Stroke is the fifth leading cause of death and affects approximately 0.8 million Americans annually1. The causes, duration, localization, and injury severity, as well as the presence of comorbidity factors, impact the overall outcome and likelihood of survival. The current therapeutical approach for acute ischemic stroke includes thrombolysis and mechanical thrombectomy. Reperfusion with intravenous Alteplase (recombinant tissue plasminogen activator; Activase® and Actilyse®) remains the mainstay treatment for ischemic stroke, and a recent pooled analysis of 9 clinical trials showed that this therapy is beneficial regardless of patient age and stroke severity, as long as it can be administered within 4.5 hours of onset2. Although Alteplase increases the risk of early hemorrhage, the number of patients with a good outcome exceeds the ones with a fatal intracranial hemorrhage2.
Nevertheless, immediate reperfusion therapy does not directly address the secondary neurological sequelae that lead to continued brain injury after stroke. Depending on injury severity, complex cascades of biochemical events are activated, generating deleterious responses, such as excitotoxicity, oxidative stress, and inflammation that affect both the central nervous system (CNS) and the overall stroke outcome. Homeostatic cellular functions governing ATP-dependent ion gradients, calcium influx, endoplasmic reticulum, and mitochondrial function, membrane stability, redox balance, and blood-brain barrier permeability all become dysfunctional after ischemic stroke3. Pharmacological interventions targeting one or more steps of this cascade have led to the development of many neuroprotective drugs over the last two decades4. While some of the drugs produced encouraging results, particularly when combined with Alteplase therapy, many proved ineffective in clinical trials5. Consequently, no neuroprotective treatment options currently exist to improve neurological outcome after ischemic stroke, making it imperative to conduct new research for novel therapies.
The Endocannabinoid System
In recent years, the endocannabinoid system (ECS) has become subject of great interest in neurobiology and neuropharmacology, mainly due to its predominant distribution in the CNS6. The ECS is composed of endocannabinoids, endogenous lipid-based retrograde neurotransmitters that bind to the cannabinoid receptors CB1 and CB2, as well as cannabinoid receptor proteins expressed throughout the CNS and peripheral nervous system (Fig. 1). Importantly, modulating the activity of the ECS turned out to hold therapeutic promise in a wide range of pathological conditions and neurological disorders6.
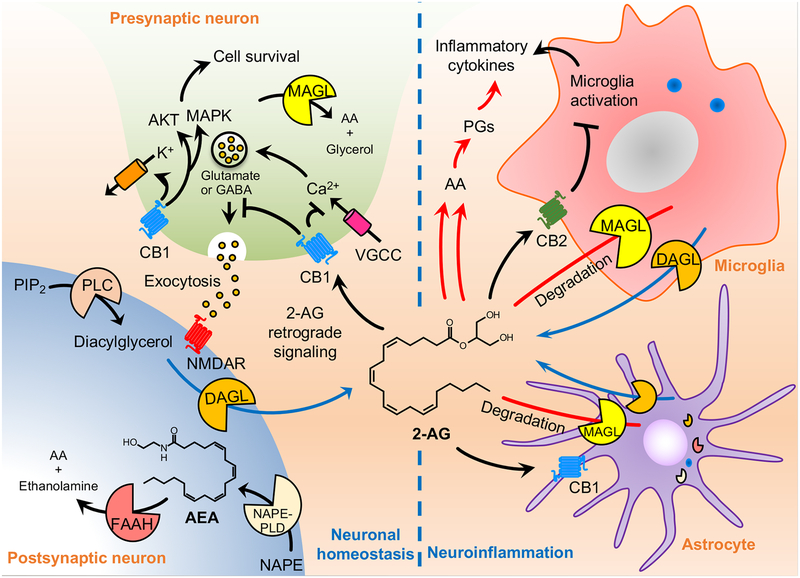
Figure 1.
Overview of biosynthesis and degradation of endocannabinoids and its multiple cellular actions in the brain. The endocannabinoid system is composed of cannabinoid receptors (CB1 and CB2), endocannabinoids (2-AG and AEA), and its synthesizing (DAGL and NAPE-PLD) and degrading enzymes (MAGL, and FAAH). CB1 receptors are abundant in the central nervous system, particularly in the cortex, basal ganglia, hippocampus, hypothalamus, and cerebellum. CB2 receptors are primarily present in microglia and immune system and are highly inducible following tissue injury or during neuroinflammation. As a lipid signaling molecule, 2-AG readily cross the membrane and activate CB1 receptors located in the presynaptic neurons. Activated CB1 receptor inhibits neurotransmitter release through the suppression of calcium influx and activates potassium (K+) channels, as well as induces the activation of AKT and MAPK survival pathway. Although 2-AG mainly mediates retrograde endocannabinoid signaling, AEA also activates presynaptic CB1 receptors and intracellular CB1 receptors as well. 2-AG functions as the primary cannabinoid receptor signaling molecule. 2-AG is also a significant precursor for AA and therefore plays a substantial role in pro-inflammatory pathways. 2-AG is synthesized from DAG by DAGL, while AEA is synthesized from NAPE by NAPE-PLD. MAGL is the rate-limiting enzyme in the degradation of 2-AG. 2-AG also modulates the activity of microglia and astrocytes. These glial cells also are a source for brain endocannabinoids. Increased number of microglia and astrocytes are typically found in the ischemic brain and result in increased production of proinflammatory cytokines. This process leads to a change towards a more proinflammatory milieu in the brain.
Abbreviations: 2-AG, 2-arachidonoyl glycerol; AA, arachidonic acid; AEA, N-aradhidonoylethanolamine; AKT, protein kinase B; CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2; DAGL, diacylglycerol lipase; FAAH, fatty acid amide hydrolase; GABA, gamma aminobutyric acid; MAGL, monoacylglycerol lipase; MAPK, mitogen-activated protein kinase; NAPE-PLD, N-arachidonoyl phosphatidyl ethanol-preferring phospholipase D; NMDAR, N-methyl-D-aspartate receptor; PI3K, phosphoinositide 3-kinase; PLC, phospholipase C; PIP2, phosphatidylinositol 4,5-bisphosphate; VGCC, voltage-gated calcium channel.
The ECS has emerged as a new therapeutic target in a variety of neurological disorders with a robust neuroinflammatory component that leads to brain tissue injury7. There is convincing evidence that the components of the endocannabinoid system are altered during ischemic stroke in both animals and humans, indicating that this system may contribute to the consequences of ischemic stroke8. Although numerous studies have examined the effects of cannabinoids and the role of the ECS in experimental models of ischemic stroke, results have been somewhat conflicting in support of either a beneficial or detrimental role. Nevertheless, a recent systematic review and meta-analysis of the currently available preclinical studies reported neuroprotective effects from a range of approaches to use of cannabinoids9. The main advantage of cannabinoids in neuroprotection is their broad-spectrum activity at multiple cellular and molecular mechanisms that involve not only the ECS itself but also the immune system10. Cannabinoids can limit excitotoxicity, oxidative stress, and neuroinflammation, and to enhance the trophic and metabolic support of neurons by acting through either specific cannabinoid receptor-mediated signaling pathways or via direct interactions with transcription factors. There are no previous or current clinical trials using cannabinoids in stroke, but their pleiotropic effects on the ischemic penumbra and cerebral vasculature after stroke, combined with their excellent tolerability, make them promising candidates for future treatment development.
Neurological benefits of cannabis and cannabinoid use
Cannabis (Cannabis sativa), also known as marijuana, has been used for centuries as a medicinal and recreational drug. It contains more than 120 different cannabinoids that have been identified, but the role and importance of most of them are yet to be fully understood11. Broadly, cannabinoids are defined as phytocannabinoids, synthetic cannabinoids, and endogenous cannabinoids. The two most notable and thoroughly investigated phytocannabinoids are the delta-9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD). Studies of Δ9-THC and CBD led to the discovery of the two endogenous cannabinoid receptors type 1 (CB1R) and type 2 (CB2R)12, 13. THC exerts its well-known psychoactive effects, including relaxation, euphoria, dreaminess, feelings of anxiety and paranoia, through the CB1R. THC has also been demonstrated to impair cognition and psychomotor performance14, 15. However, recent studies have shown that THC promotes hippocampal neurogenesis and restores memory and cognitive function in aged animals16, 17. Despite the beneficial effects of THC, its psychoactive effects have limited the medical use of cannabis.
On the other hand, CBD is known as the main non-psychoactive component of cannabis and has shown anti-inflammatory, immunosuppressive, analgesic, and anxiolytic effects. In particular, CBD has a very low affinity in the micromolar range for the cannabinoid receptors and was found to be an anticonvulsant in animal models and humans with epilepsy18. Although cannabis is still listed by the U.S. Drug Enforcement Administration (DEA) as a Schedule I substance of the Controlled Substances Act, the U.S. Food and Drug Administration (FDA) has approved on June 25, 2018, the first prescription drug derived from cannabis for treating two rare and severe forms of epilepsy19. The Epidiolex® oral solution contains highly purified plant-derived CBD. In September 2018, the DEA classified Epidiolex as a Schedule V substance, clearing the final hurdle for its legal prescription in the USA. While Epidiolex is the first FDA-approved drug directly derived from the cannabis plant, it is not the first cannabinoid-based drug approved in the USA. The FDA approved three synthetic cannabinoid-based drugs: Marinol®, Syndros®, and Cesamet®. Marinol and Syndros include the active component dronabinol, a synthetic form of THC. Cesamet includes the active ingredient nabilone, which is synthetically derived and has a chemical structure similar to THC. All three synthetic compounds are prescribed to adults for the treatment of nausea and vomiting associated with chemotherapy, while Marinol and Syndros are also indicated for the treatment of anorexia associated with weight loss in AIDS patients. Although Epidiolex is currently only approved for two rare forms of epilepsy, this sets a precedent that may benefit the world of cannabinoid research.
Accumulating preclinical studies suggest that cannabinoids have significant therapeutic value in stroke. A recent systemic review and meta-analysis by England et al. demonstrated that all subclasses of cannabinoids, cannabis-derived, synthetic, specific CB1R, and CB2R agonists, significantly reduced infarct volume in transient and permanent ischemia and improve both early and late functional outcome in experimental stroke when given after stroke onset9. CBD showed trends to infarct reduction with delayed administration up to 6 hours after stroke onset9. Repeated treatment with CBD from day 1 or day 3 to day 14 improved functional outcome and survival rates, which suggest that CBD may have neuroprotective effects not only at the early phase but also at the late time point20. Multiple targets have been proposed to mediate the neuroprotective effects of CBD such as a combination of a potent antioxidant, immunosuppression, and anti-inflammatory actions21. CBD also counteracts the cerebral hemodynamic impairment and produces beneficial cardiac effects. Sultan et al. conducted a systemic review and meta-analysis with 25 studies and concluded that CBD is associated with changes in hemodynamics in vivo22. Acute and chronic administration of CBD did not affect blood pressure or heart rate under control conditions, but reduced blood pressure and heart rate in stressful conditions. Jadoon et al. found that acute administration of CBD reduced resting systolic blood pressure and blunted the blood pressure response to stress in humans23. In mouse and piglet models of stroke, CBD significantly increased cerebral blood flow24, 25.
Moreover, CBD reduced brain edema and blood-brain barrier permeability associated with ischemic condition26. CBD has negligible activity on cannabinoid receptors but may interfere with the endocannabinoid system and directly or indirectly stimulate 5-hydroxytryptamine 1A receptors, adenosine receptors, transient receptor potential vanilloid subtype 1, and nuclear receptors of the peroxisome proliferator-activated receptor family21. THC also showed trends to infarct reduction with delayed administration up to 4 hours9. The neuroprotective effect of THC is related to the CB1R-mediated inhibition of voltage-sensitive calcium channels, which reduces calcium influx, excessive glutamate release, hypothermia, and enhances cerebral blood flow27, 28. Also, THC, when acting on the CB2R found in the immune system, decreased the severity of stroke. Oral treatment with a low dose of THC inhibited atherosclerosis progression in a murine model of established atherosclerosis, through pleiotropic immunomodulatory effects on lymphoid and myeloid cells. CBD was also effective in diabetic complications and related atherosclerosis29. Administration of the CB1R agonist HU-210 significantly reduced motor disability and infarct volume in a dose-dependent manner and was useful 4 hours after stroke onset30. This neuroprotection is associated with the indirect protective effects of hypothermia.
Despite a large number of preclinical studies, there are no previous clinical trials using cannabinoids in stroke, while relevant clinical trials using cannabinoids in other neurological disorders already exist31. THC/CBD oromucosal spray (Sativex®) is the first cannabis-based medicine to be licensed in the U.K. and currently available in numerous countries worldwide for the treatment of multiple sclerosis-related spasticities not responded adequately to other medication32. Spasticity is common after stroke and other neurological conditions and causes significant limitations on activities of daily living33. Importantly, a clinical trial investigating THC:CBD oromucosal spray efficacy and safety for post-stroke spasticity has been registered34. Also, another relevant clinical trial was efficacy and safety evaluation of a single intravenous dose of dexanabinol (HU-211), a synthetic and non-psychoactive cannabinoid derivate, in patients suffering from severe traumatic brain injury35. This phase III trial concluded that dexanabinol was safe but was not efficacious for the treatment of traumatic brain injury.
Neurological complications of cannabis and cannabinoid use
Cannabis is the most commonly produced and consumed illicit substance in the world and the contents of THC and CBD varied widely in street cannabis. As THC is the primary psychoactive component cannabinoid, cannabis users prefer the strains of the plant with higher THC content. Potter et al. carried out the potency of THC and CBD seized by police in England in 2005 and found that the median content of THC was significantly higher than the one recorded ten years before36. In contrast, CBD content was found to be extremely low in more recent cannabis. Cascini et al. also performed a meta-analysis to assess the potency of THC from 1970 to 2009 and reported a temporal trend of increasing potency worldwide37. These findings indicate that trends for preferring higher THC content variants carry significant health risks, particularly to those who are susceptible to its harmful effects.
The growing popularity of recreational consumption of cannabis, especially among the young population, raises immediate concerns regarding its safety. Recent case-control studies and systemic reviews have shown that cannabis use can significantly affect physical and mental health and lead to substance dependence38. Mainly, ischemic stroke is the most commonly reported adverse neurovascular effect of cannabis use in the young population. Kalla et al. conducted a study in patients aged 18–55 years and found that cannabis use was independently associated with a 26% increase in the risk of stroke after corrected for known risk factors, such as obesity, hypertension, smoking, and alcohol use39. This study drew data from the Nationwide Inpatient Sample, which includes the health records of patients admitted over 1,000 hospitals comprising about 20% of U.S. medical centers.
Similarly, Jouanjus et al. conducted a systemic review with 116 cases published between January 2011 to March 2016 in patients aged 18–44 years and concluded that although cannabis use is linked to several adverse cardiovascular events, the evidence is most persuasive for ischemic stroke38. Similar results were also reported from the U.S. nationwide inpatient sample, showing that recreational use of cannabis was independently associated with a 2.25-fold increase in the risk of acute ischemic stroke among aged 25–34 years40. On the contrary, a recent population-based cohort and long-term follow-up study of 45,000 Swedish men carried out by Falkstedt et al. analyzed the effects of cannabis, tobacco, and alcohol use on the risk of early stroke41. They found no apparent association between cannabis use in young adult and stroke, including strokes occurring before 45 years of age. However, tobacco smoking showed a clear association with stroke. Since most studies were based on hospital discharge records, the findings may not be reflective of the general population regarding cannabis use and early stroke. Furthermore, epidemiological studies have not performed an individual analysis of patients without other cardiovascular risk factors, which may limit the estimation of the risk of stroke associated with cannabis use alone. Nevertheless, there are currently limited data, inadequate assessment of cannabis use, and few population-based studies to confirm or reject the hypothesis of the effects of cannabis use and early stroke.
It is critically important to identify all factors that may play a role in the recent increase in the incidence of stroke among the young population. One striking element reported in the majority of the case studies was a temporal relationship between cannabis use and the occurrence of stroke. However, a temporal correlation does not mean causation, and other factors may be involved. Currently, reversible cerebral vasoconstriction triggered by cannabis use may be a possible mechanism of stroke. Chronic cannabis use leads to impairment in cerebrovascular function, which has been associated with an increased risk for stroke42. Cannabis-related angiopathy has also been linked with ischemic stroke in heavy users43. Another possible mechanism of acute ischemic stroke due to cannabis use could be an increase in pro-coagulant effects, as THC increases the expression of glycoprotein IIb-IIIa and P-selectin on human platelets in a concentration-dependent manner44. Platelet aggregation is a significant risk factor for acute ischemic stroke45, and it is relatively more important in younger than in older stroke patients46. Earlier research has indicated that THC induces tachycardia47. It has been suggested that CBD reduces THC-induced adverse cardiovascular effects48. Jamil et al. reported a case of possible concurrent use of cannabis and other drugs on the development of stroke49. Cannabis often precedes or is used along with other substances.
Besides this vascular role of cannabis in the occurrence of stroke, a cellular effect of cannabis on brain mitochondrial respiratory chain dysfunction and oxidative stress was recently suggested as a potential mechanism involved in cannabis-related stroke50. Indeed, despite the widespread use of cannabis, the low frequency of neurovascular complications after their use may be due to a genetic predisposition to neurovascular toxicity in some individuals.
There is still debate about the possible behavioral and pathological consequences of cannabis use. Since several questions remain unsolved, further research is still needed to assess the pathophysiological mechanisms involved in young cannabis users with stroke.
New approaches to targeting the endocannabinoid system with selective inhibitors of monoacylglycerol lipase
In recent decades, multiple lines of research have provided insights into the biochemical regulation, pathophysiological roles, and therapeutic potential of the endogenous cannabinoid 2-arachidonoylglycerol (2-AG)51. 2-AG is synthesized “on-demand” and acts as a full agonist of cannabinoid receptors, but its rapid degradation by monoacylglycerol lipase (MAGL) results in short-lived actions. Many physiological and pathological processes involve 2-AG, which behaves as a retrograde signaling lipid that inhibits neurotransmitter release at both excitatory and inhibitory synapse. Several neurological disorders with an inflammatory involvement, including ischemic stroke, show elevated levels of 2-AG52. Although the exact role of 2-AG is not clear, the neuroprotection exerted by exogenous 2-AG suggests that 2-AG contributes to neuroprotection not only by reducing neuronal excitotoxicity and inhibiting the production of proinflammatory cytokines and reactive oxygen species but also by lowering cerebral vasoconstriction53.
Inactivation of MAGL leads to the elevation of the level of 2-AG, thus resulting in an enhancement of the endocannabinoid signaling. Beyond this critical role, the study of Nomura et al. provides compelling evidence linking both the endocannabinoid and eicosanoid signaling pathways through MAGL, which hydrolyzes 2-AG to arachidonic acid (AA)54. AA is of particular interest as the precursor of the eicosanoid family that includes proinflammatory prostaglandins and leukotrienes. It is possible that pharmacological inhibition of MAGL might be a promising therapeutic target not only by enhancing anti-inflammatory and neuroprotective 2-AG signaling through cannabinoid receptor-dependent mechanisms but also by reducing proinflammatory eicosanoid production. Although early MAGL inhibitors had poor selectivity and low potency, they contributed significantly to advancing the understanding of the pathophysiological roles of MAGL55. Inhibition of MAGL activity with JZL184 reduced 2-AG hydrolysis by ~85% in the mouse brain and led to dramatic elevations in brain 2-AG levels. A single dose of JZL184 was capable of inhibiting MAGL for up to 24 hours, with a maximal 8-fold elevation of brain 2-AG levels for at least 8 hours55.
Most importantly, acute MAGL blockade with JZL184 has been shown to exhibit a wide range of beneficial effects in neurodegenerative diseases54. Recent studies demonstrated that the MAGL inhibitor JZL184 significantly reduces infarct volume in both transient and permanent models of ischemic stroke, and improve the functional outcome when administered it after stroke onset56. CPD-4645, a newly characterized MAGL inhibitor, modified the transcription profile of brain vasculature and restored its functional homeostasis in the photothrombotic model of ischemic stroke57. These results highlight a bidirectional mechanism of action – simultaneous enhancement of cannabinoid signaling through elevation of 2-AG and reduction of AA and downstream eicosanoids - that can achieve therapeutic efficacy through either cannabinoid receptor-dependent or independent mechanisms.
Over the past decades, considerable efforts were made for developing novel MAGL inhibitors with improved selectivity and cross-species activity compared to JZL184. KML29, an analog of JZL184, was the most selective for MAGL over other serine hydrolase family of enzymes58. MJN110, another recently developed selective MAGL inhibitor, showed markedly increased potency and no significant cross-reactivity with fatty acid amide hydrolase59. Interestingly, those MAGL inhibitors do not cause cannabimimetic activity such as catalepsy and hypothermia. Clinical research in the field of MAGL inhibitors is still at early stages. During the past five years, several academic groups and pharmaceutical companies have patented about 20 MAGL inhibitors for a large number of therapeutic uses, such as pain, inflammation, metabolic, and neurodegenerative diseases, as well as the treatment of cancer, anxiety, and epilepsy60. Recently, a potent human MAGL inhibitor ABX1431 (Abide Therapeutics) has completed a placebo-controlled phase I for safety and tolerability61. ABX-1431 is currently subjected to phase II trials in patients with Tourette Syndrome and neuropathic pain. Although the translational potential of MAGL inhibitors still needs basic and preclinical studies, the results of ongoing and future clinical trials will hopefully unveil the MAGL inhibitors as a new drug class for the treatment of human diseases, including stroke.
Conclusions
Neurovascular complications, such as reversible cerebral vasoconstriction syndrome, intracranial hemorrhages, and ischemic strokes, and their association with cannabis and cannabinoids are an emerging area of research as more states legalize cannabis for medical and recreational use. However, several questions remain unanswered about the pathophysiological role of cannabis and cannabinoids in such complications. Epidemiological studies must provide detailed information concerning not only the quantity and the frequency of cannabis use but also the type of cannabis used. Longitudinal studies are needed in order to clarify the impact of cannabis on the severe consequences associated with their use. Despite the controversy of cannabis use, the first cannabis-derived Epidiolex® received the U.S. FDA approval as a treatment for some types of epilepsy and opened new horizons for medical use of cannabis. This approval highlights the importance of critical benefit-risk analysis and careful evaluation in the drug development process.
Supplementary Material
Acknowledgments
We acknowledge several relevant studies used to prepare this article that could not be cited because of word count restrictions.
Sources of Funding
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Footnotes
Disclosures
None.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: A report from the american heart association. Circulation. 2018;137:e67–e492 [DOI] [PubMed] [Google Scholar]
- 2.Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, Bluhmki E, et al. Risk of intracerebral hemorrhage with Alteplase after acute ischaemic stroke: A secondary analysis of an individual patient data meta-analysis. Lancet Neurol. 2016;15:925–933 [DOI] [PubMed] [Google Scholar]
- 3.Rink C, Khanna S. Significance of brain tissue oxygenation and the arachidonic acid cascade in stroke. Antioxid Redox Signal. 2011;14:1889–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamorro A, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881 [DOI] [PubMed] [Google Scholar]
- 5.Gibson LM, Brazzelli M, Thomas BM, Sandercock PA. A systematic review of clinical trials of pharmacological interventions for acute ischaemic stroke (1955–2008) that were completed, but not published in full. Trials. 2010;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moris D, Georgopoulos S, Felekouras E, Patsouris E, Theocharis S. The effect of the endocannabinoid system in ischemia-reperfusion injury: A friend or a foe? Expert Opin Ther Targets. 2015;19:1261–1275 [DOI] [PubMed] [Google Scholar]
- 8.Hillard CJ. Role of cannabinoids and endocannabinoids in cerebral ischemia. Curr Pharm Des. 2008;14:2347–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.England TJ, Hind WH, Rasid NA, O’Sullivan SE. Cannabinoids in experimental stroke: A systematic review and meta-analysis. J Cereb Blood Flow Metab. 2015;35:348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Ruiz J, Moro MA, Martinez-Orgado J. Cannabinoids in neurodegenerative disorders and stroke/brain trauma: From preclinical models to clinical applications. Neurotherapeutics. 2015;12:793–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner SE, Williams CM, Iversen L, Whalley BJ. Molecular pharmacology of phytocannabinoids. Prog Chem Org Nat Prod. 2017;103:61–101 [DOI] [PubMed] [Google Scholar]
- 12.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564 [DOI] [PubMed] [Google Scholar]
- 13.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65 [DOI] [PubMed] [Google Scholar]
- 14.Fletcher JM, Page JB, Francis DJ, Copeland K, Naus MJ, Davis CM, et al. Cognitive correlates of long-term cannabis use in costa rican men. Arch Gen Psychiatry. 1996;53:1051–1057 [DOI] [PubMed] [Google Scholar]
- 15.Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131 [DOI] [PubMed] [Google Scholar]
- 16.Bilkei-Gorzo A, Albayram O, Draffehn A, Michel K, Piyanova A, Oppenheimer H, et al. A chronic low dose of delta(9)-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat Med. 2017;23:782–787 [DOI] [PubMed] [Google Scholar]
- 17.Suliman NA, Taib CNM, Moklas MAM, Basir R. Delta-9-tetrahydrocannabinol ((9)-THC) induce neurogenesis and improve cognitive performances of male Sprague Dawley rats. Neurotox Res. 2018;33:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with lennox-gastaut syndrome (gwpcare4): A randomized, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:1085–1096 [DOI] [PubMed] [Google Scholar]
- 19.Rubin R. The path to the first FDA-approved cannabis-derived treatment and what comes next. JAMA. 2018;320:1227–1229 [DOI] [PubMed] [Google Scholar]
- 20.Hayakawa K, Irie K, Sano K, Watanabe T, Higuchi S, Enoki M, et al. Therapeutic time window of cannabidiol treatment on delayed ischemic damage via high-mobility group box1-inhibiting mechanism. Biol Pharm Bull. 2009;32:1538–1544 [DOI] [PubMed] [Google Scholar]
- 21.Hayakawa K, Mishima K, Fujiwara M. Therapeutic potential of non-psychotropic cannabidiol in ischemic stroke. Pharmaceuticals (Basel). 2010;3:2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sultan SR, Millar SA, England TJ, O’Sullivan SE. A systematic review and meta-analysis of the hemodynamic effects of cannabidiol. Front Pharmacol. 2017;8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jadoon KA, Tan GD, O’Sullivan SE. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized cross-over study. JCI Insight. 2017;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, et al. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1a receptor-dependent mechanism. Stroke. 2005;36:1077–1082 [DOI] [PubMed] [Google Scholar]
- 25.Alvarez FJ, Lafuente H, Rey-Santano MC, Mielgo VE, Gastiasoro E, Rueda M, et al. Neuroprotective effects of the non-psychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr Res. 2008;64:653–658 [DOI] [PubMed] [Google Scholar]
- 26.Hind WH, England TJ, O’Sullivan SE. Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via ppargamma and 5-ht1a receptors. Br J Pharmacol. 2016;173:815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parmentier-Batteur S, Jin K, Mao XO, Xie L, Greenberg DA. Increased severity of stroke in cb1 cannabinoid receptor knock-out mice. J Neurosci. 2002;22:9771–9775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayakawa K, Mishima K, Nozako M, Hazekawa M, Ogata A, Fujioka M, et al. Delta9-tetrahydrocannabinol (delta9-THC) prevents cerebral infarction via hypothalamic-independent hypothermia. Life Sci. 2007;80:1466–1471 [DOI] [PubMed] [Google Scholar]
- 29.Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Drel VR, et al. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol. 2007;293: H610–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leker RR, Gai N, Mechoulam R, Ovadia H. Drug-induced hypothermia reduces ischemic damage: Effects of the cannabinoid hu-210. Stroke. 2003;34:2000–2006 [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Sendon Moreno JL, Garcia Caldentey J, Trigo Cubillo P, Ruiz Romero C, Garcia Ribas G, Alonso Arias MA, et al. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with sativex in huntington’s disease. J Neurol. 2016;263:1390–1400 [DOI] [PubMed] [Google Scholar]
- 32.Keating GM. Delta-9-tetrahydrocannabinol/cannabidiol oromucosal spray (sativex((r))): A review in multiple sclerosis-related spasticity. Drugs. 2017;77:563–574 [DOI] [PubMed] [Google Scholar]
- 33.Urban PP, Wolf T, Uebele M, Marx JJ, Vogt T, Stoeter P, et al. Occurrence and clinical predictors of spasticity after ischemic stroke. Stroke. 2010;41:2016–2020 [DOI] [PubMed] [Google Scholar]
- 34.Marinelli L, Balestrino M, Mori L, Puce L, Rosa GM, Giorello L, et al. A randomized controlled cross-over double-blind pilot study protocol on thc: Cbd oromucosal spray efficacy as an add-on therapy for post-stroke spasticity. BMJ Open. 2017;7:e016843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maas AI, Murray G, Henney H 3rd, Kassem N, Legrand V, Mangelus M, et al. Efficacy and safety of dexanabinol in severe traumatic brain injury: Results of a phase iii randomized, placebo-controlled, clinical trial. Lancet Neurol. 2006;5:38–45 [DOI] [PubMed] [Google Scholar]
- 36.Potter DJ, Clark P, Brown MB. Potency of delta 9-THC and other cannabinoids in cannabis in England in 2005: Implications for psychoactivity and pharmacology. J Forensic Sci. 2008;53:90–94 [DOI] [PubMed] [Google Scholar]
- 37.Cascini F, Aiello C, Di Tanna G. Increasing delta-9-tetrahydrocannabinol (delta-9-THC) content in herbal cannabis over time: Systematic review and meta-analysis. Curr Drug Abuse Rev. 2012;5:32–40 [DOI] [PubMed] [Google Scholar]
- 38.Jouanjus E, Raymond V, Lapeyre-Mestre M, Wolff V. What is the current knowledge about the cardiovascular risk for users of cannabis-based products? A systematic review. Curr Atheroscler Rep. 2017;19:26. [DOI] [PubMed] [Google Scholar]
- 39.Kalla A, Krishnamoorthy PM, Gopalakrishnan A, Figueredo VM. Cannabis use predicts risks of heart failure and cerebrovascular accidents: Results from the national inpatient sample. J Cardiovasc Med (Hagerstown). 2018;19:480–484 [DOI] [PubMed] [Google Scholar]
- 40.Rumalla K, Reddy AY, Mittal MK. Recreational marijuana use and acute ischemic stroke: A population-based analysis of hospitalized patients in the united states. J Neurol Sci. 2016;364:191–196 [DOI] [PubMed] [Google Scholar]
- 41.Falkstedt D, Wolff V, Allebeck P, Hemmingsson T, Danielsson AK. Cannabis, tobacco, alcohol use, and the risk of early stroke: A population-based cohort study of 45 000 Swedish men. Stroke. 2017;48:265–270 [DOI] [PubMed] [Google Scholar]
- 42.Thomas G, Kloner RA, Rezkalla S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: What cardiologists need to know. Am J Cardiol. 2014;113:187–190 [DOI] [PubMed] [Google Scholar]
- 43.Tsivgoulis G, Lachanis S, Papathanasiou MA, Chondrogianni M, Brountzos EN, Voumvourakis K. Cannabis-associated angiopathy: An uncommon cause of crescendo transient ischemic attacks. Circulation. 2014;130:2069–2070 [DOI] [PubMed] [Google Scholar]
- 44.Deusch E, Kress HG, Kraft B, Kozek-Langenecker SA. The procoagulatory effects of delta-9-tetrahydrocannabinol in human platelets. Anesth Analg. 2004;99:1127–1130, table of contents [DOI] [PubMed] [Google Scholar]
- 45.Fateh-Moghadam S, Htun P, Tomandl B, Sander D, Stellos K, Geisler T, et al. Hyperresponsiveness of platelets in ischemic stroke. Thromb Haemost. 2007;97:974–978 [PubMed] [Google Scholar]
- 46.Couch JR, Hassanein RS. Platelet aggregation, stroke, and transient ischemic attack in middle-aged and elderly patients. Neurology. 1976;26:888–895 [DOI] [PubMed] [Google Scholar]
- 47.Beaconsfield P, Ginsburg J, Rainsbury R. Marihuana smoking. Cardiovascular effects in man and possible mechanisms. N Engl J Med. 1972;287:209–212 [DOI] [PubMed] [Google Scholar]
- 48.Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of delta 9 - tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28:172–177 [DOI] [PubMed] [Google Scholar]
- 49.Jamil M, Zafar A, Adeel Faizi S, Zawar I. Stroke from vasospasm due to marijuana use: Can cannabis synergistically with other medications trigger cerebral vasospasm? Case Rep Neurol Med. 2016;2016:5313795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolff V, Schlagowski AI, Rouyer O, Charles AL, Singh F, Auger C, et al. Tetrahydrocannabinol induces brain mitochondrial respiratory chain dysfunction and increases oxidative stress: A potential mechanism involved in cannabis-related stroke. Biomed Res Int. 2015;2015:323706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baggelaar MP, Maccarrone M, van der Stelt M. 2-arachidonoylglycerol: A signaling lipid with manifold actions in the brain. Prog Lipid Res. 2018;71:1–17 [DOI] [PubMed] [Google Scholar]
- 52.Naccarato M, Pizzuti D, Petrosino S, Simonetto M, Ferigo L, Grandi FC, et al. Possible anandamide and palmitoylethanolamide involvement in human stroke. Lipids Health Dis. 2010;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panikashvili D, Shein NA, Mechoulam R, Trembovler V, Kohen R, Alexandrovich A, et al. The endocannabinoid 2-ag protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol Dis. 2006;22:257–264 [DOI] [PubMed] [Google Scholar]
- 54.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi SH, Arai AL, Mou Y, Kang B, Yen CC, Hallenbeck J, et al. Neuroprotective effects of magl (monoacylglycerol lipase) inhibitors in experimental ischemic stroke. Stroke. 2018;49:718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piro JR, Suidan GL, Quan J, Pi Y, O’Neill SM, Ilardi M, et al. Inhibition of 2-ag hydrolysis differentially regulates blood-brain barrier permeability after injury. J Neuroinflammation. 2018;15:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang JW, Niphakis MJ, Lum KM, Cognetta AB 3rd, Wang C, Matthews ML, et al. Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem Biol. 2012;19:579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Niphakis MJ, Cognetta AB 3rd, Chang JW, Buczynski MW, Parsons LH, Byrne F, et al. Evaluation of NHS carbamates as a potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chem Neurosci. 2013;4:1322–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Granchi C, Caligiuri I, Minutolo F, Rizzolio F, Tuccinardi T. A patent review of monoacylglycerol lipase (magl) inhibitors (2013–2017). Expert Opin Ther Pat. 2017;27:1341–1351 [DOI] [PubMed] [Google Scholar]
- 61.Cisar JS, Weber OD, Clapper JR, Blankman JL, Henry CL, Simon GM, et al. Identification of abx-1431, a selective inhibitor of monoacylglycerol lipase and clinical candidate for the treatment of neurological disorders. J Med Chem. 2018;61:9062–9084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.