Abstract
Focal segmental glomerulosclerosis is a major cause of end stage renal disease. Many patients prove unresponsive to available therapies. An improved understanding of the molecular basis of the disease process could provide insights leading to novel therapeutic approaches. In this study we carried out an RNA-seq analysis of the altered gene expression patterns of podocytes, mesangial cells and glomerular endothelial cells of the bigenic Cd2ap+/-, Fyn-/- mutant mouse model of FSGS. In the podocytes we observed upregulation of many genes related to the Tgfβ family/pathway, including Gdnf, Tgfβ1, Tgfβ2, Snai2, Vegfb, Bmp4, and Tnc. The mutant podocytes also showed upregulation of Acta2, a marker of smooth muscle and associated with myofibroblasts, which are implicated in driving fibrosis. GO analysis of the podocyte upregulated genes identified elevated protein kinase activity, increased expression of growth factors, and negative regulation of cell adhesion, perhaps related to the observed podocyte loss. Both podocytes and mesangial cells showed strong upregulation of aldehyde dehydrogenase genes involved in the synthesis of retinoic acid. Similarly, the Cd2ap+/-, Fyn-/- mesangial cells, as well as podocytes in other genetic models, and the glomeruli of human FSGS patients, all show upregulation of the serine protease Prss23, with the common thread suggesting important functionality. Another gene with strong upregulation in the Cd2ap+/-, Fyn-/- mutant mesangial cells as well as multiple other mutant mouse models of FSGS was thrombospondin, which activates the secreted inactive form of Tgfβ. The Cd2ap+/-, Fyn-/- mutant endothelial cells showed elevated expression of genes involved in cell proliferation, angioblast migration, angiogenesis, and neovasculature, all consistent with the formation of new blood vessels in the diseased glomerulus. The resulting global definition of the perturbed molecular pathways in the three major cell types of the mutant glomerulus provide deeper understanding of the molecular pathogenic pathways.
Introduction
Focal segmental glomerulosclerosis (FSGS) is a histologic pattern that is the most common glomerular cause of end stage renal disease (ESRD) in the United States [1]. It is characterized by sclerosis of parts (segmental) of some (focal) glomeruli. There is typically evidence of collapse of capillary loops and increased mesangial matrix deposition in segments of a portion of glomeruli. FSGS histologic lesion is found in about 35% of all cases, and 50% of African Americans, with nephrotic syndrome [2]. There is a trend of increasing incidence of FSGS caused ESRD in the United States [3] and world [4].
The Columbia morphological classification defines the collapsing variant, tip variant, perihilar variant, cellular variant and classical or NOS (not otherwise specified) FSGS [5, 6]. It is also possible to cluster FSGS into six clinical types based on etiology [7], which includes the less common medication or infection associated FSGS. Adaptive FSGS results from glomerular hyperfiltration, which can be associated with low birth weight, morbid obesity or sickle cell anemia. Another type is idiopathic, or primary FSGS, with no known cause. In addition, one can define two types of FSGS with a clear genetic basis. First, there are high penetrance genetic causes, with over 38 genes now identified, where homozygous mutation of a single gene results in FSGS [7]. These are more likely seen in childhood nephrotic syndrome (~60%) than in older children or adolescents (~5%), with still lower rates in adults [8, 9]. Causative genes include Actn4, Inf2, Trpc6, and Nphs2, to name a few [7].
A second genetic cause category involves multiple mutant allele combinations that contribute to FSGS with lower penetrance. For example, genetic variants of Apol1 are a major contributing factor to FSGS in individuals of sub-Saharan descent, being associated with 72% of cases [10]. The effect is mostly recessive, with two risk alleles required, but penetrance is low, as most individuals with two risk alleles will not develop FSGS. Presumably additional environmental and/or genetic contributions are required. Indeed, it is generally thought that monogenic disease is relatively rare compared to multifactorial (multiple mutant genes combined with environmental causes) and polygenic (mutations in multiple genes) disease. The cumulative effects of several mutations in different genes can combine to cause FSGS or modulate its severity. For example, homozygous MYO1E mutation is associated with childhood FSGS [11], while coinheritance of mutations in both COl4A5 and MYO1E can dramatically accentuate disease severity [12].
It has also been shown in mouse models that there can be combined polygenic contributions to FSGS. Cd2ap is a scaffold protein located in the slit diaphragms of podoctyes where it interacts with nephrin and podocin [13, 14]. Homozygous mutation of Cd2ap has been shown to cause high penetrance FSGS in humans [15, 16]. Mice with homozygous mutation of Cd2ap also develop FSGS like disease, with severe nephrotic syndrome, extracellular matrix deposition, glomerulosclerosis, extensive podocyte foot process effacement, and death within weeks of birth [13]. The phenotype of heterozygous mice with only one Cd2ap mutation, however, is “relatively unremarkable” [17], with some glomerular changes noted at 9 months of age [18]. Fyn encodes a tyrosine kinase, related to Src, that phosphorylates the slit diaphragm component Nephrin [19]. Heterozygous mutation of Fyn gives rise to very rare proteinuria, while homozygous Fyn mutation results in proteinuria in only 31% of mice at an average onset of 8 months [17]. Of interest, however, combined Cd2ap+/-, Fyn-/- mice develop proteinuria in 100% of mice at average onset of only 5 months [17]. This synergy of phenotype represents a combined susceptibility allele model. A fraction of human FSGS cases also likely result from multiple mutant alleles, in various mixes of susceptibility genes, together increasing the chances of FSGS. In this report we further study the Cd2ap+/-, Fyn-/- bigenic murine model of FSGS, examining gene expression changes that take place in the glomerular podocyte, mesangial and endothelial cells.
Podocyte injury plays a key role in the initiation and progression of glomerular disease, including FSGS [20]. The first detectable morphological characteristics of FSGS are found in podocytes [21], which undergo hypertrophy, detachment from the glomerular basement membrane (GBM), effacement of foot processes and depletion in number. The other two main glomerular cell types, however, the mesangial and endothelial cells, also undergo dramatic changes during FSGS. There is, for example, mesangial expansion with increased extracellular matrix, and endothelial cells can show increased leukocyte recruitment [22] as well as de novo angiogenesis, which can result in leaky vessels [23]. A comprehensive analysis of FSGS, therefore, requires examination of mesangial cells and endothelial cells as well as podocytes.
The current Kidney Disease: Improving Global Outcome (KDIGO) practice guidelines link therapy to pathology. Initial treatments include inhibitors of the renin-angiotensin system and corticosteroids. Steroid resistant patients can be treated with cyclosporine, mycophenolate mofetil, or tacrolimus, with responses varying for different types of FSGS. Nevertheless, a high percentage of patients prove unresponsive to all available therapies, emphasizing the need for a deeper understanding of FSGS to guide the development of improved treatment options. In this report we define the activated pathogenic and protective molecular pathways in each major cell type of the glomerulus in the bigenic Cd2ap+/-, Fyn-/- mouse model of FSGS, thereby providing a global view the disease process that might aid in the identification of novel therapeutic targets.
Materials and methods
Mouse strains
The Cd2ap mutant (B6.129X1-Cd2aptm1Shaw/J), Tie2-GFP (Tg[TIE2GFP]287Sato/J) and Fyn (B6.129-Fyntm1Sor/J) mice were obtained from the Jackson Laboratory. MafB-GFP, Tg (MafB-EGFP) FT79Gsat and Meis1-GFP Tg (Meis1-EGFP) FO156Gsat, were from GENSAT/MMRC (http://www.gensat.org).
Animal ethics
All animal experiments were carried out according to protocols approved by the Cincinnati Children's Medical Center Institutional Animal Care and Use Committee (protocol title "mouse models of focal segmental glomerulosclerosis", number 2016–0020).
Single cell prep for FACS sorting
Mice were euthanized via cervical dislocation after sedation with isoflurane, kidneys were isolated and placed into ice-cold PBS. Glomeruli were isolated as previously described [24].
For isolating mesangial and endothelial cells, the glomeruli were pelleted, rinsed with ice-cold PBS, and then re-suspended in 200 μL TrypLE Select 10x, incubated at 37° C for 10–15 min, and triturated vigorously every 3 min. Cell digestion was monitored by taking a small aliquot and visualizing with a microscope. After digestion ice-cold 10% FBS/PBS was added and the cells were again triturated vigorously. The cells were then pelleted, washed with ice-cold 1% FBS/PBS and filtered using a 35-μM filter mesh (Falcon, cat. # 352235) before FACS sorting. GFP positive cells were collected into lysis buffer containing 0.1% SDS (in H20). After collection, the lysate was vortexed and placed on dry ice.
For isolating podocytes, a single cell suspension was derived from the glomeruli as previously described [24]. Briefly, the glomeruli were incubated for 40 min in 2 mL enzymatic digest buffer (Containing Type 2 collagenase 300 U/mL, 1 mg/mL pronase E, 50 U/mL DNAse 1) at 37° C while shaking at 1400 RPM/min in a thermomixer. Every 10 min the digestion mix was passaged two times with a 27-gauge needle [25]. After digestion, equal volume ice-cold 10% FBS/PBS was added and the cells were triturated. The cells were filtered using a 40 μM filter to remove clumps, pelleted by centrifugation, rinsed with ice-cold 1% FBS/PBS, re-suspended in 400 μL 1% FBS/PBS and filtered using a 35-μM filter mesh prior to FACS sorting. GFP-positive cells were collected in Buffer RL. After collection, the lysate was vortexed and placed on dry ice.
Target cell isolation
The Meis1-GFP, MafB-GFP, and Tie2-GFP transgene reporters enabled FACS-sorting purification of mesangial cells, podocytes and endothelial cells, respectively, from single-cell suspensions derived from the glomeruli of control (wild type or one-allele mice), and Cd2ap+/- Fyn-/- (3-allele) mice. Although 3-allele Cd2ap+/- Fyn-/- mice developed albuminuria at 5 months of age, both the 3-allele and control mice were sacrificed at an average age of approximately 10–14 months, which coincided with 3-allele mice having significantly elevated blood urea nitrogen (BUN) and increased pathological evidence of FSGS compared to control mice. From 5–9 months of age, the average BUN of 3-allele mice was 29.13 ± 1.2 compared to 26.46 ± 0.97 for control mice. From 10–14 months of age, the average BUN of 3-allele mice was 35.98 ± 2.9 compared to 27.22 ± 1.4 for control mice. The mice sacrificed were all adult (> = 5 months). The first two mice, aged 5 months, (Mesangial cells: 3-allele and control) that we sacrificed did not show substantial differences in the RNA-Seq gene profiles, so subsequently we used older mice ranging in age from 8 months to 1.5 years that showed significant proteinuria as measured by a protein gel. The average age for 3-allele and control mice was as follows in Table 1.
Table 1. Average ages of mice used for analysis.
| Cell Type | Control (Age, months) | 3-allele (Age, months) |
|---|---|---|
| Podocyte | 12 | 12 |
| Mesangial | 10 | 11 |
| Endothelial | 14 | 13 |
RNA purification, amplification and sequencing
For mesangial and endothelial samples, we purified the RNA using Zymo ZR MicroPrep Kit (cat. # R1061) with in-solution DNAse treatment. Due to a lower yield of cells for 3-allele MafB-GFP selected podocytes (due to podocyte cell loss), we used the Norgen Single Cell RNA Purification Kit (cat. # 51800) with on-column DNAse treatment for both the podocyte control and 3-allele samples. A minimum of 4K cells were used for each preparation. (Podocytes mutant 4K, 8.4K, 6.7K and ctrl 10K, 42K, 21K; mesangial mutant 48.7K, 58.5K, 53.6K and ctrl 14.8K, 42.2K 30.6K; endothelial mutant 130K, 500K, 318 K and ctrl 155K, 213K and 184K).
The initial amplification step for all samples was done with the NuGEN Ovation RNA-Seq System v2. The assay was used to amplify RNA samples to create double stranded cDNA. The concentrations were measured using the Qubit dsDNA BR assay. Libraries were then created for all samples using the Illumina Nextera XT DNA Sample Preparation Kit. The concentrations were measured using the Qubit dsDNA HS assay. The size of the libraries for each sample was measured using the Agilent HS DNA chip. The samples were placed in a pool. The concentration of the pool was optimized to acquire at least 15–20 million reads per sample. Sequencing was performed on the Illumina HiSeq2500, single-end 75 base pair. Sequencing data is available in the Gene Expression Omnibus [26], accession number GSE123179.
RT-qPCR validations
Glomeruli were isolated from 3-allele and control mice using the sieving method described previously [24]. Glomeruli were then lysed by addition of Qiagen Buffer RLT, vortexing and then placing the tubes on dry ice. RNA was purified using the Qiagen RNEasy Micro Kit (cat. # 74004). RNA was quantified using a NanoDrop spectrophotometer (NanoDrop Technologies, DE, USA). The quality of the RNA was analyzed using an Agilent Bioanalyzer (nano-chip). RNA Integrity Numbers (RIN) were > 8.0. Reverse transcription reactions were set up using the RT-VILO SuperScript cDNA synthesis kit from Invitrogen (cat. # 11754–050). The cDNA was then precipitated by adding 2 μL of 3 M sodium acetate, 1 μL glycogen and 57.5 μL 100% ETOH for a total volume of 80.5 μL. Following overnight incubation at -20°C, the cDNA was pelleted at 14,000 RPM for 20 minutes at 4° C. The pellet was rinsed with 70% ETOH, decanted and air dried. The cDNA was re-suspended in 51 μL H20 and then quantified using a NanoDrop spectrophotometer set to measure single-stranded DNA. RT-qPCR was performed using the PowerUp SYBR Green Master Mix (Applied Biosystems, cat. # A25742) and the StepOnePlus using the relative standard curve method. Samples were normalized to beta-actin (mesangial and endothelial genes) or nephrin (podocyte genes).
RT-qPCR primer design
Genes were selected based on restricted up-regulation in one sorted glomerular 3-allele cell type compared to control cells, or in the case of Serpine1 up-regulation in several cell-types. RT-qPCR primers were designed using Primer-Blast to amplify a product size between 75 and 200 bp using the mRNA RefSeq database of Mus musculus. Where possible, the primers were designed to span an intron. Primers used are listed below in Table 2.
Table 2. Primer sequences used for RT-qPCR validations.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Actb | TTTGCAGCTCCTTCGTTGCC | ACCCATTCCCACCATCACAC |
| Aldh1a2 | TCCCTAAATGGCGGTAAGCC | CCACGGGATGATCTGTCCAC |
| Cpe | AGGCGGTCCTAACAATCACC | GTACCGCTCCGTGTCTCATC |
| Cpne7 | GGACCCATTGACCAAGTCCG | TCTGCACCCCCTCGAAGTAG |
| Endou | CAGGGAGGTCATGAAGACGG | TCGAGTACAGCCCAAACCAC |
| FrzB | GTGGAAGGATCGGCTTGGTA | CTGGCCGGGGATTAGAGTTC |
| Nphs1 | TAATGTGTCTGCAGCCCAGG | TCCACTCCAGTCCTACCGAG |
| Parm1 | TTGGAGGTGCAGCATACCTG | GACCCGTAGTCATGGTCGTC |
| Rspo1 | TCTGAGCTGGACACACATCG | GCAGAATGAAGAGCTTGGGC |
| Serpine1 | ACAGGCACTGCAAAAGGTCA | TCAAGGCTCCATCACTTGGC |
| Spon2 | ACCGACAGTGGTTTCACCTT | CGAAGGTCACTTTGGCGATG |
| Thbs1 | CCAGAGCATCTTCACCAGGG | ACCACGTTGTTGTCAAGGGT |
| Tnc | CCACATCTCAGGGCTTCCAC | GAAACCGTCTGGAGTGGCAT |
RNA-seq data analysis
RNA-seq data was analyzed using Strand NGS 3.2. The reads were aligned to mm9. Reads were quantified using DeSeq with normalization threshold of 1. The baseline was set to the median of all samples.
Filtering gene lists (all samples)
Reads were filtered based on genic region; spliced, partial genic, exonic, exon intron junction and genic reads were retained; intergenic and intronic reads were removed. Reads were further filtered on read quality metrics with the following parameters: Quality threshold > = 20, N's allowed in read < = 0, number of multiple matches allowed < = 1, and reads were removed that failed vendor's QC. Duplicate reads were removed, with a threshold of 4.
Generating gene lists comparing podocytes, mesangial cells, endothelial cells and controls
At least 5.0 NRPKM (very similar to the standard RPKM or FPKM) in 3 out of 6 samples was required. A moderated t-test was then used to compare 3-allele and control samples with a corrected p-value cut-off of 0.05. The p-value computation was asymptotic and no multiple testing correction was used. This list was then used to perform a fold-change analysis between 3-allele and control samples, with a fold-change cut-off of > = 1.5. Y-linked genes, associated with sex differences between the samples, were removed from the final gene list.
Filtering endothelial genes from MafB-GFP podocyte samples
For the podocytes, in order to remove the effects of a small percentage of contaminating endothelial cells, the WT glomerular endothelial cells were compared with WT podocytes. At least 80 NRPKM was required in 2 out of 6 samples (podocyte or endothelial); the cell types were compared with Audic Claverie test with no multiple-testing correction and a p-value cut off of 0.05. A fold change> = 4 in endothelial cells compared to podocytes was used to generate a list of genes with high specific expression in endothelial cells. This list of genes was compared using a Venn diagram with differentially expressed podocyte (MAFB) genes. Genes were excluded from the list of differentially expressed MAFB genes which were found in both the MAFB list and the list of genes up-regulated in endothelial cells.
Heat map parameters
The heat map was generated within Strand NGS software using a hierarchical clustering algorithm and clustered by normalized intensity values. The heat map was clustered on entities and conditions. The similarity measure is Pearson Centered. The linkage rule is Ward’s. There was no clustering within conditions.
Blood analyses
Following anesthetization with isoflurane, serum was collected from mice via submandibular blood draw using 5.5 mm Goldenrod animal lancets. Serum BUN, creatinine and albumin were analyzed. Blood was collected using BD Microtainer serum collection tubes from the puncture site and then centrifuged at 1000 g for 10 min at 4°C to isolate serum; the serum was then shipped to IDEXX laboratories for analysis. Average lab values between 3 allele and control mice were compared with using a two-tailed t-test with unequal variance (total n = 44 3 allele mutants, 36 CTRL for phenotypic comparison).
Results and discussion
In this study we used RNA-seq to define the altered gene expression patterns of each major cell type of the glomerulus in the Cd2ap+/-, Fyn-/- bigenic mouse model of FSGS. To isolate the podocytes, mesangial cells and endothelial cells from the control and mutant FSGS kidneys we used three different transgenic reporter mouse lines. MafB-GFP, Meis1-GFP and Tie2-GFP which drive cell type restricted GFP expression in the podocytes, mesangial cells and endothelial cells of the glomerulus, respectively [24]. We first isolated glomeruli, using a sieving protocol, and then used further enzymatic dissociation to produce single cell suspensions. By combining the three mutant alleles (Cd2ap+/-, Fyn-/-) with the appropriate GFP transgene reporter it was possible to purify the podocytes, mesangial cells and endothelial cells by fluorescent activated cell sorting (FACS). We then examined the FSGS altered gene expression patterns of all three major glomerular cell types using RNA-seq to begin to better understand the underlying pathogenic molecular pathways.
Cd2ap+/-, Fyn-/- Phenotype
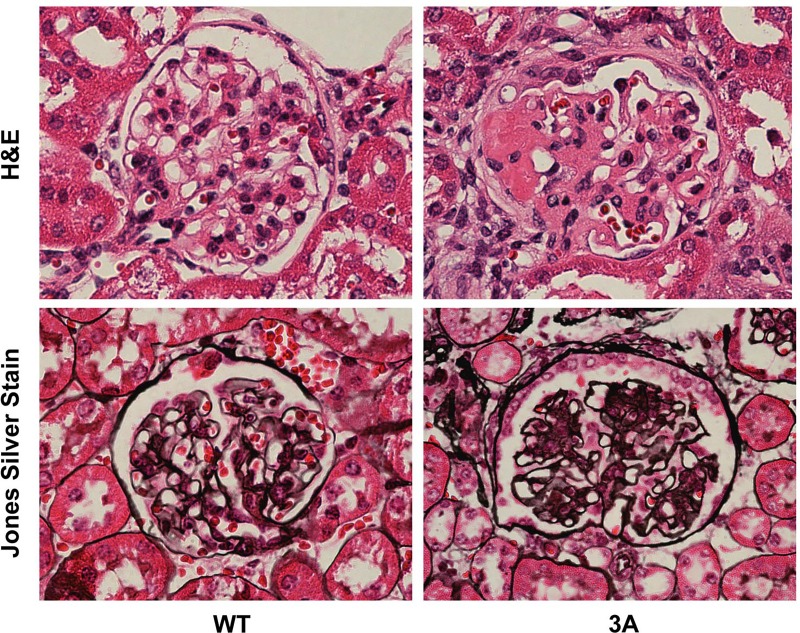
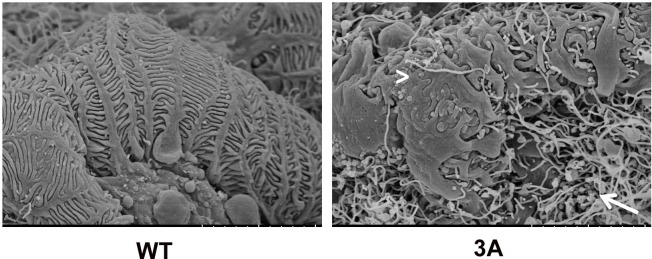
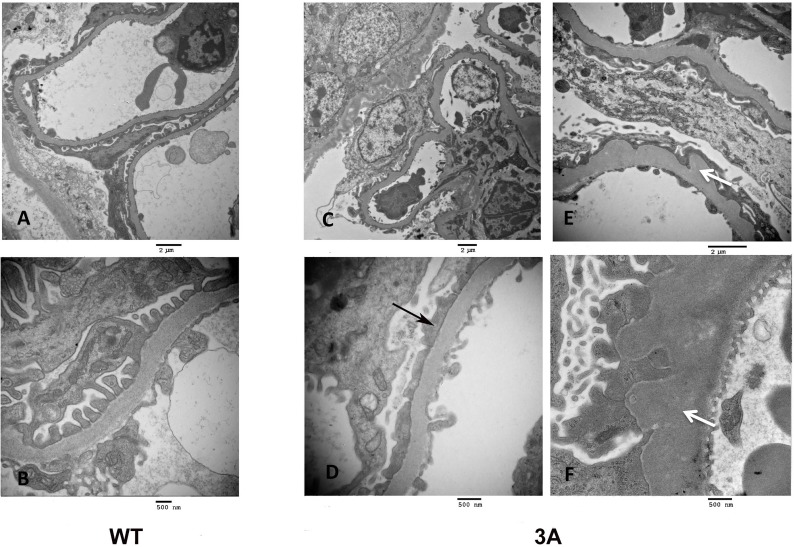
In agreement with previous studies, we observed the onset of proteinuria at around 5 months of age in the Cd2ap+/-, Fyn-/- mice, with near 100% penetrance [17]. H&E and Jones Silver Stain were used to characterize the FSGS like pathology observed in the Cd2ap+/-, Fyn-/- glomeruli (Fig 1). There were localized regions of scarring in some glomeruli, as well as capillary lumen loss and increased mesangial matrix. Scanning EM of the Cd2ap+/-, Fyn-/- glomeruli showed podocyte depletion, fusion of foot processes and general disorganization of remaining podocytes (Fig 2). Transmission EM further showed the altered podocyte foot process structures and, of interest, regions of expanded GBM (Fig 3). At the time of sacrifice, around 11 months, the Cd2ap+/-, Fyn-/- mice showed normal serum creatinine, elevated serum BUN (33.6 mg/dL versus 26.2 mg/dL for control, P = 0.0001), and decreased serum albumin (2.58 g/dL versus 2.89 g/dL for control, P = 0.03).
Fig 1. Histology of wild type (WT) and Cd2ap+/-, Fyn-/- three allele mutant (3A) glomeruli.
H&E staining, top panels, show partial blockage of mutant capillaries and regions of scarring, particularly pronounced on the left extreme. (3A was 14 months, Ctrl was 10 months). Bottom panels show Jone’s Silver stain, with increased staining of glomerular basement membranes in 3A glomerulus. (3A was 15 months, Ctrl was 10 months).
Fig 2. Scanning electron microscopy of wild type (WT) and three allele mutant (3A) glomeruli.
Note uniform capillary coverage by wild type podocytes, with evenly spaced foot processes and slit diaphragms. In contrast the mutant podocytes are disorganized, partially detached, with foot processes sometimes fused (arrowhead). In addition, the mutant glomerulus showed abundant fibrils (arrow). (Both 3A and Ctrl were 14 months).
Fig 3.
Transmission electron microscopy of glomeruli of wild type (WT), left panels, and mutant (3A), middle and right panels. Upper panels are lower magnification and lower panels are higher magnification (see size bars). Note evenly spaced foot processes and slit diaphragms in the WT, with the mutant showing fused foot processes (black arrow) and expanded glomerular basement membrane (white arrow). (Both 3A and Ctrl were 14 months).
Assaying cell type purity
The isolation of strongly enriched populations of glomerular cell types can be challenging. The podocytes, in particular, are tightly wrapped around capillary loops with extensively interdigitated foot processes attached to the glomerular basement membrane. The mutant podocytes, within partially sclerotic glomeruli, were particularly challenging to detach.
We used two metrics to assay enrichment levels for the three cell types following FACS. The first was enriched expression levels of expected cell type specific marker genes. By this measure all FACS cell preparations showed strongly elevated expression of predicted markers, indicating robust enrichment of desired cell types. For example, Nphs2 expression is an excellent marker for the podocyte, and all six podocyte cell preparations, including three Cd2ap+/-, Fyn-/- mutant and three control, showed very high expression levels of Nphs2, in the range of 1,400–2,300 RPKM.
A second and more stringent metric is to look for the expression levels of marker genes associated with potential contaminating cell types. By this measure, for example, the endothelial and mesangial cell preparations were very free of podocyte contamination, with podocyte marker Nphs2 expression levels of only 0, 0, 0, 0, 4 and 1 RPKM in the six endothelial cell preparations and 0, 2, 0, 1, 1, and 11 RPKM in the mesangial cell preparations. In similar manner the mesangial cells were essentially free of endothelial contamination and the endothelial cells also free of mesangial contamination. Nphs2 was used as a marker of possible podocyte contamination because of its extremely restricted expression in podocytes. We have observed that some other genes whose transcription is widely considered podocyte specific actually show low but significant levels of expression in other cell types. For example, Wt1 is expressed not only in podoctyes, but also in mesangial cells, albeit at a level about six fold lower than in podocytes [27].
The podocytes, however, showed detectable levels of cross contamination. For example, the endothelial expressed gene Kdr, gave an average expression level of 336 RPKM in endothelial cell preparations and 15 RPKM in podocytes, suggesting a low level of contamination. The bioinformatics analysis pipeline was therefore modified for podocytes to take this into account, as described in Material and Methods.
Podocytes
As previously mentioned, to define the gene expression pattern of mutant podocytes we made Cd2ap+/-, Fyn-/- mice that also carried the MafB-GFP transgene, which gives GFP expression specifically in podocytes in the developing kidney [26]. Glomeruli were purified, from mutants and controls, dissociated to give single cell suspensions, and podocytes isolated by FACS. RNA was purified and used for RNA-seq, and the resulting data analyzed with Strand NGS software.
There were many significant gene expression changes in the Cd2ap+/-, Fyn-/- podocytes, compared to controls, with 90 genes up-regulated greater than 2 fold change (FC) and 29 genes down-regulated (> 2 FC) (S1 Table). A heatmap showing gene expression changes in bigenic mutant podocytes compared to controls is shown in Fig 4, with an expandable version including gene names provided (S1 Fig).
Fig 4. Heatmap showing gene expression changes in control (ctrl) versus Cd2ap+/-, Fyn-/- three allele mutant (3A) podocytes.
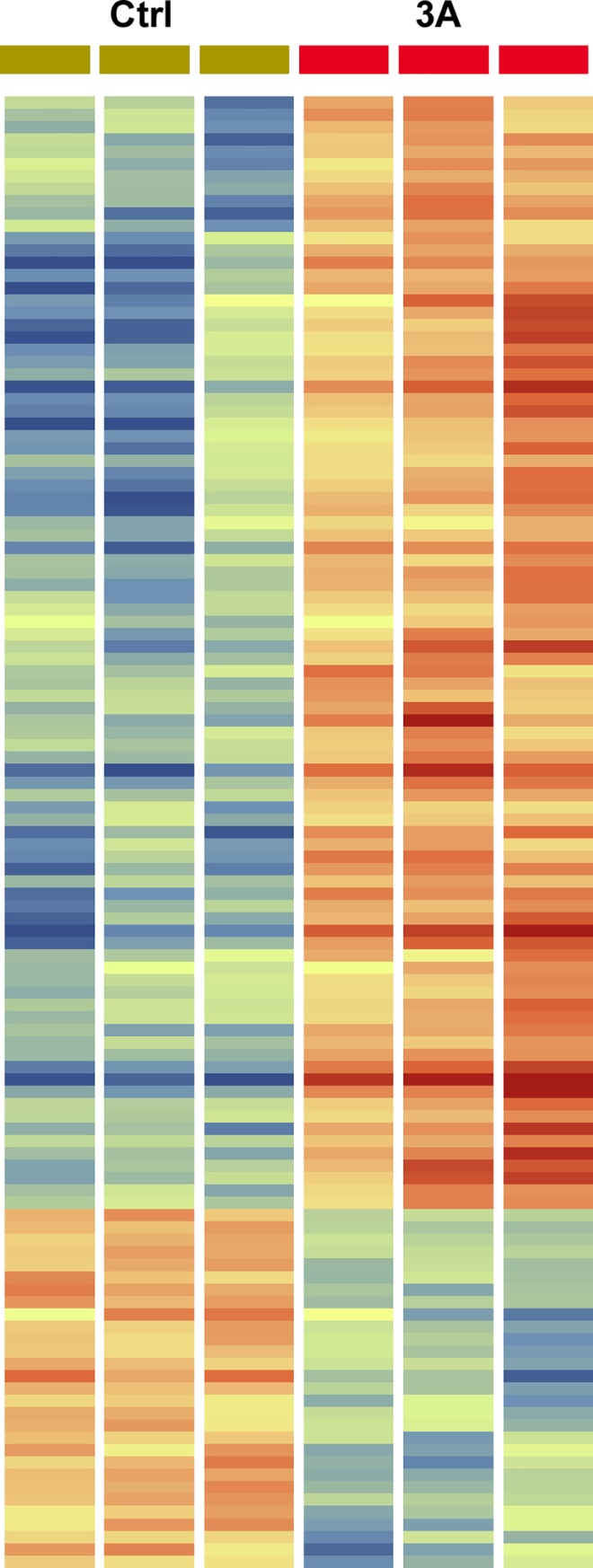
There were three samples of each, with red indicating stronger expression and blue indicating weaker expression. Genes with greater than 2 fold change are shown. An expandable version including gene names is included in supplementary data (S1 Fig). Lists of genes with fold change are included in S1 Table.
Growth factor related genes with elevated expression in mutant podocytes included Gdnf (22 FC), Tnc (22 FC), Bmp4 (5 FC), Tgfβ1 (2.5 FC), Tgfβ2 (3.5 FC), Cxcl12 (1.7 FC), Nrp2 (3.0 FC) Rspo1 (3.0 FC), Ctgf (1.5 FC), and Vegfb (2.0 FC). This group of genes includes a very strong Tgfβ family signature, as detailed below.
Gdnf, glial derived neurotrophic factor, showed 22 FC increased expression in podocytes of mutants. During kidney development Gdnf is expressed by cap mesenchyme nephron progenitors as well as stromal cells [28], driving branching morphogenesis of the ureteric bud, which expresses the Gdnf receptor Ret. Gdnf is a member of the Tgfβ superfamily. Of particular interest, Gdnf has been shown to be a survival factor in injured podocytes, acting in an autocrine manner [29].
The increased expression of Tgfβ1, Tgfβ2, Vegfb and Bmp4, additional members of the Tgfβ family, give evidence for a role for podocytes in driving fibrosis, since Tgfβ has been shown to be a major mediator of fibrosis [30, 31]. Bmp4 has been shown to play a profibrotic role in the liver [32], and to drive expression of the fibrosis related gene Acta2 in hepatocyte stellate cells [33]. Of note, we did also observe in the mutant podocytes strong upregulation (8.2 FC) of Acta2, a classic smooth muscle marker associated with myofibroblasts. During fibrosis many cell types can differentiate into myofibroblasts [34].
We also observed the upregulation of Arid5b (1.6 FC) in mutant podocytes. This gene encodes a component of a histone demethylase complex, resulting in target gene activation. Overexpression of Arid5b in fibroblasts can result in induction of smooth muscle genes, including smooth muscle actin, Acta2 [35].
Also connected to the Tgfβ pathway, we observed upregulation of Snai2 (5.8 FC), encoding the transcriptional repressor Snail. Tgfβ has been shown to induce Snail expression [36]. Further, expression of Snail has been shown to activate Tgfβ signaling in breast cancer cells [37], providing a feed forward loop for this pathogenic pathway.
Tenascin-C (Tnc) showed ~22 fold elevated expression in mutant podocytes. Tenascin-C is a multifunctional extracellular matrix protein that is upregulated during wound healing, with persistent expression observed in a variety of chronic pathological conditions [38]. Importantly, Tnc expression in fibroblasts drives a fibrotic response, once again including upregulation of Acta2 expression [39]. Further, there is a dramatic attenuation of lung fibrosis in bleomycin treated mice carrying homozygous mutation of Tnc [39]. Tnc expression is upregulated by Tgfβ in fibroblasts through Alk5 mediated Smad2/3 signaling [39]. Tnc promotes secretion of Tgfβ, which in turn promotes expression of Tnc, resulting in yet another a feed forward loop [39].
Tnc transcripts undergo extensive alternative splicing, potentially giving rise to hundreds of different isoforms [40]. In addition, the Tnc protein undergoes many variable post translational modifications, further contributing to its observed considerable functional diversity. Tnc can bind multiple ligands including fibronectin, contactin, glypican, and integrins [40], and can also activate epidermal grown factor receptor [41] and the Toll like receptor TLR-4 [42]. Tnc is a poor adhesive substrate and blocks fibronectin mediated adhesion and is therefore anti-adhesive. The dramatic upregulation of Tnc in the mutant podocytes and its multiple functions including pro-fibrotic and anti-adhesive suggest a significant role in the disease process.
The observed upregulation of Rspo1 (3 FC) is also of interest in relation to fibrosis/sclerosis. R-spondins are extracellular Wnt agonists that promote Wnt signaling by reducing degradation of Frizzled and LRP co-receptors [43]. Further, canonical Wnt signaling has been shown to play a central role in multiple fibrosing diseases, including pulmonary, renal and liver fibrosis [44–46].
The mutant podocytes also showed strong evidence of elevated retinoic acid (RA) related pathways. Expression of Aldh1a1, encoding an enzyme driving the final step of RA synthesis [47], the conversion of retinaldehyde to retinoic acid, was increased 7 fold in Cd2ap+/-, Fyn-/- mutants. RA can induce expression of Ripply3 as well as Tbx1 in the pre-placodal ectoderm [48]. Retinoic acid signaling can also induce Tbx3 during limb development [49]. Of interest, we observed upregulation of both Ripply3 (7 FC) and Tbx3 (5.2 FC) in mutant podocytes, consistent with elevated RA signaling. Ripply3 is a transcriptional repressor that can interact with the transcription factor Tbx1, converting it from an activator to a repressor [48].
Several lines of evidence suggest that the observed elevated RA synthesis in the Cd2ap+/-, Fyn-/- mutant podocytes represents a protective response [50]. It has been shown in the rat model of membrane nephropathy that RA can help restore podocyte number, with an increase in the number of parietal epithelial cells expressing podocyte markers, suggesting their transitional recruitment to the visceral epithelial podocyte population [51]. Conversely, vitamin A deficiency, which results in reduced RA levels, delays podocyte recovery in a puromycin treatment model of podocytopathy [52]. In the adriamycin murine model of FSGS reducing RA synthesis resulted in increased proteinuria and glomerulosclerosis, while treatment with RA reduced proteinuria and reduced podocyte loss [53]. RA has also been shown to have an anti-inflammatory effect on podocytes [54]. In addition, RA has been shown to reduce apoptosis in cultured podocyte model systems [55]. Despite these promising results, the RA treatment of glomerular disease has been hindered by its toxicity, with multiple side effects including liver toxicity, hyperlipidemia, myalgia, and athralgias [56]. Efforts are underway to develop RA based therapeutics with reduced toxicity [50].
Another highly upregulated gene in the mutant podocytes was Spon2 (135 FC), which encodes an extracellular matrix protein that is critical for inflammatory cell recruitment [57], efficient dendritic cell priming of T-lymphocytes [58] and trafficking of eosinophils [59], suggesting an important role in driving the immune response. Fxyd5 (9.6 FC), encoding the gamma subunit of the Na,K-ATPase, as well as Atp1b1 (9.4 FC), encoding the beta chain of Na,K-ATPase were also increased in expression in mutant podocytes. Sost, encoding sclerostin, was also strongly upregulated (10 FC) in mutant podocytes. It is a BMP antagonist, perhaps counteracting in some measure the observed upregulation of Bmp4.
GO analysis of the list of genes upregulated in the mutant podocytes identified elevated protein kinase activity and increased expression of growth factors as the most significant molecular functions. The most strongly impacted biological process was negative regulation of cell adhesion (P = 4.26E-9), likely related to FSGS loss of podocytes. Other upregulated biological processes were cell motility (P = 7.8E-9) and programmed cell death (P = 3.5E-8). The observed apoptosis gene expression signature in the mutant podocytes, with for example elevated expression of Casp4 (5.8 FC), is also consistent with the well-documented podocyte depletion in FSGS [60, 61]. Fig 5 shows a cytoscape with multiple identified functionalities and associated genes. Complete lists of molecular functions and biological processes found in the GO analysis are listed in S1 Table.
Fig 5. Cytoscape showing some of the genes upregulated in Cd2ap+/-, Fyn-/- mutant podocytes and their associated functionalities.
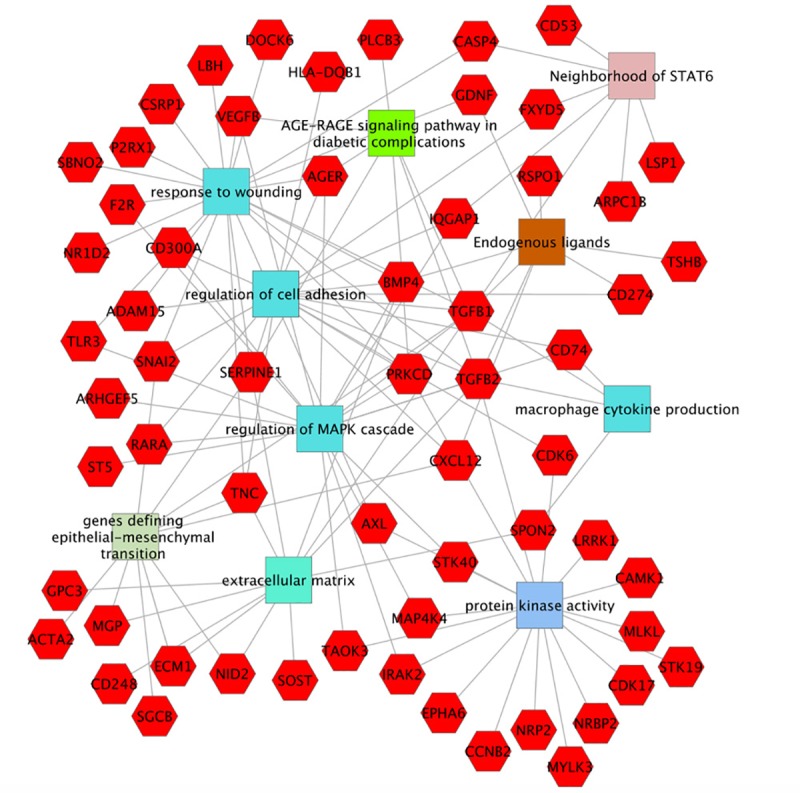
Red hexagons represent upregulated genes in mutant podocytes. Molecular functions (Protein kinase activity), Biological processes (response to wounding, regulation of cell adhesion, regulation of MAPK cascade, macrophage cytokine production), Cellular components (extracellular matrix), Pathways (AGE-RAGE signaling pathway in diabetic complications), Computational (neighborhood of STAT6) and Co-expression (genes defining epithelial-mesenchymal transition), as determined using ToppGene (Toppgene.cchmc.org).
There were fewer genes showing strong downregulation compared to upregulation for the mutant podocytes (90 genes upregulated > 2FC versus 29 downregulated > 2FC). Among downregulated genes were Robo2 (1.5 FC) and Dpysl2 (1.6 FC), both involved in axon guidance, and Ncam1 (3.2 FC), encoding neural cell adhesion molecule, all perhaps reflecting altered neural character of the mutant podocytes. Reduced expression of NCAM1 could contribute to FSGS associated podocyte loss.
Perhaps surprising, we did not observe the downregulation of accepted podocyte differentiation markers, such as Wt1, Nphs1, Nphs2, and Podx1, which has been previously reported [62, 63]. In some earlier studies the reduced expression of these markers could have been the result of reduced podocyte numbers. Our results confirm other studies which examined isolated podocytes in murine FSGS models and failed to detect reduced expression of these podocyte differentiation genes [24, 64]. These combined observations call into question the conclusion that podocyte dedifferentiation is associated with FSGS.
GO analysis of the list of down regulated genes gave the strongest Biological Processes signature for reduced protein ubiquitination (uncorrected P = 3.4E-5), with seventeen associated genes. Also of interest, four genes of the mediator complex were down regulated (Med10, Med11, Med28, Med31). The mediator complex is absolutely required for pol II transcription, but in addition to its role as a general transcription factor it functions as a coactivator and corepressor in the regulation of transcription [65]. Little else emerged with statistical significance in the GO analysis, perhaps due to the relatively small number of podocyte genes with downregulated expression.
The Actn4-/- genetic murine model of FSGS was previously transcriptionally profiled using the Translating Ribosome Affinity Purification (TRAP) method, with cell type specific expression of transgenic Collagen-1α1-eGFP-L10a allowing affinity enrichment of podocyte expressed RNAs [64]. Of interest, the podocytes of Actn4-/- mice also showed elevated expression of aldehyde dehydrogenase, similar to the Cd2ap+/-, Fyn-/- mutant podocytes in this report. Further, the podocytes of Actn4-/- mice showed increased expression of Pamr1, which also gave elevated expression in the Cd2ap+/-, Fyn-/- mutant podocytes (3.6 FC, S1 Table). The Pamr1 gene has been associated with muscle regeneration [66], which is of interest given the contractile nature of the podocyte [67], perhaps playing a role in counteracting the perfusion pressure of the capillaries. Nevertheless, the gene expression signature of the podocyte shows only a weak muscle signature [26]. The complete list of commonly upregulated podocyte genes in the Actn4-/- and Cd2ap+/-, Fyn-/- mice includes Aldh1a1, Atp1b1, Axl, Casp4, Ccnb2, Cdk6, Cldn1, Csrp1, Ctgf, Fam26e, Filip1, Lbh, Nkd1, Pamr1, Peg3, S100a6, Tgfb1, and Tgfb2. The commonly down regulated podocyte genes in these two studies included Abca5, Aifm3, Fbxo3, Ggt5, Glcci1, Itpr3, Ncam1, and Spnb1.
Mesangial cells
Mesangial expansion is a hallmark of FSGS, with increased extracellular matrix and mesangial cell proliferation. The Cd2ap+/-, Fyn-/- mutant mesangial cells showed upregulation of 55 genes (>1.5 FC) compared to control (S2 Table). One of the upregulated genes (4.3 FC) with strong expression, over 100 RPKM in mutants, was Aldh1a2, involved in the synthesis of retinoic acid, similar to Aldh1a1, which was upregulated in podocytes.
Thrombospondin (Thbs1, 3.8 FC) was strongly upregulated in mutant mesangial cells. Thbs plays an important role in the activation of Tgfβ, which is secreted in inactive pro-cytokine form. The inflammatory phenotype of Thbs1 mutants closely resembles that of Tgfβ mutants [68]. Given the key role of Tgfβ in fibrosis the elevated Thbs1 expression in mutant mesangial cells is likely pro-fibrotic.
Upregulated mesangial cell genes in Cd2ap+/-, Fyn-/- mutants also included ccdc68 (3.9 FC), encoding a centriole protein, Frzb (4.1 FC) encoding a secreted Wnt binding protein involved in the regulation of bone development, Tnnt2 (4.1 FC) involved in muscle contraction, Col8a1 (1.9 FC) involved in extracellular matrix, and F2r (1.6 FC), encoding a G-protein coupled thrombin receptor involved in the thrombotic response.
It is interesting to again compare the altered gene expression observed in Cd2ap+/-, Fyn-/- mutant mesangial cells in this report with previously observed gene expression changes in other mouse genetic models of kidney disease. Mesangial cell upregulated genes in Cd2ap+/-, Fyn-/- mice that were also upregulated in the mesangial cells of the db/db diabetic nephropathy mouse model include Cpe, Anxa3, Thbs1, Pmp22, Tspan2 and Akap12, and closely related gene family members for Ppap2c, Mmp3 and Adam22 [27]. Some of the top genes upregulated in Cd2ap-/- mesangial cells, and also upregulated in Cd2ap+/-, Fyn-/- mutant mesangial cells include Col8a1, Ccdc68, Thbs1, Tnnt2, Frzb, Pmp22, and Aldh1a2 [24]. The Thbs1 gene stands out as strongly upregulated in all three genetic models. The robust correlation for Cd2ap-/- and Cd2ap+/-, Fyn-/- mutant mesangial cells is perhaps not surprising given the overlapping genetics.
It was interesting to find that in some cases the same pathway was activated in both podocytes and mesangial cells. For example, aldehyde dehydrogenase activity, reflecting increased RA synthesis, was elevated in both Cd2ap+/-, Fyn-/- podocytes and mesangial cells. Further, Prss23 (4.7 FC) was one of the most strongly upregulated genes in Cd2ap+/-, Fyn-/- mesangial cells, and also one of the most strongly upregulated genes in podocytes of the Cd2ap-/- FSGS model [24], although, interestingly, it was not strongly upregulated in Cd2ap+/-, Fyn-/- podocytes. In addition, Prss23 is upregulated in the glomeruli of human patients with FSGS [69]. This common thread of upregulated Prss23 in multiple FSGS mouse models, and cell types, as well as in human FSGS gives evidence for a significant role. The serine protease encoded by Prss23 can activate Par2 (Protease-Activated Receptor 2), which has been implicated in TGFβ1 induced podocyte injury in the Adriamycin model of nephropathy in rats [70]. It has also been shown that Prss23 can promote TGFβ signaling during zebrafish cardiac valve formation as well as in human aortic endothelial cell assays [71]. These observations give evidence for a pathogenic role for Prss23 in FSGS and suggest a novel therapeutic target. Protease inhibitors have proven very effective in the treatment of HIV, where viral specific proteases are required for viral replication.
There were relatively few downregulated genes in Cd2ap+/-, Fyn-/- mutant mesangial cells, with only six with greater than 2 FC, and all of these showing low expression levels, even in controls (S2 Table).
GO analysis of the mesangial up regulated genes, again using Toppgene (Toppgene.cchmc.org), gave relatively few hits with FDR (Benjamini and Hochberg) corrected P values below 0.05. These included the molecular functions proteoglycan binding and protein kinase A binding, and the cellular component extracellular space.
Endothelial cells
The endothelial cells are the third major cell type of the glomerulus. They can also play a role in the progression of FSGS through the production of growth factors and cytokines, leaky angiogenesis, and the recruitment of macrophages and leukocytes. The observed gene expression changes in the Cd2ap+/-, Fyn-/- mutant endothelial cells were modest in number, with only 45 genes showing greater than 1.5 FC and 15 genes with greater than 2 FC upregulation. There were even fewer downregulated genes in mutants, with 26 showing >1.5 FC and only 5 with >2.0 FC (S3 Table).
Upregulated genes included Apnlr encoding a G-coupled receptor for Apelin that inhibits adenylate cyclase and is implicated in angioblast migration and regulation of blood vessel formation. Also upregulated was Kctd10, which binds proliferating cell nuclear antigen (PCNA) and may be involved in DNA synthesis and cell proliferation and is also involved in ubiquitination.
Another upregulated gene in mutant endothelial cells was Nestin, which encodes an intermediate filament protein and is normally associated with neural stem/progenitor cells. It is, however, also expressed in endothelial cells in pancreas [72], in ovary and placenta [73], as well as vascular neoplasms [74] and cancers [75], where it is a marker of neovasculature.
GO analysis for mutant endothelial cell upregulated genes found biological processes including negative regulation of response to oxidative stress, and negative regulation of apoptosis in response to hydrogen peroxide.
QPCR validations
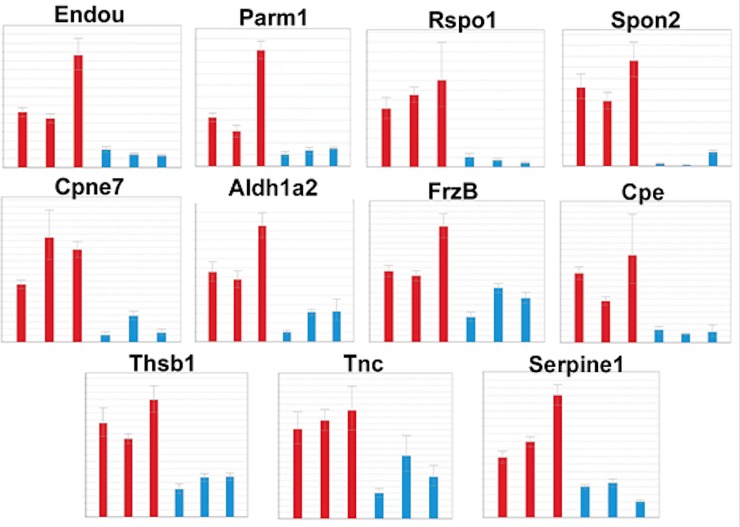
The RNA-seq results were validated using RT-QPCR. Glomeruli from three mutant allele Cd2ap+/-, Fyn-/- and control mice were isolated using a sieving procedure [24], RNA purified and used for RT-QPCR. Genes were selected based on restricted up-regulation in mutants in one sorted glomerular cell type compared to control cells. Samples were normalized to beta actin (actb) for mesangial genes, and nephrin (Nphs2) for podocyte genes. RT-QPCR validated upregulation of Endou, Parm1, Serpine1, Spon2, and Rspo1 in mutant podocytes, and Cpe, Aldh1a2, Tnc, Cpne7, Frzb, and Thbs1 in mutant mesangial cells (Fig 6).
Fig 6. RT-QPCR validation of gene expression changes.
Using RNA from isolated glomeruli the RNA-seq predicted changes in expression of Endou, Parm1, Serpine1, Spon2, and Rspo1 in mutant podocytes, and Cpe, Aldh1a2, Tnc, Cpne7, Frzb, and Thbs1 in mutant mesangial cells were validated.
Conclusions
FSGS is a major cause of ESRD, with a high percentage of patients unresponsive to available therapies. Improved understanding of the molecular underpinnings of the disease process could provide insights leading to novel therapeutic approaches. In this study we carry out an RNA-seq analysis of the altered gene expression patterns of podocytes, mesangial cells and glomerular endothelial cells of the bigenic Cd2ap+/-, Fyn-/- mutant mouse model of FSGS. The podocytes showed the most dramatic changes, with upregulation of many genes related to the Tgfβ family/pathway, including Gdnf, Tgfβ1, Tgfβ2, Snai2, Vegfb, Bmp4, and Tnc. The mutant podocytes also showed upregulation of Acta2, a marker of smooth muscle and associated with myofibroblasts, which are implicated in driving fibrosis. GO analysis of the podocyte upregulated genes identified elevated protein kinase activity, increased expression of growth factors, and negative regulation of cell adhesion, perhaps related to the observed podocyte loss in FSGS.
Both podocytes and mesangial cells showed strong upregulation of aldehyde dehydrogenase genes involved in the synthesis of RA. Similarly, the Cd2ap+/-, Fyn-/-mesangial cells, as well as podocytes in other genetic models, and the glomeruli of human FSGS patients, all show upregulation of the serine protease Prss23, with the common thread suggesting important functionality. Another gene with strong upregulation in the Cd2ap+/-, Fyn-/- mutant mesangial cells as well as multiple other mutant mouse models of FSGS was thrombospondin, which activates the secreted inactive form of Tgfβ.
The Cd2ap+/-, Fyn-/- mutant endothelial cells showed elevated expression of genes involved in cell proliferation, angioblast migration, angiogenesis, and neovasculature, all consistent with the formation of new blood vessels in the diseased glomerulus. In total the data herein provide a global definition of the pathogenic and protective molecular pathways that are activated in the three major cell types of the glomerulus in the bigenic Cd2ap+/-, Fyn-/- mouse model of FSGS.
Supporting information
List of genes up and down regulated in mutant podocytes compared to control, including fold change and nRPKM expression levels in each mutant and control sample. GO analysis results for molecular functions and biological processes, including P values and lists of associated genes.
(XLSX)
List of genes up and down regulated in mutant mesangial cells compared to control, including fold change and nRPKM expression levels in each mutant and control sample.
(XLSX)
List of genes up and down regulated in mutant endothelial cells compared to control, including fold change and nRPKM expression levels in each mutant and control sample.
(XLSX)
Red indicates high expression and blue corresponds to low expression levels.
(TIF)
Acknowledgments
We thank Hung Chi Liang for carrying out amplification reactions for RNA-seq analysis. We thank Dominic Distasio for assistance in managing the mouse colony.
Data Availability
Sequencing data is available in the Gene Expression Omnibus (GEO), accession number GSE123179.
Funding Statement
This work was supported by the National Institutes of Health grant P50 DK096418 to SSP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kitiyakara C, Kopp JB, Eggers P. Trends in the epidemiology of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23(2):172–82. Epub 2003/04/22. 10.1053/snep.2003.50025 . [DOI] [PubMed] [Google Scholar]
- 2.Sprangers B, Meijers B, Appel G. FSGS: Diagnosis and Diagnostic Work-Up. Biomed Res Int. 2016;2016:4632768 Epub 2016/06/18. 10.1155/2016/4632768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitiyakara C, Eggers P, Kopp JB. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44(5):815–25. Epub 2004/10/20. . [PubMed] [Google Scholar]
- 4.Asinobi AO, Ademola AD, Okolo CA, Yaria JO. Trends in the histopathology of childhood nephrotic syndrome in Ibadan Nigeria: preponderance of idiopathic focal segmental glomerulosclerosis. BMC Nephrol. 2015;16:213 Epub 2015/12/17. 10.1186/s12882-015-0208-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43(2):368–82. Epub 2004/01/30. 10.1053/j.ajkd.2003.10.024 . [DOI] [PubMed] [Google Scholar]
- 6.Meehan SM, Chang A, Gibson IW, Kim L, Kambham N, Laszik Z. A study of interobserver reproducibility of morphologic lesions of focal segmental glomerulosclerosis. Virchows Arch. 2013;462(2):229–37. Epub 2012/12/25. 10.1007/s00428-012-1355-3 . [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg AZ, Kopp JB. Focal Segmental Glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12(3):502–17. Epub 2017/03/01. 10.2215/CJN.05960616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadowski CE, Lovric S, Ashraf S, Pabst WL, Gee HY, Kohl S, et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2015;26(6):1279–89. Epub 2014/10/29. 10.1681/ASN.2014050489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trautmann A, Bodria M, Ozaltin F, Gheisari A, Melk A, Azocar M, et al. Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin J Am Soc Nephrol. 2015;10(4):592–600. Epub 2015/01/31. 10.2215/CJN.06260614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22(11):2129–37. Epub 2011/10/15. 10.1681/ASN.2011040388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mele C, Iatropoulos P, Donadelli R, Calabria A, Maranta R, Cassis P, et al. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med. 2011;365(4):295–306. Epub 2011/07/16. 10.1056/NEJMoa1101273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lennon R, Stuart HM, Bierzynska A, Randles MJ, Kerr B, Hillman KA, et al. Coinheritance of COL4A5 and MYO1E mutations accentuate the severity of kidney disease. Pediatr Nephrol. 2015;30(9):1459–65. Epub 2015/03/06. 10.1007/s00467-015-3067-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shaw AS. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol. 2001;159(6):2303–8. Epub 2001/12/06. 10.1016/S0002-9440(10)63080-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, et al. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108(11):1621–9. Epub 2001/12/26. 10.1172/JCI12849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gigante M, Pontrelli P, Montemurno E, Roca L, Aucella F, Penza R, et al. CD2AP mutations are associated with sporadic nephrotic syndrome and focal segmental glomerulosclerosis (FSGS). Nephrol Dial Transplant. 2009;24(6):1858–64. Epub 2009/01/10. 10.1093/ndt/gfn712 . [DOI] [PubMed] [Google Scholar]
- 16.Lowik MM, Groenen PJ, Pronk I, Lilien MR, Goldschmeding R, Dijkman HB, et al. Focal segmental glomerulosclerosis in a patient homozygous for a CD2AP mutation. Kidney Int. 2007;72(10):1198–203. Epub 2007/08/24. 10.1038/sj.ki.5002469 . [DOI] [PubMed] [Google Scholar]
- 17.Huber TB, Kwoh C, Wu H, Asanuma K, Godel M, Hartleben B, et al. Bigenic mouse models of focal segmental glomerulosclerosis involving pairwise interaction of CD2AP, Fyn, and synaptopodin. J Clin Invest. 2006;116(5):1337–45. Epub 2006/04/22. 10.1172/JCI27400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, et al. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003;300(5623):1298–300. Epub 2003/05/24. 10.1126/science.1081068 . [DOI] [PubMed] [Google Scholar]
- 19.Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, et al. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem. 2003;278(23):20716–23. Epub 2003/04/02. 10.1074/jbc.M301689200 . [DOI] [PubMed] [Google Scholar]
- 20.Reiser J, Altintas MM. Podocytes. F1000Res. 2016;5 Epub 2016/02/27. 10.12688/f1000research.7255.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim BJ, Yang JW, Do WS, Fogo AB. Pathogenesis of Focal Segmental Glomerulosclerosis. J Pathol Transl Med. 2016;50(6):405–10. Epub 2016/10/18. 10.4132/jptm.2016.09.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol. 2006;17(2):368–77. Epub 2006/01/06. 10.1681/ASN.2005080859 . [DOI] [PubMed] [Google Scholar]
- 23.Zent R, Pozzi A. Angiogenesis in diabetic nephropathy. Semin Nephrol. 2007;27(2):161–71. Epub 2007/04/10. 10.1016/j.semnephrol.2007.01.007 . [DOI] [PubMed] [Google Scholar]
- 24.Brunskill EW, Potter SS. Pathogenic pathways are activated in each major cell type of the glomerulus in the Cd2ap mutant mouse model of focal segmental glomerulosclerosis. BMC Nephrol. 2015;16:71 Epub 2015/05/15. 10.1186/s12882-015-0063-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boerries M, Grahammer F, Eiselein S, Buck M, Meyer C, Goedel M, et al. Molecular fingerprinting of the podocyte reveals novel gene and protein regulatory networks. Kidney Int. 2013;83(6):1052–64. Epub 2013/02/01. 10.1038/ki.2012.487 . [DOI] [PubMed] [Google Scholar]
- 26.Brunskill EW, Georgas K, Rumballe B, Little MH, Potter SS. Defining the molecular character of the developing and adult kidney podocyte. PLoS One. 2011;6(9):e24640 Epub 2011/09/21. 10.1371/journal.pone.0024640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunskill EW, Potter SS. Changes in the gene expression programs of renal mesangial cells during diabetic nephropathy. BMC Nephrol. 2012;13:70 Epub 2012/07/31. 10.1186/1471-2369-13-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magella B, Adam M, Potter AS, Venkatasubramanian M, Chetal K, Hay SB, et al. Cross-platform single cell analysis of kidney development shows stromal cells express Gdnf. Dev Biol. 2018;434(1):36–47. Epub 2017/12/01. 10.1016/j.ydbio.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsui CC, Shankland SJ, Pierchala BA. Glial cell line-derived neurotrophic factor and its receptor ret is a novel ligand-receptor complex critical for survival response during podocyte injury. J Am Soc Nephrol. 2006;17(6):1543–52. Epub 2006/05/05. 10.1681/ASN.2005080835 . [DOI] [PubMed] [Google Scholar]
- 30.Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect. 1999;1(15):1349–65. Epub 1999/12/28. . [DOI] [PubMed] [Google Scholar]
- 31.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29(5):196–202. Epub 2011/07/12. 10.3109/08977194.2011.595714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan J, Shen H, Sun Y, Li P, Burczynski F, Namaka M, et al. Bone morphogenetic protein 4 mediates bile duct ligation induced liver fibrosis through activation of Smad1 and ERK1/2 in rat hepatic stellate cells. J Cell Physiol. 2006;207(2):499–505. Epub 2006/02/01. 10.1002/jcp.20593 . [DOI] [PubMed] [Google Scholar]
- 33.Shen H, Huang G, Hadi M, Choy P, Zhang M, Minuk GY, et al. Transforming growth factor-beta1 downregulation of Smad1 gene expression in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2003;285(3):G539–46. Epub 2003/06/07. 10.1152/ajpgi.00436.2002 . [DOI] [PubMed] [Google Scholar]
- 34.Bergmann C, Distler JH. Canonical Wnt signaling in systemic sclerosis. Lab Invest. 2016;96(2):151–5. Epub 2016/01/12. 10.1038/labinvest.2015.154 . [DOI] [PubMed] [Google Scholar]
- 35.Watanabe M, Layne MD, Hsieh CM, Maemura K, Gray S, Lee ME, et al. Regulation of smooth muscle cell differentiation by AT-rich interaction domain transcription factors Mrf2alpha and Mrf2beta. Circ Res. 2002;91(5):382–9. Epub 2002/09/07. 10.1161/01.res.0000033593.05545.7b . [DOI] [PubMed] [Google Scholar]
- 36.Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278(23):21113–23. Epub 2003/04/01. 10.1074/jbc.M211304200 . [DOI] [PubMed] [Google Scholar]
- 37.Dhasarathy A, Phadke D, Mav D, Shah RR, Wade PA. The transcription factors Snail and Slug activate the transforming growth factor-beta signaling pathway in breast cancer. PLoS One. 2011;6(10):e26514 Epub 2011/10/27. 10.1371/journal.pone.0026514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Udalova IA, Ruhmann M, Thomson SJ, Midwood KS. Expression and immune function of tenascin-C. Crit Rev Immunol. 2011;31(2):115–45. Epub 2011/05/06. . [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharyya S, Wang W, Morales-Nebreda L, Feng G, Wu M, Zhou X, et al. Tenascin-C drives persistence of organ fibrosis. Nat Commun. 2016;7:11703 Epub 2016/06/04. 10.1038/ncomms11703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Midwood KS, Chiquet M, Tucker RP, Orend G. Tenascin-C at a glance. J Cell Sci. 2016;129(23):4321–7. Epub 2016/11/23. 10.1242/jcs.190546 . [DOI] [PubMed] [Google Scholar]
- 41.Swindle CS, Tran KT, Johnson TD, Banerjee P, Mayes AM, Griffith L, et al. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol. 2001;154(2):459–68. Epub 2001/07/27. 10.1083/jcb.200103103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Midwood KS, Orend G. The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal. 2009;3(3–4):287–310. Epub 2009/10/20. 10.1007/s12079-009-0075-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485(7397):195–200. Epub 2012/05/12. 10.1038/nature11019 . [DOI] [PubMed] [Google Scholar]
- 44.Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, et al. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun. 2012;3:735 Epub 2012/03/15. 10.1038/ncomms1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20(4):765–76. Epub 2009/03/20. 10.1681/ASN.2008060566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119(4):772–87. Epub 2009/03/17. 10.1172/JCI33950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowles J, Feng CW, Miles K, Ineson J, Spiller C, Koopman P. ALDH1A1 provides a source of meiosis-inducing retinoic acid in mouse fetal ovaries. Nat Commun. 2016;7:10845 Epub 2016/02/20. 10.1038/ncomms10845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janesick A, Shiotsugu J, Taketani M, Blumberg B. RIPPLY3 is a retinoic acid-inducible repressor required for setting the borders of the pre-placodal ectoderm. Development. 2012;139(6):1213–24. Epub 2012/02/23. 10.1242/dev.071456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ballim RD, Mendelsohn C, Papaioannou VE, Prince S. The ulnar-mammary syndrome gene, Tbx3, is a direct target of the retinoic acid signaling pathway, which regulates its expression during mouse limb development. Mol Biol Cell. 2012;23(12):2362–72. Epub 2012/04/27. 10.1091/mbc.E11-09-0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mallipattu SK, He JC. The beneficial role of retinoids in glomerular disease. Front Med (Lausanne). 2015;2:16 Epub 2015/04/09. 10.3389/fmed.2015.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Pippin JW, Vaughan MR, Krofft RD, Taniguchi Y, Romagnani P, et al. Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron Exp Nephrol. 2012;121(1–2):e23–37. Epub 2012/10/31. 10.1159/000342808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki A, Ito T, Imai E, Yamato M, Iwatani H, Kawachi H, et al. Retinoids regulate the repairing process of the podocytes in puromycin aminonucleoside-induced nephrotic rats. J Am Soc Nephrol. 2003;14(4):981–91. Epub 2003/03/28. 10.1097/01.asn.0000057857.66268.8f . [DOI] [PubMed] [Google Scholar]
- 53.Peired A, Angelotti ML, Ronconi E, la Marca G, Mazzinghi B, Sisti A, et al. Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J Am Soc Nephrol. 2013;24(11):1756–68. Epub 2013/08/21. 10.1681/ASN.2012090950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han SY, So GA, Jee YH, Han KH, Kang YS, Kim HK, et al. Effect of retinoic acid in experimental diabetic nephropathy. Immunol Cell Biol. 2004;82(6):568–76. Epub 2004/11/20. 10.1111/j.1440-1711.2004.01287.x . [DOI] [PubMed] [Google Scholar]
- 55.Moreno-Manzano V, Mampaso F, Sepulveda-Munoz JC, Alique M, Chen S, Ziyadeh FN, et al. Retinoids as a potential treatment for experimental puromycin-induced nephrosis. Br J Pharmacol. 2003;139(4):823–31. Epub 2003/06/19. 10.1038/sj.bjp.0705311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orfanos CE, Ehlert R, Gollnick H. The retinoids. A review of their clinical pharmacology and therapeutic use. Drugs. 1987;34(4):459–503. Epub 1987/10/01. 10.2165/00003495-198734040-00003 . [DOI] [PubMed] [Google Scholar]
- 57.Jia W, Li H, He YW. The extracellular matrix protein mindin serves as an integrin ligand and is critical for inflammatory cell recruitment. Blood. 2005;106(12):3854–9. Epub 2005/08/18. 10.1182/blood-2005-04-1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H, Oliver T, Jia W, He YW. Efficient dendritic cell priming of T lymphocytes depends on the extracellular matrix protein mindin. EMBO J. 2006;25(17):4097–107. Epub 2006/08/19. 10.1038/sj.emboj.7601289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Z, Garantziotis S, Jia W, Potts EN, Lalani S, Liu Z, et al. The extracellular matrix protein mindin regulates trafficking of murine eosinophils into the airspace. J Leukoc Biol. 2009;85(1):124–31. Epub 2008/09/27. 10.1189/jlb.0208135 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 60.Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69(12):2131–47. Epub 2006/05/12. 10.1038/sj.ki.5000410 . [DOI] [PubMed] [Google Scholar]
- 61.Jefferson JA, Shankland SJ. The pathogenesis of focal segmental glomerulosclerosis. Adv Chronic Kidney Dis. 2014;21(5):408–16. Epub 2014/08/30. 10.1053/j.ackd.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hodgin JB, Borczuk AC, Nasr SH, Markowitz GS, Nair V, Martini S, et al. A molecular profile of focal segmental glomerulosclerosis from formalin-fixed, paraffin-embedded tissue. Am J Pathol. 2010;177(4):1674–86. Epub 2010/09/18. 10.2353/ajpath.2010.090746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JH, Kim BK, Moon KC, Hong HK, Lee HS. Activation of the TGF-beta/Smad signaling pathway in focal segmental glomerulosclerosis. Kidney Int. 2003;64(5):1715–21. Epub 2003/10/09. 10.1046/j.1523-1755.2003.00288.x . [DOI] [PubMed] [Google Scholar]
- 64.Grgic I, Hofmeister AF, Genovese G, Bernhardy AJ, Sun H, Maarouf OH, et al. Discovery of new glomerular disease-relevant genes by translational profiling of podocytes in vivo. Kidney Int. 2014;86(6):1116–29. Epub 2014/06/19. 10.1038/ki.2014.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kornberg RD. The molecular basis of eukaryotic transcription. Proc Natl Acad Sci U S A. 2007;104(32):12955–61. Epub 2007/08/03. 10.1073/pnas.0704138104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakayama Y, Nara N, Kawakita Y, Takeshima Y, Arakawa M, Katoh M, et al. Cloning of cDNA encoding a regeneration-associated muscle protease whose expression is attenuated in cell lines derived from Duchenne muscular dystrophy patients. Am J Pathol. 2004;164(5):1773–82. Epub 2004/04/28. 10.1016/S0002-9440(10)63735-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andrews PM, Coffey AK. Cytoplasmic contractile elements in glomerular cells. Fed Proc. 1983;42(14):3046–52. Epub 1983/11/01. . [PubMed] [Google Scholar]
- 68.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93(7):1159–70. Epub 1998/07/10. 10.1016/s0092-8674(00)81460-9 . [DOI] [PubMed] [Google Scholar]
- 69.Bennett MR, Czech KA, Arend LJ, Witte DP, Devarajan P, Potter SS. Laser capture microdissection-microarray analysis of focal segmental glomerulosclerosis glomeruli. Nephron Exp Nephrol. 2007;107(1):e30–40. Epub 2007/08/09. 10.1159/000106775 . [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, He Y, Wang M, Lv P, Liu J, Wang J. Role of Protease-Activated Receptor 2 in Regulating Focal Segmental Glomerulosclerosis. Cell Physiol Biochem. 2017;41(3):1147–55. Epub 2017/03/01. 10.1159/000464121 . [DOI] [PubMed] [Google Scholar]
- 71.Chen IH, Wang HH, Hsieh YS, Huang WC, Yeh HI, Chuang YJ. PRSS23 is essential for the Snail-dependent endothelial-to-mesenchymal transition during valvulogenesis in zebrafish. Cardiovasc Res. 2013;97(3):443–53. Epub 2012/12/06. 10.1093/cvr/cvs355 . [DOI] [PubMed] [Google Scholar]
- 72.Klein T, Ling Z, Heimberg H, Madsen OD, Heller RS, Serup P. Nestin is expressed in vascular endothelial cells in the adult human pancreas. J Histochem Cytochem. 2003;51(6):697–706. Epub 2003/05/20. 10.1177/002215540305100601 . [DOI] [PubMed] [Google Scholar]
- 73.Mokry J, Cizkova D, Filip S, Ehrmann J, Osterreicher J, Kolar Z, et al. Nestin expression by newly formed human blood vessels. Stem Cells Dev. 2004;13(6):658–64. Epub 2005/02/03. 10.1089/scd.2004.13.658 . [DOI] [PubMed] [Google Scholar]
- 74.Shimizu T, Sugawara K, Tosaka M, Imai H, Hoya K, Takeuchi T, et al. Nestin expression in vascular malformations: a novel marker for proliferative endothelium. Neurol Med Chir (Tokyo). 2006;46(3):111–7. Epub 2006/03/28. 10.2176/nmc.46.111 . [DOI] [PubMed] [Google Scholar]
- 75.Teranishi N, Naito Z, Ishiwata T, Tanaka N, Furukawa K, Seya T, et al. Identification of neovasculature using nestin in colorectal cancer. Int J Oncol. 2007;30(3):593–603. Epub 2007/02/03. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of genes up and down regulated in mutant podocytes compared to control, including fold change and nRPKM expression levels in each mutant and control sample. GO analysis results for molecular functions and biological processes, including P values and lists of associated genes.
(XLSX)
List of genes up and down regulated in mutant mesangial cells compared to control, including fold change and nRPKM expression levels in each mutant and control sample.
(XLSX)
List of genes up and down regulated in mutant endothelial cells compared to control, including fold change and nRPKM expression levels in each mutant and control sample.
(XLSX)
Red indicates high expression and blue corresponds to low expression levels.
(TIF)
Data Availability Statement
Sequencing data is available in the Gene Expression Omnibus (GEO), accession number GSE123179.