Abstract
Purpose:
It’s been shown that intensive monocular perceptual learning can improve visual acuity, contrast sensitivity, and vernier acuity in the amblyopic eye in adults with amblyopia. It is however not clear how much monocular training can enhance binocular visual functions. In the current study, we aimed to evaluate effects of monocular training on a variety of binocular functions.
Methods
Nineteen anisometropic amblyopes (18.5±1.26 yrs) were trained in a grating contrast detection task near each individual’s cutoff spatial frequency for 6 to 10 days (630 trials/day). Visual acuity, stereoacuity, monocular and binocular contrast sensitivity functions (CSF), binocular phase combination and binocular rivalry were tested before and after training.
Results
Training substantially improved contrast sensitivity at the trained spatial frequency (by 67.8%) and a wide range of untrained spatial frequencies (84.0% on average), logMAR acuity (from 0.51 to 0.34; about 2 lines) in the amblyopic eye, and stereoacuity from 929.1” to 80.4”. Training also significantly improved the dominance duration of the amblyopic eye (from 9% to 15%) in binocular rivalry through elongation of each dominant phase without changing switching frequency between the two eyes. On the other hand, training didn’t significantly improve the ratio of the areas under CSF between binocular and monocular (fellow eye) viewing (1.16 vs 1.11, p>0.1) and the interocular balance point, i.e. the contrast ratio at which the two eyes contribute equally to binocular phase combination (0.14 vs 0.16, p>0.10). There was no significant correlation between improvements in visual acuity, stereoacuity, and binocular rivalry.
Conclusions
Although monocular training can improve visual acuity and contrast sensitivity and eye dominance of the amblyopic eye, the magnitudes of improvements didn’t correlate with each other; the impact of monocular training on binocular phase combination was not significant. The results strongly suggest that structured monocular and binocular training is needed to fully recover deficient visual functions in anisometropic amblyopia.
1. Introduction
Amblyopia, a type of visual degradation in the absence of any detectable structural or pathologic ocular abnormalities, affects 2–4% of the population1–3. Caused by abnormal visual experience during development, it leads to many spatial vision deficits, including decreased visual acuity, vernier acuity, contrast sensitivity, and motion sensitivity4–10. Although most studies have focused on monocular deficits in the amblyopic eye11–16, amblyopia is intrinsically a binocular disorder. The imbalance between the two eyes during abnormal development affects the visual pathway associated not only with the amblyopic eye but also the fellow eye17–20. Many studies have documented abnormal binocular vision in amblyopia, including abnormal binocular combination17, 21–24, interocular interaction12, 13 and stereopsis25. Several studies have concluded that the degree of binocularity is a good predictor of the abnormalities in monocular tasks26–29. Several theoretical studies found that the observed abnormalities in binocular phase and contrast in anisometropic amblyopia can be explained by a combination of both monocular and binocular deficits30–32. Specifically, Huang et al (2010) concluded that deficits in binocular combination in observers with anisometropic amblyopia were caused by attenuated monocular signal in the amblyopic eye, stronger interocular contrast gain control from the fellow eye to the signal in the amblyopic eye (direct interocular inhibition), and stronger interocular contrast gain control from the fellow eye to the contrast gain control signal from the amblyopic eye (indirect interocular inhibition). These results suggest that both monocular and binocular functions must be examined when evaluating visual deficits and treatment outcomes in amblyopia.
In clinical practice, amblyopia is treated as a monocular disorder, with occlusion or penalization of the fellow eye as the most popular treatment choice33. Although such monocular treatments can recover visual acuity in the amblyopic eye for about 2/3 of the patients and improve stereoacuity to some degree25, 34, several other visual functions remain deficient in clinically treated amblyopia (defined as 20/20 vision in the amblyopic eye following treatment), including contrast sensitivity at high spatial frequencies2, 7, 15, 35 and eye–hand coordination36. These existing results suggest that traditional treatments focusing on monocular deficits in the amblyopic eye cannot fully restore deficient monocular and binocular functions.
Recently, a number of perceptual learning paradigms have been developed to improve visual performance of observers with amblyopia. Many have focused on monocular training in the amblyopic eye, including vernier offset discrimination37, 38, contrast detection with flankers39–41, contrast detection at the cutoff spatial frequency40, contrast discrimination40, 42, video game43, and de-suppression44, and found that monocular training significantly improved visual acuity in the amblyopic eye. A few studies evaluated the relationship between the magnitudes of visual acuity improvements in the amblyopic eye and enhancement of binocular vision measured in terms of stereoacuity, interocular suppression, lateral interactions and Gabor grating resolution under dichoptic viewing41, 45–48, but found no significant correlation. One study49 found a significant correlation between the magnitudes of the improvements in contrast sensitivity and binocular combination following monocular training in contrast detection.
Most recently, there have been growing interests in binocular training methods for amblyopia. Studies have evaluated the effects of stereo training47, dichoptic training50, and virtual reality training51 on monocular visual acuity and contrast sensitivity and binocular functions. It has been shown that, although monocular visual functions such as visual acuity improved through binocular training, the magnitudes of monocular and binocular improvements were not significantly correlated42, 43, 52. Taken together, results from the perceptual learning studies suggest that both monocular and binocular training are necessary in amblyopia treatment, and monocular and binocular visual functions must be systematically evaluated to fully characterize the efficacy of any training paradigm.
In this study, we systematically investigated the effects of monocular contrast detection training in the amblyopic eye on a variety of monocular and binocular functions, including visual acuity, stereoacuity, monocular and binocular contrast sensitivity functions (CSF), binocular phase combination, and binocular rivalry. Our aim was to directly test the effects of monocular training on both monocular and binocular functions and evaluate the relationship between improvements of monocular and binocular functions following monocular amblyopia treatment. The observers in our study had stronger imbalance between the amblyopic and fellow eyes than those in Chen et al., (2016). We found that although monocular training improved visual acuity, contrast sensitivity and eye dominance of the amblyopic eye, the magnitudes of improvements didn’t significantly correlate with each other; the impact of training on binocular combination was not significant.
Methods
2.1. Subjects
Nineteen subjects (18.5±1.26 yrs) with anisometropic amblyopia participated in the study. Detailed characteristics of the subjects, including age, sex, optical correction, corrected visual acuity, stereoacuity, and interocular balance point in binocular phase combination (see below for details) are listed in Table 1. All subjects were referred to the study from the ophthalmology/optometry clinics in the People’s Hospital of Guangxi Zhuang Autonomous Region, were naive to psychophysical experiments, and underwent at least 16 weeks’ spectacle treatment prior to the study. Written informed consent was obtained from each subject and/or their guardians/parents after explanation of the nature of the study. The experimental protocol was approved by the Institutional Review Board of the Institute of Psychology, Chinese Academy of Sciences. This study adhered to the tenets of the Declaration of Helsinki.
Table 1.
Amblyopic eye’s characteristics of subjects
| No. | Sex | Age (yr) | AE Correction | AE Acuity(LogMAR) | FE Acuity(LogMAR) | Stereoacuity(Titmus) | Balance point * | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||||
| 1 | M | 21 | +4.50DS:+0.5DC×130 | 0.50 | 0.25 | −0.03 | −0.03 | 160 | 63 | 0.11 | 0.10 |
| 2 | M | 22 | +5.75DS:+1.25DC×100 | 0.68 | 0.40 | −0.13 | −0.17 | 400 | 200 | 0.08 | 0.06 |
| 3 | M | 20 | +6.25 DS:+1.50 DC×55 | 0.78 | 0.63 | −0.03 | −0.11 | 4800 | 400 | 0.08 | 0.09 |
| 4 | M | 17 | +3.75 DS:+1.75 DC×95 | 0.58 | 0.50 | −0.03 | −0.03 | 400 | 100 | 0.12 | 0.10 |
| 5 | M | 24 | +2.50 DS:+0.50 DC×95 | 0.78 | 0.55 | −0.03 | −0.03 | 400 | 100 | 0.12 | 0.08 |
| 6 | F | 17 | +5.50 DS | 0.55 | 0.40 | −0.01 | −0.03 | 400 | 40 | 0.07 | 0.11 |
| 7 | M | 18 | +4.25 DS:+0.75 DC×60 | 0.58 | 0.38 | −0.03 | −0.05 | 160 | 100 | 0.04 | 0.09 |
| 8 | F | 12 | +4.5 DS +2.0 DC×70 | 0.88 | 0.66 | −0.13 | −0.13 | 100 | 20 | 0.09 | 0.10 |
| 9 | M | 21 | +4.5 DS +2.0 DC×70 | 0.78 | 0.50 | −0.13 | −0.15 | 4800 | 50 | 0.10 | 0.06 |
| 10 | F | 18 | +5.25 DS:+0.50 DC×75 | 0.38 | 0.18 | −0.03 | −0.13 | 160 | 40 | 0.30 | 0.37 |
| 11 | M | 14 | +2.75 DS +1.25 DC×60 | 0.88 | 0.63 | −0.03 | −0.03 | 100 | 40 | 0.05 | 0.08 |
| 12 | F | 13 | +0.75DS:+3.50DC×80 | 0.36 | 0.29 | −0.03 | −0.11 | 200 | 50 | 0.27 | 0.19 |
| 13 | M | 22 | +1.00DS | 0.11 | 0.03 | −0.03 | −0.03 | 50 | 50 | 0.22 | 0.21 |
| 14 | F | 16 | +3.50DS:+1.00DC×100 | 0.38 | 0.23 | −0.03 | −0.13 | 160 | 50 | 0.14 | 0.10 |
| 15 | M | 27 | +1.0 DS:+3.50 DC×85 | 0.63 | 0.40 | −0.03 | −0.03 | 4800 | 100 | 0.11 | 0.15 |
| 16 | F | 31 | +5.50DS:+0.75 DC×145 | 0.29 | 0.17 | −0.03 | −0.03 | 160 | 25 | 0.10 | 0.08 |
| 17 | M | 10 | +0.50DS:+0.75DC×170 | 0.19 | 0.07 | −0.03 | −0.03 | 40 | 20 | 0.32 | 0.33 |
| 18 | F | 10 | +5.50DS:+0.75DC×115 | 0.28 | 0.19 | −0.13 | −0.23 | 100 | 40 | 0.07 | 0.07 |
| 19 | M | 18 | +2.75DS:+0.50DC×95 | 0.18 | 0.07 | −0.03 | −0.05 | 63 | 40 | 0.35 | 0.42 |
balance point in binocular phase combination.
2.2. Apparatus
All stimuli were generated using a computer running Matlab 8.0 based on Psychtoolbox extensions 3.038, 39 and presented on a gamma-corrected Sony G220 color monitor (21 inch, P22 phosphor; Sony, Tokyo, Japan) with a spatial resolution of 1600×1200 pixels and a refresh rate of 85 Hz. A special circuit was used to produce 14-bit gray-level resolution53. The mean luminance of the display was 28.3 cd/m2. A chin rest was used to constrain head movements during the experiment. The stimuli were centrally displayed in a dimly light room at a distance of 1.38 m.
2.3. Battery of tests
The following tests were administered before and after monocular contrast sensitivity training at each individual subject’s cutoff spatial frequency.
2.3.1. Monocular visual acuity
Monocular visual acuity (VA) was measured in both the amblyopic and fellow eyes using a Chinese Tumbling E chart (decimal chart)54 and specified as LogMAR55. The untested eye was covered by an opaque patch.
2.3.2. Monocular and binocular contrast sensitivity functions (CSF)
We used the quick CSF56 procedure to measure the contrast sensitivity function in the amblyopic and fellow eyes, and under binocular viewing. The quick CSF method (qCSF) was developed by Lesmes et al. (2010) to accurately estimate contrast sensitivity function with greatly reduced testing times (e.g., 50 trials in a 2IFC task56). As shown in Figure 1, the quick CSF method characterized the CSF with a truncated log parabola function57–59: peak gain (CSmax), peak spatial frequency (fc), truncation in the low spatial frequencies (δ), and bandwidth (full-width at half-maximum, β). Different combinations of parameter values were assigned initial probabilities, creating a four-dimensional probability density function (pdf). The pdf was updated using Bayes rule based on subject’s response in each trial56, 60. The spatial frequency and contrast of the stimulus in the next trial is chosen from all possible combinations of spatial frequency and contrast conditions such that the expected outcome will result in greatest information gain on the pdf.
Figure 1.
CSF Parameterization. The contrast sensitivity function, which describes the reciprocal of contrast threshold as a function of spatial frequency, can be described by four parameters: (1) peak gain CSmax, (2) peak frequency fc, (3) bandwidth (fullwidth at half-maximum) β, and (4) truncation in low spatial frequencies δ. The quick CSF method rapidly estimates the CSF by directly estimating the posterior distribution of the four parameters.
In the current implementation of the quick CSF procedure, the stimuli were 2.5°×2.5° vertical sine-wave gratings. To minimize edge effects, a half-Gaussian ramp (σ=0.25°) was added to the edges of the gratings to blend them into the background. The test procedure was exactly the same as that described in previous researches56,56. Briefly, the stimulus space consisted of gratings with contrasts ranging from 0.1% to 99% in steps of 1.5 dB and spatial frequencies from 0.5 to 16 cycles per degree (c/deg) in steps of 3 dB. The estimated CSF was obtained after 100 quick CSF trials. Each trial consisted of an initial 294 ms fixation in the center of the display and two 153 ms stimulus intervals separated by an inter-stimulus interval (ISI) of 588 ms. A brief tone signaled the onset of each interval. The grating was only presented in one of the two intervals. Subjects were asked to indicate the interval that contained the grating using the computer keyboard. No feedback was provided.
Two indexes, the area under contrast sensitivity function (AUCSF) and cutoff spatial frequency (coSF), were derived from the estimated CSFs. AUCSF was calculated by integrating the CSF over spatial frequency from 0.5 to 16 c/deg; Cutoff frequency was defined as the spatial frequency that corresponds to a contrast sensitivity of 2.5 which corresponds to a contrast threshold of 0.40. Following tradition, we termed the ratio in the area under CSF (AUCSF) between the binocular and the fellow eye’s CSF’s as binocular summation ratio21, 61. Previous studies showed that the binocular summation ratio is about 1.4 in normal subjects61, and near 1.0 in patients with amblyopia62.
2.3.3. Stereoacuity
Stereoacuity was assessed with the Fly Stereo Acuity Test that consisted of 10 circles ranging from 400 to 20 arcsec (Fly Stereo Acuity Test; Vision Assessment Corporation, IL). We administered the tests according to the manufacture’s guidelines. Subjects looked directly at the test material at a viewing distance of 40 cm. They started the task with the easiest fly of 4800”, and moved to more and more difficult conditions. In the test, subjects were asked to report which circle was out of the plane of the other three (zero plane). After normal test procedure, the materials were rotated 90 degrees and displayed to subjects to ensure that the judgments were based on stereo perception.
2.3.4. Worth 4-dot test
The Worth 4-Dot test was used to assess sensory eye dominance. Subjects wore anaglyph glasses with the red filter over the right eye and the green filter over the left eye. They were instructed to fixate on the Worth 4-Dot target (diameter=8.5 mm; 2 green, 1 yellow, 1 red with equal luminance, with the yellow dot at the bottom) while it was held slightly below the participant’s line of sight at a distance of 33 cm. The eye dominance was qualitatively determined based on the perceived color of the yellow dot. Subjects were asked to first report the number of dots they perceived and then make a three-alternative, forced-choice decision about whether the bottom dot appeared yellow (equal dominance), red (right eye dominant), or green (left eye dominant). To minimize bias, we flipped the red/green anaglyph glasses and repeated the test. The dominance index was determined based on the scores in the two tests: 0 means equal dominance, 1 means partial dominance, and 2 means full dominance. The test took about 3~5 minute for each subject.
2.3.5. Binocular phase combination
To quantify binocular interaction between the two eyes, we adopted the suprathreshold cyclopean phase combination paradigm22, 63, 64. The test stimuli consisted of two monocular horizontal sine-wave gratings of 1.0 c/deg, each subtending 2×2 deg2 at a viewing distance of 1.38 m that differed in phase by 45 deg. The contrast of the grating in the amblyopic eye CA was fixed at 50%. The contrast of the grating in the fellow eye varied systematically, with interocular contrast ratio CF/CA∈[0, 0.1, 0.2, 0.4, 0.8, 1]. A stereo-scope was used to direct the two images to the two eyes. To assist binocular fusion, the grating in each eye was placed in the center of a large (6×6 deg2), high-contrast frame with clearly marked white diagonals (Figure 2). Subjects were asked to adjust the stereoscope to fuse the frames, the fixation crosses, and the monocular fixation dots in the beginning of the experiment. They were instructed to check fusion before each trial and only initiate the test when it was stable. A total of 96 trials were used in each test, allocated to the 12 conditions (6 interocular contrast ratios × 2 phase configurations) with 8 repetitions per condition.
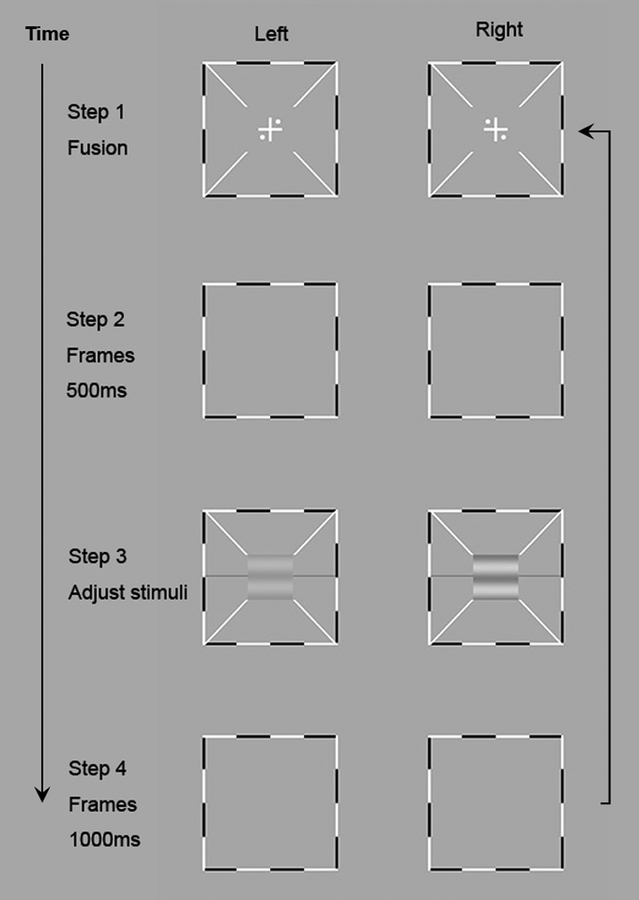
Figure 2.
Binocular phase combination procedure22. The left column shows the stimuli in the left eye and the right column shows the stimuli in the right eye. In step 1, subjects adjusted the two frames to fuse the images displayed to the left and right eyes. After perceiving one cross with four dots in the four quadrants, they could press a key to start the trial. A blank frame appeared for 500ms in Step 2. In Step 3 horizontal sine-wave gratings were presented to the two eyes and subjects adjusted the reference line to indicate the “darkest” part of the cyclopean grating. A blank frame was displayed for 1000 ms after the subject finished this trial (Step 4).
Each trial began with the presentation of the binocular fixation crosses, the high contrast frames, and the monocular fixation dots. This was followed by a 500 ms presentation of the frames, and then sine-wave gratings in the two eyes. Subjects were asked to adjust the location of the horizontal reference line to indicate the perceived phase of the cyclopean sine-wave grating, defined as the location of the center of the dark stripe of the grating, and press the “Enter” key after they finished the task. Each trial was followed by a 1-sec blank display. A typical trial lasted about 5 seconds.
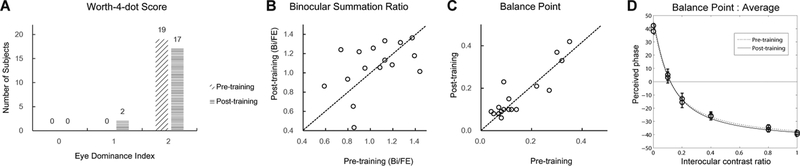
The procedure was used to generate the “PvC” curve, i.e., the perceived phase of the cyclopean grating versus the contrast ratio of the gratings in the two eyes (Figure 2c). Since gratings in the two eyes were in opposite phase (±22.5), a perceived 0 deg phase of the cyclopean grating signaled equal contributions from the two eyes during binocular combination. We thus defined the interocular contrast ratio at which the perceived phase was zero as the interocular balance point (IBP) of the subject. The lower the IBP is, the more severe the imbalance between the two eyes. A IBP of 0 indicates full dominance of the fellow eye and a IBP of 1 indicates perfect balance of the two eyes.
2.3.6. Binocular rivalry
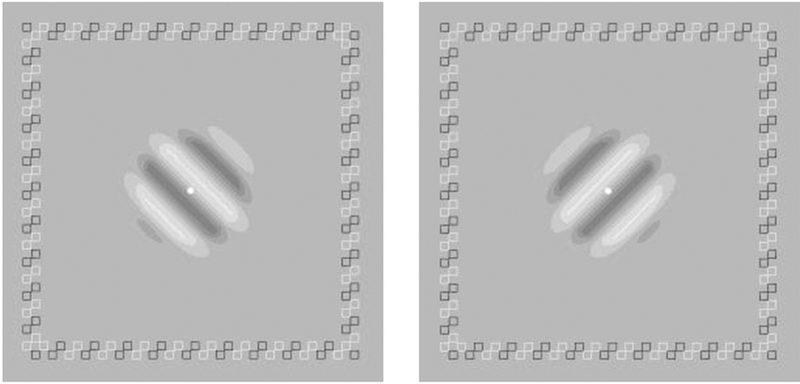
The rivalry stimuli consisted of two orthogonal sinewave gratings (±45 degrees) at 40% contrast, each subtending 3×3 deg2 (0.25 deg half-Gaussian ramp + 2.5 deg plateau), and viewed dichoptically through a stereo-scope. To help fuse the dichoptic gratings at corresponding retinal points in the two eyes, a high-contrast frame of small open squares was used (Figure 3). The center fixation dot (56.6 cd/m2) was displayed throughout the experiment. There were 8 trials, allocated to two frequencies, 1 c/deg and the cutoff spatial frequency before training. In each trial, subjects viewed the dichoptic gratings for 120 seconds and reported their dominant percept by pressing one of two keys to indicate the perceived grating orientation. The detailed time stamps of all dominant phases were recorded. The ratio between the total durations (collapsed across trials) of dominance of the amblyopic and fellow eyes was used to index the degree of rivalry between the two eyes. A dominance ratio of 0 means complete dominance of the fellow eye and 1 means equal dominance between the two eyes. The number of switches between the two eyes was also recorded. Trials for different spatial frequency conditions were intermixed.
Figure 3.
Stimuli used in binocular rivalry measurement.
2.5. Training
Each subject was trained monocularly in the amblyopic eye near his/her cutoff spatial frequency, defined as the spatial frequency at which the contrast threshold from the pre-training CSF measurement of the amblyopic eye was 0.4. Similar to the CSF test, each training trial started with an initial 294 ms fixation in the center of the display and followed by two 153 ms stimulus intervals separated by an inter-stimulus interval (ISI) of 588 ms. A brief tone signaled the onset of each interval. The grating was only presented in one of the two intervals. Subjects were asked to indicate the interval that contained the grating. An auditory beep followed each correct response. In each session, there were 630 trials, allocated in 7 blocks with 90 trials/block. Training lasted 6~10 sessions (6 sessions for 1 subject and 8~10 sessions for all other subjects), leading to a total of 3780~6300 trials. A 3-down 1-up staircase was used to keep subjects’ performance around 79.4% correct, in which three consecutive correct responses resulted in a 10% decrease of grating contrast (i.e. Ct+1=Ct×90%) and a single incorrect response resulted in a 10% contrast increase (i.e. Ct+1=Ct×110%).
3. Results
3.1. Monocular functions: contrast sensitivity and visual acuity
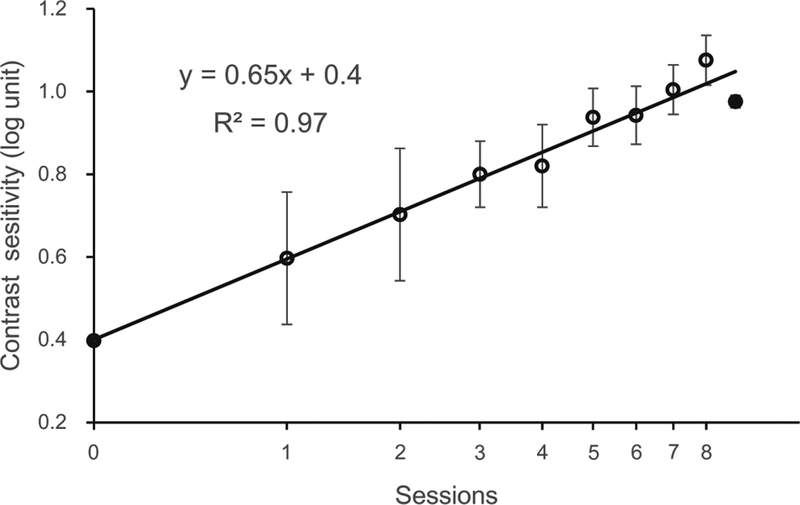
Training significantly improved contrast sensitivity at the trained spatial frequency in the amblyopic eye, from 2.5 to 9.46, an improvement of 278.4% (t(18)=18.85, p<0.01). For the average subject, the learning rate was 0.65 log10 contrast sensitivity per log10 session (R2=0.97, p<0.01).
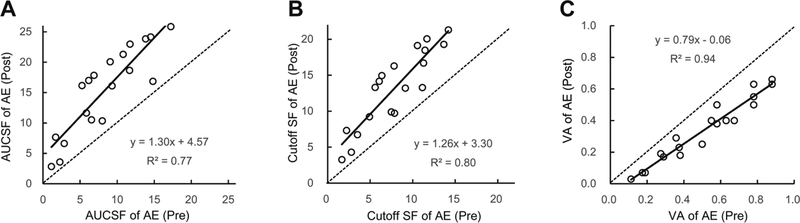
Training at the cutoff spatial frequency also significantly improved contrast sensitivity at other untrained spatial frequencies (F(1,5)=3.66, p<0.01) in the amblyopic eye. The Area Under CSF (AUCSF) increased from 8.41±1.09 (mean±S.E.) to 15.48±1.61 after training (t(18)=8.45, p<0.01). The cutoff spatial frequency also increased from 7.86±0.88 to 13.19±1.25 c/deg (t(18)=8.78, p<0.01).
Contrast sensitivity in the untrained fellow eye also increased (F(1,5)=2.22, p=0.05), with AUCSF increased from 21.67±2.2 to 25.29±1.72 (Figure 5A). The cutoff spatial frequency in the fellow eye didn’t change significantly (from 19.85±2.1 to 22.23±1.55 c/deg (Figure 5B), t(18)=1.99, p=0.06).
Figure 5.
AUCSF (A), cutoff spatial frequency (B), and logMAR visual acuity (C) before and after training.
The magnitude of AUCSF improvement was greater in the amblyopic eye than that in the fellow eye (F(1,18)=24.26, p<0.01), and there was a significant interaction between interocular improvement and training effect (F(1,8)=9.31, p<0.01). The magnitude of cutoff spatial frequency improvement was also greater in amblyopic eye than that in the fellow eye (F(1,18)=26.98, p<0.01) and the interaction also exist between interocular improvements and training effect (F(1,18)=7.28, p<0.01).
Consistent with previous studies using the cutoff spatial frequency training method40, 42, visual acuity in the amblyopic eye also significantly improved, from 0.51 (logMAR) to 0.34 (Figure 5C; t(18)=10.53, p<0.01) on average, an improvement of about 2 lines. Visual acuity in the fellow eye also significantly improved (though mild), from −0.05 logMAR to −0.08 (t(18)=3.36, p<0.01) on average. The regression line (r2 = 0.94, p < 0.01) had a slope of 0.79, indicating that the worse the initial acuity was, the greater the improvement41, 65.
3.2. Binocular functions
Stereoacuity.
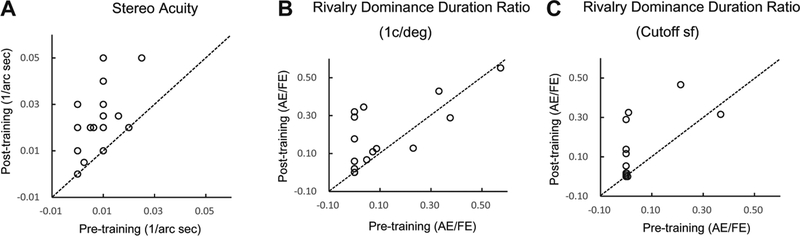
After about 8 days of monocular training in contrast detection in the amblyopic eyes, stereo threshold decreased remarkably from 929.11” to 80.42” (Figure 6A; t(18)=2.17, p<0.05).
Figure 6.
stereoacuity (A), rivalry dominance duration ratio at 1c/deg(B) and dominance duration ratio at cutoff spatial frequency (C);
Dominance Ratio in Binocular Rivalry.
Training also significantly increased the dominance duration ratio of the amblyopic eye at 1 c/deg (from 9% to 15%; p<0.05, Figure 6B) and the cutoff spatial frequency (from 3% to 9%, p<0.05, Figure 6C). Detailed analysis revealed that it was the average duration in each dominant phase of the amblyopic eye that was increased (from 1.14±0.36 s to 2.29±0.42 s at 1 c/deg, p<0.05, and from 0.57±0.28 s to 1.64±0.44 s at the cutoff spatial frequency, p<0.05); training did not significantly increase the number of dominant phase of the amblyopic eye (21.74±10.06 vs 21.42±5.04 at 1 c/deg, p>0.1; 8.89±6.17 vs 13.68±4.51 at cutoff spatial frequency, p>0.1).
Worth 4-dot Test.
The Worth 4-dot test scores didn’t improve significantly (Figure 7A, p=0.29, Pearson’s chi-square test). Only two subjects demonstrated a trend for change—their fellow eyes showed full dominance in pre-test (score of 2) and partial dominance in the post-test (score of 1).
Figure 7.
Worth 4-dot scores(A), pre- and post-training binocular summation ratios(B), interocular balance point(C), and the average phase vs contrast ratio curve in phase combination(D).
Summation Ratio and Interocular Balance Point.
Training did not significantly improve binocular summation ratio (1.16 vs 1.11, p>0.1; Figure 7B), nor the interocular balance point (IBP) in binocular phase combination (0.14 vs 0.15, p>0.10; Figure 7C).
3.3. Correlations
Two monocular functions, visual acuity and contrast sensitivity, and two binocular functions, dominance ratio in binocular rivalry and stereoacuity, improved after intensive monocular training. We evaluated the relationships between the magnitudes of these improvements.
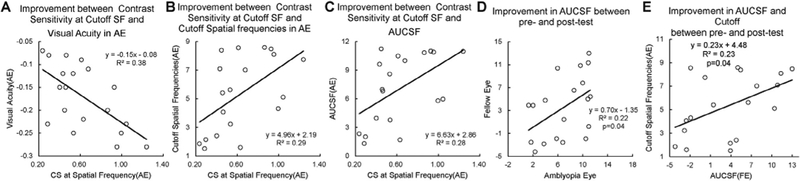
Significant correlations were found only between the magnitudes of contrast sensitivity improvement at the cutoff spatial frequency (i.e., training frequency) in the amblyopic eye and improvement in visual acuity, cutoff spatial frequency, AUCSF in the amblyopic eye (Figures 8ABC); the magnitudes of AUCSF improvements in the amblyopic and fellow eyes (Figure 8D), and between the magnitudes of AUCSF improvements and the cutoff spatial frequency improvements in the amblyopic eye (Figure 8E). None of the other correlations was significant (Table 2). The results suggest that monocular and binocular improvements following intensive monocular training were not correlated, consistent with several reports in the literature30, 47, 48.
Figure 8.
Correlation between the magnitudes of contrast sensitivity improvement at the cutoff spatial frequency and the visual acuity improvement in the amblyopic eye (A), the magnitudes of contrast sensitivity improvement at the cutoff spatial frequency and the cutoff spatial frequency improvements in the amblyopic eye (B), the magnitudes of contrast sensitivity improvement at the cutoff spatial frequency and the magnitudes of AUCSF improvements in the amblyopic eye (C), the magnitudes of AUCSF improvements in the amblyopic and fellow eyes (D), the magnitudes of AUCSF improvements and the cutoff spatial frequency improvements in the amblyopic eye(E)
Table 2.
Correlation between improvements in different measures
| VA_AE | VA_FE | Cutoff SF | CutSF CS | AUCSF | Stereopsis | BP | Rivalry Duration (RD) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AE | FE | Bi | AE | FE | Bi | 1 c/deg | Cutoff | ||||||
| VA_AE | |||||||||||||
| VA_FE | 0.259(0.284) | ||||||||||||
| Cutoff SF_AE | 0.106(0.666) | 0.332(0.164) | |||||||||||
| Cutoff SF_FE | 0.208(0.392) | 0.004(0.986) | 0.414(0.078) | ||||||||||
| Cutoff SF_Bi | 0.049(0.843) | 0.153(0.531) | 0.144(0.556) | 0.043(0.861) | |||||||||
| CutSF CS | 0.620(0.005) ** | 0.075(0.760) | 0.543(0.016) * | 0.283(0.241) | 0.024(0.922) | ||||||||
| AUCSF_AE | 0.069(0.778) | 0.315(0.189) | 0.982(<0.01) ** | 0.407(0.084) | 0.082(0.738) | 0.527(0.021) * | |||||||
| AUCSF_FE | 0.08(0.744) | 0.055(0.822) | 0.483(0.036) * | 0.964(<0.01) ** | 0.138(0.547) | 0.254(0.294) | 0.47(0.043)* | ||||||
| AUCSF_Bi | 0.075(0.759) | 0.104(0.670) | 0.248 (0.306) | 0.051(0.837) | 0.954(<0.01) ** | 0.027(0.912) | 0.181(0.458) | 0.160(0.512) | |||||
| Stereopsis | 0.005(0.984) | 0.202(0.407) | 0.032(0.896) | 0.26(0.283) | 0.175(0.475) | 0.087(0.725) | 0.012(0.962) | 0.213(0.381) | 0.262(0.278) | ||||
| BP | 0.113(0.645) | 0.135(0.581) | 0.309(0.199) | 0.267(0.27) | 0.235(0.333) | 0.059(0.811) | 0.278(0.248) | 0.213(0.382) | 0.119(0.627) | 0.014(0.955) | |||
| RD_1 c/deg | 0.053(0.829) | 0.342(0.152) | 0.121(0.622) | 0.075(0.76) | 0.121(0.621) | 0.310(0.196) | 0.131(0.592) | 0.025(0.918) | 0.19(0.436) | 0.234(0.336) | 0.411(0.08) | ||
| RD_cutoff | 0.056(0.82) | 0.039(0.874) | 0.278(0.249) | 0.385(0.103) | 0.251(0.3) | 0.506(0.027) | 0.25(0.303) | 0.433(0.064) | 0.238(0.327) | 0.233(0.336) | 0.337(0.158) | 0.437(0.061) | |
Pearson correlation were performed and was expressed as “R(p)”.
Correlation is significant;
highly significant.
VA_AE: Visual acuity of previous amblyopic eye;
VA_FE: Visual acuity of previous fellow eye;
Cutoff SF_AE: Cutoff spatial frequency of amblyopic eye;
Cutoff SF_FE: Cutoff spatial frequency of fellow eye;
Cutoff SF_Bi: Cutoff spatial frequency of binocular test;
CutSF CS: Contrast sensitivity at cutoff spatial frequency of amblyopic eye;
AUCSF_AE: Area under contrast sensitivity function of amblyopic eye;
AUCSF_FE: Area under contrast sensitivity function of fellow eye;
AUCSF_Bi: Area under contrast sensitivity function of binocular test;
Stereopsis: Stereoacuity in Titmus Fly test;
BP: Interocular balance point in binocular phase combination;
RD_1 c/deg: Binocular rivalry duration of amblyopic eye at 1c/deg;
RD_cutoff: Binocular rivalry duration of amblyopic eye at cutoff spatial frequency.
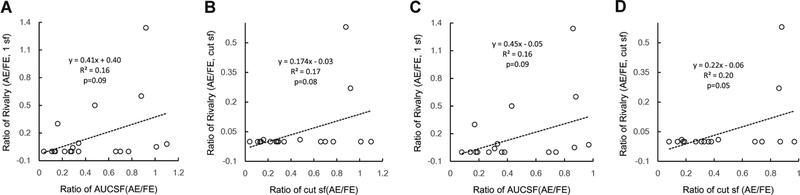
We also compared AE/FE ratios in CSF (AUCSF and cut sf) and rivalry tests before training. The ratios of AUCSF (AE/FE) and rivalry dominant duration (AE/FE) were marginally significant correlation (0.05<p<0.1, Figure 9), and so were the ratios of cutoff spatial frequencies (AE/FE) and rivalry dominant duration (AE/FE).
Figure 9.
Correlation between ratios of AUCSF (AE/FE) and rivalry dominant duration (AE/FE)(A,C); ratios of cutoff spatial frequencies (AE/FE) and rivalry dominant duration (AE/FE)(B,D)
4. Discussion
In the current study, we found that monocular contrast detection training near individual’s cutoff spatial frequency in the amblyopic eye significantly improved its contrast sensitivity and visual acuity and dominance duration during binocular rivalry. It also improved steroacuity. However, the training did not significantly improve binocular summation and the interocular balance point of binocular phase combination. The finding that monocular training is only beneficial to some but not all binocular functions may reflect limitations of monocular perceptual learning in recovering visual functions in anisometropic amblyopia. It is also possible that the recovery of different visual functions may require different amount of training66–69; since our monocular training procedure lasted only eight days, it would be interesting to evaluate whether there is extra benefit of extended monocular training on a range of visual functions, especially those without significant improvement following eight days of training.
We didn’t find significant correlation among the magnitudes of improvements in visual acuity of the amblyopic eye, its dominance duration in binocular rivalry, and stereoacuity. The results are consistent with previous findings, including no significant correlations between improved visual acuity and stereoacuity47, between decrease interocular suppression and improved stereopsis45, 46, and between improved dichoptic Gabor resolution and improvements of visual acuity or stereopsis48. Significant correlation was only found between indexes derived from the same tests, e.g., improvements in AUCSF and cutoff spatial frequency. These findings suggest that recovery of different visual functions may require different types of treatment; structured monocular and binocular training with a range of tasks are necessary to treat amblyopia.
Why is monocular training effective in improving stereoacuity and increasing the dominance of the amblyopic eye during binocular rivalry but not in increasing the contribution of the amblyopic eye in binocular phase combination? Stereoacuity threshold was found to be inversely proportional to the product of the square root of contrast energy in the two eyes70, 71. In this vein, improvement of contrast sensitivity may lead to enhanced contrast energy in the amblyopic eye and thus lower the stereo threshold. Dominance duration and/or switch rate may be closely related to the contrasts of the images in the two eyes. Previous studies found that binocular rivalry of two incompatible contours strongly depended on the contrasts of the two contours; the completeness of rivalry and the amount of exclusive visibility usually increased with the contrasts of rivaling targets72, 73. It’s interesting to note that we also found marginally significant correlation between ratios of AUCSF (AE/FE) and rivalry dominant duration (AE/FE), ratios of cutoff spatial frequencies (AE/FE), and rivalry dominant duration (AE/FE) (all 0.05<p<.01, Figure 9) before training, indicating monocular mechanism(s) may have prominent roles in binocular rivalry. It’s thus possible that improved monocular contrast sensitivity elevated the dominance of the amblyopic eye in binocular rivalry. On the other hand, both monocular (e.g. attenuation) and binocular (e.g. interocular inhibition) mechanisms contribute to deficient binocular phase summation in amblyopia and more importantly, abnormal inhibition from the fellow eye to the amblyopic eye is the more dominant factor than attenuation of the signal in the amblyopic eye. Although monocular training led to improved contrast signals in the amblyopic eye, it may not significantly affect the stronger inhibition from the fellow eye to the amblyopic eye.
Our results are inconsistent with Chen et al. (2016), who found a modest (0.43±0.21 to 0.57±0.22) improvement of interocular balance point (IBP), and a significant correlation between the magnitudes of AUCSF and IBP improvements following monocular contrast detection training in the amblyopic eye49. Although visual acuity in the amblyopic eye was comparable in the two studies, the extent of interocular imbalance was much lower in ours’ (VA: 0.51±0.06 vs 0.48±0.26 LogMAR; IBP: 0.43± 0.21 vs 0.14±0.02). The 8–10 sessions’ monocular training might work better for amblyopic subject with more balanced eyes. Whether more extensive training can improve IBP in amblyopes with more severely imbalanced eyes remained to be investigated.
In dichoptic amblyopia training paradigms, two different images are presented to the two eyes simultaneously, using a stereoscope, a pair of red–green glasses, a head-mounted video display like the Oculus Rift, or an iPad, often with the stimuli projected to amblyopic eye cued or at a higher contrast, and stimuli projected to fellow eye blurred or with a lower contrast30, 47, 51, 69. The paradigms are designed to reduce interocular suppression69, 74, or re-establish stereopsis47, 75 and visual acuity76. However, binocular training paradigms may not completely replace other therapies including traditional patching therapy and monocular training because studies have found that a given training method, whether monocular or dichoptic, could only improve a certain limited range of visual functions and there was no significant correlation between improvements of monocular and binocular visual functions following a single procedure47, 51, 75.
To conclude, monocular perceptual learning is effective in improving a range of monocular functions and some but not all binocular functions. Structured monocular and binocular functions are required to cover a full range of visual functions in amblyopia treatment.
Figure 4.
Average learning curve. The first and last data points (filled circles) were derived from pre-training and post-training CSF measurements, respectively. Data from the training phase are represented by open circles. The number of training sessions varied between observers, from 6 to 10 (9.2 ± 1.1 SD) sessions (only one observer trained for 6 sessions). We illustrated eight common sessions for all but one subjects here. Data were fitted with a linear function with a slope of 0.65 and r2 of 0.97 (p < 0.01). Error bars represent SEM.
Acknowledgments
Supported by National Natural Science Foundation of China (NSFC 31230032 and 31470983), the Knowledge Innovation Program of the Chinese Academy of Sciences (Y3CX102003), Institute of Psychology, CAS to Chang-Bing Huang, and NEI (EY021553, EY017491) to Zhong-Lin Lu, and The Guangxi Zhuang Autonomous Region Health and Family Planning Commission Foundation (Z2013336 and Z2016600) to Wuxiao Zhao.
Footnotes
Financial disclosure
ZLL owns intellectual property rights on the qCSF technology. ZLL has equity interest in Adaptive Sensory Technology, Inc. ZLL, and CBH have equity interest in Jiangsu Juehua Medical Technology Co., LTD.
References
- 1.Levi DM, Knill DC, Bavelier D. Stereopsis and amblyopia: A mini-review. Vision research 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciuffreda KJ, Levi DM, Selenow A. Amblyopia: Basic and clinical aspects: Stoneham, MA: Butterworth-Heinemann; 1991. [Google Scholar]
- 3.McKean-Cowdin R, Cotter SA, Tarczy-Hornoch K, et al. Prevalence of amblyopia or strabismus in asian and non-Hispanic white preschool children: multi-ethnic pediatric eye disease study. Ophthalmology 2013;120:2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gstalder R, Green D. Laser interferometric acuity in amblyopia. Journal of Pediatric Ophthalmology 1971;8:251–256. [Google Scholar]
- 5.Hess R, Campbell F, Greenhalgh T. On the nature of the neural abnormality in human amblyopia; neural aberrations and neural sensitivity loss. Pflügers Archiv 1978;377:201–207. [DOI] [PubMed] [Google Scholar]
- 6.Levi DM, Klein S. Hyperacuity and amblyopia. Nature 1982;298:268–270. [DOI] [PubMed] [Google Scholar]
- 7.Bradley A, Freeman R. Contrast sensitivity in anisometropic amblyopia. Investigative ophthalmology & visual science 1981;21:467–476. [PubMed] [Google Scholar]
- 8.Levi D, Harwerth RS. Spatio-temporal interactions in anisometropic and strabismic amblyopia. Investigative ophthalmology & visual science 1977;16:90–95. [PubMed] [Google Scholar]
- 9.Bedell HE, Flom MC. Monocular spatial distortion in strabismic amblyopia. Invest Ophthalmol Visual Sci 1981. [PubMed] [Google Scholar]
- 10.Polat U, Sagi D, Norcia AM. Abnormal Long-range Spatial Interactions in Amblyopia. Vision research 1997;Vol. 37. [DOI] [PubMed] [Google Scholar]
- 11.Baker DH, Meese TS, Hess RF. Contrast masking in strabismic amblyopia: attenuation, noise, interocular suppression and binocular summation. Vision research 2008;48:1625–1640. [DOI] [PubMed] [Google Scholar]
- 12.Levi DM, Klein SA. Sampling in spatial vision. Nature 1986;320:360–362. [DOI] [PubMed] [Google Scholar]
- 13.Hess RF, Wang Y-Z, Demanins R, Wilson HR. A deficit in strabismic amblyopia for global shape detection. Vision research 1999;39. [DOI] [PubMed] [Google Scholar]
- 14.Roelfsema PR, Konig P, Engel AK, Sireteanu R, Singer W. Reduced synchronization in the visual cortex of cats with strabismic amblyopia. The European journal of neuroscience 1994;6:1645–1655. [DOI] [PubMed] [Google Scholar]
- 15.Huang C, Tao L, Zhou Y, Lu ZL. Treated amblyopes remain deficient in spatial vision: a contrast sensitivity and external noise study. Vision research 2007;47:22–34. [DOI] [PubMed] [Google Scholar]
- 16.Levi DM, Klein SA. Noise provides some new signals about the spatial vision of amblyopes. The Journal of neuroscience : the official journal of the Society for Neuroscience 2003;23:2522–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrad R, Hess R. Binocular integration of contrast information in amblyopia. Vision research 1992;32:2135–2150. [DOI] [PubMed] [Google Scholar]
- 18.Harwerth RS, Levi DM. Psychophysical studies on the binocular processes of amblyopes. Am J Optom Physiol Opt 1983;60:454–463. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell DE, Kind PC, Sengpiel F, Murphy K. Brief daily periods of binocular vision prevent deprivation-induced acuity loss. Current biology : CB 2003;13:1704–1708. [DOI] [PubMed] [Google Scholar]
- 20.Smith SL, Trachtenberg JT. Experience-dependent binocular competition in the visual cortex begins at eye opening. Nature neuroscience 2007;10:370–375. [DOI] [PubMed] [Google Scholar]
- 21.Baker DH, Meese TS, Mansouri B, Hess RF. Binocular summation of contrast remains intact in strabismic amblyopia. Investigative ophthalmology & visual science 2007;48:5332–5338. [DOI] [PubMed] [Google Scholar]
- 22.Huang CB, Zhou J, Lu ZL, Feng L, Zhou Y. Binocular combination in anisometropic amblyopia. Journal of vision 2009;9:17 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lema SA, Blake R. Binocular summation in normal and stereoblind humans. Vision research 1977;17:691–695. [DOI] [PubMed] [Google Scholar]
- 24.Levi DM, Harwerth RS, Manny RE. Suprathreshold spatial frequency detection and binocular interaction in strabismic and anisometropic amblyopia. Investigative ophthalmology & visual science 1979;18:714–725. [PubMed] [Google Scholar]
- 25.Lee SY, Isenberg SJ. The relationship between stereopsis and visual acuity after occlusion therapy for amblyopia. Ophthalmology 2003;110:2088–2092. [DOI] [PubMed] [Google Scholar]
- 26.Kiorpes L, McKee SP. Neural mechanisms underlying amblyopia. Current opinion in neurobiology 1999. [DOI] [PubMed] [Google Scholar]
- 27.McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. Journal of vision 2003;3:380–405. [DOI] [PubMed] [Google Scholar]
- 28.Weakley DR. The association between nonstrabismic anisometropia, amblyopia, and subnormal binocularity. Ophthalmology 2001;108:163–171. [DOI] [PubMed] [Google Scholar]
- 29.Brooks SE, Johnson D, Fischer N. Anisometropia and binocularity. Ophthalmology 1996;103:1139–1143. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Spiegel DP, Hess RF, et al. Dichoptic training improves contrast sensitivity in adults with amblyopia. Vision research 2015. [DOI] [PubMed] [Google Scholar]
- 31.Huang C-B, Zhou J, Zhou Y, Lu Z-L. Contrast and Phase Combination in Binocular Vision. PloS one 2010;Volume 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding J, Levi DM. Rebalancing binocular vision in amblyopia. Ophthalmic and Physiological Optics 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loudon S, Simonsz H. The history of the treatment of amblyopia. Strabismus 2005;13:93–106. [DOI] [PubMed] [Google Scholar]
- 34.Group PEDI. Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Archives of ophthalmology 2005;123:149. [DOI] [PubMed] [Google Scholar]
- 35.Yang S, Wu DZ, Tian N, Wu L. CONTRAST SENSITIVITY IN AMBLYOPIA. Yan ke xue bao = Eye science / “Yan ke xue bao” bian ji bu 1991;7:25–28. [PubMed] [Google Scholar]
- 36.Suttle CM, Melmoth DR, Finlay AL, Sloper JJ, Grant S. Eye–hand coordination skills in children with and without amblyopia. Investigative ophthalmology & visual science 2011;52:1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levi DM, Polat U. Neural plasticity in adults with amblyopia. PNAS 1996;93:6830–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levi DM, Polat U, Hu Y-S. Improvement in Vernier acuity in adults with amblyopia. Practice makes better. IOVS 1997;38. [PubMed] [Google Scholar]
- 39.Bonneh YS, Sagi D, Polat U. Local and non-local deficits in amblyopia: acuity and spatial interactions. Vision research 2004;44:3099–3110. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Y, Huang C, Xu P, et al. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision research 2006;46:739–750. [DOI] [PubMed] [Google Scholar]
- 41.Polat U Restoration of underdeveloped cortical functions: evidence from treatment of adult amblyopia. Restorative neurology and neuroscience 2008;26:413–424. [PubMed] [Google Scholar]
- 42.Zhang J-Y, Cong L-J, Levi DM, Klein SA, Yu C. Perceptual learning improves adult amblyopic vision through rule-based cognitive compensation. Investigative ophthalmology & visual science 2014;IOVS-13–13739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vedamurthy I, Nahum M, Huang SJ, et al. A dichoptic custom-made action video game as a treatment for adult amblyopia. Vision research 2015;114:173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hess RF, Mansouri B, Thompson B. A new binocular approach to the treatment of amblyopia in adults well beyond the critical period of visual development. Restorative neurology and neuroscience 2010;28:793–802. [DOI] [PubMed] [Google Scholar]
- 45.Hess RF, Thompson B. Amblyopia and the binocular approach to its therapy. Vision research 2015. [DOI] [PubMed] [Google Scholar]
- 46.Hess RF, Babu RJ, Clavagnier S, Black J, Bobier W, Thompson B. The iPod binocular home‐based treatment for amblyopia in adults: efficacy and compliance. Clinical and Experimental Optometry 2014;97:389–398. [DOI] [PubMed] [Google Scholar]
- 47.Xi J, Jia W, Feng L, Lu Z-L, Huang C-B. Perceptual Learning Improves Stereoacuity in Amblyopia. Investigative ophthalmology & visual science 2014;IOVS-13–12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vedamurthy I, Nahum M, Bavelier D, Levi DM. Mechanisms of recovery of visual function in adult amblyopia through a tailored action video game. Sci Rep 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Z, Li J, Liu J, et al. Monocular perceptual learning of contrast detection facilitates binocular combination in adults with anisometropic amblyopia. Scientific reports 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Thompson B, Deng D, Chan LY, Yu M, Hess RF. Dichoptic training enables the adult amblyopic brain to learn. Current biology : CB 2013;23:R308–309. [DOI] [PubMed] [Google Scholar]
- 51.Vedamurthy I, Knill DC, Huang SJ, et al. Recovering stereo vision by squashing virtual bugs in a virtual reality environment. 2016;371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li SL, Reynaud A, Hess RF, et al. Binocular movie treatment of amblyopia improves visual acuity in children. Journal of American Association for Pediatric Ophthalmology and Strabismus 2015;19:e14. [Google Scholar]
- 53.Li X, Lu Z-L, Xu P, Jin J, Zhou Y. Generating high gray-level resolution monochrome displays with conventional computer graphics cards and color monitors. Journal of neuroscience methods 2003;130:9–18. [DOI] [PubMed] [Google Scholar]
- 54.Mou T Logarithmic visual acuity chart and five-score recording. Chinese Journal of Ophthalmology 1966;13:96–106. [Google Scholar]
- 55.Jia W, Zhou J, Lu ZL, Lesmes LA, Huang CB. Discriminating anisometropic amblyopia from myopia based on interocular inhibition. Vision research 2015;114:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lesmes LA, Lu ZL, Baek J, Albright TD. Bayesian adaptive estimation of the contrast sensitivity function: the quick CSF method. Journal of vision 2010;10:17 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watson AB, Ahumada AJ. A standard model for foveal detection of spatial contrast. Journal of Vision 2005;5:717–740. [DOI] [PubMed] [Google Scholar]
- 58.Hou F, Huang CB, Lesmes L, et al. qCSF in clinical application: efficient characterization and classification of contrast sensitivity functions in amblyopia. Invest Ophthalmol Vis Sci 2010;51:5365–5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lesmes LA, Lu Z-L, Baek J, Albright TD. Bayesian adaptive estimation of the contrast sensitivity function: The quick CSF method. Journal of Vision 2010;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kontsevich LL, Tyler CW. Bayesian adaptive estimation of psychometric slope and threshold. Vision research 1999;39:2729–2737. [DOI] [PubMed] [Google Scholar]
- 61.Campbell F, Green D. Monocular versus binocular visual acuity. 1965. [DOI] [PubMed]
- 62.Pardhan S, Gilchrist J. Binocular contrast summation and inhibition in amblyopia. The influence of the interocular difference on binocular contrast sensitivity. Documenta ophthalmologica Advances in ophthalmology 1992;82:239–248. [DOI] [PubMed] [Google Scholar]
- 63.Ding J, Sperling G. A gain-control theory of binocular combination. Proceedings of the National Academy of Sciences of the United States of America 2006;103:1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang C-B, Zhou J, Lu Z-L, Zhou Y. Deficient binocular combination reveals mechanisms of anisometropic amblyopia: Signal attenuation and interocular inhibition. Journal of vision 2011;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.The Pediatric Eye Disease Investigator G. The course of moderate amblyopia treated with atropine in children: experience of the amblyopia treatment study. American journal of ophthalmology 2003;136:630–639. [DOI] [PubMed] [Google Scholar]
- 66.Levi DM, Li RW. Perceptual learning as a potential treatment for amblyopia: a mini-review. Vision research 2009;49:2535–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hess RF, Thompson B, Baker DH. Binocular vision in amblyopia: structure, suppression and plasticity. Ophthalmic and Physiological Optics 2014;34:146–162. [DOI] [PubMed] [Google Scholar]
- 68.Polat U, Ma-Naim T, Belkin M, Sagi D. Improving vision in adult amblyopia by perceptual learning. Proceedings of the National Academy of Sciences of the United States of America 2004;101:6692–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ooi TL, Su YR, Natale DM, He ZJ. A push-pull treatment for strengthening the ‘lazy eye’ in amblyopia. Current biology : CB 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hou F, Lu ZL, Huang CB. The external noise normalized gain profile of spatial vision. Journal of vision 2014;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pelli DG, Farell B. Why use noise? Journal of the Optical Society of America A, Optics, image science, and vision 1999;16:647–653. [DOI] [PubMed] [Google Scholar]
- 72.Whittle P Binocular rivalry and the contrast at contours. Quarterly Journal of Experimental Psychology 1965;17:217–226. [DOI] [PubMed] [Google Scholar]
- 73.Hollins M The effect of contrast on the completeness of binocular rivalry suppression. Perception & Psychophysics 1980;27:550–556. [DOI] [PubMed] [Google Scholar]
- 74.Noah S, Bayliss J, Vedamurthy I, Nahum M, Levi D, Bavelier D. Comparing dichoptic action video game play to patching in adults with amblyopia. Journal of vision 2014;14:691–691. [Google Scholar]
- 75.Ding J, Levi DM. Recovery of stereopsis through perceptual learning in human adults with abnormal binocular vision. Proceedings of the National Academy of Sciences 2011;108:E733–E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li SL, Reynaud A, Hess RF, et al. Dichoptic movie viewing treats childhood amblyopia. Journal of American Association for Pediatric Ophthalmology and Strabismus 2015;19:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]