Abstract
Objective
The aim of this study was to investigate the underlying mechanism whereby HBx modulates the targeting of NUSAP1 by miR-18b to enhance hepatocarcinogenesis.
Methods
We employed an integrated approach of bioinformatics analysis and molecular experiments in hepatoma cells, HBV transgenic mice, and clinical liver cancer tissues to investigate the role of HBx-regulated miR-18b in the development of liver cancer.
Results
In this study, we report that the HBx-mediated tumor suppressor miR-18b modulates hepatocarcinogenesis during the host-HBV interaction. The expression levels of miR-18b were lower in clinical HBV-positive liver cancer tissues and liver tissues of HBV-transgenic mice. Interestingly, HBx inhibited miR-18b expression by inducing the methylation of CpG islands in its promoter. Accordingly, we tested the hypothesis that HBx enhanced hepatocarcinogenesis by increasing the expression of target genes of miR-18b. Moreover, we identified nucleolar spindle-associated protein 1 (NUSAP1) as one of the target genes of miR-18b. NUSAP1 was expressed at high levels in liver cancer tissues. Interestingly, HBx up-regulated NUSAP1 by suppressing miR-18b. Functionally, miR-18b significantly inhibited the proliferation of hepatoma cells by depressing NUSAP1 levels in vivo and in vitro.
Conclusions
Thus, we conclude that the targeting of NUSAP1 mRNA by the tumor suppressor miR-18b is controlled by HBx-modulated promoter methylation during the host-virus interaction, leading to hepatocarcinogenesis. Our findings provide new insights into the mechanism by which HBx-mediated miRNAs modulate hepatocarcinogenesis.
Keywords: Hepatitis B virus, HBx, miR-18b, NUSAP1, HCC
Introduction
Hepatocellular carcinoma (HCC) is a common cancer worldwide and the third leading cause of cancer-related deaths globally1. Chronic hepatitis B virus (HBV) infection is closely related to chronic hepatitis, cirrhosis, and HCC2-4. As a functional trans-activating protein, HBV X protein (HBx) plays important roles in the development of liver cancer5-7. HBx can stimulate transcription, signal transduction, and cell cycle progression by binding to different transcription factors or components of various signaling pathways8-10. In addition, HBx participates in genetic and epigenetic regulation during the development of liver cancer11,12. However, the underlying mechanism by which HBx modulates hepatocarcinogenesis is poorly understood.
MicroRNAs (miRNAs) are endogenous, 21–24 nucleotide RNAs that can play regulatory roles by targeting mRNAs. As a result, the targeted mRNAs are cleaved or posttranscriptionally repressed13-15. More than half of all mRNAs are regulated by miRNAs and each miRNA is estimated to regulate hundreds of mRNAs16-18. Studies of specific miRNAs have shown that they play distinct roles in cellular functions, such as proliferation, development, differentiation, and apoptosis19-23. A previous study reported that miRNAs are critical for essential cellular processes, host-virus interactions, and virus life cycles24. Many miRNAs have been reported to be involved in the host-virus interactions of more than 50 different infections, including those involving dengue virus, Ebola virus, hepatitis B virus, hepatitis C virus, and rabies virus25. These findings demonstrate the important role of miRNAs in host immunity and the defense against viral infections. Immune dysregulation, instance, during chronic inflammation, can lead to chronic illness and even death26-28. Therefore, a balanced immune response is essential. MiRNAs play an important role in innate immunity by directly targeting viral transcripts29-31. Various miRNAs, such as miR-141, miR-501, and miR-125a-5p have been implicated in the regulation of HBV replication32,33.
It has been reported that miR-18b is able to suppress cell migration and invasiveness in melanoma34. MiR-18b can suppress high-glucose-induced proliferation of human retinal endothelial cells (HRECs) by targeting the IGF-1/IGF1R signaling pathways35. However, the role of miR-18b in HBV-related HCC is still unclear. Some miRNAs are decreased in human tumors due to aberrant hypermethylation of cytosine-phosphate-guanosine (CpG) islands of miRNA genes36,37. Aberrant DNA methylation is also associated with the development of HCC. CpG hypermethylation serves as a mechanism for miRNA silencing, but this field remains largely unexplored. Our group reported that HBx was able to inhibit the tumor suppressor, miR-205 and enhance hepatocarcinogenesis by inducing methylation of the miR-205 promoter38. However, the effect of HBx on miR-18b is undefined. Nucleolar spindle associated protein 1 (NUSAP1) is an important mitotic regulator that has been reported to be essential for many cellular processes39. NUSAP1 plays crucial roles in embryogenesis and cancer40-42 and is up-regulated in HCC when compared to nontumor liver tissues43. However, the significance of NUSAP1 in HBV-related liver cancer progression remains poorly understood.
In the present study, we investigated the mechanism by which HBx modulates hepatocarcinogenesis during the host-virus interaction. Interestingly, we identified that the targeting of NUSAP1 mRNA by the tumor suppressor miR-18b was controlled by HBx during the host-virus interaction, leading to hepatocarcinogenesis. Thus, our finding provides new insights into the mechanism by which HBx-mediated miRNAs modulate hepatocarcinogenesis.
Materials and methods
Patient samples
Thirty liver tissues were obtained immediately after surgical resection from randomly selected HCC patients at Tianjin First Center Hospital (Tianjin, China) and Tianjin Medical University Cancer Institute and Hospital (Tianjin, China). Clinicopathological information for the patients was collected from hospital patient records (Supplementary Table S1). Written informed consent for the use of their tissues for research purposes was obtained from all patients in the study. The Institute Research Ethics Committee at the Nankai University approved the study protocol.
S1.
The characteristics of patients
| No. | Age | Gender | Organ | HBV |
| 1 | 59 | F | Liver | + |
| 2 | 60 | M | Liver | + |
| 3 | 65 | M | Liver | + |
| 4 | 43 | M | Liver | + |
| 5 | 60 | F | Liver | + |
| 6 | 41 | M | Liver | + |
| 7 | 45 | M | Liver | + |
| 8 | 56 | M | Liver | + |
| 9 | 70 | M | Liver | + |
| 10 | 67 | M | Liver | + |
| 11 | 59 | M | Liver | + |
| 12 | 57 | M | Liver | + |
| 13 | 61 | F | Liver | + |
| 14 | 51 | F | Liver | + |
| 15 | 56 | M | Liver | + |
| 16 | 60 | M | Liver | + |
| 17 | 54 | M | Liver | + |
| 18 | 36 | M | Liver | + |
| 19 | 46 | M | Liver | + |
| 20 | 60 | M | Liver | + |
| 21 | 59 | M | Liver | + |
| 22 | 57 | F | Liver | + |
| 23 | 51 | M | Liver | + |
| 24 | 38 | M | Liver | + |
| 25 | 56 | M | Liver | + |
| 26 | 49 | M | Liver | + |
| 27 | 58 | F | Liver | + |
| 28 | 46 | M | Liver | + |
| 29 | 56 | M | Liver | + |
| 30 | 54 | M | Liver | + |
Cell lines and cell culture
The HepG2, HepG2-X (cell line with stable HBx transfection), HepAD38 (cell line stably producing HBV), and HepG2.2.15 (a hepatoma HepG2 cell line with integrated full-length HBV DNA), were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA). For HBV replication, HepG2.2.15 cells were seeded at optimal densities and HepAD38 cells were treated without tetracycline for 8 days. LO2 (human immortalized normal liver cell line), H7402 (hepatoma cell line), and H7402-X (cell line with stable HBx- transfection) cells were maintained in RPMI 1640 medium (Gibco) supplemented with 10% fetal calf serum, 100 IU/mL penicillin, and 100 μg/mL streptomycin in 5% CO2 at 37°C. HepG2-X and H7402-X cells were maintained in 200 μg/mL G418.
HBV transgenic mice and tissue analysis
BALB/c mice and HBV transgenic BALB/c mice (HBV-Tg) containing the HBV genome S, pre-S, and X domains were purchased from Vital River Laboratory Animal Technology (Beijing, China). Animals were maintained under specific pathogen-free conditions. Mice were sacrificed at the end of the experimental period and serum and liver tissues were obtained.
In vivo tumorigenicity assays
Animal transplantation experiments were performed in compliance with the principles of the Declaration of Helsinki. Nude mice were housed and treated according to the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal experiments were approved by the Institute Research Ethics Committee at Nankai University. Tumor transplantation experiments were performed as previously described44,45. Briefly, HepG2-X cells were pretreated with control, miR-18b, miR-18b, or pcDNA3.1-NUSAP1 for 24 h. Cells were then resuspended at 2 × 107 cells/mL in sterile PBS. Four-week-old male BALB/c athymic nude mice (Experimental Animal Center of Peking, China) were grouped (n = 6 per group) and injected subcutaneously on the shoulder with 0.2 mL of the appropriate cell suspension. Tumor growth was measured 6 days after the injection and thereafter, every 4 days. Tumor volume was evaluated by measuring the length (L) and width (W) with calipers and was then calculated according to the formula, (L× W2)/2. On the thirtieth day, mice were sacrificed and tumors were excised and measured.
Statistical analysis
The correlation between NUSAP1 (or miR-18b) and HBx mRNA/pregenomic (pg) RNA levels in tumor tissues was determined by calculating the Pearson’s correlation coefficient. NUSAP1 expression levels in tumor tissues and adjacent nontumorous tissues were compared using Wilcoxon’s signed-rank test. Statistical significance was assessed by comparing mean values (± standard deviation, SD) using a Student’s t-test for independent groups, with significance assumed at P < 0.05. Each experiment was repeated at least three times.
Results
HBx suppressed miR-18b by inducing hypermethylation of the miR-18b promoter
It has been reported that miR-18b suppresses cell migration and invasiveness in melanoma34. However, the role of miR-18b in HBV-related liver cancer remains unclear. Therefore, we hypothesized that HBx modulates miR-18b during hepatocarcinogenesis. Therefore, we measured miR-18b expression in 30 paired HCC and adjacent nontumorous liver tissues using qRT-PCR. MiR-18b expression was normalized to the expression of an endogenous control (U6 RNA). Our data showed that the expression levels of miR-18b were significantly lower in clinical HBV-related HCC samples, relative to their adjacent noncancerous liver tissues (Figure 1A; P < 0.01, Wilcoxon’s signed rank test). Next, we examined the relationship between HBx and miR-18b in HCC tissues using qRT-PCR. Intriguingly, we found that the expression levels of miR-18b were inversely correlated with those of HBx mRNA/pgRNA (Figure 1B; Pearson’s correlation coefficient, r = –0.5771; P < 0.01). Moreover, our data showed that the expression levels of miR-18b were lower in the livers of HBV-transgenic mice relative to the livers of wild-type mice (Figure 1C), suggesting that HBx may suppress the expression of miR-18b during HBV infection and HCC development. The levels of HBx/pgRNA were also determined in wild-type mice and HBV-transgenic mice using qRT-PCR (Supplementary Figure S1A). Based on these results, we proposed that HBx directly down-regulates miR-18b in liver cells. Next, we transiently transfected HepG2 and H7402 cells with an HBx-expression plasmid (pcDNA3.1-HBx). The overexpression of HBx was validated in the two cell lines (Supplementary Figure S1B). We showed that HBx decreased miR-18b expression in a dose-dependent manner (Figure 1D), suggesting that HBx is able to inhibit miR-18b in HCC cells. Given that miR-18b was down-regulated in clinical HCC tissues, we sought to investigate the mechanism by which HBx down-regulates miR-18b in hepatoma cells. It has been reported that HBx downregulates miRNA expression through methylation of the promoter region38. We evaluated methylation levels in the promoter region of miR-18b in HCC cells and clinical HCC tissues (Figure 1E) using bisulfite-sequencing polymerase chain reaction (PCR). Our data showed that the methylation levels of the miR-18b promoter CpG islands were higher in HepG2-X, HepAD38, and HepG2.2.15 cells than in LO2 cells (Figure 1F), suggesting that the miR-18b gene promoter is hypermethylated in HBV-infected hepatoma cells relative to normal liver cells (Supplementary Figure S1C). MiR-18b levels were then measured by qRT-PCR in LO2, HepG2-X, HepAD38, and HepG2.2.15 cells. Interestingly, the expression levels of miR-18b were negatively correlated with the methylation status of the miR-18b promoter in all four cell lines (Supplementary Figure S1D). Eight tumor tissues showed high DNA methylation levels (Figure 1G) and DNA methylation levels of all peritumoral tissues were low, suggesting that the miR-18b promoter is hypermethylated in HCC tissues relative to adjacent nontumorous liver tissues (Supplementary Figure S1E). Thus, we conclude that HBx down-regulates miR-18b by inducing the methylation of CpG islands in the miR-18b gene promoter.
1.
HBx suppresses miR-18b through inducing DNA hypermethylation of miR-18b promoter. (A) Relative mRNA levels of miR-18b were examined by qRT-PCR analysis in 30 pairs of HCC tissues and corresponding nontumorous tissues. **P < 0.01; Wilcoxon’s signed-rank test. (B) The correlation between HBx mRNA level and miR-18b mRNA level was analyzed by qRT-PCR analysis in 30 cases of HCC tissues (**P < 0.01, r = –0.5771; Pearson’s correlation coefficient). (C) MiR-18b expression was tested by qRT-PCR in HBV-transgenic mice (n = 6). (D) The expression of miR-18b was measured by qRT-PCR analysis after treatment with pcDNA3.1-HBx in HepG2 and H7402 cells. (E) Scheme depicting the genomic localization of miR-18b. The regions analyzed by BSP are indicated. The CpG dinucleotide within the miR-18b promoter regions (–2563 to –1534, 172bp) were numbered as 1-12. (F) The methylation status of themiR-18b promoter in LO2, HepG2-X, HepAD38 and HepG2.2.15 cell lines. The open and filled circles indicate the unmethylated and methylated CpGs, respectively. (G) The methylation status of the miR-18b promoter was examined by MSP analysis in HCC tissues (T).
S1.
HBx suppresses miR-18b through inducing DNA hypermethylation of miR-18b promoter. (A) The levels of HBx/pgRNA were tested by qRT-PCR in HBV-transgenic mice (n=6). (B) The levels of HBx were examined by Western blot analysis in HepG2 and H7402 cells transfected with pcDNA3.1 or pcDNA3.1-HBx. (C) The methylation status of the miR-18b promoter was examined by MSP analysis in LO2, HepG2-X, HepAD38 and HepG2.2.15 cells after treatment with DZNep (10 μM) for 72 h. (D) The levels of miR-18b were determined by qRT-PCR in the cell lines of LO2, HepG2-X, HepAD38 and HepG2.2.15. (E) The methylation status of the miR-18b promoter was measured by MSP analysis in HCC peritumor tissues (P). Error bars indicate SD. Student’s t-test, ** P< 0.01,***P < 0.001.
MiR-18b inhibited the expression of NUSAP1 by directly targeting the 3'UTR of its mRNA
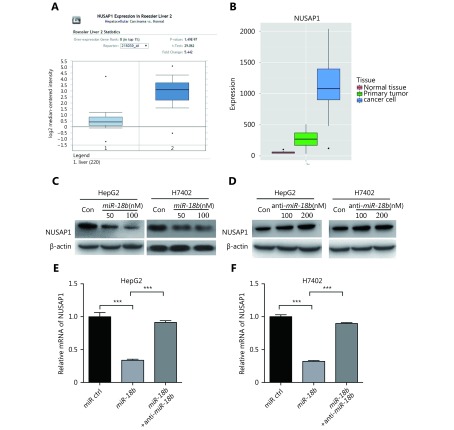
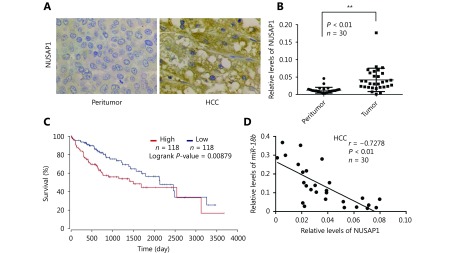
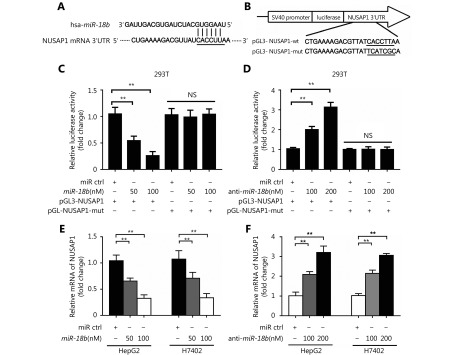
To further elucidate the molecular mechanism whereby miR-18b affects the pathogenesis of HCC, we used the online miRNA target gene prediction tool, Targetscan (http://www.targetscan.org/) and found that NUSAP1 was a potential target gene for miR-18b. However, the significance of NUSAP1 in HCC is poorly understood. Therefore, we used the cancer microarray database, Oncomine (https://www.oncomine.org/) and Metabolic gEne RApid Visualizer (MERAV, http://merav.wi.mit.edu/) to predict the expression level of NUSAP1 in HCC. These results showed that the expression levels of NUSAP1 were higher in HCC tissues relative to those in normal tissues, suggesting that NUSAP1 is a potential oncogene (Supplementary Figure S2A and S2B). Next, we analyzed the expression of NUSAP1 in clinical HCC tissues using immunohistochemical (IHC) staining of tissue arrays. In HCC tissues, NUSAP1 staining was observed in the cytoplasm and nucleus (Figure 2A). NUSAP1 staining was observed in 68.61% (129/188) of all HCC tissues and in 77.41% (24/31) of grade III HCC tissues, suggesting that NUSAP1 is closely associated with the malignancy of HCC. QPCR analysis revealed that NUSAP1 expression levels were significantly increased in 30 clinical HCC samples, relative to their adjacent nontumor tissues (P < 0.01, n = 30, Wilcoxon’s signed-rank test, Figure 2B). Using the OncoLnc database (http://www.oncolnc.org), we found that a higher level of NUSAP1 expression was associated with a lower survival rate in HCC patients (Figure 2C). Moreover, we found that miR-18b expression levels were negatively correlated with NUSAP1 mRNA levels in HCC tissues (Pearson’s correlation coefficient, r = –0.7278; P < 0.01; Figure 2D), suggesting that NUSAP1 may be one of the target genes of miR-18b. To confirm the site-specific repression of NUSAP1 by miR-18b, we constructed a luciferase reporter vector containing the 3'UTR of NUSAP1 mRNA (Figure 3A and 3B). Luciferase reporter gene assays showed that miR-18b directly targeted the NUSAP1 mRNA 3'UTR in HepG2 cells, in a dose-dependent manner, but it did not target a mutated NUSAP1 mRNA 3'UTR (Figure 3C). Conversely, an anti-miR-18b oligonucleotide increased the luciferase activity of pGL3-NUSAP1-3'UTR-wt in a dose-dependent manner, but had no effect on the mutant (Figure 3D), suggesting that miR-18b is able to directly bind to the 3'UTR of NUSAP1 mRNA. Furthermore, the over-expression of miR-18b suppressed the expression of NUSAP1 in HepG2 and H7402 cells, at the mRNA and protein level, in a dose-dependent manner, and the reverse effect was seen when cells were treated with an anti-miR-18b oligonucleotide (Figure 3E and 3F, Supplementary Figure S2C and S2D). Our data showed that an anti-miR-18b oligonucleotide significantly rescued NUSAP1 levels that were decreased by miR-18b in HCC cells (Supplementary Figure S2E and S2F). Collectively, we conclude that miR-18b is able to inhibit NUSAP1 expression by directly targeting the 3'UTR of its mRNA.
S2.
MiR-18b inhibits the expression of NUSAP1 through directly targeting its 3'UTR. (A and B) the expression level of NUSAP1 was predicted in cancer microarray Database Oncomine (https://www.oncomine.org/) and Metabolic gEne RApid Visualizer (MERAV) (http://merav.wi.mit.edu/). (C and D) The effect of miR-18b (or anti-miR-18b) on the expression of NUSAP1 was measured by Western blot analysis in HepG2 and H7402 cells. (E and F) The expression of NUSAP1 mRNA was examined by qRT-PCR in HepG2 and H7402 cells transfected with miR-18b (100 nM) or co-transfected with both miR-18b (100 nM)) and anti-miR-18b (100 nM). Error bars indicate SD. Student’s t-test, **P < 0.01, ***P < 0.001.
2.
MiR-18b inhibits the expression of NUSAP1 through directly targeting its mRNA 3'UTR. (A) The expression of NUSAP1 was examined by IHC staining in normal tissues and HCC tissues using tissue array, respectively. (B) Relative mRNA levels of NUSAP1 were measured by qRT-PCR analysis in 30 pairs of HCC tissues and corresponding nontumorous tissues. **P<0.01; Wilcoxon’s signed-rank test. (C) The survival rate of HCC patients analyzed by OncoLnc database (http://www.oncolnc.org/). (D) The correlation between NUSAP1 mRNA level andmiR-18b mRNA level was analyzed by qRT-PCR analysis in 30 cases of HCC tissues (**P<0.01,r=–0.7278; Pearson’s correlation coefficient).
3.
MiR-18b inhibits the expression of NUSAP1 through directly targeting its 3'UTR. (A,B) MiR-18b sequence and the potential MiR-18b -binding sites at the 3'UTR of NUSAP1 mRNA. Seed sequences and mutant are marked. (C,D) The effect of miR-18b (or anti-miR-18b) on the pGL3-NUSAP1-3'UTR-wt and pGL3-NUSAP1-3'UTR-mut reporters was measured by luciferase reporter gene assays in HepG2 cells. (E,F) The effect of miR-18b (or anti- miR-18b) on the expression of NUSAP1 was examined by qRT-PCR analysis in HepG2 and H7402 cells. Error bars indicate SD. Student’s t-test, **P<0.01, NS, not significant.
HBx up-regulated NUSAP1 in hepatoma cells
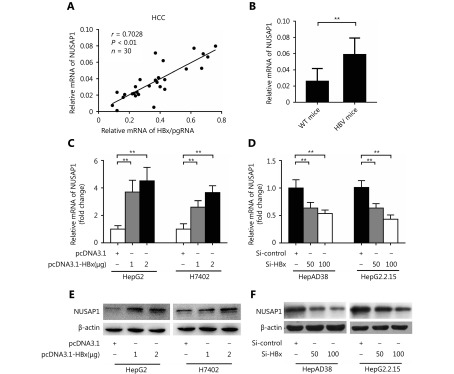
Given that HBx was able to down-regulate the targeting of NUSAP1 by miR-18b, we investigated whether HBx could down-regulate NUSAP1 by inhibiting miR-18b. The mRNA levels of NUSAP1 and HBx/pgRNA were examined by qRT-PCR in 30 HCC liver tissues. Our data showed that the expression levels of HBx/pgRNA were positively correlated with those of NUSAP1 mRNA in HCC tissues (Pearson’s correlation coefficient, r = 0.7028; P < 0.01; Figure 4A), suggesting that HBx is able to up-regulate NUSAP1. Moreover, our data showed that the expression levels of NUSAP1 were higher in HBV-transgenic mice relative to wild-type mice (Figure 4B), suggesting that HBx may up-regulate the expression of NUSAP1 during HBV infection. Furthermore, we confirmed that HBx was able to up-regulate the expression of NUSAP1, in a dose-dependent manner, in HepG2 and H7402 cells transfected with HBx (Figure 4C and 4E). Conversely, HBx knockdown by si-HBx abolished the up-regulation of NUSAP1 in HepAD38 and HepG2.2.15 cells, at both the RNA and protein level, in a dose-dependent manner (Figure 4D and 4F). Thus, we conclude that HBx is able to up-regulate NUSAP1 in hepatoma cells.
4.
HBx is able to up-regulate NUSAP1 in hepatoma cells. (A) The correlation between NUSAP1 mRNA level and HBx mRNA level was examined by qRT-PCR analysis in 30 cases of HCC tissues (**P < 0.01, r = 0.7028; Pearson’s correlation coefficient). (B) MiR-18b expression was measured by qRT-PCR in HBV-transgenic mice (n = 6). (C-F) The expression of NUSAP1 was analyzed by qRT-PCR and Western blot analysis in the cells transfected with pcDNA3.1-HBx plasmid (or si-HBx). Error bars indicate SD. Student’s t-test. **P < 0.01.
HBx-induced NUSAP1 promoted the proliferation of hepatoma cells in vitro
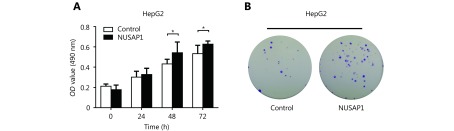
NUSAP1 expression has been reported to promote cell proliferation in renal cell carcinoma46. We sought to determine whether NUSAP1 could also influence cell proliferation in HCC. MTT and colony-formation assays showed that NUSAP1 promoted cell proliferation in HepG2 cells (Supplementary Figure S3A and S3B). Next, we determined the effect of NUSAP1 targeting by miR-18b on cell proliferation using MTT assays, colony formation assays, and cell cycle analysis in HepG2, HepG2-X, H7402, and H7402-X cells. We found that the proliferation of HepG2-X and H7402-X cells was significantly reduced by miR-18b. However, the over-expression of NUSAP1 could rescue the proliferation of these cells (Figure 5A–5C and Supplementary Figure S4A–4D), suggesting that miR-18b targeting of NUSAP1 is able to suppress the HBx-induced proliferation of hepatoma cells. Taken together, we conclude that HBx-induced NUSAP1 increases the proliferation of hepatoma cells in vitro.
S3.
HBx-elevated NUSAP1 targeted by miR-18b promotes the proliferation of hepatoma cells in vitro. (A) The effect of NUSAP1 on cell proliferation was determined by MTT assays in HepG2 cells. (B) The effect of NUSAP1 on cell proliferation was determined by colony formation assays in HepG2 cells. Error bars indicate SD. Student’s t-test, *P < 0.05, **P < 0.01, NS, not significant.
5.
HBx-elevated NUSAP1 promotes the proliferation of hepatoma cells in vitro. (A) The effect of miR-18b and NUSAP1 on HBx-induced cell proliferation was determined by MTT assays in HepG2-X cells. (B) The effect of miR-18b and NUSAP1 on HBx-induced cell proliferation was measured by colony formation assays in HepG2-X cells. (C) The effect of miR-18b and NUSAP1 on HBx-induced cell proliferation was examined by cell cycle assays in HepG2-X cells. Error bars indicate SD. Student’s t-test, *P<0.05,**P<0.01, NS, not significant.
S4.
HBx-elevated NUSAP1 targeted by miR-18b promotes the proliferation of hepatoma cells in vitro. (A) The effect of miR-18b and NUSAP1 on HBx-induced cell proliferation was determined by MTT assays in H7402-X cells. (B) The effect of miR-18b and NUSAP1 on HBx-induced cell proliferation was determined by colony formation assays in H7402-X cells. (C) The effect of miR-18b and NUSAP1 on HBx-induced cell proliferation was determined by cell cycle assays in H7402-X cells. (D) The statistical results of cell cycle assays from Figure 5C and Figure S4C were shown. Error bars indicate SD. Student’s t-test *P < 0.05, **P < 0.01, NS, not significant.
HBx-induced NUSAP1 facilitated the growth of liver cancer in vivo
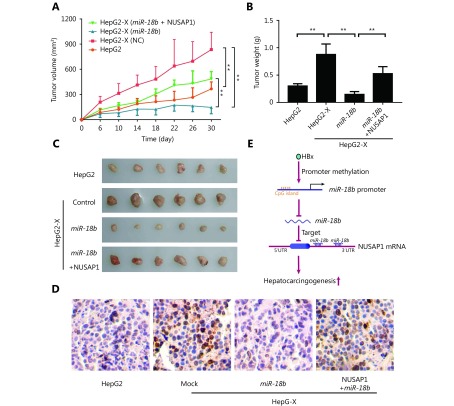
To further evaluate the effect of NUSAP1 on tumor growth, we subcutaneously injected pretreated cells into 4-week-old BALB/c athymic nude mice. Interestingly, tumor weight and volume in mice injected with miR-18b-transfected HepG2-X cells were significantly lower than in mice injected with negative control-transfected HepG2-X cells. Over-expression of NUSAP1 rescued the decrease in tumor size in mice injected with miR-18b-transfected HepG2-X cells (Figure 6A–6C), suggesting that the targeting of NUSAP1 by miR-18b is able to suppress the HBx-induced growth of hepatoma cells. In addition, IHC staining was used to determine the expression levels of Ki-67, a marker of proliferation, in tumor tissues from athymic nude mice. Ki-67 expression results were consistent with the results of tumor growth analysis (Figure 6D). Thus, we conclude that HBx-induced NUSAP1 facilitates the growth of liver cancer in vivo.
6.
HBx-elevated NUSAP1 facilitates the growth of liver cancer in vivo. (A) Growth curve of tumors transplanted with HepG2 and HepG2-X cells pretreated with miR-18b or co-transfected MiR-18b and NUSAP1 in nude mice. (B) Diagram of average weight of tumors. (C) The photographs of dissected tumors from nude mice were shown. (D) Ki-67, a biomarker of proliferation, was examined by IHC staining in tumor tissues from nude mice, respectively (scale bar: 50μm). (E) A model shows that HBx enhances hepatocarcinogenesis through depressing miR-18b targeting NUSAP1 mRNA.
Discussion
HBV is a widespread virus and chronic HBV infection is a major risk factor for HCC47,48. HBx is a key factor involved in hepatocarcinogenesis. Host miRNAs play an important role in the development of liver cancer associated with HBV infection49. However, the underlying mechanism by which HBx-modulated miRNAs promote liver cancer during the host-virus interaction is still unclear. In this study, we investigated the mechanism by which HBx modulates hepatocarcinogenesis.
In the early stage of HBV infection, the host can use its own miRNA as a defense against HBV. Some miRNAs, including hsa-miR-210, hsa-miR-199-3p, hsa-miR-125a-5p, and hsa-miR-151-5p, have been shown to affect HBV gene expression in cultured cells by direct binding to viral transcripts50-52. However, the role of miR-18b in hepatocarcinogenesis remains unclear. It has been reported that miR-18b is down-regulated in HCC tissues compared to normal liver tissue53. In addition, miR-18b expression levels decrease during chronic HBV infection54. However, we observed that miR-18b expression levels were low in clinical HBV-related HCC samples, relative to their adjacent noncancerous hepatic tissues and in HBV-transgenic mice, relative to wild-type mice. This implies that HBV may suppress the expression of miR-18b during HBV infection and the subsequent development of HCC.
Our group previously reported that HBx can inhibit the tumor suppressor, miR-205, by inducing methylation of the miR-205 gene promoter38. Accordingly, we hypothesized that HBx may be a key factor in overcoming the host defense against HBV. As expected, HBx was shown to down-regulate the expression of miR-18b by inducing the hypermethylation of the miR-18b gene promoter in hepatoma cells. Therefore, we conclude that HBx can inhibit miR-18b. Next, we evaluated the significance of HBx-inhibited miR-18b in hepatocarcinogenesis. Using a miRNA target gene prediction website, we identified NUSAP1 as a potential target gene of miR-18b. Using the gene prediction database, we found that NUSAP1 was expressed at high levels in liver cancer. It has been reported that NUSAP1 is a microtubule- and chromatin-binding protein. The physiological function of NUSAP1 is to stabilize microtubules, maintain spindle integrity, and further format spindle networks55. NUSAP1 can promote the invasion and metastasis of prostate cancer and increase the aggressiveness of astrocytoma through the Hedgehog signaling pathway56,57. It has been previously reported that NUSAP1 plays crucial roles in various types of cancer40-42. NUSAP1 is up-regulated in HCCs, when compared to nontumor liver tissues43. Our findings in the present study are consistent with these previous reports. It has been reported that miR-18b can modulate many biological functions by targeting different genes. Interestingly, in this study we demonstrated that miR-18b was capable of targeting the 3'UTR of NUSAP1 mRNA, resulting in the down-regulation of NUSAP1 in HCC cells. Therefore, we hypothesized that HBx may up-regulate NUSAP1 expression by down-regulating miR-18b, to promote the development of liver cancer. qRT-PCR analysis showed that the level of NUSAP1 expression was higher in HBV-transgenic mice than in wild-type mice. Functionally, we found that the overexpression of miR-18b significantly inhibited the growth of HepG2-X and H7402-X cells in vitro and in vivo. Thus, miR-18b can down-regulate NUSAP1 in hepatoma cells and HBx can promote the proliferation of hepatoma cells by inhibiting miR-18b. Thereby, HBx can promote the development of liver cancer by also inhibiting other target genes of miR-18b.
Conclusions
We conclude that the targeting of NUSAP1 mRNA by the tumor suppressor miR-18b is controlled by HBx-modulated promoter methylation during the host-virus interaction, leading to hepatocarcinogenesis (Figure 6E).
Acknowledgements
This work was supported in part by grants from National Basic Research Program of China (973 Program, Grant No. 2015CB553703), National Natural Science Foundation of China (Grant No. 31670769 and 31470756). All authors would like to acknowledge the First Center Hospital (Tianjin, China) and Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) for providing the patient samples.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Supplementary material
Materials and methods
Plasmid construction and treatment
The CDS sequence of NUSAP1 was obtained by PCR from the cDNA of HepG2 using specific primers and was cloned into the pcDNA3.1 vector. The plasmid was named pcDNA3.1-NUSAP1. To construct luciferase reporter plasmids of NUSAP1, the wild type or mutant of NUSAP1 mRNA 3′UTR, which were targeted by miR-18b, were amplified by PCR and were inserted into the pGL3-Control vector to generate pGL3-NUSAP1-wt or pGL3-NUSAP1-mut, respectively. The miR-18b mimics and anti-miR-18b were purchased from RIBOBIO (Guang Zhou, China).
RNA extraction, reverse-transcription, and quantitative real-time polymerase chain reaction (qRT-PCR)
Using Trizol reagent (Invitrogen, Carlsbad, CA), we got total RNAs from cells (or liver tissues from mice and clinical HCC patient tissues). First-strand cDNA was synthesized by using first strand cDNA synthesis kit (Thermo fisher, Shanghai, China), according to the manufacturer’s instructions. To analyze miR-18b expression, total RNA were polyadenylated by poly (A) polymerase (Ambion, Austin, TX, USA). Reverse transcription was performed using poly (A)-tailed total RNA and reverse transcription primer with ImPro-II Reverse Transcriptase (Promega, Madison, WI, USA) according to the manufacturer’s instructions. QRT-PCR was performed by a Bio-Rad sequence detection system according to the manufacturer’s instructions. Relative transcriptional folds were calculated as 2-ΔΔCt. Experiments were performed in three independent assays. β-actin was used as an internal control for normalization. U6 was used as an internal control to normalize miR-18b levels. All primers used in this paper were listed in Supplementary Table S2.
S2.
List of primers used in this paper
| Primer | Forward primer (5′-3′) | Reverse primer (5′-3′) |
| Primers for NUSAP1 3′UTR | ||
| pGL3-NUSAP1-3′UTR-wt | GCTCTAGAGCACATCTTGTAAATATTCCTG | GGGGGCCGGCCAACAGAATTTTAGGATGGGT |
| pGL3-NUSAP1-3′UTR-mut | CTCTGAAAAGACGTTATTCATCGCAAGCTCAAATTCTTTG | CAAAGAATTTGAGCTTGCGATGAATAACGTCTTTTCAGAG |
| pGL3-HBx-wt | GCTCTAGAGCATGGCTGCTAGGCTGTGCT | GGGGGCCGGCCTTAGGCAGAGGTGAAAAAG |
| pGL3-HBx-mut | CGTCCGACCACGGGGCTAGTTGCTCTTTACGCGGTCTCCC | GGGAGACCGCGTAAAGAGCAACTAGCCCCGTGGTCGGACG |
| Primers for CDS cloning | ||
| NUSAP1 | CGGGATCCCGATGATCATCCCCTCTCTAGAGG | CCGCTCGAGCGGTTAATCTTCAGCCAAAATGAGG |
| Primers for RT-PCR and qRT-PCR | ||
| NUSAP1 | AAACTTACAAACAACCCCATCTCC | GTTTCTTCGGTTGCTCTTCCTTT |
| mNUSAP1 | ACCTTAGACTCGGAGGACCC | GTCCAATGAAGACGCAGGGA |
| mGAPDH | AGAGGGATGCTGCCCTTACC | ATCCGTTCACACCGACCTTC |
| HBx | ATGGCTGCTAGGGTGTGCT | TAAATCTCCTCCCCCAACTC |
| miR-18b | TAAGGTGCATCTAGTGCAGTTAG | GCGAGCACAGAATTAATACGAC |
| mmu-miR-18b | TAAGGTGCATCTAGTGCTGTTAG | GCGAGCACAGAATTAATACGAC |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| GAPDH | ATCACCATCTTCCAGGAGCGA | CCTTCTCCATGGTGGTGAAGAC |
| Primers for miR-18b promoter methylation | ||
| miR-18b BSP | ATTTTAGTTTGGGGGAAGAGAGA | CCCTCTACCTTTAATAACCCAAAA |
| miR-18b M | AGCGCGGGGATTTCGTAC | GACCCCGAAACGCTCG |
| miR-18b U | TGGAGAGTGTGGGGATTTTGTATG | CACCCAACCCCAAAACACTCA |
| siRNAs sequences | ||
| siHBx | AAUGUCAACGACCGACCUUGA | |
| siNUSAP1 | CAGCCAACGACGCUCGCAA | |
Cell proliferation assay
HepG2/H7402, HepG2-X/H7402-X cells were plated into 96 well plates (1000 cells/well) for 24 hours before transfection and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to assess cell proliferation.
Luciferase reporter gene assay
The Dual-Luciferase Reporter Assay System (Promega, USA) was used to perform the luciferase reporter gene assay according to the manufacturer’s instructions. Cells were plated into 24 well plates at 3× 104 cells per well. After 24 hours, the cells were transiently co-transfected with 50 ng/well of pRL-TK plasmid (Promega, USA) containing the Renilla luciferase gene used for internal normalization, pGL3-NUSAP1-wt or pGL3-NUSAP1-mut (100 ng/well). All experiments were performed at least three times.
Western blot analysis
Cells were disrupted in cell lysis buffer. Proteins in the same amount were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto PVDF membranes. After incubation with specific antibodies for NUSAP1 (Proteintech, USA), HBx (Santa Cruz Biotechnology, Santa Cruz, CA) and β-actin (Sigma, USA), the blots were incubated with goat anti-rabbit or anti-mouse secondary antibody (Sigma, USA) and visualized with enhanced chemiluminescence. The results were obtained by using Luminescent Imaging Workstation (Tanon, Shanghai, China).
Genomic DNA extraction and bisulfite treatment
The Axygen genomic DNA purification kit (Axygen Biotechnology, Hangzhou, China) was used to extract genomic DNA was from cells. Treat Genomic DNA (0.5 μg) with sodium bisulfite with the DNA methylation quantification kit (Epigentek, Farmingdale, NY) according to the manufacturer’s instructions.
Bisulfite genomic sequencing
Treated DNA, which extracted from LO2, HepG2-X, HepAD38 and HepG2.2.15 cells with bisulfite. Then the DNA was amplified with specific primers to the gene promoters by PCR. The genomic PCR products were then gel-extracted, cloned into pEASY-T vectors (TransGen, Beijing, China), transformed into Escherichia coli for sequencing. The sequence results were shown in Supplementary Table S3.
S3.
BSP mthylation analysis of miR-18b promoter CpG islands sequences
| >Genome |
| ACTCCAGCTTGGGGGAAGAGAGAAGGGAAAATGCCGACTGCGGTCCGCCCGTGGGTAACTGGGGCTCGGTGGGGTTTTCGCTTTGGCGGGGTGGGGGTTCCGTTTCCTAATTCCTGTGCGGGCCGGGTGGGCGGGGGCGGGCTTTTTTCCTTGGGCTATTAAAGGCAGAGGG |
| >LO2-1 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTTGATTGCGGTTTGTTTGTGGGTAATTGGGGTTTGGTGGGGTTTTTGTTTTGGCGGGGTGGGGGTTTTGTTTTTTAATTTTTGTGCGGGTTGGGTGGGTGGGGGTGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >LO2-2 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTTGATTGCGGTTTGTTTGTGGGTAATTGGGGTTTGGTGGGGTTTTTGTTTTGGCGGGGTGGGGGTTTTGTTTTTTAATTTTTGTGCGGGTTGGGTGGGTGGGGGCGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >LO2-3 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTTGATTGCGGTTTGTTTGTGGGTAATTGGGGTTTGGTGGGGTTTTTGTTTTGGTGGGGTGGGGGTTTTGTTTTTTAATTTTTGTGTGGGTTGGGTGGGCGGGGGTGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >LO2-4 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTTGATTGTGGTTTGTTTGTGGGTAATTGGGGTTTGGTGGGGTTTTTGTTTTGGTGGGGTGGGGGTTTTGTTTTTTAATTTTTGTGTGGGTCGGGTGGGTGGGGGTGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >HepG2-X-1 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTCGATTGCGGTTCGTTCGTGGGTAATTGGGGTTTGGTGGGGTTTTCGTTTTGGTGGGGTGGGGGTTTCGTTTTTTAATTTTTGTGCGGGTCGGGTGGGTGGGGGTGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >HepG2-X-2 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTCGATTGCGGTTCGTTCGTGGGTAATTGGGGTTCGGTGGGGTTTTTGTTTTGGTGGGGTGGGGGTTTCGTTTTTTAATTTTTGTGCGGGTCGGGTGGGCGGGGGTGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >HepG2-X-3 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTTGATTGCGGTTCGTTTGTGGGTAATTGGGGTTCGGTGGGGTTTTCGTTTTGGTGGGGTGGGGGTTTCGTTTTTTAATTTTTGTGCGGGTCGGGTGGGCGGGGGTGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >HepG2-X-4 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTTGATTGCGGTTCGTTTGTGGGTAATTGGGGTTCGGTGGGGTTTTTGTTTTGGCGGGGTGGGGGTTTCGTTTTTTAATTTTTGTGTGGGTCGGGTGGGCGGGGGCGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >HepAD38-1 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTCGATTGCGGTTCGTTCGTGGGTAATTGGGGTTCGGTGGGGTTTTTGTTTTGGCGGGGTGGGGGTTTCGTTTTTTAATTTTTGTGCGGGTCGGGTGGGCGGGGGCGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >HepAD38-2 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTTGATTGTGGTTCGTTTGTGGGTAATTGGGGTTCGGTGGGGTTTTCGTTTTGGCGGGGTGGGGGTTTCGTTTTTTAATTTTTGTGCGGGTCGGGTGGGCGGGGGTGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >HepAD38-3 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTCGATTGCGGTTCGTTCGTGGGTAATTGGGGTTCGGTGGGGTTTTTGTTTTGGTGGGGTGGGGGTTTCGTTTTTTAATTTTTGTGCGGGTCGGGTGGGCGGGGGCGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >HepAD38-4 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTTGATTGCGGTTCGTTCGTGGGTAATTGGGGTTCGGTGGGGTTTTTGTTTTGGCGGGGTGGGGGTTTCGTTTTTTAATTTTTGTGCGGGTCGGGTGGGCGGGGGCGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >HepG2.2.15-1 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTCGATTGCGGTTCGTTCGTGGGTAATTGGGGTTCGGTGGGGTTTTCGTTTTGGCGGGGTGGGGGTTTCGTTTTTTAATTTTTGTGCGGGTCGGGTGGGTGGGGGCGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >HepG2.2.15-2 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTTGATTGCGGTTCGTTCGTGGGTAATTGGGGTTCGGTGGGGTTTTCGTTTTGGCGGGGTGGGGGTTTCGTTTTTTAATTTTTGTGTGGGTCGGGTGGGCGGGGGCGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >HepG2.2.15-3 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTCGATTGCGGTTCGTTCGTGGGTAATTGGGGTTCGGTGGGGTTTTCGTTTTGGCGGGGTGGGGGTTTCGTTTTTTAATTTTTGTGCGGGTCGGGTGGGCGGGGGCGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
| >HepG2.2.15-4 |
| ATTTTAGTTTGGGGGAAGAGAGAAGGGAAAATGTCGATTGCGGTTCGTTCGTGGGTAATTGGGGTTCGGTGGGGTTTTTGTTTTGGCGGGGTGGGGGTTTTGTTTTTTAATTTTTGTGCGGGTCGGGTGGGCGGGGGCGGGTTTTTTTTTTTGGGTTATTAAAGGTAGAGGG |
Colony formation
48 hours after transfection, about 1,000 viable transfected cells were placed into 6-well plates and grew in complete medium for 2 weeks. Colonies were fixed with methanol for 20 minutes and then stained with methylene blue.
References
- 1.Tsai WL, Chung RT Viral hepatocarcinogenesis. Oncogene. 2010;29:2309–24. doi: 10.1038/onc.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang XD, Wang Y, Ye LH Hepatitis B virus X protein accelerates the development of hepatoma. Cancer Biol Med. 2014;11:182–90. doi: 10.7497/j.issn.2095-3941.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu C, Zhou WC, Wang YM, Qiao L Hepatitis B virus-induced hepatocellular carcinoma. Cancer Lett. 2014;345:216–22. doi: 10.1016/j.canlet.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Sinn DH, Lee J, Goo J, Kim K, Gwak GY, Paik YH, et al Hepatocellular carcinoma risk in chronic hepatitis B virus-infected compensated cirrhosis patients with low viral load. Hepatology. 2015;62:694–701. doi: 10.1002/hep.27889. [DOI] [PubMed] [Google Scholar]
- 5.Arzumanyan A, Sambandam V, Clayton MM, Choi SS, Xie GH, Diehl AM, et al Hedgehog signaling blockade delays hepatocarcinogenesis induced by hepatitis B virus X protein. Cancer Res. 2012;72:5912–20. doi: 10.1158/0008-5472.CAN-12-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang T, Zhang JP, You XN, Liu Q, Du YM, Gao YE, et al Hepatitis B virus x protein modulates oncogene yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology. 2012;56:2051–59. doi: 10.1002/hep.25899. [DOI] [PubMed] [Google Scholar]
- 7.Geng X, Huang CH, Qin Y, McCombs JE, Yuan Q, Harry BL, et al Hepatitis B virus X protein targets Bcl-2 proteins to increase intracellular calcium, required for virus replication and cell death induction. Proc Natl Acad Sci USA. 2012;109:18471–6. doi: 10.1073/pnas.1204668109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.You XN, Liu FB, Zhang T, Li YH, Ye LH, Zhang XD Hepatitis B virus X protein upregulates oncogene Rab18 to result in the dysregulation of lipogenesis and proliferation of hepatoma cells. Carcinogenesis. 2013;34:1644–52. doi: 10.1093/carcin/bgt089. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Shan CL, Cui WJ, You XN, Du YM, Kong GY, et al Hepatitis B virus X protein protects hepatoma and hepatic cells from complement-dependent cytotoxicity by up-regulation of CD46. FEBS Lett. 2013;587:645–51. doi: 10.1016/j.febslet.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Feng JY, Yang G, Zhang SQ, Liu YX, Bu YN, et al Hepatitis B virus X protein-elevated MSL2 modulates hepatitis B virus covalently closed circular DNA by inducing degradation of APOBEC3B to enhance hepatocarcinogenesis. Hepatology. 2017;66:1413–29. doi: 10.1002/hep.29316. [DOI] [PubMed] [Google Scholar]
- 11.Park ES, Park YK, Shin CY, Park SH, Ahn SH, Kim DH, et al Hepatitis B virus inhibits liver regeneration via epigenetic regulation of urokinase-type plasminogen activator. Hepatology. 2013;58:762–76. doi: 10.1002/hep.26379. [DOI] [PubMed] [Google Scholar]
- 12.Song K, Han C, Wu T Epigenetic regulation of miR-122 expression by PPAR gamma/RXR alpha complex and HBx in hepatocellular carcinoma. Hepatology. 2012;56:609a–10a. doi: 10.1016/j.jhep.2011.09.012. [DOI] [Google Scholar]
- 13.Bartel DP MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.Soifer HS, Rossi JJ, Sætrom P MicroRNAs in disease and potential therapeutic applications. Mol Ther. 2007;15:2070–9. doi: 10.1038/sj.mt.6300311. [DOI] [PubMed] [Google Scholar]
- 15.Finnegan EF, Pasquinelli AE MicroRNA biogenesis: regulating the regulators. Crit Rev Biochem Mol Biol. 2013;48:51–68. doi: 10.3109/10409238.2012.738643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurtan AM, Sharp PA The role of miRNAs in regulating gene expression networks. J Mol Biol. 2013;425:3582–600. doi: 10.1016/j.jmb.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory RI, Chendrimada TP, Shiekhattar R. MicroRNA biogenesis: <italic>Isolation and characterization of the microprocessor complex</italic>. In: Ying SY. MicroRNA Protocols. Totowa, NJ: Humana Press; 2006; 33-47.
- 18.Jinek M, Doudna JA A three-dimensional view of the molecular machinery of RNA interference. Nature. 2009;457:405–12. doi: 10.1038/nature07755. [DOI] [PubMed] [Google Scholar]
- 19.Chen RJ, Kelly G, Sengupta A, Heydendael W, Nicholas B, Beltrami S, et al MicroRNAs as biomarkers of resilience or vulnerability to stress. Neuroscience. 2015;305:36–48. doi: 10.1016/j.neuroscience.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 20.Haflidadottir BS, Ceder Y Exosomal microRNAs as potential biomarkers in castration-resistant prostate cancer. Eur Urol. 2015;67:42–3. doi: 10.1016/j.eururo.2014.08.067. [DOI] [PubMed] [Google Scholar]
- 21.Kriegel AJ, Baker MA, Liu Y, Liu PY, Cowley AW Jr, Liang MY Endogenous microRNAs in human microvascular endothelial cells regulate mRNAs encoded by hypertension-related genes. Hypertension. 2015;66:793–9. doi: 10.1161/HYPERTENSIONAHA.115.05645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YH, Chen J, Lin ZY, Cao J, Huang HL, Jiang Y, et al Role of deregulated microRNAs in non-small cell lung cancer progression using fresh-frozen and formalin-fixed, paraffin-embedded samples. Oncol Lett. 2016;11:801–8. doi: 10.3892/ol.2015.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakravarthi BVSK, Pathi SS, Goswami MT, Cieślik M, Zheng H, Nallasivam S, et al The miR-124-prolyl hydroxylase P4HA1-MMP1 axis plays a critical role in prostate cancer progression. Oncotarget. 2014;5:6654–69. doi: 10.18632/oncotarget.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan Gana NH, Onuki T, Victoriano AFB, Okamoto T MicroRNAs in HIV-1 infection: an integration of viral and cellular interaction at the genomic level. Front Microbiol. 2012;3:306. doi: 10.3389/fmicb.2012.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drury RE, O'Connor D, Pollard AJ The clinical application of microRNAs in infectious disease. Front Immunol. 2017;8:1182. doi: 10.3389/fimmu.2017.01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou J, Wang P, Lin L, Liu XG, Ma F, An HZ, et al MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol. 2009;183:2150–8. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- 27.Rossato M, Curtale G, Tamassia N, Castellucci M, Mori L, Gasperini S, et al IL-10-induced microRNA-187 negatively regulates TNF-α, IL-6, and IL-12p40 production in TLR4-stimulated monocytes. Proc Natl Acad Sci USA. 2012;109:E3101–10. doi: 10.1073/pnas.1209100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtale G, Mirolo M, Renzi TA, Rossato M, Bazzoni F, Locati M Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci USA. 2013;110:11499–504. doi: 10.1073/pnas.1219852110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lecellier CH, Dunoyer P, Arar K, Lehmann-Che J, Eyquem S, Himber C, et al A cellular microRNA mediates antiviral defense in human cells. Science. 2005;308:557–60. doi: 10.1126/science.1108784. [DOI] [PubMed] [Google Scholar]
- 30.Nathans R, Chu CY, Serquina AK, Lu CC, Cao H, Rana TM Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol Cell. 2009;34:696–709. doi: 10.1016/j.molcel.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai XT, Nicot C miR-28-3p is a cellular restriction factor that inhibits human T cell leukemia virus, type 1(HTLV-1) replication and virus infection. J Biol Chem. 2015;290:5381–90. doi: 10.1074/jbc.M114.626325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu W, Wang XJ, Ding XR, Li Y, Zhang XJ, Xie PW, et al MicroRNA-141 represses HBV replication by targeting PPARA. PLoS One. 2012;7:e34165. doi: 10.1371/journal.pone.0034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin J, Tang SH, Xia L, Du R, Xie HH, Song JG, et al MicroRNA-501 promotes HBV replication by targeting HBXIP. Biochem Biophys Res Commun. 2013;430:1228–33. doi: 10.1016/j.bbrc.2012.12.071. [DOI] [PubMed] [Google Scholar]
- 34.Dar AA, Majid S, Rittsteuer C, de Semir D, Bezrookove V, Tong S, et al The role of miR-18b in MDM2-p53 pathway signaling and melanoma progression. J Natl Cancer Inst. 2013;105:433–42. doi: 10.1093/jnci/djt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu JH, Wang YH, Wang W, Shen W, Sang YZ, Liu L, et al MiR-18b suppresses high-glucose-induced proliferation in HRECs by targeting IGF-1/IGF1R signaling pathways. Int J Biochem Cell Biol. 2016;73:41–52. doi: 10.1016/j.biocel.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Lujambio A, Calin GA, Villanueva A, Ropero S, Sánchez-Céspedes M, Blanco D, et al A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci USA. 2008;105:13556–61. doi: 10.1073/pnas.0803055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Serra P, Esteller M DNA methylation-associated silencing of tumor-suppressor microRNAs in cancer. Oncogene. 2012;31:1609–22. doi: 10.1038/onc.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang T, Zhang JP, Cui M, Liu FB, You XN, Du YM, et al Hepatitis B virus X protein inhibits tumor suppressor miR-205 through inducing hypermethylation of miR-205 promoter to enhance carcinogenesis. Neoplasia. 2013;15:1282–91. doi: 10.1593/neo.131362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanden Bosch A, Raemaekers T, Denayer S, Torrekens S, Smets N, Moermans K, et al NuSAP is essential for chromatin-induced spindle formation during early embryogenesis. J Cell Sci. 2010;123:3244–55. doi: 10.1242/jcs.063875. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto A, Higo M, Shiiba M, Nakashima D, Koyama T, Miyamoto I, et al Down-regulation of nucleolar and spindle-associated protein 1(NUSAP1) expression suppresses tumor and cell proliferation and enhances anti-tumor effect of paclitaxel in oral squamous cell carcinoma. PLoS One. 2015;10:e0142252. doi: 10.1371/journal.pone.0142252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon CA, Gulzar ZG, Brooks JD NUSAP1 expression is upregulated by loss of RB1 in prostate cancer cells . Prostate. 2015;75:517–26. doi: 10.1002/pros.v75.5. [DOI] [PubMed] [Google Scholar]
- 42.Chen LD, Zhuo DQ, Chen JK, Yuan HY Screening feature genes of lung carcinoma with DNA microarray analysis. Int J Clin Exp Med. 2015;8:12161–71. [PMC free article] [PubMed] [Google Scholar]
- 43.Satow R, Shitashige M, Kanai Y, Takeshita F, Ojima H, Jigami T, et al Combined functional genome survey of therapeutic targets for hepatocellular carcinoma. Clin Cancer Res. 2010;16:2518–28. doi: 10.1158/1078-0432.CCR-09-2214. [DOI] [PubMed] [Google Scholar]
- 44.Liu YW, Xia R, Lu K, Xie M, Yang F, Sun M, et al LincRNAFEZF1-AS1 represses p21 expression to promote gastric cancer proliferation through LSD1-Mediated H3K4me2 demethylation . Mol Cancer. 2017;16:39. doi: 10.1186/s12943-017-0588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu T, Zhu Y, Xiong YJ, Ge YY, Yun JP, Zhuang SM MicroRNA-195 suppresses tumorigenicity and regulates G1/S transition of human hepatocellular carcinoma cells. Hepatology. 2009;50:113–21. doi: 10.1002/hep.22919. [DOI] [PubMed] [Google Scholar]
- 46.Fang L, Zhang M, Chen L, Xiong H, Ge YK, Lu W, et al Downregulation of nucleolar and spindle-associated protein 1 expression suppresses cell migration, proliferation and invasion in renal cell carcinoma. Oncol Rep. 2016;36:1506–16. doi: 10.3892/or.2016.4955. [DOI] [PubMed] [Google Scholar]
- 47.Bimonte S, Leongito M, Barbieri A, Del Vecchio V, Falco M, Giudice A, et al The therapeutic targets of miRNA in hepatic cancer stem cells. Stem Cells Int. 2016;2016:1065230. doi: 10.1155/2016/1065230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braconi C, Henry JC, Kogure T, Schmittgen T, Patel T The role of MicroRNAs in human liver cancers. Semin Oncol. 2011;38:752–63. doi: 10.1053/j.seminoncol.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coppola N, Potenza N, Pisaturo M, Mosca N, Tonziello G, Signoriello G, et al Liver microRNA hsa-miR-125a-5p in HBV chronic infection: correlation with HBV replication and disease progression. PLoS One. 2013;8:e65336. doi: 10.1371/journal.pone.0065336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang RQ, Deng L, Zhao L, Li XC, Zhang F, Xia YX, et al miR-22 promotes HBV-related hepatocellular carcinoma development in males. Clin Cancer Res. 2011;17:5593–603. doi: 10.1158/1078-0432.CCR-10-1734. [DOI] [PubMed] [Google Scholar]
- 51.Khokhar A, Noorali S, Sheraz M, Mahalingham K, Pace DG, Khanani MR, et al Computational analysis to predict functional role of hsa-miR-3065-3p as an antiviral therapeutic agent for treatment of triple infections: HCV, HIV-1, and HBV . Libyan J Med. 2012;7:19774. doi: 10.3402/ljm.v7i0.19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li BB, Li DL, Chen C, Liu BH, Xia CY, Wu HJ, et al Potentials of the elevated circulating miR-185 level as a biomarker for early diagnosis of HBV-related liver fibrosis. Sci Rep. 2016;6:34157. doi: 10.1038/srep34157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katayama Y, Maeda M, Miyaguchi K, Nemoto S, Yasen M, Tanaka S, et al Identification of pathogenesis-related microRNAs in hepatocellular carcinoma by expression profiling. Oncol Lett. 2012;4:817–23. doi: 10.3892/ol.2012.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang ZZ, Liu X, Wang DQ, Teng MK, Niu LW, Huang AL, et al Hepatitis B virus and hepatocellular carcinoma at the miRNA level. World J Gastroenterol. 2011;17:3353–8. doi: 10.3748/wjg.v17.i28.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen L, Yang L, Qiao F, Hu X, Li S, Yao L, et al High levels of nucleolar spindle-associated protein and reduced levels of BRCA1 expression predict poor prognosis in triple-negative breast cancer. PLoS One. 2015;10:e0140572. doi: 10.1371/journal.pone.0140572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu XQ, Xu BK, Yang C, Wang WT, Zhong DQ, Zhao Z, et al Nucleolar and spindle associated protein 1 promotes the aggressiveness of astrocytoma by activating the Hedgehog signaling pathway. J Exp Clin Cancer Res. 2017;36:127. doi: 10.1186/s13046-017-0597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gordon CA, Gong X, Ganesh D, Brooks JD NUSAP1 promotes invasion and metastasis of prostate cancer. Oncotarget. 2017;8:29935–50. doi: 10.18632/oncotarget.15604. [DOI] [PMC free article] [PubMed] [Google Scholar]