkensmeyer DJ. 2007. Motor adaptation as a greedy optimization of error and effort, J NeurophysioW: 3997–4006. [DOI] [PubMed] [Google Scholar]
- [17].Fischer HC, Stubblefield K, Kline T, Luo X, Kenyon RV, Kamper DG. 2007. Hand rehabilitation following stroke: a pilot study of assisted finger extension training in a virtual environment, Top Stroke Rehabil 14: 1–12. [DOI] [PubMed] [Google Scholar]
- [18].Gottlieb GL, Song Q, Hong DA, Almeida GL, Corcos D. 1996. Coordinating movement at two joints: a principle of linear covariance, J Neurophysiol75: 1760–1764. [DOI] [PubMed] [Google Scholar]
- [19].Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. 1987. Arm function after stroke: measurement and recovery over the first three months, J Neurol Neurosurg Psychiat 50: 714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hermsdörfer J, Hagl E, Nowak DAQ, Marquardt C. 2003. Grip force control during object manipulation in cerebral stroke, Clin Neurophysiol 114: 915–929. [DOI] [PubMed] [Google Scholar]
- [21].Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. 2008. Enhanced gait-related improvements after therapist-versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study, Stroke 39: 1786–1792. [DOI] [PubMed] [Google Scholar]
- [22].Israel JF, Campbell DD, Kahn JH, Hornby TB. 2006. Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury, Phys Ther 86: 1466–1478. [DOI] [PubMed] [Google Scholar]
- [23].Johanson ME, Valero-Cuevas FJ, Hentz VR. 2001. Activation patterns of the thumb muscles during stable and unstable pinch tasks, J Hand Surg Am 26: 698–705. [DOI] [PubMed] [Google Scholar]
- [24].Kahn LE, Zygman ML, Rymer WZ, Reinkensmeyer DJ. 2006a. Robot-assisted reaching exercise promotes arm movement recovery in chronic hemiparetic stroke: a randomized controlled pilot study, J Neuroeng Rehabil 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kahn LE, Lum PS, Rymer WZ, Reinkensmeyer DJ. 2006b. Robot-assisted movement training for the stroke-impaired arm: Does it matter what the robot does? J Rehabil Res Dev 43: 619–630. [DOI] [PubMed] [Google Scholar]
- [26].Kwakkel G, Kollen BJ, Krebs HI. 2008. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review, Neurorehabil Neural Repair 22: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kawato M, Kuroda T, Imamizu H, Nakano E, Miyauchi S, Yoshioka T. 2003. Internal forward models in the cerebellum: fMRI study on grip force and load force coupling. Prog Brain Res 142: 171–188. [DOI] [PubMed] [Google Scholar]
- [28].Kawato M, Gomi H. 1992. A computational model of four regions of the cerebellum based on feedback-error learning,Biol Cybern 68: 95–103. [DOI] [PubMed] [Google Scholar]
- [29].Kitago T, Liang J, Huang VS, Hayes S, Simon P, Tenteromano L, Lazar RM, Marshall RS, Mazzoni P, Lennihan L, Krakauer JW. 2012. Improvement after constraint-induced movement therapy: recovery of normal motor control or task-specific compensation? Neurorehabil Neural Repair 27: 99–109. [DOI] [PubMed] [Google Scholar]
- [30].Krakauer JW. 2005. Arm function after stroke: from physiology to recovery, Semin Neurol 25: 384–395. [DOI] [PubMed] [Google Scholar]
- [31].Krebs HI, Palazzolo JJ, Dipietro L, Ferraro M, Krol J, Rannekleiv K, Volpe BT, Hogan N. 2003. Rehabilitation robotics: Performance-based progressive robot-assisted therapy, Auton. Robots 15: 7–20. [Google Scholar]
- [32].Lang CE, DeJong SL, Beebe JA. 2009. Recovery of thumb and finger extension and its relation to grasp performance after stroke, J Neurophysiol 102: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee SW, Chen H, Towles JD, Kamper DG. 2008. Effect of finger posture on the tendon force distribution within the finger extensor mechanism, J Biomech Eng 130: 051014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee SW, Kamper DG. 2009. Modeling of multiarticular muscles: importance of inclusion of tendon-pulley interactions in the finger, IEEE Trans Biomed Eng 56: 2253–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lee SW, Landers KA, Park HS. 2014. Development of a biomimetic hand exotendon device (BiomHED) for restoration of functional hand movement post-stroke, IEEE Trans Neural Syst Rehabil Eng 22: 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lee SW, Vermillion BC, Geed S, Dromerick AW, Kamper DG. 2018. Impact of targeted assistance of multiartimular finger musculotendons on the coordination of finger muscles during isometric force production, IEEE Trans Neural Syst Rehabil Eng 26: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Levin MF, Kleim JA, Wolf SL. 2009. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair 23: 313–319. [DOI] [PubMed] [Google Scholar]
- [38].Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. 2010. Robot-assisted therapy for long-term upper-limb impairment after stroke, N Eng J Med 367: 2375–2384. [Google Scholar]
- [39].Marchal-Crespo L, Reinkensmeyer DJ. 2009. Review of control strategies for robotic movement training after neurologic injury, J Neuroeng Rehabil 16: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mayo NE,Wood-Dauphinee S, Ahmed S, Gordon C, Higgins J, McEwen S, Salbach N. 1999. Disablement following stroke, Disabil Rehabil 21: 258–268 [DOI] [PubMed] [Google Scholar]
- [41].Mehrholz J, Platz T, Kugler J, Pohl M. 2008. Electromechanical and robot-assisted arm training for improving arm function and activities of daily living after stroke, Cochrane Database Syst Rev 8: CD006876. [DOI] [PubMed] [Google Scholar]
- [42].Michaelsen SM, Luta A, Roby-Brami A, Levin MF. 2001. Effect of trunk restraint on the recovery of reaching movements in hemiparetic patients, Stroke 32: 1875–1883. [DOI] [PubMed] [Google Scholar]
- [43].Michaelsen SM, Dannenbaum R, Levin MF. 2006. Task-specific training with trunk restraint on arm recovery in stroke: randomized control trial, Stroke 37: 186–192. [DOI] [PubMed] [Google Scholar]
- [44].Nef T, Mihelj M, Riener R. 2007. ARMin: a robot for patient-cooperative arm therapy, Med Biol Eng Comput 45: 887–900. [DOI] [PubMed] [Google Scholar]
- [45].Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. 2005. Factors influencing stroke survivors’ quality of life during subacute recovery, Stroke 36: 1480–1484. [DOI] [PubMed] [Google Scholar]
- [46].Nimbarte AD, Kaz R, Li ZM. 2008. Finger joint motion generated by individual extrinsic muscles: a cadaveric study, J Orthop Surg Res 3: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Patton JL, Stoykov ME, Kovic M, Mussa-Ivaldi FA. 2006. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors, Exp Brain Res 168: 368–383. [DOI] [PubMed] [Google Scholar]
- [48].Pehlivan AU, Losey DP, O’Malley MK. 2016. Minimal assist-as-needed controller for upper limb robotic rehabilitation, IEEE Trans Robot 32: 113–124. [Google Scholar]
- [49].Platz T, Denzler P, Kaden B, Mauritz K-H. 1994. Motor learning after recovery from hemiparesis. Neuropsychologia 32:1209–1223. [DOI] [PubMed] [Google Scholar]
- [50].Prange GB, Jannink MJ, Groothuis-Oudshoorn CG, Hermens HJ, Ijzer-man MJ. 2006. Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke, J Rehabil Res Dev 43: 171–184. [DOI] [PubMed] [Google Scholar]
- [51].Raghavan P 2007. The nature of hand motor impairment after stroke and its treatment, Curr Treat Options Cardiovasc Med 9: 221–228. [DOI] [PubMed] [Google Scholar]
- [52].Rohrer B, Fasoli S, Krebs HI, Hughes R, Volpe B, Frontera WR, Stein J, Hogan N. 2002. Movement smoothness changes during stroke recovery, J Neurosci 22: 8297–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Roby-Brami A, Feydy A, Combeaud M, Biryukova EV, Bussel B, Levin MF. 2003. Motor compensation and recovery for reaching in stroke patients. Acta Neurol Scand 107: 369–381. [DOI] [PubMed] [Google Scholar]
- [54].Saladin LK. 1996. Cerebrovascular Disease: Stroke In: Fredricks CM, Saladin LK (Eds) Pathophysiology of the Motor Systems: Principles and Clinical Presentation. Philadelphia: F.A Davis: 486–512. [Google Scholar]
- [55].Santello M, Soechting JF. 1998. Gradual molding of the hand to object contours, J Neurophysiol 79: 1307–1320. [DOI] [PubMed] [Google Scholar]
- [56].Schabowsky CN, Godfrey SB, Holley RJ, Lum PS. 2010. Development and pilot testing of HEXORR: hand EXOskeleton rehabilitation robot, J Neuroeng Rehabil 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Scott SH, Loeb GE. 1994. The computation of position sense from spindles in mono-and multiarticular muscles, J Neurosci 14: 7529–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Seo NJ, Rymer WZ, Kamper DG. 2010. Altered digit force direction during pinch grip following stroke, Exp Brain Res 202: 891–901. [DOI] [PubMed] [Google Scholar]
- [59].Seo NJ, Fischer HC, Bogey RA, Rymer WZ, Kamper DG. 2011. Use of visual force feedback to improve digit force direction during pinch grip in persons with stroke: a pilot study, Arch Phys Med Rehabil 92: 24–30. [DOI] [PubMed] [Google Scholar]
- [60].Susanto EA, Tong RKY, Ockenfeld C, Ho NSK. 2015. Efficacy of robot-assisted fingers training in chronic stroke survivors: a pilot randomized-controlled trial,J Neuroeng Rehabil 12: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer CC. 2008. Robot-based hand motor therapy after stroke, Brain 131: 425–437. [DOI] [PubMed] [Google Scholar]
- [62].Twitchell TE. 1951. The restoration of motor function following hemi-plegia in man, Brain 74: 443–480. [DOI] [PubMed] [Google Scholar]
- [63].Valero-Cuevas FJ, Towles JD, Hentz VR. 2000. Quantification of fin-gertip force reduction in the forefinger following simulated paralysis of extensor and intrinsic muscles, J Biomech 33: 1601–1609. [DOI] [PubMed] [Google Scholar]
- [64].Veerbeek JM, Langbroek-Amersfoort AC, van Wegen EE, Meskers CG, Kwakkel G. 2017. Effects of robot-assisted therapy for the upper limb after stroke, Neurorehabil Neural Repair 31: 107–121. [DOI] [PubMed] [Google Scholar]
- [65].Welmer AK, Holmqvist LW, Sommerfeld DK. 2008. Limited fine hand use after stroke and its association with other disabilities, J Rehabil Med 40: 603–608. [DOI] [PubMed] [Google Scholar]
- [66].Wing AM, Lederman SJ. 1998. Anticipating load torques produced by voluntary movements, J Exp Psychol Hum Percept Perform 24: 1571–1581. [DOI] [PubMed] [Google Scholar]