Abstract
Heightened responsivity to unpredictable, and perhaps predictable, threat characterizes some internalizing disorders and may be vulnerability factors for psychopathology as well. However, few studies have directly tested whether individual differences in unpredictable and/or predictable threat responding longitudinally predict symptoms of psychopathology and functional outcomes. Examining functioning is particularly important given that functioning is separable from symptoms of psychopathology. The present study examined whether electromyography startle measures of predictable and/or unpredictable threat responding was associated with interviewer-assessed symptoms of internalizing psychopathology and functional impairment at baseline (n = 409) and one-year follow-up (n = 104). Elevated startle responding to unpredictable and predictable threat longitudinally predicted a worsening of functioning over time and this effect was independent of change of symptoms over time. Importantly, threat responding at baseline predicted functional impairment during the follow-up independent of the effects of DSM-defined fear-based (e.g., panic disorder) or distress-misery (e.g., major depressive disorder) internalizing disorders. These findings provide initial support for the incremental validity of neurobiological vulnerability markers of threat responding over and above DSM disorders and highlight the importance of distinguishing functional outcomes from symptom outcomes.
Keywords: functioning, internalizing disorders, electromyography startle, predictability, RDoC
1. Introduction
Internalizing disorders (e.g., depression, anxiety disorders), which have been shown to be phenotypically and genotypically distinct from externalizing disorders (e.g., substance use disorders; Kendler et al., 2011, Krueger et al., 2003), are associated with high economic and disease burden (Greenberg et al. 2003; Kessler et al., 2009). Depression and anxiety disorders are estimated to yield $36 billion and $4.1 billion in productivity loss, respectively, in the United States (Greenberg et al., 1999; Kessler et al., 2006). It is therefore vital to identify vulnerability factors that can be targeted by preventative interventions.
Two vulnerability factors for internalizing psychopathology are sensitivity to unpredictable threat (SUT) and sensitivity to predictable threat (SPT) threat (often labeled ‘potential threat’ and ‘acute threat’, respectively, in RDoC parlance; see Cuthbert and Insel, 2013 for a discussion of NIMH’s RDoC initiative). SUT is characterized as defensive responding to threat that is ambiguous, or less certain to occur, and SPT is characterized as defensive responding to present or immediate threat (Davis, 2006). While SUT and SPT are both forms of threat responding, multiple human and animal studies have shown they have different physiological, neural, and behavioral correlates (Alvarez et al., 2011; Davis, 2006; Grillon et al., 2006; although see Shackman and Fox, 2016). Whereas predictable threats elicit the fight-flight-freeze response, unpredictable threats yield sustained vigilance or defensive preparedness (Davis, 1998). Importantly, although the threatening stimuli vary across disorders (Taylor et al., 2007), elevated sensitivity to threat has been shown to be a key mechanism for multiple internalizing psychopathologies (McEvoy et al., in press).
To experimentally differentiate predictable versus unpredictable threat responding, Grillon and colleagues developed the No-Predictable-Unpredictable (NPU)-threat task (Schmitz and Grillon, 2012). The NPU-threat task assesses defensive responding during three conditions: (1) no threat, (2) shock occurring at a signaled time (i.e., acute/predictable threat), and (3) uncued shocks that may occur at any time (i.e., potential/unpredictable threat). Numerous studies have demonstrated that defensive responding, indexed by startle eye-blink, is enhanced during the threat conditions compared to during no threat (e.g., Gorka et al., 2017a; Grillon et al., 2006).
Using the NPU-threat task, several studies indicate that startle to unpredictable threat is elevated in multiple internalizing disorders (Grillon et al., 2008; Nelson and Hajcak, 2017; Shankman et al., 2013). The importance of predictable threat responding is more mixed. Most studies show no differences between those with internalizing disorders and controls (Grillon et al., 2008; Grillon et al., 2009; Gorka et al., 2017a). Two studies, however, have shown heightened response to predictable threats for individuals with panic disorder (Shankman et al., 2013) and depression with prior suicide attempts (Ballard et al., 2014).
SUT, but not SPT, has also been shown to connote vulnerability for some forms of psychopathology. For example, individuals with a family history of panic disorder, but not depression, exhibited elevated unpredictable, but not predictable threat responding (Nelson et al., 2013). Importantly, this association was independent of probands’ psychopathology, suggesting that even those at familial risk who are not symptomatic evidence abnormal threat responding. This may be particularly true of individuals with certain disorders. Across multiple investigations, internalizing disorders cluster into two factors: fear (e.g., panic disorder, specific phobia, social phobia) and distress-misery (e.g., generalized anxiety disorder [GAD], major depressive disorder [MDD]) disorders. This distinction is supported by studies of the phenotypic factor structure of psychopathology (Krueger, 1999; Shankman and Klein, 2003; Watson, 2005) and twin studies examining genotypic structure (Kendler et al., 2003). In line with this distinction, heightened unpredictable threat responding has been shown to characterize fear disorders, whereas distress-misery disorders exhibit no or blunted unpredictable threat responding (Gorka et al., 2017a; Kaviani et al., 2004). Thus, threat responding, perhaps specifically to unpredictable threats, may represent a transdiagnostic feature of internalizing disorders, especially fear disorders, akin to Caspi et al.’s (2014) concept of a “p” factor that underlies psychopathology more broadly. However, no longitudinal studies (which provide more direct tests of vulnerability; Raulin and Lilienfeld, 2009) have examined these questions. The first goal of the present study was therefore to prospectively examine the relationship between psychophysiological indicators of sensitivity to threat and clinical outcomes.
Typically, longitudinal studies on the relationship between psychophysiological indices and clinical outcomes focus on changes in symptoms or diagnoses. However, functional impairment is a critically important clinical outcome. Functional impairment refers to difficulties in carrying out routine activities in a person’s roles at home, work, school, or in other social areas (Üstün et al. 2010). Functional impairment is an important clinical outcome in psychopathology research given that individuals with internalizing disorders report comparable functional impairment to individuals with medical illnesses (Bieling et al., 2001). Additionally, symptoms and functional impairment, although correlated, are not equivalent (McKnight and Kashdan, 2009; McKnight et al., 2016), as individuals can be highly symptomatic and functioning adequately or, conversely, minimally symptomatic but functioning poorly. Identifying predictors of functional impairment is especially important as functional impairment is one of the strongest predictors of treatment-seeking (Mojtabai et al., 2002) and predicts disorder relapse more than symptom severity (Ishak et al., 2013). Thus, a second goal of this study is to examine whether sensitivity to threat connotes vulnerability for subsequent symptoms and functioning.
There are reasons to expect associations between sensitivity to threat and multiple domains of functioning. Responding to threat entails activation of defensive motivational neural circuitry, including the central extended amygdala and bed nucleus of the stria terminalis (Shackman and Fox, 2011), which are linked to both the physiological and emotional experience of fear/anxiety as well as to enhanced threat-related cognitive processes (e.g., attention, appraisal) and disruptions in adaptive behavioral responding (e.g., chronic avoidance; Grupe and Nitschke, 2013). People with these cognitive and behavioral abnormalities are likely to have difficulties understanding and communicating with others, which will ultimately impact, for example, social and occupational functioning (Grupe and Nitschke, 2013; McTeague and Lang 2012; Mathews and MacLeod, 2005). Indeed, increased physiological threat responding has been found to be associated with reduced responsive social behavior (Peters et al., 2018), a critical aspect of interpersonal functioning. Additionally, response to perceived threat (and subsequent avoidance) has been shown to contribute to impaired work functioning and reduced community participation (Antony et al., 1998; Bieling et al., 2001; McKnight and Kashdan, 2009; McKnight et al., 2016), although these outcomes are likely more distally linked. Thus, sensitivity to threat may exhibit widespread associations with functioning in a variety of domains.
If sensitivity to threat, or any psychophysiological measures, are to be useful markers of vulnerability, they should provide additional information about the biological correlates and/or underpinnings of disorders above that explained by symptom measures. If they cannot, it would be more economical to not include psychophysiological assessments and just use diagnostic or other self-report measures to predict outcomes. Assuming that psychophysiological markers represent vulnerability to developing psychopathology, and therefore exist prior to disorder onset (Graver, 1987), they might contribute predictive power beyond measures of symptoms in predicting functional outcomes. Another goal of including psychophysiological measures is to aid in construct validation and refinement, as examining patterns of co-variation with other constructs can enhance understanding of mechanisms of psychopathology. In the current study, we incorporate measures of both symptoms of psychopathology and functional impairment to aid in validating the constructs of heightened responsivity to unpredictable and predictable threat. Consistent with the mission of RDoC, these insights can ultimately be integrated into a more comprehensive framework that captures the interface between neurobiology and psychopathology.
In sum, the aim of the present study is to examine whether sensitivity to unpredictable and/or predictable threat are associated cross-sectionally and longitudinally with symptoms of psychopathology and functional impairment. Furthermore, this study will examine whether sensitivity to unpredictable and/or predictable threat predict symptoms and functioning over a one-year follow-up independent of psychiatric diagnoses.
2. Methods
2.1. Participants and Procedure
Participants (N = 409) were recruited as part of a larger investigation on cognitive and affective responding in internalizing psychopathology (Correa et al., in press; Weinberg et al., 2015). Participants were 18–30 and exclusion criteria included personal or family history of mania or psychosis, major medical or neurological illness, and history of serious head trauma. As the broader study examined neural measures, left-handed individuals were excluded. Participants were recruited irrespective of diagnoses, but as discussed below, current and lifetime psychopathology were assessed. All participants provided informed consent after reviewing the study procedures. The study was conducted in accordance with the Declaration of Helsinki and approved by the University Institutional Review Board (protocol #2012–0646).
At the baseline visit, participants completed several laboratory tasks in a counterbalanced order, including the NPU-threat task and interviewer-administered assessments of diagnoses and functional impairment. As a secondary aim of the study, a subset of participants (n = 144; 35.2%) returned for a 12-month follow-up, during which symptoms and functioning were reassessed. Of participants who completed the follow-up visit, 40 were excluded due to missing (n = 32) or unusable (n = 8) startle data, leaving 104 participants. There were no differences between participants who did versus did not complete the follow-up on age, gender, ethnicity, medication status, current or lifetime psychiatric diagnoses, or baseline functioning (ps > 0.14), nor SUT or SPT (ps > 0.395). Participant demographic and clinical characteristics are in Table 1.
Table 1.
Sample demographic and clinical characteristics
| Followed Up (n = 104) | Not Followed Up (n = 265) | Total (n = 369) | |
|---|---|---|---|
| Measure | M (SD) | M (SD) | M (SD) |
| Sex (% Female) | 61.7 | 59.2 | 60.4 |
| Age | 21.87 (3.15) | 22.52 (3.16) | 22.34 (3.13) |
| Race/Ethnicity (%) | |||
| White | 40.6 | 44.3 | 43.7 |
| Black | 9.4 | 13.9 | 11.9 |
| Asian | 16.0 | 11.1 | 12.9 |
| Hispanic | 26.4 | 20.1 | 22.1 |
| Middle Eastern | 3.8 | 2.5 | 3.0 |
| Mixed Race or Other | 3.7 | 7.5 | 6.4 |
| Education (%) | |||
| High school graduate or less | 4.8 | 11.3 | 9.5 |
| Some college or 2-year degree | 63.4 | 51.1 | 54.6 |
| 4-year college degree | 15.4 | 18.9 | 17.9 |
| Some graduate school | 13.5 | 14.4 | 14.1 |
| Graduate degree | 2.9 | 4.2 | 3.8 |
| On Psychiatric Medication (%) | 4.8 | 10.9 | 9.2 |
| Psychotherapy, no Medication (%) | 1.9 | 1.1 | 1.4 |
| Current Diagnosis (n, %) | 29 (27.1) | 77 (29.1) | 100 (26.9) |
| MDD | 5 (4.8) | 13 (4.9) | 18 (4.9) |
| Panic Disorder | 4 (3.8) | 6 (2.3) | 10 (2.7) |
| Agoraphobia | 0 (0.0) | 3 (1.1) | 3 (0.8) |
| Social Phobia | 9 (8.7) | 29 (10.9) | 36 (9.8) |
| Specific Phobia | 12 (11.5) | 39 (14.7) | 46 (12.5) |
| GAD | 4 (3.8) | 6 (2.3) | 10 (2.7)+ |
| OCD | 6 (5.8) | 13 (4.9) | 16 (4.3) |
| PTSD | 2 (1.9) | 2 (0.8) | 4 (1.1) |
| Fear | 24 (23.1) | 59 (22.3) | 83 (22.6) |
| Distress-Misery | 9 (8.7) | 17 (6.4) | 26 (7.0) |
| Lifetime Diagnosis (n, %) | 51 (47.7) | 159 (60.0) | 203 (54.6) |
| MDD | 28 (26.9) | 98 (37.0) | 126 (34.1) |
| Panic Disorder | 10 (9.6) | 20 (7.5) | 29 (7.9) |
| Agoraphobia | 1 (1.0) | 5 (1.9) | 5 (1.4) |
| Social Phobia | 16 (15.4) | 60 (22.6) | 71 (19.3) |
| Specific Phobia | 18 (17.3) | 56 (21.1) | 69 (18.8) |
| GAD | 8 (7.7) | 24 (9.1) | 32 (8.7) |
| OCD | 9 (8.7) | 19 (7.2) | 25 (6.8) |
| PTSD | 7 (6.7) | 20 (7.5) | 25 (6.8) |
| Number of Current Diagnoses | 0.39 (0.77) | 0.53 (0.90) | 0.38 (0.74) |
| Number of Lifetime Diagnoses | 0.91 (1.25) | 1.69 (1.73) | 1.03 (1.25) |
| Baseline Functioning | |||
| WHODAS | |||
| General Disability | 42.91 (10.78) | 44.14 (13.37) | 43.77 (12.66) |
| Cognition | 7.60 (2.47) | 7.90 (3.16) | 7.81 (2.98) |
| Getting Along with Others | 5.73 (1.72) | 6.03 (2.19) | 5.95 (2.07) |
| Life Activities | 10.24 (3.94) | 10.43 (4.44) | 10.37 (4.29) |
| Participation in Society | 9.58 (3.09) | 10.20 (3.96) | 10.02 (3.73) |
| Self-Care | 4.22 (0.87) | 4.29 (0.94) | 4.27 (0.92) |
| Mobility | 5.58 (1.39) | 5.75 (2.05) | 5.70 (1.88) |
| Symptoms during Follow-Up (LIFE) | |||
| Any Internalizing Disorder | 0.64 (0.58) | N/A | N/A |
| Fear Disorders | 0.63 (0.61) | N/A | N/A |
| Distress-Misery Disorders | 0.85 (0.87) | N/A | N/A |
| Functioning during Follow-Up (WHODAS12) | 1.71 (7.81) | N/A | N/A |
Note: MDD = major depressive disorder; SAD = social anxiety disorder; GAD = generalized anxiety disorder; OCD = obsessive compulsive disorder; PTSD = post-traumatic stress disorder
2.2. Measures of Functioning and Psychopathology
2.2.1. Structured Clinical Interview for DSM-5 (SCID-5).
Current (i.e., past month) and lifetime Axis I psychopathology at baseline was assessed using the SCID-5 (First et al., 2015). Interviewers were trained to criterion by viewing the SCID-101 training videos (Biometrics Research Department, New York, NY), observing two SCIDs with an experienced interviewer, and completing three SCIDs observed by advanced interviewers (and the senior author) in which diagnoses were in full agreement with observers. In a subset of the sample, retest reliability was moderate to strong for all diagnoses (Shankman et al., 2018).
2.2.2. World Health Organization Disability Assessment Schedule 2.0 (WHODAS).
The 36-item WHODAS interview is a gold standard assessment of disability comprised of six domains of current (past month) functioning - Cognition (understanding, communicating), Getting Along with Others, Life Activities (household, work, or school), Participation in Society, Mobility, and Self-Care. Higher scores reflect greater functional impairment. Domain-level scores have excellent internal consistency and test-retest reliability, as well as concurrent, construct, and discriminant validity (Ustün et al., 2010). Cronbach’s alphas for WHODAS domains in this study were 0.81–0.92.
2.2.3. WHODAS 12-item Short Form (WHODAS12).
The WHODAS12 interview was administered one year after the initial SCID and retrospectively assessed impairment since the baseline visit. The WHODAS12 accounts for 81% of the variance in the WHODAS (Ustün et al., 2010), but only produces an overall disability score and not domain-level scores. Interviewers determined WHODAS12 impairment ratings for each month during the follow-up period. Given that functioning might change over time with changing circumstances, functioning during the follow-up was operationalized as the average of the maximum WHODAS12 item scores from each month, irrespective of domain. This allowed us to capture participants’ worst functioning over time regardless of which domain was impaired. Additionally, we chose to examine average functioning over the follow-up rather than WHODAS12 scores at 12 months given that the latter represents a snapshot and may not reflect dysfunction that occurred during the followup period but was resolved at the 12-month assessment. Internal consistency of the 12 monthly maximum scores was excellent (α = 0.94).
2.2.4. Longitudinal Interval Follow-up Evaluation (LIFE; Keller et al., 1987).
The LIFE is a semi-structured interview that retrospectively assesses the severity and course of disorders. For each week since baseline, a 1 (absent) to 6 (severe) rating (psychiatric status rating) was assigned for each disorder assessed, reflecting more severe symptoms. Ratings of 5 or 6 signified that full diagnostic criteria were met for the disorder during that week, with 6 indicating more severe symptoms than a 5. Symptom severity was operationalized as the average psychiatric status ratings for the relevant disorders over the follow up. The LIFE has exhibited good to excellent inter-rater reliability and excellent test-retest reliability over one-year periods (Keller et al., 1987; Warshaw et al., 1994).
2.3. NPU-Threat Task
Participants completed a modified version of the NPU-threat task, used repeatedly in our laboratory (Gorka et al., 2017a; Sarapas et al., 2014). The task included three within-subject conditions: no shock (N), predictable shock (P), and unpredictable shock (U). Each condition lasted 145s, during which six 4s visual countdowns were presented. Inter-stimulus intervals (ISIs; i.e., time between countdowns) ranged from 15–21s (M = 18s) during which only text describing the condition was visible. During N, no shocks were delivered. During P, participants received a shock every time the countdown reached 1. During U, shocks were administered during the countdown or ISI. Text at the bottom of the screen informed participants of the current condition by displaying “no shock” (N), “shock at 1” (P), or “shock at anytime” (U). Startle probes were presented during countdowns (1–2s following countdown onset) and ISIs (4–13s following ISI onset). The time between shocks (or startle probes) and the following startle probe was always more than 10s to ensure that the subsequent startle response was not affected by immediately preceding stimuli. Each condition was presented twice in randomized order (counterbalanced across participants). Participants received 24 electric shocks (12 in P; 12 in U) and 60 startle probes (20 in each of N, P, and U).
2.4. Electromyography Startle Data Collection and Processing
Electromyography data were acquired using BioSemi Active Two (Amsterdam, Netherlands), and stimuli were delivered with PSYLAB (Contact Precision Instruments, London, UK). Acoustic startle probes were 40 ms, 103dB bursts of white noise with near-instantaneous rise time presented binaurally through headphones. Electric shocks lasted 400ms. Startle responses were recorded from two 4mm Ag/AgCl electrodes placed over the left orbicularis oculi muscle. The ground electrode was the frontal pole of an electroencephalography cap. Data were collected using a bandpass filter of DC-500Hz at a sampling rate of 2000Hz.
Blinks were scored according to published guidelines (Blumenthal et al., 2005). Data processing included applying a 28Hz high-pass filter, rectifying, then smoothing using a 40Hz low-pass filter. Blinks were defined as the peak amplitude of electromyography activity within 20–150ms following startle probe onset relative to baseline (i.e., the 50ms preceding startle probes). Peaks were identified by software but verified by hand. Blinks were scored as nonresponses (coded as zero) and missing using published guidelines and definitions (Blumenthal et al., 2005). Blink magnitude (i.e., including nonresponses in condition averages) values were used in analyses.
2.5. Data Analytic Plan
The larger study from which this sample was drawn was a family study; thus, many participants were part of the same family, which violates assumptions of independence required for many traditional statistical tests. Analyses were therefore mixed regression models, which account for shared variance between siblings nested within the same family (e.g., aggregation of symptoms). Prior to analyses, outliers in all variables defined as data below or above the first or third quartile by more than 1.5 times the interquartile range (IBM Corp, 2017) were removed to prevent biased results. Then, all predictors were standardized via z-scores. First, to examine the effects of the NPU-threat task on electromyography startle, we conducted a 3 (Condition: N, P, U) × 2 (Cue: countdown, ISI) repeated-measures mixed model with Condition and Cue as within-subjects factors. Second, we examined the predictive power of baseline SUT and SPT on outcomes. Two types of models were run: (1) predicting concurrent diagnosis or functioning (WHODAS) and (2) predicting follow-up symptoms (average severity for corresponding disorders on the LIFE) and functioning (WHODAS12). Third, to examine whether prospective relationships between threat responding and functioning could be explained by symptoms during the follow-up period, we added average follow-up symptom severity to the previous models. In follow-up models, baseline WHODAS General Disability and any current1 internalizing diagnoses (MDD, panic disorder, social phobia, specific phobia, GAD, obsessive compulsive disorder, post-traumatic stress disorder [PTSD]) were included as covariates. This was done to (a) control for baseline levels of functioning and (b) more broadly, examine the independent effects of sensitivity to threat from diagnoses. Given the heterogeneity of internalizing disorders, and that fear disorders are more consistently linked to aberrant threat responding (Gorka et al., 2017a), separate models were conducted covarying fear (panic, social phobia, specific phobia, PTSD) and distress-misery (GAD, MDD)2 disorders.
SUT was defined as startle during UCD adjusted for NCD3 and SPT was defined as startle during PCD adjusted for NCD. Residualized change scores have better psychometric properties than difference scores (e.g., UCD-NCD) (see Meyer et al., 2017 for further discussion)4. Additionally, residualized scores eliminate questions of whether observed differences in potentiation are due to differences in the active condition or differences at baseline.
3. Results
3.1. NPU-Threat Task Effects
Consistent with prior studies, there were main effects of Condition [F(2, 180.32) = 124.96, p < 0.001, ηp2 = 0.58] and Cue [F(1, 235.40) = 24.31, p < 0.001, ηp2 = 0.17], which were qualified by a Condition x Cue interaction [F(2, 312.34) = 19.21, p < 0.001, ηp2 = 0.11]. As in prior studies, to follow up this interaction, we subtracted startle magnitude during the no shock condition from that of the threat conditions. Both scores were significantly greater than zero, demonstrating threat-potentiated startle: unpredictable [t(309) = 13.33, p < 0.001, d = 1.52] and predictable [t(309) = 4.83, p < 0.001, d = 0.55]. Additionally, startle potentiation was greater during unpredictable than predictable threat [t(309) = 7.55, p < 0.001, d = 0.40].
3.2. Associations between Baseline Threat Responding, SCID Diagnoses, and Functioning
As shown in Table 2, consistent with prior studies, greater SUT (p = 0.028), but not SPT (p = 0.295), was associated with fear disorders, but neither SUT (p = 0.767) nor SPT (p = 0.596) was associated with distress-misery disorders. There was no association between threat responding and any internalizing disorder (ps > 0.25) when fear and distress-misery disorders were combined.
Table 2.
Cross-sectional associations of baseline symptoms, functioning, and threat responding
| Diagnosis | Threat Responding | ||||
|---|---|---|---|---|---|
| Any Internalizing | Fear | Distress-misery | Unpredictable | Predictable | |
| B [95%CI] | B [95%CI] | B [95%CI] | B [95%CI] | B [95%CI] | |
| Threat Responding | |||||
| SUT | 0.06** [0.01, 0.11] | 0.01 [−0.03, 0.04] | |||
| SPT | 0.04 [−0.04, 0.12] | 0.01 [−0.03, 0.06] | |||
| WHODAS | |||||
| General Disability | 0.74*** [0.50, 0.99] | 0.76*** [0.50, 1.02] | 1.75*** [1.35, 2.15] | −0.03 [−0.16, 0.10] | −0.02 [−0.19, 0.15] |
| Getting Along | 0.70*** [0.47, 0.94] | 0.73*** [0.48, 0.99] | 1.62*** [1.23, 2.01] | 0.13* [0.02, 0.25] | 0.13* [0.01, 0.24] |
| Cognition | 0.70*** [0.46, 0.94] | 0.60*** [0.35, 0.85] | 1.68*** [1.31, 2.05] | 0.17** [0.05, 0.28] | 0.21*** [0.10, 0.32] |
| Life Activities | 0.53*** [0.28, 0.78] | 0.56*** [0.29, 0.83] | 1.48*** [1.06, 1.90] | −0.07 [−0.19, 0.06] | 0.003 [−0.17, 0.17] |
| Participation | 0.73*** [0.49, 0.96] | 0.65*** [0.38, 0.91] | 1.59*** [1.19, 1.99] | −0.02 [−0.13, 0.09] | −0.03 [−0.14, 0.09] |
| Self-Care | 0.60*** [0.36, 0.85] | 0.57*** [0.30, 0.83] | 1.24*** [0.82, 1.66] | 0.02 [−0.09, 0.13] | −0.006 [−0.12, 0.11] |
| Mobility | 0.28* [0.03, 0.52] | 0.36** [0.11, 0.61] | 0.57** [0.16, 0.98] | 0.05 [−0.06, 0.16] | 0.17** [0.06, 0.28] |
0.05 < p < 0.10
p < 0.05
p < 0.01
p < 0.001
Note: Diagnosis was coded categorically as whether or not participants met criteria for one or more disorders per the SCID. Higher scores on the WHODAS indicate greater functional impairment. Although unusual, Betas exceeding 1.0 have been observed, but may indicate high multi-collinearity (Jöreskog, 1999).
At baseline, greater SUT was associated with impairment in WHODAS Cognition (p = 0.004) and Getting Along with Others (p = 0.019). Greater SPT was associated with greater impairment in Cognition (p < 0.001), Getting Along with Others (p = 0.027), and Mobility (p = 0.003). SCID diagnosis of any internalizing disorder (and both fear and distress-misery disorders) was associated with greater baseline functional impairment, including WHODAS General Disability and all six subscales (ps ≤ 0.028).
3.3. Threat Responding and Diagnoses as Independent Predictors of Follow-Up Outcomes
As shown in Table 3, baseline diagnosis of any internalizing disorder (and fear and distress-misery disorders) predicted average overall symptoms of psychopathology on the LIFE during follow-up (ps < 0.001), whereas SUT (ps > 0.332) and SPT (ps > 0.126) did not.
Table 3.
Independence of diagnosis and threat responding at baseline in predicting average symptoms of psychopathology during the one-year follow-up
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Diagnosis at Baseline | Unpredictable Threat Responding at Baseline | Predictable Threat Responding at Baseline | Unpredictable Threat Responding Independent of Diagnosis at Baseline | Predictable Threat Responding Independent of Diagnosis at Baseline | |
| B [95%CI] | B [95%CI] | B [95%CI] | B [95%CI] | B [95%CI] | |
| Any | 1.29 [0.94, 1.64]*** | 0.06 [−0.15, 0.26] | 0.22 [−0.07, 0.50] | 0.08 [−0.09, 0.25] | 0.18 [−0.05, 0.42] |
| Fear | 1.30 [0.92, 1.68]*** | 0.03 [−0.18, 0.23] | 0.07 [−0.22, 0.36] | 0.004 [−0.17, 0.18] | −0.004 [−0.27, 0.26] |
| Distress-Misery | 2.18 [1.62, 2.74]*** | 0.06 [−0.15, 0.27] | 0.25 [−0.04, 0.54]+ | 0.02 [−0.16, 0.19] | 0.15 [−0.11, 0.42] |
0.05 < p < 0.10
p < 0.05
p < 0.01
p < 0.001
Note: Although unusual, Betas exceeding 1.0 have been observed, but may indicate high multi-collinearity (Jöreskog, 1999).
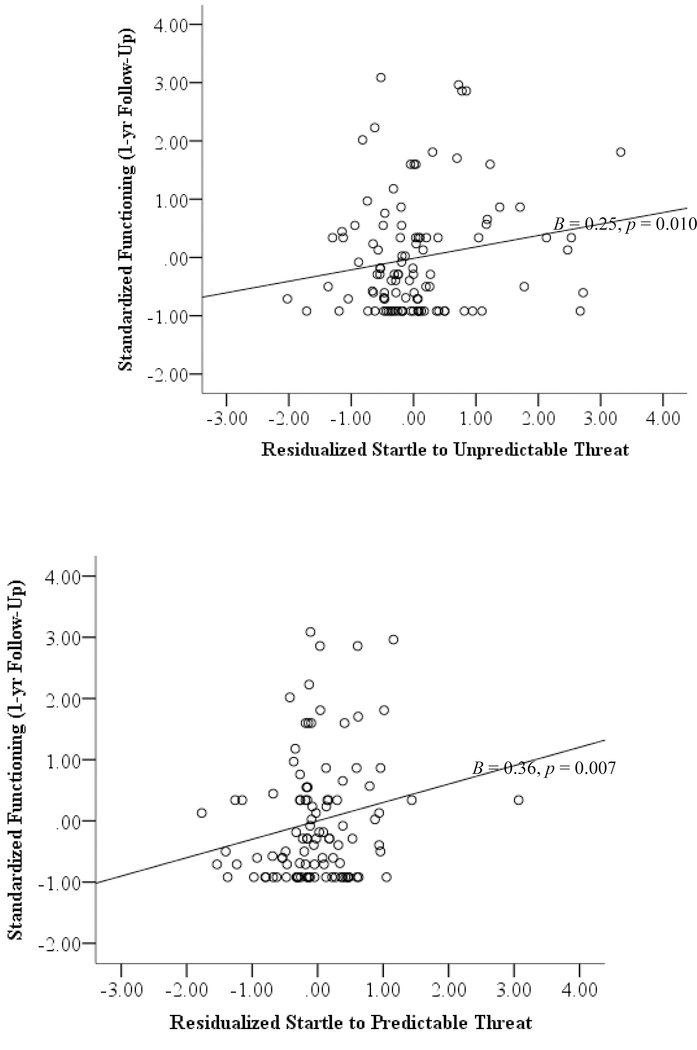
As shown in Table 4, SUT (p = 0.009) independently predicted worse WHODAS12 functioning during the follow-up over and above baseline functioning and any internalizing disorder at baseline.5 The effect of SUT was similar when fear or distress-misery disorders were examined separately. SPT (p = 0.007) also independently predicted worse WHODAS12 functioning during the follow-up over and above baseline WHODAS functioning and any internalizing diagnosis and exhibited comparable results to SUT when fear or distress-misery disorders were examined separately.
Table 4.
Independence of diagnosis and threat responding in predicting functional impairment during the one-year follow-up (adjusting for baseline functioning)
| Diagnosis at Baseline (Univariate) | Multivariate Models with Baseline Diagnosis and Unpredictable Threat Respondinga | Multivariate Models with Baseline Diagnosis and Predictable Threat Respondingb | Fisher’s Z c | ||
|---|---|---|---|---|---|
| B [95%CI] | B [95%CI] | B [95%CI] | |||
| Any | 0.17 [−0.26, 0.61] | Diagnosis | 0.20 [−0.22, 0.63] | 0.18 [−0.25, 0.60] | |
| NPU-Threat | 0.25 [0.06, 0.44]** | 0.36 [0.10, 0.63]** | 1.06 | ||
| Fear | 0.01 [−0.44, 0.46] | Diagnosis | 0.01 [−0.44, 0.43] | −0.04 [−0.47, 0.39] | |
| NPU-Threat | 0.28 [0.08, 0.48]** | 0.50 [0.20, 0.80]*** | 1.76+ | ||
| Distress- | 1.08 [0.34, 1.81]** | Diagnosis | 1.05 [0.33, 1.77]** | 0.98 [0.26, 1.70]** | |
| Misery | NPU-Threat | 0.27 [0.08, 0.46]** | 0.45 [0.17, 0.74]** | 1.45 | |
0.05 < p < 0.10
p < 0.05
p < 0.01
p < 0.001
Note: All models adjusted for baseline functioning indexed by the WHODAS General Disability scores, which significantly predicted WHODAS12 functioning during the follow-up (ps < .015). Diagnosis was coded categorically as whether or not participants met criteria for one or more disorders. Higher scores on the WHODAS12 indicate greater functional impairment. Although unusual, Betas exceeding 1.0 have been observed, but may indicate high multi-collinearity (Jöreskog, 1999).
In univariate models, SUT predicted worse functioning, B = 0.25, p = 0.010, 95% CI [0.06, 0.44]
In univariate models, SPT predicted worse functioning, B = 0.36, p = 0.007, 95% CI [0.10, 0.63]
Fisher’s Z test compared the strength of the associations between (a) follow-up functioning and unpredictable threat sensitivity with (b) follow-up functioning and predictable threat sensitivity.
3.4. Predicting Follow-Up Functioning Independent of Follow-Up Symptoms
Finally, when symptoms during the follow-up were added as covariates to the models predicting follow-up WHODAS12 functioning, SUT continued to predict greater functional impairment adjusting for any baseline internalizing (p = 0.028), fear (p = 0.009), or distress-misery (p = 0.010) disorder. SPT also independently predicted functional impairment during follow-up irrespective of any internalizing (p = 0.026), as well as fear (p = 0.001), or distress-misery (p = 0.005) disorder (Table 5).
Table 5.
Predicting follow-up functioning independent of follow-up symptoms (adjusting for baseline functioning).
| Models Including Sensitivity to Unpredictable Threat | Models Including Sensitivity to Predictable Threat | Fisher’s Z a | ||||
|---|---|---|---|---|---|---|
| B [95% CI] | p | B [95% CI] | p | |||
| Any | Baseline diagnosis | −0.23 [−0.71, 0.20] | 0.317 | −0.24 [−0.69, 0.22] | 0.307 | |
| NPU-Threat | 0.20 [0.02, 0.38] | 0.028 | 0.28 [0.03, 0.54] | 0.026 | 0.67 | |
| Follow-up symptoms | 0.43 [0.21, 0.65] | < 0.001 | 0.42 [0.19, 0.64] | < 0.001 | ||
| Fear | Baseline diagnosis | −0.38 [−0.87, 0.12] | 0.137 | −0.43 [−0.91, 0.06] | 0.082 | |
| NPU-Threat | 0.26 [0.07, 0.45] | 0.009 | 0.50 [0.22, 0.79] | 0.001 | 1.85+ | |
| Follow-up symptoms | 0.33 [0.10, 0.56] | 0.005 | 0.34 [0.12, 0.57] | 0.003 | ||
| Distress | Baseline diagnosis | 0.34 [−0.46, 1.13] | 0.402 | 0.29 [−0.50, 1.09] | 0.467 | |
| Misery | NPU-Threat | 0.24 [0.06, 0.42] | 0.010 | 0.40 [0.13, 0.68] | 0.005 | 1.26 |
| Follow-up symptoms | 0.37 [0.16, 0.59] | 0.001 | 0.36 [0.14, 0.58] | 0.001 | ||
Note: All models adjusted for baseline functioning indexed by the WHODAS General Disability scores, which significantly predicted WHODAS12 functioning during the follow-up (ps < 0.027). Follow-up symptoms were indexed by the LIFE. Diagnosis was coded categorically as whether or not participants met criteria for one or more disorders. Higher scores on the WHODAS12 indicate greater functional impairment.
Fisher’s Z test compared the strength of the associations between (a) follow-up functioning and unpredictable threat sensitivity with (b) follow-up functioning and predictable threat sensitivity.
4. Discussion
The present study found that sensitivities to both unpredictable and predictable threat predicted functional impairment at both baseline and during the follow-up, even when accounting for baseline differences in functioning. Importantly, threat responding demonstrated predictive validity on outcomes over and above diagnosis. However, whereas elevated SUT was associated with baseline diagnosis of fear disorders, neither SUT nor SPT predicted symptoms during the follow-up.
These findings demonstrate that threat responding, perhaps particularly unpredictable threat responding, connotes vulnerability for certain psychopathologies (Gorka et al., 2017a; Nelson et al., 2013) and extend prior findings to functional outcomes. This study is one of the first to demonstrate a longitudinal relationship between neurobiological vulnerability markers of threat responding and clinically relevant functional impairment beyond symptoms. If RDoC, or any model that utilizes expensive neurobiological measures, is to ultimately help refine our diagnostic system, it is essential that RDoC constructs demonstrate predictive power for important outcomes such as functioning. That threat responding remained an equal or stronger predictor of functioning independent of diagnosis reflects its potential validity and added utility.
The findings also highlight the distinction between symptoms and functioning. Threat responding and baseline diagnoses were each associated with concurrent functioning in multiple domains. However, sensitivity to threat was associated with more circumscribed domains (cognition, getting along with others), suggesting that threat responding may be more relevant for some functional domains than others. The results for cognition are particularly notable as evidence has linked neural circuits involved in threat responding to attention to and appraisal of threat (Grupe and Nitschke, 2013), which may be more directly associated with cognitive domains of functioning. Threat responding may also impact domains such as community participation, but more distally (or mediated by other deficits). Notably, diagnosis and physiological threat responding exhibited different predictive relationships with symptoms and functional impairment. Although baseline diagnosis was a strong predictor of symptoms during the follow-up, only distress-misery disorders predicted subsequent functional impairment, a finding consistent with larger associations between symptoms and functioning for depression relative to most anxiety disorders (McKnight and Kashdan, 2009; McKnight et al., 2016). Similarly, although sensitivity to threat evidenced weaker associations with follow-up symptoms compared to diagnosis, it generally evidenced stronger associations with functioning at follow-up than diagnosis, even independent of symptoms during the follow-up. This suggests that symptoms and functioning, while correlated, reflect distinguishable outcomes that each provide unique, valuable information.
There are several reasons that sensitivity to threat might evidence stronger longitudinal associations with functioning than symptoms. First, startle responding to threats represents a more proximal index of emotion generation and regulation (given its associations with brain regions such as the dorsal anterior cingulate cortex; Gorka et al., 2017b) than self-reported symptoms, and thus may play a larger role in functioning than symptom severity (Graver, 1987). Second, follow-up symptoms were assessed for each DSM disorder using the LIFE’s psychiatric status ratings. While DSM disorders were grouped into fear and distress-misery disorders, using transdiagnostic measures specifically designed to reflect the factor structure of psychopathology (e.g., Hierarchical Taxonomy of Psychopathology [HiTOP]) or trait-like measures (e.g., anxiety sensitivity; Stevens et al., 2017) may have yielded different results. Finally, it is possible that relatively low symptom severity during the follow-up restricted the variance in symptoms that could be explained by threat responding. As this is one of the few psychophysiological studies that have attempted to disambiguate symptoms from functioning, further studies are needed to differentiate mechanisms that lead to symptoms versus functional impairment.
It is also interesting that sensitivity to both unpredictable and predictable threat predicted these outcomes. Threat responding in general, not just SUT, may be an important marker of vulnerability for psychopathology. Given the abundant evidence specifically linking SUT to personal (Gorka et al., 2017a; Shankman et al., 2013) and familial (Nelson et al., 2013) vulnerability for internalizing psychopathology as well as physiological distinctions between unpredictable versus predictable threat responding (Davis, 1998, 2006), an alternative interpretation is that unpredictable and predictable threat responding may operate via separate mechanisms but have final common neural circuitry (Shackman and Fox, 2016) that leads to functional outcomes. Indeed, McEvoy et al. (in press) speculate that mechanisms of unpredictable threat responding may have substantial overlap with predictable threat responding. Given that the neurobiology of the startle response is well-documented, it is possible that the disruptions in the overlapping neural circuitry of SUT and SPT is involved in the pathways leading from neurophysiological vulnerability to functional impairment, especially in cognitive, and to some extent, behavioral domains (Grupe and Nitschke, 2013).
The NPU-threat task is well suited for future investigations of sensitivity to threat. The NPU-threat task produces reliable responses (Kaye et al., 2016; Lieberman et al., 2017) and can be used to assess other neurobiological constructs of interest via event-related potentials (e.g., Nelson et al., 2015; Stevens et al., 2017) and magnetic resonance imaging (Gorka et al., 2017b; Gorka et al., 2014). Adaptations of the task (e.g., aversive images, air puffs) have been successfully used in various populations, including children (Grillon and Ameli, 1998; Nelson and Hajcak, 2017), enabling examination of the development of symptoms and functioning. Additionally, the NPU-threat task measures sensitivity to unpredictable and predictable threat within the same paradigm, reducing burden on participants and maintaining internal validity. Given the substantial variability in the relationship between sensitivity to threat and functioning (Figure 1), threat responding may be a particularly important vulnerability factor for some individuals more than others. These findings represent an important step toward identifying individuals at greater risk for future psychopathology, which would enable clinicians to intervene before symptoms or impairment worsen.
Figure 1.
Unpredictable threat sensitivity (top) and predictable threat sensitivity (bottom) predicting functioning during the one-year follow-up (adjusting for functioning at baseline). Higher functioning scores indicate greater impairment.
Although the present study had several notable strengths (e.g., interviewer-assessed diagnoses of multiple psychopathologies, follow-up assessment), these findings should be interpreted in light of several limitations. First, all participants were young adults, limiting generalizability to individuals of other ages and aspects of functioning that are less pertinent to this age group (e.g., mobility). Second, although a substantial number of individuals in the sample met criteria for one or more diagnoses, the number of individuals meeting criteria for some diagnoses was small, precluding analyses of specific diagnoses. This may be particularly true of distress-misery diagnoses, leading to greater error variance and unreliable coefficient estimates for the models testing the predictive power of these disorders. Relatedly, because follow-up assessment was a secondary aim of the larger study, a substantially smaller sample of participants completed the follow-up. However, baseline diagnoses evidenced similar relationships with functioning across fear and distress-misery disorders and both were distributed evenly among samples, providing some evidence of generalizability across disorders and samples. Third, although the WHODAS and WHODAS12 are validated, widely-used assessments of functioning and assess a variety of domains, they do not necessarily assess all relevant aspects of functioning and may conflate impairment with core symptoms of some psychopathologies (e.g., engagement in social situations in social phobia). Use of the WHODAS12 also limited our ability to examine specific aspects of functioning during the follow-up. Future studies may consider behavioral or observer-reported indices of functioning to complement the WHODAS and assess additional domains. Finally, given that this research was exploratory in nature, we chose not to employ alpha value corrections for multiple comparisons in our analyses, thus our findings should be interpreted with caution.
In summary, sensitivity to both unpredictable and predictable threat predicted concurrent functioning and functional impairment during a one-year follow-up period independent of both baseline diagnosis and symptoms of psychopathology during the follow-up. In contrast, although baseline diagnosis consistently predicted current functioning and symptoms during the follow-up, only distress-misery disorder diagnoses longitudinally predicted functioning. These findings demonstrate the added clinical utility of assessing sensitivity to threat as vulnerability markers for internalizing psychopathology in addition to traditional diagnostic assessments and support using the NPU-threat task to assess the RDoC constructs of acute and potential threat responding. Furthermore, these findings highlight the distinction between symptom-based and functional outcomes and support the assessment of both types of outcomes in psychopathology research.
Highlights.
Heightened response to unpredictable threats characterizes internalizing disorders
Functioning is important and separable from symptoms, but rarely assessed
Threat responding predicted worse functioning over time independent of symptoms
Neurobiological vulnerability markers have added utility in predicting functioning
Acknowledgements
This work was supported by the National Institute of Mental Health grant R01 MH098093 awarded to Dr. Shankman.
Footnotes
Declarations of interest: none.
Given that assessment of baseline functioning was past-month and threat responding was a current snapshot, current diagnoses were used in all models. Patterns of effects were similar, albeit weaker, for lifetime diagnoses.
Evidence is mixed as to whether PTSD is a fear or distress-misery disorder (Watson, 2005). Analyses included PTSD as a fear disorder, although the pattern of findings was identical when it was a distress-misery disorder (see McTeague and Lang, 2012).
SUT can also be computed as the average of UCD and UISI minus the average of NCD and NISI. Since the countdown and ISI cannot be averaged in the predictable condition given that the two phases are qualitatively different, similar to our prior studies (Gorka and Shankman, 2017) only countdown phases were examined. This allowed SUT and SPT to be matched on the number of startle probes in each condition average and the visual stimuli (i.e., countdowns) on the screen.
The pattern of results was identical, albeit less robust, when potentiation was analyzed using difference scores rather than residualized scores.
As discussed above, we were interested in capturing functioning across the one-year follow-up period rather than solely a snapshot (e.g., functioning at 12 months), as the latter approach may mask impairment that occurred during the year but that resolved by 12 months. Indeed, after adjusting for baseline diagnoses, the association between threat responding and functioning varied a great deal when functioning during each of the 12 months was analyzed separately (range of ps = .001-.747).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C, 2011. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 1, 389–400. doi: 10.1016/j.neuroimage.2010.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony MM, Roth D, Swinson RP, Huta V, Devins GM, 1998. Illness intrusiveness in individuals with panic disorder, obsessive-compulsive disorder, or social phobia. J. Nerv. Ment. Dis 186(5), 311–315. doi: 10.1097/00005053-199805000-00008 [DOI] [PubMed] [Google Scholar]
- Ballard ED, Ionescu DF, Vande Voort JL, Slonena EE, Franco-Chaves J, Zarate CA Jr., et al. , 2014. Increased fear-potentiated startle in major depressive disorder patients with lifetime history of suicide attempt. J. Affect. Disord 162, 34–38. doi: 10.1016/j.jad.2014.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieling PJ, Rowa K, Antony MM, Summerfeldt LJ, Swinson RP, 2001. Factor structure of the illness intrusiveness rating scale in patients diagnosed with anxiety disorders. J. Psychopathol. Behav. Assess 23(4), 223–230. doi: 10.1023/A:1012723318964 [DOI] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A, 2005. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 42, 1–15. doi: 10.1111/j.1469-8986.2005.00271.x [DOI] [PubMed] [Google Scholar]
- Carleton RN, 2016. Into the unknown: A review and synthesis of contemporary models involving uncertainty. J. Anxiety Disord 39, 30–43. doi: 10.1016/j.janxdis.2016.02.007 [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor S, Harrington H, Israel S, et al. , 2014. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clin. Psychol. Sci 2(2), 119–137. doi: 10.1177/2167702613497473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa K, Liu H, Shankman SA (In press). The role of intolerance of uncertainty in current and remitted internalizing and externalizing psychopathology. J. Anxiety Disord doi: 10.1016/j.janxdis.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR, 2013. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11(1), 126. doi: 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, 1998. Are different parts of the extended amygdala involved in fear versus anxiety? Biol. Psychiatry. 44, 1239–1247. doi: 10.1016/S0006-3223(98)00288–1 [DOI] [PubMed] [Google Scholar]
- Davis M, 2006. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am. Psychol 61, 741–56. doi: 10.1037/0003-066X.61.8.741 [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL, 2015. Structured Clinical Interview for DSM-5 Disorders, Clinician Version (SCID-5-CV). American Psychiatric Association Publishing, Arlington, VA. [Google Scholar]
- Gorka SM, Lieberman L, Shankman SA, Phan KL, 2017a. Startle potentiation to uncertain threat as a psychophysiological indicator of fear-based psychopathology: An examination across multiple internalizing disorders. J. Abnorm. Psychol 126(1), 8–18. doi: 10.1037/abn0000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Shankman SA, Phan KL, 2017b. Association between neural reactivity and startle reactivity to uncertain threat in two independent samples. Psychophysiology 54(5), 652–662. doi: 10.1111/psyp.12829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Phan KL, Shankman SA (2014). Insula response to unpredictable and predictable aversiveness in individuals with panic disorder and comorbid depression. Biol. Mood Anxiety Disord 4, 9. doi: 10.1186/2045-5380-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Shankman SA, 2017. Preliminary evidence that reactivity to uncertain threat is an endophenotype for alcohol use disorder. Drug Alcohol Depend 180, 265–271. doi: 10.1016/j.drugalcdep.2017.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graver DL, 1987. Methodological issues facing the interpretation of high-risk studies: biological heterogeneity. Schizophr. Bull 13, 525–529. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. , 2003. The economic burden of depression in the united states: How did it change between 1990 and 2000? J. Clin. Psychiatry 64(12), 1465–1475. doi: 10.4088/JCP.v64n1211 [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JRT, et al. , 1999. The economic burden of anxiety disorders in the 1990s. J. Clin. Psychiatry 60(7), 427–435. doi: 10.4088/JCP.v60n0702 [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, 1998. Effects of threat and safety signals on startle during anticipation of aversive shocks, sounds, or airblasts. J. Psychophysiol 12(4), 329–337. doi: 10.1017/sjp.2014.106 [DOI] [Google Scholar]
- Grillon C, Baas JP, Pine DS, Lissek S, Lawley M, Ellis V, et al. , 2006. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol. Psychiatry 60, 760–766. doi: 10.1016/j.biopsych.2005.11.027 [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS, 2008. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am. J. Psychiatry 165, 898–904. doi: 10.1176/appi.ajp.2007.07101581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M, 2009. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol. Psychiatry 66(1), 47–53. doi: 10.1016/j.biopsych.2008.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB, 2013. Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat. Rev. Neurosci 14(7), 488–501. doi: 10.1038/nrn3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp (2017). IBM SPSS Statistics for Windows, Version 25.0 Armonk, NY: IBM Corp. [Google Scholar]
- Ishak WW, Greenberg JM, Cohen RM, 2013. Predicting relapse in major depressive disorder using patient-reported outcomes of depressive symptom severity, functioning, and quality of life in the individual burden of illness index for depression (IBI-D). J. Affect. Disord 151(1), 59–65. doi: 10.1016/j.jad.2013.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöreskog KG (1999, June 22). How large can a standardized coefficient be? Retrieved February 15, 2019 from http://www.ssicentral.com/lisrel/column2.htm
- Kaviani H, Gray JA, Checkley SA, Raven PW, Wilson GD, Kumari V, 2004. Affective modulation of the startle response in depression: Influence of the severity of depression, anhedonia, and anxiety. J. Affect. Disord 83(1), 21–31. doi: 10.1016/j.jad.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Curtin JJ, 2016. Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks. Psychophysiology 53(8), 1241–1255. doi: 10.1111/psyp.12663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, et al. , 1987. The longitudinal interval follow-up evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Arch. Gen. Psychiatry 44(6), 540–548. doi: 10.1001/archpsyc.1987.01800180050009 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC, 2003. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch. Gen. Psychiatry 60(9), 929–937. doi: 10.1001/archpsyc.60.9.929 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, et al. , 2009. The global burden of mental disorders: An update from the WHO world mental health (WMH) surveys. Epidemiol. Psichiatr. Soc 18(1), 23–33. doi: 10.1017/S1121189X00001421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Akiskal HS, Ames M, Birnbaum H, Greenberg P, Hirschfeld RMA, et al. , 2006. Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. Am. J. Psychiatry 163(9), 1561–1568. doi: 10.1176/appi.ajp.163.9.1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, et al. , 2017. The hierarchical taxonomy of psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J. Abnorm. Psychol 126(4), 454–477. doi: 10.1037/abn0000258 [DOI] [PubMed] [Google Scholar]
- Krueger RF, 1999. The structure of common mental disorders. Arch. Gen. Psychiatry 56(10), 921–926. doi: 10.1001/archpsyc.56.10.921 [DOI] [PubMed] [Google Scholar]
- Lieberman L, Stevens ES, Funkhouser CJ, Weinberg A, Sarapas C, Huggins AA, et al. , 2017. How many blinks are necessary for a reliable startle response? A test using the NPU-threat task. Int. J. Psychophysiol 114, 24–30. doi: 10.1016/j.ijpsycho.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, MacLeod C, 2005. Cognitive vulnerability to emotional disorders. Annu. Rev. Clin. Psychol 1(1), 167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916 [DOI] [PubMed] [Google Scholar]
- McEvoy PM, Carleton RN, Correa K, Shankman S, Shihata S, in press. Intolerance of uncertainty: diagnoses to dimensions, in Olatunji B (Ed.) Handbook of Anxiety and Related Disorders. Cambridge University Press, Cambridge, UK. [Google Scholar]
- McKnight PE, Kashdan TB, 2009. The importance of functional impairment to mental health outcomes: A case for reassessing our goals in depression treatment research. Clin. Psychol. Rev 29(3), 243–259. doi: 10.1016/j.cpr.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight PE, Monfort SS, Kashdan TB, Blalock DV, Calton JM, 2016. Anxiety symptoms and functional impairment: A systematic review of the correlation between the two measures. Clin. Psychol. Rev 45, 115–130. doi: 10.1016/j.cpr.2015.10.005 [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, 2012. The anxiety spectrum and the reflex physiology of defense: from circumscribed fear to broad distress. Depress. Anxiety 29(4), 264–281. doi: 10.1002/da.21891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, Hajcak G, 2017. Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiol. 54(1), 114–122. doi: 10.1111/psyp.12664 [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M, Mechanic D, 2002. Perceived need and help-seeking in adults with mood, anxiety, or substance use disorder. Arch. Gen. Psychiatry 59(1), 77–84. doi: 10.1001/archpsyc.59.1.77 [DOI] [PubMed] [Google Scholar]
- Nelson BD, Hajcak G, 2017. Anxiety and depression symptom dimensions demonstrate unique relationships with the startle reflex in anticipation of unpredictable threat in 8 to 14 year-old girls. J. Abnorm. Child Psychol 45(2), 397–410. doi: 10.1007/s10802-016-0169-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, et al. , 2013. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. J. Abnorm. Psychol 122(3), 662–671. doi: 10.1037/a0033982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BJ, Reis HT, Jamieson JP, 2018. Cardiovascular indexes of threat impair responsiveness in situations of conflicting interests. Int. J. Psychophysiol 123, 1–7. doi: 10.1016/j.ijpsycho.2017.12.005 [DOI] [PubMed] [Google Scholar]
- Raulin ML, Lilienfeld SO, 2009. Studying psychopathology, in: Blaney PH, Millon T (Eds.), Oxford Textbook of Psychopathology, 2nd edition Oxford University Press, New York, pp. 86–115. [Google Scholar]
- Sarapas C, Katz AC, Nelson BD, Campbell ML, Bishop JR, Robison-Andrew E, et al. , 2014. Are individual differences in appetitive and defensive motivation related? A psychophysiological examination in two samples. Cogn. Emot 28(4), 636–655. doi: 10.1080/02699931.2013.848787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Grillon C, 2012. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nat. Protoc 7(3), 527–532. doi: 10.1038/nprot.2012.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, 2016. Contributions of the central extended amygdala to fear and anxiety. J. Neurosci 36(31), 8050–8063. doi: 10.1523/JNEUROSCI.0982-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Funkhouser CJ, Klein DN, Davila J, Lerner D, Hee DE, 2018. Reliability and validity of severity dimensions of psychopathology assessed using the Structured Clinical Interview for DSM-5 (SCID). Int. J. Methods in Psychiatr. Res 27(1), 1–12. doi: 10.1002/mpr.1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, 2003. The relation between depression and anxiety: An evaluation of the tripartite, approach-withdrawal and valence-arousal models. Clin. Psychol. Rev 23(4), 605–637. doi: 10.1016/S0272-7358(03)00038-2 [DOI] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, et al. , 2013. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. J. Abnorm. Psychol 122(2), 322–338. doi: 10.1037/a0030747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, et al. , 2007. Robust dimensions of anxiety sensitivity: Development and initial validation of the anxiety sensitivity index-3. Psychol. Assess 19(2), 176–188. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- Üstün TB, Chatterji S, Kostanjsek N, Rehm J, Kennedy C, Epping-Jordan J, et al. , 2010. Developing the World Health Organization disability assessment schedule 2.0. Bull. World Health Org 88(11), 815–823. doi: 10.2471/BLT.09.067231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üstün TB, Kostanjsek N, Chatterji S, Rehm J (2010). Measuring Health and Disability: Manual for WHO Disability Assessment Schedule (WHODAS 2.0). World Health Organization. [Google Scholar]
- Warshaw MG, Keller MB, Stout RL, 1994. Reliability and validity of the longitudinal interval follow-up evaluation for assessing outcome of anxiety disorders. J. Psychiatr. Res 28(6), 531–545. doi: 10.1016/0022-3956(94)90043-4 [DOI] [PubMed] [Google Scholar]
- Watson D, 2005. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. J. Abnorm. Psychol 114(4), 522–536. doi: 10.1037/0021-843X.114.4.522 [DOI] [PubMed] [Google Scholar]
- Weinberg A, Liu H, Hajcak G, Shankman SA, 2015. Blunted neural response to rewards as a vulnerability factor for depression: Results from a family study. J. Abnorm. Psychol 124(4), 878–889. doi: 10.1037/abn0000081 [DOI] [PMC free article] [PubMed] [Google Scholar]