Abstract
4, 4’-methylenedianiline (MDA) is used extensively as a curing agent in the production of elastomers and is classified as reasonably anticipated to be a human carcinogen based on sufficient evidence in animal experiments. Human N-acetyltransferase 1 (NAT1) and 2 (NAT2) catalyze the N-acetylation of aromatic amines and NAT2 is subject to a common genetic polymorphism in human populations separating individuals into rapid, intermediate, and slow acetylator phenotypes. Although MDA is known to undergo N-acetylation to mono- and di-acetyl metabolites, very little is known regarding whether this metabolism is subject to the NAT2 genetic polymorphism. We investigated the N-acetylation of MDA by recombinant human NAT1, NAT2, genetic variants of NAT2, and cryoplateable human hepatocytes obtained from rapid, intermediate and slow acetylators. MDA N-acetylation was catalyzed by both recombinant human NAT1 and NAT2 exhibiting a 5-fold higher affinity for human NAT2. N-acetylation of MDA was acetylator genotype dependent as evidenced via its N-acetylation by recombinant human NAT2 genetic variants or by cryoplateable human hepatocytes. MDA N-acetylation to the mono-acetyl or di-acetyl MDA was highest in rapid, lower in intermediate, and lowest in slow acetylator human hepatocytes. MDA-induced DNA damage in the human hepatocytes was dose-dependent and also acetylator genotype dependent with highest levels of DNA damage in rapid, lower in intermediate, and lowest in slow acetylator human hepatocytes under the same MDA exposure level. In summary, the N-acetylation of MDA by recombinant human NAT2 and cryopreserved human hepatocytes support an important role for the NAT2 genetic polymorphism in modifying MDA metabolism and genotoxicity and potentially carcinogenic risk.
Keywords: Human N-acetyltransferase; 4, 4’-Methylenedianiline; NAT2 polymorphism; Genotoxicity; Carcinogenic risk
Introduction
4, 4’-Methylenedianiline (MDA) (CAS 101-77-9) is a primary aromatic amine used as a chemical intermediate in the production of isocyanates and polyisocyanates (McQueen and Williams 1990). This chemical has an annual world production estimated to exceed 4 million metric tons per year (Carvajal-Diaz 2015). Approximately 98% of this production is used as an intermediate for the production of methylenediphenyl diisocyanate (MDI) (Schupp et al. 2018). These chemicals are used extensively in the manufacture of rigid polyurethane foams for thermal insulation and the production of semiflexible polyurethane foams for automobile safety cushioning (National Toxicology Program, 1983). MDA also is used extensively as a curing agent in the production of elastomers.
MDA is listed by the United States Environmental Protection Agency Clean Air Act as a hazardous air pollutant and its manufacture is subject to certain provisions to control emissions of volatile organic compounds (National Toxicology Program, 2016). MDA is listed as a possible occupational carcinogen by the United States National Institute for Occupational Safety and Health (National Toxicology Program, 2016). In its 1992 survey, it estimated that nearly 4000 workers in 11 principal industry sectors were exposed to MDA at air concentrations up to 250 ppb and for average annual exposures up to 250 days. MDA air concentration was up to 31 mg/m3 inside facilities where it was produced and up to 1.6 mg/m3 inside facilities while it was being used (OSHA, 1992). MDA is classified as category 2B by the International Agency for Research on Cancer because it is reasonably anticipated to be a human carcinogen based on sufficient evidence in animal experiments (Agency for Toxic Substances and Disease Registry 1998). The liver and thyroid gland are the main target organs for MDA in rat and mice. MDA induced neoplastic nodules in the liver of Fischer 344 rats and carcinomas in the liver of B6C3F1 mice, as well as thyroid carcinomas in both species in a carcinogenicity study with MDA added to the drinking water (National Toxicology Program 1983; National Toxicology Program, 2011).
The primary routes of potential human exposure to MDA are inhalation, dermal contact and ingestion. Several studies have described the health effects of MDA exposure to be related to contact dermatitis (Hamada et al. 2017; Zuliani et al. 2017), acute myocardial damage (Brooks et al. 1979; Hebert et al. 2011), jaundice (Dunn and Guirguis 1979; Nichols 2004), photosensitivity (LeVine 1983) and hepatitis (Bastian 1984; Giouleme et al. 2011). Dialysis patients may be exposed to MDA through polyurethane-containing medical devices from which MDA is released during gamma-ray or autoclave sterilization (Shintani and Nakamura 1989). Occupational exposure during the production of these chemicals or elastomers occurs in the workplace. N-acetyl-MDA is a major metabolite in urine samples of workers exposed to MDA (Cocker et al. 1988; Robert et al. 1995; Schutze et al. 1995) or to MDI. Individuals exposed to MDI are in turn exposed to MDA since it is a major metabolite of MDI (Kaaria et al. 2001; Sepai et al. 1995a). In addition, MDA is genotoxic (McQueen and Williams 1990), forms DNA adducts in the liver (Schutze et al. 1996) and induces DNA damage in primary cultures of rat and human hepatocytes (Martelli et al. 2002).
N-acetylation of aromatic amines is catalyzed by two human N-acetyltransferases, NAT1 and NAT2, which are encoded by the two genes NAT1 and NAT2 that are located chromosome region 8p22 (Hein et al. 2000). Human NAT2 is polymorphic and possesses different combinations of single nucleotide polymorphisms (SNPs) some of them affect the stability, amount and activity of NAT2 protein (Hein 2002). For human NAT2, there is a well-established gene-dosage relationship between genotype and phenotype. Homozygotes for rapid alleles (NAT2*4, NAT2*12, NAT2*13) are rapid acetylators, homozygotes for slow alleles (NAT2*5, NAT2*6, NAT2*7, NAT2*14) are slow acetylators and heterozygotes possessing a rapid and a slow acetylator allele are intermediate acetylators. Human populations divide into rapid, intermediate, and slow acetylator phenotypes. Human epidemiology studies have shown that NAT2 genetic polymorphism modifies susceptibility to various cancers following exposure to aromatic amine and heterocyclic amine carcinogens (Hein et al. 2000).
An industrial outbreak of occupational MDA exposure suggested that MDA hepatotoxicity was modified by individual susceptibility (McGill and Motto 1974). Both the N-acetyl and the N, N’-diacetyl-MDA metabolites have been identified in urine following administration of MDA (Tanaka et al. 1985) or MDI (Sepai et al. 1995b) to rats. Whereas N-acetyl-MDA represents more than half of all MDA metabolites in human urine in MDA-exposed workers, the N, N’-diacetyl-MDA metabolite represents less than 3%. Furthermore, the individual ratio of N-acetyl-MDA to total MDA in workers exposed to MDA (Robert et al. 1995) or MDI (Sepai et al. 1995a) varied widely, suggestive of genetic polymorphism in the N-acetylation of MDA in human populations.
Although MDA is known to undergo N-acetylation to mono- and di-acetyl metabolites, very little is known regarding whether this metabolism is subject to the NAT2 genetic polymorphism. We investigated the N-acetylation of MDA by recombinant human NAT1, NAT2, genetic variants of NAT2, and cryoplateable human hepatocytes. Finally, the genotoxic damage of MDA exposure was evaluated in cryoplateable human hepatocytes obtained from rapid, intermediate and slow acetylator subjects.
Materials and Methods
Expression of Recombinant Human NAT2 Allozymes
NAT1*4 and NAT2*4, the reference human NAT1 and NAT2 alleles, and numerous NAT2 genetic variants were expressed recombinantly in E. coli JM105 as previously described (Hein et al. 1995; Hein et al. 1994). Briefly, bacteria harboring different NAT2 plasmids were grown up overnight in Luria-Bertani medium containing 100 μg/ml ampicillin at 37°C. Fresh Luria-Bertani ampicillin broth was reinoculated, and NAT2-expressing bacteria were grown to approximately A600=0.5. Isopropyl-β-D-thiogalactopyranoside (1 mM) was added to the broth for induction, and the cultures were grown for an additional 3 h. The cells were harvested by centrifugation at 5000×g for 10 min. Cell pellets were suspended in 20 volumes of homogenization buffer (20 mM sodium phosphate, pH 7.4, containing EDTA (1 mM), DTT (1 mM), and protease inhibitors aprotinin (1 μg/ml), PMSF (100 μM), and pepstatin A (1 μM). The suspension was lysed by sonication and the lysed suspension was then subjected to centrifugation at 15,000×g for 20 min at 4°C. Supernatants were mixed, aliquoted and stored at −80°C until use.
Source and processing of cryoplateable human hepatocytes
Cryoplateable human hepatocyte samples obtained from BioIVT (Baltimore, MD, USA) were stored in liquid nitrogen until use. Hepatocyte samples were collected from consented donors under IRB approved protocols at their FDA licensed donor center (http://www.bioivt.com/). Hepatocytes were prepared from fresh human tissue with hepatocytes isolated and frozen within 24 hr of organ removal. All hepatocytes are human transplant rejected and tested negative for hepatitis B and C and HIV 1 and 2. Upon removal from liquid nitrogen, hepatocytes were thawed according to the manufacturer’s instructions by warming a vial of the hepatocytes at 37°C for 90 s and transferring to a 50 mL conical tube containing 45 mL of InVitroGRO HT medium (BioIVT, Baltimore, MD, USA). The cell suspension was centrifuged at 50×g at room temperature for 5 min. The supernatant was discarded, and cells washed once in ice-cold phosphate-buffered saline (PBS) before lysing the cells in ice-cold 20 mM sodium phosphate, 1 mM DTT, 1 mM EDTA, 0.2% Triton X-100, 100 μM PMSF, 1 μM pepstatin A, and 1 μg/mL aprotinin. The lysate was centrifuged at 15,000×g for 20 min and the supernatant was aliquoted and stored at −80°C. Protein concentrations in the lysates were determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). Hepatocyte used for all in vitro experiments were categorized as either cryosuspension or cryoplateable hepatocytes. Hepatocytes used for in situ studies were categorized as cryoplateable and approved for studies involving drug metabolism.
NAT2 genotyping and assignment of acetylator phenotype
Genomic DNA was isolated from pelleted cells prepared from human cryoplateable hepatocyte samples as described above by using the QIAamp DNA Mini Kit (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instructions. NAT2 genotypes and deduced phenotypes were determined as described previously (Doll and Hein 2001). Controls (no DNA template) were run to ensure that there was no amplification of contaminating DNA. Individuals possessing two NAT2 alleles associated with rapid acetylation activity (NAT2*4, NAT2*12, and NAT2*13) were classified as rapid acetylators; individuals possessing one of these alleles and one allele associated with slow acetylation activity (NAT2*5, NAT2*6, NAT2*7, and NAT2*14) were classified as intermediate acetylators, and those individuals that possessed two slow acetylation alleles were classified as slow acetylators. Cryoplateable hepatocytes with rapid, intermediate and slow NAT2 acetylator genotype were selected at random for experiments described below.
Measurement of MDA N-acetyltransferase Activity
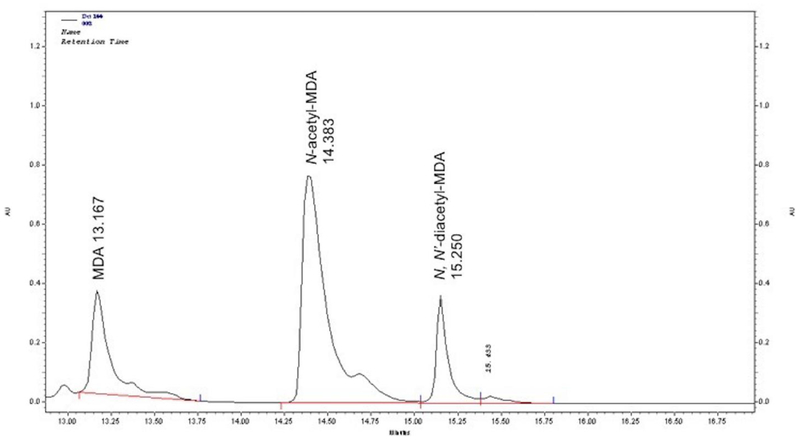
MDA N-acetyltransferase activities were measured using modifications of high-performance liquid chromatography (HPLC) assays as previously described (Zhang et al. 2006). Suitably diluted hepatocyte or bacterial lysate, acetyl coenzyme A (1000 μM) and MDA (15 μM to 1000 μM) were incubated at 37°C. The reaction was stopped by the addition of 1 M perchloric acid. Following centrifugation to precipitate protein, reaction supernatants were injected (40 μl) onto an EM Science 125 mm × 4 mm Lichrocart C18 (5 μm) column fitted with a similar Lichrocart guard column (4 mm × 4 mm) (Merck, Darmstadt, GER). Reactants and products were eluted from the column with a 10-minute linear gradient (2 ml/min) from 100% sodium perchlorate (pH 2.5) to 100% acetonitrile. Under the conditions of this assay, MDA eluted at 13.1 min, N-acetyl-MDA at 14.4 min, and N, N’-diacetyl-MDA at 15.1 min (Fig. 1). Protein concentrations in the lysates were determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). N-acetyltransferase activities were calculated as nanomoles of N-acetyl-MDA or N, N’-diacetyl-MDA product per minute per mg of cytosolic protein.
Fig. 1.
HPLC separation of MDA, N-acetyl-MDA and N,N-diacetyl-MDA as outlined in Materials and Methods.
Measurement of DNA damage from MDA
Cryoplateable human hepatocyte samples previously identified as rapid, intermediate, or slow NAT2 acetylator genotypes were thawed according to manufacturer’s instructions. Contents of the vial were transferred into a 15 mL conical tube containing 12 mL of InVitroGRO CP (BioIVT, Baltimore, MD, USA) media pre-warmed to 37°C. Cells (100 μL/well) were plated into Biocoats collagen coated black/clear bottom 96-well plates (Corning, Corning, NY, USA) and allowed to attach overnight. The next morning media was removed (cell debris and non-adherent cells were washed away and removed) and attached cells washed with PBS and replaced with fresh pre-warmed InVitroGRO CP (BioIVT, Baltimore, MD, USA) media containing 10–1000 μM MDA. Hepatocytes were incubated for up to 24 h after which media was removed and γH2AX in-cell western (ICW) staining protocol was performed as follows; cells were fixed to the plate using 3.7% chloroform and incubating at room temperature for 20 min. Then, the cells were permeabilized by washing five times with 0.1% Triton X-100 in TBS. After permeabilization, the cells were blocked using Odyssey® Blocking Buffer (LI-COR, Lincoln, NE, USA) for 90 min at room temperature with constant agitation. Primary antibody Anti-phospho-Histone H2A.X (Millipore-Sigma, Burlington, MA, USA) was diluted to 2 μg/mL and added to the cells and then incubated overnight at 4°C. The next morning cells were washed with 0.1% Tween 20 in TBS for five minutes, five times. Secondary antibody IRDye® 800CW Goat anti-Mouse IgG (H+L) (LI-COR, Lincoln, NE, USA) was used at a 1:1000 dilution and DNA dye RedDot™ 2 diluted to 1X (Biotium, Fremont, CA, USA) to normalize for DNA content. Cells were incubated with this combination for 60 min and washed again with the Tween 20 solution as previously described. DNA and the γH2AX were simultaneously visualized using an Odyssey CLx imaging system (LI-COR, Lincoln, NE, USA) with the 680 nm fluorophore (red) and the 800 nm fluorophore (green). Relative fluorescence units from the scanning allowed a quantitative analysis. Relative fluorescent units for γH2AX per cell (as determined by γH2AX divided by DNA content) were divided by untreated cells to determine percent change in phosphorylation of H2AX levels relative to control. Cell viability was measured with the Alamar blue method (Page et al. 1993); no changes in cell viability due to the MDA treatment were observed in the cells.
Statistical analyses
Differences between rapid, intermediate, and slow NAT2 acetylator genotypes or among NAT2 genetic variants were tested for significance by one-way ANOVA followed by Tukey-Kramer multiple comparisons tests. Student’s t-test was used to analyze the significance of differences between two groups. All statistical analyses were performed using GraphPad Prism v6.0c (GraphPad Software, La Jolla, CA, USA). The results are expressed as the mean ± the standard error of the mean (SEM). Values of p < 0.05 were considered to be statistically significant.
Results
MDA N-acetyltransferase activity catalyzed by Human Recombinant NAT1 and NAT2
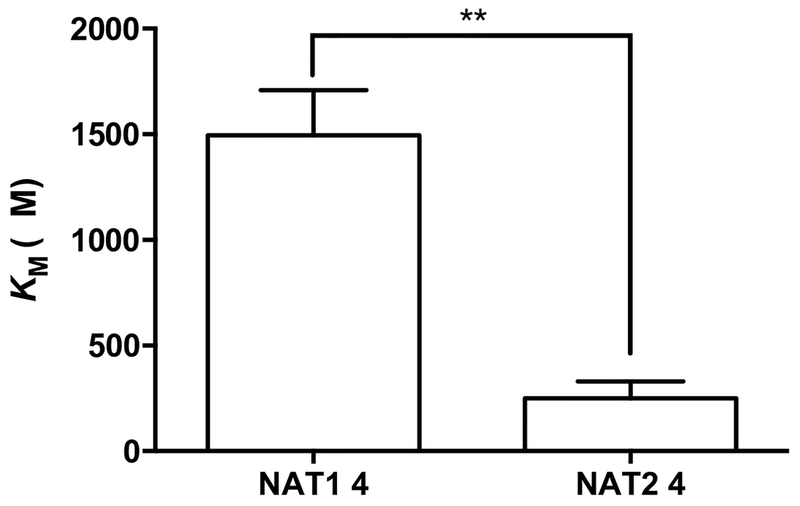
Human NAT1*4 and NAT2*4 were recombinantly expressed in E. coli. As shown in Fig. 2, MDA exhibited a 6-fold lower (p < 0.01) apparent KM toward recombinant human NAT2 (251 ±80 μM) than recombinant human NAT1 (1496 ±213 μM).
Fig. 2. Apparent Km of MDA towards recombinant human NAT1 4 and NAT2 4.
Each bar illustrates the mean ± SEM of MDA apparent KM from three individual determinations carried out at a fixed concentration of 1000 μM AcCoA. MDA exhibited a 6-fold lower (**p < 0.01) apparent KM toward recombinant human NAT2 4 (251 ±80 μM) than recombinant human NAT1 4 (1496 ±213 μM).
MDA N-acetyltransferase activity catalyzed by Human Recombinant NAT2 variants
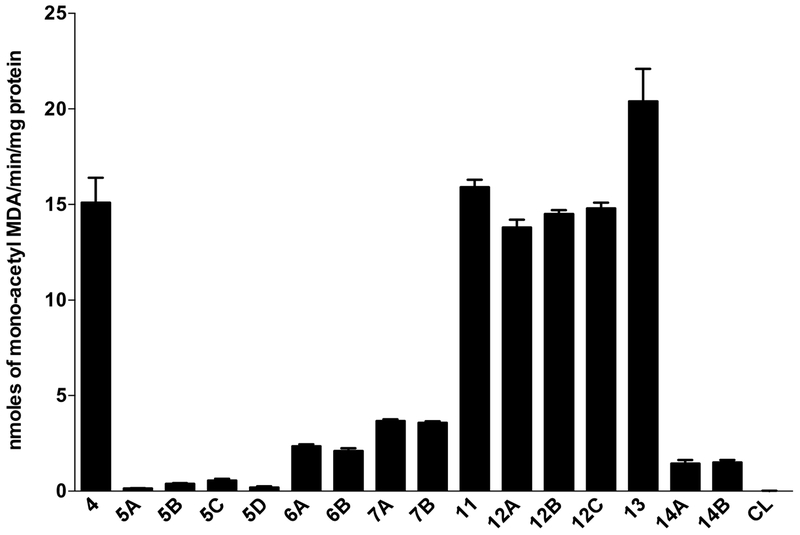
Human NAT2*4 and 15 NAT2 genetic variant alleles possessing different SNPs were recombinantly expressed in E. coli to assess catalytic activities towards the N-acetylation of MDA. Human NAT2 5, NAT2 6, NAT2 7 and NAT2 14 clusters catalyzed MDA N-acetylation at rates significantly (p < 0.05) lower than human NAT2 11, NAT2 12, NAT2 13 and NAT2 4 clusters (Fig. 3).
Fig. 3. N-acetylation of MDA by recombinant human NAT2 allozymes.
Each bar illustrates the mean ± SEM of MDA N-acetyltransferase activity from three individual determinations carried out at a fixed concentration of 1000 μM AcCoA. Human NAT2 allozymes associated with slow acetylators (NAT2 5, NAT2 6, NAT2 7 and NAT2 14) showed markedly lower MDA N-acetyltransferase activities than reference NAT2 4 and other genetic variants (NAT2 11, NAT2 12, and NAT2 13) associated with rapid acetylators.
MDA N-acetyltransferase activity in human cryopreserved human hepatocytes
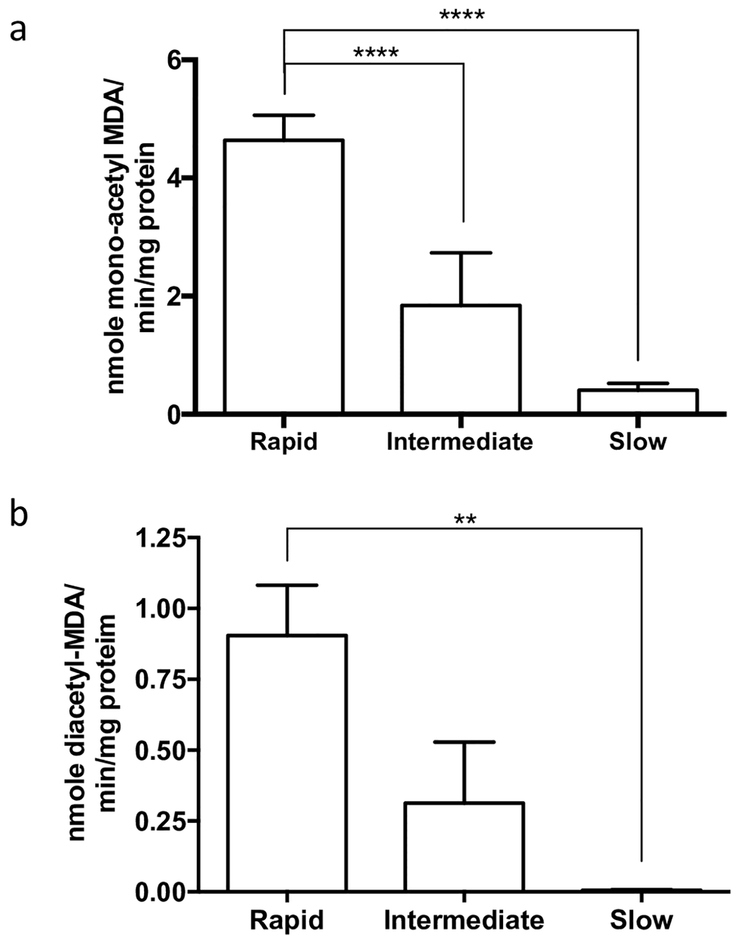
MDA N-acetylation to mono-acetyl MDA and N-acetylation of mono-acetyl MDA to diacetyl MDA exhibited a gene dose-response with highest levels in homozygous rapid acetylator, lower levels in heterozygous intermediate acetylator, and lowest levels in homozygous slow acetylator cryopreserved human hepatocytes (Fig. 4).
Fig. 4. N-acetylation of MDA in vitro in cryopreserved human hepatocytes.
Each bar illustrates the mean ± SEM of MDA N-acetyltransferase activity from three individual determinations carried out at a fixed concentration of 1000 μM AcCoA. a. MDA N-acetylation to mono-acetyl MDA differed significantly among the rapid, intermediate, and slow acetylator cryopreserved human hepatocytes (ANOVA p < 0.0001). b. N-acetyl-MDA N-acetylation to diacetyl-MDA differed significantly between rapid and slow acetylator cryopreserved hepatocytes (ANOVA p = 0.006). **p < 0.01, ****p < 0.0001
Damage induced by MDA in Cryopreserved Human Hepatocytes
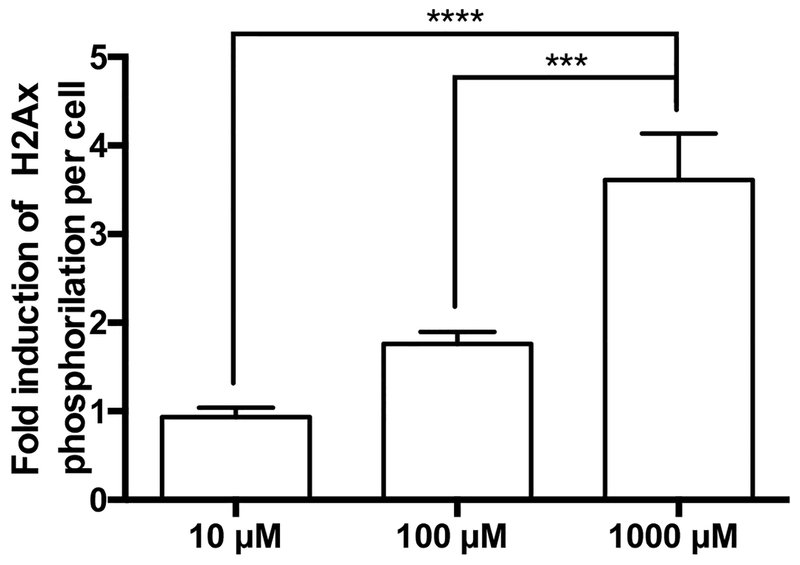
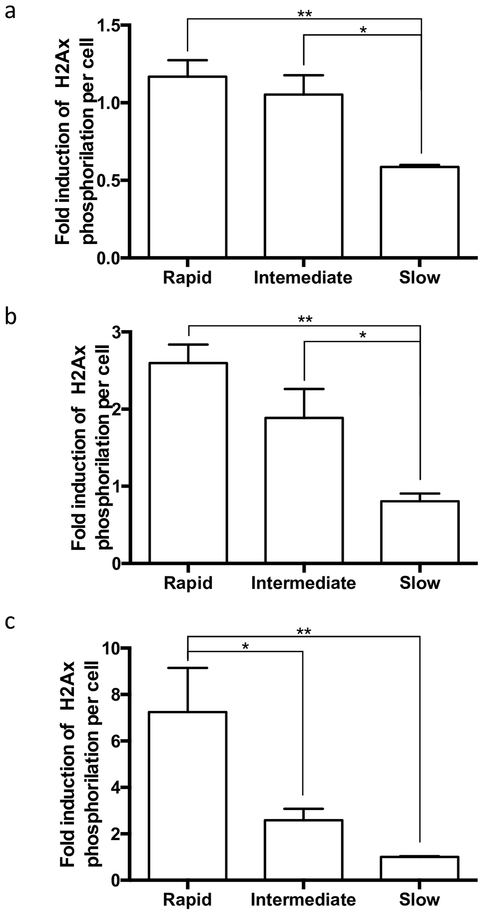
Cryoplatable human hepatocytes previously genotyped were incubated with increasing concentrations of MDA to assess the dose dependency of MDA-induced DNA damage. Following the quantification of the γH2AX (green) and the DNA (red) signals, fold induction of γH2AX per cell was calculated in MDA-treated cells versus untreated cells. H2AX phosphorylation induced by MDA was dose-dependent at concentrations up to 1000 μM with significantly (p < 0.0001, Fig. 5) higher induction observed at higher MDA concentrations. MDA-induced DNA damage was assessed at multiple doses in rapid, intermediate, and slow acetylator hepatocytes. At 10 μM MDA, rapid acetylator hepatocytes showed twice the DNA damage compared to the slow acetylator hepatocytes (p = 0.004; Fig. 6a), this observation was similar for the intermediate vs. slow acetylator hepatocytes (p = 0.036). At the 100 μM MDA concentration, DNA damage was 3-fold higher in the rapid vs slow acetylator hepatocytes (p = 0.003) and 2-fold higher in intermediate versus slow acetylator hepatocytes (p = 0.089; Fig. 6b). Finally, at 1 mM MDA (Fig. 6c), DNA damage induction in rapid acetylator hepatocytes was 3-fold higher (p = 0.027) than intermediate acetylator and 7-fold higher (p = 0.009) than slow acetylator hepatocytes.
Fig. 5. DNA damage in cryopreserved human hepatocytes by MDA.
Each bar illustrates the mean ± SEM of MDA induction of γH2Ax from three individual determinations performed in NAT2 rapid acetylator cryoplateable hepatocytes carried out at concentrations up to 1000 μM MDA. H2Ax phosphorylation induced by MDA was dose-dependent at concentrations up to 1000 μM (ANOVA p < 0.0001) ***p < 0.001, ****p < 0.0001.
Fig. 6. Genotype-dependent DNA damage in cryopreserved human hepatocytes by MDA.
Each bar illustrates mean ± SEM of MDA induction of γH2Ax from four individual determinations performed in rapid, intermediate and slow N-acetylation cryoplateable hepatocytes carried out at concentrations up to 1000 μM MDA a. Cryoplateable human hepatocytes were incubated with 10 μM MDA, rapid and intermediate acetylator hepatocytes showed significantly increased γH2Ax induction, compared to the slow acetylator hepatocytes (ANOVA p = 0.0042). b. Cryoplateable human hepatocytes were incubated with 100 μM MDA, rapid and intermediate acetylator hepatocytes showed increased γH2Ax induction, compared to the slow acetylator hepatocytes (ANOVA p = 0.0032). c. Cryoplateable human hepatocytes were incubated with 1000 μM MDA, rapid acetylator hepatocytes showed increased γH2Ax induction, compared to the intermediate and slow acetylator hepatocytes (ANOVA p = 0.0094) *p < 0.05, **p < 0.01.
Discussion
Because of widespread use and large-scale production of both MDA and MDI, the potential for human exposure to MDA is significant (Castelain et al. 2018; Schupp et al. 2018). Short-term oral administration of MDA to rats causes necrotizing cholangitis with periportal necrosis (Bailie et al. 1993; Gohlke and Schmidt 1974), subchronic exposure elicits inflammatory cell infiltrate, severe bile duct proliferation, portal fibrosis, and cirrhosis (Fukushima et al. 1979), and long-term exposure is associated with liver tumors and hepatic lesions consisting of midzonal focal necrosis (Schoental 1968). MDA is carcinogenic in both rats and mice with primary tumor sites in the thyroid and liver (National Toxicology 1983; National Toxicology 2011). Liver damage was also observed in humans after exposure to MDA (Giouleme et al. 2011).
A previous report (Dalene et al. 1996) measured MDA in hydrolyzed human plasma and urine, and whether these levels were modified by NAT2 genotype. Blood and urine samples were drawn from 30-pipe-layers who had been welding polyurethane insulated pipes during the preceding 3 months. MDA in plasma was detected in 18 of the 30 pipe-layers with plasma concentrations ranging from 0.05 to 8.48 μg/l that did not correlate with NAT2 genotype. In the present study, we demonstrated higher affinity of MDA for human NAT2 than NAT1 and differences in MDA N-acetylation among recombinant human NAT2 genetic variants. We also clearly showed that polymorphism in human NAT2 modifies MDA N-acetylation and genotoxic damage in a human hepatocyte model at concentrations ranging from 10 to 1000 μM.
MDA can be metabolized by the P450 system and by peroxidases. The first step of the P450 metabolism involves N-oxidation to N-hydroxy-MDA, mainly by the hepatic 1A2 isoenzyme (Guengerich and Shimada 1991). The resulting metabolites can react with cellular thiol groups of proteins, resulting in albumin and hemoglobin adducts. Other route of MDA metabolism include N- and O-acetylation by N-acetyltransferases and conjugation by UDP-glucuronosyltransferases and sulfotransferases (Kautiainen et al. 1998). Highly variable levels of N-acetyl-MDA are detected in the urine and hemoglobin adducts derived from N-acetyl-MDA are detected; however, the metabolites directly responsible for the hepatotoxicity remain unclear.
In humans, elimination of MDA or MDI takes place mainly via the urine, the three compounds found are N-acetyl-MDA > MDA > N, N’-diacetyl-MDA, in order of abundance (Dalene et al. 1996; Robert et al. 1995; Schutze et al. 1995). Thus, we hypothesized that NAT2 acetylation polymorphism would affect MDA metabolism and genotoxicity. Variation in N-acetyltransferase activity in the human population divides humans into rapid, intermediate and slow acetylator phenotypes (Hein 2002). Since humans express both, hepatic NAT1 and NAT2 studying their relative ability to catalyze MDA N-acetylation is required to understand the role of NAT2 genetic polymorphisms in MDA and MDI toxicity. .For example, the N-acetylation of benzidine is selectively catalyzed by human NAT1 (Zenser et al., 1996) whereas the N-acetylation of 4, 4’-methylene bis (2-chloroaniline) is selectively catalyzed by human NAT2 (Hein et al., 2018). We found that N-acetylation of MDA is selectively catalyzed by human NAT2, given its lower KM, compared to human NAT1 a finding consistent with previous findings for aromatic amine carcinogens such as 4-aminobiphenyl (Hein et al. 1993).
Human NAT2 genotype and phenotype relationship are well established in previous studies (Hein 2002), and it is documented as a gene dosage relationship. NAT2*4 allele is the reference allele with high activity. We found that the NAT2*5 cluster (all possessing T341C), NAT2*14 cluster (all possessing G191A), NAT2*6 cluster (all possessing G590A) and the NAT2*7 cluster (all possessing G857A) showed reductions in N-acetylation of MDA, similar to previous findings for the N-acetylation of mono-arylamines such as 4-aminobiphenyl (Hein et al., 1995) and di-arylamines such as 4, 4’-methylene bis (2-chloroaniline) (Hein et al., 2018). The other variant NAT2 alleles did not differ significantly from reference NAT2*4. The results suggest that the individual’s susceptibility to genotoxicity or toxicity is modified by human NAT2 genotype. MDA hepatotoxicity was higher in rapid than slow acetylator rats (Zhang et al. 2006). Rapid and slow acetylator rats differ in the catalytic activity of rat Nat2, (Hein et al. 1991a; Hein et al. 1991b); an ortholog of human NAT1 (Hein et al., 1997). Although N-acetylation of MDA is catalyzed by both rat Nat1 and rat Nat2 (Zhang et al., 2006), the relative affinities of rat Nat1 and Nat2 for MDA is unknown.
Since MDA or MDI exposure is highly variable between subjects, and previous data shows that NAT2 N-acetylation polymorphism affects hepatotoxicity in rats; we tested our hypothesis in cryopreserved human hepatocytes with a known N-acetylation polymorphism. Our data shows a genotype-dependent relationship for the N-acetylation of MDA and N-acetyl-MDA. The abundance of N-acetyl-MDA is considerably higher than N, N’-diacetyl-MDA. Previous reports show N-acetylation to increase and decrease toxicities of different xenobiotic compounds (Hein 2002). MDA possesses two amino groups subject to N-acetylation, the N-acetylation of one of them may enhance metabolic activation of the second amino group. One example of this phenomenon is the effect of aromatic amines to induce urinary bladder cancer. For mono-arylamines, N-acetylation competes with N-oxidation, resulting in an increased risk of urinary bladder cancer in NAT2 slow acetylation phenotype subjects, particularly in European and Caucasian populations with documented aromatic amine exposures (Golka et al., 2002; Garcia-Closas et al. 2005; Rothman et al., 2007; Moore et al., 2011). On the other hand, in the case of di-arylamines N-acetylation does not compete and very likely enhances the oxidation of the second amine, increasing the risk for NAT2 rapid acetylators (Carreon et al. 2006). Workers exposed to benzidine show relatively high levels of N-acetyl-benzidine in the urine, but extremely low levels of N, N’-diacetyl-benzidine (Hsu et al. 1996; Rothman et al. 1996). DNA adducts derived from N-acetyl-benzidine, suggesting that N-acetylation is an activation pathway for benzidine.
Several reports have established the genotoxic effect of MDA exposure through the formation of DNA adducts (Gries and Leng 2013; Kautiainen et al. 1998; Schutze et al. 1996). As a bifunctional electrophile, MDA has the potential to form DNA-DNA crosslinks which could result in DNA double-strand breaks (DSB) upon repair. Previous studies described DSB formation from MDI (Vock et al. 1998) and MDA in human white blood cells (Marczynski et al. 1992) and human hepatocytes (Martelli et al. 2002), where the authors described DNA damage in concentrations up to 180 μM using a comet assay. We exposed cryoplateable human hepatocytes to increasing concentrations of MDA and then assessed the genotoxic damage using γH2AX in-cell western assays which have been demonstrated to be more sensitive than Hprt mutation assays for this purpose (Chevereau et al. 2017). Our data shows dose-dependent genotoxic damage in the presence of MDA in concentrations from 10 to 1000 μM; this finding is consistent with previous studies (Martelli et al. 2002). Very interestingly, this genotoxic damage is 2-fold to 7-fold higher, depending on the dose, in the rapid N-acetylation phenotype hepatocytes compared to the slow N-acetylation samples. This is also consistent with previous findings with benzidine (Carreon et al. 2006), suggesting the N-acetylation of MDA acts as an activation pathway.
In summary, we found that N-acetylation of MDA is modified by the NAT2 acetylation polymorphism which affects genotoxic damage caused by the activation of MDA. Since the NAT2 acetylation polymorphism is widely distributed in human populations and the risk of MDA or MDI exposure is relatively high in the workplace, further studies on NAT2 gene-environment interactions are warranted.
Acknowledgments
We appreciate our collaboration with BioIVT in providing cryoplateable human hepatocytes. The research was supported in part by USPHS grants P20-GM113226 and P42-ES023716. The authors gratefully acknowledge the contribution of the Erasmus+ International Credit Mobility programme for faculty and student exchanges funded by the European Union. A preliminary report of this work was presented at the annual meeting of the Society of Toxicology, Baltimore, Maryland, March 2019.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- Agency for Toxic Substances and Disease Registry (1998) Toxicological Profile for 4,4’-methylenedianiline. U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA [Google Scholar]
- Bailie MB, Mullaney TP, Roth RA (1993) Characterization of acute 4,4’-methylene dianiline hepatotoxicity in the rat. Environ Health Perspect 101(2):130–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian PG (1984) Occupational hepatitis caused by methylenedianiline. Med J Aust 141(8):533–5 [DOI] [PubMed] [Google Scholar]
- Brooks LJ, Neale JM, Pieroni DR (1979) Acute myocardiopathy following tripathway exposure to methylenedianiline. JAMA : the journal of the American Medical Association 242(14):1527–8 [PubMed] [Google Scholar]
- Carreon T, Ruder AM, Schulte PA, et al. (2006) NAT2 slow acetylation and bladder cancer in workers exposed to benzidine. Int J Cancer 118(1):161–168 [DOI] [PubMed] [Google Scholar]
- Carvajal-Diaz J (2015) IHS chemical economics handbook: aniline. IHS, Englewood [Google Scholar]
- Castelain F, Girardin P, Penven E, Pelletier F (2018) Occupational contact dermatitis caused by polyurethane foam: 6 cases. Contact Dermatitis 79(1):52–54 [DOI] [PubMed] [Google Scholar]
- Chevereau M, Glatt H, Zalko D, Cravedi JP, Audebert M (2017) Role of human sulfotransferase 1A1 and N-acetyltransferase 2 in the metabolic activation of 16 heterocyclic amines and related heterocyclics to genotoxicants in recombinant V79 cells. Arch Toxicol 91(9):3175–3184 [DOI] [PubMed] [Google Scholar]
- Cocker J, Boobis AR, Davies DS (1988) Determination of the N-acetyl metabolites of 4,4’-methylene dianiline and 4,4’-methylene-bis(2-chloroaniline) in urine. Biomedical & environmental mass spectrometry 17(3):161–7 [DOI] [PubMed] [Google Scholar]
- Dalene M, Jakobsson K, Rannug A, Skarping G, Hagmar L (1996) MDA in plasma as a biomarker of exposure to pyrolysed MDI-based polyurethane: correlations with estimated cumulative dose and genotype for N-acetylation. Int Arch Occup Environ Health 68(3):165–9 [DOI] [PubMed] [Google Scholar]
- Doll MA, Hein DW (2001) Comprehensive human NAT2 genotype method using single nucleotide polymorphism-specific polymerase chain reaction primers and fluorogenic probes. Anal Biochem 288(1):106–8 [DOI] [PubMed] [Google Scholar]
- Dunn G, Guirguis S (1979) pp’ - Methylene dianiline (MDA) as an occupational health problem a suggested time-weighted average exposure level and medical program. Archives of Industrial Hygene and Toxicology 30:639–645 [Google Scholar]
- Fukushima S, Shibata M, Hibino T, Yoshimura T, Hirose M, Ito N (1979) Intrahepatic bile duct proliferation induced by 4,4’-diaminodiphenylmethane in rats. Toxicol Appl Pharmacol 48(1 Pt 1):145–55 [DOI] [PubMed] [Google Scholar]
- Garcia-Closas M, Malats N, Silverman D, et al. (2005) NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet 366(9486):649–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giouleme O, Karabatsou S, Hytiroglou P, et al. (2011) 4,4’-Methylenedianiline-induced hepatitis in an industrial worker: case report and review of the literature. Hum Exp Toxicol 30(7):762–7 [DOI] [PubMed] [Google Scholar]
- Gohlke R, Schmidt P (1974) [4,4’-Diaminodiphenylmethane--histological, enzyme histochemical and autoradiographic investigations in acute and subacute experiments in rats, with and without the additional stress of heat (author’s transl)]. Int Arch Arbeitsmed 32(3):217–31 [PubMed] [Google Scholar]
- Golka K, Prior V, Blaszkewicz M, Bolt HM (2002) The enhanced bladder cancer susceptibility of NAT2 slow acetylators towards aromatic amines: a review considering ethnic differences. Toxicol Lett 128(1–3):229–41 [DOI] [PubMed] [Google Scholar]
- Gries W, Leng G (2013) Analytical determination of specific 4,4’-methylene diphenyl diisocyanate hemoglobin adducts in human blood. Anal Bioanal Chem 405(23):7205–13 [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Shimada T (1991) Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzymes. Chemical research in toxicology 4(4):391–407 [DOI] [PubMed] [Google Scholar]
- Hamada H, Bruze M, Zimerson E, Isaksson M, Engfeldt M (2017) Sensitization and cross-reactivity patterns of contact allergy to diisocyanates and corresponding amines: investigation of diphenylmethane-4,4’-diisocyanate, diphenylmethane-4,4’-diamine, dicyclohexylmethane-4,4’-diisocyanate, and dicylohexylmethane-4,4’-diamine. Contact Dermatitis 77(4):231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert VY, Jones BC, Mifflin RC, Dugas TR (2011) Role of COX-2 in the bioactivation of methylenedianiline and in its proliferative effects in vascular smooth muscle cells. Cardiovasc Toxicol 11(4):316–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein DW (2002) Molecular genetics and function of NAT1 and NAT2: role in aromatic amine metabolism and carcinogenesis. Mutat Res 506–507: 65–77 [DOI] [PubMed] [Google Scholar]
- Hein DW, Doll MA, Fretland AJ, et al. (1997) Rodent models of the human acetylation polymorphism: comparisons of recombinant acetyltransferases. Mutat Res 376(1–2):101–6 [DOI] [PubMed] [Google Scholar]
- Hein DW, Doll MA, Rustan TD, Ferguson RJ (1995) Metabolic activation of N-hydroxyarylamines and N-hydroxyarylamides by 16 recombinant human NAT2 allozymes: effects of 7 specific NAT2 nucleic acid substitutions. Cancer Res 55(16):3531–6 [PubMed] [Google Scholar]
- Hein DW, Doll MA, Rustan TD, et al. (1993) Metabolic activation and deactivation of arylamine carcinogens by recombinant human NAT1 and polymorphic NAT2 acetyltransferases. Carcinogenesis 14(8):1633–8 [DOI] [PubMed] [Google Scholar]
- Hein DW, Ferguson RJ, Doll MA, Rustan TD, Gray K (1994) Molecular genetics of human polymorphic N-acetyltransferase: enzymatic analysis of 15 recombinant wild-type, mutant, and chimeric NAT2 allozymes. Hum Mol Genet 3(5):729–34 [DOI] [PubMed] [Google Scholar]
- Hein DW, McQueen CA, Grant DM, Goodfellow GH, Kadlubar FF, Weber WW (2000) Pharmacogenetics of the arylamine N-acetyltransferases: a symposium in honor of Wendell W. Weber. Drug Metab Dispos 28(12):1425–32 [PubMed] [Google Scholar]
- Hein DW, Rustan TD, Bucher KD, Furman EJ, Martin WJ (1991a) Extrahepatic expression of the N-acetylation polymorphism toward arylamine carcinogens in tumor target organs of an inbred rat model. J Pharmacol Exp Ther 258(1):232–6 [PubMed] [Google Scholar]
- Hein DW, Rustan TD, Bucher KD, Martin WJ, Furman EJ (1991b) Acetylator phenotype-dependent and -independent expression of arylamine N-acetyltransferase isozymes in rapid and slow acetylator inbred rat liver. Drug Metab Dispos 19(5):933–7 [PubMed] [Google Scholar]
- Hein DW, Zhang X, Doll MA (2018) Role of N-acetyltransferase 2 acetylation polymorphism in 4, 4’-methylene bis (2-chloroaniline) biotransformation. Toxicol Lett 283:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FF, Lakshmi V, Rothman N, et al. (1996) Determination of benzidine, N-acetylbenzidine, and N,N’-diacetylbenzidine in human urine by capillary gas chromatography/negative ion chemical ionization mass spectrometry. Anal Biochem 234(2):183–9 [DOI] [PubMed] [Google Scholar]
- Kaaria K, Hirvonen A, Norppa H, Piirila P, Vainio H, Rosenberg C (2001) Exposure to 4,4’-methylenediphenyl diisocyanate (MDI) during moulding of rigid polyurethane foam: determination of airborne MDI and urinary 4,4’-methylenedianiline (MDA). The Analyst 126(4):476–9 [DOI] [PubMed] [Google Scholar]
- Kautiainen A, Wachtmeister CA, Ehrenberg L (1998) Characterization of hemoglobin adducts from a 4, 4’-methylenedianiline metabolite evidently produced by peroxidative oxidation in vivo. Chemical research in toxicology 11(6):614–21 [DOI] [PubMed] [Google Scholar]
- LeVine MJ (1983) Occupational photosensitivity to diaminodiphenylmethane. Contact Dermatitis 9(6):488–90 [DOI] [PubMed] [Google Scholar]
- Marczynski B, Czuppon AB, Hoffarth HP, Marek W, Baur X (1992) DNA damage in human white blood cells after inhalative exposure to methylenediphenyl diisocyanate (MDI)--case report. Toxicology letters 60(2):131–8 [DOI] [PubMed] [Google Scholar]
- Martelli A, Carrozzino R, Mattioli F, Brambilla G (2002) DNA damage induced by 4,4’-methylenedianiline in primary cultures of hepatocytes and thyreocytes from rats and humans. Toxicology and applied pharmacology 182(3):219–25 [DOI] [PubMed] [Google Scholar]
- McGill DB, Motto JD (1974) An industrial outbreak of toxic hepatitis due to methylenedianiline. N Engl J Med 291(6):278–82 [DOI] [PubMed] [Google Scholar]
- McQueen CA, Williams GM (1990) Review of the genotoxicity and carcinogenicity of 4,4’-methylene-dianiline and 4,4’-methylene-bis-2-chloroaniline. Mutat Res 239(2):133–42 [DOI] [PubMed] [Google Scholar]
- Moore LE, Baris DR, Figueroa JD, et al. (2011) GSTM1 null and NAT2 slow acetylation genotypes, smoking intensity and bladder cancer risk: results from the New England bladder cancer study and NAT2 meta-analysis. Carcinogenesis 32(2):182–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program (1983) NTP Carcinogenesis Studies of 4,4’-Methylenedianiline Dihydrochloride (CAS No. 13552-44-8) in F344/N Rats and B6C3F1 Mice (Drinking Water Studies). Natl Toxicol Program Tech Rep Ser 248:1–182 [PubMed] [Google Scholar]
- National Toxicology Program (2011) 4,4’Methylenedianiline and its dihydrochloride. Rep Carcinog 12:265–7 [PubMed] [Google Scholar]
- National Toxicology Program (2016) Report on Carcinogens, Fourteenth Edition. Research Triangle Park NC: U.S. Department of Health and Human Services, Public Health Service. https://ntp.niehs.nih.gov/go/roc14 [Google Scholar]
- Nichols L (2004) The Epping Jaundice outbreak: mortality after 38 years of follow-up. Int Arch Occup Environ Health 77(8):592–4 [DOI] [PubMed] [Google Scholar]
- OSHA (1992). Occupational exposure to 4/4’-methylenedianiline (MDA) (1910.1050); Final rule. Fed Regist 57: 35630 [Google Scholar]
- Page B, Page M, Noel C (1993) A new fluorometric assay for cytotoxicity measurements in-vitro. Int J Oncol 3(3):473–6 [PubMed] [Google Scholar]
- Robert A, Ducos P, Francin JM (1995) Determination of urinary 4,4’-methylenedianiline and its acetylated metabolites by solid-phase extraction and HPLC analysis with UV and electrochemical detection. Int Arch Occup Environ Health 68(1):44–51 [DOI] [PubMed] [Google Scholar]
- Rothman N, Bhatnagar VK, Hayes RB, et al. (1996) The impact of interindividual variation in NAT2 activity on benzidine urinary metabolites and urothelial DNA adducts in exposed workers. Proc Natl Acad Sci USA 93(10):5084–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman N, Garcia-Closas M, Hein DW (2007) Commentary: Reflections on G. M. Lower and colleagues’ 1979 study associating slow acetylator phenotype with urinary bladder cancer: meta-analysis, historical refinements of the hypothesis, and lessons learned. Int J Epidemiol 36(1):23–8 [DOI] [PubMed] [Google Scholar]
- Schoental R (1968) Pathological lesions, including tumors, in rats after 4,4’-diaminodiphenylmethane and gamma-butyrolactone. Isr J Med Sci 4(6):1146–58 [PubMed] [Google Scholar]
- Schupp T, Allmendinger H, Boegi C, et al. (2018) The Environmental Behavior of Methylene-4,4’-dianiline. Rev Environ Contam Toxicol doi: 10.1007/398_2018_13 [DOI] [PubMed] [Google Scholar]
- Schutze D, Sagelsdorff P, Sepai O, Sabbioni G (1996) Synthesis and quantification of DNA adducts of 4,4’-methylenedianiline. Chemical research in toxicology 9(7):1103–12 [DOI] [PubMed] [Google Scholar]
- Schutze D, Sepai O, Lewalter J, Miksche L, Henschler D, Sabbioni G (1995) Biomonitoring of workers exposed to 4,4’-methylenedianiline or 4,4’-methylenediphenyl diisocyanate. Carcinogenesis 16(3):573–82 [DOI] [PubMed] [Google Scholar]
- Sepai O, Henschler D, Sabbioni G (1995a) Albumin adducts, hemoglobin adducts and urinary metabolites in workers exposed to 4,4’-methylenediphenyl diisocyanate. Carcinogenesis 16(10):2583–7 [DOI] [PubMed] [Google Scholar]
- Sepai O, Schutze D, Heinrich U, Hoymann HG, Henschler D, Sabbioni G (1995b) Hemoglobin adducts and urine metabolites of 4,4’-methylenedianiline after 4,4’-methylenediphenyl diisocyanate exposure of rats. Chemico-biological interactions 97(2):185–98 [DOI] [PubMed] [Google Scholar]
- Shintani H, Nakamura A (1989) Analysis of a carcinogen, 4,4’-methylenedianiline, from thermosetting polyurethane during sterilization. J Anal Toxicol 13(6):354–7 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ino T, Sawahata T, Marui S, Igaki H, Yashima H (1985) Mutagenicity of N-acetyl and N,N’-diacetyl derivatives of 3 aromatic amines used as epoxy-resin hardeners. Mutat Res 143(1–2):11–5 [DOI] [PubMed] [Google Scholar]
- Vock EH, Vamvakas S, Gahlmann R, Lutz WK (1998) Investigation of the induction of DNA double-strand breaks by methylenediphenyl-4–4’-diisocyanate in cultured human lung epithelial cells. Toxicological sciences 46(1):83–9 [DOI] [PubMed] [Google Scholar]
- Zenser TV, Lakshmi VM, Rustan TD, et al. (1996) Human N-acetylation of benzidine: role of NAT1 and NAT2. Cancer Res 56(17):3941–7 [PubMed] [Google Scholar]
- Zhang X, Lambert JC, Doll MA, Walraven JM, Arteel GE, Hein DW (2006) 4,4’-methylenedianiline-induced hepatotoxicity is modified by N-acetyltransferase 2 (NAT2) acetylator polymorphism in the rat. J Pharmacol Exp Ther 316(1):289–94 [DOI] [PubMed] [Google Scholar]
- Zuliani F, Prodi A, Fortina AB, Corradin MT, Bovenzi M, Filon FL (2017) Diaminodiphenylmethane Sensitization in north-eastern Italy from 1996 to 2012. J Eur Acad Dermatol Venereol 31(5):833–836 [DOI] [PubMed] [Google Scholar]