Abstract
Introduction:
Conventional coagulation assays (CCAs), PT/INR (prothrombin time/international normalized ratio) and aPTT (activated partial thromboplastin time), detect clotting factor (CF) deficiencies in hematologic disorders. However, there is controversy about how these CCAs should be used to diagnose, treat and monitor trauma-induced coagulopathy. Study objectives were to determine whether CCA abnormalities are reflective of deficiencies of coagulation factor activity in the setting of severe injury.
Methods:
Patients without previous CF deficiency within a prospective database at an ACS verified Level 1 trauma center had CF activity levels, PT/INR, aPTT, and fibrinogen levels measured upon Emergency Department arrival from 2014–2017. Linear regression assessed how CF activity explained the aPTT and PT/INR variation. Prolonged CCA values were set as INR>1.3 and aPTT>34sec. CF deficiency was defined as <30% activity, except for fibrinogen, defined as <150mg/dL.
Results:
Sixty patients with a mean age of 35.8 (std dev:13.6) years and median new injury severity score (NISS) of 32 (IQR:12–43) were included; 53.3% sustained blunt injuries, 23.3% required massive transfusion, and mortality was 11.67%. Overall, 44.6% of the PT/INR variance and 49.5% of the aPTT variance remained unexplained by CF activity. Deficiencies of CFs were: common pathway 25%; extrinsic pathway 1.7%, and intrinsic pathway 6.7%. The positive predictive value for CF deficiencies were: 1)PT/INR>1.3:4.4% for extrinsic pathway, 56.5% for the common pathway; 2) aPTT>34 sec:16.7% for the intrinsic pathway, 73.7% for the common pathway.
Conclusion:
Almost half of the variances of PT/INR and aPTT were unexplained by CF activity. Prolonged PT/INR and aPTT were poor predictors of deficiencies in the intrinsic or extrinsic pathways, however, they were indicators of common pathway deficiencies.
Keywords: trauma-induced coagulopathy, coagulation factor activity, trauma, aPTT, PT/INR, hemorrhagic shock
Introduction
The use of conventional coagulation assays (CCA) such as the prothrombin time/international normalized ratio (PT/INR) and activated partial thromboplastin time (aPTT) were developed for the monitoring of anticoagulation with warfarin and heparin respectively, as well as the diagnosis of hemophilia due to isolated clotting factor deficencies (1, 2). The use of INR allows for inter-laboratory and inter-patient comparisons (1). Assessment and management of hemostasis in the injured patient has commonly relied on CCAs, based primarily on PT/INR and aPTT with the addition of platelet count and fibrinogen level (3). The PT/INR is used to assess the extrinsic or tissue factor pathway, while the aPTT is used to assess the function of the intrinsic or contact pathway of coagulation (4). Deficiencies in the common pathway are associated with prolongations of both the PT/INR and aPTT (4).
The driving mechanisms of trauma-induced coagulopathy (TIC) is thought to be predominantly due to coagulation factor activity deficiencies, resulting in reduced thrombin generation and fibrinogen depletion, with the added effects of platelet dysfunction and dysregulated systemic fibrinolysis (5–8). Based on studies evaluating patients with hereditary coagulopathies, a “critical” clotting factor deficiency has been defined as <30% activity for any coagulation factor (8–11), and clotting factor activity of <30% has been identified as the minimum level required for normal in vivo hemostasis (8). Rizoli et al. found that injured patients with <30% activity of any coagulation factor were more severely injured, had a higher prevalence of elevated PT/INR or aPTT, and required increased blood product transfusions (8).
PT/INR and aPTT have been used as clinical correlates to evaluate coagulation factor activity (8), and as such, one would expect patients with prolonged CCAs to have abnormal CF activity.. However, there are significant limitations to relying on CCAs. PT/INR and aPTT are plasma-based tests and only provide information on a fraction of the clotting process, e.g., the liquid or plasma phase(12). Specifically, they do not provide information on in vivo interaction with cellular components (10). Activated platelets are critical for localizing coagulation factors at the injury site, and the extent of bleeding with a prolonged PT/INR or aPTT may vary according to platelet function (10). Furthermore, it is not possible to estimate the overall stability of a thrombus using PT/INR or aPTT, as both tests are terminated before fibrin is polymerized by active factor XIII (10).
PT/INR and aPTT were initially designed to test for heritable coagulopathy, hemophilias, and factor VII deficiency (13), and standard reference ranges were generated using data from healthy volunteers (14). As such, concerns have been raised about the use of these tests as a standard benchmark for managing TIC (15). Despite the increased adoption of viscoelastic assays, which have been shown to improve survival guiding TIC treatment, the definition of TIC is often defined by prolonged PT/INR and aPTT, specifically an INR>1.3 and/or an aPTT>34 seconds (15–17). While PT/INR and aPTT have been identified as predictors of bleeding and mortality in trauma (15–17), the specific changes in coagulation factor activity in trauma and how this accounts for changes to postinjury PT/INR and aPTT has not been fully evaluated. Therefore, our objective was to assess the variability in PT/INR and aPTT based on deficiencies in CF activity in the injured patient.
Methods
Study Design
This is a nested study within our Trauma Activation Protocol (TAP) database in adult (age ≥18 years) patients with hemorrhagic shock (evidence of hemorrhage with field systolic blood pressure (SBP) <70mmHg or SBP of 70–90mmHg plus heart rate, HR, >108bpm) transported by ambulance from 2014 to 2017 to the at the Ernest E Moore Shock Trauma Center at Denver Health an American College of Surgeons verified and Colorado state certified Level 1 trauma center affiliated with the University of Colorado Denver. Whole blood was collected from patients under waiver of consent immediately upon arrival to the emergency department. This clinical study was approved by the Colorado Multiple Institutional Review Board.
Exclusion criteria included visibly or verbally reported pregnant women, age<18 years, known prisoners, unsalvageable injuries (defined as asystole or CPR at the scene prior to randomization), isolated GSW to the head with a GCS < 5 (a highly lethal injury that is not primarily due to acute blood loss), and known or religious objection to blood products. Patients on anticoagulation were not included in this study.
Some of these patients were in a trial that tested the effectiveness of pre-hospital plasma, conducted under waiver of consent for emergency research. In the patients who were part of the study evaluating effectiveness of pre-hospital plasma, there were no significant differences between study groups in regards to coagulation factor activity upon ED arrival (post plasma administration), due to the low volume of plasma given and short transport times. The smallest t-test p-value for differences in clotting factors (CF) between the study groups upon ED arrival was p=0.15 for factor X; the differences between the two groups did not exceed 0.4 standard deviations for any of the factors, with higher levels observed consistently in the control (no plasma) group. In comparison to other patients, the patients with coagulation factor measurements were similar in age, NISS, SBP and HR, as well as massive transfusion (MT), and mortality (p>0.05 for all).
Clinical data included demographic characteristics, injury mechanism, injury severity (Abbreviated Injury Scale, AIS; New Injury Severity Score, NISS), and physiologic derangement indicators.
Blood Samples from Healthy Volunteers
Trained study staff collected samples from volunteers at an outpatient clinic after obtaining an informed consent. The study was open to patients and hospital staff not taking antiplatelet or anticoagulant medications. Volunteers with diabetes, morbid obesity, renal disease, or liver disease were excluded. Blood samples were collected in tubes containing sodium citrate (3.5mL, 3.2% sodium citrate, Greiner Bio-One).
Blood Samples from Trauma Patients
Samples were collected during trauma activations upon arrival to the ED in tubes in citrated tubes (3.5 mL, 3.2% sodium citrate, Greiner Bio-One). A team of trained PRAs on a 24/7 call schedule for prospective studies completed viscoelastic assays within two hours after blood collection. Citrated blood samples were analyzed using the TEG 5000 Thrombelastography Hemostasis Analyzer (Haemonetics, Niles, IL, USA), as previously described (18). The following indices were obtained from the tracings of the TEG: activated clotting time (ACT [sec]), angle (°), maximum amplitude (MA [mm]), and lysis 30 min after MA (LY30 [%]). The contribution of fibrinolysis (clot breakdown) toward the variance of both aPTT and PT/INR was also evaluated by an elevated LY30 on TEG, LY30 was included in the analysis because it has been suggested to prolong the PT/INR in some clinical scenarios (19).
Conventional coagulation assays (CCA)
Values for conventional coagulation assays (PT/INR and aPTT) were determined by the clinical laboratory at DHMC by standard operating procedures. Fibrinogen levels were also measured by the clinical laboratory after initial blood draw using the von Clauss functional assay based on the time to fibrin clot formation (20). Prolonged values for CCA were set as INR>1.3 and aPTT>34 sec as previously defined (16, 17, 21).
Coagulation Factor (CF) Activity
Factors II, V, VII, VIII, IX, X, and XI activity levels were determined using coagulation factor activity assays (Stago, Parsippany, NJ) on STA Compact Max (Stago, Parsippany, NJ). In brief, the assay is a measurement of clot time in the presence of the STA-Neoplastine® kit and reagent and excess CF. The CF of interest is absent from reagents and supplied from patient sample plasma. Analysis was performed by comparing samples to standard human plasma assays of CF and results were expressed as a percentage of standard activity. If immediate testing of clotting factor activity was not possible, specimens were snap frozen and stored at −80 °C until the final analysis could be performed. CF deficiency was defined as previously published as <30%, except for fibrinogen, defined as <150mg/dL (5, 8–10). Factor XIII was measured qualitatively (normal/abnormal) with a clot-solubility test, in which plasma is clotted with calcium and/or thrombin, and resistance to lysis by urea or acetic acid is determined. Only two patients had a qualitative deficiency of Factor XIII and were excluded from this analysis.
Statistical Analysis
SAS 9.4 (SAS Institute Inc., Cary, NC) was used for statistical analysis. Non-normally distributed variables were expressed as median and interquartile range (IQR) and normally distributed variables as mean and standard deviation (SD). Continuous normally distributed variables were compared with two-sampled t-test using the Satterthwaite method(22) to allow for possibly unequal variances between the two groups being compared, while non-normally distributed variables were compared employing the Wilcoxon test. Significance was determined at p < 0.05.
The squared semi-partial correlation coefficient type II via linear regression was calculated to estimate the percent of the PT/INR variance explained by factor VII (extrinsic pathway) and factors in the common pathway (factors X, V, II and fibrinogen), individually and collectively. Using the same procedure, we estimated the percent of the aPTT variance explained by factors in the intrinsic pathway (factors XI, IX, and VIII), as well as those in the common pathway. The distribution of all CFs approximated normality, thus transformation was not needed. The sensitivity, specificity, positive, and negative predictive values were also determined for prolonged conventional assays ability to predict deficiencies in the extrinsic, intrinsic, or common coagulation pathways.
Results
Patient Characteristics
Healthy volunteers (n=122), aged 31.8 ± 7.62 years, were 51.6% male. Supplemental Digital Content 1, Table 1, http://links.lww.com/TA/B417 shows the baseline hematology and coagulation test values for these healthy volunteers, as well as DHMC reference ranges for these coagulation tests, demonstrating that these healthy controls coagulation tests fell within the normal values of the DHMC clinical laboratory.
The 60 study patients were severely injured, with a median NISS of 32 (IQR: 12–43). Characteristics of these patients are summarized in Table 1. Of these patients, 23.3% required a massive transfusion (MT) and the mortality was 11.7%. Compared to healthy volunteers, the patients were of similar age (31.8 ± 7.62 vs 35.8 ± 13.6 years, p=0.32) but had prolonged PT/INR (1.01 ± 0.06 vs 1.4 ± 0.5, p<0.0001), prolonged aPTT (31.1 ± 2.67 vs 33.45 ± 15.76 seconds, p=0.0107), and lower fibrinogen count (278 ± 48 vs 215.4 ± 78 mg/dL, p<0.0001).
Table 1 –
Characteristics of patients with hemorrhagic shock (n=60)
| Variable | % | Mean | Std Dev |
Median | Lower Quartile |
Upper Quartile |
|---|---|---|---|---|---|---|
| Age (years) | 35.8 | 13.6 | 32 | 24.5 | 47 | |
| Sex=male | 75.00% | |||||
| Blunt trauma | 53.30% | |||||
| NISS | 30 | 21 | 32 | 12 | 43 | |
| Max AIS head | 1.1 | 1.8 | 0 | 0 | 2.5 | |
| Max AIS chest | 2 | 1.8 | 2 | 0 | 3 | |
| Max AIS abdomen/pelvis | 1.9 | 1.9 | 2 | 0 | 3 | |
| Max AIS extremities | 1.4 | 1.7 | 0.5 | 0 | 3 | |
| Field SBP (mmHg) | 60.2 | 31.3 | 70 | 50 | 85 | |
| Field Heart Rate (bpm) | 105.4 | 30.2 | 110 | 100 | 120 | |
| Field GCS | 10.5 | 4.6 | 12.5 | 7 | 15 | |
| ED SBP (mmHg) | 96.4 | 34.7 | 91 | 79 | 112 | |
| ED Heart Rate (bpm) | 109.2 | 27 | 116 | 93.5 | 127.5 | |
| ED GCS | 11.3 | 4.9 | 14 | 9 | 15 | |
| Coagulation Assays | ||||||
| ED INR | 1.4 | 0.5 | 1.2 | 1.1 | 1.4 | |
| ED INR>1.3 | 38.30% | |||||
| ED aPTT (sec) | 33.1 | 15 | 27.5 | 24.2 | 37.9 | |
| ED aPTT>34sec | 31.70% | |||||
| Blood Product | ||||||
| Transfusions | ||||||
| RBC units/6hrs | 5.7 | 7.9 | 2 | 0 | 9.5 | |
| Received RBC/6hrs | 65% | |||||
| Plasma units/6hrs | 2.7 | 4 | 1 | 0 | 5 | |
| Received Plasma/6hrs | 55% | |||||
| Platelet units/6hrs | 0.6 | 1.2 | 0 | 0 | 1 | |
| Received Platelets/6hrs | 30% | |||||
| Cryoprecipitate units/6hrs | 0.4 | 0.9 | 0 | 0 | 0 | |
| Received Cryoprecipitate/6hrs |
15% | |||||
| Coagulation Factors | ||||||
| Fibrinogen (mg/dL) | 215.4 | 78 | 199 | 158 | 265.5 | |
| Factor II (% activity) | 71.7 | 21 | 72 | 59.5 | 87 | |
| Factor V (% activity) | 64.8 | 25.6 | 68.5 | 42 | 87 | |
| Factor VIII (% activity) | 331.9 | 175.4 | 326.7 | 178.2 | 471.9 | |
| Factor X (% activity) | 93.7 | 32.5 | 91.5 | 73 | 113.5 | |
| Factor IX (% activity) | 125.1 | 52 | 123 | 93 | 152 | |
| Factor XI (% activity) | 102 | 49.1 | 105.5 | 67 | 132 | |
| Factor VII (% activity) | 75.7 | 24.8 | 71 | 55.5 | 94.5 |
Abbreviations: NISS (new injury severity score), AIS (Abbreviated Injury Scale), SBP (Systolic Blood Pressure), GCS (Glasgow Coma Scale), INR (International Normalized Ratio), aPTT (activated Partial Thromboplastin Time); RBC: red blood cells.
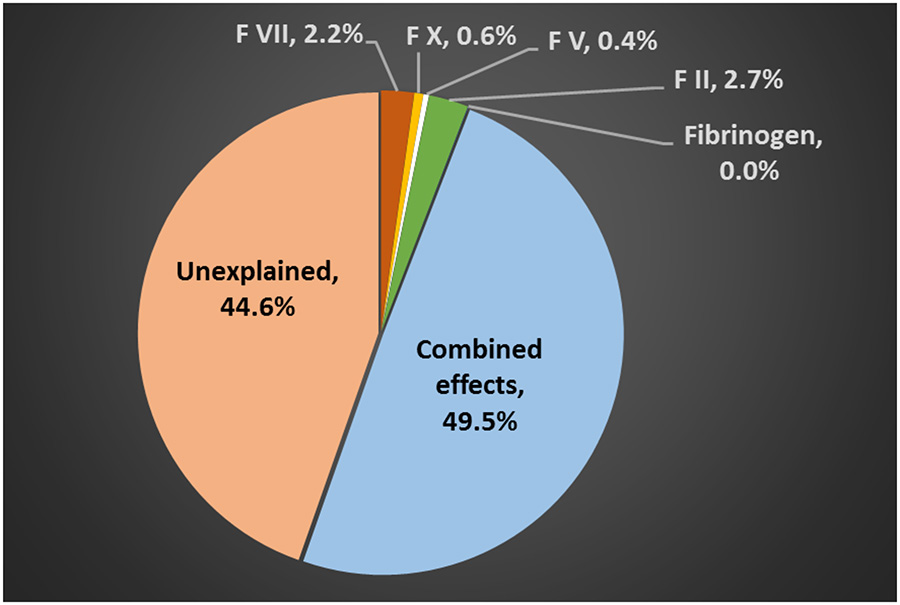
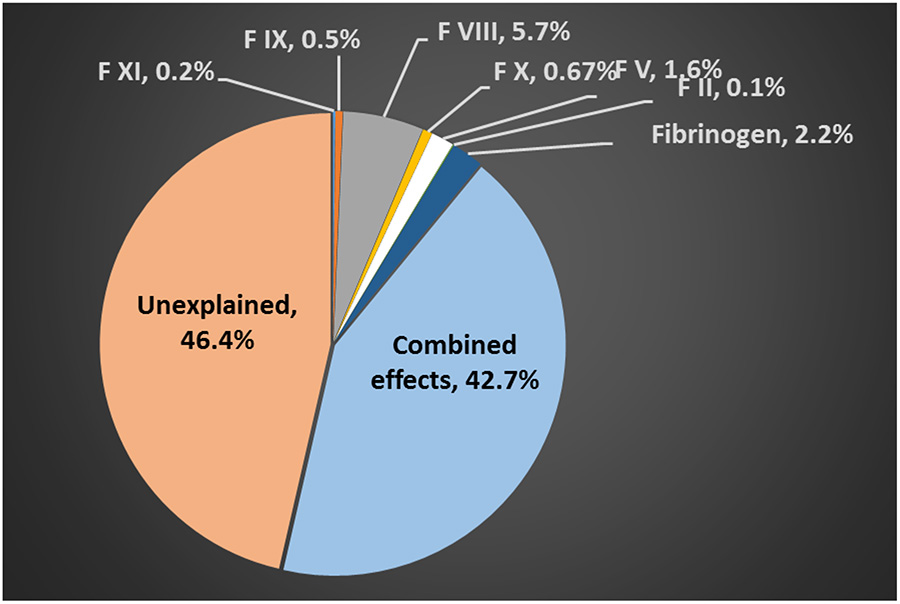
The percent of the variance explained by CFs was evaluated for both PT/INR and aPTT. For PT/INR, the percent variance explained by Factor VII (extrinsic pathway) and the common pathway (factors X, V, II, and fibrinogen), as well as their combined contribution upon hospital arrival, are shown in Figure 1. Collectively, these CFs explained 50.1% of the PT/INR variance. The percent of the aPTT variance explained by the intrinsic pathway factors (factors VIII, IX, and XI), the common pathway factors, as well as their combined contribution are shown in Figure 2. Together, these factors explained 53.6% of aPTT variance. Factor II was the single largest contributor to PT/INR variance while Factor VIII was the single largest contributor to aPTT variance. Adding platelet count and LY30 did not contribute to explain the PT/INR variance (0.05% and 0.6%, respectively). Overall, 44.6% of the PT/INR variance and 49.5% of the aPTT variance were unexplained.
Figure 1–
Percent variance of the arrival international normalized ratio (PT/INR) explained by coagulation factors in the extrinsic (Factor VII) and common pathways (Factors X, V, II, and Fibrinogen).
Figure 2 -.
Percent of variance of the arrival activated Partial Thromboplastin Time (aPTT) explained by coagulation factors in the Intrinsic (Factors XI, IX, and VIII) and Common Pathways (Factors X, V, II, and Fibrinogen).
CF Deficiencies
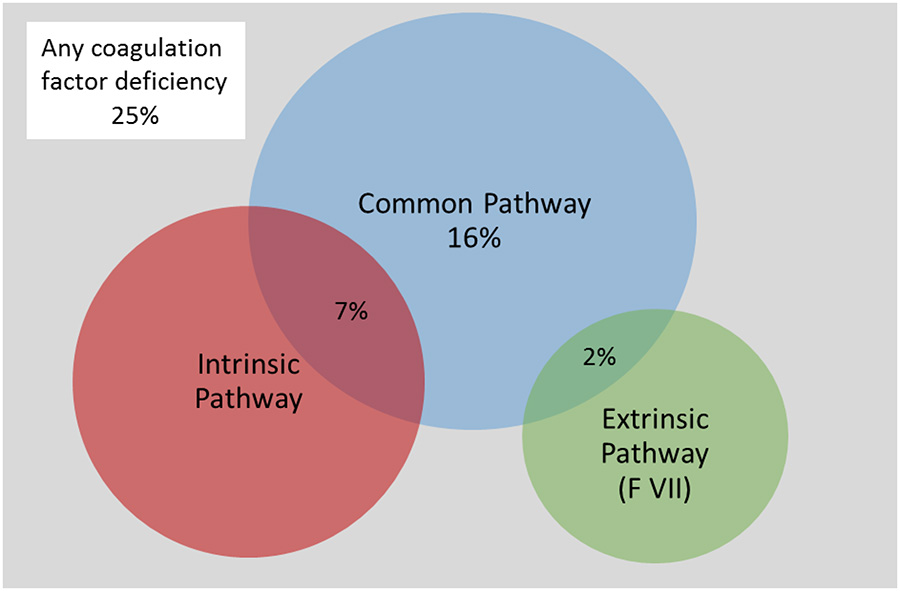
CF deficiencies occurred in 25.0% of the patients with hemorrhagic shock, most frequently affecting exclusively the common pathway (16% of all patients) followed by a combination with intrinsic pathway (7%), as shown in the Venn diagram in Figure 3. Eighty percent of the common pathway deficiencies were related to low fibrinogen. All CF deficiencies of the intrinsic pathway were related to low factor XI, while no patients had factor VIII or IX levels lower than 30%. Having at least one CF deficiency was associated with a higher likelihood of requiring MT (46.7% vs. 15.6%, p=0.03) and trend toward higher mortality (26.7% vs. 6.7%, p=0.06).
Figure 3:
Venn diagram depicting the proportions of patients with coagulation factor deficiencies
CCAs and Coagulation Factors
The mean coagulation factors by PT/INR and aPTT strata are summarized in Tables 2 and 3. Prolonged CCAs were associated with lower mean CF activity and higher proportion of patients with prolonged CF. Furthermore, the presence of prolonged PT/INR and aPTT did not invariably equate to abnormal CF activity. Of those with a prolonged PT/INR alone (n=5), all patients had a normal factor VII activity (extrinsic pathway). Similarly, of those injured patients with both a prolonged PT/INR and prolonged aPTT (n=18), 94.4% had normal factor VII activity. There were five patients (27.8%) who had both increased PT/INR and aPTT and normal CF activity of the common pathway, and no patients with isolated prolonged INR had abnormal CF activity of the common pathway. There were 15 patients (25%) who had both a prolonged INR and aPTT and normal CF activity in the intrinsic pathway. Finally, there were no patients who had an isolated prolonged aPTT and no deficiency in CF activity of the intrinsic pathway.
Table 2 –
Coagulation Factor Activity by PT/INR strata
| INR<=1.3 (n=37, 61.7%) |
INR>1.3 (n=23, 38.3%) |
P-value for means |
P -value for % with deficiency |
|||
|---|---|---|---|---|---|---|
| Mean (SD) |
% with deficiency* |
Mean (SD) |
% with deficiency* |
|||
| Fibrinogen (md/dL) |
252.2 (71.4) |
2.70% | 156 (45) |
47.80% | <0.0001 | <0.0001 |
| Factor II (% activity) |
82 (15.7) | 0 | 55.2 (17.9) |
13.00% | <0.0001 | 0.05 |
| Factor V (% activity) |
77.6 (20.6) |
2.70% | 44.2 (18.4) |
13.00% | <0.0001 | 0.15 |
| Factor X (% activity) |
109.2 (26) |
0 | 68.6 (25.6) |
4.40% | <0.0001 | 0.38 |
| Factor VII (% activity) |
85.5 (22.8) |
0 | 59.9 (19.2) |
4.40% | <0.0001 | 0.38 |
<30% activity or <150mg/dL for fibrinogen. Significance set at p<0.05 by two tailed t-test.
Table 3 -.
Coagulation Factor Activity by activated Partial Thromboplastin Time (aPTT)
| aPTT<=34sec (n=41, 68.3%) |
aPTT>34sec (n=19, 31.7%) |
P -value for % with deficiency |
|||||
|---|---|---|---|---|---|---|---|
| Mean (SD) |
% with deficiency* |
Mean (SD) |
% with deficiency* |
P-value for means |
|||
| Common Pathway |
Fibrinogen (md/dL) | 245.5 (70.9) |
2.40% | 150.4 (47.3) |
52.94% | <0.0001 | <0.0001 |
| Factor II (% activity) | 81.4 (15) |
0 | 50.8 (16.7) |
11.76% | <0.0001 | 0.03 | |
| Factor V (% activity) | 77.6 (18.9) |
0 | 37.2 (13.2) |
17.65% | <0.0001 | 0.008 | |
| Factor X (% activity) | 106.4 (26.3) |
0 | 66.1 (27.2) |
5.88% | <0.0001 | 0.32 | |
| Intrinsic Pathway |
Factor VIII (% activity) | 397.7 (152.3) |
0 | 190 (134.8) |
0 | <0.0001 | NA |
| Factor IX (% activity) | 146 (45.6) |
0 | 79.9 (33.3) |
0 | <0.0001 | NA | |
| Factor XI (% activity) |
124.1 (40.3) |
0 | 54.1 (27.5) |
23.53% | <0.0001 | 0.008 | |
<30% activity or <150mg/dL for fibrinogen. Significance set at p<0.05 by two tailed t-test.
The predictive accuracy of a prolonged PT/INR, prolonged aPTT, and combined prolonged PT/INR and aPTT for deficiencies in the intrinsic, extrinsic and common pathways is shown in Table 4. Overall, having prolonged values for both CCAs tests had a high positive predictive value for common pathway deficits. This represented a modest gain over an PT/INR>1.3, which had a positive predictive value (PPV) of 56.5%. Conversely, PT/INR>1.3 had a low PPV (4.4%) for factor VII (extrinsic pathway) deficiency and aPTT>34 sec had low PPV for deficits in the intrinsic pathway (21.1%). The CCA tests’ negative predictive values were consistently high.
Table 4 –
Predictive Accuracy of Prolonged Conventional Coagulation Assays (CCA) for Deficiency of Coagulation Factors
| CCA | Deficiency of: | Sensitivity % |
Specificity % |
PPV % |
NPV % |
|---|---|---|---|---|---|
| INR>1.3 | Extrinsic Pathway (Factor VII) |
100% | 62.70% | 4.40% | 100% |
| Common Pathway | 86.70% | 77.80% | 56.50% | 94.60% | |
| aPTT>34 sec | Intrinsic Pathway | 100% | 73.20% | 21.10% | 97.20% |
| Common Pathway | 93.30% | 88.90% | 73.70% | 100% | |
| INR>1.3 and aPTT>34 sec |
Intrinsic Pathway | 75.00% | 73.20% | 16.70% | 97.60% |
| Extrinsic Pathway (Factor VII) |
100% | 73.20% | 5.60% | 100% | |
| Common Pathway | 86.70% | 88.90% | 72.20% | 95.20% |
PPV: positive predictive value; NPV: negative predictive value; INR: International Normalized Ratio; aPTT: activated Partial Thromboplastin Time
Discussion
This study of critically injured patients indicates that a large proportion of PT/INR and aPTT variance is not explained solely by decreased coagulation factor activities. Furthermore, platelet count and fibrinolysis, as measured by the TEG LY30, did not explain the variance. On the other hand, prolonged values of CCA were predictive of deficiencies in the common pathway (factors II, V, X, and fibrinogen), which included the most commonly deranged factors. However, there were a number of patients who had prolonged CCAs who did not have deficiencies in CF activity. Both CCAs had lower predictive value for deficits in factor VII and for factors in the intrinsic pathway (factors XI, IX and VIII).
Several studies have focused on defining the phenotypes of coagulopathy. Two separate principal component analyses (PCA) identified distinct phenotypes of coagulopathy, with the most prevalent phenotype being CF depletion (23, 24). Hence, depletion of clotting elements, assumed in the setting of prolonged CCAs, remains the basis for early transfusion of plasma in trauma patients (7). A recent study evaluating the prevalence of combined or isolated elevations of PT/INR and aPTT revealed discordant phenotypes demonstrating differential factor deficiencies consistent with dysfunction of contact versus tissue factor pathways and additive effects from the common pathway (25). In our patient population, the most common coagulation assay phenotype was a PT/INR of <1.3 and an aPTT of <34 sec followed by elevations in both assays. Abnormalities in PT/INR or aPTT alone were infrequent with six (10%) patients having either a prolonged PT/INR or aPTT and 14 patients having combined abnormalities in PT/INR and aPTT.
Another recent PCA identified two distinct phenotypes within the global clotting factor abnormalities, and these findings substantiated the association of Factors V and VIII deficiency on mortality following injury (21). In our study, common pathway deficiencies were most commonly associated with deficits in Factor V. However, no patients had a critical Factor VIII deficiency (< 30%); in fact, these patients had very high levels of Factor VIII activity (>300% activity in those with aPTT <34 second and 200% in those with aPTT> 34 seconds). Previous studies have emphasized that these early elevated Factor VIII levels, may represent endothelial release (26, 27). Furthermore, Factor VIII is an acute phase reactant which may be elevated in response to the inflammatory response to trauma(26, 27). Because Factor VIII is associated with mortality (21), questions regarding the driving mechanism of adverse outcomes with Factor VIII deficiencies continue. It may be that the absolute level of Factor VIII activity is not the primary driver, rather the relative decrease in Factor VIII activity is a biomarker of adverse outcomes.
In our study, there was significant unexplained variation in the CCA from coagulation factors alone. Patients have an isolated aPTT abnormality could have factor XII deficiency. However, Factor XII deficiency is rare (1/1,000,000) and causes an isolated increased aPTT without an in vivo bleeding diathesis (28–31). It is inherited in an autosomal recessive mechanism, and heterozygotes express 20–60% of activity (28–31). Individuals of Asian heritage are more likely to have Factor XII deficiency rather than other ethnic groups (28–30). In the presented data, there were no patients who presented with an isolated prolonged aPTT, normal PT/INR, and a normal TEG.
Some of this unexplained variation may be accounted for by interactions between coagulation factors, and in a limited number of patients, PT/INR and aPTT may remain normal when bleeding is secondary to increased fibrin breakdown as is seen with congenital or acquired deficiency of α2-antiplasmin (32). Furthermore, other clinical studies demonstrate that the infusion of tPA can prolong INR (19). However, our analysis did not identify fibrinolysis as a contributor to the observed variation. Moreover, in vivo, the local effects of coagulation factors may be substantially different than the plasma-based assays that determine PT/INR and aPTT as activated platelets are capable of locally accumulating coagulation factors (10).
There are multiple mechanisms that could explain deficits of CF after trauma. These range from impaired generation, increased consumption, dilution, and inactivation through inhibitors such as activated Protein C. If consumption was the dominant mechanism we would expect similar decreases in activity of all coagulation factors. However, as shown in Table 1, there are variable levels of CF activity, with Factor V having the lowest activity of 65% activity. Furthermore, those with either an elevated PT/INR or elevated aPTT have significantly lower Factor V activity than those with normal values for these coagulation assays (Tables 2 and 3). This may be partially explained by activated Protein C, which has been shown to be elevated after trauma and cleaves activated Factor V and increases the time to clot initiation (15, 16). This is also suggested by a decreased Factor VIII activity in patients with prolonged PT/INR or aPTT (Tables 2 and 3). Studies have shown that through cleaving activated Factor V and VIII, activated protein C can increase the viscoelastic measurements of clot initiation and increase PT/INR and PTT in a dose-dependent manner (33, 34). Finally, there is evidence that the time of day affects coagulation factor activity and thus circadian rhythms may account for some of the unexplained variation (35).
Each coagulation factor has a unique site of synthesis and storage (Supplemental Digital Content 2, Table 2, http://links.lww.com/TA/B418). Many CFs are synthesized in the liver. Factor V and VIII also have unique sites of storage that are extrahepatic: Factor V is partially stored in platelets while factor VIII is synthesized and stored in endothelial cells (36–38). These sites of storage may also affect their ability to be fully activated during injury. Thus, it is likely a combination of the above-mentioned mechanisms that ultimately contribute to deficiencies in CF activity following injury.
The clinical implications of these findings are diverse, as there is a large proportion of PT/INR and aPTT variation unexplained in injured patients. While these CCAs may be strong indicators of injury, there is a large amount of variability that is not explained by CFs. The definition of TIC is most commonly defined with CCAs, specifically, prolonged PT/INR (>1.3) and aPTT (>34 seconds) (16, 17). McCully et al (39) demonstrated that despite a prolongation in PT/INR, median TEG values remained within normal limits, and clotting factor levels retained adequate function to produce normal clotting before and following the transfusion of plasma in injured and surgical patients. This would indicate that the use of CCAs such as PT/INR might overestimate coagulopathy in injured and surgical patients. Furthermore, the absence of CF deficiency in those with prolonged CCA indicates that another process may be affecting the determination of plasma aPTT and PT/INR, such as presence of inhibitors. The aPTT and PT/INR are plasma-based tests and do not account for cellular contributions to clotting (40). The cellular components of whole blood have been shown to have an important contribution to hemostasis, including the tightly packed array of red blood cells in a contracted clot that resists dissolution (41). Analysis of the trauma proteome may identify factors that contribute to CCA variation other than CF activity alone.
While there remains a large amount of variation that is unexplained by conventional coagulation assays, PT/INR and aPTT remain predictors of coagulation factor deficiencies in the intrinsic, extrinsic, and common pathways of the coagulation cascade (Table 4). While isolated prolongation of PT/INR and aPTT reflect deficiencies in the extrinsic and intrinsic pathway respectively, common pathway deficiencies prolonged both PT/INR and aPTT. However, both PT/INR and aPTT had better predictive value for deficits in the common pathway than the extrinsic or intrinsic pathways, which these tests are most commonly used to evaluate. Not surprisingly, the combination of PT/INR and aPTT enhanced the predictive capability of identifying coagulation factor pathway deficiencies than either test alone.
Limitations
There are several limitations to this study. First, we did not measure Factor XIII activity in our analysis, but only two patients in our data set had qualitative abnormal Factor XIII activity and were excluded. Furthermore, Factor XII was not measured as described above. However, no patient had an isolated aPTT without either TEG or PT/INR evidence of coagulopathy. Patients were also not tested for Vitamin K deficiency. While Vitamin K mainly affects PT/INR, it can also have a lesser effect on aPTT (42). In addition, these data reflect a single time point in a dynamic process and do not take into account the temporal changes of the coagulation process associated with ongoing bleeding. Moreover, it is possible that the traditional cutoffs for coagulation factor deficiency are not applicable to trauma patients, and there was no specific testing completed to screen for coagulation factor inhibitors: mixing studies with normal plasma or specific inhibitor assays. A small sample size precludes subgroup analysis of different types of patients with hemorrhagic shock such as those with organ specific injuries or traumatic brain injury. Further, pre-injury baseline coagulation characteristics were not available and thus we could not account for normal variability. Unfortunately, our study did not have a sufficient number of patients to address the question of whether viscoelastic assays should replace CCAs. Conventional coagulation assays are commonly used at many institutions to guide resuscitation in the injured patient based on presumed CF deficiencies, and therefore our goal was to determine if these assays reflected deficits in CF activity. We did not focus on how the variability in TEG is explained by CF deficits in this study but plan to continue to obtain additional data to attempt to ascertain this. While our data does not explicitly point to TEG being superior to PT/INR and PTT, a previous randomized study from our group compared outcomes of patients resuscitated with convectional coagulation assays vs TEG based resuscitation and showed improved survival with fewer blood products in the TEG based group.
Conclusion
Conventional coagulation assays are commonly used to stratify patients at risk for TIC and guide coagulation factor replacement. We believe these data suggest that administration of plasma should be based on TEG/ROTEM rather than PT/INR or aPTT as our previous randomized trial indicated. But the current study was not powered sufficiently to allow that conclusion. While these conventional coagulation tests are strong biomarkers of injury severity, they do not represent CF deficiencies for which they were designed. A better understanding of the responsibility factors, possibly through metabolomics or proteomics, may identify targets for more efficient management of TIC..
Supplementary Material
Disclosure:
Research reported in this publication was supported in part by the National Institute of General Medical Sciences grants: T32-GM008315 and P50-GM49222, the National Heart Lung and Blood Institute UM1-HL120877, in addition to the Department of Defense USAMRAA and W81XWH-12-2-0028. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Heart, Lung, and Blood institute, or the Department of Defense. Additional research support was provided by Haemonetics (Haemonetics, Niles, IL, USA) with shared intellectual property.
Footnotes
Level of Evidence: Level III - Prognostic
References
- 1.Rose DK, Bar B. Direct Oral Anticoagulant Agents: Pharmacologic Profile, Indications, Coagulation Monitoring, and Reversal Agents. J Stroke Cerebrovasc Dis. 2018. August;27(8):2049–2058 [DOI] [PubMed] [Google Scholar]
- 2.Kaplan A, Raut P, Totoe G, Morgan S, Zantek ND. Management of systemic unfractionated heparin anticoagulation during therapeutic plasma exchange. J Clin Apher. 2016. December;31(6):507–515 [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez E, Pieracci FM, Moore EE, Kashuk JL. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Semin Thromb Hemost. 2010. October;36(7):723–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruttmann T Coagulation for the clinician. S Afr J Surg. 2006. February;44(1):22, 24–6, 28–30. [PubMed] [Google Scholar]
- 5.Fries D, Martini WZ. Br J Anaesth. 2010. August;105(2):116–21 [DOI] [PubMed] [Google Scholar]
- 6.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, Silliman CC, Ploplis V, Castellino FJ, Walsh M. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012. May;214(5):739–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore HB, Moore EE, Chapman MP, Huebner BR, Einersen PM, Oushy S, Silliman CC, Banerjee A, Sauaia A. Viscoelastic Tissue Plasminogen Activator Challenge Predicts Massive Transfusion in 15 Minutes. J. Am. Coll. Surg 2017;225(1):138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizoli SB, Scarpelini S, Callum J, Nascimento B, Mann KG, Pinto R, Jansen J, Tien HC. Clotting factor deficiency in early trauma-associated coagulopathy. J Trauma. 2011. November;71(5 Suppl 1):S427–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burggraf M, Payas A, Kauther MD, Schoeneberg C, Lendemans S. Evaluation of clotting factor activities early after severe multiple trauma and their correlation with coagulation tests and clinical data. World J Emerg Surg. 2015. September 22;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka KA, Key NS, Levy JH. Blood coagulation: hemostasis and thrombin regulation. Anesth Analg. 2009. May;108(5):1433–46. [DOI] [PubMed] [Google Scholar]
- 11.Mattox KL, Moore EE, Feliciano DV. Trauma. 7th ed. New York: McGraw-Hill Medical; 2013. 1224 p. p. [Google Scholar]
- 12.Goodnight SH, Hathaway WE. Disorders of hemostasis and thrombosis: a clinical guide 2nd ed. New York: McGraw-Hill, Medical Pub; Division; 2001. xiv, 622 p. p. [Google Scholar]
- 13.Brinkhous KM. Hemophilia. Bull N Y Acad Med. 1954. May;30(5):325–42. [PMC free article] [PubMed] [Google Scholar]
- 14.Eckman MH, Erban JK, Singh SK, Kao GS. Screening for the risk for bleeding or thrombosis. Ann Intern Med. 2003. February 4;138(3):W15–24 [DOI] [PubMed] [Google Scholar]
- 15.Cohen MJ, Christie SA. Coagulopathy of Trauma. Crit Care Clin. 2017;33(1):101–18. [DOI] [PubMed] [Google Scholar]
- 16.Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, Pittet JF. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012;255(2):379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44. [DOI] [PubMed] [Google Scholar]
- 18.Moore HB, Moore EE, Gonzalez E, Wiener G, Chapman MP, Dzieciatkowska M, Sauaia A, Banerjee A, Hansen KC, Silliman C. Plasma is the physiologic buffer of tissue plasminogen activator-mediated fibrinolysis: rationale for plasma-first resuscitation after life-threatening hemorrhage. J. Am. Coll. Surg 2015;220(5):872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee VH, Conners JJ, Cutting S, Song SY, Bernstein RA, Prabhakaran S. Elevated international normalized ratio as a manifestation of post-thrombolytic coagulopathy in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23(8):2139–44. [DOI] [PubMed] [Google Scholar]
- 20.Tan V, Doyle CJ, Budzynski AZ. Comparison of the kinetic fibrinogen assay with the von Clauss method and the clot recovery method in plasma of patients with conditions affecting fibrinogen coagulability. Am J Clin Pathol. 1995;104(4):455–62. [DOI] [PubMed] [Google Scholar]
- 21.Kunitake RC, Howard BM, Kornblith LZ, Christie SA, Conroy AS, Cohen MJ, Callcut RA. Individual clotting factor contributions to mortality following trauma. J Trauma Acute Care Surg. 2017;82(2):302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satterthwaite FE. Synthesis of variance. Psychometrika. 1941;6(5):309–16. [Google Scholar]
- 23.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74(5):1223–9; discussion 9–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chin TL, Moore EE, Moore HB, Gonzalez E, Chapman MP, Stringham JR, Ramos CR, Banerjee A, Sauaia A. A principal component analysis of postinjury viscoelastic assays: clotting factor depletion versus fibrinolysis. Surgery. 2014;156(3):570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christie SA, Kornblith LZ, Howard BM, Conroy AS, Kunitake RC, Nelson MF, Hendrickson CM, Calfee CS, Callcut RA, Cohen MJ. Characterization of distinct coagulopathic phenotypes in injury: Pathway-specific drivers and implications for individualized treatment. J Trauma Acute Care Surg. 2017;82(6):1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noe DA, Murphy PA, Bell WR, Siegel JN. Acute-phase behavior of factor VIII procoagulant and other acute-phase reactants in rabbits. Am J Physiol Cell Physiol. 1989;257(1 Pt 2):R49–56. [DOI] [PubMed] [Google Scholar]
- 27.Begbie M, Notley C, Tinlin S, Sawyer L, Lillicrap D. The Factor VIII acute phase response requires the participation of NFkappaB and C/EBP. Thromb Haemost. 2000;84(2):216–22. [PubMed] [Google Scholar]
- 28.Gailani D, Renne T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol. 2007;27(12):2507–13. [DOI] [PubMed] [Google Scholar]
- 29.The Saito H. “contact system” in health and disease. Adv Intern Med. 1980;25:217–38. [PubMed] [Google Scholar]
- 30.Colman RW, Wong PY. Participation of Hageman factor dependent pathways in human disease states. Thromb Haemost. 1977;38(4):751–75. [PubMed] [Google Scholar]
- 31.National Organization for Rare Disorders. Factor XII Deficiency.
- 32.Aoki N, Saito H, Kamiya T, Koie K, Sakata Y, Kobakura M. Congenital deficiency of alpha 2-plasmin inhibitor associated with severe hemorrhagic tendency. J Clin Invest. 1979;63(5):877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard BM, Kornblith LZ, Cheung CK, Kutcher ME, Miyazawa BY, Vilardi RF, Cohen MJ. Inducing Acute Traumatic Coagulopathy In Vitro: The Effects of Activated Protein C on Healthy Human Whole Blood. PLoS One. 2016;11(3):e0150930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen MJ, Kutcher M, Redick B, Nelson M, Call M, Knudson MM, Schreiber MA, Bulger EM, Muskat P, Alarcon LH, et al. Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soulban G, Labrecque G. Circadian rhythms of blood clotting time and coagulation factors II, VII, IX and X in rats. Life Sci. 1989;45(25):2485–9. [DOI] [PubMed] [Google Scholar]
- 36.Camire RM. Platelet factor V to the rescue. Blood. 2010;115(4):753–4. [DOI] [PubMed] [Google Scholar]
- 37.Levine JD, Harlan JM, Harker LA, Joseph ML, Counts RB. Thrombin-mediated release of factor VIII antigen from human umbilical vein endothelial cells in culture. Blood. 1982;60(2):531–4. [PubMed] [Google Scholar]
- 38.Jaffe EA. Endothelial cells and the biology of factor VIII. NEJM. 1977;296(7):377–83. [DOI] [PubMed] [Google Scholar]
- 39.McCully SP, Fabricant LJ, Kunio NR, Groat TL, Watson KM, Differding JA, Deloughery TG, Schreiber MA. The International Normalized Ratio overestimates coagulopathy in stable trauma and surgical patients. J Trauma Acute Care Surg. 2013;75(6):947–53. [DOI] [PubMed] [Google Scholar]
- 40.Feng L, Zhao Y, Zhao H, Shao Z. Effects of storage time and temperature on coagulation tests and factors in fresh plasma. Scientific Reports. 2014;4:3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Litvinov RI, Weisel JW. Role of red blood cells in haemostasis and thrombosis. ISBT Sci Ser. 2017;12(1):176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berkner KL. Vitamin K-dependent carboxylation. Vitam Horm. 2008;78:131–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.