Abstract
Background/Objective:
We investigated interhemispheric interactions in stroke survivors by measuring TMS-evoked cortical coherence. We tested the effect of TMS on interhemispheric coherence during rest and active muscle contraction and compared coherence in stroke and older adults. We evaluated the relationships between interhemispheric coherence, paretic motor function, and the ipsilateral cortical silent period (iSP).
Methods:
Participants with (n=19) and without (n=14) chronic stroke either rested or maintained a contraction of the ipsilateral hand muscle during simultaneous recordings of evoked responses to TMS of the ipsilesional/nondominant (i/ndM1) and contralesional/dominant (c/dM1) primary motor cortex with EEG and in the hand muscle with EMG. We calculated pre and post-TMS interhemispheric beta coherence (15–30Hz) between motor areas in both conditions and the ipsilateral silent period (iSP) duration during the active condition.
Results.
During active i/ndM1 TMS, interhemispheric coherence increased immediately following TMS in controls but not in stroke. Coherence during active cM1 TMS was greater than iM1 TMS in the stroke group. Coherence during active iM1 TMS was less in stroke participants and was negatively associated with measures of paretic arm motor function. Paretic iSP was longer compared to controls and negatively associated with clinical measures of manual dexterity. There was no relationship between coherence and. iSP for either group. No within or between-group differences in coherence were observed at rest.
Conclusions.
TMS-evoked cortical coherence during hand muscle activation can index interhemispheric interactions associated with post-stroke motor function and potentially offer new insights into neural mechanisms influencing functional recovery.
Keywords: Interhemispheric inhibition, transcallosal inhibition, transcranial magnetic stimulation, electroencephalography, stroke, GABA
Introduction
The extent of motor recovery following stroke is influenced by both direct loss of neurons and widespread neural network reorganization both local and distant to the site of the lesion.1,2 Post-stroke motor recovery may be influenced by atypical levels of interhemispheric inhibition between the lesioned and non-lesioned motor cortices resulting from a disruption of transcallosal neural network communication.3,4 However, our limited understanding of the complex neural mechanisms underlying stroke recovery may explain, in part, why post-stroke rehabilitation approaches aimed at normalizing interhemispheric imbalance, such as those using therapeutic non-invasive brain stimulation, have been ineffective in reducing post-stroke impairment.5
Following stroke, changes in structural neuroanatomy and cortical activity patterns may occur in regions remote from the lesion in both the ipsilesional and contralesional hemisphere, implicating the role of expansive cortical networks in the recovery process.1,2 In contrast to unilateral hemisphere activation observed in healthy controls, bilateral activation of the ipsilesional primary motor cortex (iM1) and contralesional primary motor cortex (cM1) is often observed during paretic hand movement in stroke survivors and has been associated with poorer motor function.6–8 Stronger bilateral activation of motor cortical areas is correlated with the structural integrity of the corpus callosum and poor motor performance after stroke.7 Findings from these neuroimaging studies support the notion that interhemispheric interactions between neural networks via transcallosal pathways play an important role in post-stroke motor function.
Though interhemispheric interactions between neuronal networks in iM1 and cM1 have been implicated in post-stroke motor recovery, measuring functional interhemispheric interactions directly is difficult. Previously, the primarily inhibitory9 interhemispheric interactions between M1s have been assessed indirectly using electromyography (EMG) responses in arm and hand muscles elicited by transcranial magnetic stimulation (TMS) using either a paired-pulse paradigm (two TMS coils overlying M1s)10 or a single-pulse paradigm during sustained muscle contraction in the target muscle ipsilateral to the site of TMS.11 In the single-pulse paradigm, TMS over the ipsilateral M1 generates a brief interruption in volitional muscle activity, the ipsilateral cortical silent period (iSP), that is thought to reflect interhemispheric inhibition from the ipsilateral to the contralateral M112 and mediated by inhibitory GABAA (type A γ-aminobutyric acid) and GABAB receptor activity.13 After stroke, greater magnitude and duration of iSP in the paretic limb has been observed4,14,15 that was negatively correlated with motor function, suggesting greater interhemispheric inhibition from cM1 to iM1 in the most severely impaired individuals.16 Increased interhemispheric inhibition from iM1 to cM1 is positively correlated with improved motor performance following bilateral upper limb rehabilitation.17 Together, these findings suggest that excessive levels of inhibition from cM1 to iM1 coupled with reduced inhibition from iM1 to cM1 may impede post-stroke motor recovery.11 Despite the conflicting findings of other studies,16,18 the interhemispheric competition model of stroke recovery has been commonly used as a theoretical framework for the development of rehabilitation strategies, including the use of noninvasive brain stimulation in attempt to upregulate iM1or downregulate cM1 in stroke survivors.19,20 However, the often conflicting findings of these therapeutic paradigms used in interventional studies are minimally effective and limited progress has been made to reduce upper limb motor disability for stroke survivors.5
Non-invasive evaluation of cortical neuronal network reactivity to TMS using electroencephalography (EEG) recordings at the level of the scalp to measure evoked cortical responses is now possible.21 Concurrent TMS-EEG permits a more direct probe into neural network connectivity at highly specific time-scales, thus overcoming some of the previous technological limitations of interhemispheric connectivity measures (e.g. EMG MEPs and iSPs, fMRI, dynamic causal modeling) and potentially bridging the gap in our understanding of the functional role of post-stroke interhemispheric interactions. Additionally, cortical recordings can be obtained from individuals with a limited or absent corticospinal tract and lacking peripheral motor responses to TMS. Because structural reserve within the lesioned hemisphere may be an important factor in the functionality of post-stroke interhemispheric imbalance to recovery,22 the use of direct cortical recordings may offer important insights into the neural recovery mechanisms of these individuals who are often the most severely impaired.
Coherence is a common EEG measure used to study neural interactions between brain regions and to characterize synchronous oscillatory neural network activity.23,24 Neural network activity in the beta frequency band (15–30Hz) is closely tied to sensorimotor behavior and appears to be directly controlled by GABAergic activity; thus coherence in the beta frequency provides an index for inhibitory neural interactions.25,26 Similar to EMG measures of the iSP, EEG measures of interhemispheric coherence between M1s in the beta frequency band are thought to reflect, in part, GABAergic activity associated with interhemispheric inhibition required for skilled movement.9 After stroke, desynchronization of beta frequency range activity typically observed in neurologically-intact participants was reduced post-stroke during paretic hand muscle activation.27 EEG coherence within iM1 may provide a useful biomarker of upper limb motor recovery28 and response to rehabilitation.29 The addition of TMS to EEG paradigms could infer unique information regarding the directionality of cortical network connectivity through local and remote evoked cortical responses. Such information could be particularly useful to enhance the clinical translation of post-stroke neurophysiologic research.
Recent findings from our group demonstrated the feasibility of using concurrent TMS-EEG in stroke30 by showing that interhemispheric coherence between iM1 and cM1 was greater in a small sample of chronic stroke survivors compared to controls when the hand muscle was active.31 However, whether TMS offers a unique contribution to assessments of interhemispheric coherence using resting state EEG alone has not been investigated. Additionally, the relationship between TMS-evoked interhemispheric coherence, motor function and iSP measures after stroke is unclear. The purposes of this study were to: 1) test the effect of TMS on measures of interhemispheric coherence in stroke and age-matched neurologically-intact controls; 2) investigate the relationship between interhemispheric coherence and paretic arm and hand motor behavior in stroke survivors; and 3) evaluate the relationship between interhemispheric coherence and EMG measures of iSP.
Methods
Nineteen individuals (age: 66±11years, 8 females) with chronic stroke (> 6 mo.) and 14 neurologically-intact age-matched individuals (age: 53±14years, 6 females) participated in the single-session, cross-sectional study (Table 1). All participants were between the ages of 44–81 years old. Participants with stroke had a single ischemic cortical or subcortical stroke confirmed by magnetic resonance imaging (MRI). Participants were excluded if they had hemorrhagic stroke, a stroke directly affecting the upper extremity region of the primary motor cortex or the corpus callosum, history of multiple strokes, neurodegenerative disorder or psychiatric diagnosis, or contraindications to TMS (Supplemental FigureS-1).32 All participants gave written informed consent and the experimental protocol was approved by the Emory University Institutional Review Board.
Table 1:
Stroke participant characteristics.
| ID | Gender | Age (y) | PSD (mo) | iM1 | Lesion Location | FM score (/66) | iM1 RMT (% MSO) | cM1 RMT (% MSO) | iM1 iSP | cM1 iSP |
|---|---|---|---|---|---|---|---|---|---|---|
| S01 | M | 81 | 35 | L | MCA | 61 | 42 | 47 | + | + |
| S02 | M | 73 | 33 | R | MCA | 58 | 40 | 37 | + | − |
| S03 | F | 62 | 145 | L | IC | 61 | 61 | 44 | + | + |
| S04 | M | 54 | 35 | R | IC, CR | 14 | 801 | 62 | − | − |
| S05 | M | 68 | 54 | L | Pontine | 58 | 46 | 45 | + | + |
| S06 | M | 66 | 33 | L | Pontine | 61 | 32 | 31 | + | + |
| S07 | M | 81 | 35 | R | MCA | 35 | 44 | 41 | + | − |
| S08 | F | 66 | 31 | R | IC, striatum | 62 | 62 | 59 | + | + |
| S09 | F | 64 | 42 | R | ACA | 58 | 55 | 50 | + | + |
| S10 | M | 66 | 133 | L | BG | 66 | 38 | 38 | + | + |
| S11 | F | 76 | 54 | R | BG | 54 | 681 | 56 | − | − |
| S12 | M | 59 | 18 | R | Medulla | 24 | >1002 | 74 | − | − |
| S13 | F | 46 | 30 | L | BG, IC | 62 | 49 | 39 | + | + |
| S14 | M | 75 | 12 | R | Thalamus, cerebral | 54 | 69 | 70 | + | + |
| S15 | F | 65 | 18 | L | peduncles, BG Parietal | 65 | 34 | 40 | + | + |
| S16 | M | 73 | 9 | R | IC/BG | 45 | 64 | 54 | + | + |
| S17 | M | 49 | 8 | L | BG | 20 | >1002 | 60 | + | − |
| S18 | F | 48 | 11 | R | IC/BG | 60 | 79 | 71 | + | + |
| S19 | F | 74 | 14 | R | IC/BG | 40 | 66 | 77 | + | + |
| F = 8 | 66 ± 11 | 45 ± 36 | LH=8 | 60 ± 20a | 53 ± 14 | |||||
RMT: resting motor threshold; iM1: ipsilesional M1; cM1: contralesional M1; MSO: maximal stimulator output; PSD: post-stroke duration; MCA: middle cerebral artery; ACA: anterior cerebral artery; IC: internal capsule; CR: corona radiata; BG: basal ganglia; +, iSP present; -, iSP absent; n/a, not assessed.
Group values are expressed as means ± SD.
Mean RMT values include an RMT of 100% for S12 and S20 due to an inability to elicit an MEP at maximum stimulator output with active muscle contraction.
Ipsilateral active motor thresholds (AMTs) were used to determine stimulating intensities when RMT was not determinable.
Contralesional 110% RMT was used to determine stimulating intensities, as AMT was not determinable.
Assessment of Motor Behavior
Upper extremity motor behavior was assessed using the Fugl-Meyer Assessment (UE-FM) and the Wolf Motor Function Test (WFMT).33 Additionally, hand grip strength was assessed using a hand grip dynamometer. Hand motor dexterity was assessed using the Nine Hole Peg Test (NHPT). The WMFT and NHPT were both converted to a rate indicating how many times the participant could complete the task in 60 seconds. The stroke group underwent clinical assessments at the beginning of the session by a licensed physical therapist.
TMS Experimental Procedures
Single monophasic TMS pulses (Magstim 2002, MagStim, Wales, UK) were delivered though a figure-of-eight coil (70mm). Participants were fitted with earplugs and seated upright in an arm chair with both hands resting on a table. Stereotactic neuronavigation software (BrainSight®, Rogue Research Inc.) was used to identify the target location of the abductor pollicis brevis (APB) muscle representation within the ipsilesional/nondominant(i/nd) and contralesional/dominant(c/d)M1. A high-resolution T1 anatomical MRI image (TR=7.4ms,TE=3.7ms,flip angle θ=6°,FOV=256mm,160 slices,1mm thickness) was collected prior to the testing session and used to guide TMS delivery. At the beginning of the session, the optimal site for eliciting a motor evoked potential (MEP) in the contralateral APB muscle was determined using standard procedures and used for navigated TMS during the session.34 Resting motor threshold (RMT) was determined bilaterally using standard procedures.34
TMS-evoked EEG responses were assessed during resting and active motor states. During the resting assessment, participants kept both hands at rest while the experimenters monitored real-time bilateral APB EMG activity. During the active assessment, participants maintained a volitional contraction at 50% of their maximal force output using a handheld dynamometer in the hand ipsilateral to the site of TMS.14 At each assessment, 50 TMS pulses were delivered over the M1 APB location at a jittered rate of 0.1 to 0.25 Hz. TMS intensities were set at 120% and 150% RMT for rest and active conditions, respectively.14
Assessment of TMS-evoked cortical responses.
During each assessment, EEG signals (sampling rate 5kHZ, filter range DC to 1000Hz) were continuously recorded using a 32-channel TMS-compatible electrode cap (Easy Cap, Brain Products, Germany) connected to a BrainAmp DC amplifier (Brain Products, GmbH). Signals were recorded using Recorder software (Brain Products, GmbH) and saved for offline analysis. The reference channel location was chosen as the FCz electrode position and the ground was in the AFz position. An additional electrode was attached to the infraorbital area of the right eye and used to record eye blinks. Prior to assessments, impedance levels were lowered to ≤10 kΩ for all channels and checked periodically to maintain this impedance level.
Assessment of EMG corticomotor excitability measures.
EMG activity was recorded from bilateral APB muscles during each assessment. Two disposable conductive adhesive hydrogel electrodes were attached over each APB muscle and a ground electrode was placed over the dorsum of each hand following standard preparation procedures. BrainSight software (v. 2.2.14) was used to visualize and record EMG signals using a 2-channel EMG device (Rogue Research Inc., Canada).
Data reduction and analysis
Quantification of interhemispheric coherence.
All EEG data were pre-processed in EEGLAB.35 This software was used to epoch (−1000 to 4000ms relative to TMS) and re-reference (linked to FCz electrode position) all data. Interhemispheric coherence was calculated as the imaginary part of coherency (IPC)36 value between electrodes overlying the scalp location of M1 of each hemisphere (left: C3, right: C4) within the beta frequency range (15–30Hz) pre (−1000–0ms) and post (0–300ms) TMS. While coherence measures the linear dependency of two signals at a specific frequency, IPC requires a time-lag in the evaluation of these signals and reflects true interaction.36 Utilizing IPC minimizes overestimation biases that artificially influence coherence from volume conduction and other artifacts (stimulation, movement, etc.). IPC is a particularly useful approach to evaluate TMS perturbation-evoked EEG signals due to the insensitivity to instantaneous sources of artifact, such as that from the TMS. Perturbation-evoked interhemispheric beta IPC values were calculated during rest and active conditions and for each hemisphere of TMS delivery for all participants. IPC analyses were performed using custom MATLAB routines.36
Quantification of the iSP.
The iSP was determined using EMG data from each participant during the active iM1 and cM1 conditions. Ipsilateral APB muscle activity was rectified and averaged over 50 trials. The mean pre-stimulus EMG activity over 50 milliseconds prior to TMS (mean pre-TMS EMG) was calculated and used to normalize the EMG data and in defining the iSP duration. iSP onset was defined as the post-stimulus point at which EMG activity dropped lower than 2 standard deviations below the mean pre-TMS EMG and remained so for at least 2 milliseconds (ms).37 iSP offset was defined as the time at which the post stimulus EMG activity resumed its mean pre-TMS EMG activity for at least 2ms; iSP duration was then calculated as the time point of the iSP offset minus that of the iSP onset.37 Study team members were blinded to group and lesion location during data processing and analysis.
Statistical analysis
All clinical motor behavior, coherence, iSP and data were tested for normality and homogeneity of variance using Kolmogorov-Smirnov and Levene’s tests, respectively. Parametric testing procedures were used if the data met assumptions of normality and homogeneity of variance as indicated by a nonsignificant test result. All analyses were performed using Statistical Package for Social Sciences (Chicago, IL) version 24 with a critical α level set to 0.05. For all significant interactions and main effects, Bonferroni post-hoc testing was performed. Groups were matched for stimulation hemisphere (ipsilesionalM1/non-dominantM1 and contralesionalM1/dominantM1). To test the effect of TMS on interhemispheric coherence, two separate 2×2 (group-by-time) mixed-design analyses of variance (ANOVAs) were performed during i/ndM1 and c/dM1 TMS in both the active and rest conditions. Two-way mixed ANOVAs were performed to test if interhemispheric coherence and iSP duration for each group differed between hemisphere (i/ndM1 vs. c/dM1) during the active and rest condition. The relationships between interhemispheric coherence and iSP duration versus paretic arm motor behavior and relationships between interhemispheric coherence and iSP for each limb and corresponding hemisphere of stimulation were tested using Pearson product-moment correlation coefficients.
Results
Complete datasets of EEG coherence measures were collected for all participants in the control group. During the active condition in the stroke group, complete data sets were obtained for 18 of 19 participants; one participant trial (S11 cM1 TMS) had excessive (>50ms duration) TMS artifact in the EEG recording (C3 and C4 electrodes) and was subsequently discarded from all active condition coherence analyses. During the resting condition in the stroke group, complete data sets were obtained from 16 of 19 participants; two participants (S01 and S03) did not complete testing during the resting condition due to length of the testing session; three participants in the stroke group were discarded from all resting IPC analyses due to prolonged TMS artifact resolution (>50ms duration) in the EEG signal during iM1 (S04) and cM1 (S08 and S11) TMS.
Complete datasets of EMG iSP measures were calculated in 12 of 14 total control participants; a suppression of EMG activity was not present during ndM1 stimulation (C01) and dM1 stimulation (C02), and these participants were removed from the iSP analysis. Bilateral iSP measures were present in 13 of 19 stroke participants; the presence of an iSP could not be detected for 6 participants in the paretic hand (cM1 TMS) (S02, S04, S07, S11, S12, S20) and 3 participants in the nonparetic hand (iM1 TMS) (S04, S11, S12). Participants for whom iSP could not be calculated were discarded from iSP analyses. All data met assumptions of normality and homogeneity of variance.
Effect of TMS on interhemispheric coherence.
Comparison of pre- and post-TMS coherence during the active i/ndM1 TMS condition showed a group-by-time interaction (F1,31=6.00, p=.020), where coherence increased immediately following TMS in controls (p=.0006) but not in stroke (p=.15) (Figure 1A). Between group comparisons showed that coherence was greater in controls post-TMS (p=.004) but not pre-TMS (p=.27). During the rest i/ndM1 TMS condition, no interaction (F1,28=1.22, p=.278) or main effect of time (F1,28=1.67, p=.206) was observed. During both the active and rest c/dM1 TMS conditions, no interactions were observed (active: F1,30=0.33, p=.568; rest: F1,27=0.08, p=.777), but there was a main effect of time with post-TMS greater than pre-TMS coherence (active: F1,30=41.72, p<.0001; rest: F1,27=17.12, p<.0001) (active condition shown in Figure 1B).
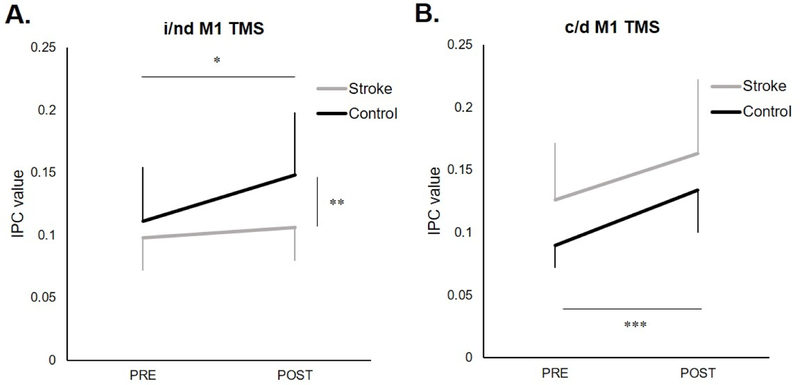
Figure 1.
Coherence values (mean ± SD) during the active condition (A) ipsilesional (i)/nondominant (nd) M1 and (B) contralesional (c)/dominant (d) M1 TMS at pre and post time points relative to TMS. (A) During ndM1 TMS in controls, coherence increased immediately following TMS (*p=.0006). During iM1 TMS in stroke, coherence was not increased following stimulation(p=.15). While there was no difference in coherence between groups before TMS (p=.27), coherence was greater in controls post-TMS (**p=.004). (B) During c/d M1 TMS there was an increase in coherence immediately following TMS in both groups (***p<.0001).
Between-group and hemisphere comparisons of interhemispheric coherence during muscle contraction.
When testing the effects of group and hemisphere of stimulation during the active condition, we observed a significant group-by-hemisphere interaction (F1,30=8.06, p=.008). Post hoc analyses revealed that coherence during cM1 TMS was greater than iM1 TMS in the stroke group (p=.007) while there was no between-hemisphere difference in the control group (p=.18). When comparing coherence between groups there was lower coherence during iM1 TMS in the stroke compared to ndM1 TMS in the controls (p=.004) (Figure 2). There was no difference in coherence between cM1 TMS in the stroke compared to dM1 TMS in the controls (p=.11). During the rest condition, no interaction effects (F1,26=.11, p=.949) or main effects of group (F1,26=2.155, p=.154) or hemisphere (F1,26=2.16, p=.154) were observed.
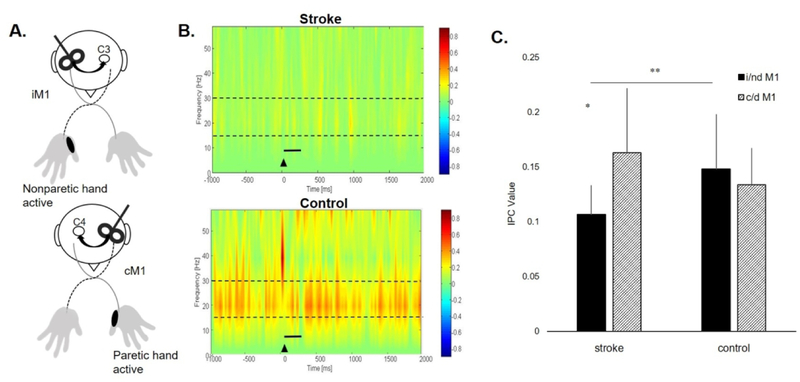
Figure 2.
(A) Experimental paradigm for the active condition during ipsilesional (i)M1 and contralesional (c)M1 TMS. TMS was delivered to the motor hotspot of the APB muscle contralateral to the site of stimulation while the APB muscle ipsilateral to the site of stimulation maintained a consistent contraction at 50% maximal volitional isometric contraction. iM1 and cM1 TMS were matched to nondominant M1 and dominant M1 TMS in the control group. (B) Time-frequency plot during the active iM1 TMS condition showing greater TMS-evoked interhemispheric beta IPC values (warmer colors) in a representative participant post-stroke (top) and a control participant (bottom). Black triangle denotes TMS onset. Broken lines indicate the frequency range (15–30Hz) of interest. Solid black line represents time bin of data (0–300ms) used for analysis. (C) Coherence values (mean ± SD) for i/nd M1 and c/d M1 TMS in stroke and control groups during the active condition. There was a significant group-by-hemisphere interaction (p=.008). There was greater coherence during cM1 TMS compared to iM1 TMS in the stroke group (*p=.007). There was lower coherence during iM1 TMS in the stroke group compared to ndM1 TMS in controls (**p=.004).
Associations between interhemispheric coherence and motor behavior.
During the active iM1 TMS condition, we observed a negative relationship between coherence and UE-FM score (r=−0.674, p=.0002), paretic WMFT rate (r=−.61, p=.0045), and paretic grip strength (r=−0.68, p=.0009) (Figure 3A-D). For the participants in the stroke group who could complete the NHPT with the paretic hand, there was no relationship between active iM1 coherence and NHPT rate of the paretic limb (Figure 3E). During the active cM1 TMS condition, no significant relationships were detected between coherence and UE-FM, WMFT score or paretic NHPT rate. There was a positive relationship between coherence and paretic grip strength (r=0.52, p=.022) during cM1 TMS; however, this relationship appeared to be driven by a single participant. Pre-TMS coherence was negatively associated with UE-FM (r=−0.53, p=.0196) during iM1 TMS; pre-TMS coherence during iM1 and cM1 TMS were not associated with any other clinical measure (see Supplemental Table 2).
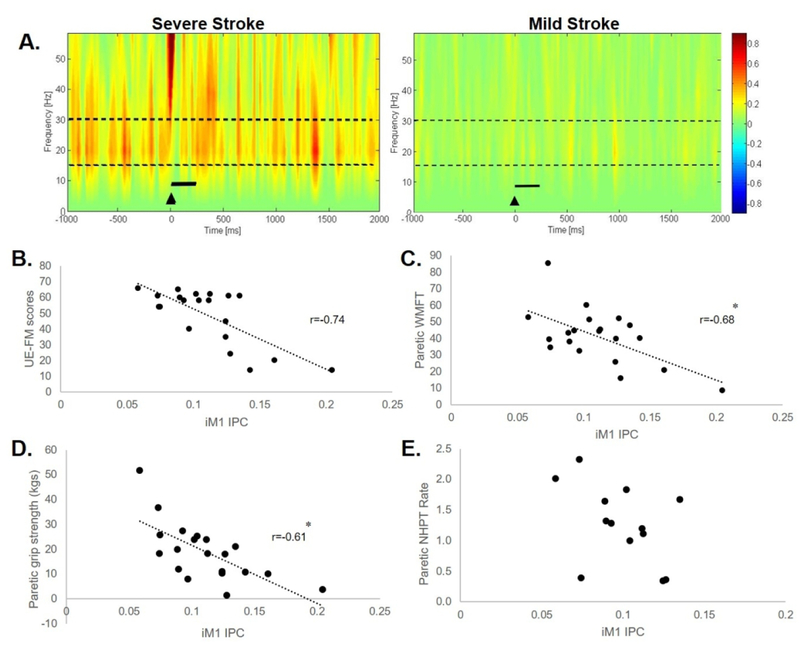
Figure 3.
(A) Time-Frequency plots during the active iM1 TMS condition showing greater TMS-evoked interhemispheric beta IPC values (warmer colors) in a participant post-stroke with severe arm impairment (S12, UE-FM score=24) compared to a participant with mild arm impairment (S14, UE-FM score: 54). Black triangle indicates TMS onset. Broken lines indicate the frequency band range (15–30Hz) of interest. Solid black line represents bin of data (0–300ms) used for analysis. During the active iM1 condition, relationships between IPC versus (B) UE-FM score, (C) paretic WMFT, (D) paretic grip strength, and (E) paretic NHPT rate in the stroke group. There was a negative relationship between IPC values vs. UE-FM (r=−0.74, p=.0002), paretic WMFT rate (r=−.61, p=.0045), and paretic grip strength (r=−0.68, p=.0009). *denotes statistical significance (p<.05).
Between-group and limb comparisons of iSP duration and magnitude.
When testing the effects of group (stroke vs. control) and limb (paretic/nondominant vs nonparetic/dominant) during the active condition on iSP measures, there was a main effect of group; individuals with stroke showed longer iSP duration compared to controls (F1,54 =6.11, p=0.02). No effect of limb was observed (F1,54 =0.56, p=0.46), nor was there a significant group-by-limb interaction (F1,54=0.02, p=.90) (Figure 4).
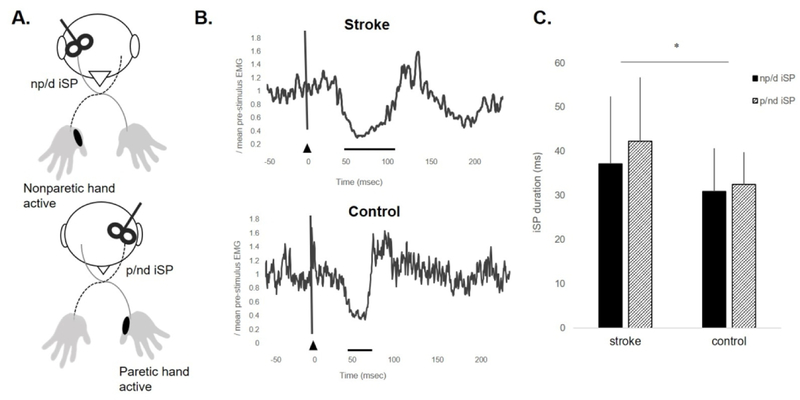
Figure 4.
(A) Experimental paradigm during ipsilateral cortical silent period (iSP) data acquisition for the nonparetic (np)/dominant (d) hand and paretic (p)/nondominant (nd) hand. TMS was delivered to the motor hotspot of the APB muscle contralateral to the site of stimulation while the APB muscle ipsilateral to the site of stimulation maintained a consistent contraction at 50% maximal volitional isometric contraction. (B) Electromyographic (EMG) activity representing the paretic iSP of a participant in the stroke group (S13, iSP duration=62.3ms) and the nondominant iSP of a participant in the control group (iSP duration=28.97ms). Black triangle indicates TMS onset. Solid black line represents iSP duration. (C) Mean±SD iSP duration for the np/d and p/nd hand in stroke and control groups. Individuals post-stroke showed longer iSP durations compared to controls (*p=.02) regardless of limb.
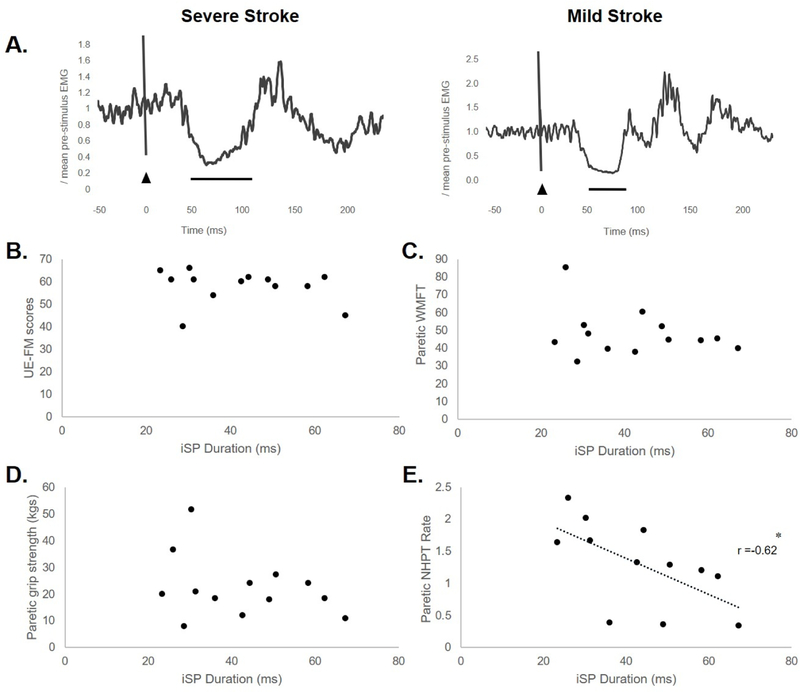
Associations between iSP duration and motor behavior.
There were no significant relationships between paretic iSP duration and UE-FM score (r=−0.19, p=.52), paretic grip strength (r=−0.31, p=.29), or paretic WMFT (r=−.26, p=.31) (Figure 5B-D). A negative relationship between paretic iSP duration vs. paretic NHPT rate (r=−0.62, p=.03) was observed (Figure 5A and E). During the active iM1 TMS condition, no significant relationships were detected between nonparetic iSP duration and nonparetic NHPT rate (r=−.45, p=.11), UE-FM score (r=−0.02, p=.94), nonparetic WMFT score (r=.14, p=.60) or nonparetic grip strength (r=.22, p=.41) (Supplemental Table 2).
Figure 5.
(A) Electromyographic activity representing the paretic iSP of a severely affected participant (S13, NHPT rate = 1.11, iSP duration=62.3ms) and a mildly affected participant (S10, NHPT rate = 2.01, iSP duration=30.3ms) in the stroke group. Black triangle indicates TMS onset. Solid black line represents iSP duration. Relationships between paretic iSP duration versus (B) UE-FM score, (C) paretic WMFT, (D) paretic grip strength, and (E) paretic NHPT rate in the stroke group. There was a negative relationship between iSP duration paretic NHPT rate (r=−0.62, *p=.03).
Associations between interhemispheric coherence and iSP.
During the active nd/iM1 TMS condition, there was no relationship between coherence and iSP duration in the nonparetic/dominant or in the paretic/nondominant hand for either group (stroke nonparetic r=.08, p=.77; paretic r=−.22, p=.40) (control dominant r=.17, p=.56; nondominant r=−.10, p=.73).
Discussion
Here we provide novel evidence that TMS-evoked measures of interhemispheric interactions between ipsilesional and contralesional motor cortices during motor activity are atypical and negatively associated with paretic hand motor behavior. Our findings show that the typical increase in interhemispheric coherence occurring immediately following TMS in neurologically-intact controls and the cM1 in stroke was not observed following iM1 stimulation in the stroke group. This observation supports the addition of TMS to assessments of cortical connectivity as a unique contribution to enhance our understanding of neural connectivity post-stroke. Interestingly, interhemispheric coherence measures were not associated with transcallosal inhibition assessed with the EMG-based iSP during the same task. This finding suggests there may be distinct neuromechanistic contributions to interhemispheric interactions indexed by interhemispheric coherence and iSP. Further supporting this concept, each measure was associated with different clinical measures of paretic arm motor behavior. Thus, TMS-evoked EEG and EMG measures of interhemispheric interactions may reflect unique neural mechanisms contributing to different aspects of abnormal post-stroke paretic arm and hand motor behavior.
Interhemispheric interactions were dependent on motor activity, as differences between groups and relationships to post-stroke clinical function were observed only during muscle contraction but not at rest. Beta frequency oscillations have cortical generators in M1, appear to be directly controlled by inhibitory GABAergic neuronal activity,38 and are modulated by motor state.39,40 In our previous work, differences in amplitudes and latencies of cortical TMS-evoked-potentials between stroke and controls were observed during both active and rest conditions,30 whereas, here we observed group differences in interhemispheric connectivity and relationships to clinical function measures only during an active motor state. Additionally, we observed interactions between stroke and control groups before and immediately following TMS during the active condition but not at rest. Here, higher interhemispheric beta coherence in the most severely-impaired stroke survivors may suggest increased communication between iM1 and cM1 during sustained motor activity38,41–43 likely reflecting post-stroke neural network reorganization.21 This finding is also consistent with previous studies demonstrating atypical modulation of interhemispheric inhibition during volitional motor activity after stroke3,31 and the presence of bilateral cortical activation patterns during motor activity in the most severely affected stroke survivors.6–8 Although causality is difficult to determine, bilateral cortical activation patterns in these individuals may reflect stronger interhemispheric GABAergic interactions engaged during muscle activation triggered by lesion-induced neural dysfunction within the lesioned hemisphere.44,45 Together, these results inform future studies that the state of the motor system represents an important consideration when assessing measures of functional cortical connectivity and may even influence neuromodulatory effects of noninvasive brain stimulation paradigms.46
Our findings, for the first time, revealed that higher TMS-evoked interhemispheric beta coherence during sustained ipsilateral nonparetic hand muscle contraction was associated with greater impairment of paretic arm motor behavior in stroke survivors. While decreased interhemispheric inhibition from cM1 to iM1 may occur with recovery of function following rehabilitation,47 others have found no relationship between change in interhemispheric inhibition and post-stroke motor function48,49 or even showed greater cM1 activity with the recovery of motor function after stroke.50 In support of the latter finding, when the activity in cM1 is suppressed, stroke survivors with severe impairment show poorer reaction times51 and animals with large lesions lose the ability to perform a reaching task.52 Interestingly, when comparing coherence between groups and hemispheres, our results imply that lower interhemispheric inhibition was present during iM1 TMS in stroke; this was a result of the lack of typical increase in interhemispheric interactions that occurred post-TMS with muscle contraction in cM1 and controls (Figure 1A). Further, stroke survivors with lower post-TMS interhemispheric coherence showed higher levels of paretic arm motor function (Figure 3). These collective findings suggest that this atypical reduction of interhemispheric inhibition potentially serves a compensatory role that may support better post-stroke motor function. Future studies investigating the effect of interhemispheric beta coherence modulation through rehabilitation on changes in motor function in stroke survivors will help elucidate the contribution of interhemispheric interactions to post-stroke motor recovery.
Findings from this study indicate TMS-evoked cortical responses may augment information available with standalone TMS and EEG and potentially index unique features of reactivity and connectivity in the corticomotor system not previously available. Our findings support findings of previous studies implicating the functional role of cortical connectivity in post-stroke motor recovery.14,28,29 Additionally the concurrent TMS-EEG paradigm may also provide insight into the directionality of interhemispheric interactions and distinct hemispheric contributions to cortical connectivity strength. Coherence during iM1 but not cM1 TMS was negatively associated with post-stroke motor behavior suggesting that neural excitability and connectivity of iM1 may be the primary contributor to persistent upper limb motor impairment after stroke. This notion is in agreement with previous literature.53 Associations between interhemispheric coherence and motor behavior generally did not occur in the absence of TMS, which may further support iM1 connectivity as the origin for atypical interhemispheric interactions after stroke. Additionally, structural reserve of the lesioned hemisphere and corticospinal tract may influence the strength of interhemispheric connectivity54 and whether interhemispheric inhibition plays a maladaptive or functional role over the course of post-stroke motor recovery.22 This possibility may be supported by the data distribution of interhemispheric coherence and motor impairment in Figure 3B; the negative correlation is driven primarily by individuals with UE-FM scores <50. Given that iSPs could not be elicited and quantified in participants primarily with more severe impairment (Table 1), using TMS-evoked cortical responses provided an opportunity to evaluate interhemispheric interactions across a wider range of post-stroke motor disability. Categorizing patients according to impairment level may further clarify different functional roles of cortical reorganization patterns salient to motor recovery in individuals post-stroke. Improved understanding of the mechanisms of cortical connectivity offered by multimodal neuroimaging approaches such as TMS-EEG could be important for the development of more effective post-stroke treatments, particularly when developing therapeutic noninvasive brain stimulation paradigms. These paradigms include cortico-cortical paired associative stimulation to strengthen or weaken transcallosal pathway connectivity.55
Interhemispheric coherence was not associated with iSP suggesting that distinct neural mechanisms contribute to each of these measures. Interestingly, our previous study found that latencies of early TMS-evoked potentials correlated with the contralateral silent period duration in the paretic hand of stroke survivors. Differences between these findings and those of the present study could be because local TMS-evoked cortical responses and the contralateral SP reflect the excitability of similar intracortical neural circuits while interhemispheric coherence and iSP reflect different neural mechanisms of inhibition. Likewise, our findings show that interhemispheric coherence was associated with overall paretic arm motor behavior (UE-FM, grip strength, WMFT) but not with fine motor control (paretic NHPT rate) (Figure 3); however the iSP was negatively associated with fine motor control but not with overall paretic arm motor behavior (Figure 5). This dichotomy supports the notion that distinct neural mechanisms of interhemispheric interactions are reflected in each of these measures and are uniquely associated with different clinical features of post-stroke paretic arm and hand motor behavior. The iSP reflects neural activity of not only cortical but also subcortical sources including rubrospinal projections involved in fine motor control of the hand.56 The influence of such subcortical pathways could explain iSP as an index of fine motor skill function in stroke survivors. Given its lack of muscle specificity, interhemispheric coherence measured with EEG may serve as a better index of interhemispheric contributions to overall upper limb motor behavior after stroke. We propose that interhemispheric coherence and the ipsilateral silent period are complimentary measures of interhemispheric interactions that can be used in tandem to understand the contributions of typical and atypical interhemispheric communication to normal and dysfunctional motor control.
Limitations
There are several limitations to the present study that could influence the interpretation of the observed results. Given the limited sample size, we were unable to stratify the heterogeneous stroke cohort based on lesion location and demographic characteristics. Because iSP is associated with mid-callosal volume,57 future studies could employ measures of transcallosal anatomy to further characterize brain structure-function relationships mediating stroke recovery. Limited spatial resolution of scalp-based EEG recordings restricts the interpretation of the cortical generators contributing to the interhemispheric coherence measures evaluated in the present study. Nonetheless, there is evidence to indicate the primary motor cortex as a main generator of cortical activity recorded in the electrodes of interest (C3/C4) and sensorimotor cortical activity is likely the primary source of beta oscillatory activity.58,59 TMS-evoked potentials possess cortical and peripheral-evoked components;60 thus we cannot rule out the influence of peripheral contributions (e.g. somatosensory and auditory potentials) to coherence measures. In the present study design, the possibility that differences observed in the active condition were influenced by the higher stimulation intensities should also be considered.
Conclusions
TMS-evoked cortical coherence during hand muscle activation may advance findings from previous investigations of post-stroke interhemispheric interactions and offer new insights into neural network connectivity that inform and extend our previous understanding of the neural mechanisms underlying post-stroke arm and hand motor function. The findings of this study support future investigations exploring the effect of rehabilitation on TMS-evoked interhemispheric coherence and coincident changes in paretic limb motor function. Further research utilizing multi-modal approaches should inform models of post-stroke recovery that have implications for the clinical prognosis and rehabilitation of persistent upper limb motor impairment in stroke survivors.
Supplementary Material
Acknowledgments
We would like to acknowledge Kamal Shadi for assisting in data analyses. This research was supported by the American Heart Association [AHA00035638] and the Eunice Kennedy Shriver National Institutes of Child Health & Human Development of the National Institutes of Health [K12HD055931].
References
- 1.Takatsuru Y, Fukumoto D, Yoshitomo M, Nemoto T, Tsukada H, Nabekura J. Neuronal circuit remodeling in the contralateral cortical hemisphere during functional recovery from cerebral infarction. J Neurosci 2009;29(32):10081–10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohajerani MH, Aminoltejari K, Murphy TH. Targeted mini-strokes produce changes in interhemispheric sensory signal processing that are indicative of disinhibition within minutes. Proc Natl Acad Sci U S A 2011;108(22):E183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 2004;55(3):400–409. [DOI] [PubMed] [Google Scholar]
- 4.Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage 2005;28(4):940–946. [DOI] [PubMed] [Google Scholar]
- 5.Hao Z, Wang D, Zeng Y, Liu M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst Rev 2013;5:CD008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol 2008;63(2):236–246. [DOI] [PubMed] [Google Scholar]
- 7.Wang LE, Tittgemeyer M, Imperati D, et al. Degeneration of corpus callosum and recovery of motor function after stroke: A multimodal magnetic resonance imaging study. Hum Brain Mapp 2012;33(12):2941–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 2002;125(Pt 12):2731–2742. [DOI] [PubMed] [Google Scholar]
- 9.Irlbacher K, Brocke J, Mechow JV, Brandt SA. Effects of GABA(A) and GABA(B) agonists on interhemispheric inhibition in man. Clin Neurophysiol 2007;118(2):308–316. [DOI] [PubMed] [Google Scholar]
- 10.Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol 1992;453:525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertolucci F, Chisari C, Fregni F. The potential dual role of transcallosal inhibition in post-stroke motor recovery. Restor Neurol Neurosci 2018;36(1):83–97. [DOI] [PubMed] [Google Scholar]
- 12.Correa F, Gauberti M, Parcq J, et al. Tissue plasminogen activator prevents white matter damage following stroke. The Journal of Experimental Medicine 2011;208(6):1229–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziemann U, Reis J, Schwenkreis P, et al. TMS and drugs revisited 2014. Clin Neurophysiol 2015;126(10):1847–1868. [DOI] [PubMed] [Google Scholar]
- 14.Mang CS, Borich MR, Brodie SM, et al. Diffusion imaging and transcranial magnetic stimulation assessment of transcallosal pathways in chronic stroke. Clinical Neurophysiology 2015;126(10):1959–71. [DOI] [PubMed] [Google Scholar]
- 15.Harris-Love ML, Chan E, Dromerick AW, Cohen LG. Neural substrates of motor recovery in severely impaired stroke patients with hand paralysis. Neurorehabil Neural Repair 2016;30(4):328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takechi U, Matsunaga K, Nakanishi R, et al. Longitudinal changes of motor cortical excitability and transcallosal inhibition after subcortical stroke. Clin Neurophysiol 2014;125(10):2055–2069. [DOI] [PubMed] [Google Scholar]
- 17.Stinear CM, Barber PA, Coxon JP, Fleming MK, Byblow WD. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain 2008;131(Pt 5):1381–1390. [DOI] [PubMed] [Google Scholar]
- 18.McCambridge AB, Stinear JW, Byblow WD. Revisiting interhemispheric imbalance in chronic stroke: A tDCS study. Clin Neurophysiol 2017;129(1):42–50. [DOI] [PubMed] [Google Scholar]
- 19.Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: Brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair 2009;23(7):641–656. [DOI] [PubMed] [Google Scholar]
- 20.Di Lazzaro V, Dileone M, Capone F, et al. Immediate and late modulation of interhemipheric imbalance with bilateral transcranial direct current stimulation in acute stroke. Brain Stimul 2014;7(6):841–848. [DOI] [PubMed] [Google Scholar]
- 21.Sato S, Bergmann TO, Borich MR. Opportunities for concurrent transcranial magnetic stimulation and electroencephalography to characterize cortical activity in stroke. Front Hum Neurosci 2015;9(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nat Rev Neurol 2014;10(10):597–608. [DOI] [PubMed] [Google Scholar]
- 23.Pellegrino G, Tomasevic L, Tombini M, et al. Inter-hemispheric coupling changes associate with motor improvements after robotic stroke rehabilitation. Restor Neurol Neurosci 2012;30(6):497–510. [DOI] [PubMed] [Google Scholar]
- 24.Vecchio F, Lacidogna G, Miraglia F, Bramanti P, Ferreri F, Rossini PM. Prestimulus interhemispheric coupling of brain rhythms predicts cognitive-motor performance in healthy humans. J Cogn Neurosci 2014;26(9):1883–1890. [DOI] [PubMed] [Google Scholar]
- 25.Hall SD, Stanford IM, Yamawaki N, et al. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage 2011;56(3):1506–1510. [DOI] [PubMed] [Google Scholar]
- 26.Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. On the human sensorimotor-cortex beta rhythm: Sources and modeling. Neuroimage 2005;26(2):347–355. [DOI] [PubMed] [Google Scholar]
- 27.Rossiter HE, Boudrias MH, Ward NS. Do movement-related beta oscillations change after stroke? J Neurophysiol 2014;112(9):2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu W, Sun J, Jin Z, et al. Impaired neuronal synchrony after focal ischemic stroke in elderly patients. Clin Neurophysiol 2011;122(1):21–26. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Quinlan EB, Dodakian L, et al. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain 2015;138(Pt 8):2359–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray WA, Palmer JA, Wolf SL, Borich MR. Abnormal EEG responses to TMS during the cortical silent period are associated with hand function in chronic stroke. Neurorehabil Neural Repair 2017;31(7):666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borich MR, Wheaton LA, Brodie SA, Lakhani B, Boyd LA. Evaluating interhemispheric cortical responses to transcranial magnetic stimulation in chronic stroke: A TMS-EEG investigation. Neurosci Lett 2016;3(618):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossini PM, Burke D, Chen R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. an updated report from an I.F.C.N. committee. Clinical Neurophysiology 2015;126(6):1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodbury M, Velozo CA, Thompson PA, et al. Measurement structure of the wolf motor function test: Implications for motor control theory. Neurorehabil Neural Repair 2010;24(9):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 1997;114(2):329–338. [DOI] [PubMed] [Google Scholar]
- 35.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004;134(1):9–21. [DOI] [PubMed] [Google Scholar]
- 36.Nolte G, Bai O, Wheaton L, Mari Z, Vorbach S, Hallett M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin Neurophysiol 2004;115(10):2292–2307. [DOI] [PubMed] [Google Scholar]
- 37.Jung P, Ziemann U. Differences of the ipsilateral silent period in small hand muscles. Muscle Nerve 2006;34(4):431–436. [DOI] [PubMed] [Google Scholar]
- 38.Hall SD, Barnes GR, Furlong PL, Seri S, Hillebrand A. Neuronal network pharmacodynamics of GABAergic modulation in the human cortex determined using pharmaco-magnetoencephalography. Hum Brain Mapp 2010;31(4):581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakarov V, Naranjo JR, Schulte-Monting J, Omlor W, Huethe F, Kristeva R. Beta-range EEG-EMG coherence with isometric compensation for increasing modulated low-level forces. J Neurophysiol 2009;102(2):1115–1120. [DOI] [PubMed] [Google Scholar]
- 40.Pfurtscheller G, Berghold A. Patterns of cortical activation during planning of voluntary movement. Electroencephalogr Clin Neurophysiol 1989;72(3):250–258. [DOI] [PubMed] [Google Scholar]
- 41.Stefanou MI, Desideri D, Belardinelli P, Zrenner C, Ziemann U. Phase synchronicity of µ-rhythm determines efficacy of interhemispheric communication between human motor cortices. Journal of Neuroscience 2018;5(38):10525–10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fries P Rhythms for cognition: Communication through coherence. Neuron 2015;88(1):220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muthukumaraswamy SD, Myers JF, Wilson SJ, et al. The effects of elevated endogenous GABA levels on movement-related network oscillations. Neuroimage 2013;66:36–41. [DOI] [PubMed] [Google Scholar]
- 44.Grefkes C, Fink GR. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol 2014;13(2):206–216. [DOI] [PubMed] [Google Scholar]
- 45.Rehme AK, Eickhoff SB, Rottschy C, Fink GR, Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. Neuroimage 2012;59(3):2771–2782. [DOI] [PubMed] [Google Scholar]
- 46.Palmer JA, Halter A, Gray WA, Wolf SL, Borich MR. Modulatory effects of motor state during paired associative stimulation on motor cortex excitability and motor skill learning. Frontiers in human neuroscience 2019;13(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris-Love ML, Morton SM, Perez MA, Cohen LG. Mechanisms of short-term training-induced reaching improvement in severely hemiparetic stroke patients: A TMS study. Neurorehabil Neural Repair 2011;25(5):398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stinear CM, Petoe MA, Byblow WD. Primary motor cortex excitability during recovery after stroke: Implications for neuromodulation. Brain Stimul 2015;8(6):1183–1190. [DOI] [PubMed] [Google Scholar]
- 49.Cunningham DA, Machado A, Janini D, et al. Assessment of inter-hemispheric imbalance using imaging and noninvasive brain stimulation in patients with chronic stroke. Arch Phys Med Rehabil 2015;96(4 Suppl):S94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaechter JD, Perdue KL. Enhanced cortical activation in the contralesional hemisphere of chronic stroke patients in response to motor skill challenge. Cereb Cortex 2008;18(3):638–647. [DOI] [PubMed] [Google Scholar]
- 51.Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A 2002;99(22):14518–14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur J Neurosci 2005;21(4):989–999. [DOI] [PubMed] [Google Scholar]
- 53.McDonnell MN, Stinear CM. TMS measures of motor cortex function after stroke: A meta-analysis. Brain Stimul 2017;10(4):721–734. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, Qin W, Zhang J, Zhang X, Yu C. Enhanced interhemispheric functional connectivity compensates for anatomical connection damages in subcortical stroke. Stroke 2015;46(4):1045–1051. [DOI] [PubMed] [Google Scholar]
- 55.Borich MR, Wolf SL, Tan AQ, Palmer JA. Targeted neuromodulation of abnormal interhemispheric connectivity to promote neural plasticity and recovery of arm function after stroke: A randomized crossover clinical trial study protocol. Neural plasticity 2018. [DOI] [PMC free article] [PubMed]
- 56.van Kuijk AA, Pasman JW, Geurts AC, Hendricks HT. How salient is the silent period? the role of the silent period in the prognosis of upper extremity motor recovery after severe stroke. J Clin Neurophysiol 2005;22(1):10–24. [DOI] [PubMed] [Google Scholar]
- 57.Borich MR, Neva JL, Boyd LA. Evaluation of differences in brain neurophysiology and morphometry associated with hand function in individuals with chronic stroke. Restor Neurol Neurosci 2015;33(1):31–42. [DOI] [PubMed] [Google Scholar]
- 58.Pfurtscheller G, Stancak A Jr, Neuper C. Post-movement beta synchronization. A correlate of an idling motor area? Electroencephalogr Clin Neurophysiol 1996;98(4):281–293. [DOI] [PubMed] [Google Scholar]
- 59.Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. J Neurosci 2009;29(24):7679–7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conde V, Tomasevic L, Akopian I, et al. The non-transcranial TMS-evoked potential is an inherent source of ambiguity in TMS-EEG studies. Neuroimage 2019;185:300–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.