Abstract
Background
Tuberculosis is an infectious bacterial disease that is spread via respiratory droplets from infected individuals to susceptible contacts. To eliminate this disease from low‐ and medium‐incidence settings, people who are most likely to be infected (contacts) must be identified. Recently, study authors have examined alternate approaches to contact tracing methods that demonstrate improved detection and prioritization of contacts. The comparative benefit of these methods has not been established.
Objectives
To assess the effectiveness of novel methods of contact tracing versus current standard of care to identify latent and active cases in low‐ to moderate‐incidence settings.
Search methods
We searched CENTRAL, MEDLINE, Embase, LILACS, Web of Science, and CINAHL up to 15 July 2019. We also searched for clinical trials and examined reference lists and conference proceedings.
Selection criteria
Randomized controlled trials (RCTs) and cluster‐RCTs of contact tracing strategies that included alternate approaches (other than standard practice).
Data collection and analysis
Two review authors independently assessed identified articles for eligibility and quality using prespecified criteria.
Main results
No trials met the inclusion criteria of this review. Several study authors described an alternate method for examining contacts and performing social network analysis but did not compare this with the current contact tracing approach.
Authors' conclusions
This Cochrane Review highlights the lack of research in support of the current contact tracing method and the need for RCTs to compare new methods such as social network analysis to improve contact tracing processes.
16 September 2019
Up to date
All studies incorporated from most recent search
We performed the last search up to 15 Jul, 2019, and did not identify any trials for inclusion.
Plain language summary
Contact tracing methods for tuberculosis
What is the aim of this review?
This Cochrane Review aims to establish whether any evidence is available to support the current approach to contact tracing (the process of identifying individuals exposed to an infectious case of tuberculosis), and whether alternate options could result in a higher rate of infection detection in contacts. We searched for all relevant studies to answer this question.
Key messages
Contact tracing is an important method to further reduce the rates of tuberculosis. Cochrane Review authors identified no studies addressing this question. Therefore further research is needed to determine whether alternate contact tracing approaches could produce a greater yield in the number of contacts detected and the proportion of individuals with disease.
What was studied in the review?
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis bacteria. Globally, tuberculosis infects an estimated 1.7 billion people, with 1.3 million deaths and 10 million new cases each year. Tuberculosis is transmitted via droplets coughed up from infected patients to susceptible contacts. The World Health Organization (WHO) aims to eliminate this disease by 2035. To achieve this ambitious task, the current decline in new cases must be at a faster rate. In high‐income countries with low rates of tuberculosis, contact tracing is the primary method used to find those at risk of developing tuberculosis.
What are the main results of the review?
The review authors found that no suitable randomized controlled trials have been conducted to answer this question. There is insufficient high‐certainty evidence comparing current contact tracing methods used against alternate options; further research is therefore needed.
How up‐to‐date is this review
We searched for studies published up to 15 July 2019.
Background
Description of the condition
Mycobacterium tuberculosis is a species of bacteria that causes tuberculosis infection in susceptible individuals. The World Health Organization (WHO) estimates that 1.7 billion people in the world are potentially infected with M tuberculosis. As part of the sustainable development goal (SDG) 3, the WHO aims to achieve an 80% reduction in tuberculosis incidence (new cases per 100,000 population per year) in 2030 compared with levels in 2015 (WHO 2018). For this to be achieved, the current decline in new cases must be at a faster rate. This requires an evaluation of current practice (Yates 2016).
Transmission of infection
M tuberculosis is transmitted through expectoration of respiratory droplets. Disease can be transmitted to other individuals (contacts) by infected persons (index cases) before treatment interventions are provided. Exposure to tuberculosis can result in no infection or in active disease (symptomatic and infectious state) or in latent tuberculosis infection (LTBI). The latter represents an asymptomatic, preinfectious state with no clinical evidence of active disease (Lee 2016; Young 2016). In this way, the endemic nature of tuberculosis is propagated. Tuberculosis infection is currently thought to affect 10 million people annually, with severe disease resulting in approximately 1.3 million deaths a year (WHO 2018).
Tuberculosis incidence and control approaches
Tuberculosis incidence does not have an equal distribution globally. Areas of high (> 100/100,000), moderate (30 to 100/100,000), and low (< 30/100,000) incidence have been reported (WHO 2018).
Prevention and control methods differ depending on the level of incidence, with different approaches adopted for high‐ versus low‐incidence settings (WHO 2012; WHO 2014). High‐incidence settings allocate resources to infected (active) cases, given the large numbers in these settings and the lack of resources for intensive examination of contacts. In low‐incidence settings, more resources are allocated in trying to identify latent cases to prevent development of the infectious stage and further propagation of infection (WHO 2014). This approach relies on effective contact tracing approaches, as contacts in a low‐incidence setting have a higher relative risk than the general population of acquiring tuberculosis disease (Sloot 2014).
Identifying contacts of infected patients
‘Contact tracing’ refers to a process involving a set of interventions usually instituted by a tuberculosis service once an active case of tuberculosis has been identified. This normally occurs once a patient has presented to services but occur as part of the contact tracing process itself (Abubakar 2012; NICE 2016).
Although several defined screening methods are available to identify latent and active disease, methods used to identify individuals deemed to be contacts are less obvious.
Contacts in close proximity with infected patients for prolonged periods of time have long been recognized as being at greater risk of being infected (Jones‐Lopez 2016). Minimum amount of time and absolute proximity are not well‐established.
The currently adopted contact tracing approach utilizes a ‘stone‐in‐pond’ model (Veen 1992), with each ‘ripple’ representing a social circle with varying degrees of physical proximity to the index case, thus suggesting a way to limit screening size in contact tracing scenarios. In this model, contact tracing progresses to the next possible circle of contacts only if a predefined proportion of positive contacts are included in the preceding circle. The utility of this approach compared to alternate approaches has not been evaluated.
Description of the intervention
Contact tracing
This screening process can identify a substantial group of contacts depending on home, work, and travel arrangements of the index cases. Current approaches rely heavily on subjective reports from patients and occupational health teams to generate a list of prospective contacts. This approach does not necessarily capture all individuals at risk (Munang 2016).
These individuals are then assigned to different contact strata relative to the patient, reflecting presumed degrees of exposure. Thus, family is the most proximal relationship, followed by close friends, casual contacts, etc. Screening contacts therefore proceeds from the innermost (most proximal) social circle to the least related, depending on the proportion of positive contacts detected (Veen 1992).
Alternative approaches take into account not only the list of contacts volunteered by the presenting patient, but also their social relationships and geospatial movements outside of clearly defined contact strata. These may include work colleagues as well as opportunistic contacts such as those sharing recreational spaces (for example, bars, shops, transport). Current guidance does not advise routine screening of these social contacts (NICE 2016). However, methods that do take into account these social interactions have demonstrated evidence of disease in these populations. Furthermore, difficult to control outbreaks can often be seen to have connections between previously unidentified or poorly defined contacts (McElroy 2003; Andre 2007; Cook 2007; Gardy 2011; Munang 2016).
Uncertainties in current approaches
The ‘stone‐in‐pond’ approach relies on consistent social relationships for all infected patients, as a negative screen in a closer contact circle results in the cessation of further contact tracing. This consistency in social relationships and presumed proximity for all individuals may not be universally applicable nor representative of contemporary social relationships. Furthermore, social relationships tend to vary with age and occupation ‐ factors not taken into account by current methods (Middlekoop 2009; Middlekoop 2014).
Traditional contact tracing methods may not take into account areas of congregation that have been shown to be potential sources of transmission for tuberculosis, especially for at‐risk populations groups (Barnes 1996; WHO 2014).
Once a more systematized approach is adopted, the number of contacts identified increases, demonstrating the deficiency in effective contact identification through current methods (Gardy 2011; Munang 2016).
Systematized approaches include social network analysis (SNA), which differs from traditional contact tracing approaches by seeking to identify geospatial distributions of individuals. This information, garnered from questionnaires, places patients in areas where they may have spread disease but for which they have deliberately or accidentally omitted mentioning contacts. In this way, additional contacts can be identified (Gardy 2011; Munang 2016). Geospatial data can also assist in identifying key transmission points, significant for congregate (that is, non‐household areas of public or social aggregation) settings (Yates 2016; Hella 2017; Huang 2017; Patterson 2017).
We are not aware of any reviews examining alternative contact tracing approaches for a higher rate of latent case detection.
How the intervention might work
Assessing for alternative contact tracing methods could provide a much‐needed evidence base for current clinical practice or, if lacking, could demonstrate the need for more evidence. Given the development of new tracing methods including SNA, there is a need to identify the most cost‐effective methods that provide a high yield of case detection whilst not allowing tuberculosis incidents to propagate. Proposed new methods for contact tracing would involve social network questionnaires that draw on areas of congregation and therefore demonstrate previously unidentified links and transmission nodes. When combined with genetic techniques such as whole genome sequencing, this could result in identification of an increased number of contacts (Gardy 2011; Munang 2016).
Why it is important to do this review
To reduce cases of tuberculosis in low‐ and moderate‐incidence settings, the most effective contact tracing method should be adopted. We look to review the evidence for any contact tracing strategies used to identify latent cases of tuberculosis in low‐ and moderate‐incidence settings and the economic viability of any existing strategies to better inform resource allocation.
This Cochrane Review will add to the evidence base of meaningful strategies for early detection and prevention of tuberculosis targeting low‐ and moderate‐incidence settings. In addition, it will assist healthcare providers in providing an evidence base to better inform contact tracing and screening strategies. Improved strategies for identification of latent tuberculosis will help to reduce the endemic presence of tuberculosis and will help to achieve the WHO tuberculosis elimination goal.
Objectives
To assess the effectiveness of novel methods of contact tracing versus current standard of care to identify latent and active cases in low‐ to moderate‐incidence settings.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) and cluster‐RCTs.
Types of participants
People of any age, gender, and ethnicity living in low‐ (< 30 per 100,000) and moderate‐incidence (30 to 100 per 100,000 population) tuberculosis settings identified as potential tuberculosis contacts.
Types of interventions
Intervention
Alternative approaches to identify potential tuberculosis contacts, for example, use of SNA to identify contacts prospectively.
Controls
Traditional contact screening methods, such as the ‘stone‐in‐pond’ approach (standard care).
The stone‐in‐pond method describes the contact tracing approach of prioritizing contacts by risk‐stratifying cases based on assumed proximity. Household contacts therefore have the highest presumed risk and form the closest circle to be screened, followed by the next ‘ripple’, which may include close friends then casual friends, and so on. This set of outwardly expanding concentric circles is similar to the appearance of a stone being dropped in a pond, with the ripples generated representing concentric circles of risk proximity.
Types of outcome measures
Primary outcomes
Contacts with latent tuberculosis infection out of all contacts screened.
Secondary outcomes
Contacts with diagnosis of proven M tuberculosis infection or active clinical tuberculosis disease out of all contacts screened.
Search methods for identification of studies
We sought to include all relevant studies regardless of language or stage of publication (published, unpublished, in press, and ongoing).
Electronic searches
The CIDG Information Specialist, Vittoria Lutje, performed electronic searches of the following databases up to 15 July 2019, using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register; Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library (Issue 7, July 2019); MEDLINE (PubMed, from 1966); Embase (OVID, from 1947); Latin American Caribbean Health Sciences Literature (LILACS) (BIREME, from 1982); Cumulative Index to Nursing and Allied Health Literature (CINAHL; EBSCOHost, from 1982); Science Citation Index Expanded, and Social Sciences Citation Index (Web of Science, from 1900). She also searched the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/en/), ClinicalTrials.gov (clinicaltrials.gov/ct2/home), and the Clinical Trials Unit of the International Union Against Tuberculosis and Lung Disease (IUATLD; www.theunion.org) for trials in progress, using ‘tuberculosis', ‘contact tracing', and ‘contact screening' as search terms.
Searching other resources
We searched the following conference proceedings for abstracts of relevant studies: World Congress on TB, World Lung Conferences of the International Union Against Tuberculosis Lung Disease (IUATLD), American Thoracic Society Meetings Proceedings, and the British Society for Antimicrobial Therapy. Where relevant, we sought to contact researchers and experts in the field to identify additional eligible studies. We also checked the bibliography and reference list of all identified studies for other relevant studies (Lefebvre 2011).
Data collection and analysis
In the event that we identified relevant trials for inclusion, we planned to contact trial authors for unpublished data or results.
Selection of studies
Two review authors (DBM and BM) independently screened the titles and abstracts of citations identified by the search strategy using a study selection form. We planned to obtain the full texts of studies meeting the eligibility criteria. Two review authors (DBM and BM) would then have independently assessed these and recorded any articles excluded and reasons for exclusions in a Characteristics of excluded studies table. When discrepancy between review authors arose, we planned to address this through discussion and consensus. When disagreement was ongoing, we would have consulted a third review author (MD). When clarification of study methods was required, we planned to contact the study authors for further information. We illustrated the study selection process in a PRISMA diagram.
Data extraction and management
Two review authors planned to independently extract data using a data extraction form. When disagreements arose, we would have resolved these through discussion or by consultation with a third review author (MD). Data to be extracted included start and end dates of the study, study locations, study design, funding, tuberculosis prevalence (as stated by study authors), and conflict of interests. We also planned to extract data on participants, including demographic details (age and sex), geographical location, index case description, and description of the contact relationship. We planned to extract details of the intervention regarding how these individuals were identified as contacts as well as the outcomes (disease development as active or latent disease).
We planned to extract details of any co‐interventions (that is, what method was used to identify individuals as contacts, whether this formed part of a cost‐effectiveness analysis, and if methods differed depending on the setting (congregate versus household)). Details of the control (that is, the standard of care employing stone‐in‐pond model of contact tracing) were also to be extracted. We aimed to extract the number of contacts identified from each described outbreak or incident event. We planned to generate through extracted data the number of contacts identified and screened for tuberculosis infection (active or latent disease ‐ numerator) over the total number of contacts identified and screened (denominator).
For cluster‐RCTs, we planned to record the number, size, and method used for clustering. The clustered measure of effect and variance would have been recorded, if this was adjusted for by the study authors. If no adjustment for clustering was made, we planned to extract details on the number of participants experiencing the event and the number randomized to each group (for dichotomous outcomes). For continuous outcomes, we aimed to extract the summary effect (mean or median) and the measure of variance (standard deviation or range).
After data extraction, two review authors (DBM and BM) would enter these data into Review Manager 5 (RevMan 2014). We would have contacted trial authors to clarify any unclear data or in the event of missing or incomplete data. For continuous outcomes, we would have recorded the measure of effect (mean or median) and variance (SD or range). For dichotomous outcomes, we planned to record the number of patients with the outcome event and the total number of patients in each intervention group.
Assessment of risk of bias in included studies
Two review authors (DBM and BM) planned to independently assess the risk of bias of each included study using an assessment form as per the Cochrane ‘Risk of bias' tool (RevMan 2014). We planned to resolve any differences of opinion through discussion and, if needed, by consulting a third review author (MD). When data were missing, unclear, or incomplete, we would have contacted the trial authors for further details.
We planned to use the Cochrane approach to assess risk of bias across six domains: sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other potential biases. For each domain, we planned to record the methods used by study authors to reduce the risk of bias and to assign a judgement of ‘low risk of bias', ‘high risk of bias', or ‘unclear risk of bias'.
For cluster‐RCTs, we would have considered baseline imbalance in the appraisal of selection bias and loss of clusters in the appraisal of attrition bias, and we would have further considered the risk of contamination bias (where people living in the control areas also benefit from the intervention).
We aimed to summarize results for the assessment of risk of bias using the ‘Risk of bias' summary and the ‘Risk of bias' graph, in addition to ‘Risk of bias' tables (Higgins 2017).
Measures of treatment effect
To assess the treatment effect, we planned to examine continuous and dichotomous data separately. For continuous data, we would have assessed effects by mean differences; for dichotomous data, we would have used risk ratios. We planned to present 95% confidence intervals (CIs) and ranges where relevant.
Unit of analysis issues
When cluster‐RCTs had not adjusted their results for effects of the cluster design, we planned to adjust sample sizes using the methods described in Section 16.3.4 or 16.3.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using an estimate of the intracluster correlation coefficient (ICC). When possible, we aimed to derive the ICC from the trial itself, or from a similar trial. If an appropriate ICC was unavailable, we planned to conduct sensitivity analyses to investigate potential effects of clustering by imputing a range of values for the ICC.
Dealing with missing data
We planned to contact trial authors when we deemed data were missing or incomplete. Further than this, we did not plan any imputation measures for missing data.
Assessment of heterogeneity
We planned to assess statistical heterogeneity between trials by visually inspecting forest plots to detect overlapping CIs and by applying the Chi² test and the I² statistic (Higgins 2003). We would have regarded a Chi² P value < 0.05 as statistically significant, and an I² statistic value > 75% as representing considerable heterogeneity (Deeks 2017).
Assessment of reporting biases
We aimed to examine the likelihood of reporting bias using funnel plots, provided there were at least 10 included trials (Sterne 2017).
Data synthesis
Two review authors (DBM and BM) planned to analyse the data using Review Manager 5 (RevMan 2014). We planned to stratify the primary analysis by study design, and we did not plan to perform a meta‐analysis across different trial designs.
Outcomes were to be stratified according to numbers of cases detected at a particular time point per contact tracing strategy. When appropriate, we planned to group time points together and perform a meta‐analysis (for example, changing number of contacts over time in a single contact tracing episode).
We aimed to tabulate results from cluster‐RCTs that could not be adjusted for clustering. We also aimed to use a random‐effects model in the presence of significant statistical heterogeneity and a fixed‐effect model in the absence of heterogeneity.
Subgroup analysis and investigation of heterogeneity
We planned to investigate potential causes of heterogeneity by performing subgroup analyses by study setting (congregate versus home), screening test used, risk factors in demography (drug and alcohol use, immunosuppressive states), occupation, age of participants, and tuberculosis prevalence in the study area.
Sensitivity analysis
If a minimum of 10 trials met our inclusion criteria, we planned to conduct a sensitivity analysis. Sensitivity analyses would test the robustness of results to the risk of bias components.
Certainty of the evidence
We aimed to use the GRADE approach to assess the certainty of evidence, and when appropriate, we planned to create ‘Summary of findings' tables and Evidence Profiles (GRADEpro 2015).
Results
Description of studies
See Characteristics of excluded studies.
Results of the search
We identified 755 articles using the prespecified search strategy, with an additional 16 clinical trials in progress. Of these, none met the inclusion criteria for this review. We identified no conference proceedings or abstracts. Several trial authors examined the feasibility and effectiveness of network‐based approaches to contact tracing, an alternative method. These studies did not meet the inclusion criteria for the reasons below (Figure 1). They are further explored in the Discussion section.
1.
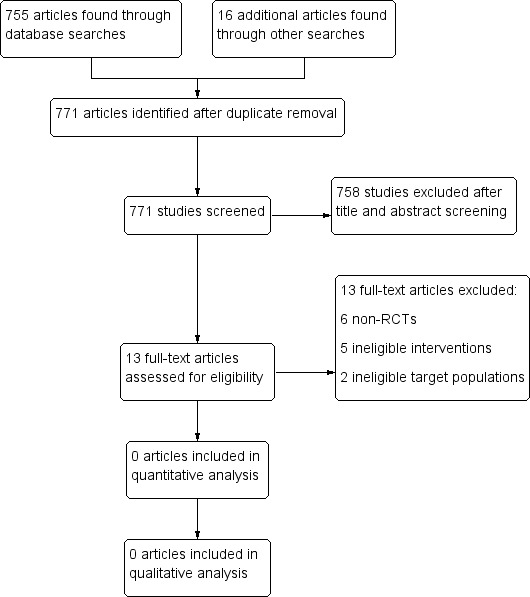
PRISMA flow diagram.
Included studies
No trials met the inclusion criteria of this review.
Excluded studies
We listed the reasons for exclusion of relevant studies in the Characteristics of excluded studies table.
Risk of bias in included studies
This was not applicable.
Effects of interventions
This was not applicable.
Discussion
We did not identify any randomized controlled trials (RCTs) or quasi‐RCTs that compared alternative contact tracing methods to the standard approach.
To eliminate tuberculosis in low‐incidence settings, high‐risk groups must be scrutinized as part of an effective screening process. Contacts of patients who have active, infectious tuberculosis represent a higher‐risk group than the general population (Diel 2006; Morrison 2008; WHO 2014).
Effective contact tracing processes are therefore key to reducing and eventually eliminating tuberculosis (WHO 2014). Several trial authors have demonstrated the effectiveness of alternative strategies for contact tracing in a variety of settings (McElroy 2003; Andre 2007; Cook 2007; Gardy 2011; Andrews 2014; Munang 2016; Yates 2016; Hella 2017; Patterson 2017).
Andrews 2014 undertook a modelling study in a high‐incidence area. Using a modified Wells‐Riley model, researchers described transmission patterns in an endemic setting. They demonstrated the significance of non‐household settings for transmission of infection and for correlation with age (adolescents 15 to 19 responsible for school transmission). They included no control group; this was a modelling study examining congregate settings in a high‐incidence environment.
Cook 2007 examined the role of SNA in detecting tuberculosis transmission to prioritize contacts. Using a structured questionnaire and supplementing this with patient records, review authors extracted demographic data and information about social aggregation, patients, and their contacts. This was augmented with molecular genotyping. Trial authors found that patients who were not identified through conventional contact investigations were connected through areas of social aggregation or via mutual contacts. These authors found a positive correlation between contact screening tests (positive tuberculin skin tests) and location in the denser portions of constructed networks (P < 0.1). This was not an RCT, and trial authors included no comparison group. These trial findings were supported by another study (Andre 2007), whose authors again examined the role of network analysis in prioritizing contacts by supplementing epidemiological data collected through hospital records and patient interviews with genotyping isolates. They found that contacts prioritized using a network analysis approach were more likely to have latent disease (LTBI) than non‐prioritized contacts (odds ratio (OR) 7.8). This again was not a review article and did not include a comparison group for analysis. A further network‐based study was identified (McElroy 2003). These trial authors retrospectively reviewed outbreak events in a US low‐incidence state, applying social network analytical methods. This involved re‐interviewing patients and contacts. Researchers discovered previously unrecorded patterns of drug use propagating infection.
Fox 2012 conducted a systematic review to look at the outcomes of contact investigations for tuberculosis. These review authors established the risk to contacts of developing disease and the lack of evidence to support current contact tracing methods. They performed subgroup analyses according to index and contact characteristics. These review authors established the prevalence of contact outcomes according to setting income; low‐ and middle‐income countries have a latent disease prevalence of 45.9%, with tuberculosis disease in 3.1% of all contacts and 3.6% of household contacts. In high‐income countries (correlating with low‐incidence settings), tuberculosis disease was 1.0%, and amongst household contacts 3.5%, with 26.3% of latent cases detected. There was no comparison of contact tracing methods, and low‐quality observational studies provided the basis for conclusions.
Review authors found no clear evidence for the current contact tracing method used ‐ the ‘stone‐in‐pond’ approach (Veen 1992; Fox 2012). This approach involves assessment of contacts in prescribed proximity rings to the index case, with the most proximal ring traditionally representing household contacts. Several trial authors have demonstrated the shortcomings of this approach, with transmission zones changing depending on age and social activities (Middlekoop 2014; Yates 2016; Hella 2017; Patterson 2017; Worrell 2017). The search conducted for our review revealed no RCTs addressing a comparison of the standard contact tracing approach ‐ the ‘stone‐in‐pond' model ‐ versus a new approach such as a network analysis approach ‐ social network analysis (SNA).
Summary of main results
Our review did not find any RCTs that compared the standard contact tracing approach (the ‘stone‐in‐pond’ model) to alternative approaches (SNA).
Overall completeness and applicability of evidence
Several identified studies demonstrated the benefit of a network analysis approach; however these were not RCTs, and they did not include a comparison group (McElroy 2003; Andre 2007; Cook 2007).
From contemporary research studies, it appears that a more effective approach could possibly be adopted, and that previous assumptions about transmission zones for tuberculosis need to be revisited, with non‐household transmission playing a key role. Transmission of tuberculosis is also influenced by demographic factors such as age and gender; therefore an approach that takes into account geospatial location, activity spaces, and these demographic factors could better identify contacts and help prioritize tracing opportunities while identifying contacts at highest risk of disease development.
Policies advising contact tracing processes should take into account the growing research evidence in support of a network‐based approach to contact tracing, considering factors that influence different transmission zones in low‐incidence settings. Improved contact tracing can help to further reduce incidence rates and prevent ongoing active case development in high‐risk groups such as contacts of infectious patients. Furthermore, once identified, these factors can inform control and prevention measures in congregate settings to prevent ongoing occurrence of incidents.
Certainty of the evidence
This is not applicable.
Potential biases in the review process
We attempted to minimize bias by using a previously described search criterion. We also searched clinical trials in progress to minimize the risk of missed studies; however this review process may have missed studies that have not yet been published. We conducted a broad search including participants and environments studied, and it is unlikely that we missed any published RCTs. Rather, lack of studies is a reflection of the lack of evidence on this topic. Furthermore, it may reflect differences in the description of interventions used, as well as less clearly defined use of methods by clinical teams (that is, a degree of overlap in contact tracing approaches).
Studies identified through our search have been discussed above and reflect the difficulty involved in conducting a prospective RCT. Trial authors suggest it may be possible to conduct such a trial via a cross‐over method using comparative sites.
Agreements and disagreements with other studies or reviews
We did not identify any papers as part of this review that compared the standard contact tracing approach (‘stone‐in‐pond' approach) with alternative approaches such as a network analysis approach.
Several trial authors have suggested the benefit that could be gained from using a social network approach; however this was not compared to the standard contact tracing approach (McElroy 2003; Andre 2007; Cook 2007; Gardy 2011; Andrews 2014; Munang 2016; Yates 2016; Hella 2017; Patterson 2017). Additionally, trial authors have previously described deficiencies in the current contact tracing approach, lending support to the need for a comparative analysis (Middlekoop 2014; Yates 2016; Hella 2017; Patterson 2017; Worrell 2017).
Authors' conclusions
Implications for practice.
The current contact tracing method (‘stone‐in‐pond' approach) does not appear to be based on a comparative evidence‐based approach and instead has been adopted from its early proposal as a pragmatic process.
Implications for research.
This Cochrane Review has identified the dearth of evidence for contact tracing methods. It highlights the need for further research into optimal contact tracing methods to evaluate the most efficient and effective approach.
Contact tracing methods need to take into account the limited resource allocation provided and should be able to effectively identify those at risk of disease development. Ideally, these methods should balance the accurate identification of exposed contacts, whilst allowing prioritization of those at highest risk of disease development.
Future trials should examine the benefit of network‐based approaches to contact tracing using current methods as a benchmark. It is likely that the background incidence of study setting will affect outcomes, and trials should take this into account. Furthermore, designing studies to examine the effectiveness of alternative methods for contact tracing (for example, network approach) could take into account novel molecular techniques (for example, whole genome sequencing). This would allow studies to examine the relative decline in the number of molecularly linked active cases over a specified time period. Finally, to fully inform future policy decisions, a cost‐effectiveness analysis should ideally be included.
Our review demonstrates that there is limited high‐quality evidence for the current contact tracing method and for consideration of alternative approaches. RCTs are therefore needed to assess the best approach to effective contact tracing methods. Ideally these trials would compare a network analysis approach using standard care (the ‘stone‐in‐pond’ approach).
New approaches to molecular diagnostics could assist with a network analysis approach. Whole genome sequencing has been shown to provide higher resolution for investigations in outbreaks of tuberculosis infection (Gardy 2011). In addition, several trial authors have demonstrated the benefit of assessing ventilation when congregate settings are considered in contact tracing processes; this has so far been done using carbon dioxide (CO2) monitors (Jones‐Lopez 2016; Hella 2017; Patterson 2017).
Acknowledgements
The Academic Editor is Professor Mical Paul.
We are grateful to our affiliated institutions and organizations, and we thank the referees and editors for their comments and encouragement.
The editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials
#1 tuberculosis:ti,ab,kw (Word variations have been searched)
#2 TB
#3 MeSH descriptor: [Mycobacterium tuberculosis] explode all trees
#4 MeSH descriptor: [Tuberculosis] explode all trees
#5 #1 or #2 or #3 or #4
#6 MeSH descriptor: [Contact Tracing] explode all trees
#7 "contact tracing"
#8 "contact screening" or "contact management"
#9 "contact investigation*"
#10 "transmission dynamics"
#11 referral
#12 "stone in pond"
#13 "household screening"
#14 "social network*"
#15 #7 or #8 or #9 or #10 or #10 or #11 or #12 or #13 or #14
#16 #15 and #5
PubMed (MEDLINE)
| Search | Query |
| #1 | tuberculosis [MesH] |
| #2 | Mycobacterium tuberculosis [MesH] |
| #3 | tuberculosis or TB Field: Title/Abstract |
| #4 | ((Mycobacterium tuberculosis [MesH]) OR #2) OR #1 |
| #5 | "Contact Tracing"[Mesh] |
| #6 | "Contact Tracing" Field: Title/Abstract |
| #7 | "contact screening" or "contact management" Field: Title/Abstract |
| #8 | "contact investigation*" Field: Title/Abstract |
| #9 | "transmission dynamics" Field: Title/Abstract |
| #10 | referral Field: Title/Abstract |
| #11 | "stone in pond" Field: Title/Abstract |
| #12 | "household screening" Field: Title/Abstract |
| #13 | "social network*" Field: Title/Abstract |
| #14 | (((((((( #13) OR #12) OR #11) OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 |
| #15 | (#15) AND #4 |
| #16 | "Randomized Controlled Trial" [Publication Type] OR "Controlled Clinical Trial" [Publication Type] |
| #17 | randomized or placebo Field: Title/Abstract |
| #18 | randomly or trial or groups Field: Title/Abstract |
| #19 | "drug therapy" [Subheading] |
| #20 | (((#19) OR #18) OR #17 OR #16 |
| #21 | "Animals"[Mesh] |
| #22 | "Humans"[Mesh] |
| #23 | (#21) NOT #22 |
| #27 | (#20) NOT #23 |
| #28 | #15 AND #27 |
Embase
1 (tuberculosis or TB).mp.
2 limit 1 to human
3 Mycobacterium tuberculosis/
4 2 or 3
5 "contact tracing".mp. or contact examination/
6 ("contact screening" or "contact management").mp.
7 "contact investigation".mp.
8 "transmission dynamics".mp.
9 patient referral/
10 "stone in pond".mp.
11 "household screening".mp.
12 social network/ or "social network*".mp.
13 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12
14 4 and 13
15 controlled clinical trial.mp. or Controlled Clinical Trial/
16 randomized controlled trial.mp. or Randomized Controlled Trial/
17 single blind procedure/
18 double blind procedure/
19 crossover procedure/
20 placebo.ti. or placebo.ab.
21 "randomly allocated".mp.
22 (randomized or placebo or double‐blind* or single‐blind*).mp
23 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24 14 and 23
LILACS
Search on : tuberculosis or TB [Words] and "contact tracing" or "contact screening" [Words] and randomized or trial or groups [Words]
CINAHL (EBSCOHost)
Query
S7 S5 AND S6
S6 TX ( randomized controlled trial or rct ) OR MH controlled clinical trial OR TX ( "double blind*" or "single blind" or placebo or crossover )
S5 S1 AND S4
S4 S2 OR S3
S3 MH contact tracing OR TX "transmission dynamics" OR TX referral OR TX "household screening" OR TX "social network"
S2 TX "contact tracing" OR TX "contact screening" OR TX "contact investigation"
S1 TX ( tuberculosis or TB ) OR MH mycobacterium tuberculosis
Web of Science
# 5 #4 AND #3
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
# 4 TOPIC: (randomized trial or clinical trial) OR TOPIC: (double‐blind* or single‐blind* or placebo)
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
# 3 #2 AND #1
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
# 2 TOPIC: ("contact tracing" or "contact screening" or "contact investigation") OR TOPIC: ("household screening" or "transmission dynamics" or "social network*")
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
# 1 TOPIC: (tuberculosis or tb or "mycobacterium tuberculosis")
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andre 2007 | Cluster analysis study. No control group used. |
| Andrews 2014 | No control group. Modelling study examining congregate settings in a high‐incidence environment. |
| Bayona 2003 | Cohort study. Investigated multidrug‐resistant tuberculosis. Different interventions; study authors examined treatment options and secondary contact cases. |
| Cook 2007 | No control group. Cluster analysis. |
| de Vries 2006 | Cluster analysis study. No control group. Narrow study population. |
| Driver 2003 | Prospective observational study. Ineligible comparison group. Different assessments and outcomes. |
| Fatima 2016 | Different interventions. Examined active and passive case finding. Retrospective study. |
| Fox 2012 | Systematic review and meta‐analysis looking at the overall effectiveness of contact tracing. Did not examine alternate methods of contact tracing. Looked at the prevalence of latent tuberculosis infection. |
| Fox 2013 | Different intervention. Examined active case finding. Looked at high incidence setting. Examined only household contacts. |
| Fox 2018 | Different intervention. Examined active case finding. Looked at high incidence setting. Examined only household contacts. |
| Jensen 2016 | Retrospective study. Different intervention. Examined active case finding. |
| McElroy 2003 | Retrospective study. No control group. Outbreak, cluster analysis. |
| Ribeiro 2015 | Spatial and genotypic cluster analysis of tuberculosis transmission events. High incidence setting. |
Differences between protocol and review
We amended the Methods section of the published protocol, Menezes 2018, regarding inclusion of studies. We changed the text from "any contact tracing strategy to identify tuberculosis infection cases other than a stone‐in‐pond screening approach (standard care), e.g. the use of SNA to identify contacts prospectively" to "any contact tracing strategy to identify tuberculosis infection cases versus traditional or alternative approaches such as the stone‐in‐pond screening approach, e.g. the use of SNA to identify contacts prospectively". We made this change on the basis that we found no trials comparing the ‘stone‐in‐pond' approach to another intervention, and we wanted to expand the search.
We amended the outcomes section to further clarify the differences between primary and secondary outcomes and to provide a clear denominator. The primary outcome has been changed from "the proportion of contacts with tuberculosis infection identified through screening strategies" to "contacts with latent TB infection out of all contacts screened". The secondary outcome has been changed from "the proportion of contacts with disease (latent and active tuberculosis versus non‐infected contacts) identified between the two screening approaches" to "contacts diagnosed with proven Mycobacterium tuberculosis infection or active clinical tuberculosis disease out of all contacts screened". Neither of these changes affected the search criteria or the number of studies found or investigated.
Contributions of authors
All review authors contributed to the development of this protocol. DBM drafted the original manuscript with input from BM and MD. DBM and BM screened studies, and MD acted as an arbitrator where necessary. All review authors read and approved the final review version.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development (DFID), UK.
Project number 300342‐104
Declarations of interest
DBM has no known conflicts of interest. BM has no known conflicts of interest. MD has no known conflicts of interest.
Unchanged
References
References to studies excluded from this review
Andre 2007 {published data only}
- Andre M, Ijaz K, Tillinghast JD, Krebs VE, Diem LA, Metchock B, et al. Transmission network analysis to complement routine tuberculosis contact investigations. American Journal of Public Health 2007;97(3):470‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrews 2014 {published data only}
- Andrews JR, Morrow C, Walensky RP, Wood R. Integrating social contact and environmental data in evaluating tuberculosis transmission in a South African township. Journal of Infectious Diseases 2003;7(12):S486‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayona 2003 {published data only}
- Bayona J, Chavez‐Pachas AM, Palacios E, Llaro K, Sapag R, Becerra MC. Contact investigations as a means of detection and timely treatment of persons with infectious multidrug‐resistant tuberculosis. International Journal of Tuberculosis and Lung Disease 2003;7(12):S501‐9. [PubMed] [Google Scholar]
Cook 2007 {published data only}
- Cook VJ, Sun SJ, Tapia J, Muth SQ, Arguello DF, Lewis BL, et al. Transmission network analysis in tuberculosis contact investigations. Journal of Infectious Diseases 2007;196(10):1517‐27. [DOI] [PubMed] [Google Scholar]
de Vries 2006 {published data only}
- Vries G, Hest RA. From contact investigation to tuberculosis screening of drug addicts and homeless persons in Rotterdam. European Journal of Public Health 2005;16(2):133‐6. [DOI] [PubMed] [Google Scholar]
Driver 2003 {published data only}
- Driver CR, Balcewicz‐Sablinska MK, Kim Z, Scholten J, Munsiff SS. Contact investigations in congregate settings, New York city. International Journal of Tuberculosis and Lung Disease 2003;7(12):S432‐8. [PubMed] [Google Scholar]
Fatima 2016 {published data only}
- Fatima R, Qadeer E, Yaqoob A, Haq MU, Majumdar SS, Shewade HD, et al. Extending ‘contact tracing' into the community within a 50‐metre radius of an index tuberculosis patient using Xpert MTB/RIF in urban, Pakistan: did it increase case detection?. PLOS ONE 2016;11(11):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2012 {published data only}
- Fox GJ, Barry S, Marks GB. Outcomes of contact investigation for tuberculosis: a systematic review and meta‐analysis. American Journal of Respiratory and Critical Care Medicine 2012;185:140‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2013 {published data only}
- Fox GJ, Nhung NV, Sy DN, Britton WJ, Marks GB. Cluster randomized controlled trial of household contact investigation in 8 provinces in Vietnam. American Journal of Respiratory and Critical Care Medicine 2013;187(Meetings/ Abstracts):342. [Google Scholar]
Fox 2018 {published data only}
- Fox GJ, Nhung NV, Sy DN, Hoa NLP, Anh LTN, Anh NT, et al. Household‐contact investigation for detection of tuberculosis in Vietnam. New England Journal of Medicine 2018;378(3):221‐9. [DOI] [PubMed] [Google Scholar]
Jensen 2016 {published data only}
- Jensen SG, Lillebaek T, Wilcke T, Pedersen MK, Andersen PH, Olsen NW, et al. Impact of contact investigation and tuberculosis screening among high‐risk groups in Denmark. International Journal of Tuberculosis and Lung Disease 2016;20(12):1580‐7. [DOI] [PubMed] [Google Scholar]
McElroy 2003 {published data only}
- McElroy PD, Rothenberg RB, Varghese R, Woodruff R, Minns GO, Muth SQ, et al. A network‐informed approach to investigating a tuberculosis outbreak: implications for enhancing contact investigations. International Journal of Tuberculosis and Lung Disease 2003;7(12):S486‐93. [PubMed] [Google Scholar]
Ribeiro 2015 {published data only}
- Ribeiro FK, Pan W, Bertolde A, Vinhas SA, Peres RL, Riley L, et al. Genotypic and spatial analysis of Mycobacterium tuberculosis transmission in a high‐incidence urban setting. Clinical Infectious Diseases 2015;61(5):758‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abubakar 2012
- Abubakar I, Griffiths C, Ormerod P, Guideline Development G. Diagnosis of active and latent tuberculosis: summary of NICE guidance. British Medical Journal 2012;345:6828. [DOI] [PubMed] [Google Scholar]
Barnes 1996
- Barnes PF, El‐Hajj H, Preston‐Martin S, Cave MD, Jones BE, Otaya M, et al. Transmission of tuberculosis among the urban homeless. JAMA 1996;275(4):305‐7. [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9. Analysing data and undertaking meta‐analyses.In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook.
Diel 2006
- Diel R, Nienhaus A, Lange A, Schaberg T. Cost‐optimisation of screening for latent tuberculosis in close contacts. European Respiratory Journal 2006;28:35‐44. [DOI] [PubMed] [Google Scholar]
Gardy 2011
- Gardy JL, Johnston JC, Ho Sui SJ, Cook VJ, Shah L, Brodkin E, et al. Whole‐genome sequencing and social‐network analysis of a tuberculosis outbreak. New England Journal of Medicine 2011;364(8):730‐9. [DOI] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 14 May 2018. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Hella 2017
- Hella J, Morrow C, Mhimbira F, Ginsberg S, Chitnis N, Gagneux S, et al. Tuberculosis transmission in public locations in Tanzania: a novel approach to studying airborne disease transmission. Journal of Infection 2017;75(3):191‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16. Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8. Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook.
Huang 2017
- Huang L, Li XX, Abe EM, Xu L, Ruan Y, Cao CL, et al. Spatial‐temporal analysis of pulmonary tuberculosis in the northeast of the Yunnan province, People's Republic of China. Infectious Diseases of Poverty 2017;6(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones‐Lopez 2016
- Jones‐Lopez EC, Acuna‐Villaorduna C, Ssebidandi M, Gaeddert M, Kubiak RW, Ayakaka I, et al. Cough aerosols of Mycobacterium tuberculosis in the prediction of incident tuberculosis disease in household contacts. Clinical Infectious Diseases 2016;63(1):10‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2016
- Lee SH. Tuberculosis infection and latent tuberculosis. Tuberculosis and Respiratory Disease (Seoul) 2016;79(4):201‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6. Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Middlekoop 2009
- Middelkoop K, Bekker LG, Morrow C, Zwane C, Wood R. Childhood tuberculosis infection and disease: a spatial and temporal transmission analysis in a South African township. South African Medical Journal 2009;99(10):738–43. [PMC free article] [PubMed] [Google Scholar]
Middlekoop 2014
- Middelkoop K, Bekker LG, Morrow C, Lee NC, Wood R. Decreasing household contribution to TB transmission with age: a retrospective geographic analysis of young people in a South African township. BMC Infectious Diseases 2014;14(221):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 2008
- Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low‐income and middle‐income countries: a systematic review and meta‐analysis. Lancet. Infectious Diseases 2008;8(6):359‐68. [DOI] [PubMed] [Google Scholar]
Munang 2016
- Munang ML, Browne C, Evans JT, Smith EG, Hawkey PM, Welch SB, et al. Programmatic utility of tuberculosis cluster investigation using a social network approach in Birmingham, United Kingdom. International Journal of Tuberculosis and Lung Disease 2016;20(10):1300‐5. [DOI] [PubMed] [Google Scholar]
NICE 2016
- National Institute for Clinical Excellence. Tuberculosis. NICE guideline [NG33]. Published date: January 2016. Last updated: May 2016. www.nice.org.uk/guidance/ng33 (accessed prior to 27 August 2019).
Patterson 2017
- Patterson B, Morrow CD, Kohls D, Deignan C, Ginsburg S, Wood R. Mapping sites of high TB transmission risk: integrating the shared air and social behaviour of TB cases and adolescents in a South African township. Science of the Total Environment 2017;583:97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sloot 2014
- Sloot R, Schim van der Loeff MF, Kouw PM, Borgdorff MW. Risk of tuberculosis after recent exposure. A 10‐year follow‐up study of contacts in Amsterdam. American Journal of Respiratory and Critical Care Medicine 2014;190(9):1044‐52. [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JAC, Egger M, Moher D, Boutron I (editors). Chapter 10. Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook.
Veen 1992
- Veen J. Microepidemics in tuberculosis: the stone‐in‐pond principle. International Journal of Tuberculosis and Lung Disease 1992;73:73‐6. [DOI] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. Recommendations for investigating contacts of persons with infectious tuberculosis in low‐ and middle‐income countries. 2012. Available at www.who.int/tb/publications/2012/contact_investigation2012/en/ (accessed prior to 27 August 2019). [PubMed]
WHO 2014
- World Health Organization. Towards TB elimination: an action framework for low‐incidence countries. 2014. Available at www.who.int/tb/publications/elimination_framework/en/ (accessed prior to 27 August 2019).
WHO 2018
- World Health Organization. Global tuberculosis report 2018. Available at www.who.int/tb/publications/global_report/en/ (accessed prior to 27 August 2019).
Worrell 2017
- Worrell MC, Kramer M, Yamin A, Ray SM, Goswami ND. Use of activity space in a tuberculosis outbreak: bringing homeless persons into spatial analyses. Open Forum Infectious Diseases 2017;4(1):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yates 2016
- Yates TA, Tanser F, Abubakar I. Plan beta for tuberculosis: it's time to think seriously about poorly ventilated congregate settings. International Journal of Tuberculosis and Lung Disease 2016;20(1):5‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2016
- Young KH, Ehman M, Reves R, Peterson Maddox BL, Khan A, Chorba TL, et al. Tuberculosis contact investigations ‐ United States, 2003‐2012. MMWR. Morbidity and Mortality Weekly Report 2016;64(50‐1):1369‐74. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Menezes 2018
- Braganza Menezes D, Menezes B, Dedicoat M. Contact tracing strategies in household and congregate environments to identify cases of tuberculosis in low‐ and moderate‐incidence populations. Cochrane Database of Systematic Reviews 2018, Issue 7. [DOI: 10.1002/14651858.CD013077] [DOI] [PMC free article] [PubMed] [Google Scholar]


