Abstract
Old age and Cx43 deletion in osteocytes are associated with increased osteocyte apoptosis and osteoclastogenesis. We previously demonstrated that apoptotic osteocytes release elevated concentrations of the pro-inflammatory cytokine, high mobility group box1 protein (HMGB1) and apoptotic osteocyte conditioned media (CM) promotes osteoclast differentiation. Further, prevention of osteocyte apoptosis blocks osteoclast differentiation and attenuates the extracellular release of HMGB1 and RANKL. Moreover, sequestration of HMGB1, in turn, reduces RANKL production/release by MLO-Y4 osteocytic cells silenced for Cx43 (Cx43def), highlighting the possibility that HMGB1 promotes apoptotic osteocyte-induced osteoclastogenesis. However, the role of HMGB1 signaling in osteocytes has not been well studied. Further, the mechanisms underlying its release and the receptor(s) responsible for its actions is not clear. We now report that a neutralizing HMGB1 antibody reduces osteoclast formation in RANKL/MCSF treated bone marrow cells (BMC). In bone marrow macrophages (BMMs), TLR4 inhibition with LPS-RS, but not RAGE inhibition with Azeliragon attenuated osteoclast differentiation. Further, inhibition of RAGE but not of TLR4 in osteoclast precursors reduced osteoclast number, suggesting that HGMB1 produced by osteoclasts directly effects differentiation by activating TLR4 in BMMs and RAGE in pre-osteoclasts. Our findings also suggest that increased osteoclastogenesis induced by apoptotic osteocytes CM is not mediated through HMGB1/RAGE activation and that direct HMGB1 actions in osteocytes stimulate pro-osteoclastogenic signal release from Cx43def osteocytes. Based on these findings, we propose that HMGB1 exerts dual effects on osteoclasts, directly by inducing differentiation through TLR4 and RAGE activation and indirectly by increasing pro-osteoclastogenic cytokine secretion from osteocytes.
Keywords: osteoclast, osteocyte, HMGB1, RAGE, TLR4, apoptosis, cytokine
1. Introduction
Over the last decade the interconnected bidirectional link between the skeleton and immune system has been extensively demonstrated, leading to creation of the osteoimmunology field (Mori, D’Amelio, Faccio, & Brunetti, 2013; Terashima & Takayanagi, 2018). In particular, numerous studies have reported that inflammatory conditions increase the risk of developing osteoporosis (Lin et al., 2017; Souza & Lerner, 2013) Consistent with this, emerging evidence suggests that inflammatory cytokines function as local regulators of bone cell function under physiological conditions and in disease states (Souza & Lerner, 2013; Zhou & Xiong, 2011).
In particular, cytokines and growth factors produced and released by osteocytes control osteoblast and osteoclast differentiation and activity, thereby regulating bone remodeling. It has long been proposed that maintenance of osteocyte viability is a key process by which osteocytes control bone remodeling (Bonewald, 2011). Osteocyte viability is reduced in conditions of increased bone fragility (Aguirre et al., 2006; Charoonpatrapong et al., 2006a; Kousteni et al., 2001; Tomkinson, Reeve, Shaw, & Noble, 1997; Weinstein, Jilka, Parfitt, & Manolagas, 1998), and, conversely, agents that preserve bone strength prevent osteocyte apoptosis (Jilka et al., 1999; Kousteni et al., 2001; Plotkin et al., 2008; Plotkin et al., 1999). Moreover, osteocyte apoptosis and the prevalence of empty lacunae are increased in old mice and humans (Almeida et al., 2007; Qiu, Rao, Palnitkar, & Parfitt, 2002).
In previous work, we demonstrated that deletion of the gap junction protein, connexin (Cx) 43 from osteocytes increases osteocyte apoptosis and leads to the accumulation of osteoclasts on the adjacent bone surfaces (Bivi, Condon, et al., 2012). Consistent with these findings, Cx43-deficient mice exhibit a skeletal phenotype resembling that of bones from old mice and humans, with bone marrow cavity widening, periosteal expansion, and defective bone material properties (Bivi, Condon, et al., 2012; Bivi, Nelson, et al., 2012). Further, more osteoclasts developed from bone marrow cells (BMCs) cultured in the presence of MLO-Y4 osteocytic cells silenced for Cx43 (Cx43def), or treated with conditioned media (CM) from Cx43def cells, compared to scramble (scr) control cells (Davis et al., 2017). Consistent with this, Cx43def osteocytic cells undergo accelerated apoptosis and release increased concentrations of soluble (s)RANKL and high mobility group box 1 (HMGB1), which promote osteoclast resorption (Davis et al., 2017).
The pro-inflammatory cytokine HMGB1 is a multifunctional redox sensitive protein expressed and released by most cell types including bone cells (osteoclasts, osteoblasts, osteocytes), which exerts various cellular compartment-specific functions (Charoonpatrapong et al., 2006b). Additionally, following its extracellular secretion, HMGB1 operates as a damage associated molecular pattern (DAMP) protein, and mediates a variety of cellular processes by interacting with two receptors, the receptor for advanced glycation end products (RAGE) and the toll-like receptor 4 (TLR4) (Scaffidi, Misteli, & Bianchi, 2002).
In bone cells, HMGB1 signaling has been shown to modulate key cellular processes including differentiation, proliferation, apoptosis, and autophagy, and subsequently regulate bone tissue homeostasis, turnover, and repair (Charoonpatrapong et al., 2006a; Davis et al., 2017; Feng et al., 2016; Meng et al., 2008; Taniguchi et al., 2007; Yang et al., 2008; Zhou et al., 2008). For example, in mesenchymal stem cells (MSCs) and osteoblasts, HMGB1 signaling stimulates the release of cytokines and osteogenic MSC differentiation (Feng et al., 2016; Li, Yu, & Yang, 2015). Consistent with these findings, HMGB1 has beneficial effects on bone repair and promotes facture healing (Hurtgen et al., 2017; Palumbo et al., 2004; Taniguchi et al., 2007). In osteoclasts, HMGB1-RAGE signaling regulates RANKL-induced osteoclast differentiation and activity (Zhou et al., 2008). Further, epidermal growth factor-mediated HMGB1 release induces RANK expression via CD68 in patients with autoimmune disease, suggesting that extracellular HMGB1 promotes osteoclast differentiation in human diseases (Hou, Luan, & Ren, 2018). In osteocytes, aside from the reports by our groups (Davis et al., 2017; Yang et al., 2008), showing that HMGB1 is secreted from dying osteocytes, the role of HMGB1 signaling in osteocytes has not been well studied. Further, the mechanisms underlying its release and the receptor(s) responsible for its actions is not clear.
In the current study, we assessed the effects of HMGB1 signaling in osteoclasts and osteocytes. Further, we tested whether RAGE signaling in osteoclasts regulates the stimulatory effects of apoptotic osteocytes on osteoclastogenesis. Overall our results suggest that, in osteoclasts, direct HMGB1 actions affect early stages of osteoclast differentiation through TLR4 activation in bone marrow macrophages (BMMs), followed by RAGE activation in pre-osteoclasts. Further, in osteocytes, direct HMGB1 actions stimulate pro-osteoclastogenic signal release from Cx43def osteocytes. These findings demonstrate that HMGB1 exerts dual effects on bone cells, stimulating osteoclast differentiation through RAGE and TLR4 activation in osteoclasts and inducing pro-osteoclastogenic cytokine secretion from osteocytes. Thus, direct actions of HMGB1 signaling in osteoclasts and osteocytes, rather than indirect effects of apoptotic osteocyte-derived extracellular HMGB1 appear to underlie the stimulatory effects of apoptotic osteocytes on osteoclasts.
2. Materials and Methods
2.1. Cell culture
MLO-Y4 osteocytic cells stably silenced for Cx43 (Cx43def) or scramble shRNA controls were produced using lentivial shRNA transfection, as previously published (Davis et al., 2017; Plotkin, Vyas, Gortazar, Manolagas, & Bellido, 2006). 2 × 104 cells/cm2 MLO-Y4 osteocytic cells were seeded on collagen-coated 48-well plates and and cultured for 12 hours. RAGE-deficient non-adherent BMCs were isolated from two global RAGE knockout mouse models. BMCs isolated from global RAGE knockout mice, previous reported by Philip et al. (Philip et al., 2010), were co-cultured with MLO-Y4 osteocytic cells. Additionally, BMCs were isolated from RAGE knockout mice generated by CRISPR/Cas9 as detailed in the supplemental information (Suppl. Fig. 1). The efficiency of the knock out was determined in genomic DNA by PCR and in lung lysates by Western blotting (Suppl. Fig. 2). BMCs were cultured with conditioned media from ex vivo long bone cultures of young and old female C57BL/6 mice.
2.2. RNA extraction and real-time PCR (qPCR)
RNA was isolated and purified using TRIzol, as published (Davis et al., 2017). Briefly, a high-capacity cDNA kit was used to perform reverse transcription and then Gene Expression Assay Mix TaqMan Universal Master Mix with an ABI 7900HT real-time PCR system was used to perform qPCR. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the house-keeping gene. Primers and probes were already available at the vendor site, or designed with the Assay Design Center (Roche Applied Science, Indianapolis, IN, USA).
2.3. HMGB1 receptor inhibitors
The small molecular RAGE inhibitor, Azeliragon (DC Chemicals, cat.# DC8338) and LPS-RS ultrapure (InvivoGen, cat.# tlrl-prslps) were used at a concentration of 100ng/mL to inhibit RAGE and TLR4, respectively.
2.4. In vitro HMGB1 neutralization
Following overnight culture, Cx43 or scramble control silenced MLO-Y4 osteocytic cells were exposed to 0.5μg/ml non-immune (ni) rabbit IgG or neutralizing rabbit anti-HMGB1 antibodies for 24h and CM was collected then concentrated 4x using centricon, as published (Davis et al., 2017). For cultures with immunoglobulins, CM was cultured with 10μl/ml Protein A agarose (Sigma-Aldrich, cat.#11134515001) overnight to remove the immunoglobulins. IgG-depleted CM was then collected, 1M HEPES was added and CM was stored at −20°C until used for the osteoclastogenesis assays.
2.5. Ex vivo bone organ cultures
Long bones were isolated from young (4-month-old) and old (21-month-old) female C57BL/6 mice obtained from National Institute on Aging (NIA). BMCs were flushed out with α-minimal essential medium (MEM). Osteocyte-enriched marrow-flushed long bones were then cultured ex vivo in α-MEM containing 10% FBS and 1% penicillin/streptomycin (P/S) for 48h. Conditioned media was collected 1M HEPES was added and stored at −20°C until used for the osteoclastogenesis assays.
2.6. Osteoclastogenesis assays: HMGB1 receptor inhibitor treatment
BMCs were collected from wildtype C57BL/6 mice and cultured for 48h with α-MEM supplemented with 10% FBS and 1% P/S (Davis et al., 2017). Next, non-adherent BMCs were collected and seeded at a density of 2×104 cells/cm2 on 96-well plates and cultured with sub-optimal levels of RANKL (40 ng/ml) and M-CSF (20 ng/ml). Inhibitors of the HMGB1 receptors RAGE (Azeliragon) or TLR4 (LPS-RS) were added at 100ng/ml in BMMs (added during day 1–3) and pre-osteoclasts (added during day 3–5).
2.7. Osteoclastogenesis assays: in co-culture and with osteocytic conditioned medium
BMCs were collected from wildtype C57BL/6 mice and cultured for 48h with α-MEM supplemented with 10% FBS and 1% P/S (Davis et al., 2017). Next, non-adherent BMCs, wildtype or RAGE-deficient, were collected and 2×104 cells/cm2 were plated on 96-well plates and exposed to conditioned media collected from scramble control and Cx43-deficient MLO-Y4 osteocytic cells or ex vivo cultures of osteocyte-enriched marrow-flushed long bones isolated from 4- and 21-month old female C57BL/6 mice. RANKL (80 ng/ml) and M-CSF (20 ng/ml) were added to facilitate osteoclast differentiation and media was changed at day 3. For the co-culturing assays BMCs were isolated from C57BL/6 mice and cultured for 24–48 h. Non-adherent BMCs (2×105 cells/cm2) were seeded onto Cx43 and scramble control silenced MLO-Y4 osteocytic cells and treated with 10nM 1.25(OH)2 vitamin D3 and 1M PGE2. Medium was changed every 2 days for 5 days, as previously published (Miyazaki, Neff, Tanaka, Horne, & Baron, 2003). Cells were stained using a commercially available TRAPase kit (Sigma-Aldrich) and mature osteoclasts with ≥ 3 or more nuclei were quantified. A Zeiss Axiovert 35 microscope with a digital camera was used to obtain images.
2.8. Statistical analysis
Data were analyzed with SigmaPlot (Systat Software Inc., San Jose, CA, USA). All results are presented as the mean ± standard deviation. Differences were assessed using two-way ANOVA, and Tukey Method for post-hoc analysis or Student’s t-test, as appropriate. Differences of p<0.05 were regarded as significant.
3. Results
3.1. Inhibition of HMGB1 and its receptors alters osteoclast differentiation.
Consistent with previous reports (Zhou et al., 2008), direct inhibition of HMGB1 signaling during the process of osteoclast differentiation with anti-HMGB1 neutralizing antibodies reduced the number of mature osteoclasts generated in vitro from non-adherent BMCs compared to cells treated with control rabbit IgG antibodies (Fig. 1A). Therefore, we tested effects of the HMGB1 receptors, RAGE and TLR4, on different stages of the osteoclast differentiation process. For this, the inhibitors for the receptors were supplemented at early stages of differentiation (day 1–3), later in differentiation (day 3–5), or throughout the experiment (day 1–5) (Fig. 1B). We found that in osteoclasts precursors, inhibition of TLR4, but not RAGE, reduced osteoclast number; whereas, in later stages of differentiation, RAGE, but not TLR4 inhibition, decreased osteoclast formation (Fig. 1C). Further, when added throughout differentiation (day 1–5), osteoclast number was reduced by the individual inhibitors and additively suppressed with a combination of both Azeliragon and LPS-RS. Taken together, these data stress the critical function of HMGB1 signaling at different stages of the osteoclast maturation process, and suggest that HGMB1 produced by osteoclasts has direct effects on differentiation via TLR4 activation in BMMs and RAGE activation in pre-osteoclasts.
Figure 1. Inhibition of HMGB1 and its receptors alters osteoclast differentiation in a stage-dependent manner.
(A) Non-adherent BMCs treated with rabbit IgG control or anti-HMGB1 antibodies. Bars represent mean ± S.D. (n=6). *p<0.05 vs vehicle rabbit IgG control treated cells by t-test. Representative osteoclast images are shown. (B) Illustration of the experimental design testing the effects of inhibitor treatment at different stages of osteoclast differentiation and (C) osteoclast numbers reported as fold changes. Inhibitors or vehicle were added to BMMs (day 1-3) or pre-osteoclasts (day 3-5) HMGB1 receptor inhibitor- (Azeliragon and LPS-Rs) treated non-adherent BMCs at different stages of differentiation. *p<0.05 vs vehicle control treated non-adherent BMCs by one-way ANOVA. Bars represent mean ± S.D. (n=6).
3.2. Apoptotic osteocyte induced increases in osteoclast differentiation are not mediated through RAGE-signaling in osteoclasts.
Consistent with our previous findings that conditioned media from apoptotic Cx43def MLO-Y4 osteocytic cells induces osteoclastogenesis (Davis et al., 2017), more osteoclasts were produced when conditioned media from osteocyte-enriched long bone cultures of old (21-mo) compared to young (4-mo) mice was added to wildtype BMCs; an effect that was attenuated by pre-treatment with the apoptosis inhibitor, DEVD-CHO (Fig. 2A and B).
Figure 2. Apoptotic osteocyte induced increases in osteoclast differentiation are not mediated through RAGE signaling in osteoclasts.
(A) Scheme illustrating experimental design. (B) Non-adherent BMCs treated with conditioned media (CM) collected from ex vivo osteocyte-enriched bone cultures of young (4-month-old) and old (21-month-old) female mice treated with vehicle or DEVD-CHO. *p<0.05 vs young bone culture CM-treated osteoclasts by two-way ANOVA, n=6. (C-E) Osteoclast differentiation was induced in non-adherent BMCs (C) Wildtype and RAGE-deficient BMCs treated with CM from ex vivo osteocyte-enriched bone cultures of young and old female mice. Bars represent mean ± S.D. *p<0.05 versus young bone culture CM-treated osteoclasts by two-way ANOVA, n=6. (D) Wildtype and RAGE-deficient BMCs co-cultured with scramble or Cx43def MLO-Y4 osteocytic cells. Bars represent mean ± S.D. *p<0.05 versus osteoclasts co-cultured with scramble MLO-Y4 cells by two-way ANOVA, n=6. (n=6). (E) Wildtype BMCs treated with CM from scramble or Cx43def MLO-Y4 osteocytic cells in the presence of vehicle or Azeliragon (100ng/mL). Bars represent mean ± S.D. *p<0.05 versus scramble CM treated osteoclasts precursors +/− Azeliragon by two-way ANOVA, n=6.
Based on our previous findings that apoptotic osteocytes release elevated levels of HMGB1, we next evaluated whether RAGE signaling in osteoclasts facilitates the stimulatory effects of apoptotic osteocyte conditioned media on osteoclastogenesis. Similar to previous reports (Souza & Lerner, 2013; Zhou & Xiong, 2011), both genetic and pharmacological RAGE inhibition in BMCs decreased osteoclast number (Fig 2C-E). However, despite the inhibitory effects of RAGE inhibition in osteoclasts with control media, the addition of conditioned media from old bones to BMCs from wildtype or global RAGE-knockout mice enhanced osteoclast number by 1.3- and 1.7-fold, respectively, vs. conditioned media from young bones (Fig. 2C). Moreover, co-culturing Cx43def osteocytic cells with wildtype or RAGE-knockout BMCs increased osteoclast number 2- and 4-fold, respectively compared to cells co-cultured with scramble control osteocytic cells (Fig. 2D). Additionally, Cx43def conditioned media induced 1.6-fold more osteoclasts than conditioned media from scramble control osteocytic cells, when cultured with or without the small-molecule RAGE inhibitor, Azeliragon (Fig. 2E). Taken together, our findings suggest that even though RAGE deficiency/inhibition reduces osteoclast differentiation, apoptotic osteocyte conditioned media-induced increases in osteoclastogenesis are not mediated through HMGB1/RAGE activation in osteoclasts.
3.3. Autocrine actions of apoptotic osteocyte-derived HMGB1 in osteocytes, rather than direct signaling in osteoclasts promotes osteoclastogenesis.
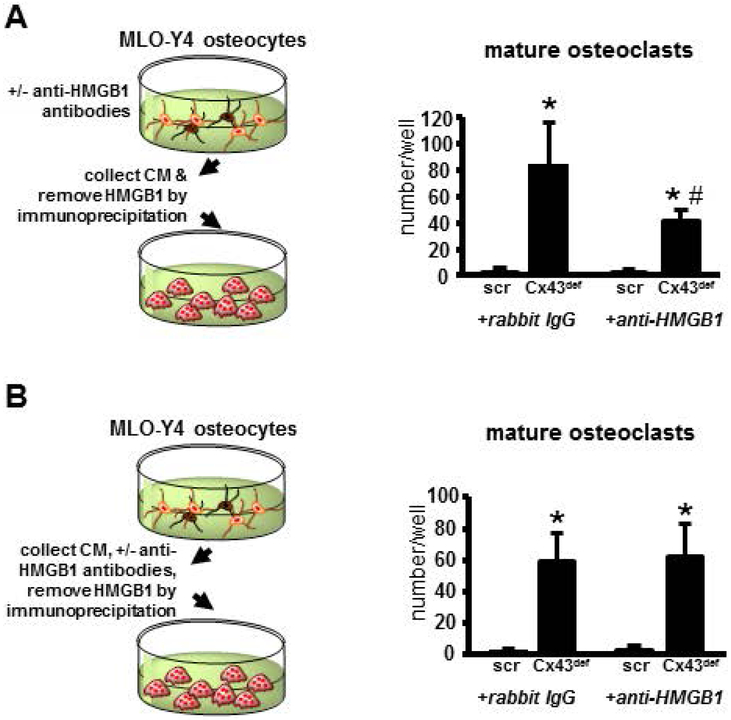
We next tested whether HMGB1 released by osteocytes has autocrine effects on the osteocytic cells and/or mediates the effect of the osteocytic cells on osteoclasts. We first cultured MLO-Y4 osteocytic cells silenced for Cx43 or scramble controls with an anti-HMGB1 antibody to block the autocrine effects of secreted HMGB1 on osteocytes. The conditioned media was collected and then anti-HMGB1 antibody was removed by immunoprecipitation. Alternatively, conditioned media from MLO-Y4 cells was immunodepleted with an anti-HMGB1 antibody after collection. Both conditioned media preparations were then used in osteoclastogenesis assays. We found that conditioned media prepared from MLO-Y4 cells that had been cultured in HMGB1-deficient conditions yielded fewer osteoclasts than control media (Fig. 3A). Conversely, and consistent with the results included in Fig. 2, immunodepletion of HMGB1 from Cx43def CM after collecting the media, did not prevent osteoclast formation (Fig. 3B). Together, these data suggest that direct HMGB1 actions in osteocytes stimulates osteoclast differentiation.
Figure 3. Direct actions of apoptotic osteocyte-derived HMGB1 in osteocytes, rather than indirect signaling in osteoclasts promotes osteoclastogenesis.
Mature osteoclasts generated in vitro from non-adherent BMCs treated with conditioned media from (A) scramble and Cx43-deficient MLO-Y4 osteocytic cells or (B) conditioned media from scramble and Cx43def MLO-Y4 osteocytic cells treated with rabbit IgG or anti-HMGB1 neutralizing antibodies, then removed by immunoprecipitation. Bars represent mean ± S.D. *p<0.05 versus scramble cells, #<0.05 versus corresponding IgG-treated cells by two-way ANOVA, n=4.
4. Discussion
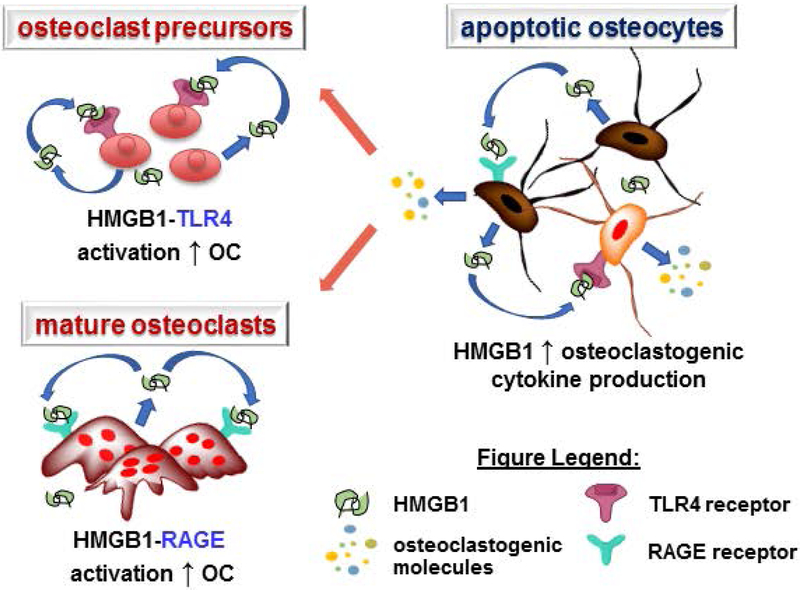
Numerous reports have shown that inflammatory cytokines function as local signals that influence bone cell function under both physiological and pathological conditions (Bidwell, Yang, & Robling, 2008; Karsenty & Mera, 2017). In particular, HMGB1 has been shown to be involved in mediating various cellular processes in bone (Charoonpatrapong et al., 2006b). In the current study, we demonstrate that HMGB1 exerts effects on both osteocytes and osteoclasts, altering osteoclast differentiation (Fig. 4).
Figure 4. Schematic Summary.
Dual effects exerted by HMGB1 on bone cells, resulting in increased osteoclastogenesis, directly by promoting osteoclast differentiation via activation of TLR4 and RAGE in osteoclasts and indirectly by increasing the release of pro-osteoclastogenic cytokine from osteocytes.
Our current findings provide additional evidence that HMGB1 receptor signaling regulates osteoclastogenesis. As predicted by Zhou et. at., our findings demonstrate that during osteoclast differentiation TLR4 is required for early HMGB1 signaling, whereas RAGE is required for later HMGB1 signaling (Zhou et al., 2008). These studies showed that HMGB1-TLR4 signaling is required for early osteoclast differentiation stages including ERK and NF-κB activation, whereas, HMGB1-RAGE signaling is required for integrin signaling and proper actin ring formation during later osteoclast differentiation signaling events.
Based on our previous findings that apoptotic osteocytes release elevated levels of extracellular HMGB1, we next tested whether RAGE signaling in osteoclasts mediates the stimulatory effects of apoptotic osteocyte conditioned media on osteoclastogenesis. However, while osteoclastogenesis was decreased with RAGE deficiency, neither genetic nor pharmacological RAGE inhibition blocked the apoptotic osteocyte-induced increases in osteoclastogenesis. Taken together, these pieces of evidence suggest that apoptotic osteocytes do not increase osteoclastogenesis by directly activating RAGE signaling in osteoclasts.
In support of this notion, we also found that while HMGB1 immunodepletion from Cx43def conditioned media did not inhibit osteoclast formation, treatment of Cx43def osteocytes with HMGB1 neutralizing antibody followed by HMGB1 immunodepletion, prevented osteoclast differentiation stimulated by Cx43def conditioned media, suggesting direct HMGB1 actions in osteocytes, rather than indirect actions on osteoclasts, stimulates osteoclast differentiation induced by apoptotic osteocytes. This idea is consistent with our previously published findings showing that HMGB1 neutralizing antibody treatment in Cx43def osteocytes attenuates the release of the pro-osteoclastogenic cytokine RANKL from Cx43def osteocytes (Davis et al., 2017).
Additionally, other inflammatory cytokines known to activate RAGE have also been shown to have similar effects on cell viability and cytokine production/release in osteoblasts and osteocytes (Kim et al., 2017; Yoshida, Flegler, Kozlov, & Stern, 2009). For example, MLO-Y4 osteocytic cell apoptosis is stimulated by advanced glycation end products (AGEs) (Notsu et al., 2017) and promote pro-inflammatory cytokine release (IL-6, TNFα, RANKL, VEGFA) from both osteoblasts and osteocytes (Chen et al., 2017; Tanaka, Yamaguchi, Kanazawa, & Sugimoto, 2015; Yang et al., 2008), which may subsequently increase osteoclastogenesis. Further, high levels of AGEs in bone are associated with increased osteoclast activity in humans, despite a lack of effect of AGEs on osteoclast activity in vitro (Dong, Qin, Xu, & Wang, 2011). In addition, S100A9, another ligand for RAGE, has similar effects on cell viability and cytokine secretion in osteoblasts and osteocytes (Davis et al., 2017; Kim et al., 2017; Yoshida et al., 2009). Specifically, S100A9 treatment in osteoblasts stimulates RAGE expression and promotes cytokine release; and S100A9-treated osteoblast CM increases osteoclast differentiation/activity, whereas when added directly to osteoclasts S100A9 inhibits osteoclastogenesis (Yoshida et al., 2009).
Overall, these data suggest that in bone HMGB1 signaling in osteocytes stimulates pro-inflammatory cytokine release, which subsequently induces osteoclastogenesis. Future studies will be carried out aiming to elucidate the specific effects of HMGB1 signaling in osteocytes and identify the receptor responsible for these effects.
In summary, our findings demonstrate that HMGB1 directly promotes osteoclastogenesis through RAGE and TLR4 stimulation in osteoclasts, and increases pro-osteoclastogenic cytokine release from apoptotic osteocytes (Fig. 4). Thus, direct actions of HMGB1 signaling in osteoclasts and osteocytes, rather than indirect effects of apoptotic osteocyte-derived extracellular HMGB1 appear to underlie the stimulatory effects of apoptotic osteocytes on osteoclasts.
Supplementary Material
Acknowledgements
This research project was funded by the National Institutes of Health R01-AR067210 to LIP. HMD received funding from the Cagiantas Scholarship from Indiana University School of Medicine. LG received funding from the Life-Health Sciences Internship Program. SV received funding from the Louis Stokes Alliances for Minority Participation and Diversity Scholar Research Programs at IUPUI. Services in support of the research project were provided by the VCU Massey Cancer Center Transgenic/Knockout Mouse Core, supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059. The authors thank Padmini Deosthale for technical support.
Footnotes
Conflict of interest None
References
- Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, & Bellido T (2006). Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Min. Res, 21(4), 605–615. [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, . . . Manolagas SC (2007). Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem, 282(37), 27285–27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell JP, Yang J, & Robling AG (2008). Is HMGB1 an osteocyte alarmin? J. Cell Biochem, 103(6), 1671–1680. doi: 10.1002/jcb.21572 [doi] [DOI] [PubMed] [Google Scholar]
- Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun L, . . . Plotkin LI (2012). Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J. Bone Min. Res, 27(2), 374–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivi N, Nelson MT, Faillace ME, Li J, Miller LM, & Plotkin LI (2012). Deletion of Cx43 from osteocytes results in defective bone material properties but does not decrease extrinsic strength in cortical bone. Calcif. Tissue Int, 91(3), 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF (2011). The Amazing Osteocyte. J. Bone Miner. Res, 26(2), 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoonpatrapong K, Shah R, Robling AG, Alvarez M, Clapp DW, Chen S, . . . Bidwell JP (2006a). HMGB1 expression and release by bone cells. J Cell Physiol, 207(2), 480–490. [DOI] [PubMed] [Google Scholar]
- Charoonpatrapong K, Shah R, Robling AG, Alvarez M, Clapp DW, Chen S, . . . Bidwell JP (2006b). HMGB1 expression and release by bone cells. J Cell Physiol, 207(2), 480–490. doi: 10.1002/jcp.20577 [DOI] [PubMed] [Google Scholar]
- Chen H, Liu W, Wu X, Gou M, Shen J, & Wang H (2017). Advanced glycation end products induced IL-6 and VEGF-A production and apoptosis in osteocyte-like MLO-Y4 cells by activating RAGE and ERK½, P38 and STAT3 signalling pathways. Int Immunopharmacol, 52, 143–149. doi: 10.1016/j.intimp.2017.09.004 [DOI] [PubMed] [Google Scholar]
- Davis HM, Pacheco-Costa R, Atkinson EG, Brun LR, Gortazar AR, Harris J, . . . Plotkin LI (2017). Disruption of the Cx43/miR21 pathway leads to osteocyte apoptosis and increased osteoclastogenesis with aging. Aging Cell, 16(3), 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XN, Qin A, Xu J, & Wang X (2011). In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone, 49(2), 174–183. doi: 10.1016/j.bone.2011.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Xue D, Chen E, Zhang W, Gao X, Yu J, . . . Pan Z (2016). HMGB1 promotes the secretion of multiple cytokines and potentiates the osteogenic differentiation of mesenchymal stem cells through the Ras/MAPK signaling pathway. Exp Ther Med, 12(6), 3941–3947. doi: 10.3892/etm.2016.3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C, Luan L, & Ren C (2018). Oxidized low-density lipoprotein promotes osteoclast differentiation from CD68 positive mononuclear cells by regulating HMGB1 release. Biochemical and Biophysical Research Communications, 495(1), 1356–1362. doi: 10.1016/j.bbrc.2017.11.083 [DOI] [PubMed] [Google Scholar]
- Hurtgen BJ, Ward CL, Leopold Wager CM, Garg K, Goldman SM, Henderson BEP, . . . Corona BT (2017). Autologous minced muscle grafts improve endogenous fracture healing and muscle strength after musculoskeletal trauma. Physiol Rep, 5(14). doi: 10.14814/phy2.13362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, & Manolagas SC (1999). Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J. Clin. Invest, 104(4), 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G, & Mera P (2017). Molecular bases of the crosstalk between bone and muscle. Bone. doi: 10.1016/j.bone.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee YD, Kim MK, Kwon JO, Song MK, Lee ZH, & Kim HH (2017). Extracellular S100A4 negatively regulates osteoblast function by activating the NF-kappaB pathway. BMB Rep, 50(2), 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, . . . Manolagas SC (2001). Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell, 104(5), 719–730. [PubMed] [Google Scholar]
- Li Q, Yu B, & Yang P (2015). Hypoxia-induced HMGB1 in would tissues promotes the osteoblast cell proliferation via activating ERK/JNK signaling. Int J Clin Exp Med, 8(9), 15087–15097. [PMC free article] [PubMed] [Google Scholar]
- Lin TH, Pajarinen J, Lu L, Nabeshima A, Cordova LA, Yao Z, & Goodman SB (2017). NF-kappaB as a Therapeutic Target in Inflammatory-Associated Bone Diseases. Adv Protein Chem Struct Biol, 107, 117–154. doi: 10.1016/bs.apcsb.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng E, Guo Z, Wang H, Jin J, Wang J, Wang H, . . . Wang L (2008). High mobility group box 1 protein inhibits the proliferation of human mesenchymal stem cells and promotes their migration and differentiation along osteoblastic pathway. Stem Cells Dev, 17(4), 805–813. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Neff L, Tanaka S, Horne WC, & Baron R (2003). Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J. Cell Biol, 160(5), 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori G, D’Amelio P, Faccio R, & Brunetti G (2013). The Interplay between the bone and the immune system. Clin Dev Immunol, 2013, 720504. doi: 10.1155/2013/720504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notsu M, Kanazawa I, Takeno A, Yokomoto-Umakoshi M, Tanaka KI, Yamaguchi T, & Sugimoto T (2017). Advanced Glycation End Product 3 (AGE3) Increases Apoptosis and the Expression of Sclerostin by Stimulating TGF-beta Expression and Secretion in Osteocyte-Like MLO-Y4-A2 Cells. Calcif. Tissue Int. doi: 10.1007/s00223-017-0243-x [doi];10.1007/s00223–017-0243-x [pii] [DOI] [PubMed] [Google Scholar]
- Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, . . . Bianchi ME (2004). Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. The Journal of Cell Biology, 164(3), 441–449. doi: 10.1083/jcb.200304135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip BK, Childress PJ, Robling AG, Heller A, Nawroth PP, Bierhaus A, & Bidwell JP (2010). RAGE supports parathyroid hormone-induced gains in femoral trabecular bone. Am. J Physiol Endocrinol. Metab, 298(3), E714–E725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Lezcano V, Thostenson J, Weinstein RS, Manolagas SC, & Bellido T (2008). Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J. Bone Miner. Res, 23(11), 1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Vyas K, Gortazar AR, Manolagas SC, & Bellido T (2006). barrestin complexes with connexin (Cx) 43 and anchors ERKs outside the nucleus: a requirement for the Cx43/ERK-mediated anti-apoptotic effect of bisphosphonates in osteocytes. J. Bone Miner. Res, 21(Suppl 1), S65. [Google Scholar]
- Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, & Bellido T (1999). Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Invest, 104(10), 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Rao DS, Palnitkar S, & Parfitt AM (2002). Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone. Bone, 31(2), 313–318. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, & Bianchi ME (2002). Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature, 418, 191. doi: 10.1038/nature00858 [DOI] [PubMed] [Google Scholar]
- Souza PP, & Lerner UH (2013). The role of cytokines in inflammatory bone loss. Immunol. Invest, 42(7), 555–622. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Yamaguchi T, Kanazawa I, & Sugimoto T (2015). Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem. Biophys. Res. Commun, 461(2), 193–199. doi:S0006–291X(15)00319–8 [pii]; 10.1016/j.bbrc.2015.02.091 [doi] [DOI] [PubMed] [Google Scholar]
- Taniguchi N, Yoshida K, Ito T, Tsuda M, Mishima Y, Furumatsu T, . . . Asahara H (2007). Stage-specific secretion of HMGB1 in cartilage regulates endochondral ossification. Mol. Cell Biol, 27(16), 5650–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A, & Takayanagi H (2018). Overview of Osteoimmunology. Calcif Tissue Int, 102(5), 503–511. doi: 10.1007/s00223-018-0417-1 [DOI] [PubMed] [Google Scholar]
- Tomkinson A, Reeve J, Shaw RW, & Noble BS (1997). The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J. Clin. Endocrinol. Metab, 82(9), 3128–3135. [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Parfitt AM, & Manolagas SC (1998). Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J. Clin. Invest, 102(2), 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Shah R, Robling AG, Templeton E, Yang H, Tracey KJ, & Bidwell JP (2008). HMGB1 is a bone-active cytokine. J. Cell Physiol, 214(3), 730–739. doi: 10.1002/jcp.21268 [doi] [DOI] [PubMed] [Google Scholar]
- Yoshida T, Flegler A, Kozlov A, & Stern PH (2009). Direct inhibitory and indirect stimulatory effects of RAGE ligand S100 on sRANKL-induced osteoclastogenesis. J Cell Biochem, 107(5), 917–925. doi: 10.1002/jcb.22192 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Han JY, Xi CX, Xie JX, Feng X, Wang CY, . . . Xiong WC (2008). HMGB1 regulates RANKL-induced osteoclastogenesis in a manner dependent on RAGE. J. Bone Miner. Res, 23(7), 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, & Xiong WC (2011). RAGE and its ligands in bone metabolism. Front Biosci (Schol Ed), 3, 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.