Abstract
Objective:
To identify the number of cumulative medication exposures and most frequently used medications in infants with severe BPD.
Study Design:
We performed a retrospective cohort study in infants with severe BPD admitted to United States children’s hospitals. We measured cumulative medication exposures in individual subjects and between-center variation after adjustment for infant characteristics. We then identified the specific medications and therapeutic classes with the highest rates of use.
Results:
In 3252 subjects across 43 hospitals, we identified a median (interquartile range) of 30 (17– 45) cumulative medication exposures per infant. The adjusted mean number of medication exposures varied between centers (p < 0.0001), with a range of 22 – 50. Diuretics and furosemide were the most frequently prescribed therapeutic class and specific medication for the management of severe BPD.
Conclusions:
Infants with severe BPD are exposed to alarming number of medications of unclear efficacy and safety, with marked variation between center.
Introduction
Bronchopulmonary dysplasia (BPD) is the most common chronic morbidity of premature birth.1 BPD strongly predicts death, disability and poor cardiorespiratory health in childhood.2-4 The prognosis is particularly poor for infants with severe BPD (sBPD), defined by the use of positive airway pressure or supplemental oxygen of 30% or greater at 36 weeks postmenstrual age (PMA).5 Prolonged initial hospitalizations, life-threatening comorbidities such as pulmonary hypertension, and tracheostomy placement for prolonged positive-pressure ventilation are common.6,7 Over 60% of infants surviving with sBPD are cognitively impaired at 2 years PMA.8
There is an urgent need to identify pharmacotherapies that safely improve disease course in sBPD. The importance of this unmet need is highlighted by the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act, legislation motivated by the need to reduce medication-related harms and prioritize medications for pediatric-specific study.9,10 Infants with sBPD are at particularly high risk of exposure to off-label medications of unclear efficacy or safety as a result of their prolonged hospitalizations and high disease severity.11,12
Research to address medication use in sBPD is hindered by two key knowledge gaps. First, the scope of medication use in this population remains unclear. Second, the most frequently used medications and classes have not been adequately characterized. We therefore sough to: (1) provide an overall measure of, and evaluate between-center variation in, cumulative medication exposure; and (2) identify the most frequently used medications and therapeutic classes among infants with sBPD admitted to United States children’s hospitals.
Methods
We performed a retrospective cohort study using the Pediatric Health Information System (PHIS) database, a national administrative database from freestanding children’s hospitals affiliated with the Children’s Hospital Association (Kansas City, KS). Most major United States metropolitan areas are represented by participating hospitals. For each hospital encounter, PHIS captures patient demographics and resource utilization codes inclusive of daily respiratory support and medication administrations.
We included subjects born between January 1, 2007 and August 1, 2016 who were admitted to a participating PHIS hospital neonatal intensive care unit and diagnosed with sBPD per the National Institutes of Health consensus definition for premature infants of birth gestational age (GA) < 32 weeks: treatment with supplemental oxygen for ≥ 28 days and need for ≥ 30% oxygen and/or positive pressure at 36 weeks postmenstrual age (PMA).5 We excluded infants of a GA ≥ 32 completed weeks, infants admitted after 36 weeks PMA and those admitted for less than one week. As PHIS data does not include the amount of supplemental oxygen administered, we restricted the cohort to subjects requiring invasive or non-invasive positive pressure support at 36 weeks PMA. This was identified by the presence of clinical service codes for the following types of respiratory support: non-invasive continuous positive airway pressure, non-invasive bi-level positive airway pressure, non-invasive positive pressure ventilation, conventional mechanical ventilation, or high frequency ventilation. To ensure subjects were not classified with sBPD due to a transient respiratory support needs, we limited inclusion to infants with a qualifying respiratory support code for at least 4 days in the week preceding 36 weeks PMA.
We examined medication use in the management of infants with established sBPD, an infant population requiring distinct care from younger premature neonates.11 Therefore, in each subject, evaluation of medication exposures was restricted to the period between diagnosis at 36 weeks PMA and the first occurrence of either hospital discharge or one year of age, which defined the study period. Specific medications were identified by their generic names as reported in PHIS. Therapeutic classes were categorized as described by PHIS using the Red Book classification (Truven Health Analytics; Ann Arbor, Michigan), with adjustments made by a neonatal clinical pharmacist and study author (H.M.M) when necessary to reflect pharmacotherapeutic use in infants with sBPD.
We defined cumulative medication exposure as the total number of unique, distinct medication exposures observed in a subject over the course of the study period. We measured variation in cumulative medication exposure between children’s hospitals that contributed at least fifteen subjects to the cohort. To adjust for differences in case-mix, we considered the following subject-level characteristics that are plausibly associated with disease severity or the number of medication exposures as covariates for inclusion in multivariable analyses: GA in completed weeks, birth weight, sex, maternal race, maternal ethnicity, type of respiratory support at 36 weeks PMA, infection during the hospitalization and the presence of operating room charges during the hospitalization.
We then identified the most frequently used medications and therapeutic classes through two measures of medication use. We defined any study-period exposure as the use of a medication or class at any time during the study period (yes or no binary outcome). Cumulative exposure days was defined as the sum of medication exposure days (calendar days in which the medication or class was prescribed to subjects) divided by the total number of patient-days for the full cohort, with units of medication days/100 patient-days.
We applied the approach described above with and without restriction of medications administered as part of routine health care maintenance (e.g. immunizations, ophthalmic drops for retinopathy of prematurity (ROP) examinations) or as part of fluid, electrolyte and nutritional management. The PHIS data used for this study were determined to not meet the definition of human subjects research by the institutional review board of the Children’s Hospital of Philadelphia.
Statistical Analyses
Cohort characteristics were summarized with standard descriptive statistics. To measure between-center variation, we first performed bivariable analyses between subject-level covariates and the outcome of cumulative medication exposure. All covariates associated with the outcome at p < 0.15 were then included in a multivariable linear regression model using robust sandwich variance estimates. Treatment center (each individual children’s hospital) was included in the model using a center-specific indicator variable to identify cumulative medication exposure estimates by center. A Wald test provided an adjusted global test of difference between centers. Graphical displays of cumulative medication exposures by center, adjusting for subject characteristics, were produced by plotting estimated marginal means following multivariable analyses. Model fit was compared for multivariable analyses with and without treatment center using coefficient of determination (R2) values and Akaike information criterion (AIC).13 AIC is a measure of the relative quality of fit between models that penalizes for inclusion of additional explanatory variables. We used AIC to ensure that an observed increase in R2 following the inclusion of center did not simply reflect additional model parameters. All analyses were performed with Stata 14 (StataCorp, College Station, Texas, USA).
Results
We identified 3252 subjects admitted to 43 United States children’s hospitals between 2007 and 2016 (Table 1). Subjects had a median birth GA of 26 weeks and a median birth weight of 790 grams. Greater than 90% of the study cohort received conventional mechanical ventilation (53%) or non-invasive continuous positive airway pressure (40%) at 36 weeks PMA, with the remainder receiving either non-invasive bi-level support or high frequency mechanical ventilation. We identified a median (interquartile range) of 30 (17–45) unique cumulative medication exposures per subject during the study period.
Table 1.
Cohort Characteristics
| Variable | (N = 3252) |
|---|---|
| Gestational age, median [IQR], wk | 26 [24-28] |
| Birth weight, median [IQR], g | 790 [640-1040] |
| Sex, No. (%)1 | |
| Female | 1283 (40) |
| Male | 1967 (60) |
| Maternal ethnicity, No. (%) | |
| Not Hispanic or Latino | 2240 (69) |
| Hispanic or Latino | 394 (12) |
| Other or unknown | 618 (19) |
| Maternal race, No. (%) | |
| White | 1576 (49) |
| Black | 819 (25) |
| Asian | 78 (2) |
| Other or unknown | 779 (24) |
| Type of respiratory support at 36 wk postmentrual age | |
| Non-invasive continuous positive airway pressure | 1296 (40) |
| Non-invasive bi-level support | 93 (3) |
| Conventional mechanical ventilation | 1739 (53) |
| High frequency mechanical ventilation | 124 (4) |
| Documented infection | 2703 (83) |
| Documented operating room charges, No (%) | 2420 (74) |
Abbreviations: IQR, interquartile range
n = 3250; represents greatest degree of missingness for subject characteristics
We compared cumulative medication exposures across United States children’s hospitals that contributed > 15 subjects to the cohort. This constraint resulted in a total of 3214 subjects at 37 United States children’s hospitals. The number of cumulative medication exposures varied significantly between centers despite adjustment for infant characteristics (p < 0.0001; Figure 1). The adjusted mean number of cumulative medication exposures per subject at different centers ranged from a low of 22 to a high of 50. The restricted analysis (without medications administered as part of routine health care maintenance or as part of fluid, electrolyte and nutritional management) had analogous findings, with significant variation between centers and an adjusted mean number of cumulative medication exposures between 12 and 34, (p < 0.0001), (Figure 2). In both models, the coefficient of determination (R2) increased while the AIC decreased following the addition of center to the models, confirming improvement in the model’s explanatory power with the inclusion of center as a covariate. Statistically significant associations between infant characteristics and greater cumulative medication exposures in both unrestricted and restricted multivariable analyses were observed for: sex, maternal race, type of respiratory support at 36 weeks PMA and the presence of a documented infection or operating room charges (Table 2).
Figure 1. Adjusted Cumulative Medication Exposures in Infants with Severe Bronchopulmonary Dysplasia Across United States Children’s Hospitals.
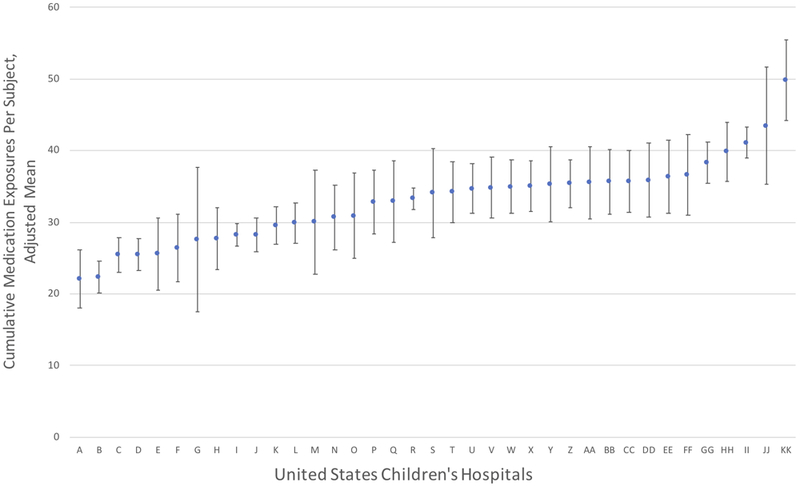
Plot depicts estimated marginal means and 95% confidence intervals for each hospital, ordered from lowest (A) to highest (KK) adjusted mean cumulative medication exposures during the study period (from 36 weeks postmenstrual age to the first of either hospital discharge or one year of age). Variation analysis was restricted to the 37 hospitals with >15 observations. Estimated marginal means were obtained following adjustment for the following infant characteristics in multivariable linear regression analysis: gestational age in completed weeks, sex, maternal race, maternal ethnicity, type of respiratory support at 36 weeks postmenstrual age, infection during the hospitalization and the presence of operating room charges during the hospitalization. P < 0.0001 for Wald global test of difference among centers.
Figure 2. Adjusted Cumulative Medication Exposures in Infants with Severe Bronchopulmonary Dysplasia Across United States Children’s Hospitals, Restricted Analysis.
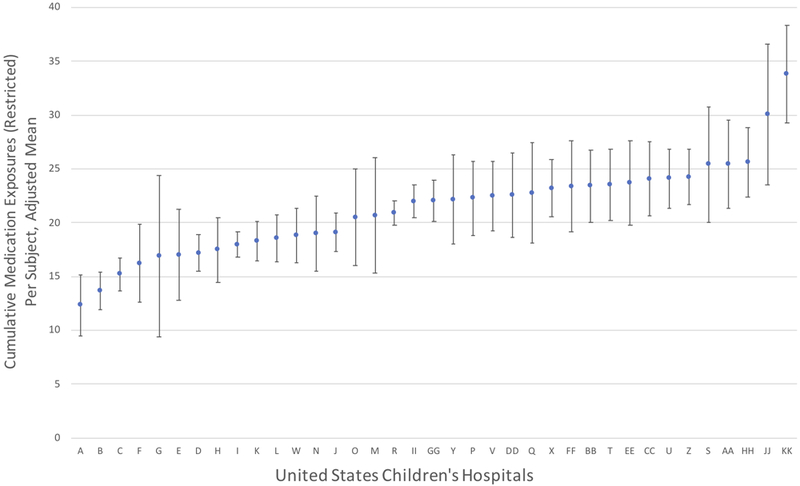
Plot depicts estimated marginal means and 95% confidence intervals for each hospital, ordered from lowest (A) to highest (KK) adjusted mean cumulative medication exposures during the study period (from 36 weeks postmenstrual age to the first of either hospital discharge or one year of age). Medications associated with routine health care maintenance and fluid, electrolyte and nutrition management were restricted from this analysis. Hospital labels (A, B, etc.) correspond to ordering from unrestricted analysis (Figure 1) for consistency and comparison. Variation analysis was restricted to the 37 hospitals with >15 observations. Estimated marginal means were obtained following adjustment for the following infant characteristics in multivariable linear regression analysis: gestational age in completed weeks, sex, maternal race, type of respiratory support at 36 weeks PMA, infection during the hospitalization and the presence of operating room charges during the hospitalization. P < 0.0001 for Wald global test of difference among centers.
Table 2.
Association Between Infant Characteristics and Cumulative Medication Exposure in Infants with Severe Bronchopulmonary Dysplasia
| Adjusted Multivariable Associations, Including Center, All Medications1 |
Adjusted Multivariable Associations, Including Center, Restricted Medications2 |
|
|---|---|---|
|
Cumulative Medication Exposures, Estimated Mean Difference (95% CI) |
Cumulative Medication Exposures, Estimated Mean Difference (95% CI) |
|
| Variable | ||
| Gestational age, weeks | ||
| 22 (reference) | - | |
| 23 | 0.86 (−6.78, 8.49) | −0.13 (−5.85,5.59) |
| 24 | −0.57 (−8.04, 6.90) | −1.21 (−6.80, 4.38) |
| 25 | 0.42 (−7.05, 7.88) | −0.19 (−5.77, 5.39) |
| 26 | 0.21 (−7.29, 7.71) | −0.51 (−6.12, 5.10) |
| 27 | 1.42 (−6.11, 8.95) | −0.04 (−5.67, 5.60) |
| 28 | 1.01 (−6.54, 8.56) | −0.17 (−5.83, 5.49) |
| 29 | 5.18 (−2.55, 12.90) | 3.13 (−2.68, 8.93) |
| 30 | 3.95 (−3.75, 11.65) | 2.83 (−2.94, 8.61) |
| 31 | 2.19 (−5.52, 9.90) | 1.42 (−4.35, 7.20) |
| Sex | ||
| Female (reference) | ||
| Male | 1.83 (0.70, 2.97) | 1.50 (0.64, 2.37) |
| Maternal ethnicity | ||
| Hispanic or Latino (reference) | - | NA |
| Not Hispanic or Latino | 0.20 (−1.80, 2.21) | - |
| Other or unknown | −1.10 (−3.54, 1.34) | - |
| Maternal race | ||
| White (reference) | - | |
| Black | 1.78 (0.32, 3.24) | 1.32 (0.22, 2.43) |
| Asian | 5.35 (1.87, 8.84) | 2.98 (0.31, 5.65) |
| Other or unknown | 0.32 (−1.52, 2.17) | 0.02 (−1.11, 1.14) |
| Type of respiratory support at 36 wk PMA | ||
| Non-invasive CPAP (reference) | - | - |
| Non-invasive bi-level support | 7.32 (2.98, 11.67) | 5.39 (2.24, 8.55) |
| Conventional mechanical ventilation | 6.47 (5.08, 7.86) | 5.35 (4.28, 6.41) |
| High frequency mechanical ventilation | 9.65 (5.80, 13.50) | 7.97 (5.09, 10.85) |
| Documented infection | ||
| No (reference) | - | - |
| Yes | 7.40 (5.97, 8.84) | 5.63 (4.55, 6.70) |
| Documented operating room charges | ||
| No (reference) | - | - |
| Yes | 17.06 (15.92, 18.19) | 12.09 (11.25, 12.92) |
Abbreviations: CPAP, continuous positive airway pressure; CI, confidence intervals; NA, not applicable; PMA, postmenstrual age,
Multivariable analysis included all co-variates associated with cumulative medication exposures at the 0.15 level in bivariable analysis. Only birth weight did not meet criteria for inclusion. Considers all medication exposures as reported by the Pediatric Health Information database. N = 3212, two values missing for sex.
Multivariable analysis included all co-variates associated with cumulative medication exposures at the 0.15 level in bivariable analysis. Only birth weight and maternal ethnicity did not meet criteria for inclusion. Restricts medications associated with routine health care maintenance or fluid, electrolyte and nutrition management. N = 3212, two values missing for sex.
The top twenty specific medications (Table 3) are listed in rank order for both the proportion of subjects with any study-period exposure and the number of cumulative exposure days during the study period. Among all medications, sodium chloride (79%), furosemide (74%) and potassium chloride (69%) occupied the top three positions for any study-period exposure. The same three medications, in a different order, occupied the top three positions for cumulative exposure days, with potassium chloride (35 exposure days per 100 patient-days) followed by sodium chloride and furosemide (each with 33 exposure days per each 100 patient-days). In the restricted analysis, furosemide (74%) was followed by acetaminophen (64%) and heparin (56%) for any study-period exposure. Furosemide (33 exposure days per 100 patient-days) was followed by chlorothiazide and heparin (19 and 18 exposure days per 100 patient-days, respectively) for cumulative exposure days.
Table 3.
Top Twenty Specific Medication Exposures in Infants with Severe Brochopulmonary Dysplasia Admitted to United States Children's Hospitals
| Any study-period exposure | Cumulative exposure days | Any study-period exposure | Cumulative exposure days | ||||
|---|---|---|---|---|---|---|---|
| Medication, No (%) | (n = 3252) | Medication, days/100 patient-days | Medication - restricted1, No (%) | (n = 3252) | Medication - restricted1, days/100 patient-days | ||
| Sodium chloride | 2557 (79) | Potassium chloride | 35 | Furosemide | 2391 (74) | Furosemide | 33 |
| Furosemide | 2391 (74) | Sodium chloride | 33 | Acetaminophen | 2079 (64) | Chlorothiazide | 19 |
| Potassium chloride | 2229 (69) | Furosemide | 33 | Heparin | 1831 (56) | Heparin | 18 |
| Cyclopentolate & phenylephrine | 2172 (67) | Vitamin combinations with iron/minerals | 26 | Fentanyl | 1815 (56) | Lorazepam | 13 |
| Acetaminophen | 2079 (64) | Ferrous sulfate | 21 | Morphine | 1778 (55) | Morphine sulfate | 12 |
| Dextrose in water | 1928 (59) | Chlorothiazide | 19 | Caffeine | 1551 (48) | Budesonide | 12 |
| Heparin | 1831 (56) | Heparin | 18 | Glycerin | 1404 (43) | Caffeine | 12 |
| Fentanyl | 1815 (56) | Fat emulsions | 17 | Vancomycin | 1388 (43) | Albuterol | 11 |
| Diphtheria/tetanus/pertussis/hepatitis vaccine | 1814 (56) | Hyperalimentation solutions unspecified | 16 | Midazolam | 1355 (42) | Ranitidine | 11 |
| Vitamin combinations with iron/minerals | 1806 (56) | Dextrose in water | 13 | Lorazepam | 1238 (38) | Lansoprazole | 11 |
| Morphine | 1778 (55) | Lorazepam | 13 | Palivizumab | 1229 (38) | Ursodiol | 10 |
| Pneumococcal 7-valent conjugate vaccine | 1753 (54) | Morphine | 12 | Gentamicin | 1228 (38) | Spironolactone | 9 |
| Ferrous sulfate | 1575 (48) | Budesonide | 12 | Albuterol | 1222 (38) | Levothyroxine | 8 |
| Caffeine | 1551 (48) | Caffeine | 12 | Nystatin | 1138 (35) | Sildenafil | 8 |
| Haemophilus B conjugate vaccine | 1535 (47) | Multiple vitamins | 12 | Dexamethasone | 1131 (35) | Hydrocortisone | 8 |
| Fat emulsions | 1406 (43) | Albuterol | 11 | Lidocaine | 1078 (33) | Midazolam | 7 |
| Glycerin | 1404 (43) | Ranitidine | 11 | Rocuronium | 1040 (32) | Phenobarbital | 7 |
| Vancomycin | 1388 (43) | Lansoprazole | 11 | Chlorothiazide | 1032 (32) | Acetaminophen | 6 |
| Midazolam | 1355 (42) | Cholecalciferol | 11 | Ranitidine | 1005 (31) | Vancomycin | 6 |
| Sterile water | 1309 (40) | Ursodiol | 10 | Propofol | 997 (31) | Diuretic combinations2 | 6 |
Medication exposures were evaluated in the period between 36 weeks postmenstrual age and the first occurrence of either hospital discharge or one year of age
Restricting medications given as part of routine health maintenance or fluid, electrolyte and nutrition management
Diuretic combinations are hydrochlorothiazide and either amiloride, spironolactone or triamterene
The top ten medication therapeutic classes are listed in rank-order in Table 4. Considering all medications, anesthetics/analgesics/sedatives (90%), vitamins/minerals/metals (88%) and electrolyte and replenishment agents (87%) were the most common for any study-period exposure. For cumulative exposure days, vitamins/minerals/metals (62 exposure days per 100 patient-days), electrolyte and replenishment agents, and diuretics (each with 57 exposure days per 100 patient-days) occupied the top three positions. In the restricted analysis, diuretics (57 exposure days per 100 patient-days) were used most frequently, followed by anesthetics/analgesics/sedatives (37 exposure days per 100 patient-days) and anti-reflux and promotility agents (33 exposure days per 100 patient-days) for cumulative exposure days. Anesthetics/analgesics/sedatives (90%) were followed by diuretics (82%) and anti-infective agents (80%) for any study-period exposure.
Table 4.
Top Ten Medication Therapeutic Class Exposures in Infants with Severe Brochopulmonary Dysplasia Admitted to United States Children's Hospitals
| Any study-period exposure | Cumulative exposure days | Any study-period exposure | Cumulative exposure days | ||||
|---|---|---|---|---|---|---|---|
| Class - unrestricted, No (%) | (n = 3252) | Class - unrestricted, days/100 patient-days | Class - restricted1, No (%) | (n = 3252) | Class - restricted*, days/100 patient-days | ||
| Anesthetics/Analgesics/Sedatives | 2915 (90) | Vitamins/Minerals/Metals | 62 | Anesthetics/Analgesics/Sedatives | 2915 (90) | Diuretics | 57 |
| Vitamins/Minerals/Metals | 2868 (88) | Electrolyte and replenishment agents | 57 | Diuretics | 2676 (82) | Anesthetics/Analgesics/Sedatives | 37 |
| Electrolyte and replenishment agents | 2846 (87) | Diuretics | 57 | Anti-infective agents | 2598 (80) | Anti-reflux and promotility agents | 33 |
| Ophthalmic preparations | 2823 (87) | Anesthetics/Analgesics/Sedatives | 37 | Gastrointestinal agent | 1896 (58) | Anti-infective agents | 27 |
| Diuretics | 2676 (82) | Anti-reflux and promotility agents | 33 | Anticoagulants | 1855 (57) | Anticoagulants | 20 |
| Anti-infective agents | 2598 (80) | Anti-infective agents | 27 | Corticosteroids (Systemic) | 1803 (55) | Corticosteroids (Systemic) | 16 |
| Vaccinations | 2453 (75) | Parenteral nutrition | 22 | Anti-reflux and promotility agents | 1767 (54) | Bronchodilators | 15 |
| Dextrose containing solutions | 2278 (70) | Anticoagulants | 20 | Neuromuscular blocking agents | 1643 (51) | Corticosteroids (Inhaled) | 13 |
| Gastrointestinal agents | 1896 (58) | Dextrose containing solutions | 16 | Skin preparation | 1616 (50) | Stimulants | 12 |
| Anticoagulants | 1855 (57) | Corticosteroids (Systemic) | 16 | Stimulants | 1586 (49) | Gastrointestinal agents | 11 |
Medication exposures were evaluated in the period between 36 weeks postmenstrual age and the first occurrence of either hospital discharge or one year of age
Restricting medications given as part of routine health maintenance or fluid, electrolyte and nutrition management
Discussion
This comprehensive report of medication use in infants with sBPD admitted to United States children’s hospitals provides several important findings. Despite limited evidence to inform their use, we identified a large number of cumulative medication exposures and marked variation in use between centers. Medications associated with routine health care maintenance and fluid, electrolyte and nutrition management were common. Excluding these, diuretics and furosemide were the most frequently used therapeutic class and specific medication, respectively.
We identified a median of 30 cumulative medication exposures per subject during the study period. In comparison, a report of infants discharged from neonatal intensive care units managed by the Pediatrix Medical Group describes a median of 4 medication courses per subject. This difference represents an approximately 7-fold increase in medication exposures among infants with sBPD compared to typical neonates admitted to the intensive care unit.14 The number of cumulative medication exposures for sBPD infants identified in this analysis is also higher than what has been reported for children admitted to US children’s hospital’s pediatric intensive care units (an average of 20 cumulative medication exposures during hospitalization) and patients less than one year of age admitted to US children’s hospitals for at least 30 days (a median of 25 cumulative medication exposures by hospital day 30).15,16 This high degree of medication exposure underscores the high disease severity and complexity of sBPD, and the importance of dedicated pharmacotherapeutic research in this population.
In addition to an overall high number of cumulative medication exposures, we identified significant variation in this measure between centers. This was noted despite adjustment for differences in case-mix, making it unlikely that it simply reflects differences in patient complexity or disease severity between centers. We noted a greater than two-fold difference in adjusted mean cumulative medication exposures between the center with the least and greatest number of exposures. Our findings are consistent with prior findings of high variation in the use of specific therapeutic classes for infants with BPD.17,18 The absence of research evidence to guide professional consensus is known to facilitate unwarranted practice variation; we speculate that the uncertain value of nearly all medications in sBPD contributes to our findings.19 Whether this variation in medication use represents underuse or overuse at specific centers remains uncertain.
Not surprisingly, medications associated with routine health care maintenance and fluid, electrolyte and nutrition management were common. These accounted for eleven and nine of the top twenty medications for any study-period exposure and cumulative exposure days, respectively. These medications may be considered less relevant research targets than medications used to treat or manage chronic lung disease or common co-morbidities of sBPD. We chose to retain them in our primary analyses and instead conduct secondary analyses that restricted these medications, for two reasons. First, our objective was to provide a comprehensive report of medication use in infants with sBPD. Removing specific exposures that we deemed less relevant would introduce subjective bias. Second, nearly all medication exposures may have important and/or unintended effects. Frequently used medications, however routine, may benefit from dedicated study. For example, sodium chloride supplementation influences fluid retention and water balance. As pulmonary edema may contribute to the pathophysiology of BPD, differences in sodium chloride use could influence clinically relevant outcomes.20-22 The impact of hypokalemia and hypochloremia on infants with BPD, and the optimal use of supplements such as potassium chloride to manage these electrolyte derangements remains uncertain.23 Vaccinations accounted for three of the top twenty medications observed for any study-period exposure. While efforts to avoid unnecessary vaccination delays are critical, concerns for transient cardiorespiratory deterioration following vaccination may justify brief delays in unstable infants with sBPD.24,25 These are examples of routinely used medications that would nonetheless benefit from dedicated research to better define best practice.
Diuretics were the therapeutic class most frequently used to manage sBPD, ranking first in cumulative exposure days in the restricted analysis. Subjects in our cohort were exposed to a diuretic on 57 of every 100 patient-days, considerably more than the next therapeutic class (anesthetics/analgesics/sedatives; 37 of every 100 patient-days). Among individual medications, the loop diuretic furosemide occupied the top rank for both any study-period exposure and cumulative exposure days in restricted analysis. It is worth noting that both sodium chloride and potassium chloride are often used in response to electrolyte depletion following diuretic use, further highlighting the prominent influence of this therapeutic class. In addition to furosemide, the diuretics chlorothiazide, spironolactone and “diuretic combinations” (hydrochlorothiazide with either amiloride, spironolactone or triamterene) were each among the top twenty medications for cumulative exposure days. This frequent use of diuretics in sBPD is not supported by clinical research evidence. We are unaware of any comparative effectiveness data in infants with established sBPD. In turn, a systematic review in infants with evolving BPD recommends against the use of loop diuretics, citing a lack of effect on important clinical outcomes and the potential for adverse effects.26 An analogous systematic review on diuretics acting on the distal renal tubule such as thiazides urges caution in their routine use, citing a need for further research.27 A clinical trial assessing the safety and preliminary effectiveness of furosemide in infants at risk of BPD is currently enrolling subjects.28 Targeted research specific to infants with established sBPD is also needed.
Comparisons with the existing literature regarding the most commonly identified medications and classes are limited. The most applicable comparison is a report of the single-day point prevalence of five specifically selected therapeutic classes among eight centers participating in the BPD Collaborative Group, a multicenter effort to improve outcomes in sBPD infants.29 The rank order of single-day point prevalence among considered classes was diuretics (56%), inhaled corticosteroids (35%), inhaled beta-agonists (32%), anti-reflux medications (22%) and systemic corticosteroids (13%). This has partial concordance with our cumulative exposure days ranking when restricted to analogous or similar classes: diuretics (57% of patient-days), anti-reflux and promotility agents (33%), systemic corticosteroids (16%), bronchodilators (15%) and inhaled corticosteroids (13%).
There are several limitations to our study. The longitudinal nature of our dataset allowed us to evaluate medication exposures for each subject day. However, data allowing accurate calculation of total cumulative dose was not available. Further, we are unable to link medication exposures with clinical indications. Dedicated studies using more granular clinical data sources could help clarify why specific medications are used frequently. Our findings do not generalize to all sBPD patients in the United States. First, our cohort is limited to infants admitted to United States children’s hospitals. Second, due to limitations in available data surrounding oxygen supplementation, we restricted our cohort to subjects requiring positive airway pressure at 36 weeks PMA. Both result in selection of a higher disease severity cohort than the broader sBPD population. Lastly, our between-center variation analysis may be limited by residual, unmeasured confounding.
Our study underscores that infants with severe BPD are a compelling example of a pediatric population in need of dedicated pharmacotherapeutic study, as mandated by the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act.9,10 Specific medication targets for research should be informed, in part, by common use as reported here. Other considerations include the potential for benefits and harms in the presence or absence of treatment. Diuretics, analgesics and sedatives, anti-reflux medications and corticosteroids are examples of frequently used therapeutic classes with strongly plausible benefits and/or harms. Pulmonary vasodilators were less commonly observed in our cohort. However, the high likelihood of serious morbidity or mortality resulting from inadequately managed pulmonary hypertension justifies the prioritization of this medication class despite lower rates of observed use.
In summary, we provide a comprehensive report of medication use in infants with sBPD admitted to United States children’s hospitals, facilitating subsequent research. We identified an overall high number of medication exposures with marked variation between centers. Diuretics are the most frequently used therapeutic class and should be prioritized in future research for this vulnerable pediatric population.
Acknowledgments
Conflict of Interest Disclosures: The authors have no financial interests to disclose. This work was supported by the National Institutes of Health Institutional National Research Service Award 2T32HD060550–06 to Dr. Bamat and National Heart, Lung, and Blood Institute Award K23HL136843 to Dr. Jensen. The funding sources had no role in the study design; the collection, analysis and interpretation of the data; the writing of the report or the decision to submit for publication. Dr. Bamat wrote the first draft of the manuscript. No compensation honorarium, grant, or other form of payment was given to produce the manuscript.
Abbreviations:
- AIC
Akaike information criterion
- BPD
bronchopulmonary dysplasia
- GA
gestational age
- PHIS
Pediatric Health Information System
- PMA
postmenstrual age
- R2
coefficient of determination
- sBPD
severe bronchopulmonary dysplasia
References
- 1.Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012; 129:1019–26. [DOI] [PubMed] [Google Scholar]
- 2.Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med 2015; 372: 331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt B, Asztalos E V, Roberts RS, Robertson CMT, Sauve RS, Whitfield MF, et al. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA 2003; 289:1124–9. [DOI] [PubMed] [Google Scholar]
- 4.Gough A, Spence D, Linden M, Halliday HL, McGarvey LPA. General and respiratory health outcomes in adult survivors of bronchopulmonary dysplasia: a systematic review. Chest 2012; 141:1554–67. [DOI] [PubMed] [Google Scholar]
- 5.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163:1723–9. [DOI] [PubMed] [Google Scholar]
- 6.Lagatta JM, Hysinger EB, Zaniletti I, Wymore EM, Vyas-Read S, Yallapragada S, et al. The impact of pulmonary hypertension in preterm infants with severe bronchopulmonary dysplasia through 1 year. J Pediatr 2018; 203: 218–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murthy K, Savani RC, Lagatta JM, Zaniletti I, Wadhawan R, Truog W, et al. Predicting death or tracheostomy placement in infants with severe bronchopulmonary dysplasia. J Perinatol 2014; 34: 543–8. [DOI] [PubMed] [Google Scholar]
- 8.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005; 116:1353–60. [DOI] [PubMed] [Google Scholar]
- 9.Government Publishing Office. Public Law 107–109 - January 4, 2002. Best Pharmaceuticals for Children Act. Available from: https://www.govinfo.gov/content/pkg/PLAW-107publ109/pdf/PLAW-107publ109.pdf
- 10.Government Publishing Office. Public Law 108–155 - December 3, 2003. Pediatric Research Equity Act of 2003. Available from: https://www.govinfo.gov/content/pkg/PLAW-108publ155/pdf/PLAW-108publ155.pdf
- 11.Abman SH, Collaco JM, Shepherd EG, Keszler M, Cuevas-Guaman M, Welty SE, et al. Interdisciplinary care of children with severe bronchopulmonary dysplasia. J Pediatr 2017; 181:12–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donn SM. Bronchopulmonary dysplasia: Myths of pharmacologic management. Semin Fetal Neonatal Med 2017; 22:354–8. [DOI] [PubMed] [Google Scholar]
- 13.Akaike H A new look at the statistical model identification. IEEE Trans Automat Contr 1974; 19:716–23. [Google Scholar]
- 14.Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK, Smith PB, et al. Medication use in the neonatal intensive care unit. Am J Perinatol 2014; 31:811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feudtner C, Dai D, Hexem KR, Luan X, Metjian TA. Prevalence of polypharmacy exposure among hospitalized children in the United States. Arch Pediatr Adolesc Med 2012; 166:9–16. [DOI] [PubMed] [Google Scholar]
- 16.Dai D, Feinstein JA, Morrison W, Zuppa AF, Feudtner C. Epidemiology of polypharmacy and potential drug-drug interactions among pediatric patients in ICUs of U.S. children’s hospitals. Pediatr Crit Care Med 2016;17:e218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slaughter JL, Stenger MR, Reagan PB. Variation in the use of diuretic therapy for infants with bronchopulmonary dysplasia. Pediatrics. 2013;131:716–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slaughter JL, Stenger MR, Reagan PB, Jadcherla SR. Inhaled bronchodilator use for infants with bronchopulmonary dysplasia. J Perinatol. 2015; 35:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman DC. Unwarranted Variation in Pediatric Medical Care. Pediatr Clin North Am. 2009;56:745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown ER, Stark A, Sosenko I, Lawson EE, Avery ME. Bronchopulmonary dysplasia: possible relationship to pulmonary edema. J Pediatr 1978; 92:982–84. [DOI] [PubMed] [Google Scholar]
- 21.Northway WH, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967; 276:357–68. [DOI] [PubMed] [Google Scholar]
- 22.Abman SH, Rosenberg AA, Lum GM. Management of hyponatremia in infants with bronchopulmonary dysplasia. J Pediatr 1988; 113:789–90. [DOI] [PubMed] [Google Scholar]
- 23.Perlman JM, Moore V, Siegel MJ, Dawson J. Is chloride depletion an important contributing cause of death in infants with bronchopulmonary dysplasia? Pediatrics 1986; 77:212–6. [PubMed] [Google Scholar]
- 24.Meinus C, Schmalisch G, Hartenstein S, Proquitté H, Roehr CC. Adverse cardiorespiratory events following primary vaccination of very low birth weight infants. J Pediatr (Rio J) 2012; 88:137–42. [DOI] [PubMed] [Google Scholar]
- 25.Montague EC, Hilinski JA, Williams HO, McCracken CE, Giannopoulos HT, Piazza AJ. Respiratory decompensation and immunization of preterm infants. Pediatrics 2016; 137: e20154225. [DOI] [PubMed] [Google Scholar]
- 26.Stewart A, Brion LP. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev 2011; 9:CD001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart A, Brion LP, Ambrosio-Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev 2011; 9:CD001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ClinicalTrials.gov. Safety of furosemide in premature infants at risk of bronchopulmonary dysplasia (BPD). Available from: https://clinicaltrials.gov/ct2/show/NCT02527798
- 29.Guaman MC, Gien J, Baker CD, Zhang H, Austin ED, Collaco JM. Point prevalence, clinical characteristics, and treatment variation for infants with severe bronchopulmonary dysplasia. Am J Perinatol 2015; 32:960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


