Abstract
Objective
Patients with BRAF-mutant colorectal cancer (CRC) have a poor prognosis. Molecular status is not currently used to select which drug to use in combination with radiotherapy. Our aim was to identify drugs that radiosensitise CRC cells with known BRAF status.
Methods
We screened 298 oncological drugs with and without ionising radiation in colorectal cancer cells isogenic for BRAF. Hits from rank product analysis were validated in a 16-cell line panel of human CRC cell lines, using clonogenic survival assays and xenograft models in vivo.
Results
Most consistently identified hits were drugs targeting cell growth/proliferation or DNA damage repair. The most effective class of drugs that radiosensitised wild-type and mutant cell lines was PARP inhibitors. In clonogenic survival assays, talazoparib produced a radiation enhancement ratio of 1.9 in DLD1 (BRAF-wildtype) cells and 1.8 in RKO (BRAF V600E) cells. In DLD1 xenografts, talazoparib significantly increased the inhibitory effect of radiation on tumour growth (P ≤ 0.01).
Conclusions
Our method for screening large drug libraries for radiosensitisation has identified PARP inhibitors as promising radiosensitisers of colorectal cancer cells with wild-type and mutant BRAF backgrounds.
Keywords: Radiosensitizer, colorectal cancer, PARP inhibitor, radiotherapy
Introduction
Colorectal cancer (CRC) is one of the most common forms of cancer, accounting for approximately 1 in 10 new cancer diagnoses worldwide in 20121. Radiotherapy is commonly used to treat rectal cancers prior to surgery or to treat inoperable colorectal metastases, in the form of stereotactic body radiotherapy or selective internal radiotherapy2-4.
International standard combination therapy for rectal cancer, radiotherapy delivered with 5-fluorouracil (5FU) as a radiosensitiser, is given either as an infusion or as an oral prodrug (capecitabine). There is currently no molecular basis for the selection of patients for radiotherapy, nor for the selection of any alternative drug to use as a radiosensitiser. With the current standard, sufficient downsizing by chemoradiotherapy is obtained by approximately half of patients treated5. There is scope for improving the radiotherapy approaches currently offered to patients. Clinical trials have added additional drugs to 5FU as a combination radiosensitising approach6,7 without molecular selection, but these trials have not changed the international standard.
Colorectal tumours have a heterogeneous molecular background8. Commonly occurring CRC mutations that may be prognostic or can affect treatment decisions include KRAS, BRAF and PIK3CA mutations, which are found in 42%, 9% and 13% of CRC patients respectively9. KRAS, BRAF and PIK3CA are vital components of two main cellular signalling pathways; RAS/MEK/ERK and PI3K/AKT/mTOR; strongly inter-connected pathways that play central roles in tumorigenesis by regulating cell survival, proliferation, metabolism, and motility. The KRAS gene is a member of the oncogenic RAS gene family and binds to effector kinases including BRAF and phosphatidylinositol 3-kinase (PI3K). The PIK3CA gene encodes the PI3K p110α subunit, which interacts with RAS proteins10.
The commonest BRAF mutation in colorectal cancer, the V600E substitution, results in elevated kinase activity and constitutive downstream MEK and ERK phosphorylation11,12. The presence of BRAF V600E in advanced CRC correlates with poor prognosis with markedly worse progression after chemotherapy13-15. BRAF mutation is predictive of poor response to cetuximab in metastatic CRC, also observed for KRAS and PIK3CA mutations16-18. Although patients with BRAF-mutant cancers do less well with chemotherapy, anti-EGFR therapies and surgery19, there is currently no suggestion that they benefit less from radiotherapy. Although BRAF mutation is relatively rare in rectal cancer, radiotherapy can also be used to treat inoperable liver metastases from CRC. It has been suggested that CRC liver metastases respond less well to radiotherapy than liver metastases from other primary malignancies20, hence the addition of a radiosensitising drug may be of value to improve the therapeutic index during radiotherapy21.
Our aim was to develop a radiosensitiser drug discovery assay enabling identification of drugs that will enhance radiotherapy more effectively than the current standard, 5FU, and demonstrate activity in defined molecular backgrounds. Firstly, we developed a high throughput screen (HTS), in CRC cell lines, to identify drugs that could be effective radiosensitisers in the context of BRAF V600E activating mutations. The drugs identified during the screen were validated across an extensive panel of human CRC cell lines, selected to represent aspects of the molecular landscape of CRC; including BRAF V600E in both MSI and MSS backgrounds, and a spectrum of KRAS, PIK3CA and p53 mutations. Such cell line panels recapitulate the different subtypes found in CRC, are representative of genetic alterations found in primary cancers and are good predictors of clinical efficacy during drug development programmes22. Here, we use this model to test new drug-radiotherapy combinations for the first time, identifying PARP inhibitors as the most strongly radiosensitising class of agent before validating by clonogenic survival assays and in vivo xenograft studies.
Materials and methods
Cell lines, drug library and irradiations
The parental CRC cell lines RKO (BRAF V600E/V600E/WT) and VACO432 (V600E/WT) and their isogenic pairs RKO-T29 (BRAF WT/-/-) and VACO432-VT1 (BRAF WT/-) were a gift from Sandra Van Schaeybroeck, Queens University, Belfast, UK (mutation status confirmed by sequencing). The panel of colorectal cancer cell lines utilised for cell proliferation assays was obtained from Prof. Walter Bodmer, University of Oxford, UK. The cell line panel is listed in Supplementary Table S1, and has been previously described22. Non-malignant cell lines were obtained from Prof. Gillies McKenna, University of Oxford, UK. All cell lines were used within 12 passages, or where necessary, replenished using frozen aliquots of the initial passage. Isogenic cell lines were grown in McCoy's 5A (Modified) Medium, and other cell lines in DMEM; both supplemented with 10% Fetal Bovine Serum and 1 × penicillin/streptomycin (Thermofisher Scientific Inc., MA, USA), in a 37°C, 5% CO2, humidified incubator. The small compound anti-cancer drug library was provided in 384-well plate format (Target Discovery Institute, University of Oxford), and contained 222 drugs from the TDI Extended Oncology Drugs Library (ODL) and 76 from the NCI Developmental Therapeutics Program (DTP) Approved Oncology Drug set (Supplementary Table S2).
A GSR D1 irradiator (Gamma-Service Medical GmbH, Leipzig, Germany) a Cs-137 source, (dose rate 1.5 Gy/min) was used for cell irradiations. For xenografts, a RS320 X-ray irradiator (Gulmay Limited, Byfleet, UK) was used (1.6 Gy/min), with lead shielding to localise dose to tumor. Dosimetry was calculated from optical density of scanned Gafchromic EBT3 film (Ashland, NJ, USA), corrected and calibrated to the National Physical Laboratory (Teddington, UK) primary standard.
High-throughput drug screen with ionising radiation
Methodology and data analysis followed internationally recognised high-throughput screening guidelines23. BRAF V600E isogenic RKO and VACO432 cells were seeded in 52 μL/well by Flexdrop (PerkinElmer, MA, USA). Seeding density in 384-well plates was 300 cells/well (RKO) and 1,000 cells/well (VACO432). Eighteen hours after seeding, cells were screened with 298 oncological drugs, in 5-fold dilutions from 10 μM–16 nM. Janus workstations (PerkinElmer, MA, USA) were used to transfer 13 μL of compound from library plate to cell culture plates. Positive controls were PI103 and vorinostat, negative controls were vehicle (DMSO) alone. After 6 h, plates were either mock-irradiated, or irradiated with 4 Gy. Media was replaced 24 h following treatment, and surviving cells allowed to proliferate for five doubling times as optimised in preliminary screens. Cell viability was measured by resazurin (10 μg/mL) in phenol red-free DMEM. Metabolically viable cells reduce resazurin to fluorescent resorufin, which was quantified by PerkinElmer Envision microplate reader (540 nm excitation/590 nm emission). Control wells reached 90%–100% confluency at the time of assay performance, control irradiated wells were around 60% confluent. Raw data were normalized by rescaling to plate mean intensity and to negative controls. Quality plots were contrasted to assess artifacts and reproducibility. Normalized data Z are presented, as the applied rescaling by plate mean is effectively a z-score standardization. Selection of candidate hits was based on rank product analysis, adapting a published method24. Specifically, for each pair of conditions (i.e. with/without irradiation), the differences between normalised screen intensities were calculated for each well, hence each drug. These differences are presented as Delta-Z (ΔZ) scores. Rank product applied to these differences identified compounds producing large and consistent changes. Probability of false discovery was computed by permutation, with n = 100. Analyses were implemented in R version 2.1 (https://cran.r-project.org/); heatmaps were generated by modifying D3.js libraries (https://d3js.org/).
Cell proliferation and colony formation assays
Our method for comparison of IC50 in the presence or absence of radiation has been described previously25. Clonogenic survival was measured following a standard method, with plating efficiency and surviving fractions calculated as described26. Briefly, cells were seeded into 10 cm culture dishes, normally 500 cells/plate (for 0 Gy plates), increasing by 10-fold for each 4 Gy administered, to 500,000 cells/plate (12 Gy). After attachment (overnight), cells were drug-treated, and six hours later exposed to 0, 4, 8 or 12 Gy radiation. Culture medium was replaced 24 hours post-irradiation, plates were incubated to form visible colonies > 50 cells (10 – 15 days) and fixed with 0.4% methylene blue in methanol. Survival curves were fitted using Graphpad Prism v7.0A. Radiation enhancement ratio (RER) was obtained from the ratio of radiation dose at 1% survival of vehicle compared with drug treated cells.
Xenograft studies
Animal experiments were performed following local ethical review under licence from the UK Home Office (ASPA 1986, revised January 2013). Female Balb/c nude mice (6–8 weeks old) were anaesthetised with 2% isoflurane and subcutaneously injected with 50% matrigel containing 5x106 DLD1 cells or 5 × 106 RKO/mouse (n = 24) into the back. When tumor volume reached 100 mm3, mice were randomly placed into 4 groups (n = 6/group). Oral treatments were by gavage, in two doses on the first and fourth days of treatment. Group (1) received vehicle only, 10% dimethylacetamide/6% solutol HS/PBS (0.1mL/10 g body weight). Group (2) received talazoparib; 0.1 mg/kg in vehicle. Radiation treatments comprised 2 × 5 Gy, localised to the tumor, also on the first and fourth days of treatment. Group (3) received radiation only, 5 Gy one hour after each vehicle treatment. Group (4) received combination treatment, 5 Gy one hour after each talazoparib treatment. Tumor size was measured by caliper 3 × per week. Mice were sacrificed when tumours reached 400 mm3 or 42 days following the first treatment. Tumours were formalin fixed and stained for the hypoxia marker CA9 as previously described27.
Results
Development of a high throughput screen with ionising radiation
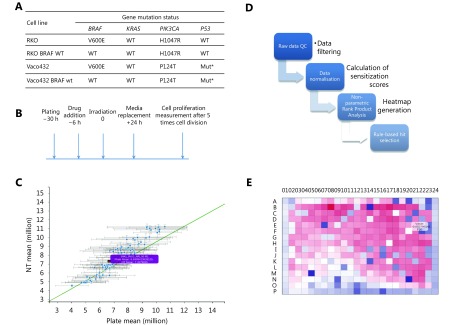
In order to identify drugs that radiosensitise CRC cells mutated for BRAF V600E, isogenic cell lines containing either BRAF V600E or BRAF WT variants were screened against a 298-compound library of approved anticancer drugs. Mutation status for KRAS, PIK3CA and p53 for these cell lines is shown in Figure 1A, with the screen protocol outlined in Figure 1B.
1.
High-throughput screening of FDA approved cancer drugs to identify which drugs should be used for radiosensitisation in the context of single gene mutations in colorectal cancer.
A prerequisite for high-throughput detection of radiosensitisers is an assay that is predictive of the effects of drug/ radiation combinations on clonogenic cell survival. Extended incubation following irradiation improves correlation with radiosensitisation28, and we incorporated 5 days incubation following radiation treatment; improving correlation to clonogenic survival, but avoiding compromises to cell metabolism and thus assay performance29. Serial dilution of cells in the presence of resazurin showed equivalent fluorescence, linear in relation to cell number, for both non-irradiated cells, and cells 5 days post-irradiation (data not shown). This indicates that the metabolic assay was a good surrogate for cell number at this timepoint.
Screens were carried out in duplicate and quality plots demonstrated good reproducibility (Figure 1C), with mean Pearson correlation between pairs of replicates of 0.88 and average Z factor of 0.58 for irradiated and 0.53 for non-irradiated plates. Cell viability was compared between normalized irradiated and non-irradiated plates, generating heatmaps of the difference, ΔZ, for each compound. Hit selection (Figure 1D) was based on rank product analysis, with the probability of false discovery computed by permutations (see Materials and methods). Potential hits were drugs that sensitised the BRAF-mutant isogenic variant, at one or more concentrations, with probability of false positive (PFP) ≤ 0.05. Some plates showed a pronounced ‘edge effect’, and for this reason, analysis was repeated considering the edge wells as a separate population (Figure 1E). Hits with significant ΔZ score between irradiated and non-irradiated samples, with radiosensitisation factor < 1 (normalised against control plates) and P-value ≤ 0.05 were selected as significant. Positive controls were consistently identified as hits, with ΔZ scores ≤ 2, comparable to results obtained in manual assays.
BRAF V600E screen in isogenic cell lines following irradiation
Drugs were ranked according to radiosensitisation against BRAF-mutated cells. The fifteen drugs with the highest significance against BRAF-mutated cells are shown in Table 1. Seven hits have previously been identified as radiosensitisers in the published literature30-36, helping to validate our methodology. Five hits were inhibitors of RAS/RAF/MEK/ERK pathway (trametinib, TAK-733, pimasertib, doramapoimod and dactolisib), predominantly acting in BRAF WT and V600E. Eight drugs reached significance in the BRAF-mutant cell line but not in BRAF WT, including the CHK1 inhibitor, PF477736. Another CHK1 inhibitor, AZD7762, radiosensitised both BRAF variants.
1.
Fifteen radiosensitisers identified for BRAF-mutant cells
| Compound | Effective concentration
in RKO (BRAF mut) (μM) |
Effective concentration
in RKO (BRAF WT) (μM) |
Mechanism of action |
| RKO colorectal cancer cells BRAF V600E or WT were screened with 298 approved oncology drugs alone or in combination with irradiation. Radiosensitisation factors were calculated from the ratio of fluorescence of irradiated versus non-irradiated plates. The most significant hits for BRAF-mutant variant RKO cells are shown; each hit has radiosensitisation factor < 1, PFP ≤0.05 and P-values ≤0.05; ‘ns’ indicates that significance was not reached in the BRAF WT cell line for the drug tested. | |||
| Dactolisib | 0.016, 0.4 | 0.016 | Dual PI3K/mTOR inhibitor |
| Panobinastat | 0.016 | ns | HDAC inhibitor |
| Trametinib | 0.016 | 0.016 | MEK inhibitor |
| ABT-199 | 0.08 | 0.08 | Bcl-2 inhibitor |
| Olaparib | 0.08 | ns | PARP inhibitor |
| Tosedostat | 0.08 | ns | Peptidase inhibitor |
| AZD 7762 | 0.08 | 0.08 | Chk inhibitor |
| Pimasertib | 0.4, 0.08 | 0.08 | MEK inhibitor |
| PF477736 | 0.08 | ns | Chk1 inhibitor |
| 17-AAG | 0.08 | ns | Hsp90 inhibitor |
| Doramapimod | 0.08 | ns | p38 MAPK inhibitor |
| Danusertib | 0.08 | ns | aurora kinase inhibitor |
| Serdametan | 0.4 | 0.4 | MDM2 inhibitor |
| Tak-733 | 0.4 | 0.4 | MEK inhibitor |
| Auranofin | 0.4 | ns | Gold complex |
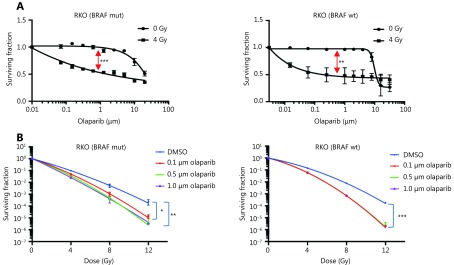
The poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib, significantly increased sensitivity to irradiation in BRAF V600E RKO cells. In a separate screen of BRAF isogenic Vaco432 cells, olaparib also radiosensitised BRAF V600E Vaco432 cells at 16 nM and 80 nM (P ≤ 0.05, data not shown). Based on these data, radiosensitisation by PARP inhibitors (PARPi) in RKO isogenic for V600E and WT, was validated by long-term proliferation assay at a broad concentration range and by clonogenic cell survival assay (Figure 2). Olaparib as a single agent had little effect on survival, but combination treatment caused a significant increase in radiation sensitivity, albeit with similar effect in both BRAF WT and V600E variants.
2.
Validation of radiosensitisation effects of olaparib in BRAF-mutant and BRAF-WT isogenic CRC cells.
Radiosensitisation in an extended CRC cell line panel
To validate the screen, we used a cell line panel inclusive of the different molecular subtypes of CRC. We specifically prioritised the drug hits with the most immediate scope for translation to clinical trials in combination with radiotherapy. The cell line panel was selected so that several cell lines exhibited each gene mutation of interest. Fifteen cell lines with defined BRAF, p53, KRAS, PIK3CA and mismatch repair status were used. The compounds chosen for further testing are shown in Table 2, along with p-values indicating whether significant IC50 shift was observed following normalisation for radiation effect. The complete IC50 results determined by these assays are shown in Supplementary Table S3.
2.
P- values for radiosensitisation by 11 drugs across a panel of 15 CRC cell lines
| Cell line | LS411 | Vaco5 | RKO | HT29 | OXCO4 | CCK81 | HCA7 | DLD1 | CW2 | C10 | HT55 | C99 | Colo678 | SW403 | SW1222 | |
| A panel of fifteen colorectal cell lines, selected for BRAF status in a heterogeneous mutational background, were treated with 11 drugs with or without 4 Gy radiation. Radiosensitisation shown is for the clinical radiosensitiser, 5-fluorouracil; two positive control drugs, SAHA and PI103; and compounds selected on the basis of primary screen P-values and potential clinical utility. Significance was determined by paired t-test on IC50 curve values following normalisation for radiation; ‘+’ indicates significant radiosensitisation, with the P-value indicated. | ||||||||||||||||
| BRAF | BRAF V600E | BRAF V600E | BRAF V600E | BRAF V600E | BRAFV600E | BRAFWT | BRAFWT | BRAFWT | BRAFWT | BRAFWT | BRAFWT | BRAFWT | BRAFWT | BRAFWT | BRAFWT | |
| MSI status | MSI | MSI | MSI | MSS | MSS | MSI | MSI | MSI | MSI | MSS | MSS | MSS | MSS | MSS | MSS | |
| KRAS | KRAS WT | KRAS WT | KRAS WT | KRAS WT | KRAS WT | KRAS WT | KRAS WT | KRAS G13D | KRAS P140H | KRAS WT | KRAS WT | KRAS WT | KRAS G12D | KRAS G12V | KRAS A146V | |
| EGFR | EGFR MUT | EGFR WT | EGFR WT | EGFR WT | Not known | EGFR Y1069C | EGFR WT | EGFR WT | EGFR G544*FS | EGFR WT | EGFR WT | EGFR WT | EGFR WT | EGFR WT | EGFR WT | |
| Compound | Target | Radiosensitisation response (+ indicates significant radiosensitisation, with P value given below) | ||||||||||||||
| 5-fluoro-
uracil |
Thymidylate synthase | ns | ns | ns | ns | ns | ns | ns | ns | +p≤0.01 | ns | ns | ns | ns | ns | ns |
| SAHA | HDAC | ns | ns | +≤ 0.05 | ns | +≤ 0.01 | ns | ns | ns | ns | ns | +≤ 0.01 | +≤ 0.05 | +≤ 0.01 | +≤ 0.01 | +≤ 0.05 |
| PI-103 | PI3K/
DNAPK/ mTOR |
+≤ 0.05 | ns | +≤ 0.01 | ns | +≤ 0.01 | +≤ 0.05 | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 | ns | ns | +≤ 0.01 | +≤ 0.01 | +≤ 0.05 | +≤ 0.01 |
| Olaparib | PARP | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 | ns | ns | +≤ 0.01 | +≤ 0.01 | ns | +≤ 0.01 | +≤ 0.01 |
| Rucaparib | PARP | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 | +≤ 0.05 | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 | ns | ns | +≤ 0.05 | +≤ 0.05 | ns | +≤ 0.01 | +≤ 0.01 |
| AZD-7762 | CHK1 and 2 | ns | ns | +≤ 0.05 | ns | +≤ 0.01 | +≤ 0.01 | +≤ 0.05 | +≤ 0.05 | +≤ 0.01 | +≤ 0.01 | ns | +≤ 0.01 | +≤ 0.01 | +≤ 0.05 | ns |
| PF477736 | CHK1 and 2 | +≤ 0.05 | +≤ 0.05 | +≤ 0.05 | +≤ 0.05 | +≤ 0.05 | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 | +≤ 0.05 | ns | ns | +≤ 0.01 | +≤ 0.05 |
| AZD-6244 | MEK1 and 2 | ns | +≤ 0.05 | +≤ 0.01 | ns | +≤ 0.01 | ns | +≤ 0.01 | ns | +≤ 0.01 | +≤ 0.05 | +≤ 0.05 | ns | +≤ 0.01 | +≤ 0.01 | +≤ 0.01 |
| Trametinib | MEK1 and 2 | +≤ 0.05 | ns | +≤ 0.01 | ns | ns | +≤ 0.05 | +≤ 0.01 | +≤ 0.01 | ns | +≤ 0.05 | ns | +≤ 0.05 | +≤ 0.01 | ns | +≤ 0.01 |
| Mitoxantrone | TOPO II | ns | ns | ns | +≤ 0.05 | ns | +≤ 0.05 | +≤ 0.05 | +≤ 0.01 | +≤ 0.01 | ns | +≤ 0.05 | ns | ns | +≤ 0.01 | ns |
| Vemurafenib | BRAF V600E | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
From these assays, olaparib and rucaparib displayed potent radiosensitising ability across multiple cell lines. IC50 curves (normalised for radiation effect) were significantly different (P ≤ 0.01) for all except three cell lines; namely, C10, CW2 and Colo678 (Table 2).
Both Chk1 inhibitors, and trametinib, were also effective radiosensitisers in the majority of cell lines tested. Vemurafenib was ineffective in BRAF WT (IC50 frequently not reached), but showed some efficacy in BRAF mutated cell lines, (not significant for radiosensitisation). This limited effect may arise from feedback activation of EGFR, PI3K or alternative signaling pathways, reducing vemurafenib efficacy in CRC when compared to melanoma37.
Validation of radiosensitisation by PARP inhibitors with clonogenic survival assays
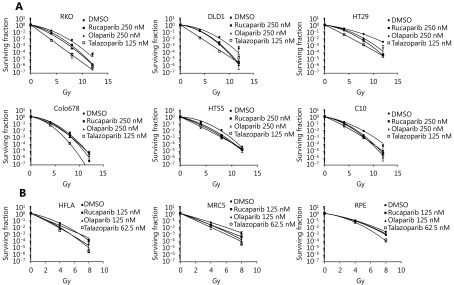
As PARPi were the most effective radiosensitisers of the CRC cell line panel, clonogenic survival assays were used to measure radiation enhancement ratios (RERs) in 3 cell lines that were strongly radiosensitised (> 10-fold IC50 shift) and 3 cell lines with IC50 shift < 10-fold. To potentially improve PARPi radiosensitisation of these resistant cell lines, a more trapping PARPi, talazoparib, was included in these assays. Survival curves ( Figure 3), and RERs (Table 3) reflected the proliferation assay results: Olaparib and rucaparib significantly radiosensitised RKO, DLD1, and HT29 compared to vehicle-treated cells, while radiosensitisation of HT55, Colo678, and C10 was limited – although significant for HT55 cells treated with rucaparib. Talazoparib significantly radiosensitised all cell lines tested, and was overall the most effective radiosensitiser (average RERs 1.21–1.92), followed by rucaparib (average RERs 1.15–1.41) and finally olaparib (average RERs 1.12–1.4).
3.
Clonogenic assays to confirm radiosensitisation of multiple cell lines by PARP inhibitors.
3.
Radiation enhancement ratios of PARP inhibitors for colorectal cancer and non-malignant cell lines
| Gene mutation status | Radiation enhancement ratio (P-value) | |||||||
| BRAF | KRAS | PIK3CA | p53 | Olaparib | Rucaparib | Talazoparib | ||
| Radiation enhancement ratios were calculated from clonogenic survival assays (normalised, by plating efficiency, for effect of drug alone) and comprise the ratio of radiation dose leading to 1% cell survival to the radiation dose producing 1% survival in the combined treatment. Significance (P ≤ 0.05), displayed by in bold, was calculated by one-way ANOVA, with multiple comparisons of each drug against the DMSO control. | ||||||||
| CRC cell lines | ||||||||
| RKO | p.V600E | WT | p.H1047R | WT | 1.48 (P ≤ 0.05) | 1.41 (P ≤ 0.05) | 1.71 (P ≤ 0.001) | |
| HT29 | p.V600E | WT | WT | R273H | 1.44 (P ≤ 0.01) | 1.28 (P ≤ 0.001) | 1.82 (P ≤ 0.001) | |
| DLD1 | WT | G13D | p.E545K | S241F | 1.18 (P ≤ 0.01) | 1.21 (P ≤ 0.01) | 1.92 (P ≤ 0.001) | |
| HT55 | WT | WT | WT | R213L | 1.21 (ns) | 1.31 (P ≤ 0.01) | 1.39 (P ≤ 0.01) | |
| C10 | WT | WT | WT | G245S | 1.12 (ns) | 1.18 (ns) | 1.48 (P ≤ 0.001) | |
| Colo678 | WT | G12D | WT | WT | 1.12 (ns) | 1.15 (ns) | 1.21 (P ≤ 0.001) | |
| Non-malignant cell lines | ||||||||
| HFLA | n/a | n/a | n/a | n/a | 1.09 (ns) | 1.3 (P ≤ 0.05) | 1.29 (ns) | |
| MRC5 | n/a | n/a | n/a | n/a | 1.35 (P ≤ 0.05) | 1.34 (P ≤ 0.05) | 1.52 (P ≤ 0.01) | |
| RPE | n/a | n/a | n/a | n/a | 1.1 (ns) | 1.07 (ns) | 1.24 (P ≤ 0.01) | |
To indicate potential normal tissue toxicity, PARPi experiments were repeated in three non-malignant cell lines, HFLA, MRC5 and RPE. In clonogenic assays (Table 3), these non-malignant cells were significantly radiosensitised by talazoparib. Radiosensitisation by rucaparib was significant for HFLA and MRC5, and radiosensitisation by olaparib was significant only for MRC5 cells (P ≤ 0.05).
Validation of PARP inhibitors as radiosensitisers in xenograft studies
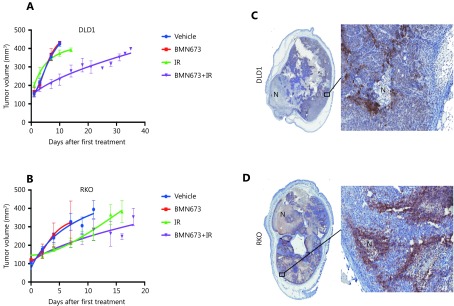
The PARP inhibitor talazoparib was the most effective radiosensitiser and had not previously been tested with radiotherapy in animal CRC models. To confirm the in vitro radiosensitisation by PARPi in an in vivo model, talazoparib was tested against two cell lines that were effectively radiosensitised by the drug in 2D assays. Mice were inoculated with subcutaneous tumors consisting of RKO or DLD1 cells, and treated with talazoparib or vehicle, either alone or one hour before each of 2 × 5 Gy radiation treatments. In DLD1 cells (Figure 4A), single treatment with talazoparib or radiation alone did not inhibit tumour growth. Combined talazoparib/radiation treatment was tolerated by the mice, and significantly reduced tumour growth compared with radiation alone (P ≤ 0.01). For the RKO cell xenograft model, there was no significant difference between the effect of radiation alone, and the radiation/talazoparib combination. Tumour histology, levels of perinecrotic hypoxia (CA9 staining) and necrosis were similar for both cell types (Figure 4B).
4.
Talazoparib significantly enhances the response of colorectal cancer cells grown in vivo to ionizing radiation.
Discussion
The aim of this study was to identify treatment options to radiosensitise colorectal cancer cells in the context of key mutations that characterise the disease. Biopsies from CRC patients are routinely screened for BRAF, KRAS and PIK3CA mutations, but this information is not currently used in treatment decisions regarding radiotherapy. There is preclinical evidence that single gene alterations in cancer can determine the extent of radiosensitisation exerted by different drugs. Examples include mammalian AMP-activated protein kinase dependence of pancreatic cancer cells to radiosensitisation by metformin38, the role of mismatch repair deficiency in radiosensitisation of CRC cell lines by gemcitabine39-40 and p53-dependent radiosensitisation by valproic acid41. Radiosensitisation drug discovery across different genetic backgrounds may enable a change from a “one size fits all” chemo- radiotherapy to the identification of the most appropriate drugs for radiotherapy based on the genetic profile of the cancer.
To address our primary aim, we developed a novel high-throughput screen to test drug library/radiotherapy combination against cell lines. For drug repurposing, which allows more rapid translation in to the clinic, we used a library of drugs already in clinical use or in clinical trials. Previous investigators using more focused library screens have successfully identified radiosensitisers of CRC42 and our study identified the same drugs with radiosensitising potential, the CHK inhibitor, AZD-7762, and the dual mTOR/PI3K inhibitor, dactosilib. We initially used isogenic cell lines to identify radiosensitisers active in a BRAF V600E background. Reassuringly, our results confirmed radiosensitisation by agents from drug classes previously shown to have radiosensitising activity in other published papers, such as inhibitors of the RAS/MEK/ERK, and PI3K/MTOR pathways. In addition, we identified compounds not previously known to be radiosensitisers (Table 1). Of the drugs targeting mutated BRAF (vemurafenib, dabrafenib, RAF265), only vemurafenib reached the threshold for hit-detection in the screen, possibly because vemurafenib is a more potent radiosensitizer, at least compared with dabrafenib43.
Cell lines manipulated by gene mutation might not be entirely representative of the molecular landscape of cancer in patients. We therefore validated results from isogenic cell lines in a panel of human colorectal cancer cell lines, inclusive of common CRC mutations and previously shown to be a useful model for drug development22,44. This approach was also novel since this cell line panel has not previously been used to test new drug-radiotherapy combinations. The results (shown in Table 2), confirmed PARPi as significant radiosensitisers, notably across a much broader range of cell lines than 5FU, the current clinical standard, suggesting that 5FU may not be the optimal treatment for all CRC patients compared to newer and more targeted drugs. This reflects data in other studies in CRC, which show that radiosensitisation by 5FU varies depending on the cell line used45,46. Additionally, the timing of 5FU exposure may influence the degree of radiosensitisation47.
In future, immunotherapy is likely to be of increasing importance in CRC treatment, although at present it is only used to treat the more immunogenic MSI-high tumours48. Despite this, radiotherapy is likely to remain an important treatment for rectal cancer and metastatic disease, particularly when the cost effectiveness of treatment is considered. The broad range of cell lines for which PARPi appear to be suitable radiosensitisers in this study may predict its potential future utility in a wide patient population.
Three PARPi, olaparib, rucaparib, and niraparib, have been approved by the US FDA for the treatment of ovarian cancer, including BRCA-deficient tumours that have deficient homologous recombination repair. PARPi function by inhibiting the binding, or enzymatic activity, of PARP to single strand breaks in DNA. The absence of SSB repair leads to double strand break (DSB) formation at the approaching replication fork, and cell death. It has been shown that PARPi have an increased radiosensitising effect on DSB- repair deficient tumour cells compared with DSB- repair proficient lines49. Compared to olaparib and rucaparib, we found that talazoparib treatment led to higher RERs. PARPi affect cell proliferation by two main actions: inhibiting PARP enzymatic function, and by binding (‘trapping’) PARP to DNA50. Olaparib and rucaparib function primarily through inhibiting enzymatic function, whereas talazoparib ‘traps’ PARP at DNA damage sites, with increased anti-proliferative effect, potentially contributing to more effective radiosensitisation51,52.
We proceeded to show that the PARP inhibitor, talazoparib, radiosensitised DLD1 xenografts in vivo. The combined treatment caused a prolonged tumour growth delay, in excess of the effects demonstrated elsewhere for combined 5FU/radiation treatment for HCT11645 and WiDr53 CRC xenografts. It is unclear why talazoparib did not significantly radiosensitise BRAF mutated RKO xenografts in vivo. It has been shown that BRAF-mutant early neoplastic lesions have upregulation of gene sets involved in aberrant DNA methylation54 and that BRAF-mutant cancers can have distinct tumour-associated-stroma and components of the extracellular matrix that are different from wild-type cancers55. These complexities may explain the discrepancy between the highly significant results we obtained in 2D culture and the non-significant results we obtained in vivo using the same cell line. Future studies should consider the use of other models, such as patient-derived xenografts or immunocompetent mouse models, to explore this discrepancy further.
Some investigators advocate preclinical comparison of non-malignant with malignant cell lines to identify cancer-specific drugs56,57. In our study, olaparib did not cause significant radiosensitisation of two non-malignant cell lines, HLA and RPE. An in vivo study of intestinal crypt damage, in which fractionated radiotherapy was combined with olaparib, did not appear to cause additional gut toxicity compared to radiotherapy without drug58. Contrastingly, clinical studies of PARPi have documented bowel toxicities as side effects of treatment59 and total body irradiation of a p21-reporter mouse has shown that olaparib can exacerbate DNA damage in normal tissues when combined with radiation60. It should be noted that, in our study, rucaparib and talazoparib caused significant radiosensitisation of 2 non-malignant cells tested by clonogenic survival assays. Although talazoparib has already completed phase I development as a single agent61, we recommend that the normal tissue toxicity from the combination of PARPi with radiotherapy should be assessed further in preclinical normal tissue toxicity models and monitored closely in early-phase clinical trials.
In conclusion, our novel approach to radiosensitisation drug discovery in cells isogenic for the BRAF V600E mutation, has led to the identification of PARPi as radiosensitisers for CRC. Validation in a broad panel of human CRC cell lines, and an in vivo xenograft model, has shown potentially broader radiosensitising activity than the current clinical standard of care, 5FU. Following toxicity evaluation of the combination of PARPi with radiotherapy in other preclinical models, we propose that PARP inhibition should be tested in combination with radiotherapy for rectal cancer or metastatic CRC treatment, with careful monitoring of potential toxicities.
Acknowledgements
This work was supported by Bowel Disease Research Foundation, Oxford Cancer Research Centre, the National Institute for Health Research University College London Hospitals Biomedical Research Centre, the Cancer Research UK University College London Experimental Cancer Medicine Centre, CRUK-UCL Centre Award (Grant No. C416/A25145), the Cancer Research UK Centers Network Accelerator Award Grant (Grant No. A21993) to the ART-NET Consortium, and the NIHR Oxford Biomedical Research Centre.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Supplementary material
S1.
Details of the cell lines
| Cell line | BRAF | KRAS | PIK3CA | P53 | MSI/MSS | CIMP |
| Table of cell lines comprising the panel for screen validation: Data is from Mouradov et al., Cancer Res. 2014; 74: 3238-47, except where indicated.
† Indicates data from Prof. Walter Bodmer, personal communication. na. Indicates information not available | ||||||
| C10 | WT | WT | WT | WT | MSS | CIMP- |
| C99 | WT | WT | WT | WT | MSS | CIMP- |
| CCK81 | WT | WT | C420R, C472Y | P278H | MSI | CIMP- |
| COLO678 | WT | G12D | WT | WT | MSS | CIMP+ |
| CW2 † | WT | P140H | P283S | WT | MSI | na. |
| DLD1 | WT | G13D | E545K | S241F | MSI | CIMP+ |
| HCA7 | WT | WT | WT | P301fs*44 | MSI | CIMP+ |
| HT29 | V600E | WT | WT | R273H | MSS | CIMP+ |
| HT55 | V600E | WT | WT | .R213L | MSS | CIMP- |
| LS411 | V600E | WT | WT | Y126* | MSI | CIMP+ |
| OXCO4 † | V600E | WT | WT | mutant | MSS | na. |
| RKO | V600E | WT | H1047R | WT | MSI | CIMP+ |
| SW1222 | WT | A146V | WT | WT | MSS | CIMP- |
| SW403 | WT | G12V | Q546K | E51* | MSS | CIMP- |
| VACO5 † | WT | WT | H1047R | mutant | MSI | na. |
S2.
Anticancer drugs comprising the small compound library for the screen
| Table of compounds tested from the combined TDI Extended Oncology Drugs Library (ODL) and the NCI Developmental Therapeutics Program (DTP) Approved Oncology Drug Library. | ||||
| (5Z)-7-Oxozeaenol | Bleomycin | FK 506_Tacrolimus | Mitomycin C | Rapamycin (sirolimus) |
| (R)-Flurbiprofen (Tarenflurbil) | BMS-754807 | FK-866 HCl_Daporinad | Mitotane | RD162 |
| 1-methyl-D-tryptophan, 95% | BMS-911543 | Floxuridine | Mitoxantrone | RDEA119_Refametinib |
| 17-AAG (Tanespimycin, Geldanamycin) | Bortezomib | Fludarabine | MK-0752 | Ridaforolimus |
| 17-DMAG (Alvespimycin) | Bosutinib | Fluorouracil | MK-2206 | Rofecoxib (Vioxx) |
| 2-methoxyestradiol (Panzem) | Brivanib | Flutamide | MK-4827, HCl salt | Romidepsin |
| 4-hydroxytamoxifen | Busulfan | Fulvestrant | MK1775 | Roscovitine_Selicilib |
| Abitrexate/Methotrexate | Cabazitaxel | Galiellalactone | MLN4924 | S-trityl-L-cysteine, 40 mM |
| ABT-199 | CAL-101 | GDC-0068 | MLN8237_Alisertib | SB 743921 |
| ABT-263 (Navitoclax) | Camptothecin | GDC-0941_Pictilisib | Motesanib Di phosphate (AMG-706) | Simvastatin |
| ABT-751 | Canertinib | GDC-0980 | Nelarabine | Sorafenib |
| ABT-869_Linifanib | Capecitabine | Gefitinib | Nilotinib | Sotrastaurin |
| ABT-888 (Veliparib) | Carboplatin | Gemcitabine HCl | Nilutamide | SR1 HCl |
| AC220_Quizartinib | Carfilzomib | Goserelin acetate | Nitrogen mustard | Stattic |
| Acrichine | Carmustine | GSK 269962 | Nutlin-3 | Streptozocin |
| AG-014699_Rucaparib | Celecoxib | GSK 650394 | NVP-AUY922 | Sunitinib |
| Allopurinol | CHIR-258 (Dovitinib) | GSK1120212_ Trametinib | NVP-BEZ235_Dactolisib | TAK-733 |
| Altretamine | Chlorambucil | GSK2126458 | NVP-BGJ398 | TAK-901 |
| Amifostine | Chloroquine diphosphate | GSK2636771 | NVP-LDE225 (Diphosphate salt) | Tamoxifen citrate |
| Aminoglutethimide | CHR 2797_Tosedostat | HA-1077 (Fasudil) | Obatoclax Mesylate (GX15-070) | Tandutinib |
| Aminolevulinic acid | CI-994_Tacedinaline | Homoharringtonine | Olaparib | Tasocitinib_Tofacitinib |
| Amonafide | Cisplatin aq | Hydroxyurea | OSI-027 | Temozolomide |
| Anagrelide | Cladribine | I-BET151 (GSK1210151A) | OSI-906_Linsitinib | Teniposide |
| Anastrozole | Clafen (Cyclophos-phamide, Endoxan) | Idarubicin HCl | Oxaliplatin | Tetramisole HCl |
| AP24534 (Ponatinib) | Clofarabine | Ifosfamide | PAC-1 | TGX-221 |
| ARQ 197_Tivantinib | Clomifene citrate | Imatinib | Paclitaxel | Thalidomide |
| ARRY-162_MEK-162 | CPI-613 | Imiquimod | Panobinostat | Thio-TEPA |
| Arsenic(III) oxide | Crenolanib | INCB018424 (free base, Ruxolitinib) | Pazopanib | Thioguanine |
| AS703026_Pimasertib | Crizotinib | Indibulin | PCI-32765_Ibrutinib | Thiotepa |
| Aspirin (Acetylsalicylic Acid) | CUDC-101 | Iniparib (BSI-201, IND-71677) | PD-0332991 | Tipifarnib (Zarnestra) |
| AT 101 | Cyclophosphamide | INK128 | Pemetrexed | Topotecan HCl |
| AT-406 | CYT-387_Momelotinib | Irinotecan | Pentostatin | Toremifene citrate |
| AT9283 | Cytarabine HCl | Ixabepilone | Perifosine aq/PBS | Tretinoin |
| Atorvastatin Ca | Dabrafenib Mesylate | JNJ 26854165 (Serdemetan) | PF 431396 | Triethylenemelamine |
| Auranofin | Dacarbazine | JNJ_26481585_Quisinostat | PF 477736 | Tubacin |
| AV-951 (Tivozanib) | Dacomitinib (monohydrate) (PF-00299804) | KX2-391 | PF-04691502 | Tubastatin A HCl |
| AVN944 | Dactinomycin | Lapatinib, di-p-toluenesulfonate salt | PF-04708671 | UCN-01 |
| Axitinib | Dasatinib | Lasofoxifene | PF-2341066 (Crizotinib) | Uracil mustard |
| AZ 3146 | Daunorubicin HCl | Lenalidomide | PF-3845 | Valproic acid |
| Azacitidine | DCC-2036_Rebastinib | Lestaurtinib | PF4800567 hydrochloride | Valrubicin |
| AZD 7762 hydrochloride | Decitabine | Letrozole | PF670462 | Vandetanib |
| AZD1152-HQPA | Decitabine (Dacogen) | Lomeguatrib | PHA-739358 (Danusertib) | Varespladib |
| AZD1480 | Deferoxamine mesylate | Lomustine, CCNU | PIK-75 HCl | Vatalanib |
| AZD2014 | Dexamethasone (Decadron) | LY 333531 mesylate- Ruboxistaurin | Pilocarpine | Vemurafenib |
| AZD4547 | Dexrazoxone | LY2157299 | Pipobroman | VER 155008 |
| AZD6244 (Selumetinib) | Dinaciclib (SCH727965) | LY2228820 (CP868569) | PKC412_Midostaurin | Vinblastine sulfate |
| AZD8055 | Docetaxel | LY2603618_Rabusertib | Plerixafor | Vincristine Sulfate (Oncovin) |
| BAY 73-4506_Regorafenib | Doxorubicin | LY2784544_Gandotinib | Plicamycin | Vinorelbine tartrate |
| Belinostat (PXD101) | Doxorubicin HCl | Masitinib | PLX4032_Vemurafenib | Vismodegib |
| Bendamustine HCl | EMD1214063 | MDV3100_Enzaluamide | Pralatrexate | Vorinostat |
| Bexarotene | Entinostat | Megestrol acetate | Pravastatin | VX-11e |
| BI 2536 | Enzastaurin | Melphalan | Prednisolone | XAV-939 |
| BI 6727_Volasertib | Epothilone B (Patupilone) | Mercaptopurine | Prednisone | XL-147 |
| BIBF 1120_Nintedanib | Erlotinib HCl | Metformin
hydrochloride aq |
Prima-1 Met | XL184_Cabozantinib |
| BIBW2992 (Tovok)_Afatinib | Estramustine sodium phosphate | Methotrexate | Procarbazine | XL880 (Foretinib) |
| Bicalutamide | Etoposide | Methoxsalen | PX-866_Sonolisib | YM155 |
| BIIB021 | Everolimus | Methylprednisolone | Quinacrine HCl | Zolendronic acid |
| Bimatoprost | Exemestane | MGCD-265 | R406_Tamatinib | ZSTK474 |
| BIRB 796 (Doramapimod) | FG-4592 | MGCD0103_Mocetinostat | RAF265 | |
| BKM-120_Buparlisib | Finasteride | Mithramycin A | Raloxifene HCl | |
S3.
IC50 (μM) for each drug at 0 and 4 Gy in a panel of colorectal cancer cell lines
| Cell line | 5-FU IC50 | Vorinostat IC50 | PI-103 IC50 | Olaparib IC50 | Rucaparib IC50 | Mitoxantrone IC50 |
| IC50 was calculated using Graphpad Prism following normalisation for radiation effect, and is shown in μM, with 95% confidence limits in parenthesis.
* Where the curve shape did not allow calculation of IC50 in Graphpad, IC50 was calculated manually by interpolation. * > indicates the highest concentration tested in cell lines where the IC 50 was not reached. | ||||||
| LS411 0 Gy | 24.67
(17.58 to 35.46) |
6.79
(3.99 to 11.91) |
5.23
(3.47 to 8.12) |
24.46
(15.71 to 38.89) |
*62.18 | 16.75
(12.32 to 22.98) |
| LS411 4 Gy | 21.95
(12.37 to 40.8) |
14.55
(9.69 to 22.27) |
2.76
(1.57 to 4.96) |
2.11
(1.09 to 4.18) |
1.72
(0.49 to 8.54) |
7.8
(5.63 to 10.89) |
| VACO5 0 Gy | 2.54
(1.95 to 3.35) |
3.45
(2.47 to 4.9) |
1.91
(1.05 to 3.58) |
10.5
(3.83 to 29.59) |
34.03
(21.2 to 58.03) |
3.49
(0.87 to 14.64) |
| VACO5 4 Gy | 0.99
(0.83 to 1.19) |
3.37
(2.85 to 4) |
0.48
(0.34 to 0.71) |
0.75
(0.43 to 1.3) |
3.07
(0.61 to 11.91) |
1.24
(0.56 to 2.99) |
| RKO 0 Gy | 2.51
(1.93 to 3.29) |
6.51
(3.89 to 11.26) |
1.55
(0.95 to 2.54) |
8.63
(4.32 to 17.22) |
61.23
(30.07 to 167.4) |
9.75
(6.21 to 15.44) |
| RKO 4 Gy | 1.15
(0.73 to 1.90) |
2.14
(1.2 to 4.15) |
0.33
(0.20 to 0.57) |
0.35
(0.15 to 0.78) |
0.3
(0.03 to 1.59) |
2.9
(1.59 to 5.46) |
| HT29 0 Gy | 9.12
(6.67 to 12.66) |
3.47
(2.42 to 5.03) |
* >20 | 17.93
(4.14 to 75.29) |
51.82
(33.61 to 86.34) |
6.58
(1.24 to 78.99) |
| HT29 4 Gy | 6.6
(5.24 to 8.36) |
4.18
(2.6 to 6.94) |
* 12.94 | 2.21
(1.25 to 3.55) |
5.48
(2.49 to 11.75) |
3.06
(0.88 to 12) |
| OXCO4 0 Gy | 16.71
(14.13 to 19.85) |
6.09
(3.69 to 10.41) |
2.42
(1.82 to 3.24) |
26.88
(16.82 to 43.79) |
13.11
(10.42 to 16.58) |
0.89
(0.61 to 1.33) |
| OXCO4 4 Gy | 9.45
(7.92 to 11.32) |
3.82
(2.69 to 5.49) |
1.13
(0.87 to 1.47) |
6.07
(4.73 to 7.82) |
2.5
(1.74 to 3.61) |
0.59
(0.44 to 0.78) |
| CW2 0 Gy | 20.19
(15.17 to 27.24) |
4.49
(2.9 to 7.08) |
4.97
(3.14 to 8.17) |
17.05
(6.48 to 44.79) |
36.91
(30.48 to 45.16) |
19.81
(11.2 to 35.76) |
| CW2 4 Gy | *20.1 | 5.33
(3.59 to 7.99) |
4.21
(1.49 to 14.87) |
* >20 | * >30 | 21.02
(12.56 to 186) |
| DLD1 0 Gy | 8.6
(6.77 to 10.99) |
6.26
(3.07 to 13.72) |
1.69
(0.94 to 3.08) |
* >100 | *30.41 | 4.08
(1.49 to 12.46) |
| DLD1 4 Gy | 7.78
(5.26 to 11.81) |
3.25
(2.02 to 5.42) |
0.52
(0.29 to 0.95) |
1.74
(0.89 to 3.5) |
0.44
(0.15 to 2.1) |
1.9
(1.33 to 2.72) |
| CCK81 0 Gy | 29.85
(23.48 to 38) |
10.4
(5.37 to 21.34) |
1.27
(0.92 to 1.76) |
>100 | *48.51 | *16.51 |
| CCK81 4Gy | 20.77
(16.64 to 26.01) |
7.84
(4.13 to 15.86) |
1.07
(0.81 to 1.44) |
13.05
(7.62 to 22.53) |
45.05
(11.83 to 105.1) |
22.6
(11.62 to 60.7) |
| C10 0 Gy | 43.38
(31.27 to 60.93) |
2.05
(0.82 to 6.16) |
0.98
(0.45 to 2.19) |
* >100 | 23.7
(20.64 to 222.2) |
3.92
(1.84 to 8.96) |
| C10 4 Gy | 39.86
(17.15 to 101.3) |
10.21
(1.3 to 74.5) |
0.53
(0.27 to 1.17) |
* >100 | 22.4
(6.87 to 130) |
2.18
(1.14 to 4.29) |
| SW403 0 Gy | 1.31
(0.86 to 2.02) |
17.71
(7.57 to 49.28) |
* >20 | 6.18
(1.46 to 26.89) |
40.51
(27.91 to 61.56) |
2.17
(0.94 to 5.27) |
| SW403 4 Gy | 0.73
(0.49 to 1.09) |
7.28
(4.53 to 12.05) |
10.81
(5.35 to 27.22) |
0.85
(0.28 to 2.46) |
12.39
(5.71 to 26.75) |
1.46
(0.66 to 3.41) |
| COLO678 0 Gy | 85
(37.7 to 197.4) |
8.68
(1.84 to 64.49) |
2.3
(1.08 to 5.15) |
* >200 | * 48.67 | 5.96
(2.98 to 12.18) |
| COLO678 4 Gy | 81.5
(70.58 to 129.3) |
*5.5 | 2.24
(1.09 to 4.85) |
* >200 | * 45.79 | 20.31
(12.29 to 34.44) |
| SW1222 0 Gy | 10.58
(5.80 to 20.42) |
41.93
(16.03 to 134) |
* >20 | 16.72
(9.97 to 28.91) |
9.78
(4.56 to 22.31) |
2.76
(1.63 to 4.8) |
| SW1222 4 Gy | 3.23
(2.19 to 4.75) |
4.07
(2.98 to 5.63) |
0.75
(0.63 to 0.90) |
0.42
(0.32 to 0.56) |
* 0.32 | 0.73
(0.32 to 1.83) |
| HCA7 0 Gy | 27.64
(22.63 to 33.87) |
1.29
(0.93 to 1.80) |
2.95
(1.71 to 5.26) |
3.99
(3.16 to 5.05) |
48.51
(35.36 to 68.98) |
1.93
(0.57 to 6.89) |
| HCA7 4 Gy | 19.82
(16.49 to 23.89) |
0.82
(0.70 to 0.97) |
1.14
(0.79 to 1.66) |
0.24
(0.18 to 0.32) |
0.36
(0.19 to 0.66) |
0.75
(0.24 to 2.53) |
| HT55 0 Gy | 10.53
(7.91 to 14.17) |
2.11
(1.51 to 3) |
2.87
(0.95 to 9.99) |
41.07(4.96 to 28.01) | 12.2
(8.31 to 18.19) |
1.26
(0.86 to 1.85) |
| HT55 4 Gy | 12.03
(8.91 to 16.48) |
3.14(1.99 to 5.10) | 2.58
(1.85 to 3.63) |
7.88
(0.88 to 3.66) |
1.47
(0.4 to 5.18) |
1.27
(0.79 to 2.08) |
| C99 0 Gy | 3.34
(2.11 to 5.72) |
3.53
(1.8 to 7.46) |
23.77
(8.94 to 31.98) |
14.01
(4.11 to 53.54) |
39.28
(21.29 to 81.87) |
1.15
(0.58 to 2.31) |
| C99 4 Gy | 4.44
(2.37 to 8.86) |
3
(1.66 to 5.92) |
0.97
(0.30 to 3.48) |
0.44
(0.22 to 0.87) |
14.2
(0.16 to 18) |
0.49
(0.27 to 0.92) |
| Cell line | AZD-7762 IC50 | PF4777 IC50 | AZD-6244 IC50 | Trametinib IC50 | Vemurafenib IC50 | |
| LS411 0 Gy | 2.69
(1.5 to 6.50) |
3.84
(2.91 to 5.08) |
11.92
(5.13 to 39.4) |
2.03
(0.08 to 25.26) |
58.81
(30.19 to 144.8) |
|
| LS411 4 Gy | 0.41
(0.25 to 0.69) |
1.49
(1.06 to 1.83) |
6.94
(2.62 to 24.83) |
1.81
(0.006 to 48.6) |
20.39
(4.63 to 210.7) |
|
| RKO 0 Gy | 0.02
(0.015 to 0.03) |
0.47
(0.31 to 0.71) |
*148.75 | 0.09
(0.03 to 0.3) |
15.14
(4.37 to 57.2) |
|
| RKO 4 Gy | 0.005
(0.004 to 0.008) |
0.19
(0.15 to 0.25) |
4.62
(0.74 to 46.47) |
0.03
(0.01 to 0.07) |
4.57
(0.99 to 29.93) |
|
| VACO5 0 Gy | 0.05
(0.03 to 0.11) |
1.5
(1.06 to 2.16) |
14.81
(7.95 to 29.18) |
0.01
(0.007 to 0.017) |
9.37
(6.57 to 13.45) |
|
| VACO5 4 Gy | 0.01
(0.004 to 0.02) |
0.28
(0.23 to 0.34) |
7.08
(4.28 to 11.86) |
0.003
(0.003 to 0.004) |
3.86
(2.6 to 5.84) |
|
| HT29 0 Gy | 0.03
(0.02 to 0.06) |
4.08
(2.58 to 6.89) |
2.34(1.02 to 5.62) | 0.02
(0.01 to 0.04) |
13.1
(5.49 to 32.24) |
|
| HT29 4 Gy | 0.01
(0.003 to 0.03) |
1.57
(1.03 to 2.45) |
1.87(0.62 to 6.39) | 0.01
(0.007 to 0.02) |
11.76
(6.68 to 20.96) |
|
| OXCO4 0 Gy | 2.14
(1.39 to 3.73) |
1.54
(1.08 to 2.22) |
3.04
(1.76 to 5.35) |
*0.15 | 14.57
(10.14 to 21.19) |
|
| OXCO4 4 Gy | 0.17
(0.13 to 0.22) |
0.76
(0.54 to 1.09) |
0.82
(0.33 to 2.4) |
*0.06 | 10.6
(4.09 to 28.44) |
|
| CW2 0 Gy | 2.16
(1.17 to 5.32) |
26.75
(21.9 to 32.78) |
1.72
(0.47 to 9.39) |
0.46
(0.2 to 1.24) |
*53.05 | |
| CW2 4 Gy | * >2 | 20.75
(6.06 to 71.96) |
* >10 | 0.18
(0.076 to 0.44) |
*49.07 | |
| DLD1 0 Gy | 0.14
(0.1 to 0.21) |
* 15.02 | * >20 | * >1 | *66.41 | |
| DLD1 4 Gy | 0.02
(0.01 to 0.05) |
5.46
(2.36 to 12.91) |
* >20 | 0.08
(0.02 to 0.59) |
33.24
(14.01 to 84.84) |
|
| CCK81 0 Gy | 0.75
(0.43 to 1.42) |
* >10 | * >20 | *>1 | * >160 | |
| CCK81 4Gy | 0.11
(0.08 to 0.17) |
* >10 | * >20 | * >1 | * >160 | |
| C10 0 Gy | 0.12
(0.1 to 0.15) |
*10.08 | *25.69 | 0.68
(0.26 to 3.31) |
53.16
(30.73 to 99.45) |
|
| C10 4 Gy | 0.12
(0.08 to 0.2) |
* >10 | * >20 | 0.29
(0.13 to 0.74) |
48.79
(23.05 to 119.8) |
|
| SW403 0 Gy | 0.26
(0.18 to 0.37) |
0.79
(0.44 to 1.41) |
6.45
(3.02 to 15.7) |
*2.03 | * >80 | |
| SW403 4 Gy | 0.13
(0.1 to 0.16) |
0.37
(0.22 to 0.62) |
1.71
(1.08 to 2.76) |
* 1.68 | * >80 | |
| COLO678 0 Gy | * >2 | *25.14 | 1.12
(0.72 to 1.78) |
0.006
(0.005 to 0.008) |
* >80 | |
| COLO678 4 Gy | * >2 | *25.39 | 1.47
(1.07 to 2.04) |
0.005
(0.002 to 0.01) |
* >80 | |
| SW1222 0 Gy | 0.07
(0.05 to 0.1) |
6.26
(4.02 to 10.64) |
3.75
(0.64 to 45.5) |
0.23
(0.09 to 0.69) |
* >160 | |
| SW1222 4 Gy | 0.02
(0.02 to 0.02) |
1.19
(0.76 to 1.91) |
0.61
(0.43 to 0.87) |
0.04
(0.02 to 0.07) |
39.5
(27.01-57.36) |
|
| HCA7 0 Gy | 0.06
(0.01 to 0.47) |
2.37
(1.76 to 3.26) |
* >20 | 0.41
(0.21 to 0.89) |
196.8
(178.93 to 231) |
|
| HCA7 4 Gy | 0.01
(0.00 to 0.14) |
0.42
(0.36 to 0.49) |
*18.77 | 0.15
(0.09 to 0.24) |
116.7
(64.29 to 256.3) |
|
| HT55 0 Gy | 0.06
(0.05 to 0.09) |
0.97
(0.73 to 1.28) |
1.55
(0.28 to 5.02) |
0.08
(0.03 to 0.29) |
49.39
(15.7 to 169.4) |
|
| HT55 4 Gy | 0.02
(0.01 to 0.02) |
0.37
(0.3 to 0.47) |
1.55
(0.41 to 3.33) |
0.05
(0.03 to 0.09) |
50.77
(22.77 to 132) |
|
| C99 0 Gy | 0.12
(0.07 to 0.22) |
3.75
(1.92 to 8.38) |
1.27
(0.32 to 5.5) |
0.01
(0.007 to 0.023) |
* >160 | |
| C99 4 Gy | 0.34
(0.07 to 2.32) |
1.43
(0.32 to 7.72) |
0.38
(0.16 to 1.08) |
0.004
(0.002 to 0.007) |
* >160 | |
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Gaertner WB, Kwaan MR, Madoff RD, Melton GB Rectal cancer: an evidence-based update for primary care providers. World J Gastroenterol. 2015;21:7659–71. doi: 10.3748/wjg.v21.i25.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolay NH, Berry DP, Sharma RA Liver metastases from colorectal cancer: radioembolization with systemic therapy. Nat Rev Clin Oncol. 2009;6:687–97. doi: 10.1038/nrclinonc.2009.165. [DOI] [PubMed] [Google Scholar]
- 4.Hong TS, Wo JY, Borger DR, Yeap BY, McDonnell EI, Willers H, et al Phase II study of proton-based stereotactic body radiation therapy for liver metastases: importance of tumor genotype. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx031. [DOI] [PubMed] [Google Scholar]
- 5.Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, et al Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29:3753–60. doi: 10.1200/JCO.2011.34.9068. [DOI] [PubMed] [Google Scholar]
- 6.Deng YH, Chi P, Lan P, Wang L, Chen WQ, Cui L, et al Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. 2016;34:3300–7. doi: 10.1200/JCO.2016.66.6198. [DOI] [PubMed] [Google Scholar]
- 7.Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–89. doi: 10.1016/S1470-2045(15)00159-X. [DOI] [PubMed] [Google Scholar]
- 8.Guinney J, Dienstmann R, Wang X, De Reyniès A, Schlicker A, Soneson C, et al The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–6. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiovitz S, Grady WM Molecular markers predictive of chemotherapy response in colorectal cancer. Curr Gastroenterol Rep. 2015;17:431. doi: 10.1007/s11894-015-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellano E, Downward J RAS interaction with PI3K: more than just another effector pathway. Genes Cancer. 2011;2:261–74. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan PTC, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/S0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 12.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature11252. [DOI] [PubMed] [Google Scholar]
- 13.Seligmann JF, Fisher D, Smith CG, Richman SD, Elliott F, Brown S, et al Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: analysis from 2530 patients in randomised clinical trials . Ann Oncol. 2017;28:562–8. doi: 10.1093/annonc/mdw645. [DOI] [PubMed] [Google Scholar]
- 14.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–74. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 15.Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, et al Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers . Cancer Res. 2005;65:6063–9. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 16.De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis . Lancet Oncol. 2010;11:753–62. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 17.Hsu HC, Thiam TK, Lu CJ, Yeh CY, Tsai WS, You JF, et al Mutations of KRAS/NRAS/BRAF predict cetuximab resistance in metastatic colorectal cancer patients . Oncotarget. 2016;7:22257–70. doi: 10.18632/oncotarget.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Souglakos J, Philips J, Wang R, Marwah S, Silver M, Tzardi M, et al Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br J Cancer. 2009;101:465–72. doi: 10.1038/sj.bjc.6605164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaeger R, Cercek A, Chou JF, Sylvester BE, Kemeny NE, Hechtman JF, et al BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer. 2014;120:2316–24. doi: 10.1002/cncr.28729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed KA, Caudell JJ, El-Haddad G, Berglund AE, Welsh EA, Yue BL, et al Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95:1399–404. doi: 10.1016/j.ijrobp.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma RA, Plummer R, Stock JK, Greenhalgh TA, Ataman O, Kelly S, et al Clinical development of new drug-radiotherapy combinations. Nat Rev Clin Oncol. 2016;13:627–42. doi: 10.1038/nrclinonc.2016.79. [DOI] [PubMed] [Google Scholar]
- 22.Mouradov D, Sloggett C, Jorissen RN, Love CG, Li S, Burgess AW, et al Colorectal cancer cell lines are representative models of the main molecular subtypes of primary cancer. Cancer Res. 2014;74:3238–47. doi: 10.1158/0008-5472.CAN-14-0013. [DOI] [PubMed] [Google Scholar]
- 23.Inglese J, Shamu CE, Guy RK Reporting data from high-throughput screening of small-molecule libraries. Nat Chem Biol. 2007;3:438–41. doi: 10.1038/nchembio0807-438. [DOI] [PubMed] [Google Scholar]
- 24.Hong FX, Breitling R, McEntee CW, Wittner BS, Nemhauser JL, Chory J RankProd: a bioconductor package for detecting differentially expressed genes in meta-analysis. Bioinformatics. 2006;22:2825–7. doi: 10.1093/bioinformatics/btl476. [DOI] [PubMed] [Google Scholar]
- 25.Carter R, Westhorpe A, Romero MJ, Habtemariam A, Gallevo CR, Bark Y, et al Radiosensitisation of human colorectal cancer cells by ruthenium(II) arene anticancer complexes. Sci Rep. 2016;6:20596. doi: 10.1038/srep20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franken NAP, Rodermond HM, Stap J, Haveman J, Van Bree C Clonogenic assay of cells in vitro . Nat Protoc. 2006;1:2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 27.Jiang YY, Verbiest T, Devery AM, Bokobza SM, Weber AM, Leszczynska KB, et al Hypoxia potentiates the radiation-sensitizing effect of olaparib in human non-small cell lung cancer xenografts by contextual synthetic lethality. Int J Radiat Oncol Biol Phys. 2016;95:772–81. doi: 10.1016/j.ijrobp.2016.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Wang M, Kern AM, Khaled S, Han J, Yeap BY, et al Adapting a drug screening platform to discover associations of molecular targeted radiosensitizers with genomic biomarkers. Mol Cancer Res. 2015;13:713–20. doi: 10.1158/1541-7786.MCR-14-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodzic J, Dingjan I, Maas MJP, Van Der Meulen-Muileman IH, De Menezes RX, Heukelom S, et al A cell-based high-throughput screening assay for radiation susceptibility using automated cell counting. Radiat Oncol. 2015;10:55. doi: 10.1186/s13014-015-0355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potiron VA, Abderrahmani R, Giang E, Chiavassa S, Di Tomaso E, Maira SM, et al Radiosensitization of prostate cancer cells by the dual PI3K/mTOR inhibitor BEZ235 under normoxic and hypoxic conditions. Radiother Oncol. 2013;106:138–46. doi: 10.1016/j.radonc.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Groselj B, Kerr M, Kiltie AE Radiosensitisation of bladder cancer cells by panobinostat is modulated by Ku80 expression. Radiother Oncol. 2013;108:429–33. doi: 10.1016/j.radonc.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senra JM, Telfer BA, Cherry KE, McCrudden CM, Hirst DG, O'Connor MJ, et al Inhibition of PARP-1 by olaparib (AZD2281) increases the radiosensitivity of a lung tumor xenograft. Mol Cancer Ther. 2011;10:1949–58. doi: 10.1158/1535-7163.MCT-11-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan MA, Parsels LA, Zhao LL, Parsels JD, Davis MA, Hassan MC, et al Mechanism of radiosensitization by the chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res. 2010;70:4972–81. doi: 10.1158/0008-5472.CAN-09-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabakov AE, Makarova YM, Malyutina YV Radiosensitization of human vascular endothelial cells through Hsp90 inhibition with 17-N-allilamino-17-demethoxygeldanamycin . Int J Radiat Oncol Biol Phys. 2008;71:858–65. doi: 10.1016/j.ijrobp.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 35.Schick U, Kyula J, Barker H, Patel R, Zaidi S, Gregory C, et al Trametinib radiosensitises RAS- and BRAF-mutated melanoma by perturbing cell cycle and inducing senescence. Radiother Oncol. 2015;117:364–75. doi: 10.1016/j.radonc.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 36.Chargari C, Leteur C, Angevin E, Bashir T, Schoentjes B, Arts J, et al Preclinical assessment of JNJ-26854165 (Serdemetan), a novel tryptamine compound with radiosensitizing activity in vitro and in tumor xenografts . Cancer Lett. 2011;312:209–18. doi: 10.1016/j.canlet.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Corcoran RB New therapeutic strategies for BRAF mutant colorectal cancers. J Gastrointest Oncol. 2015;6:650–9. doi: 10.3978/j.issn.2078-6891.2015.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fasih A, Elbaz HA, Hüttemann M, Konski AA, Zielske SP Radiosensitization of pancreatic cancer cells by metformin through the AMPK pathway. Radiat Res. 2014;182:50–9. doi: 10.1667/RR13568.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flanagan SA, Robinson BW, Krokosky CM, Shewach DS Mismatched nucleotides as the lesions responsible for radiosensitization with gemcitabine: a new paradigm for antimetabolite radiosensitizers. Mol Cancer Ther. 2007;6:1858–68. doi: 10.1158/1535-7163.MCT-07-0068. [DOI] [PubMed] [Google Scholar]
- 40.Robinson BW, Im MM, Ljungman M, Praz F, Shewach DS Enhanced radiosensitization with gemcitabine in mismatch repair-deficient HCT116 cells. Cancer Res. 2003;63:6935–41. [PubMed] [Google Scholar]
- 41.Chen XF, Wong P, Radany E, Wong JYC HDAC inhibitor, valproic acid, induces p53-dependent radiosensitization of colon cancer cells. Cancer Biother Radiopharm. 2009;24:689–99. doi: 10.1089/cbr.2009.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleiman LB, Krebs AM, Kim SY, Hong TS, Haigis KM Comparative analysis of radiosensitizers for K-RAS mutant rectal cancers. PLoS One. 2013;8:e82982. doi: 10.1371/journal.pone.0082982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hecht M, Zimmer L, Loquai C, Weishaupt C, Gutzmer R, Schuster B, et al Radiosensitization by BRAF inhibitor therapy—mechanism and frequency of toxicity in melanoma patients. Ann Oncol. 2015;26:1238–44. doi: 10.1093/annonc/mdv139. [DOI] [PubMed] [Google Scholar]
- 44.Loboda A, Nebozhyn MV, Watters JW, Buser CA, Shaw PM, Huang PS, et al EMT is the dominant program in human colon cancer. BMC Med Genomics. 2011;4:9. doi: 10.1186/1755-8794-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urick ME, Chung EJ, Shield III WP, Gerber N, White A, Sowers A, et al Enhancement of 5-fluorouracil-induced in vitro and in vivo radiosensitization with MEK inhibition . Clin Cancer Res. 2011;17:5038–47. doi: 10.1158/1078-0432.CCR-11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kjellström J, Kjellén E, Johnsson A In vitro radiosensitization by oxaliplatin and 5-fluorouracil in a human colon cancer cell line. Acta Oncol. 2005;44:687–93. doi: 10.1080/02841860500247552. [DOI] [PubMed] [Google Scholar]
- 47.Ojima E, Inoue Y, Watanabe H, Hiro J, Toiyama Y, Miki C, et al The optimal schedule for 5-fluorouracil radiosensitization in colon cancer cell lines. Oncol Rep. 2006;16:1085–91. [PubMed] [Google Scholar]
- 48.Dudley JC, Lin MT, Le DT, Eshleman JR Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. 2016;22:813–20. doi: 10.1158/1078-0432.CCR-15-1678. [DOI] [PubMed] [Google Scholar]
- 49.Löser DA, Shibata A, Shibata AK, Woodbine LJ, Jeggo PA, Chalmers AJ Sensitization to radiation and alkylating agents by inhibitors of poly (ADP-ribose) polymerase is enhanced in cells deficient in DNA double-strand break repair. Mol Cancer Ther. 2010;9:1775–87. doi: 10.1158/1535-7163.MCT-09-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murai J, Huang SYN, Renaud A, Zhang YP, Ji JP, Takeda S, et al Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther. 2014;13:433–43. doi: 10.1158/1535-7163.MCT-13-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen YQ, Aoyagi-Scharber M, Wang B Trapping poly(ADP-Ribose) polymerase. J Pharmacol Exp Ther. 2015;353:446–57. doi: 10.1124/jpet.114.222448. [DOI] [PubMed] [Google Scholar]
- 53.Glynne-Jones R, Dunst J, Sebag-Montefiore D The integration of oral capecitabine into chemoradiation regimens for locally advanced rectal cancer: how successful have we been? Ann Oncol. 2006;17:361–71. doi: 10.1093/annonc/mdj052. [DOI] [PubMed] [Google Scholar]
- 54.Mo A, Jackson S, Varma K, Carpino A, Giardina C, Devers TJ, et al Distinct transcriptional changes and epithelial-stromal interactions are altered in early-stage colon cancer development. Mol Cancer Res. 2016;14:795–804. doi: 10.1158/1541-7786.MCR-16-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perez-Villamil B, Romera-Lopez A, Hernandez-Prieto S, Lopez-Campos G, Calles A, Lopez-Asenjo JA, et al Colon cancer molecular subtypes identified by expression profiling and associated to stroma, mucinous type and different clinical behavior. BMC Cancer. 2012;12:260. doi: 10.1186/1471-2407-12-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yip KW, Mao XL, Au PYB, Hedley DW, Chow S, Dalili S, et al Benzethonium chloride: a novel anticancer agent identified by using a cell-based small-molecule screen. Clin Cancer Res. 2006;12:5557–69. doi: 10.1158/1078-0432.CCR-06-0536. [DOI] [PubMed] [Google Scholar]
- 57.Higgins GS, Prevo R, Lee YF, Helleday T, Muschel RJ, Taylor S, et al A small interfering RNA screen of genes involved in DNA repair identifies tumor-specific radiosensitization by POLQ knockdown. Cancer Res. 2010;70:2984–93. doi: 10.1158/0008-5472.CAN-09-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gani C, Coackley C, Kumareswaran R, Schütze C, Krause M, Zafarana G, et al In vivo studies of the PARP inhibitor, AZD-2281, in combination with fractionated radiotherapy: an exploration of the therapeutic ratio. Radiother Oncol. 2015;116:486–94. doi: 10.1016/j.radonc.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Brown JS, Kaye SB, Yap TA PARP inhibitors: the race is on. Br J Cancer. 2016;114:713–5. doi: 10.1038/bjc.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McMahon M, Frangova TG, Henderson CJ, Wolf CR Olaparib, monotherapy or with ionizing radiation, exacerbates DNA damage in normal tissues: insights from a new p21 reporter mouse. Mol Cancer Res. 2016;14:1195–203. doi: 10.1158/1541-7786.MCR-16-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Bono J, Ramanathan RK, Mina L, Chugh R, Glaspy J, Rafii S, et al Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers . Cancer Discov. 2017;7:620–9. doi: 10.1158/2159-8290.CD-16-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]