Abstract
Background
Multiple myeloma (MM) usually follows a clinical course leading to refractoriness and limited treatment options in advanced stages which may need bridge therapies to either autologous stem cell transplant or novel therapies. We report our experience with the high-dose chemotherapy mCBAD regimen in newly diagnosed (NDMM), relapsed/refractory myeloma (RRMM) and plasma cell leukemia (PCL) patients.
Methods
We searched our electronic records database for MM patients who received mCBAD from 2010–2016 – 28 day cycles of cyclophosphamide 350 mg/m2 IV twice daily + mesna 400mg/m2 IV daily (days 1–4), bortezomib 1.3 mg/m2 SQ/I.V. (days 1, 4, 8, 11), doxorubicin 9 mg/m2 daily continuous infusion (days 1–4) , dexamethasone 40 mg PO daily (on days 1–4, 9–12, 17–20). IMWG criteria were used for response assessment and diagnosis. Descriptive statistics, Fisher’s exact test, Chi-square, Wilcoxon rank sum, and Kaplan-Meier were used for statistical purposes.
Results
One hundred and forty patients met the inclusion criteria. A median of two cycles of therapy was administered. The overall response rate was 85% in patients with RRMM (n=116) and 100% in both NDMM (n=13) and plasma cell leukemia (n=11) patients. Respective median progression-free survival (mPFS) for NDMM, PCL, and RRMM were 19.61 months (95% CI, 5.26 - NA ), 7.56 months (95% CI, 4.7 -NA ), and 4.64 months (95% CI, 3.75 – 6.73). Patients with RRMM who used mCBAD as a bridge to autologous transplant (36.2%) had mPFS (11.48 months; 95% CI: 7.52 – 15.9 months) when compared to those who did not (mPFS: 3.19 months; 95% CI: 2.4 ~ 3.75 months). Cytopenias occurred in more than 90% of patients, and febrile neutropenia was noted in 26 %. All cases of treatment-related mortality (8%) occurred in patients with RRMM, except for one patient with PCL.
Conclusion
mCBAD results in high response rates in myeloma and PCL, however with high treatment-related mortality. Its use in RRMM should be limited to patients who have immediate need for therapy without other treatment options who have good PS (PS 0–1) or NDMM if novel agents are not available depending on practice setting. mCBAD can be a treatment option for patients with PCL.
Keywords: bortezomib, cyclophosphamide, doxorubicin, myeloma, mCBAD, plasma cell leukemia
Microabstract
We present retrospective data on 140 patients treated with high dose cyclophosphamide, bortezomib, doxorubicin and dexamethasone (mCBAD) at our institution. More than 80% of patients received ≤ 2 cycles mCBAD with best overall response ≥ 90% (very good partial response 18–23% and complete remission 7–23%). Median overall survival from start date of mCBAD and treatment-related mortality were 14 months for relapsed patients - 8% (10/116) (n=116), 16 months for plasma cell leukemia – 9% (1/11) (n=11) and 35 months for newly diagnosed myeloma −0% (n=13). mCBAD response is short-lived and has high treatment-related mortality. It may be useful as a bridge to transplantation or cell therapies when other treatments are not available.
Introduction
Over the past decade, survival of patients with multiple myeloma (MM) has drastically improved through the use of highly effective combination therapies - incorporating proteasome inhibitors (PIs), immunomodulatory agents (IMiDs), histone deacetylase inhibitors (HDACi) and, more recently, monoclonal antibodies - along with consolidation therapy and maintenance strategies1,2,3. Nevertheless, to this day, MM remains an incurable malignancy, in most patients, characterized by a course of unrelenting relapses4. The choice of salvage therapy remains rather broad in this context, considering the availability of numerous agents belonging to more than six different classes of therapeutic agents4. Despite this, some patients with relapsed/refractory MM (RRMM) need immediate control of disease due to end-organ damage5,6. Similarly, some patients newly diagnosed MM (NDMM) and those with plasma cell leukemia (PCL) may be managed with high-dose chemotherapy initially, especially if no other options are available. VDT PACE (bortezomib, dexamethasone, thalidomide with the infusion of cisplatin, doxorubicin, cyclophosphamide, and etoposide) and VDT PACE-like combination regimens have been used outside of the Arkansas Total Therapy trials/approaches7,8.
Hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD) was a chemotherapy regimen that showed a response rate of 40% and a median overall survival (mOS) of 15 months more than 20 years ago5,9. The emergence of bortezomib both in the upfront and relapsed/refractory setting, prompted our group into adding this agent to the Hyper-CVAD regimen10,11. However, due to the overlapping peripheral neuropathy, vincristine was omitted. Therefore, the regimen commonly referred to as modified CBAD (mCBAD) consists of hyper-fractionated cyclophosphamide, bortezomib, doxorubicin, and dexamethasone (table 1).
Table 1.
Details of the mCBAD (modified Cyclophosphamide, Bortezomib, Doxorubicin, and Dexamethasone) regimen - 28-day cycle.
| Cyclophosphamide | 350 mg/m2 IV every 12 hours, days 1–4 |
| Mesna | 400 mg/m2 IV every 12 hours, days 1–4 |
| Bortezomib | 1.3 mg/m2 sc/IV* days 1,4,8,11 |
| Doxorubicin | 9 mg/m2 IV continuous infusion, days 1–4 |
| Dexamethasone | 40 mg po daily on days 1–4, 9–12, 17–20 |
IV: intravenous; sc: subcutaneous; po: per os.
prior to 2012, patients received bortezomib intravenously because bortezomib was not approved as subcutaneous injection
The successful use of mCBAD as chemo-mobilization in a small cohort of 27 patients was recently reported12. In this study, we describe the largest cohort to date with this regimen.
Patients and Methods
Patients with NDMM, RRMM, or PCL who received at least one cycle of mCBAD between January 1, 2008, and December 31, 2016, at MD Anderson Cancer Center, Houston, TX were included in this retrospective analysis. Patients were identified in our institutional medical records database if they had a diagnosis of MM or PCL and had received a combination of doxorubicin, cyclophosphamide, bortezomib and dexamethasone, with the exclusion of vincristine at the time that the combination was administered. Individual medical records were subsequently reviewed to ascertain that mCBAD was effectively administered and to collect relevant data for the analysis. NDMM was defined per IWMG criteria13. Relapsed myeloma patients were defined as having had at least one prior line of therapy and refractory myeloma was defined as having progressive disease while on therapy or within 60 days of last therapy. PCL was defined as the presence of plasma cells in the peripheral blood greater than 2 × 109/l or 20% of the total leukocyte count. Patients were included if they received a minimum of one cycle of mCBAD and had at least one follow-up assessment. Patients were excluded if any of the chemotherapy agents were omitted. This study was approved by our Institutional Research Ethics committee.
Treatment regimen
Details of the individual agents comprising the mCBAD regimen, along with dosing schedules and routes of administration, are summarized in table 1. Treatments consist of 28-day cycles administered in an inpatient fashion. All patients received growth factor support, in the form of filgrastrim or pegfilgrastim. Patients also received Pneumocystis jiroveci pneumonia prophylaxis (Trimethoprim/sulfamethoxazole or other agents in case of allergies/intolerance), antiviral prophylaxis (acyclovir, valacyclovir), and anti-bacterial prophylaxis (fluoroquinolone). Patients were subsequently scheduled for twice-weekly blood tests in the outpatient setting to provide adequate transfusion support.
Outcome measures and Statistical Methods
Patient demographics, disease characteristics, treatment, and clinical outcomes were summarized through descriptive statistics. International Myeloma Working Group Uniform Criteria were used to evaluate disease response and progression14,15. The overall response rate (ORR) was defined as the rate of patients achieving a best overall response of complete response (CR), very good partial response (VGPR), or partial response (PR). Progression-free survival (PFS) was calculated from the start date of mCBAD to the date of progression or date of death. Patients alive without progression were censored on the date of the last contact. OS was calculated from the start of mCBAD to the date of death due to any cause; living patients were censored on the date of the last contact. Treatment-related mortality (TRM) was defined as death due to the toxicity of therapy, during or within one month of completion of mCBAD.
The Chi-square test or Fisher’s exact test was used to evaluate the association between two categorical variables. Wilcoxon rank sum test or Kruskal-Wallis test was used to evaluate the difference in a continuous variable between/among patient groups. Kaplan-Meier method was used to estimate the time-to-event endpoints including progression-free survival and overall survival. Median time to event in months with 95% confidence interval was calculated. The log-rank test was used to evaluate the difference in the time-to-event endpoints between/among patient groups. Univariate Cox proportional hazards model was used to evaluate the association between a continuous variable and time-to-event endpoints. Multivariate Cox proportional hazards models were fitted to include significant and important prognostic covariates in evaluating their associations with time-to-event endpoints. Collinearity diagnostics were performed and indicated no collinearity problem. Statistical software SAS 9.3 (SAS, Cary, NC) and S-Plus 8.2 (TIBCO Software Inc., Palo Alto, CA) was used for all the analyses.
Results
Patient characteristics
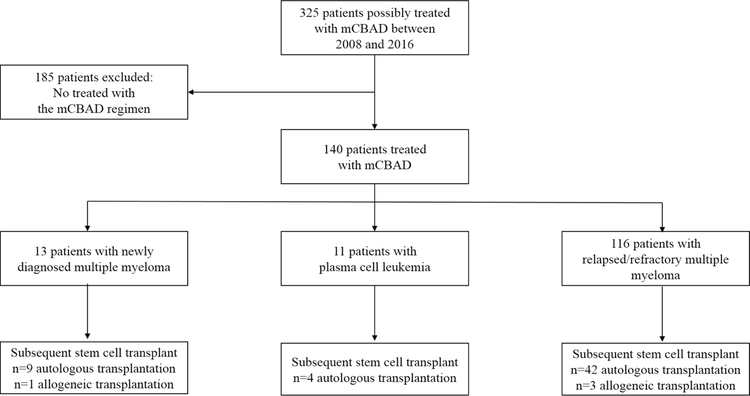
A total of 325 patients who received doxorubicin, cyclophosphamide, dexamethasone and bortezomib were initially identified. After a detailed review of individual medical records, only 140 patients had been treated with the mCBAD regimen and thus were eligible (Figure 1). Patient characteristics are listed in Table 2. Thirteen patients had NDMM, reasons for the use of mCBAD in these patients instead of novel therapies are listed in Table 3. Eleven patients received mCBAD for PCL, and 116 were treated for RRMM. Median age at treatment start time was 56 years (range, 22 – 79). More than half of the patients evaluable for staging (n=57, 51.82%) had an International Staging System (ISS) stage 3 at diagnosis. High-risk cytogenetics (defined as presence of t(4:14), t(14;16) or deletion 17p) were identified via fluorescent in situ hybridization (FISH) in 40 (36%) patients, when this data was available. 49% of patients with RRMM had received at least 3 prior lines of therapy (range, 1–12) which included an autologous stem cell transplant (ASCT) in 56 (48.7%) patients. Two patients had also received prior allogeneic stem cell transplant (Allo-SCT). The majority of patients (81.2%) had a good Eastern Cooperative Oncology Group-performance status (ECOG-PS: 0–1) before receiving mCBAD.
Figure 1.
Study design flow chart.
mCBAD: modified cyclophosphamide, bortezomib, doxorubicin, and dexamethasone
Table 2.
Characteristics and response of patients receiving mCBAD (n = 140).
| Characteristics | NDMM (n=13; 9%) | PCL (n=11; 8%) | RRMM (n=116; 83%) |
|---|---|---|---|
| Gender (male), n(%) | 11 (84.6) | 9 (81.8) | 71 (61.2) |
| Age at diagnosis, median (range), years | 56 (45 – 72) | 62 (36 – 79) | 55 (22 – 79) |
| MM subtype, n(%) | |||
| IgA lambda | 2 (15) | 2 (18) | 14 (12) |
| IgA kappa | 1 (8) | 1 (9) | 18 (15) |
| IgG kappa | 4 (31) | 1 (9) | 45 (39) |
| IgG lambda | 2 (15) | 3 (27) | 22 (19) |
| IgD lambda | 0 | 0 | 1 (0.8) |
| Lambda light chain only | 2 (15) | 2 (18) | 6 (5) |
| Kappa light chain only | 2 (15) | 1 (9) | 10 (9) |
| Non-secretory | 0 | 1 (9) | 0 |
| Disease characteristics at initial diagnosis, n(%) | |||
| Hypercalcemia | 7 (53.8) | 4 (36.4) | 26 (23.6) |
| Lytic bone lesions | 10 (76.9) | 9 (81.8) | 95 (81.9) |
| Anemia | 13 (100) | 10 (90.9) | 82 (74.5) |
| Kidney failure, creatinine ≥ 2 mg/dL | 9 (69.2) | 7 (63.6) | 34 (30.6) |
| Bone marrow plasma cells > 60% | 9 (69.2) | 10 (90.9) | 67 (59.3) |
| ISS stage*, n(%) | |||
| I | 0 | 1 (14.3) | 26 (28.6) |
| II | 2 (16.7) | 0 | 24 (26.4) |
| III | 10 (83.3) | 6 (85.7) | 41 (45.1) |
| FISH studies**, n(%) | |||
| t(4,14) | 1 (8.3) | 1 (11.1) | 9 (10) |
| t(14,16) | 0 | 0 | 2 (2.2) |
| Deletion 17p | 1 (8.3) | 3 (33.3) | 23 (25.6) |
| Number of previous lines of therapy | NA | ||
| 1 | 1 (9.1) | 26 (22.4) | |
| 2 | 1 (9.1) | 33 (28.4) | |
| 3 | 2 (18.2) | 26 (22.4) | |
| ≥ 4 | 0 | 31 (26.7) | |
| Previous ASCT, n(%) | - | 2 (18.2) | 56 (48.7) |
| Number of previous lines of therapy | |||
| < 3 regimen | NA | 2 (50) | 59 (50.7) |
| ≥ 3 regimen | 2 (50) | 57 (49.1) | |
| Number of prior therapies received, (%) | |||
| 0 | 13 (100) | 7 (63.6) | 0 |
| 1 | 0 | 1 (9.1) | 26 (22.4) |
| 2 | 0 | 1 (9.1) | 33 (28.4) |
| 3 | 0 | 2 (18.2) | 26 (22.4) |
| 4 | 0 | 0 | 14 (12.1) |
| 5 | 0 | 0 | 6 (5.2) |
| 6 | 0 | 0 | 4 (3.4) |
| 7 | 0 | 0 | 3 (2.6) |
| 8 | 0 | 0 | 3 (2.6) |
| 12 | 0 | 0 | 1 (0.9) |
| Time from diagnosis to start of mCBAD (months); median (range) | NA | 1 | |
| < 3 regimen | 0.13 and 112*** | 9 (0.33 – 86.3) | |
| ≥ 3 regimen | 9 and 52*** | 46.4 (7.23 – 744.77) | |
| Previous treatment | NA | i | |
| Bortezomib | 4 (36.4) | 109 (94) | |
| Lenalidomide/Thalidomide | 3 (27.3) | 99(85) | |
| Bortezomib plus lenalidomide | 1 (9.1) | 93 (80.2) | |
| Carfilzomib | 0 | 28 (24) | |
| Pomalidomide | 0 | 13 (11.2) | |
| ECOG-PS before mCBAD | i | ||
| 0–1 | 11 (84.6) | 6 (54.5) | 93 (80.1) |
| 2 | 2 (15.4) | 1 (9) | 18 (15.5) |
| 3–4 | 0 | 2 (18.1) | 2 (1.7) |
| Clinical rationale for use of mCBAD in NDMM | NA | NA | |
| High tumor burden with renal failure | 5 (38.5) | ||
| High tumor burden without renal failure | 1 (7.7) | ||
| Rapid change in clinical status while awaiting treatment | |||
| - Rapid increase in tumor burden with concurrent kidney failure | 4 (30.8) | ||
| -Transformation to PCL | 2(15.3) | ||
| Simultaneous diagnosis of MM and aggressive B cell lymphoma | 1 (7.7) | ||
| Number of mCBAD cycles received, (%) | 1 | ||
| ≤ 2 | 11 (84.6) | 9 (81.8) | 100 (86.2) |
| ≥ 3 | 2 (14.4) | 2 (18.2) | 16 (13.8) |
| Number of mCBAD cycles received, (%) | 1 | ||
| 1 | 8 (61.5) | 6 (63.6) | 57 (49.1) |
| 2 | 3 (23.1) | 3 (27.3) | 43 (37.1) |
| 3 | 1 (7.7) | 0 | 16 (13.8) |
| 4 | 1 (7.7) | 0 | 0 |
| 6 | 0 | 1 (9.1) | 0 |
| Subsequent transplant after treatment | |||
| No transplant | 3 (23.1) | 7 (63.7) | 71 (61.2) |
| Autologous Stem cell transplant | 9 (69.2) | 4 (36.3) | 42 (36.2) |
| Allogeneic Stem cell transplant | 1 (7.1) | 0 | 3 (2.6) |
| Maintenance Therapy | 9 (69.2) | 5 (45.5) | 54 (46.5) |
| Lenalidomide-Dex | 7 (53.8) | - | 24 (20.7) ¶ |
| PI-Dex | - | 2 (18.2) ‡ | 11 (9.5) ¶¶ |
| VRD | 2 (15.4) † | 2 (18.2) | 5 (4.3) ¶¶¶ |
| VCD | - | 1 (9.1) | 7 (6) |
| KRD/KCD | - | - | 2 (1.7)/1 (0.9) |
| Other | - | - | 4 (3.4) § |
| Type of response¥ | |||
| ORR | 13(100) | 10 (100) | 91 (85) |
| CR | 3 (23.1) | 1 (10) | 8 (7.5) |
| VGPR | 3 (23.1) | 2 (20) | 19 (17.8) |
| PR | 7 (53.8) | 7 (70) | 64 (59.8) |
| SD | 0 | 0 | 3 (2.8) |
| PD | 0 | 0 | 13 (12.1) |
mCBAD: modified Cyclophosphamide, Bortezomib, Doxorubicin, and Dexamethasone; MM: multiple myeloma; NDMM: newly diagnosed multiple myeloma; PCL: plasma cell leukemia; RRMM: relapsed refractory multiple myeloma; ISS: international staging system; FISH: fluorescent in situ; ASCT: autologous stem cell transplant; ECOG-PS: eastern cooperative oncology group - performance status, PI: proteasome inhibitor; VRD: Bortezomib, Lenalidomide, Dexamethasone; VCD: Bortezomib, Cyclophosphamide, Dexamethasone; KRD: Carfilzomib, Lenalidomide, Dexamethasone; KCD: Carfilzomib, Cyclophosphamide, Dexamethasone; ORR: overall response rate; CR: complete response; VGPR: very good partial response; PR: partial response; SD: stable disease; PD: progressive disease.
ISS staging data was available for 110 patients.
FISH studies were available for 111 patients.
2 patients each had either <3 lines or ≥ 3 lines of therapy. The other patients with PCL were newly diagnosed and had not received any therapies prior to mCBAD.
One patient received ixazomib instead of bortezomib and one patient received Elotuzumab in addition to VRD.
one patient received bortezomib and one patient received carfilzomib
4 patients received thalidomide maintenance
6 patients received bortezomib, 4 patients received carfilzomib, and 1 patients received ixazomib
One patient received thalidomide instead of lenalidomide
One patient received cyclophosphamide, one patient received a combination of daratumumab, lenalidomide, dexamethasone; one patient received carfilzomib, panobinostat, dexamethasone; one patient received an aurora A kinase inhibitor within a clinical trial.
10 patients with PCL were evaluable for response and 107 patients of RRMM patients were evaluable for response
Table 3.
Results survival analysis
| Progression free survival | |||||
| Diagnosis | N | Event | Median PFS time in months (95%CI) | PFS rate at 1 year (95%CI) | PFS rate at 3 years (95%CI) |
| NDMM | 13 | 11 | 19.61 (5.26 , NA ) | 0.62 (0.4 , 0.95 ) | 0.28 (0.11 , 0.7 ) |
| PCL | 11 | 11 | 7.56 (4.7 , NA ) | 0.27 (0.1 , 0.72 ) | 0.09 (0.01 , 0.59 ) |
| RRMM | 116 | 109 | 4.63 (3.75 , 6.73 ) | 0.21 (0.15 , 0.3 ) | 0.04 (0.01 , 0.1 ) |
| Overall survival | |||||
| Diagnosis | N | Event | Median OS time in months (95%CI) | OS rate at 1 year (95%CI) | OS rate at 3 years (95%CI) |
| NDMM | 13 | 9 | 35.41 (18.13 , NA ) | 0.83 (0.65 , 1 ) | 0.48 (0.26 , 0.88 ) |
| PCL | 11 | 10 | 16.06 (11.24 , NA ) | 0.64 (0.41 , 0.99 ) | 0.09 (0.01 , 0.59 ) |
| RRMM | 116 | 87 | 13.96 (10.91 , 17.74 ) | 0.55 (0.47 , 0.65 ) | 0.21 (0.14 , 0.31 ) |
NDMM: newly diagnosed multiple myeloma; PCL: plasma cell leukemia; RRMM: relapsed refractory multiple myeloma, PFS: progression free survival, OS: overall survival.
Response
Most patients (86.9%) received one to two cycles of mCBAD. Response rates are outlined in table 2. All patients with NDMM and PCL responded to mCBAD. The majority of patients with RRMM (85%) responded to treatment. PR was most commonly observed (60%), but up to 28% of patients had a VGPR or better after only 1–2 cycles of therapy.
Survival
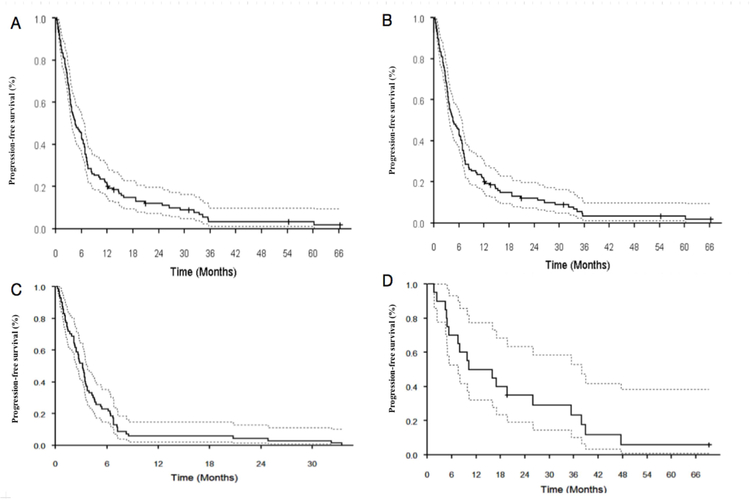
The median follow up for the censored observations was 25.1 months (range, 1.3 – 89.6 months). Survival results are summarized in table 3. For patients with RRMM, mPFS was 4.6 months (95% CI: 3.75 – 6.73 months) and mOS 13.96 months (95% CI: 10.91 – 17.74 months). However, in patients with RRMM who had subsequent ASCT, mPFS was 11.48 months (95% CI: 7.52 – 15.9 months) and mOS was 23 months (95% CI: 17.1 – 36.1 months). If no subsequent ASCT was undertaken, mPFS was 3.19 months (95% CI: 2.4 – 3.75 months) and mOS was 9 months (95% CI: 5.91 – 13.3 months) - PFS plots are depicted in figure 2.
Figure 2.
Progression free survival outcomes in patients receiving the mCBAD regimens-Kaplan Meier survival curves showing A. Progression free survival (PFS) from the initiation of mCBAD in patients with relapsed refractory multiple myeloma (RRMM) B. PFS in patients with RRMM who underwent subsequent autologous stem cell transplant (ASCT), C. PFS in patients with RRMM who did not undergo ASCT, and D. PFS in patients with newly diagnosed multiple myeloma.
Univariate Cox proportional hazards model did not identify any association between a continuous variable and PFS or OS. Multivariate analysis identified the presence of prior ASCT and the absence of maintenance therapy as detrimental to PFS with respective hazard ratios (HR) of 2.1 (95% CI: 1.4 – 3.1, p= 0.0002) and 2.7 (95% CI: 1.9 – 3.9, p < 0.0001). The absence of maintenance therapy (HR: 1.9; 95% CI: 1.3 – 2.9, p = 0.001) appeared to negatively impact mOS on multivariate analysis.
Toxicities
Toxicities, occurring in any of the 140 patients during the total of 227 patient-cycles, are listed in table 4. Hematological toxicities, in the form of cytopenias, occurred in almost all patients. Febrile neutropenia occurred in 36 (25.7%) patients, but clinically relevant bleeding was only noted in two patients (2.8%) who unfortunately passed away of central nervous system bleeding. Infections (n= 52; 37.1%), regardless of neutrophil count, and neuropathy (n=34; 24.3%) were also commonly observed. No renal toxicities were recorded. TRM occurred in 11 (7.8%) patients, only one of whom had PCL and the remainder were being treated for RRMM. TRM was due to sepsis/septic shock (n=6), multi-organ failure (n=2), central nervous system bleeding (n=2) and respiratory failure (n=1; patient with PCL). Amongst patients who had treatment-related mortality, two had a PS of 3 and two had a PS of 2. Except for the patient with PCL, whose PS was not noted, the remaining patients had a PS of 1.
Table 4.
Toxicities associated with mCBAD therapy.
| Toxicity | All grades n (%) |
|---|---|
| Anemia | 136 (97.1) |
| Neutropenia | 133 (95) |
| Thrombocytopenia | 131 (93.6) |
| Infection | 52 (37.1) |
| Febrile neutropenia | 36 (25.7) |
| Neuropathy | 34 (24.3) |
| Gastrointestinal* | 20 (13.6) |
| Thromboembolism | 4 (2.8) |
| Cardiac CHF/Arrhythmia |
4 (2.8) |
| Bleeding** | 4 (2.1) |
| Treatment-related mortality^ | 11 (7.8) |
| Sepsis/Septic Shock | 6 |
| Multi-organ failure | 2 |
| Central nervous system bleeding | 2 |
| Respiratory failure (patient with PCL) | 1 |
Reported anemia, neutropenia, and thrombocytopenia were grades 3–4 events according to the common terminology criteria for adverse events (CTCAE) version 5.0.
Diarrhea (n=10), Stomatitis (n=4), Nausea/Vomiting (n=3), Transaminitis (n=3).
Hematuria (n=2); Central nervous system bleeding (n=2).
Patient with relapsed/refractory MM (n=10) and plasma cell leukemia (n=1)
Discussion
The mCBAD regimen may be used in selected myeloma patients in need of immediate therapy with limited treatment options as a bridge to stem cell transplantation or novel therapies in clinical trials. It may also be used in areas where novel agents are not available. This regimen shows significant activity in RRMM, NDMM and PCL. By utilizing a combination of hyper-fractionated cyclophosphamide and continuously infused doxorubicin in addition to high dose dexamethasone, and bortezomib, mCBAD exposes resistant neoplastic cells to a highly efficient combination with little to no cross-resistance mechanisms overlapping with commonly used myeloma treatment strategies.
The patients presented in this study have a high proportion of high risk characteristics. For NDMM patients, 70% of the patients had kidney failure, 53% hypercalcemia, 100% anemia, 80% bony lesions, 70% had more than 70% plasma cells in the bone marrow, 16% had high risk FISH and 83% had ISS stage III. This may explain why mCBAD was selected for these patients and not other standard of care regimens frequently used for NDMM. Our own experience with KRd frontline therapy for myeloma showed that more than 80% of NDMM at our center had not relapsed after 27 months of diagnosis whereas in this study 50% of the patients had already relapsed 19 months after diagnosis16. In our KRd study, only 11% of patients had R-ISS stage III which was reflective of an overall less tumor burden for the entire population.
About half of RRMM patients underwent only 1–2 lines of therapy prior to mCBAD, which does not strictly represent a heavily pre-treated population. Despite this, these patients underwent 1–2 lines of therapy with progressive disease within 9 months of diagnosis which points towards these patients having a high frequency of either primary refractory disease or early relapse after primary therapy. The other patients with RRMM in this study had all received at least 3 lines of therapy by the time they received mCBAD with a median time of 46 months since diagnosis.
The patients included in this study had lower median age than average for both NDMM and RRMM patients and a higher proportion of high-risk cytogenetics/FISH than other studies at 37.5% vs 15 – 25%17,18,19,20,21. The median age in our study seems to be comparable to that reported in other studies that enrolled MM patients and received more aggressive regimens such as ASCT. Moreover, our study supports the notion that younger patients with myeloma may have a more aggressive clinical course22,23.
Our data illustrates the efficacy of mCBAD with an excellent ORR of 85% amongst patients with RRMM despite previous exposure to PIs and IMiDs. On the other hand, salvage combinations in multi-refractory disease, such as VD(T)PACE and VDT PACE-like regimens, have consistently produced ORR of ~ 50–60%8,24,25,26,27. Furthermore, unlike platinum-containing regimens, mCBAD can be safely administered in patients with compromised renal function as none of its individual compounds are nephrotoxic. This is particularly relevant in patients with MM where 30% - 50% of patients present with some degree of renal compromise and 25% reportedly develop renal impairment during the course of the disease28.
Despite its efficacy in terms of ORR, these responses are short-lived. Due to toxicity, this regimen is not frequently used for more than 1–3 months of therapy and underscores its use as a bridge to more permanent treatment options that can prolong the initial remission gained with mCBAD. Such bridging therapy could be conventional SCT, but alternative potentially approved newer novel agents and cellular therapies as well as enrollment in a clinical trials are also conceivable subsequent strategies29.
Although the sample size was significantly smaller for patients with NDMM and PCL, the use of mCBAD seemed beneficial in these contexts as all patients effectively responded. The majority of patients with NDMM were able to receive subsequent SCT. The use of conventional regimens and novel agents should still be the preferred approach for patients with NDMM, but specific high-risk circumstances - and possibly the lack of access to novel agents in some areas of the globe - could call for the use of mCBAD in the upfront setting.
In regards to patients with PCL, the addition of bortezomib to initial anthracycline-based induction therapy was shown to be of benefit in one retrospective study with an ORR of 69% along with significant prolongation of PFS30. Additionally, we have recently published PCL treatment outcomes after transplantation at our center. 40% (9/23) of the patients described in that study received initial therapy with either mCBAD or VDT-PACE, suggesting that mCBAD is one of our preferred regimens for initial therapy of PCL31. Our data are in line with these findings and suggest that mCBAD can be considered for treatment of PCL patients.
The use of mCBAD can be challenging if attempted in a community setting, mostly due to the adverse events profile. Therefore, close follow-up with twice-weekly blood tests and ambulatory evaluations are of paramount importance. Readily available transfusion support is also necessary to address the high incidence of treatment-related cytopenias. The common occurrence of infections and febrile neutropenia confirms the need for growth factor support and antibiotic prophylaxis. Nevertheless, other intensive regimens used in similar contexts also appear to produce high rates of febrile neutropenia (36.9% - 56%)8,24. TRM was non-negligible and reiterates the need for close follow up of patients receiving this regimen. However, TRM was almost exclusively observed in patients with RRMM and PCL, where disease relapses and exposure to numerous previous therapies undoubtedly led to patient frailty. This is probably best illustrated in the small cohort of patients who received mCBAD as chemo-mobilization prior to ASCT. In these fit patients, the rate of hospitalization due to treatment-related complications was relatively low (14.8%) and no treatment-related fatalities were reported12. Moreover, four of the eleven patients who died in our cohort had a PS >1 prior to starting mCBAD, thereby highlighting the need for careful patient selection even further.
This treatment option compares favorably in terms of efficacy to either pomalidomide/dexamethasone or daratumumab single agent32. Pomalidomide/dexamethasone had a 30% ORR and mPFS of only 4 months in RRMM. Our colleagues in the Myeloma Institute in Arkansas evaluated the clinical outcomes of high risk and low risk patients with RRMM after treatment with daratumumab single agent or in combination with pomalidomide/dexamethasone. Daratumumab single agent had an ORR of 28% in 25 patients who had received a median of 4 lines of therapy. After 3.5 months of treatment 52% of patients had already progressed. None of the patients with high risk gene expression (n=7) responded to single agent daratumumab. Daratumumab with pomalidomide/dexamethasone in 39 patients with a median of 4 lines of prior therapy had an ORR of 50% for the 25 GEP low risk patients and only 21% for the 14 GEP high risk patients33. In contrast, our reported response rate in RRMM patients after just one or two cycles of treatment is much higher around 80%.
Despite the inherent limitations associated with its retrospective nature, our study reports the largest cohort of patients treated with this regimen to date. One other report describes the use of a similar regimen, referred to as bortezomib-hyper-CAD, in a small cohort of 18 patients34. In that study bortezomib was only administered on days 1 and 4, dexamethasone was given on days 1–4, and cyclophosphamide was administered at a lower dose of 300 mg/m2. The results showed a much lower ORR of 44.4%, however, no randomized studies between this regimen and mCBAD exist.
We do not recommend the use of mCBAD for NDMM except in very limited circumstances such as in the absence of other available therapies. In 10 years we could only find 13 patients who had been treated with mCBAD at our institution, highlighting how sparingly this regimen is used in this setting. Finally, mCBAD can also be considered for initial therapy in patients with PCL.
Our data indicate that one to two cycles of mCBAD can be utilized to successfully decrease disease burden in patients with RRMM who are in dire need of therapy due to related end organ damage and who have exhausted most of the available therapeutic alternatives. Lenalidomide and pomalidomide take an average of 2 weeks to be available to patients after prescription and many patients who are developing end organ damage may not have 2 weeks to wait before starting therapy. mCBAD uses agents that can usually be given within hours and often times without requiring insurance pre-approval in the setting of care in the USA. Additionally, carfilzomib may have renal complications which may be dose-related35.
Conclusion
Thus emergent treatment in patients with increasing tumor burden and rapid development of end-organ damage, especially if no other treatment options are available, may benefit from this regimen. This therapy is optimally used as bridging therapy for subsequent SCT in eligible patients, or even enrollment in a clinical trial and as we await approval of new agents that may benefit RRMM. Patients treated with mCBAD should be monitored closely as the potential for treatment-related complications is high and the need for transfusion support should be addressed.
Clinical Practice Points.
Despite novel therapies, high dose chemotherapy regimens are still used in the treatment of myeloma and plasma cell leukemia.
mCBAD resulted in an overall response rate of 85% (7% CR and 18% VGPR); 100% (10% CR and 20% VGPR); 100% (CR 23%, VGPR 23%) in relapsed/refractory myeloma, plasma cell leukemia and newly diagnosed myeloma, respectively. Treatment-related mortality was 8%, 9% and 0%, respectively.
Due to toxicity, mCBAD cannot usually be administered for more than 2–3 cycles in a row, thus responses to this regimen are short-lived and there should be a plan for continued therapy or transplantation after mCBAD.
Acknowledgements
This work was supported in part by The MD Anderson Cancer Center Support Grant (P30 CA016672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
S. Tabchi reports no conflicts of interest.
R. Nair reports no conflicts of interest.
C. Kunacheewa no conflicts of interest.
K. Patel reports no conflicts of interest
E. Manasanch has received research support from Sanofi, Quest Diagnostics, Novartis, JW Pharma, Merck; consultant fees from Takeda, Celgene, Sanofi, Amgen, BMS.
H. Lee has received consulting fees from Adaptive Biotechnologies, Celgene, Pimera and Takeda and research support from Amgen, Daiichi Sankyo, Janssen and Takeda.
D. Weber reports no conflicts of interest
S. Thomas has received consulting fees from Amgen and research support from Acerta Pharma, Amgen, Array BioPharma, Bristol-Myers-Squibb, Celgene and Idera.
B. Amini reports no conflicts of interest.
L. Feng reports no conflicts of interest.
R. Alexanian reports no conflicts of interest.
M. Qazilbash: Research support from Janssen and BioLineRx, consulting fees from Amgen.
Q. Bashir reports no conflicts of interest.
R. Mehta reports no conflicts of interest.
S. Ahmed reports no conflicts of interest
R Orlowski has received consulting fees from Amgen, Bristol-Myers-Squibb, Celgene, Janssen, Kite Pharma, Sanofi and Takeda and research support from Amgen, BioTheryX and Spectrum Pharmaceuticals.
References
- 1.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. doi: 10.1038/leu.2013.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulte D, Jansen L, Castro FA, et al. Trends in survival of multiple myeloma patients in Germany and the United States in the first decade of the 21st century. Br J Haematol. 2015;171(2):189–196. doi: 10.1111/bjh.13537 [DOI] [PubMed] [Google Scholar]
- 3.Moreau P How I treat myeloma with new agents. Blood. 2017;130(13):1507–1513. doi: 10.1182/blood-2017-05-743203 [DOI] [PubMed] [Google Scholar]
- 4.Kumar SK, Dimopoulos MA, Kastritis E, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. 2017;31(11):2443–2448. doi: 10.1038/leu.2017.138 [DOI] [PubMed] [Google Scholar]
- 5.Griffin PT, Ho VQ, Fulp W, et al. A comparison of salvage infusional chemotherapy regimens for recurrent/refractory multiple myeloma. Cancer. 2015;121(20):3622–3630. doi: 10.1002/cncr.29533 [DOI] [PubMed] [Google Scholar]
- 6.Clark CA, Cornell RF, Scott EC, Chung J, Costa LJ. Management of relapsed and refractory multiple myeloma in modern times: Incorporating new agents into decision-making. Am J Hematol. 2016;91(10):1044–1051. doi: 10.1002/ajh.24478 [DOI] [PubMed] [Google Scholar]
- 7.Nair B, van Rhee F, Shaughnessy JD Jr., et al. Superior results of Total Therapy 3 (2003–33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006–66 with VRD maintenance. Blood. 2010;115(21):4168–4173. doi: 10.1182/blood-2009-11-255620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakshman A, Singh PP, Rajkumar SV, et al. Efficacy of VDT PACE-like regimens in treatment of relapsed/refractory multiple myeloma. Am J Hematol. 2018;93(2):179–186. doi: 10.1002/ajh.24954 [DOI] [PubMed] [Google Scholar]
- 9.Dimopoulos MA, Weber D, Kantarjian H, Delasalle KB, Alexanian R. HyperCVAD for VAD-resistant multiple myeloma. Am J Hematol. 1996;52(2):77–81. doi: [DOI] [PubMed] [Google Scholar]
- 10.Menendez P, Vargas A, Bueno C, et al. Quantitative analysis of bcl-2 expression in normal and leukemic human B-cell differentiation. Leukemia. 2004;18(3):491–498. doi: 10.1038/sj.leu.2403231 [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. doi: 10.1056/NEJMoa070594 [DOI] [PubMed] [Google Scholar]
- 12.Gettys SC, Gulbis A, Wilhelm K, et al. Modified CVAD and modified CBAD compared to high-dose cyclophosphamide for peripheral blood stem cell mobilization in patients with multiple myeloma. Eur J Haematol. 2017;98(4):388–392. doi: 10.1111/ejh.12843 [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48. doi: 10.1016/S1470-2045(14)70442-5 [DOI] [PubMed] [Google Scholar]
- 14.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284 [DOI] [PubMed] [Google Scholar]
- 15.Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215–224. doi: 10.1038/leu.2008.307 [DOI] [PubMed] [Google Scholar]
- 16.Chaudhry M, Steiner R, Claussen C, et al. Carfilzomib-based combination regimens are highly effective frontline therapies for multiple myeloma and Waldenstrom’s macroglobulinemia. Leuk Lymphoma. 2018:1–7. doi: 10.1080/10428194.2018.1508668 [DOI] [PubMed] [Google Scholar]
- 17.Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. doi: 10.1016/S1470-2045(15)00464-7 [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375(14):1319–1331. doi: 10.1056/NEJMoa1607751 [DOI] [PubMed] [Google Scholar]
- 19.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375(8):754–766. doi: 10.1056/NEJMoa1606038 [DOI] [PubMed] [Google Scholar]
- 20.Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med. 2018;378(6):518–528. doi: 10.1056/NEJMoa1714678 [DOI] [PubMed] [Google Scholar]
- 21.Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974–981. doi: 10.1182/blood-2017-05-785246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Multiple Myeloma In Patients Under 40 Years Old Is Associated With High-Risk Features and Worse Outcomes. Blood. 2013;122(21):5359 http://www.bloodjournal.org/content/122/21/5359.abstract. [Google Scholar]
- 23.Hebraud B, Leleu X, Lauwers-Cances V, et al. Deletion of the 1p32 region is a major independent prognostic factor in young patients with myeloma: the IFM experience on 1195 patients. Leukemia. 2014;28(3):675–679. doi: 10.1038/leu.2013.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orciuolo E, Galimberti S, Ghio F, Petrini M. VDTPACE As Salvage Therapy For Heavily Pretreated MM Patients. Blood. 2013;122(21):5377 http://www.bloodjournal.org/content/122/21/5377.abstract. [Google Scholar]
- 25.Gerrie AS, Mikhael JR, Cheng L, et al. D(T)PACE as salvage therapy for aggressive or refractory multiple myeloma. Br J Haematol. 2013;161(6):802–810. doi: 10.1111/bjh.12325 [DOI] [PubMed] [Google Scholar]
- 26.Beyer K, Rosner S, Woo KM, et al. Analysis of VDT-PACE Utilization in Multiple Myeloma Patients Treated at MSKCC for Relapsed Disease or Cytoreduction and Stem Cell Mobilization after Initial Induction Therapy. Blood. 2014;124(21):3459 http://www.bloodjournal.org/content/124/21/3459.abstract.25139348 [Google Scholar]
- 27.Valla K, Kaufman JL, Gleason C, et al. Bortezomib in Combination with Dexamethasone, Cyclophosphamide, Etoposide, and Cisplatin (V-DCEP) for the Treatment of Multiple Myeloma. Blood. 2014;124(21):2139 http://www.bloodjournal.org/content/124/2½139.abstract. [Google Scholar]
- 28.Dimopoulos MA, Kastritis E, Rosinol L, Blade J, Ludwig H. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008;22(8):1485–1493. doi: 10.1038/leu.2008.131 [DOI] [PubMed] [Google Scholar]
- 29.Cohen AD, Garfall AL, Stadtmauer EA, et al. Safety and Efficacy of B-Cell Maturation Antigen (BCMA)-Specific Chimeric Antigen Receptor T Cells (CART-BCMA) with Cyclophosphamide Conditioning for Refractory Multiple Myeloma (MM). Blood. 2017;130(Suppl 1):505 http://www.bloodjournal.org/content/130/Suppl_1/505.abstract. [Google Scholar]
- 30.Royer B, Minvielle S, Diouf M, et al. Bortezomib, Doxorubicin, Cyclophosphamide, Dexamethasone Induction Followed by Stem Cell Transplantation for Primary Plasma Cell Leukemia: A Prospective Phase II Study of the Intergroupe Francophone du Myélome. J Clin Oncol. 2016;34(18):2125–2132. doi: 10.1200/JCO.2015.63.1929 [DOI] [PubMed] [Google Scholar]
- 31.Gowda L, Shah M, Badar I, et al. Primary plasma cell leukemia: autologous stem cell transplant in an era of novel induction drugs. Bone Marrow Transpl. 2018. doi: 10.1038/s41409-018-0392-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. doi: 10.1016/S1470-2045(13)70380-2 [DOI] [PubMed] [Google Scholar]
- 33.Branca A, Buros A, Yoon D, et al. Daratumumab Single Agent and Daratumumab Plus Pomalidomide and Dexametasone in Relapsed/Refractory Multiple Myeloma: A Real Life Retrospective Evaluation. Blood 2016. 1284516;. [Google Scholar]
- 34.Saraceni MM, Scott E, Maziarz RT, et al. Modified HyperCVAD Versus Bortezomib-HyperCAD in Patients With Relapsed/Refractory Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2018;18(1):e77–e84. doi: 10.1016/j.clml.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 35.Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449–454. doi: 10.1182/bloodadvances.2016003269 [DOI] [PMC free article] [PubMed] [Google Scholar]