Abstract
This study examined whether HIV-associated neurocognitive impairment (NCI), assessed with the HIV-Dementia Scale (HDS), predicted mortality in an ethnically diverse sample of 209 HIV-positive adults. Participants were predominantly in the mid-range of illness at baseline, and followed over 13-years. At baseline, 31 (15%) participants scored in the NCI range (HDS ≤ 10); 58 (28%) died during follow-up. Baseline NCI was significantly associated with earlier mortality (HR = 2.10, 95% CI [1.10 - 4.00]) independent of demographic and HIV disease-related covariates. Less errors on the antisaccade task, an index of executive/attention control, was the only HDS subtest predicting earlier mortality (HR = 0.72, 95% CI [0.58 - 0.90]). In the absence of an AIDS-defining condition, NCI, particularly in the executive/attention domain, is an independent prognostic marker of morality in a diverse HIV-positive cohort. These findings highlight the clinical utility of brief screening measures in this population.
Keywords: HIV, Neurocognitive Impairment, HIV-Dementia Scale, Mortality, Antisaccade
Introduction
Neurocognitive impairment (NCI) is a prevalent and disabling consequence of Human Immunodeficiency Virus (HIV). Even in the era of widely available, highly effective antiretroviral therapy (ART), over 30% of people living with HIV continue to suffer from HIV-associated neurocognitive disorder (HAND) [1]. In fact, all but the most severe presentations of HAND (i.e., HIV-associated dementia [HAD]) are increasing in prevalence, likely as a function of rising survival rates secondary to improved viral control [2]. With the advent of these potent antiretroviral medications, the clinical presentation of HAND has shifted away from the more severe impairments characteristic of HAD towards more mild deficits in psychomotor speed, learning and prospective memory, and most notably, executive functioning (e.g., attention, working memory, executive control, mental flexibility, inhibition, etc.) [3-5]. Despite their relatively mild nature, these impairments can interfere with the ability to perform important everyday activities such as driving, cooking, medical self-care, financial management, and occupational functioning [6, 7].
In the earliest stage of infection, compromised CD4+ lymphocytes, monocytes, and macrophages of the host transport the virus through the blood brain barrier (BBB) [8, 9]. Within the central nervous system, direct viral neurotoxicity [10] and chronic immune-mediated neuroinflammation [11, 12] leave a pathological signature of glial activation, synaptodenditic simplification, and neural apoptosis and degeneration [9]. Because antiretroviral medications often have poor BBB penetration, independent viral reservoirs can proliferate in the CNS despite efficacious viral suppression the periphery [13]. While researchers have traditionally argued that the resulting imbalance of viral suppression primarily contributes to the persistence of HAND in the ART era [14], others have more recently proposed a central role for cerebrovascular disease [15].
The advent of highly effective antiretroviral therapies, which quell viral replication and allow for immune reconstitution and preservation, has substantially reduced HIV mortality rates [16, 17]. Yet, people living with HIV continue to face an increased risk of mortality compared to their non-infected counterparts, even among those with a successful response to ART [18, 19]. Early on, HIV-infected persons faced an estimated reduced life expectancy of up to 17 years compared with non-infected persons [20]. However, a recent comprehensive analysis [21] indicated a ten-year increase in life expectancy for young adults starting ART, as well as lower mortality rates for individuals starting ART between 2008–10 compared to those starting treatment in 2000–03. Nonetheless, life expectancy among HIV-infected individuals, even those initiating ART, remains lower than in the general population [21]. Thus, identifying clinical predictors of early mortality, especially among individuals whose disease progression is not severely advanced, remains a top assessment and treatment priority in the ART era.
There are several well-established independent predictors of mortality in HIV, including older age, low CD4 lymphocyte counts, presence of AIDS-defining illnesses, and lower hemoglobin concentrations [22-24]. Beyond these, there is accumulating evidence that HIV-associated NCI is an independent predictor of shortened survival time among patients living with HIV, independent of confounding risk factors [25-30]. However, to our knowledge, all but two studies to date [26, 30] included patients with a history of AIDS-defining clinical conditions, and neither study was conducted in the era of highly effective antiretroviral therapy availability.
The primary aim of the present study was to examine the relationship between baseline NCI and mortality in a sample of men and women with HIV who were predominantly in the mid-range of their illness, over a long-term follow-up period of up to 13 years. We hypothesized that global NCI, measured by a brief screening instrument, would predict earlier time to mortality independent of demographic and disease-related covariates. The secondary aim was to examine each of the four subtests of the screening tool (i.e., psychomotor speed, memory, constructional praxis, and attention/executive control) in relation to mortality, in order to elucidate which cognitive domains (if any) were most pertinent to mortality risk.
Methods
Participants
Participants were recruited from physician’s offices, specialty clinics, service organizations, and hospitals in Miami, Florida, between 1997 and 2000, shortly after the advent of the first potent antiretroviral medication therapies. Participants were included if they were HIV positive and had CD4 cell counts between 150 and 700 cells/mm3. Subjects were excluded if they were under age 18, had ever experienced an AIDS-defining (Category C) clinical condition, ever had nadir CD4 cell counts below 75 cells/mm3 (this was intended to ensure exclusion of individuals with a history of end-stage AIDS, who were unlikely to have a reasonable chance at immune reconstitution), had an additional life-threatening illness, were actively psychotic or suicidal, or had current alcohol or drug dependence (defined as meeting DSM-III-R criteria within the past six months) or intravenous (IV) drug use in the past six months. Of note, individuals with a history of lifetime (but not current) alcohol or substance dependence or IV drug use were not excluded. Participants were reimbursed for their time.
Demographic information and sample characteristics are shown in Table 1. A total of 209 men and women (71.8% male) with a mean age of 37.7 years (SD = 8.7) met the study criteria. The sample was ethnically and racially diverse, with approximately equal parts non-Hispanic White (30.6%), non-Hispanic African American (35.4%), and Hispanic (29.7%) participants. There was also a range of educational attainment in the sample; approximately one-third of participants had no more than a high school education, slightly more than one-third attended some college, and slightly less than one third obtained a college or graduate degree. Approximately 20% of participants were currently employed full-time, and nearly 44% reported receiving disability at baseline.
Table 1.
Overall Sample Characteristics and Descriptive Statistics
| Variable | n | % | |
|---|---|---|---|
| Age (M, SD) | 37.7 (8.7) | 209 | 100.0 |
| Gender | |||
| Male | 150 | 71.8 | |
| Female | 59 | 28.2 | |
| Race | |||
| Non-Hispanic White | 64 | 30.6 | |
| African American | 74 | 35.4 | |
| Hispanic | 62 | 29.7 | |
| Other | 9 | 4.3 | |
| Educational Attainment | |||
| Some high school or less | 33 | 15.8 | |
| High school graduate | 35 | 16.7 | |
| Some college | 81 | 38.8 | |
| College graduate | 40 | 19.1 | |
| Graduate degree | 19 | 9.1 | |
| Employment Status | |||
| Full-time | 43 | 20.6 | |
| Unemployed | 34 | 16.3 | |
| Disability | 91 | 43.5 | |
| Other | 41 | 19.6 | |
| CD4 cells/mm3 (M, SD) | 323.02 (122.8) | 209 | 100 |
| Viral Load copies/mL | 4171.5a | 209 | 100 |
| Antiretroviral Medication | |||
| No medication | 41 | 19.6 | |
| Combination therapyb | 65 | 31.1 | |
| HAARTc | 103 | 49.3 | |
| Beck Depression Inventory-II (M, SD) | 11.5 (8.9) | 209 | 100 |
| HIV-Dementia Scale | Range | M | SD |
| Total | 0-16 | 13.6 | 2.6 |
| Antisaccade task | 0-4 | 3.5 | 1.1 |
| Psychomotor Speed | 0-6 | 5.1 | 1.7 |
| Memory | 0-4 | 3.6 | 0.7 |
| Construction | 0-2 | 1.4 | 0.9 |
Notes.
median value.
excluding protease inhibitors.
highly active antiretroviral therapy (with protease inhibitors).
While participants’ CD4 counts ranged from 150–700 cells/mm3, the overwhelming majority (86.5%) had CD4 cell counts between 150 and 500 cells/mm3 (MCD4 = 323.02 cells/mm3). Based on this CD4 cell count range and the absence of preexisting AIDS-defining clinical conditions, we consider the sample to be predominantly in the mid-range of illness. However, it is important to recognize that at study entry, 16.7% of participants had CD4 counts below 200 cells/mm3, the current case definition for AIDS. Nearly 50% of participants reported being on HAART (highly active antiretroviral therapy, with protease inhibitors) at baseline, and an additional 31.1% reported being on combination therapy without protease inhibitors. Of note, at the time this study was initiated, the term HAART was used to distinguish “modern” antiretroviral medications from the less effective, originating AIDS medications. Since then, the terms “cART” (combination antiretroviral therapy), and more recently “ART” (single-drug therapies), have fallen into favor to describe the most contemporary, highly effective medication regimens. Hereafter, we use the term HAART only to specifically refer to the early protease inhibitor-based HIV-medications.
Procedure
All participants completed written informed consent, a clinical assessment interview to screen for exclusionary psychopathology (SCID-III-R) [31], and blood draw for CD4 and viral load assays. The neurocognitive screeners were administered by trained research staff supervised by a licensed psychiatrist. Study procedures were approved by the University of Miami institutional review board.
Measures
Global neurocognitive impairment.
The HIV-Dementia Scale [32] is a brief screening instrument that assesses global HIV-related cognitive impairment based on performance across four subtests: a timed alphabet-writing task, recall for three words after a short delay, a cube-drawing task, and an antisaccade task. Respectively, these tasks assess psychomotor speed (6 points), memory (4 points), visual-spatial constructional praxis (2 points), and executive inhibitory control (4 points). A total of 16 points is possible.
For the antisaccade task, patients are instructed to maintain a centrally fixed gaze, and volitionally produce a saccade in the opposite direction of a randomly presented stimulus in the visual periphery. For example, the examiner sits approximately one foot away facing the examinee at eye-level, with both of his/her arms outstretched to the sides. The examiner instructs the examinee to fixate on the examiner’s nose, and look in the opposite direction when the examiner’s moves one of his/her fingers. An error is scored if the subject fails to properly produce the antisaccade (e.g., looks in the direction of the stimulus or maintains a central gaze). Twenty trials are administered and a score is calculated based on the number of saccadic errors (i.e., ≤ 3 errors = 4, four errors = 3, five errors = 2, six errors=1, more than six errors = 0). This task requires inhibition of the reflexive pro-saccade toward the stimulus, and volitional response generation toward the desired opposite direction, and is thus considered a measure of executive inhibitory control [33].
Lower HDS total scores indicate greater cognitive impairment, with scores less than or equal to 10 out of 16 indicating possible HAND with excellent sensitivity and specificity [34]. Although critics of the HDS suggest that its sensitivity to detect more subtle types of neurocognitive disorder is lower than formal neuropsychological assessment [35], it has proven to be a reliable predictor of HAND in clinical samples [36] and has been used in clinical and research settings [37]. In the present study, participants were categorized based on their baseline total HDS scores; participants scoring at or below the clinical cutoff score (i.e., ≤ 10 on the HDS) were categorized as “NCI,” while the rest were categorized as cognitively “within normal limits” (WNL) [34, 37].
Depressive symptoms.
The Beck Depression Inventory-II [38] is a widely used and well-established self-rating inventory for assessing depression severity. The inventory contains 13 items, each scored from “0” indicating minimal symptomology, to “3” indicating severe symptomology. Total scores greater than 13 suggest elevated levels of depressive symptoms.
Mortality Data
Baseline interviews were conducted beginning in March of 1997. Mortality data were accessed from the Death Master File, a weekly-updated publically available data source that reflected all deaths reported to the Social Security Administration (SSA) up to the time we accessed it in April 2010. To identify any deaths that may not have been reported to the SSA, obituaries were also searched online using participants’ birthdays and full names.
Disease Markers
Plasma CD4 lymphocyte count (CD3+CD4+) was determined by whole-blood 4-color direct immunofluorescence using a coulter XL-MCL flow cytometer. Viral load was determined using the Roche Amplicor RT/PCR assay sensitive to 400 copies of viral plasma RNA.
Covariates
Demographic and disease related covariates were selected a priori due to their relevance to health outcomes in HIV and to maintain consistency with previous studies from our group. Demographic variables included age, race/ethnicity (coded 1 = African American, 0 = other), gender (0 = female, 1 = male), and education (0 = less than high school, 1 = some high school, 2 = high school graduate, 3 = some college, 4 = college graduate, 5 = graduate degree). Education, rather than income or employment, was chosen as a relatively unbiased indicator of socioeconomic status because income and employment are more likely to be influenced by progressing illness. Baseline CD4 lymphocyte counts and viral load were included as markers of initial disease status. Antiretroviral medication status was dummy coded at three levels: 1= no medication use, 2 = combination therapy without protease inhibitors, 3 = HAART.
Statistical analyses.
All analyses were performed using SPSS Version 22. Data was determined to be missing at random for four cases, and was subsequently excluded listwise. Descriptive statistics for demographic, disease-related, and neurocognitive variables were generated for the overall sample and the two subgroups. Subjects were dichotomously classified as either NCI (dummy code = 1) or WNL (dummy code = 0) based on their HDS total scores (cutoff of ≤ 10). The NCI and WNL groups were compared using independent t-tests for continuous variables, and chi-square tests for categorical variables. Next, we conducted a series of Cox proportional hazards regression analyses adjusted for age, gender, education, race/ethnicity, and baseline CD4 cell count, viral load, and antiretroviral medication status, in order to examine associations with mortality. Hazard ratios (HR) and their 95% confidence intervals (CI), were computed. First, we examined whether the presence of NCI at baseline predicted mortality over the 13-year follow-up period. A Kaplan-Meier plot was generated for descriptive purposes to depict survival curves for the NCI and cognitively WNL groups. Second, we examined the HDS total score and each of the four subtest scores (i.e., antisaccade task/executive control, psychomotor speed, memory, and construction) simultaneously in relation to mortality, with each treated as continuous. Third, we examined each covariate independently in relation to mortality in univariate analyses. To facilitate interpretation, several variables were dichotomized: subjects with a high school diploma were compared against those with some college or more; subjects with CD4 lymphocyte counts below 200 cells/mm3 were compared against those with more than 200 cells/mm3; subjects with detectable viral load were compared against those undetectable viral load; subjects on any kind of antiretroviral medication regimen were compared against subjects with no medication use. Finally, to be thorough, we conducted a post-hoc analysis with additional control for baseline depressive symptom scores to account for potential effects of depression on cognitive performance and/or mortality.
Results
Based on HDS performance, 31 (14.8%) subjects scored in the NCI range and 178 (85.2%) patients scored within normal limits (WNL) at baseline. Table 2 shows comparisons between the NCI and WNL groups on sociodemographic, disease-related, and neurocognitive measures. On average, NCI subjects were marginally older and had slightly lower CD4 lymphocyte counts. Aside from these differences, the two groups were comparable on sociodemographic variables (i.e., gender, educational attainment, and race/ethnicity). The HDS total and subtest scores were each significantly lower in the NCI group compared with the cognitively WNL group. Correlations for the HDS with sociodemographic and illness-related covariates in the overall sample (not shown) revealed that lower HDS total scores were significantly associated with older age (r = −.14, p = .04), African American race (r = −.18, p = .01), and lower educational attainment (r = .27, p < .001).
Table 2.
Sample characteristics for NCI and WNL groups
| Variable | NCIa (n = 31) | WNLb (n = 178) | ct / χ2 (df) | p-value |
|---|---|---|---|---|
| Age (M, SD) | 40.6 (8.4) | 37.2 (8.6) | −2.03 (207) | .04 |
| Gender, n (%) male | 22 (71.0) | 128 (71.9) | .01 (1) | .91 |
| Race/Ethnicity, n (%) | 6.08 (3) | .12 | ||
| Euro American | 4 (12.9) | 60 (33.7) | ||
| African American | 12 (38.7) | 62 (34.8) | ||
| Hispanic | 13 (41.9) | 49 (27.5) | ||
| Other | 2 (6.5) | 7 (3.9) | ||
| Educational Attainment, n (%) | 5.47 (4) | .24 | ||
| ≤ Some High School | 8 (25.8) | 25 (14.1) | ||
| High School Graduate | 5 (16.1) | 30 (16.9) | ||
| Some College | 13 (41.9) | 68 (38.4) | ||
| College Graduate | 2 (6.5) | 38 (21.5) | ||
| Graduate Degree | 3 (9.7) | 16 (9.0) | ||
| CD4 Count (M, SD) | 283.2 (126.5) | 330.0 (121.2) | 1.97 (207) | .05 |
| Antiretroviral Status, n (%) | 3.74 (2) | .29 | ||
| No medication | 5 (16.1) | 36 (20.2) | ||
| Combination therapye | 14 (45.2) | 51 (28.7) | ||
| HAARTf | 12 (38.7) | 91 (51.1) | ||
| Beck Depression Inventory-II (M, SD) | 13.8 (9.3) | 11.1 (8.8) | −1.57 (207) | .12 |
| HDS Total (M, SD) | 8.8 (1.4) | 14.5 (1.6) | 18.77 (207) | <.001 |
| Antisaccade Task (M, SD) | 2.7 (1.6) | 3.7 (0.9) | 3.08 (207) | .004 |
| Psychomotor Speed (M, SD) | 2.1 (2.3) | 5.6 (0.9) | 8.30 (207) | <.001 |
| Memory (M, SD) | 3.2 (1.0) | 3.7 (0.6) | 2.97 (207) | .006 |
| Construction (M, SD) | 0.7 (0.9) | 1.5 (0.8) | 4.37 (207) | <.001 |
Notes. HDS= HIV-Dementia Scale.
NCI= neurocognitive impairment group (HDS total score ≤ 10).
WNL = neurocognitively within normal limits (HDS total score >10).
Statistic is t-value for continuous variables (age, CD4 count, viral load, HDS total and subtests) and chi-square value for categorical variables (gender, race/ethnicity, income, employment status, route of infection, educational attainment, antiretroviral status).
Combination antiretroviral therapy without protease inhibitors.
Highly active antiretroviral therapy with protease inhibitors.
The median follow-up duration for the overall sample was 11 years (range, 0.92 – 13.08 years). Between study entry and the end of the 13-year follow-up period in 2010, 58 (27.8%) of subjects in the overall sample had died. In 2002, 17 (8.1%) subjects had died; in 2004, 28 (13.4%) subjects had died.
As shown in Table 3, Cox proportional hazard regression analyses controlling for age, gender, African American race, education, CD4 lymphocyte count, viral load, and antiretroviral medication status revealed that NCI at baseline was significantly associated with earlier mortality (HR = 2.10, 95% CI [1.10 – 4.00], p = .024). Baseline CD4 count (treated continuously) was the only covariate associated with mortality with NCI in the model. Survival curves for the NCI and WNL groups are illustrated in Figure 1.
Table 3:
Multivariate hazard model of neurocognitive impairment (NCI) predicting mortality, adjusted for covariates
| Variable | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Age | 1.04 | 0.99 - 1.07 | .06 |
| Gendera | 0.92 | 0.48 - 1.76 | .81 |
| Race/Ethnicityb | 1.45 | 0.78 - 2.67 | .24 |
| Education | 0.80 | 0.61 - 1.04 | .10 |
| CD4 cells/mm3 | 0.997 | 0.994 - 0.999 | <.01 |
| Log viral load | 1.28 | 0.98 - 1.68 | .07 |
| Antiretroviral statusc | 1.09 | 0.76 - 1.56 | .63 |
| NCId | 2.10 | 1.10 - 4.00 | .02 |
Notes. Covariates: age, African American race, gender, education, CD4 cell counts, viral load, antiretroviral medication status. CI= confidence interval.
Male gender was entered as the reference group.
African American ethnicity was compared against all other ethnicities.
Any medication regimen was compared against no medication use.
NCI group was compared against the within normal limits (WNL) group. All other predictors were treated as continuous variables.
Figure 1.
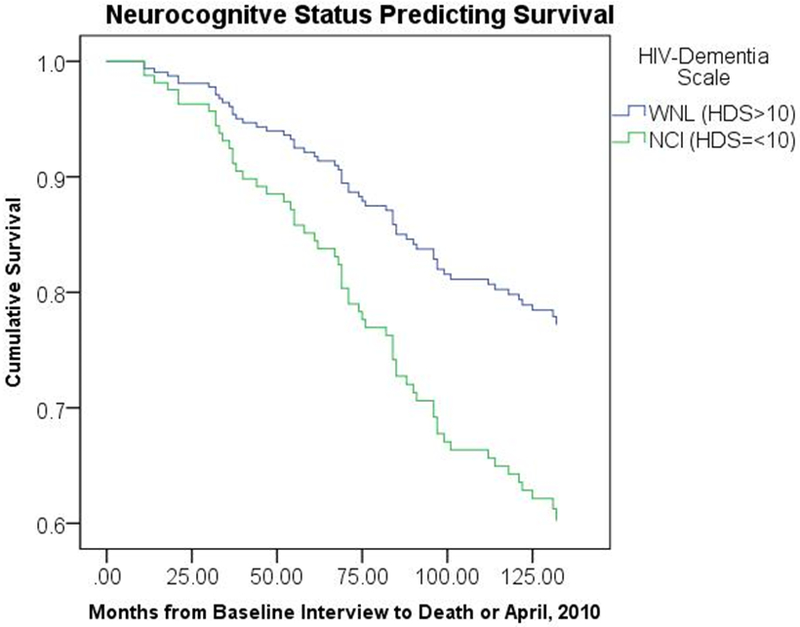
Survival curves, controlling for covariates, for participants with neurocognitive impairment (NCI) and those who were cognitively within normal limits (WNL) based on the HIV-Dementia Scale performance at study entry.
A post-hoc analysis with additional control for depressive symptoms indicated that NCI remained significantly associated with mortality, when BDI-II total score was treated continuously (HR = 2.09, 95% CI [1.09 – 4.00]) or dichotomously using a cutoff score of greater than 13 (HR = 2.13, 95% CI [1.19 – 4.12]). These findings suggest that the NCI-mortality link persisted beyond the effects of baseline depressive symptomology.
In a supplemental analysis, HDS total score and each HDS subtest score (all treated as continuous) were examined as predictors of mortality, controlling for the same sociodemographic and disease-related covariates. As shown in Table 4, superior performance on the antisaccade subtest was the only score significantly associated with time to mortality (HR = 0.72, 95% CI [0.58 – 0.90], p = .003). To further compare the predictive properties of the subtest scores, we entered all four subtests simultaneously, with covariates, in a Cox regression model. Results showed that antisaccade performance remained a significant predictor of time to mortality (HR = 0.74, 95% CI [0.60 – 0.92], p = .007) above and beyond psychomotor speed, memory, construction, and covariates.
Table 4.
Multivariate hazard models of HDS subtests predicting mortality, adjusted for covariates
| Variable | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| HDS Total | 0.93 | 0.84 - 1.03 | .17 |
| Antisaccade task | 0.72 | 0.58 - 0.90 | .003 |
| Psychomotor speed | 1.05 | 0.90 - 1.23 | .52 |
| Memory | 1.00 | 0.68 - 1.49 | .99 |
| Construction | 0.80 | 0.60 - 1.07 | .14 |
Notes. Covariates: age, African American race, gender, education, CD4 cell counts, viral load, antiretroviral medication status. HIV-Dementia Scale (HDS) total score and all subtest scores were treated as continuous. Lower scores indicate worse performance. CI = confidence interval.
Each covariate was independently examined as a predictor of mortality in univariate analyses. As shown in Table 5, older age, African American race, lower education, and CD4 cell counts below 200 cells/mm3 were each significantly associated with mortality.
Table 5.
Univariate hazard models of each covariate predicting mortality
| Variable | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Age | 1.03 | 1.00 - 1.06 | .04 |
| Gendera | 1.14 | 0.65 - 1.98 | .65 |
| Race/Ethnicityb | 1.96 | 1.17 - 3.27 | .01 |
| Educationc | 0.57 | 0.34 - 0.95 | .03 |
| CD4 cells/mm3d | 2.59 | 1.47 - 4.57 | .001 |
| Log viral loade | 1.65 | 0.89 - 3.05 | .11 |
| Antiretroviral statusf | 1.56 | 0.74 - 3.30 | .24 |
Notes. CI= confidence interval.
Male gender was entered as the reference group.
African American ethnicity was compared against all other ethnicities.
High school diploma was compared against some college or more.
CD4 counts >200 cells/mm3 were compared against CD4 counts <200 cells/mm3.
Detectable viral load was compared against undetectable viral load.
Any medication regimen was compared against no medication use. Age was treated as a continuous predictor.
Discussion
HIV-associated neurocognitive impairment (NCI) predicted earlier mortality in a sample of seropositive men and women predominantly in the mid-range of their illness over a long-term follow-up (median = 11 years). Subjects performing in the NCI range on the HIV-Dementia Scale (HDS) at baseline were over twice as likely to die during the follow-up period compared to subjects scoring within normal limits at baseline. This finding was independent of age, gender, race, and education as well as peripheral CD4 lymphocyte count, viral load, and antiretroviral medication status at baseline, suggesting that potential confounding sociodemographic factors or more advanced disease progression at the onset of the study likely did not account for the pronounced mortality hazard observed among neurocognitively impaired subjects.
In many ways, the present study serves as a conceptual replication of previous work. Firstly, univariate analyses of covariates identified more advanced disease progression (i.e., lower baseline CD4 lymphocyte counts) as well as older age, African American race, and lower educational attainment as individual predictors of earlier mortality, largely confirming findings from previously followed cohorts [25, 26, 28, 30, 39]. Secondly, the time adjusted proportional hazard ratio for mortality among patients with NCI observed in the present study (HR = 2.10) closely resembles those noted in previous investigations. In the pre-HAART era, Mayeux et al. [26] reported that HIV-positive men with neuropsychological impairment were nearly three times more likely than non-impaired subjects to die over a median of 18 months. Wilkie et al. [30] found that men scoring in the bottom 25th percentile on a composite measure of memory were 3.5 times as likely to die over a median of 24-months compared with those at the top 25th percentile. To our knowledge, these are the only other existing studies conducted exclusively with subjects absent of AIDS-defining clinical conditions across the pre or post-HAART eras, and they were both relatively small (i.e., 18/111 and 14/119 deaths/subjects, respectively) and restricted to gay men. Our findings add to this limited body of literature by showing that the link between HIV-associated NCI and mortality extends to a larger (i.e., 58 deaths/209 subjects) and more diverse sample of men and women over a considerably longer follow-up.
Our findings are also consistent with those from several larger cohort studies of mixed-gender samples. In the pre-HAART era, Ellis et al. [25] found a two-fold increase in mortality risk for subjects with mild presentations of HIV-associated NCI compared with unimpaired subjects over a median 2.4 year long follow-up (106 deaths/ 414 subjects). In their study, approximately 14.0% of subjects had a history of AIDS-defining illnesses. During the post-HAART era (i.e., after 1996), Vivithanaporn et al. [29] conducted a population based study in Canada and found that subjects with HAND were approximately three times as likely to die during a median 7.6 year follow-up than subjects without. Over 25% of subjects in their cohort had previous AIDS-defining illnesses. Sevigny et al. [28] reported a six-fold mortality hazard for subjects with HAD compared to those without, among a particularly ill sample of patients with advanced HIV infection (i.e., restricted to CD4 counts <300 cells/mm3). Tozzi et al. [39] found that Italian subjects with failed viral response to HAART exhibiting baseline cognitive impairment were 2.9 times more likely to die during the median 32.4 month long follow-up than unimpaired subjects. In this study, 38.8% of subjects met CDC criteria for Category C disease stage at baseline. Although our study included a small proportion of individuals with CD4 counts below 200 cells/mm3, falling in the AIDS-defining range, all participants had a negative history of Category C AIDS-defining clinical conditions at baseline, and therefore we consider them to be predominantly mid-range. Thus, our study augments the literature by demonstrating that HIV-associated NCI portends earlier mortality among patients predominantly in the mid-range of illness, and does so during the post-HAART era over an extended follow-up.
To our knowledge, the present study is also the first to assess HIV-associated NCI in relation to mortality using a brief and easy to administer screening measure of HIV-associated neurocognitive impairment (i.e., the HDS). Approximately 15% of subjects in the present study met our operational definition of NCI indicated by the HDS. Among other studies in the post-HAART era, this prevalence was slightly higher than that reported in Vivithanaporn et al.’s [29] Canadian population-based study (6.2%), and considerably lower than those reported by Tozzi et al. [39] or Sevigny et al. [28] (54.0% and 70.1%, respectively). It is suspected that this relatively lower prevalence may reflect our exclusion of patients with a history of more advanced disease progression, as well as the insensitivity of the HDS to more mild forms of NCI compared to the more extensive neuropsychological batteries utilized in other studies.
There are multiple plausible pathways through which HIV-associated NCI may be related to greater mortality risk. First, NCI can lead to poor fidelity to complex antiretroviral medication regimens [3, 40], which is an established independent predictor of mortality in HIV [41] presumably through inadequate viral suppression. Second, HIV-associated NCI can interfere with utilization of health care resources [42], such as those necessary for vigilant monitoring of disease progression, timely initiation of antiretroviral therapy, and interventions for potentially treatable illnesses secondary to immune compromise (e.g., additional infectious diseases, cardiometabolic syndrome, etc.). Third, some researchers posit that the development of NCI among patients on ART may reflect suboptimal viral suppression in the CNS as a result of either poor antiretroviral penetration of the BBB or medication resistant viral mutations within CNS viral reservoirs [43]. It is plausible that sustained viral toxicity within the CNS, even in the context of adequate peripheral viral control, may pose a dual threat to cognitive functioning and survival. Fourth, it is conceivable that patients exhibiting NCI may have been infected with a particularly virulent and lethal strain of HIV, or that these patients possess genetic or psychobiological vulnerabilities that are common to both the development of NCI and earlier mortality.
An underexplored mechanism potentially underlying the link between HIV-associated NCI and mortality during the ART era is increased vulnerability to vascular diseases, which are established risk-factors for both dementia and death [44]. Patients with HIV on ART face increased risk of cerebrovascular and cardiovascular diseases compared to their non-infected counterparts [45-48], likely due to the atherogenic properties of antiretroviral medications and HIV’s direct toxicity to endothelial functioning [49, 50]. In light of the growing evidence linking vascular factors to cognitive impairments in virally suppressed patients with HIV [51, 52], and considering the overlapping clinical features of vascular dementia and HAND, some researchers now hypothesize that cerebrovascular pathology may be central to the development of HAND in the ART era [15]. Thus, it is plausible that vascular disease manifested systemically and/or cerebrally may dually impact cognition and mortality in patients with HIV. Future studies are needed to elucidate the role of vascular factors within the NCI-mortality relationship.
The secondary study aim was to examine performance on the individual subtests of the HDS as predictors of mortality. Only a few studies from the pre-HAART era have reported domain-specific relationships with mortality, and those include language impairments [26], verbal memory impairments [30], and stable patterns of psychomotor slowing [53]. In the present study, performance on the antisaccade task emerged as the only significant predictor of mortality among the four HDS subtests. Specifically, a one-point decrease on this task was associated with a 28% increased mortality hazard, controlling for demographic and disease related covariates. In an additional analysis examining all four subtests simultaneously, antisaccade performance predicted mortality above and beyond psychomotor speed, memory, and construction combined, suggesting that it is a particularly robust neurocognitive predictor of mortality in HIV.
Although it is labeled as an “attention” subtest on the HDS, substantial research on the neurocognitive underpinnings of the antisaccade task suggests that it is perhaps more accurately conceptualized as a measure of executive functioning (see [33] for review), one of the earliest and most severely impacted cognitive domains among ART-medicated individuals [4]. Successful execution of this deceptively simple task requires top-down cognitive control to negotiate conflict between concurrent, competing mechanisms (inhibition of the reflexive pro-saccade toward the stimulus, and volitional response generation toward the desired opposite direction) [33, 54]. Additionally, the antisaccade task activates frontal-subcortical networks involving the frontal eye fields, dorsolateral and ventrolateral prefrontal cortices, the posterior parietal and supramarginal gyrus, and the striatum [55, 56], which also subserve the executive functions [57]. Accordingly, clinical populations evidencing fronto-subcortical dysfunction and executive deficits (e.g., patients with Huntington’s disease, Alzheimer’s disease, schizophrenia, and prefrontal lesions), exhibit impaired performance on the antisaccade task (see [58] for review).
Patients living with HIV also demonstrate saccadic dysfunction, even among those who are neurologically asymptomatic [59]. Additionally, they characteristically show compromised integrity of fronto-striatal white-matter tracts on neuroimaging [60], even among those who are cognitively asymptomatic or non-demented [61, 62]. Thus, the preliminary findings presented here suggest that the antisaccade subtest of the HDS may be particularly sensitive to early HIV-associated executive changes reflecting more advanced neurological disease burden.
Clinical Implications
Overall, our findings confirm that HIV-associated NCI is a prognostic marker that may help identify patients at risk for earlier mortality. Whereas the prognostic value of plasma viral markers (e.g., HIV RNA level) has become less clear following the advent of ART [63], objective cognitive assessments may offer much needed prognostic information for clinicians in the ART era. Although the HDS has been criticized for its relative insensitivity to milder presentations of HIV-associated cognitive impairment, our findings suggest that this brief screening measure, particularly when considering the antisaccade component, produces similar results with regard to prediction of mortality as larger neuropsychological batteries, but with the added advantage of being substantially faster and easier to administer and score. In settings seeking to minimize the strain on time or resources, or when comprehensive neuropsychological measures are unavailable, the HDS may offer clinicians rapid and objective prognostic data to complement immune and viral markers of disease progression. The installation of assessments for NCI as a part of routine care, even among ART-medicated patients with viral-control, may guide treatment planning by signaling the need for increased psychosocial support to address cognitive limitations, assessment and treatment of vascular risk factors, adherence-augmentation interventions, and/or more aggressive antiretroviral regimens.
The HDS has been criticized for containing elements that are less familiar to non-Westernized cultures (e.g., cube drawing), and because the antisaccade task may be difficult to administer without adequate training. In response, Sacktor et al. [64] created the now widely used International HIV-dementia scale (I-HDS), which omitted the antisaccade task. While there may indeed be merit to these criticisms and subsequent alterations, the present findings speak to the prognostic value of the antisaccade subtest and raise questions regarding the trade-off between improving the ease of administration with the I-HDS and neglecting to assess antisaccade performance. Interestingly, a recent systematic review comparing the I-HDS and HDS [65] found that both measures failed to achieve standard levels of accuracy to provide convincing evidence for a diagnosis of HAND; however, the HDS yielded a higher summary diagnostic odds ratio for assessing HAND than the I-HDS. It may be prudent for investigators seeking to develop new brief screening measures for HIV-associated NCI to weigh the prognostic value of the antisaccade when considering potential test elements to include.
Limitations and Future Directions
There are several limitations of the present study that should be acknowledged. First, the HDS is a measure of gross HIV-associated NCI and is less sensitive to more mild presentations of cognitive impairment [35, 66], suggesting that subjects with more mild impairments were possibly not assigned to the NCI group. Similarly, the HDS did not allow for stratification of NCI by severity, thus limiting our ability to examine how different degrees of impairment might differentially relate to mortality. Second, we only examined cognitive impairment at a single time-point (i.e., baseline), and it is possible that cognitive status, as measured by the HDS, could have changed during the course of the follow-up period (e.g., with improved antiretroviral adherence). Future studies may seek to examine patterns of cognitive decline over multiple time points in relation to mortality. Third, this study began at the turn of the HAART era when the antiretroviral options were less advanced and optimized than they are today. Fourth, this study did not account for several variables that may have been related to cognition and mortality, such as premorbid IQ, non-HIV-related neurodegenerative disease, hepatitis-C co-infection, cardiovascular disease data, or cause of death. Future studies may seek to include a more stringent set of covariates, and to specifically examine whether a more fine-grained analysis of socioeconomic status plays a role in the relationship between NCI and mortality. Fifth, a small proportion of our sample met the case definition for AIDS at baseline based on CD4 lymphocyte count. The inclusion of these individuals limited our ability to generalize findings to patients exclusively in the “mid-range” of illness or to disease stages outside the parameters of our inclusion/exclusion criteria (e.g., end-stage AIDS). Additionally, the present findings may not generalize to populations with current (i.e., past six months) substance dependence or IV drug use, given our exclusion of such individuals. Sixth, our use of the HDS precludes generalizations to the more widely used international-HDS, and it is for future studies to determine the prognostic value of the international-HDS. Finally, future studies are needed to examine potential mechanisms through which NCI is related to early mortality (e.g., medication adherence, health care utilization, biomarkers of sustained virulence within the CNS, and accelerated vascular disease).
Conclusion
HIV-associated neurocognitive impairments are becoming increasingly prevalent in the ART era. In the present study, we found that among patients predominantly in the mid-range of their illness, the presence of NCI was associated with more than twice the likelihood of death than those with scores indicting absence of cognitive impairment, independent of sociodemographic and HIV-disease related variables. This predictive effect was evident over a median of 11 years. Among the four HDS subtests, antisaccade performance was the strongest and only subtest associated with mortality. These findings indicate that performance on the HDS, and antisaccade subtest in particular, has prognostic utility for assessing mortality risk. Furthermore, this highlights the prognostic utility of brief screening measures assessing global cognitive impairment among HIV patients without an AIDS-defining clinical condition.
Acknowledgment:
This research was graciously supported by the National Institute of Mental Health (R01MH53791 and R01MH066697, PI: Dr. Ironson). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. 2016;86(4):334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychology review. 2009;19(2):169–85. [DOI] [PubMed] [Google Scholar]
- 3.Heaton R, Franklin D, Ellis R, McCutchan J, Letendre S, LeBlanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of NeuroVirology. 2011;17(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society. 2002;8(03):410–24. [DOI] [PubMed] [Google Scholar]
- 5.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychology review. 2009;19(2):152–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society : JINS. 2004;10(3):317–31. [DOI] [PubMed] [Google Scholar]
- 7.Marcotte TD, Heaton RK, Wolfson T, Taylor MJ, Alhassoon O, Arfaa K, et al. The impact of HIV-related neuropsychological dysfunction on driving behavior. The HNRC Group. Journal of the International Neuropsychological Society : JINS. 1999;5(7):579–92. [DOI] [PubMed] [Google Scholar]
- 8.Albright AV, Soldan SS, González-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. Journal of neurovirology. 2003;9(2):222–7. [DOI] [PubMed] [Google Scholar]
- 9.Hult B, Chana G, Masliah E, Everall I. Neurobiology of HIV International review of psychiatry (Abingdon, England: ). 2008;20(1):3–13. [DOI] [PubMed] [Google Scholar]
- 10.Mocchetti I, Bachis A, Avdoshina V. Neurotoxicity of human immunodeficiency virus-1: viral proteins and axonal transport. Neurotoxicity research. 2012;21(1):79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iskander S, Walsh KA, Hammond RR. Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. Journal of neuroinflammation. 2004;1(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaul M HIV-1 associated dementia: update on pathological mechanisms and therapeutic approaches. Current opinion in neurology. 2009;22(3):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Archives of neurology. 2008;65(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis RJ, Calero P, Stockin MD. HIV infection and the central nervous system: a primer. Neuropsychology review. 2009;19(2):144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brew BJ. Has HIV-associated neurocognitive disorders now transformed into vascular cognitive impairment? Aids. 2016;30(15):2379–80. [DOI] [PubMed] [Google Scholar]
- 16.Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2006;41(2):194–200. [DOI] [PubMed] [Google Scholar]
- 17.Murphy EL, Collier AC, Kalish LA, Assmann SF, Para MF, Flanigan TP, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Annals of internal medicine. 2001;135(1):17–26. [DOI] [PubMed] [Google Scholar]
- 18.Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. Jama 2008. p. 51–9. [DOI] [PubMed] [Google Scholar]
- 19.van Sighem A, Danner S, Ghani AC, Gras L, Anderson RM, de Wolf F. Mortality in patients with successful initial response to highly active antiretroviral therapy is still higher than in non-HIV-infected individuals. J Acquir Immune Defic Syndr. 2005;40(2):212–8. [DOI] [PubMed] [Google Scholar]
- 20.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146(2):87–95. [DOI] [PubMed] [Google Scholar]
- 21.Trickey A, May MT, Vehreschild J- J, Obel N, Gill MJ, Crane HM, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. The Lancet HIV. 2017;4(8):e349–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blatt SP, McCarthy WF, Bucko-Krasnicka B, Melcher GP, Boswell RN, Dolan MJ, et al. Multivariate models for predicting progression to AIDS and survival in human immunodeficiency virus-infected persons. Journal of Infectious Diseases. 1995;171(4):837–44. [DOI] [PubMed] [Google Scholar]
- 23.Luo K, Law M, Kaldor JM, McDonald AM, Cooper DA. The role of initial AIDS-defining illness in survival following AIDS. AIDS. 1995;9(1):57–64. [DOI] [PubMed] [Google Scholar]
- 24.Nightingale SD, Jockusch JD, Haslund I, Cal SX, Peterson DM, Loss SD. Logarithmic relationship of the CD4 count to survival in patients with human immunodeficiency virus infection. Archives of internal medicine. 1993;153(11):1313–8. [PubMed] [Google Scholar]
- 25.Ellis RJ, Deutsch R, Heaton RK, Marcotte TD, McCutchan JA, Nelson JA, et al. Neurocognitive impairment is an independent risk factor for death in HIV infection. Archives of neurology. 1997;54(4):416–24. [DOI] [PubMed] [Google Scholar]
- 26.Mayeux R, Stern Y, Tang M, Todak G, Marder K, Sano M, et al. Mortality risks in gay men with human immunodeficiency virus infection and cognitive impairment. Neurology. 1993;43(1 Part 1):176. [DOI] [PubMed] [Google Scholar]
- 27.McArthur JC, Hoover D, Bacellar H, Miller E, Cohen B, Becker J, et al. Dementia in AIDS patients incidence and risk factors. Neurology. 1993;43(11):2245. [DOI] [PubMed] [Google Scholar]
- 28.Sevigny JJ, Albert SM, McDermott MP, Schifitto G, McArthur JC, Sacktor N, et al. An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Archives of neurology. 2007;64(1):97–102. [DOI] [PubMed] [Google Scholar]
- 29.Vivithanaporn P, Heo G, Gamble J, Krentz HB, Hoke A, Gill MJ, et al. Neurologic disease burden in treated HIV/AIDS predicts survival: a population-based study. Neurology. 2010;75(13):1150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkie FL, Goodkin K, Eisdorfer C, Feaster D, Morgan R, Fletcher MA, et al. Mild cognitive impairment and risk of mortality in HIV-1 infection. The Journal of neuropsychiatry and clinical neurosciences. 1998;10(2):125–32. [DOI] [PubMed] [Google Scholar]
- 31.Spitzer RL, First MB, Gibbon M, Williams JB. Structured clinical interview for DSM-III-R: American Psychiatric Press; 1990. [DOI] [PubMed] [Google Scholar]
- 32.Power C, Selnes OA, Grim JA, McArthur JC. HIV Dementia Scale: a rapid screening test. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1995;8(3):273–8. [DOI] [PubMed] [Google Scholar]
- 33.Hutton SB. Cognitive control of saccadic eye movements. Brain and cognition. 2008;68(3):327–40. [DOI] [PubMed] [Google Scholar]
- 34.Ganasen K, Fincham D, Smit J, Seedat S, Stein D. Utility of the HIV Dementia Scale (HDS) in identifying HIV dementia in a South African sample. Journal of the neurological sciences. 2008;269(1):62–4. [DOI] [PubMed] [Google Scholar]
- 35.Bottiggi KA, Chang JJ, Schmitt FA, Avison MJ, Mootoor Y, Nath A, et al. The HIV Dementia Scale: predictive power in mild dementia and HAART. Journal of the neurological sciences. 2007;260(1):11–5. [DOI] [PubMed] [Google Scholar]
- 36.Hu X, Zhou Y, Long J, Feng Q, Wang R, Su L, et al. Diagnostic accuracy of the International HIV Dementia Scale and HIV Dementia Scale: A meta-analysis. Experimental and therapeutic medicine. 2012;4(4):665–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joska JA, Fincham DS, Stein DJ, Paul RH, Seedat S. Clinical correlates of HIV-associated neurocognitive disorders in South Africa. AIDS and Behavior. 2010;14(2):371–8. [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio: 1996;78(2):490–8. [Google Scholar]
- 39.Tozzi V, Balestra P, Serraino D, Bellagamba R, Corpolongo A, Piselli P, et al. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Research & Human Retroviruses. 2005;21(8):706–13. [DOI] [PubMed] [Google Scholar]
- 40.Hinkin C, Castellon S, Durvasula R, Hardy D, Lam M, Mason K, et al. Medication adherence among HIV+ adults effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59(12):1944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lima VD, Harrigan R, Bangsberg DR, Hogg RS, Gross R, Yip B, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. Journal of acquired immune deficiency syndromes (1999). 2009;50(5):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okonkwo O, Vance D, Antia L, Smith B, Blanshan S, Heirs K, et al. Service utilization and cognitive complaints in adults with HIV: results from a statewide survey. Journal of HIV/AIDS & Social Services. 2008;7(2):175–94. [Google Scholar]
- 43.Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well-controlled plasma viral load. AIDS (London, England). 2012;26(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perk J, De Backer G, Gohlke H, Graham I, Reiner Ž, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). European heart journal. 2012;33(13):1635–701. [DOI] [PubMed] [Google Scholar]
- 45.Currier JS, Lundgren JD, Carr A, Klein D, Sabin CA, Sax PE, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cruse B, Cysique LA, Markus R, Brew BJ. Cerebrovascular disease in HIV-infected individuals in the era of highly active antiretroviral therapy. Journal of neurovirology. 2012;18(4):264–76. [DOI] [PubMed] [Google Scholar]
- 47.Mateen FJ, Post WS, Sacktor N, Abraham AG, Becker JT, Smith BR, et al. Long-term predictive value of the Framingham Risk Score for Stroke in HIV-positive vs HIV-negative men. Neurology. 2013;81(24):2094–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ. Incidence and clinical features of cerebrovascular disease among HIV-infected adults in the Southeastern United States. AIDS research and human retroviruses. 2013;29(7):1068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benjamin LA, Bryer A, Emsley HC, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. The Lancet Neurology. 2012;11(10):878–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang T, Yi R, Green LA, Chelvanambi S, Seimetz M, Clauss M. Increased cardiovascular disease risk in the HIV-positive population on ART: potential role of HIV-Nef and Tat. Cardiovascular Pathology. 2015;24(5):279–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su T, Wit FW, Caan MW, Schouten J, Prins M, Geurtsen GJ, et al. White matter hyperintensities in relation to cognition in HIV-infected men with sustained suppressed viral load on combination antiretroviral therapy. Aids. 2016;30(15):2329–39. [DOI] [PubMed] [Google Scholar]
- 52.Wright E, Grund B, Robertson K, Brew B, Roediger M, Bain M, et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology. 2010;75(10):864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sacktor N, Bacellar H, Hoover D, Nance-Sproson T, Seines O, Miller E, et al. Psychomotor slowing in HIV infection: a predictor of dementia, AIDS and death. Journal of neurovirology. 1996;2(6):404–10. [DOI] [PubMed] [Google Scholar]
- 54.Massen C Parallel programming of exogenous and endogenous components in the antisaccade task. Quarterly Journal of Experimental Psychology Section A. 2004;57(3):475–98. [DOI] [PubMed] [Google Scholar]
- 55.Ettinger U, Ffytche DH, Kumari V, Kathmann N, Reuter B, Zelaya F, et al. Decomposing the neural correlates of antisaccade eye movements using event-related FMRI. Cerebral Cortex. 2007;18(5):1148–59. [DOI] [PubMed] [Google Scholar]
- 56.Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K, et al. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: cortical and subcortical networks. Psychiatry Research: Neuroimaging. 2004;131(2):147–55. [DOI] [PubMed] [Google Scholar]
- 57.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience. 2012;12(2):241–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43(3):302–13. [DOI] [PubMed] [Google Scholar]
- 59.Castello E, Baroni N, Pallestrini E. Neurotological and Auditory Brain Stem Response Findings in Human Immunodeficiency Virus—Positive Patients without Neurologic Manifestations. Annals of Otology, Rhinology & Laryngology. 1998;107(12):1054–60. [DOI] [PubMed] [Google Scholar]
- 60.Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, et al. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Archives of neurology. 2004;61(3):369–76. [DOI] [PubMed] [Google Scholar]
- 61.Castelo JMB, Sherman SJ, Courtney MG, Melrose RJ, Stern CE. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66(11):1688–95. [DOI] [PubMed] [Google Scholar]
- 62.Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV. Frontostriatal fiber bundle compromise in HIV infection without dementia. AIDS (London, England). 2009;23(15):1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarwater PM, Gallant JE, Mellors JW, Gore ME, Phair JP, Detels R, et al. Prognostic value of plasma HIV RNA among highly active antiretroviral therapy users. Aids. 2004;18(18):2419–23. [PubMed] [Google Scholar]
- 64.Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, et al. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. Aids. 2005;19(13):1367–74. [PubMed] [Google Scholar]
- 65.Haddow LJ, Floyd S, Copas A, Gilson RJC. A systematic review of the screening accuracy of the HIV Dementia Scale and International HIV Dementia Scale. PloS one. 2013;8(4):e61826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valcour V, Paul R, Chiao S, Wendelken LA, Miller B. Screening for cognitive impairment in human immunodeficiency virus. Clinical Infectious Diseases. 2011;53(8):836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


