Abstract
Bilateral cochlear implantation has provided access to some of the benefits of binaural hearing enjoyed by normal-hearing (NH) listeners. However, a gap in performance still exists between the two populations. Single-channel stimulation studies have shown that interaural place-of-stimulation mismatch (IPM) due to differences in implantation depth leads to decreased binaural fusion and lateralization of interaural time and level differences (ITDs and ILDs, respectively). While single-channel studies are informative, multi-channel stimulation is needed for good speech understanding with cochlear implants (CIs). Some multi-channel studies have shown that channel interaction due to current spread can affect ITD sensitivity. In this work, we studied the effect of IPM and channel spacing, along with their potential interaction, on binaural fusion and ITD/ILD lateralization. Experiments were conducted in adult NH listeners and CI listeners with a history of acoustic hearing. Results showed that IPM reduced the range of lateralization for ITDs but not ILDs. CI listeners were more likely to report a fused percept in the presence of IPM with multi-channel stimulation than NH listeners. However, no effect of channel spacing was found. These results suggest that IPM should be accounted for in clinical mapping practices in order to maximize bilateral CI benefits.
I. INTRODUCTION
Current cochlear implants (CIs) use multi-channel stimulation to successfully restore speech understanding in quiet (NIH, 1995). CI signal processing follows the tonotopic organization of the cochlea and converts acoustic sound into electrical stimulation by bandpass filtering an incoming signal into different frequency bands. The envelope of each frequency band is extracted and used to modulate electrical pulses that systematically stimulate the auditory nerves at different places along the cochlea. Through this mode of listening, some patients with severe to profound hearing impairment are able to regain close to full speech understanding abilities in quiet situations (Firszt et al., 2004; Wilson and Dorman, 2007).
CIs were originally designed for unilateral implantation, though bilateral implantation has become increasingly more common (Peters et al., 2010). Bilateral implantation has been shown to provide improved speech understanding in noise and sound localization ability compared to unilateral listening (e.g., van Hoesel and Tyler, 2003; Litovsky et al., 2009), although performance on these tasks is still poorer compared to normal-hearing (NH) listeners (Grantham et al., 2007; Jones et al., 2014; Kerber and Seeber, 2012; Litovsky et al., 2012; Majdak et al., 2011). The extent to which benefits from bilateral implantation can be realized may be limited by, among other factors, interaural place-of-stimulation mismatch (IPM) across the ears. For a review of a broader range of possible factors, see Kan and Litovsky (2015).
There are at least two factors that can lead to IPM in bilateral CI users. First, IPM can occur if the durations of deafness in the two ears are largely different. Prolonged durations of deafness can impact the survival of spiral ganglion cells, which leads to “dead regions” along the cochlea that may be insensitive to electrical stimulation (Kawano et al., 1998; Moore et al., 2000; Nadol, 1997). Hearing with CIs depends on innervating enough spiral ganglion cells with electrical stimulation to illicit a sensation of sound. If a pair of electrodes across the ears was intended to stimulate the same area of the cochlea in both ears but one is near a dead region, the electrode near the dead region will need to recruit spiral ganglion cells outside the intended cochlea area to create a sound sensation. In this case, different areas of the cochlea are excited in each ear, leading to IPM. A second issue arises from surgical placement of the electrode arrays in each ear. During surgery, each ear is treated separately and there is no sure method for guaranteeing that the depth of insertion of electrode arrays into the two cochleae will be precisely the same. Insertion depth differences can range from 2 to 3 mm (Ketten et al., 1998), or 14° to 171° (Landsberger et al., 2015), as revealed via radio imaging. Measurements made using psychophysical tasks and objective measures typically estimate an IPM of ∼2 mm in the majority of CI users (Hu and Dietz, 2015; Kan et al., 2015b). While both causes of IPM are interesting to study, in this paper we focus on IPM caused by insertion depth differences because this is easier to simulate and the precise identification of dead regions along the cochlea is currently difficult.
Insertion depth differences are not typically accounted for in audiological practice, for example, by adjustment of frequency allocation in the two ears. Rather, current practice is to program the CI in each ear individually. The same-numbered electrodes are assigned to the same acoustic frequency region by default if there is the same number of active electrodes in each ear (Shapiro and Bradham, 2012). Hence, if an IPM exists, the same frequency content could stimulate different cochlear places in the two ears. In this situation, IPM across the ears may lead to impaired processing of interaural time and level differences (ITD and ILD, respectively), which are two critical cues for locating the direction of a sound on the horizontal plane (Macpherson and Middlebrooks, 2002; Strutt, 1907). Processing of binaural cues will be impaired by IPM because the superior olivary complex is thought to compare interaural differences on a frequency-matched basis (Tollin and Yin, 2002; Yin and Chan, 1990).
The detrimental effects of IPM on sensitivity to binaural cues has been previously demonstrated via psychoacoustic listening tests. In NH listeners, IPM has been extensively studied using amplitude-modulated signals and narrowband noises (Blanks et al., 2007; Blanks et al., 2008; Francart and Wouters, 2007; Goupell, 2015; Goupell et al., 2013). In these studies, IPM was simulated by keeping the center frequency of the signal in one ear constant while varying the center frequency of the signal in the contralateral ear. With increasing IPM, sensitivity to ITD, ILD, and changes in interaural correlation have been found to decrease. In addition, perceived auditory objects were lateralized to one side of the head, even when ITDs and ILDs were set to zero, and large IPM led to non-fusion of the auditory objects across the ears in some listeners (Goupell, 2015; Goupell et al., 2013). Similar trends have also been observed in bilateral CI users. The approaches across studies vary somewhat, but the data indicate that by activating different pairs of electrodes across the two ears, there is a pair that yields the best sensitivity (“matched” pair), and a systematic decrease in sensitivity with increasing IPM (Goupell, 2015; Hu and Dietz, 2015; Kan et al., 2013, Kan et al., 2015b; Poon et al., 2009). Collectively, these studies showed that there is a decrease in fusion of the auditory percept and decreased sensitivity to binaural cues with IPM greater than ∼3 mm.
Although single-channel studies have been important in understanding first-order effects of IPM on binaural sensitivity, CI sound processing strategies do not operate with single channels, because multi-channel stimulation is used to provide patients with good speech understanding. A known problem with multi-channel stimulation is that monopolar stimulation results in channel interaction due to spread of current (Chatterjee and Shannon, 1998; Nelson et al., 2008), which has been shown to affect speech understanding (Stickney et al., 2006; Wilson et al., 1991). However, the effects of channel interaction on multi-electrode ITD sensitivity are less clear. A study on the effect of channel spacing on ITD sensitivity in bilateral CI users with MED-EL implants showed a trend for decreasing ITD sensitivity with decreasing separation between two electrodes (Egger et al., 2016). However, in that study, electrodes with infinitely small spacing was simulated by doubling the pulse-rate on the same electrode, and the next spacing increment between electrodes was 6 mm apart. In contrast, studies in Cochlear users where three electrodes were used and separated by ∼3 or 6 mm showed much less systematic changes in ITD sensitivity (Kan et al., 2015a, 2016).
Hence, the goal of this paper is to understand the effect of channel spacing and IPM on binaural fusion, and lateralization of ITDs and ILDs. We hypothesized that channel spacing would interact with IPM to produce outcomes that could be different to those observed in prior single-channel studies. In experiment 1, typically-developed NH listeners were tested using acoustic stimuli that simulated two channel-spacing configurations (close and far) with varying amounts of IPM to understand how the NH auditory system might respond under these conditions. Further, NH listeners are typically assumed to have more homogeneous hearing, which would allow us to determine if the effects observed in CI users are due to mismatched inputs to the binaural system or due to the inherent variability in performance of the bilateral CI population in psychophysical tasks. In experiment II, bilateral CI users were tested with electrical stimulation using similar conditions and tasks.
II. GENERAL METHODS
A. Spacing configurations and simulated IPM
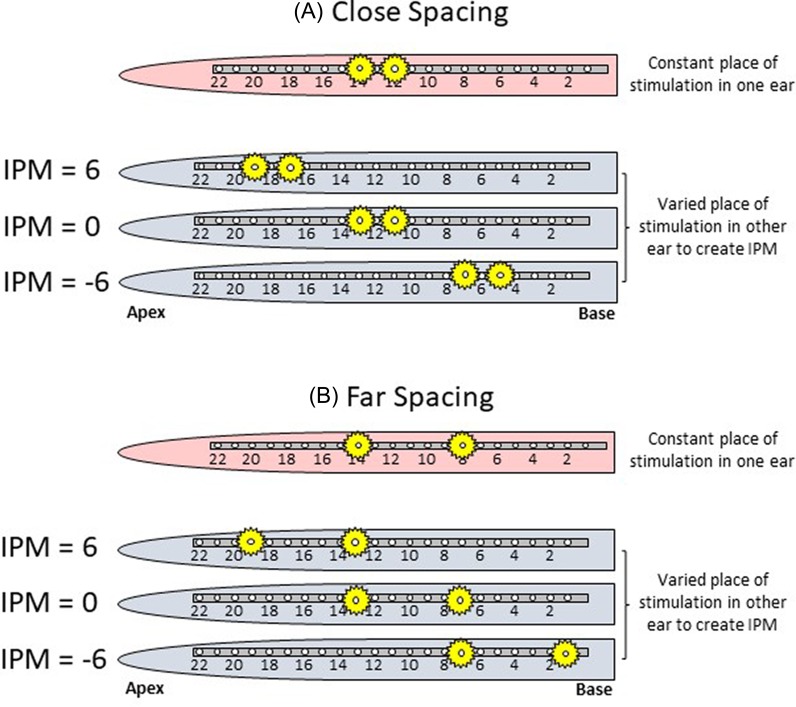
To understand the effects of channel separation and IPM on binaural fusion and lateralization, two channel-spacing configurations (close and far) were tested. Figure 1 presents a schematic of how the spacing configurations and IPMs were set up. In each spacing configuration, IPM was simulated by fixing the place-of-stimulation in one ear, and systematically varying the place-of-stimulation in the contralateral ear.
FIG. 1.
(Color online) Examples of how IPM was simulated for the two channel-spacing configurations tested. Each row shows a schematic of an unrolled cochlea with a grey 22-electrode array with different insertion depths across the ears. The yellow markers indicate the place along the cochlea being stimulated. In the close-spacing configuration (A), the place of stimulation is held consistently at the same distance apart (1.5 mm in NH and ∼2 electrode spacing in CI listeners), and the place of stimulation in the contralateral ear is varied depending on the desired amount of simulated IPM. In the far-spacing configuration (B), the places of stimulation in each ear are further apart (4.5 mm in NH and ∼6 electrode spacing in CI listeners).
B. Tasks
1. Binaural fusion
Binaural fusion was assessed in listeners for the configurations and range of IPM described in Sec. II A. In this task, ITDs and ILDs were not applied to the stimuli. Listeners responded by indicating the number and locations of perceived auditory object(s) on a graphical user interface (GUI). On each trial, the listener initiated the presentation of the stimulus by a button press. Following stimulus presentation, the listener was asked to indicate the number of perceived auditory objects, from one to four. After selecting the number of auditory objects, the listener was presented three buttons for each auditory object to indicate its location (left, center, right). Listeners were instructed to rank the dominance of the perceived auditory objects such that they indicated the perceived location of the most dominant source first. The listener could repeat the stimulus as many times as needed (typically one to three times) to make their decision. Each spacing configuration was tested separately. A block of trials for each spacing configuration consisted of 10 repetitions of each IPM condition, presented in a random order. Two blocks of trials were tested for each spacing configuration and presented in a random order for each listener.
2. Lateralization
Lateralization abilities for the configurations and range of IPMs described in Sec. II A were also assessed. On each trial, the stimulus had either an ITD or ILD applied. Negative and positive values denote ITD/ILD values pointing to the left and right, respectively. Listeners initiated presentation of the stimulus by a button press and responded by indicating the number of perceived auditory objects, and then marking the perceived lateral position of each auditory object within a visual response bar that spanned the width of a cartoon face on the GUI. If multiple auditory objects were perceived, listeners were instructed to rank the perceived dominance of each auditory object and respond with the most dominant (primary) auditory object in the topmost bar, and secondary object in the lower bar. Listeners could repeat the presentation of the stimulus as many times as needed to finish marking their responses. This task has been used extensively by the authors and others, and has been shown to yield important information about the perceptual mapping of binaural cues to spatial location (Baumgärtel et al., 2017; Kan et al., 2013; Kan et al., 2016; Litovsky et al., 2010; Stakhovskaya and Goupell, 2017). A total of ten trials were collected for each ITD/ILD and IPM combination. The application of ITD and ILD were tested separately. The order of presentation of ITD/ILD, spacing, and IPM combinations were randomized in blocks, consisting of two trials for each combination per block.
C. Data analysis
1. Binaural fusion
In this task, we were interested in understanding the stimulus conditions that form a centered auditory object in the absence of externally-applied interaural differences. Hence, the data were analyzed by calculating the proportion of trials where a single auditory object was reported for each IPM and spacing condition, and the perceived location of the fused auditory object. Statistical analysis was conducted using binomial logistic regression. Spacing configuration and IPM and their interaction were entered as fixed effects, and listener was entered as a random effect.
2. Lateralization
In this task, we were interested in understanding the stimulus conditions that facilitated lateralization of ITDs and ILDs. Our GUI for this task allowed us to capture the number and perceived lateral location of perceived auditory objects as a function of spacing configuration, IPM, and imposed interaural difference (ITD or ILD). To quantify lateralization ability, the following metrics were calculated for each listener: (1) the proportion of trials perceived as a single auditory object for each IPM; (2) the utilized lateral range (ULR) derived from the lateralization function; and (3) an estimate of the right/left discrimination threshold.
Statistical analysis was conducted on each metric using a generalized linear mixed-effects model. In each model, spacing configuration, IPM and their interaction were entered as fixed effects, and listener was entered as a random effect. For metric 1, individual trials were used and modeled as a binomial outcome variable; for metric 2 and 3, the calculated ULR and discrimination threshold, respectively, was used as the outcome variable.
To calculate the ULR, listener responses were mapped to values between 0 and 1, where 0, 0.5, and 1 represented the leftmost, center, and rightmost locations in the head, respectively. Then, for each ITD/ILD value tested, the average perceived lateral location of the primary auditory object was calculated using a 20% trimmed mean. The trimmed mean removes the highest and lowest 20% of samples prior to calculation of the mean to minimize the effect of extreme outliers. The mean perceived locations as a function of ITD/ILD was then fit with a four-parameter logistic function of the form
| (1) |
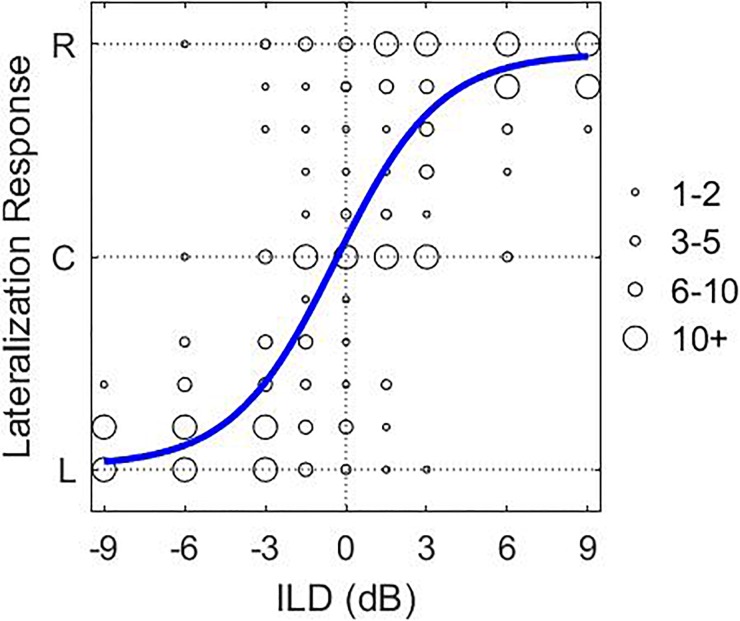
Here, cue is either the ITD or ILD value applied, A and B are the functions minimum and maximum values, respectively, and constrained to be within the range 0 to 1, m is the steepness of the curve, and shift is the bias in the responses. The ULR was calculated by taking the difference in the lateral location predicted by Eq. (1) at the leftmost and rightmost ITD/ILD value tested. The difference was then multiplied by 100 to express the ULR as a percentage. The ULR provides a conservative estimate of the perceptual range but may underestimate the actual range because the perceived lateral location of an auditory object maybe weighted more towards the center of the head because of edge effects of the lateralization GUI. Figure 2 shows an example fit of the lateralization data with ILD cues.
FIG. 2.
(Color online) Example of a fit to the lateralization data with ILD cues of one of the NH listeners. L, C, and R stand for the left, center, and right of the GUI. The size of each circle represents the number of responses in a particular location on the GUI. The solid line shows the fit to the responses.
Discrimination thresholds were estimated using the method described in Litovsky et al. (2010). Lateralization responses were first linearized by applying an arcsin transformation. Then, d′ was estimated for each left/right ITD/ILD pair of the same value (e.g., +400 and −400 μs). A line, constrained to pass through zero, was then fitted to the d′ values, and the threshold estimated as the point where the best-fit line intersected d′ = 1.
III. EXPERIMENT I: NH LISTENERS
A. Methods
The effect of IPM and channel spacing was first examined in eight NH listeners aged between 22 and 32. Listeners had pure tone thresholds at or below 20 dB hearing level and less than 10 dB difference across the ears at octave interval frequencies between 250 and 8000 Hz. One of the listeners was the first author. The rest were students from the University of Wisconsin-Madison and were paid an hourly wage for their participation. Experimental procedures followed the regulations set by the National Institutes of Health and were approved by the University of Wisconsin's Human Subjects Institutional Review Board.
Experiments were conducted in a double-walled sound booth. A computer running custom-written software programmed in matlab (Mathworks, Natick, MA) was used to generate stimuli and run the experiments. The computer was connected to a System3 Interface with RP2.1, PA5, and HB7 components (Tucker-Davis Technologies; Alachua, FL) and stimuli presented via insert headphones (ER2, Etymotic; Elk Grove Village, IL).
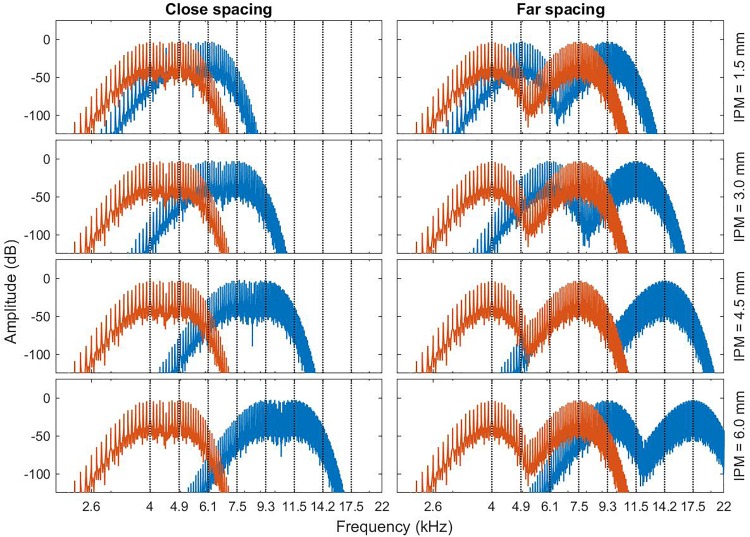
Gaussian-enveloped tone (GET) pulse trains (Goupell et al., 2010) were used as the stimulus. GET pulses had a 1.5-mm bandwidth and were played at a rate of 100 pulse-per-second (pps). The 1.5-mm bandwidth was chosen because it best approximated CI performance with monopolar stimulation in prior IPM experiments (Kan et al., 2013). Pulse trains were 300 ms in duration. Spacing conditions were simulated using carrier frequencies (CF) 4000 and 4955 Hz (close), and 4000 and 7573 Hz (far). These CFs simulate channel spacings of 1.5 and 4.5 mm according to Greenwood's place-to-frequency mapping function (Greenwood, 1990) and approximates two- and six-electrode spacings in a Cochlear straight electrode array. IPM was simulated for ±1.5, ±3, ±4.5, ±6 mm for the binaural fusion task, and for positive IPM values only in the lateralization task. Only positive IPM values were tested in lateralization because the effects of IPM were assumed to be symmetric across the ears in NH listeners. When simulating IPM, the center frequencies used in the left ear were held constant while the CFs in the right ear was varied. In Sec. III B, we will use negative values of IPM to indicate the left-ear place-of-stimulation was more basal than the right ear, and vice versa for positive values of IPM. The combinations of IPMs and CFs are shown in Table I. These CFs were the same as that used in Goupell et al. (2013). As in Goupell et al. (2013), the stimuli were calibrated such that the 4-kHz GET pulse train was presented at 70 dB sound pressure level (A-weighted). The GET pulse train at each CF was normalized to have the spectral peak at the same level which implies that the sound pressure level decreased with increasing CF but were still audible at all CFs tested. The levels at each CF can be found in Table I of Goupell et al. (2013). The spectrum of the stimuli are shown in Fig. 3.
TABLE I.
Center frequencies used for NH listeners.
| Mismatch | Common | Close spacing (1.5 mm) | Far spacing (4.5 mm) | |||
|---|---|---|---|---|---|---|
| mm | L | R | L | R | L | R |
| −6 | 9351 | 4000 | 11 538 | 4955 | 17 538 | 7573 |
| −4.5 | 7573 | 4000 | 9351 | 4955 | 14 228 | 7573 |
| −3 | 6129 | 4000 | 7573 | 4955 | 11 538 | 7573 |
| −1.5 | 4955 | 4000 | 6129 | 4955 | 9351 | 7573 |
| 0 | 4000 | 4000 | 4955 | 4955 | 7573 | 7573 |
| 1.5 | 4000 | 4955 | 4955 | 6129 | 7573 | 9351 |
| 3 | 4000 | 6129 | 4955 | 7573 | 7573 | 11 538 |
| 4.5 | 4000 | 7573 | 4955 | 9351 | 7573 | 14 228 |
| 6 | 4000 | 9351 | 4955 | 11 538 | 7573 | 17 538 |
FIG. 3.
(Color online) Spectrum of the stimulus used in the NH experiments for each IPM and spacing configuration. The red line shows the spectrum of the stimulus in the ear that was held constant and the blue line shows the spectrum of the stimulus in the ear that was changed.
Listener tasks were described in Sec. II B. For the binaural fusion task, a total of 360 trials (9 IPM conditions × 2 spacing × 20 repetitions) was tested. For the lateralization task, the ITDs and ILDs imposed on the stimuli were 0, ±200, ±400, and ±800 μs, and 0, ±1.5, ±3, ±6, and ±9 dB, respectively. This equates to 700 (7 ITDs × 5 IPM × 2 spacing × 10 repetitions) and 900 trials (9 ILDs × 5 IPM × 2 spacing × 10 repetitions) for lateralization with ITD and ILD, respectively.
B. Results
1. Binaural fusion
Figure 4 shows individual and group results for the proportion of trials when only one sound source was reported in the binaural fusion task. Listeners fell into one of two groups. Three listeners (NH2, NH3, and NH5) always reported hearing one sound regardless of spacing configuration and IPM. For the remaining five listeners, a non-fused auditory object was perceived with increasing IPM. Listeners NH4 and NH6 reported hearing three sounds but the incidence was very low (less than 1% of total trials). Statistical analysis conducted with the data from the five listeners who did not always report hearing one fused sound revealed a significant main effect of IPM [F(8,72) = 2.807, p = 0.009] but not spacing configuration [F(1,72) = 3.676, p = 0.059]. Pairwise contrast analysis with Bonferroni correction found a significant increase in the number of non-fused auditory objects when the magnitude of IPM > 3 mm (see Table II).
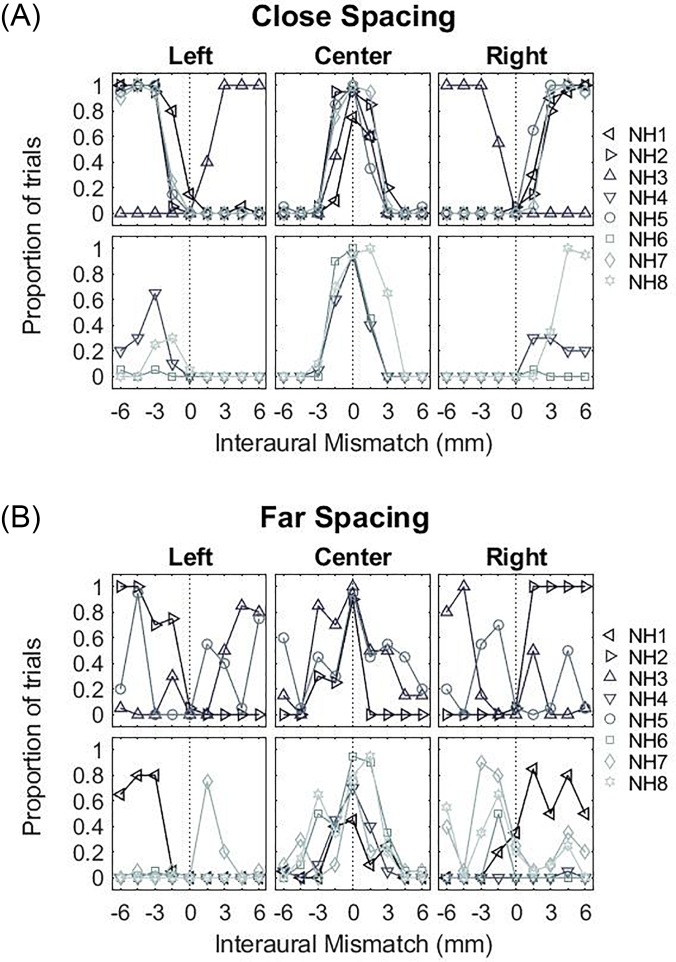
FIG. 4.
(Color online) Proportion of trials where only one auditory object was reported as a function of IPM in NH listeners. (A) shows individual data and (B) shows group averaged data for the two spacing-channel configurations tested.
TABLE II.
Pairwise contrast with Bonferroni correction examining the relationship between fusion and IPM in NH listeners. In all comparisons below, t statistics are shown, and degrees of freedom = 72.
| IPM | −4.5 | −3.0 | −1.5 | 0 | 1.5 | 3.0 | 4.5 | 6.0 |
|---|---|---|---|---|---|---|---|---|
| −6.0 | 0.14 | −1.56 | −3.20 | −4.21* | −3.06 | −0.76 | −0.05 | 0.16 |
| −4.5 | −1.71 | −3.36** | −4.38* | −3.22 | −0.90 | −0.60 | 0.03 | |
| −3.0 | −1.47 | −2.32 | −1.34 | 0.75 | 1.04 | 1.63 | ||
| −1.5 | −0.84 | 0.15 | 2.24 | 2.53 | 3.14 | |||
| 0 | 1.00 | 3.12 | 3.40** | 4.02* | ||||
| 1.5 | 2.11 | 2.40 | 3.01 | |||||
| 3.0 | 0.29 | 0.88 | ||||||
| 4.5 | 0.60 |
p ≤ 0.01.
p < 0.05.
The location of the fused auditory object is shown in Fig. 5, and is grouped by listeners who always reported hearing a single auditory object (upper panels) vs those that did not (lower panels). In the close-spacing configuration [Fig. 5(A)], there were clear systematic trends. When IPM = 0, a centered auditory object was reported by all listeners. With increasing IPM, the auditory object was lateralized towards the ear with more basal stimulation for most listeners, except NH3 who reported a lateralized auditory object towards the ear with more apical stimulation. In the far-spacing configuration [Fig. 5(B)], a centered auditory object was reported when IPM = 0. However, for other values of IPM, large inter-subject variations are seen with no systematic trends across the group.
FIG. 5.
(Color online) Perceived lateral position of the dominant auditory object for NH listeners. (A) shows the data for the close-spacing configuration where the upper panels show the data for listeners who always heard one fused auditory object and the lower panels show the data for listeners who reported hearing more than one auditory object. (B) shows the data for the far-spacing configuration arranged in a similar manner as that of (A).
2. Lateralization
Figures 6(A) and 7(A) show the proportion of trials perceived as a single auditory object when an ITD or ILD was applied to the stimulus, respectively. This analysis is like that conducted on the binaural fusion data, but here the trials may contain a non-zero ITD or ILD values. Two listeners (NH3 and NH5) always reported hearing only one sound regardless of spacing configuration and IPM. In both ITD and ILD conditions, there appears to be difference in the number of perceived auditory objects between the two spacing configurations when IPM > 3 mm. Statistical analysis conducted with the data from the remaining six listeners who did not always report hearing one fused auditory object revealed a significant main effect of IPM [F(4,50) = 3.073, p = 0.024] and spacing configuration [F(1,50) = 4.275, p = 0.044] for the ITD condition. For the ILD condition, there was only a significant main effect of IPM [F(4,50) = 3.821, p = 0.009]. In both ITD and ILD conditions, pairwise contrast analysis with Bonferroni correction found a significant increase in the number of non-fused auditory objects for IPM ≥ 3 mm (Table III).
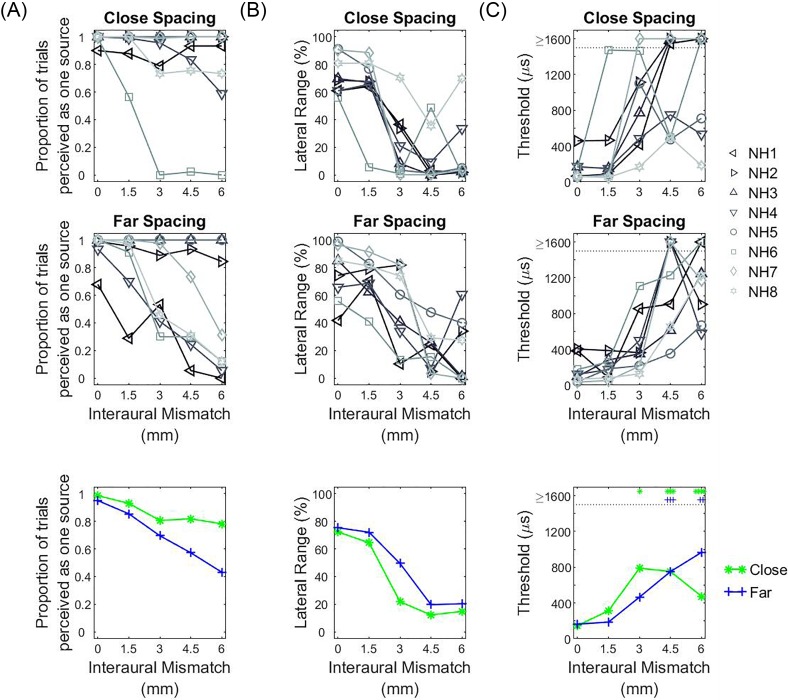
FIG. 6.
(Color online) Results from the three analysis metrics calculated from the NH lateralization data obtained when a non-zero ITD was applied to the stimulus. (A) shows the proportion of trials where one auditory object was reported, (B) shows the ULR, and (C) shows the estimated threshold. The top, middle, and bottom rows show the individual data for the close-spacing configuration, individual data for the far-spacing configuration, and group data, respectively.
FIG. 7.
(Color online) Results from the three analysis metrics calculated from the NH lateralization data obtained when a non-zero ILD was applied to the stimulus. The panels are arranged in the same way as in Fig. 6.
TABLE III.
Pairwise contrast with Bonferroni correction examining the relationship between fusion and IPM in NH listeners when a non-zero ITD or ILD is imposed on the stimulus. In all comparisons below, t statistics are shown, and degrees of freedom = 50.
| ITD | ||||
|---|---|---|---|---|
| IPM | 1.5 | 3 | 4.5 | 6.0 |
| 0 | 1.25 | 2.84* | 3.24* | 4.17** |
| 1.5 | 1.62 | 2.06 | 2.98* | |
| 3 | 0.48 | 1.39 | ||
| 4.5 | 0.89 | |||
| ILD | ||||
| IPM | 1.5 | 3 | 4.5 | 6.0 |
| 0 | 1.34 | 2.85* | 3.74** | 4.42*** |
| 1.5 | 1.53 | 2.40 | 3.04* | |
| 3 | 0.85 | 1.45 | ||
| 4.5 | 0.59 | |||
p < 0.05.
p ≤ 0.01.
p ≤ 0.001.
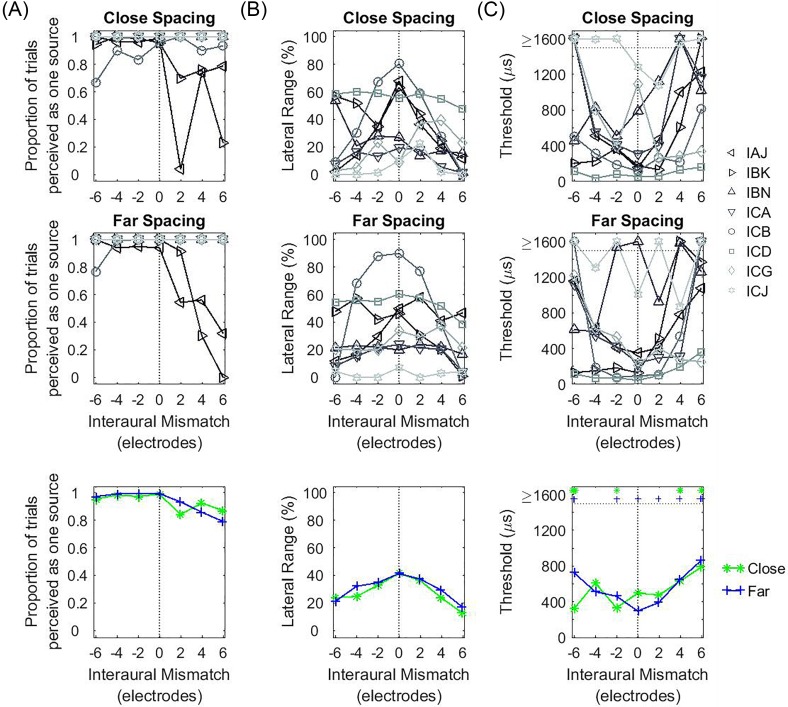
Figures 6(B) and 7(B) show the ULRs for the ITD or ILD conditions, respectively. ULRs for ITD [Fig. 6(B)] were largest when IPM = 0 mm and decreased with increasing IPM. On average, ULR for the far-spacing configuration was larger than the close-spacing configuration. Statistical analysis conducted on the ULR for the ITD condition revealed a significant main effect of IPM [F(4,70) = 38.806, p < 0.001] and spacing configuration [F(1,70) = 4.312, p = 0.042]. Pairwise contrast analysis with Bonferroni correction found a significant decrease in the ULR for IPM ≥ 3 mm (Table IV). In contrast, ULRs for ILDs [Fig. 7(B)] was not affected by IPM [F(4,70) = 0.169, p = 0.953] or spacing [F(1,70) = 0.474, p = 0.493]. Two listeners (NH1 and NH3) showed difficulty perceiving a lateral auditory object with ILDs, especially in the far-spacing condition.
TABLE IV.
Pairwise contrast with Bonferroni correction examining the relationship between IPM and (A) ULR and (B) discrimination thresholds in NH listeners when a non-zero ITD is imposed on the stimulus. In all comparisons below, t statistics are shown, and degrees of freedom = 70.
| (B) Thresholds | ||||
|---|---|---|---|---|
| IPM | 1.5 | 3 | 4.5 | 6.0 |
| 0 | −0.98 | −4.20* | −4.53* | −5.43* |
| 1.5 | −2.73** | −3.25** | −3.57*** | |
| 3 | −0.76 | −0.65 | ||
| 4.5 | 0.20 | |||
p ≤ 0.001.
p < 0.05.
p ≤ 0.01.
Figure 6(C) and 7(C) show the estimated thresholds for the ITD and ILD conditions, respectively. For non-zero ITDs [Fig. 6(C)], discrimination thresholds were lowest when IPM = 0 mm, and increased with increasing IPM for both spacing configurations. With increasing IPM, more listeners had ITD thresholds that could not be estimated in the close-spacing configuration compared with the far-spacing configuration [marked as ≥1600 μs in Fig. 6(C)]. Statistical analysis conducted on the estimated thresholds for the ITD condition revealed a significant main effect of IPM [F(4,70) = 14.272, p < 0.001] but not spacing configuration [F(1,70) = 0.016, p = 0.900]. Pairwise contrast analysis with Bonferroni correction found a significant increase in ITD discrimination threshold for IPM ≥ 3 mm (Table IV). For non-zero ILDs [Fig. 7(C)], discrimination thresholds were relatively consistent for the group as a function of IPM and statistical analysis conducted on the discrimination thresholds for the ILD condition revealed no significant effect of IPM [F(4,70) = 0.094, p = 0.984] or spacing [F(1,70) = 0.234, p = 0.630].
C. Discussion
In this experiment, we investigated the effect of IPM and channel spacing on binaural fusion and lateralization of ITDs and ILDs in NH listeners. With regards to IPM, results showed that binaural fusion and lateralization of the auditory object with ITD decreased significantly when IPM was beyond 3 mm. In contrast, ability to lateralize an auditory object with ILDs remained relatively consistent in the presence of IPM. These results are similar to those found in prior IPM studies in NH listeners where it was shown that ITD sensitivity decreased with increasing IPM (e.g., Blanks et al., 2008; Goupell et al., 2013) while ILD sensitivity remained relatively constant (e.g., Francart and Wouters, 2007; Goupell et al., 2013).
With regards to spacing configuration, a significant effect was only observed in the ITD condition. The range of lateralization for ITDs was smaller in the closer-spaced compared to the farther-spaced configuration with increasing IPM. These results are consistent with the idea that the ability to process ITD is related to the range of overlapping area of neural excitation (Blanks et al., 2008; Goupell et al., 2013) such that with closer channel spacing, the extent of overlap between the two ears decreases with increasing IPM. Hence, closer channel spacing led to a reduction in lateralization range at greater amounts of IPM.
IV. EXPERIMENT II: CI LISTENERS
A. Methods
The effect of IPM and channel spacing was examined in eight CI listeners aged between 51 and 73 years. Listener profile and etiology are shown in Table V. All listeners had CIs made by Cochlear Ltd. (Sydney, Australia), which consist of 22 intra-cochlea stimulation electrodes and two extra-cochlea ground electrodes. In these CIs, electrodes are numbered 1 (most basal) to 22 (most apical). CI listeners traveled to the University of Wisconsin-Madison for testing and participated in these tests over 2–3 days. They were paid a daily stipend for their time. Experimental procedures followed the regulations set by the National Institutes of Health and were approved by the University of Wisconsin's Human Subjects Institutional Review Board.
TABLE V.
Profile and etiology of CI listeners.
| Listener ID | Sex | Age | Implant (L/R) | Years of experience (L/R) | Etiology |
|---|---|---|---|---|---|
| IAJ | F | 68 | CI24M/CI24R(CS) | 16/9 | Childhood onset, unknown |
| IBK | M | 73 | CI24R(CS)/CI24RE | 10/4 | Adult onset, noise-induced, possibly hereditary |
| IBN | M | 67 | CI24RE/CI24R(CS) | 4/13 | Born deaf, unknown |
| ICA | F | 54 | CI24RE/CI24R(CS) | 4/11 | Childhood onset, progressive |
| ICB | F | 62 | CI24RE/CI24R(CA) | 7/10 | Childhood onset, hereditary |
| ICD | F | 57 | CI24RE/CI24R(CS) | 6/12 | Childhood onset, enlarged vestibular aqueduct |
| ICG | F | 51 | CI24R(CS)/CI24R(CS) | 10/10 | Childhood onset, unknown |
| ICJ | F | 63 | CI512/CI512 | 3/3 | Childhood onset, illness |
Direct electrical stimulation was used for conducting experiments with bilateral CI listeners. A personal computer running custom-written software programmed in matlab was used to generate stimuli and run the experiments. Listener responses were obtained using a touchscreen monitor connected to the personal computer. A pair of Laura34 speech processors (Cochlear Ltd., Sydney, Australia) was used to communicate directly to the listener's internal device and bilaterally synchronized, electrical pulse trains were used as the stimulus for these experiments. Each pulse was biphasic, with a 25-μs phase duration, 8-μs phase gap and presented via monopolar stimulation (MP1 + 2). Two-channel stimulation was achieved using continuous interleaved sampling, with the pulse on the second channel starting right after the end of the first pulse. Similar to prior work investigating the effects of IPM on binaural sensitivity (Kan et al., 2013, 2015b), stimuli were 300-ms in duration and had a constant amplitude. The stimulation rate was 100 pulses per second (pps). This stimulation rate was used because prior work has shown that CI listeners are typically most sensitive to ITDs at this rate (e.g., van Hoesel et al., 2009).
To ensure patient comfort during testing, threshold and maximum comfortable stimulation levels were measured for all electrodes (Litovsky et al., 2017), and a self-reported most comfortable (C) level within this range was used for testing. Loudness of C levels were equalized using the method described in Litovsky et al. (2012). Three pitch-matched electrode pairs were found for each subject using the techniques described in detail in Litovsky et al. (2012). Two tasks are used to identify a pitch-matched pair across the ears: (1) a pitch-magnitude estimation (PME) task and (2) a direct pitch comparison (DPC) task. In the PME task, even-numbered electrodes were played one at a time to the listener and they were asked to rate the pitch of the electrode on a scale from 1 to 100, representing low to high pitch, respectively. The results of the PME task provided a global estimate of pitch along each array in the two ears and were used as a way of estimating which electrodes might be matched in pitch for the DPC task. In the DPC task, two electrodes, one in each ear, were played sequentially to the listener, and their task was to respond whether the pitch of the second sound was “much higher,” “higher,” “same,” “lower,” or “much lower” than the first. The interaural electrode pair with the highest number of “same” ratings was chosen as the pitch-matched pair for these experiments. For some listeners, it was sometimes the case that no interaural pair of electrodes sounded the same. In these cases, there was usually an interaural pair with a bimodal response distribution such that on approximately half the trials the pitch sounded higher and for the other half the pitch sounded lower. In this case, the interaural pair with the bimodal response distribution was chosen as the pitch-matched pair. The loudness of each electrode pair was equalized and a single, centered auditory object was confirmed for each pair.
From these three pitch-matched pairs, two dual-channel configurations were created to examine the effect of multi-electrode stimulation when stimulating channels are relatively close or more distant to each other. The distance between the channels in the two configurations were approximately two-electrode spacing and six-electrode spacing, respectively (see Fig. 1). However, the choice of electrodes to match such a setup is complicated by two factors: (1) A few listeners had some electrodes deactivated in their clinical maps. Our practice, for safety reasons, is to not use deactivated electrodes in our testing (Litovsky et al., 2017). Hence, the ear with the varying electrodes needed to have the most electrodes available in order to allow us to simulate the range of interaural mismatches. (2) The pitch-matched electrodes in the contralateral ear may not always be offset by the same number of electrodes as the reference ear. To work around these limiting factors, we choose the side with the most available electrodes to be the reference side for the pitch-matching task. For most of our listeners, this was the left ear. The pitch-matching task may then identify electrodes in the contralateral ear that may or may not have the same spacing as the reference ear. In our main testing, we kept the experimentally found pitch-matched electrodes constant and varied the electrodes in the reference ear to simulate IPM. This setup allowed us to maintain the desired amount of electrode spacing (at least in the reference ear) for testing and allowed us to simulate the desired amount of interaural mismatch. Electrodes used for each listener and configuration are shown in Table VI. In the following experiments, the pitch-matched pair of electrodes was considered as IPM = 0. Artificially “mismatched” pairs were created by varying the electrodes used on the contralateral side by ±2, ±4, and ±6 electrodes. In the following, negative values of IPM means the electrodes in the variable ear was more basal than the fixed constant electrodes and vice versa for positive values of IPM. It should be noted that our CI listeners were implanted with arrays that do not have an equal spacing in millimeters between electrodes. Electrode spacing varies from about 0.8 mm at the basal end to 0.4 mm at the apical end. This design is such that the electrodes will have approximately constant angular spacing when properly inserted inside the cochlea. Hence, our simulated IPM is greater (in terms of millimeters) at the basal end compared with the apical end. Conversion between IPM from number of electrodes to millimeters is given in Table VII.
TABLE VI.
Electrodes used for testing by each CI listener in each spacing configuration and IPM combination.
| ID | Electrodes used in both configurations | Close-spacing configuration (two electrodes) | Far-spacing configuration (six electrodes) | IPM | |||
|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | # Electrodes | |
| IAJ | 8 | 13 | 6 | 12 | 2 | 11 | −6 |
| 10 | 13 | 8 | 12 | 4 | 11 | −4 | |
| 12 | 13 | 10 | 12 | 6 | 11 | −2 | |
| 14 | 13 | 12 | 12 | 8 | 11 | 0 | |
| 16 | 13 | 14 | 12 | 10 | 11 | 2 | |
| 18 | 13 | 16 | 12 | 12 | 11 | 4 | |
| 20 | 13 | 18 | 12 | 14 | 11 | 6 | |
| IBK | 14 | 20 | 12 | 18 | 10 | 14 | 6 |
| 14 | 18 | 12 | 16 | 10 | 12 | 4 | |
| 14 | 16 | 12 | 14 | 10 | 10 | 2 | |
| 14 | 14 | 12 | 12 | 10 | 8 | 0 | |
| 14 | 12 | 12 | 10 | 10 | 6 | −2 | |
| 14 | 10 | 12 | 8 | 10 | 4 | −4 | |
| 14 | 8 | 12 | 6 | 10 | 2 | −6 | |
| IBN | 8 | 18 | 6 | 16 | 2 | 14 | −6 |
| 10 | 18 | 8 | 16 | 4 | 14 | −4 | |
| 12 | 18 | 10 | 16 | 6 | 14 | −2 | |
| 14 | 18 | 12 | 16 | 8 | 14 | 0 | |
| 16 | 18 | 14 | 16 | 10 | 14 | 2 | |
| 18 | 18 | 16 | 16 | 12 | 14 | 4 | |
| 20 | 18 | 18 | 16 | 14 | 14 | 6 | |
| ICA | 8 | 14 | 6 | 13 | 2 | 10 | −6 |
| 10 | 14 | 8 | 13 | 4 | 10 | −4 | |
| 12 | 14 | 10 | 13 | 6 | 10 | −2 | |
| 14 | 14 | 12 | 13 | 8 | 10 | 0 | |
| 16 | 14 | 14 | 13 | 10 | 10 | 2 | |
| 18 | 14 | 16 | 13 | 12 | 10 | 4 | |
| 20 | 14 | 18 | 13 | 14 | 10 | 6 | |
| ICB | 8 | 13 | 6 | 12 | 2 | 8 | −6 |
| 10 | 13 | 8 | 12 | 4 | 8 | −4 | |
| 12 | 13 | 10 | 12 | 6 | 8 | −2 | |
| 14 | 13 | 12 | 12 | 8 | 8 | 0 | |
| 16 | 13 | 14 | 12 | 10 | 8 | 2 | |
| 18 | 13 | 16 | 12 | 12 | 8 | 4 | |
| 20 | 13 | 18 | 12 | 14 | 8 | 6 | |
| ICD | 8 | 13 | 6 | 11 | 2 | 7 | −6 |
| 10 | 13 | 8 | 11 | 4 | 7 | −4 | |
| 12 | 13 | 10 | 11 | 6 | 7 | −2 | |
| 14 | 13 | 12 | 11 | 8 | 7 | 0 | |
| 16 | 13 | 14 | 11 | 10 | 7 | 2 | |
| 18 | 13 | 16 | 11 | 12 | 7 | 4 | |
| 20 | 13 | 18 | 11 | 14 | 7 | 6 | |
| ICG | 14 | 20 | 12 | 18 | 8 | 14 | 6 |
| 14 | 18 | 12 | 16 | 8 | 12 | 4 | |
| 14 | 16 | 12 | 14 | 8 | 10 | 2 | |
| 14 | 14 | 12 | 12 | 8 | 8 | 0 | |
| 14 | 12 | 12 | 10 | 8 | 6 | −2 | |
| 14 | 10 | 12 | 8 | 8 | 4 | −4 | |
| 14 | 8 | 12 | 6 | 8 | 2 | −6 | |
| ICJ | 14 | 20 | 12 | 18 | 8 | 14 | −6 |
| 14 | 18 | 12 | 16 | 8 | 12 | −4 | |
| 14 | 16 | 12 | 14 | 8 | 10 | −2 | |
| 14 | 14 | 12 | 12 | 8 | 8 | 0 | |
| 14 | 12 | 12 | 10 | 8 | 6 | 2 | |
| 14 | 10 | 12 | 8 | 8 | 4 | 4 | |
| 14 | 8 | 12 | 6 | 8 | 2 | 6 | |
TABLE VII.
IPM conversion from number of electrodes to millimeters.
| IPM (electrodes) | Electrodes common to both spacing configurations (mm) | Close-spacing configuration (mm) | Far-spacing configuration (mm) |
|---|---|---|---|
| −6 | 3.32 | 3.52 | 4.01 |
| −4 | 2.16 | 2.28 | 2.58 |
| −2 | 1.04 | 1.12 | 1.24 |
| 0 | 0 | 0 | 0 |
| 2 | 0.97 | 1.04 | 1.16 |
| 4 | 1.91 | 2.01 | 2.28 |
| 6 | 2.77 | 2.95 | 3.32 |
Listener tasks were described in Sec. II B. For the binaural fusion task, a total of 280 trials (7 IPM conditions × 2 spacing × 20 repetitions) was tested. For the lateralization task, the ITDs and ILDs imposed on the stimuli were 0, ±200, ±400, and ±800 μs, and 0, ±2, ±5, and ±10 CUs, respectively. This equates to 1260 (7 ITDs/ILDs × 9 IPM × 2 spacing × 10 repetitions) trials for lateralization with ITDs or ILDs.
B. Results
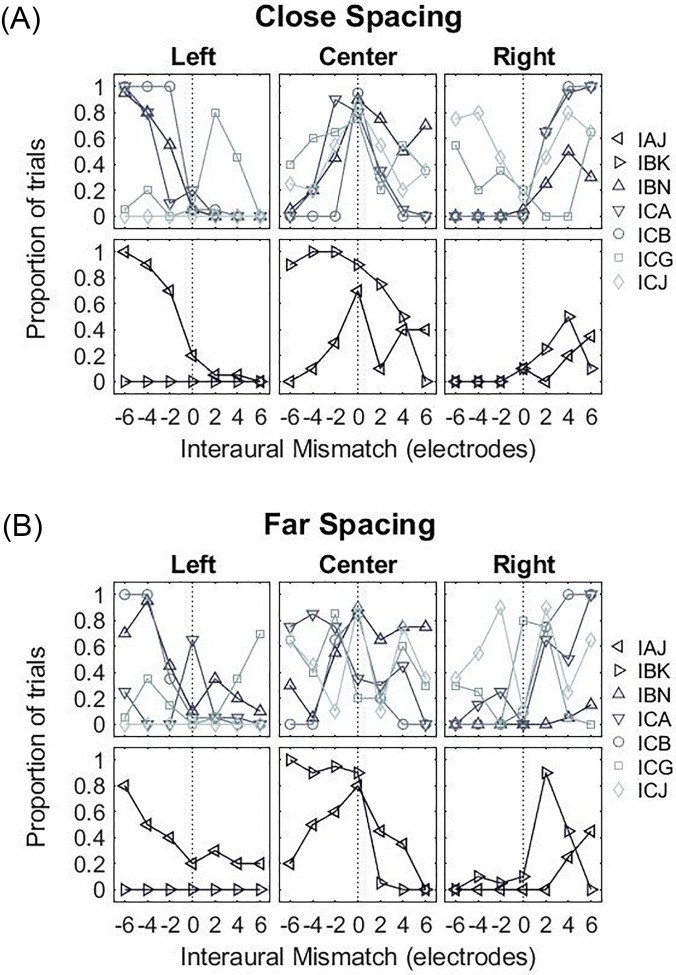
1. Binaural fusion
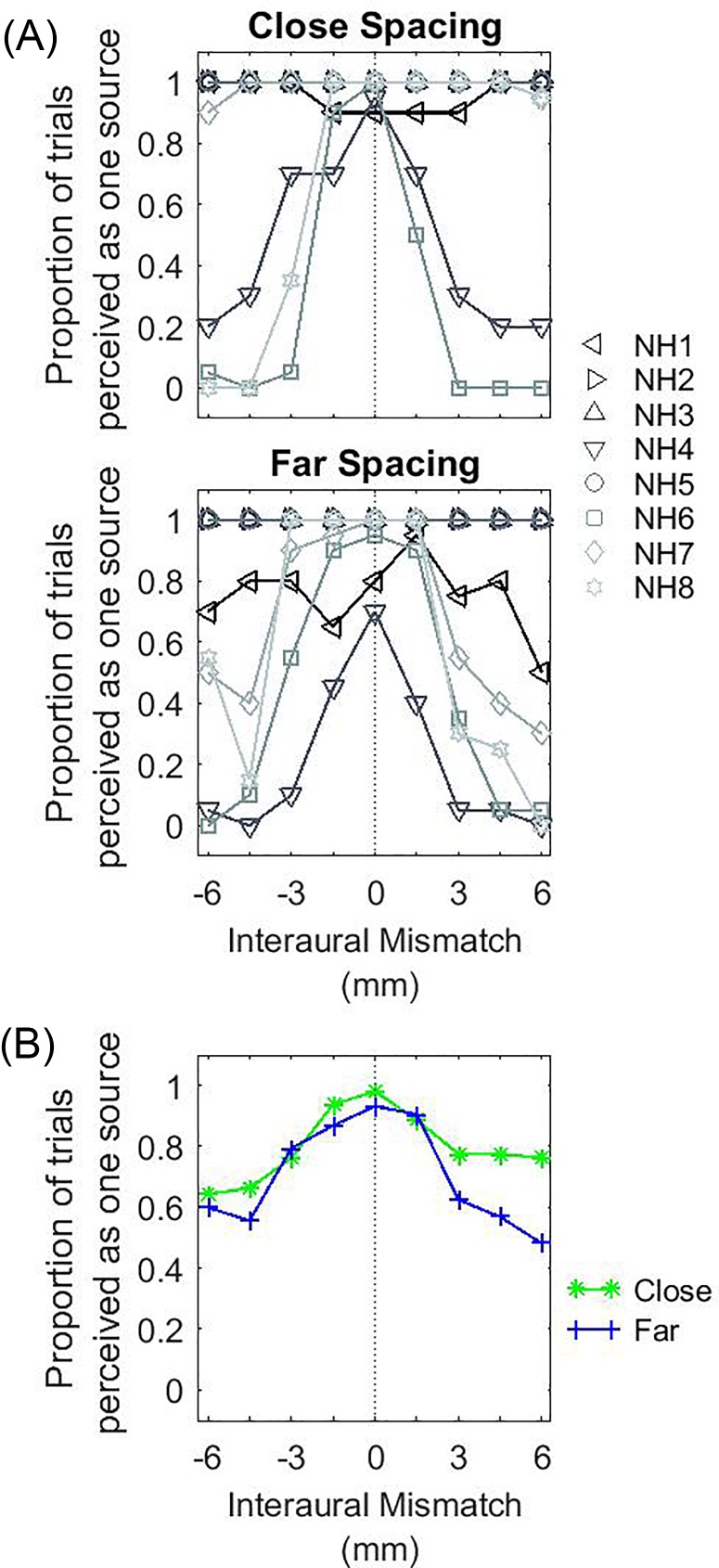
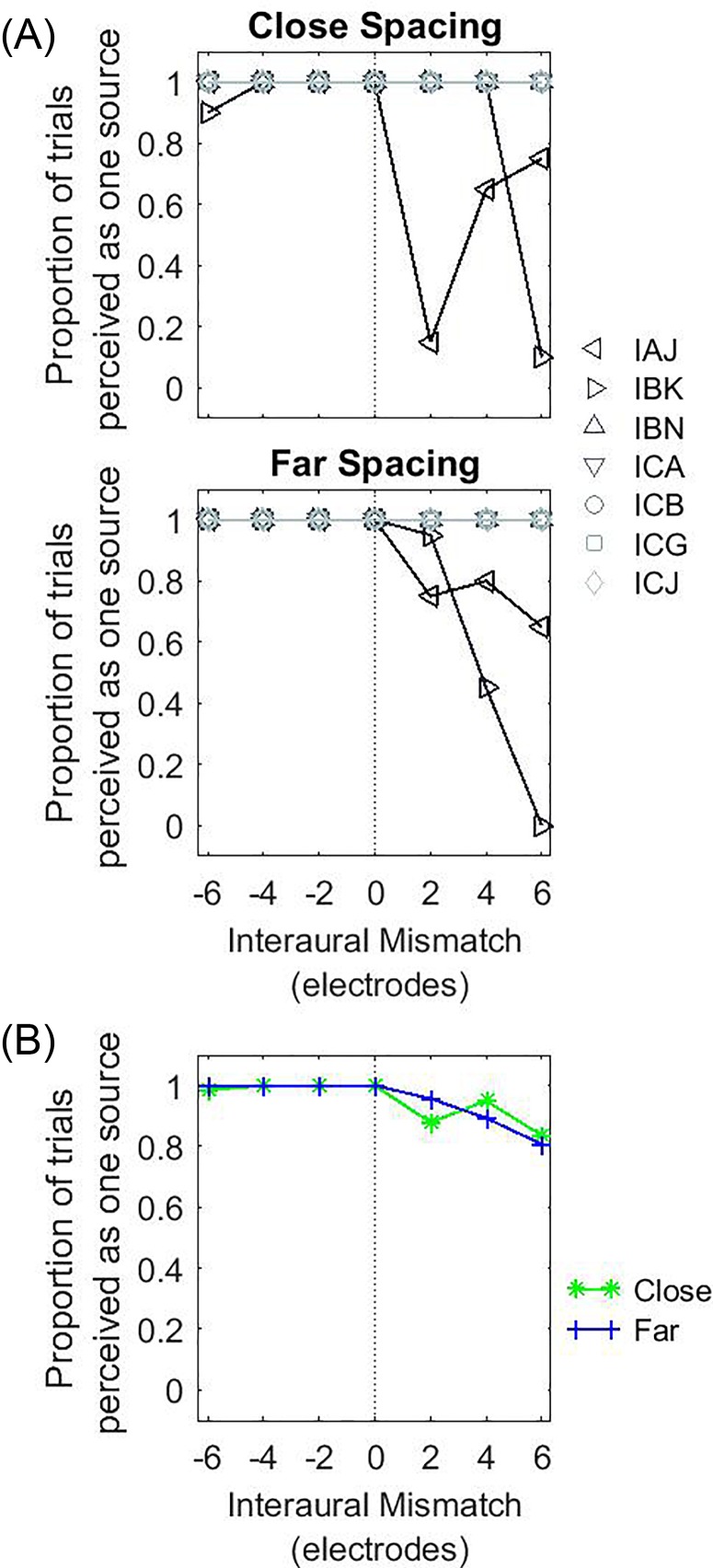
Figure 8 shows the individual and group results for the proportion of trials when the percept of a single auditory object was reported. When IPM = 0, a single fused auditory object was perceived by all bilateral CI listeners. When IPM ≠ 0, a single fused auditory object was observed in all but two bilateral CI listeners. In these listeners (IAJ and IBK; left- and right-facing triangles, respectively), there was a marked decrease in auditory fusion with positive IPM. No listener reported hearing more than two sounds. Overall, very little difference was observed between the close- and far-spacing configurations. Statistical analysis revealed no significant effect of IPM [F(6,84) = 0.574, p = 0.750] or spacing configuration [F(1,84) = 0, p = 1.000].
FIG. 8.
(Color online) Proportion of trials where only one auditory object was reported as a function of IPM in CI listeners. The panels are arranged in the same way as in Fig. 4.
The location of the dominant auditory object is shown in Fig. 9, and is grouped by listeners who always reported a fused auditory object (upper panels) vs those that did not (lower panels). In the close-spacing condition, listeners typically reported hearing a fused auditory object in the center when IPM = 0 [Fig. 9(A)]. When IPM ≠ 0, a lateralized auditory object was perceived by most listeners. In most cases, the lateralized auditory object was towards the side with more basal stimulation. In the far-spacing condition, there were less systematic trends across the group of listeners [Fig. 9(B)].
FIG. 9.
(Color online) Perceived lateral position of the dominant auditory object for CI listeners. The panels are arranged in the same way as in Fig. 5.
2. Lateralization
Figure 10(A) and 11(A) show the proportion of trials perceived as a single auditory object when a non-zero ITD or ILD was applied to the stimulus, respectively. In the ITD condition, three listeners (ICA, ICD, and ICG) always reported hearing only one auditory object regardless of spacing configuration and IPM. For the remaining listeners, there was a decrease in fusion when IPM was greater than two electrodes. Statistical analysis on the data from the listeners who did not always report hearing one fused auditory object in the ITD condition revealed no significant effect of IPM [F(6,56) = 2.058, p = 0.073] or spacing configuration [F(1,56) = 0.119, p = 0.731]. In the ILD condition, four listeners (ICA, ICD, ICG, and ICJ) always reported hearing only one auditory object. Statistical analysis on the data from the listeners who did not always report hearing one fused auditory object revealed a significant effect of IPM [F(6,42) = 9.562, p < 0.001] but not spacing configuration [F(1,42) = 0.123, p = 0.727]. Pairwise contrast analysis with Bonferroni correction revealed a significant decrease in the reporting of a single fused auditory object when IPM = +6 electrodes (Table VIII).
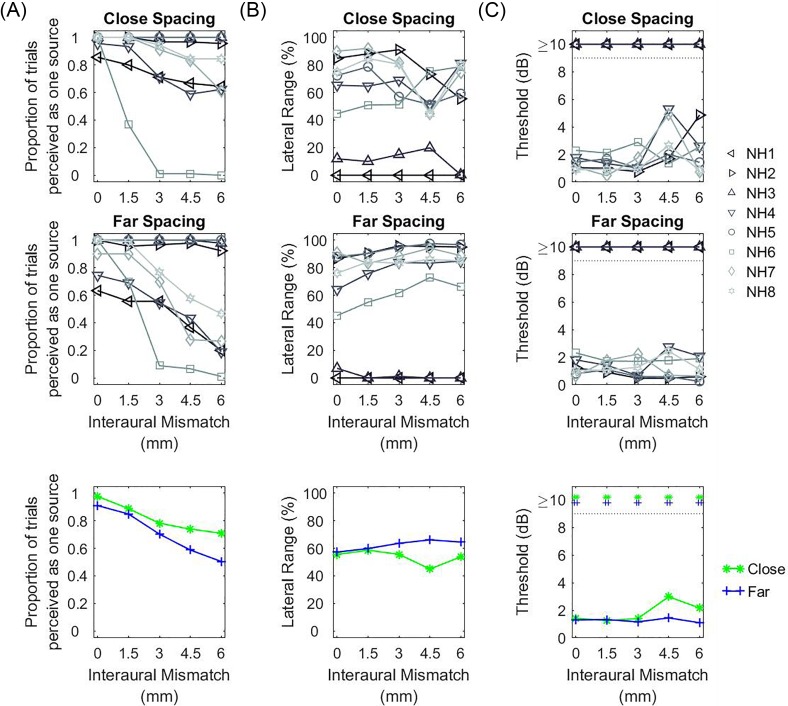
FIG. 10.
(Color online) Results from the three analysis metrics calculated from the CI lateralization data obtained when a non-zero ITD was applied to the stimulus. The panels are arranged in the same way as in Fig. 6.
FIG. 11.
(Color online) Results from the three analysis metrics calculated from the CI lateralization data obtained when a non-zero ILD was applied to the stimulus. The panels are arranged in the same way as in Fig. 6.
TABLE VIII.
Pairwise contrast with Bonferroni correction examining the relationship between binaural fusion and IPM in CI listeners when an ILD is imposed on the stimulus. In all comparisons below, t statistics are shown, and degrees of freedom = 42.
| IPM | −4 | −2 | 0 | 2 | 4 | 6 |
|---|---|---|---|---|---|---|
| −6 | −0.5 | −0.5 | 0.12 | 1.48 | 1.36 | 3.57* |
| −4 | 0.08 | 0.75 | 1.85 | 1.74 | 3.85** | |
| −2 | 0.75 | 1.85 | 1.74 | 3.85** | ||
| 0 | 1.43 | 1.31 | 3.55** | |||
| 2 | −0.14 | 2.28 | ||||
| 4 | 2.41 |
p < 0.05.
p ≤ 0.01.
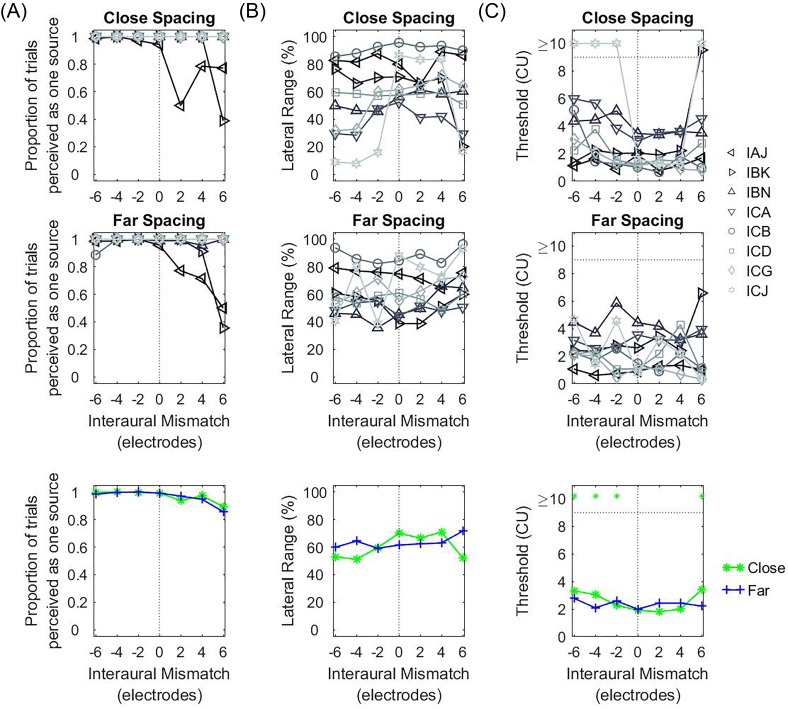
Figures 10(B) and 11(B) show the ULRs for the ITD or ILD conditions, respectively. ULRs for ITD [Fig. 10(B)] were typically largest when IPM = 0 and decreased with increasing IPM. On average, there was no difference in ULR between the two spacing configurations. Statistical analysis conducted on the ULR for the ITD condition confirmed a significant main effect of IPM [F(6,98) = 3.493, p = 0.004] but not of spacing configuration [F(1,98) = 0.001, p = 0.977]. Pairwise contrast analysis with Bonferroni correction found a significant decrease in the ULR between IPM = +6 electrodes versus IPM = 0 [t(98) = 3.283, p = 0.030] and IPM = +2 electrodes [t(98) = 3.155, p = 0.043]. In contrast, statistical analysis conducted on the ULRs for the ILD condition revealed no significant effect of IPM [F(6,98) = 0.503, p = 0.805] or spacing [F(1,98) = 1.336, p = 0.251].
Figure 10(C) and 11(C) show the estimated thresholds for the ITD and ILD conditions, respectively. For non-zero ITDs [Fig. 10(C)], discrimination thresholds appeared to be lowest when IPM = 0 and increased with increasing IPM for the far-spacing configuration. However, statistical analysis conducted on the estimated thresholds for the ITD condition revealed no significant effect of IPM [F(6,78) = 1.260, p = 0.286] or spacing configuration [F(1,78) = 0.946, p = 0.334]. For non-zero ILDs [Fig. 11(C)], statistical analysis conducted on the discrimination thresholds also revealed no significant effect of IPM [F(6,98) = 1.593, p = 0.157] or spacing [F(1,98) = 3.376, p = 0.069].
C. Discussion
This experiment examined the effects of IPM and channel spacing on bilateral fusion and ITD/ILD lateralization in bilateral CI users. Consistent with the results found in experiment I conducted in NH listeners (Sec. III), an IPM of +6 electrodes led to a significant decrease in ULR for ITDs compared to no IPM [see Figs. 6(B) and 10(B)], while ULR for ILDs remained relatively consistent for the range of IPMs tested [see Figs. 7(B) and 11(B)]. However, bilateral CI listeners had much smaller ULRs for the ITD condition compared to NH listeners in these condition [see Figs. 6(B) and 10(B)].
In our CI listeners, there was no significant effect of IPM on estimated ITD threshold [Fig. 10(C)]. This was contrary to the findings with NH listeners [Fig. 6(C)] and with CI listeners in earlier studies using a single interaural pair of electrodes. The difference between groups in the current study may be due to the limitations of not being able to simulate large IPMs in CI listeners because of the number of electrodes available on the array and individual variablilty in number of active electrodes. The largest IPM that we could simulate was ∼4 mm (see Table VII). In contrast, in NH listeners we simulated IPM of up to 6 mm. Consistent with the idea that the ability to process ITD is related to the range of overlapping area of neural excitation, the range of IPM tested in CI listeners may not have been large enough to reduce the interaural overlap.
With respect to channel spacing, no effect of spacing was found in bilateral CI listeners (Figs. 10 and 11) while NH listeners showed a decrease in ULR for ITDs with closer channel spacing [Fig. 6(B)]. One possible explanation is that the reports of binaural fusion were different between the two groups. In NH listeners, binaural fusion decreased as a function of IPM [Figs. 4 and 6(A)], while a high number of bilateral CI listeners appeared to report a higher incidence of a fused auditory object regardless of IPM [Figs. 8 and 10(A)]. It has been shown that different pitch percepts can be elicited when different areas of the cochlea are electrically stimulated in the one ear (Townshend et al., 1987). However, when two nearby channels in the same ear are stimulated simultaneously or in quick succession, the pitch of such stimulation is intermediate to the pitches illicited by stimulating each channel individually (Donaldson et al., 2005; McDermott and McKay, 1994; Townshend et al., 1987). Further, Reiss et al. (2018) showed that interaural pitch fusion in bilateral CI listeners be much greater than that of NH listeners (approximately 1 octave in CI listeners vs 0.2 octaves in NH). Hence, NH listeners were probably more sensitive to the larger inter-channel spacing and may not have binaurally fused the stimulus at large IPMs. Hence, the higher prevalence of non-fused sounds being reported at larger IPMs. This, in turn, may have interfered with binaural processing of ITDs in NH listeners.
V. GENERAL DISCUSSION
In the multi-channel stimulation results with no IPM, listeners typically reported hearing a single, fused auditory object, and could lateralize this percept when an ITD or ILD was applied (Figs. 10 and 11). This result is similar to other studies that used a priori carefully selected electrode pairs that were chosen using pitch-matching techniques as a way of approximating stimulation at similar places in the two ears (Egger et al., 2016; Kan et al., 2016). These studies have shown that with multiple, interaural electrode pairs, the application of non-zero ITDs or ILDs to the stimuli can cause a change in the location of an auditory object. Further, sensitivity to ITDs and ILDs remained relatively similar between single- and multi-channel conditions.
However, it should be noted that when IPM = 0, the full extent of lateralization was not reached for the majority of CI and NH listeners when an ITD cue was applied to the stimulus [Figs. 6(B) and 10(B)]. Small lateralization ranges have also been observed in prior studies using the lateralization task with ITDs (Baumgärtel et al., 2017; Kan et al., 2013). The data from Baumgärtel et al. suggest that the smaller ULR may be due to the bandlimited nature of the stimuli, and that broadband stimuli may be needed in order to achieve the full range of lateralization for ITDs within the physiological range of ±700 μs. While this suggests that providing ITD information along the length of the electrode array would be advantageous, full extent of lateralization is not guaranteed. Data from Kan et al. (2016) showed that 3/8 (37.5%) bilateral CI listeners did not perceive a fully lateralized auditory object when an ITD of 800 μs was applied to five channels that spanned the length of the electrode array. In contrast, ILD lateralization ranges are much greater. Further work is needed to investigate the contributions of having both ITD and ILD cues to lateralization of auditory objects in bilateral CI users.
Our results found no effect of channel spacing on ITD lateralization in bilateral CI listeners at a group level (Fig. 10). The lack of significant difference with channel spacing has also been observed in other ITD sensitivity studies with multi-channel stimulation in bilateral CI users (Kan et al., 2015a; Francart et al., 2015). The combination of these results suggests that while channel interaction may be detrimental for speech understanding (e.g., Wilson et al., 1991), it does not seem to have the same effect on ITD sensitivity. However, because all stimulating channels in these experiments have the same ITD, it is possible that the effects of IPM and channel spacing on ITD sensitivity may be more pronounced when adjacent channels have different signal envelopes and binaural cues associated with different azimuth locations.
From a practical viewpoint, the results of the present study provide additional support for the need to match interaural place of stimulation to ensure a single fused auditory object, and for maximal sensitivity to binaural differences, especially ITDs. Pitch matching has been the most common way to match place of stimulation across the ears, and has been shown to be a reasonably good method for finding a single pair of electrodes across the ears with relatively good ITD sensitivity (Kan et al., 2013; Long et al., 2003; Poon et al., 2009). However, pitch matching as a technique for identifying the best interaural pairs to maximize binaural sensitivity does have its drawbacks because of the auditory system's ability to adapt to the pitch presented via electrical stimulation (Reiss et al., 2014), non-sensory biases (Carlyon et al., 2010; Goupell et al., 2019), and work showing that pitch-matched pairs may not always yield an interaural pair with the best binaural sensitivity (Hu and Dietz, 2015). Despite these problems, pitch matching typically does yield an interaural pair that is close to the place of best binaural sensitivity.
From a functional viewpoint, this work suggests compensating for IPM should improve sound localization abilities. However, it should be noted that this is likely to only partially bridge the gap in sound localization performance between NH and bilateral CI listeners. Baumgärtel et al. (2017) suggests that a doubling of ITD magnitudes may be necessary in order to extend ITD lateralization ranges to span the entire space from left to right. In addition, compensating for IPM might improve spatial release from masking (SRM). SRM measured in bilateral CI users has often been smaller than that of NH listeners, mostly because of a lack of binaural squelch and redundancy benefits (Goupell et al., 2016; Loizou et al., 2009; Misurelli and Litovsky, 2012). Studies in NH listeners using vocoders have shown reduced binaural speech understanding benefits in the presence of IPM (Goupell et al., 2018; Yoon et al., 2013; Yoon et al., 2011), and hence compensating for IPM may bridge this gap in performance.
VI. CONCLUSIONS
In this study, the effects of IPM and channel spacing on binaural fusion and ITD/ILD lateralization were studied. Consistent with previous studies using single-channel stimulation, an increasing IPM lead to a decreased range of lateralization for ITDs and relatively little change in range of lateralization for ILDs. We found no effect of channel spacing on binaural sensitivity in CI listeners. Overall, the current study suggests that in order for ITD and ILD lateralization to be maximized, place-of-stimulation should be within 3 mm across the ears for information that has the same frequency range.
ACKNOWLEDGMENTS
We would like to thank our listeners for participating in these experiments, Zachary Smith, Sara Duran, and Aaron Parkinson from Cochlear Ltd. for providing the research hardware and technical support, Daniel Bolt for help with the statistical analysis, and Eric Bostwick and Corey Stoelb for assistance with software development and data collection. This work was supported by the NIH-NIDCD (Grant No. R01-DC003083 to R.Y.L., Grant No. R03-DC015321 to A.K., Grant No. R01-DC015798 to M.J.G.), and in part by a core grant from the NIH-NICHD (Grant No. P30-HD03352 to Waisman Center). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Baumgärtel, R. M. , Hu, H. , Kollmeier, B. , and Dietz, M. (2017). “ Extent of lateralization at large interaural time differences in simulated electric hearing and bilateral cochlear implant users,” J. Acoust. Soc. Am. 141, 2338–2352. 10.1121/1.4979114 [DOI] [PubMed] [Google Scholar]
- 2. Blanks, D. A. , Buss, E. , Grose, J. H. , Fitzpatrick, D. C. , and Hall, J. W. (2008). “ Interaural time discrimination of envelopes carried on high-frequency tones as a function of level and interaural carrier mismatch,” Ear Hear. 29, 674–683. 10.1097/AUD.0b013e3181775e03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blanks, D. A. , Roberts, J. M. , Buss, E. , Hall, J. W. , and Fitzpatrick, D. C. (2007). “ Neural and behavioral sensitivity to interaural time differences using amplitude modulated tones with mismatched carrier frequencies,” J. Assoc. Res. Otolaryngol. 8, 393–408. 10.1007/s10162-007-0088-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carlyon, R. P. , Macherey, O. , Frijns, J. H. M. , Axon, P. R. , Kalkman, R. K. , Boyle, P. , Baguley, D. M. , Briggs, J. , Deeks, J. M. , Briaire, J. J. , Barreau, X. , and Dauman, R. (2010). “ Pitch comparisons between electrical stimulation of a cochlear implant and acoustic stimuli presented to a normal-hearing contralateral ear,” J. Assoc. Res. Otolaryngol. 11, 625–640. 10.1007/s10162-010-0222-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chatterjee, M. , and Shannon, R. V (1998). “ Forward masked excitation patterns in multielectrode electrical stimulation,” J. Acoust. Soc. Am. 103, 2565–2572. 10.1121/1.422777 [DOI] [PubMed] [Google Scholar]
- 6. Donaldson, G. S. , Kreft, H. A. , and Litvak, L. (2005). “ Place-pitch discrimination of single- versus dual-electrode stimuli by cochlear implant users,” J. Acoust. Soc. Am. 118, 623–626. 10.1121/1.1937362 [DOI] [PubMed] [Google Scholar]
- 7. Egger, K. , Majdak, P. , and Laback, B. (2016). “ Channel interaction and current level affect across-electrode integration of interaural time differences in bilateral cochlear-implant listeners,” J. Assoc. Res. Otolaryngol. 17, 55–67. 10.1007/s10162-015-0542-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Firszt, J. B. , Holden, L. K. , Skinner, M. W. , Tobey, E. A. , Peterson, A. , Gaggl, W. , Runge-Samuelson, C. L. , and Wackym, P. A. (2004). “ Recognition of speech presented at soft to loud levels by adult cochlear implant recipients of three cochlear implant systems,” Ear Hear. 25, 375–387. 10.1097/01.AUD.0000134552.22205.EE [DOI] [PubMed] [Google Scholar]
- 9. Francart, T. , Lenssen, A. , Büchner, A. , Lenarz, T. , and Wouters, J. (2015). “ Effect of channel envelope synchrony on interaural time difference sensitivity in bilateral cochlear implant listeners,” Ear Hear. 36, e199–e206. 10.1097/AUD.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 10. Francart, T. , and Wouters, J. (2007). “ Perception of across-frequency interaural level differences,” J. Acoust. Soc. Am. 122, 2826–2831. 10.1121/1.2783130 [DOI] [PubMed] [Google Scholar]
- 11. Goupell, M. J. (2015). “ Interaural envelope correlation change discrimination in bilateral cochlear implantees: Effects of mismatch, centering, and onset of deafness,” J. Acoust. Soc. Am. 137, 1282–1297. 10.1121/1.4908221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goupell, M. J. , Cosentino, S. , Stakhovskaya, O. A. , and Bernstein, J. G. W. (2019). “ Interaural Pitch-discrimination range effects for bilateral and single-sided-deafness cochlear-implant users,” J. Assoc. Res. Otolaryngol. 20, 187–203. 10.1007/s10162-018-00707-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goupell, M. J. , Kan, A. , and Litovsky, R. Y. (2016). “ Spatial attention in bilateral cochlear-implant users,” J. Acoust. Soc. Am. 140, 1652–1662. 10.1121/1.4962378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goupell, M. J. , Majdak, P. , and Laback, B. (2010). “ Median-plane sound localization as a function of the number of spectral channels using a channel vocoder,” J. Acoust. Soc. Am. 127, 990–1001. 10.1121/1.3283014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goupell, M. J. , Stoelb, C. A. , Kan, A. , and Litovsky, R. Y. (2018). “ The effect of simulated interaural frequency mismatch on speech understanding and spatial release from masking,” Ear Hear. 39(5), 895–905. 10.1097/AUD.0000000000000541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goupell, M. J. , Stoelb, C. , Kan, A. , and Litovsky, R. Y. (2013). “ Effect of mismatched place-of-stimulation on the salience of binaural cues in conditions that simulate bilateral cochlear-implant listening,” J. Acoust. Soc. Am. 133, 2272–2287. 10.1121/1.4792936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grantham, D. W. , Ashmead, D. H. , Ricketts, T. A. , Labadie, R. F. , and Haynes, D. S. (2007). “ Horizontal-plane localization of noise and speech signals by postlingually deafened adults fitted with bilateral cochlear implants,” Ear Hear. 28, 524–541. 10.1097/AUD.0b013e31806dc21a [DOI] [PubMed] [Google Scholar]
- 18. Greenwood, D. D. (1990). “ A cochlear frequency-position function for several species–29 years later,” J. Acoust. Soc. Am. 87, 2592–2605. 10.1121/1.399052 [DOI] [PubMed] [Google Scholar]
- 19. Hu, H. , and Dietz, M. (2015). “ Comparison of interaural electrode pairing methods for bilateral cochlear implants,” Trends Hear. 19, 233121651561714. 10.1177/2331216515617143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones, H. , Kan, A. , and Litovsky, R. Y. (2014). “ Comparing sound localization deficits in bilateral cochlear-implant users and vocoder simulations with normal-hearing listeners,” Trends Hear. 18, 233121651455457. 10.1177/2331216514554574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kan, A. , Jones, H. G. , and Litovsky, R. Y. (2015a). “ Effect of multi-electrode configuration on sensitivity to interaural timing differences in bilateral cochlear-implant users,” J. Acoust. Soc. Am. 138, 3826–3833. 10.1121/1.4937754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kan, A. , Jones, H. G. , and Litovsky, R. Y. (2016). “ Lateralization of interaural timing differences with multi-electrode stimulation in bilateral cochlear-implant users,” J. Acoust. Soc. Am. 140, EL392–EL398. 10.1121/1.4967014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kan, A. , and Litovsky, R. Y. (2015). “ Binaural hearing with electrical stimulation,” Hear. Res. 322, 127–137. 10.1016/j.heares.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kan, A. , Litovsky, R. Y. , and Goupell, M. J. (2015b). “ Effects of interaural pitch matching and auditory image centering on binaural sensitivity in cochlear implant users,” Ear Hear. 36, e62–e68. 10.1097/AUD.0000000000000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kan, A. , Stoelb, C. , Litovsky, R. Y. , and Goupell, M. J. (2013). “ Effect of mismatched place-of-stimulation on binaural fusion and lateralization in bilateral cochlear-implant users,” J. Acoust. Soc. Am. 134, 2923–2936. 10.1121/1.4820889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawano, A. , Seldon, H. L. , Clark, G. M. , Ramsden, R. T. , and Raine, C. H. (1998). “ Intracochlear factors contributing to psychophysical percepts following cochlear implantation,” Acta Otolaryngol. 118, 313–326. 10.1080/00016489850183386 [DOI] [PubMed] [Google Scholar]
- 27. Kerber, S. , and Seeber, B. U. (2012). “ Sound localization in noise by normal-hearing listeners and cochlear implant users,” Ear Hear. 33, 445–457. 10.1097/AUD.0b013e318257607b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ketten, D. R. , Skinner, M. W. , Wang, G. , Vannier, M. W. , Gates, G. A. , and Neely, J. G. (1998). “ In vivo measures of cochlear length and insertion depth of nucleus cochlear implant electrode arrays,” Ann. Otol. Rhinol. Laryngol. Suppl. 175, 1–16. [PubMed] [Google Scholar]
- 29. Landsberger, D. M. , Svrakic, M. , Roland, J. T. , and Svirsky, M. (2015). “ The relationship between insertion angles, default frequency allocations, and spiral ganglion place pitch in cochlear implants,” Ear Hear. 36, e207–e213. 10.1097/AUD.0000000000000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Litovsky, R. Y. , Goupell, M. J. , Godar, S. , Grieco-Calub, T. , Jones, G. L. , Garadat, S. N. , Agrawal, S. , Kan, A. , Todd, A. , Hess, C. , and Misurelli, S. (2012). “ Studies on bilateral cochlear implants at the University of Wisconsin's Binaural Hearing and Speech Laboratory,” J. Am. Acad. Audiol. 23, 476–494. 10.3766/jaaa.23.6.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Litovsky, R. Y. , Goupell, M. J. , Kan, A. , and Landsberger, D. M. (2017). “ Use of research interfaces for psychophysical studies with cochlear-implant users,” Trends Hear. 21, 2331216517736464. 10.1177/2331216517736464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Litovsky, R. Y. , Jones, G. L. , Agrawal, S. , and van Hoesel, R. (2010). “ Effect of age at onset of deafness on binaural sensitivity in electric hearing in humans,” J. Acoust. Soc. Am. 127, 400–414. 10.1121/1.3257546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Litovsky, R. Y. , Parkinson, A. , and Arcaroli, J. (2009). “ Spatial hearing and speech intelligibility in bilateral cochlear implant users,” Ear Hear. 30, 419–431. 10.1097/AUD.0b013e3181a165be [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loizou, P. C. , Hu, Y. , Litovsky, R. , Yu, G. , Peters, R. , Lake, J. , and Roland, P. (2009). “ Speech recognition by bilateral cochlear implant users in a cocktail-party setting,” J. Acoust. Soc. Am. 125, 372–383. 10.1121/1.3036175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Long, C. J. , Eddington, D. K. , Colburn, H. S. , and Rabinowitz, W. M. (2003). “ Binaural sensitivity as a function of interaural electrode position with a bilateral cochlear implant user,” J. Acoust. Soc. Am. 114, 1565–1574. 10.1121/1.1603765 [DOI] [PubMed] [Google Scholar]
- 36. Macpherson, E. A. , and Middlebrooks, J. C. (2002). “ Listener weighting of cues for lateral angle: The duplex theory of sound localization revisited,” J. Acoust. Soc. Am. 111, 2219–2236. 10.1121/1.1471898 [DOI] [PubMed] [Google Scholar]
- 37. Majdak, P. , Goupell, M. J. , and Laback, B. (2011). “ Two-dimensional localization of virtual sound sources in cochlear-implant listeners,” Ear Hear. 32, 198–208. 10.1097/AUD.0b013e3181f4dfe9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McDermott, H. J. , and McKay, C. M. (1994). “ Pitch ranking with nonsimultaneous dual-electrode electrical stimulation of the cochlea,” J. Acoust. Soc. Am. 96, 155–162. 10.1121/1.410475 [DOI] [PubMed] [Google Scholar]
- 39. Misurelli, S. M. , and Litovsky, R. Y. (2012). “ Spatial release from masking in children with normal hearing and with bilateral cochlear implants: Effect of interferer asymmetry,” J. Acoust. Soc. Am. 132, 380–391. 10.1121/1.4725760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moore, B. C. , Huss, M. , Vickers, D. A. , Glasberg, B. R. , and Alcántara, J. I. (2000). “ A test for the diagnosis of dead regions in the cochlea,” Br. J. Audiol. 34, 205–224. 10.3109/03005364000000131 [DOI] [PubMed] [Google Scholar]
- 41. Nadol, J. B. (1997). “ Patterns of neural degeneration in the human cochlea and auditory nerve: Implications for cochlear implantation,” Otolaryngol. Head. Neck Surg. 117, 220–228. 10.1016/S0194-5998(97)70178-5 [DOI] [PubMed] [Google Scholar]
- 42. Nelson, D. A. , Donaldson, G. S. , and Kreft, H. (2008). “ Forward-masked spatial tuning curves in cochlear implant users,” J. Acoust. Soc. Am. 123, 1522–1543. 10.1121/1.2836786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.NIH (1995). “ Cochlear implants in adults and children,” NIH Consens. Statement 13, 1–30. [PubMed] [Google Scholar]
- 44. Peters, B. R. , Wyss, J. , and Manrique, M. (2010). “ Worldwide trends in bilateral cochlear implantation,” Laryngoscope 120, S17–S44. 10.1002/lary.20859 [DOI] [PubMed] [Google Scholar]
- 45. Poon, B. B. , Eddington, D. K. , Noel, V. , and Colburn, H. S. (2009). “ Sensitivity to interaural time difference with bilateral cochlear implants: Development over time and effect of interaural electrode spacing,” J. Acoust. Soc. Am. 126, 806–815. 10.1121/1.3158821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reiss, L. A. J. , Fowler, J. R. , Hartling, C. L. , and Oh, Y. (2018). “ Binaural pitch fusion in bilateral cochlear implant users,” Ear Hear. 39, 390–397. 10.1097/AUD.0000000000000497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reiss, L. A. J. , Turner, C. W. , Karsten, S. A. , and Gantz, B. J. (2014). “ Plasticity in human pitch perception induced by tonotopically mismatched electro-acoustic stimulation,” Neuroscience 256, 43–52. 10.1016/j.neuroscience.2013.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shapiro, W. H. , and Bradham, T. S. (2012). “ Cochlear implant programming,” Otolaryngol. Clin. North Am. 45, 111–127. 10.1016/j.otc.2011.08.020 [DOI] [PubMed] [Google Scholar]
- 49. Stakhovskaya, O. A. , and Goupell, M. J. (2017). “ Lateralization of interaural level differences with multiple electrode stimulation in bilateral cochlear-implant listeners,” Ear Hear. 38, e22–e38. 10.1097/AUD.0000000000000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stickney, G. S. , Loizou, P. C. , Mishra, L. N. , Assmann, P. F. , Shannon, R. V. , and Opie, J. M. (2006). “ Effects of electrode design and configuration on channel interactions,” Hear. Res. 211, 33–45. 10.1016/j.heares.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 51. Strutt, J. W. (1907). “ On our perception of sound direction,” Philos. Mag. Ser. 6 13, 214–232. 10.1080/14786440709463595 [DOI] [Google Scholar]
- 52. Tollin, D. J. , and Yin, T. C. T. (2002). “ The coding of spatial location by single units in the lateral superior olive of the cat I Spatial receptive fields in azimuth,” J. Neurosci. 22, 1454–1467. 10.1523/JNEUROSCI.22-04-01454.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Townshend, B. , Cotter, N. , Van Compernolle, D. , and White, R. L. (1987). “ Pitch perception by cochlear implant subjects,” J. Acoust. Soc. Am. 82, 106–115. 10.1121/1.395554 [DOI] [PubMed] [Google Scholar]
- 54. van Hoesel, R. J. M. , Jones, G. L. , and Litovsky, R. Y. (2009). “ Interaural time-delay sensitivity in bilateral cochlear implant users: Effects of pulse rate, modulation rate, and place of stimulation,” J. Assoc. Res. Otolaryngol. 10, 557–567. 10.1007/s10162-009-0175-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Hoesel, R. J. M. , and Tyler, R. S. (2003). “ Speech perception, localization, and lateralization with bilateral cochlear implants,” J. Acoust. Soc. Am. 113, 1617–1630. 10.1121/1.1539520 [DOI] [PubMed] [Google Scholar]
- 56. Wilson, B. S. , and Dorman, M. F. (2007). “ The surprising performance of present-day cochlear implants,” IEEE Trans. Biomed. Eng. 54, 969–972. 10.1109/TBME.2007.893505 [DOI] [PubMed] [Google Scholar]
- 57. Wilson, B. S. , Finley, C. C. , Lawson, D. T. , Wolford, R. D. , Eddington, D. K. , and Rabinowitz, W. M. (1991). “ Better speech recognition with cochlear implants,” Nature 352, 236–238. 10.1038/352236a0 [DOI] [PubMed] [Google Scholar]
- 58. Yin, T. C. , and Chan, J. C. (1990). “ Interaural time sensitivity in medial superior olive of cat,” J. Neurophysiol. 64, 465–488. 10.1152/jn.1990.64.2.465 [DOI] [PubMed] [Google Scholar]
- 59. Yoon, Y. , Liu, A. , and Fu, Q.-J. (2011). “ Binaural benefit for speech recognition with spectral mismatch across ears in simulated electric hearing,” J. Acoust. Soc. Am. 130, EL94–EL100. 10.1121/1.3606460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yoon, Y.-S. , Shin, Y.-R. , and Fu, Q.-J. (2013). “ Binaural benefit with and without a bilateral spectral mismatch in acoustic simulations of cochlear implant processing,” Ear Hear. 34, 273–279. 10.1097/AUD.0b013e31826709e8 [DOI] [PMC free article] [PubMed] [Google Scholar]