Abstract
The aim of this study was to explore the feasibility of using different 3D printed internal geometries as tablet formulations to obtain controlled release profiles. In order to obtain controllable release profiles, three types of tablet models (Cylinder, Horn and Reversed Horn) with controlled structures were designed. The cylinder model shows a constant release profile and can keep the drug concentration within a certain range. The horn model exhibits an increasing release profile, which is suitable for the patients who have the drug resistance in the course of medication. The reversed horn model has a decreasing release profile that would be applied to hypertension cure. Furthermore, three types of tablets were fabricated successfully by a fused deposition modeling three-dimensional (3D) printer and injected with paracetamol (APAP) -containing gels. The results of in vitro drug release demonstrate that tablets with three kinds of structures can produce constant, gradually increasing, and gradually decreasing release profiles, respectively. The release attributes can be controlled by using different 3D printed geometries as tablet formulations. More importantly, there are no residues after dissolution. The method of preparing customized tablets with distinguished release profiles presented in this study has the promising potential in the fabrication of patient-tailored medicines.
Subject terms: Drug delivery, Pharmaceutics, Engineering
Introduction
Three-dimensional (3D) printing or additive manufacturing is a rapid prototyping technology that prints 3D objects by layer-by-layer deposition approach controlled by computer-aided design software1. 3D printing has been used to generate complex structures, which are very challenge to manufacture with traditional techniques. It has been studied in the medical and pharmaceutical applications such as tissue engineering2–5, dentistry6, and implants7,8. Tablets with different structures and materials will have distinguished release profiles9. In August 2015, the application of 3D printing in the pharmaceutical industry was approved by US Food and Drug Administration (FDA)9,10, indicating that 3D printing could elaborate its advantages in personalized medicine.
Recently many researchers have paid more efforts to employ 3D printing to develop personalized medicines11–13 and drug delivery system14–21. There are multiple 3D printing technologies utilized in customized medicines, such as Stereolithography (SLA)22, Selective Laser Sintering (SLS)23,24, Fused Deposition Modelling (FDM)13,25,26, Semi-solid extrusion (SSE)27 and Powder Based (PB)28. 3D printing is used to fabricate oral dosage forms and demonstrates great potentials in the pharmaceutical industry12,13,29,30. Currently, researchers fabricated controlled-release drugs with different characteristics by 3D printing22,30–36. Tablets with different structures and materials will have distinguished release profiles9. Yu et al. employed PB 3D printing to develop doughnut-shaped multi-layered tablets with linear release profile28. Wang et al. used SLA 3D printer to manufacture drug-loaded tablets with modified-release characteristics and revealed that the release profile of the drugs was dependent on the composition of the formulations22. Trenfield et al. fabricated printlets with three different geometries using a desktop SLS printer and developed a calibration model to predict drug concentration of a different geometry37,38. Of these 3DP technologies FDM technology exhibits the excellent promise in fabricating drug products, because FDM printers are low-cost, easy to operate9 and able to refine hollow objects39. FDM 3D printing has recently attracted increasing interests as one of the most widely used techniques for developing individualized medicines in pharmaceutical applications13. Muwaffak et al. used 3D scanning to construct models of a nose and ear and printed a customized wound dressing using antimicrobial metals incorporated into polycaprolactone (PCL) to produce filaments for 3D printing40. These metals with broad-spectrum antimicrobial properties can improve the wound healing process41,42. FDM 3D printing is an effective process to fabricate tablets with modified release profiles. FDM printer has been used to produce immediate, sustained, and time-released tablets43. It is able to manufacture complex shapes and geometries to obtain different release profiles in personalized medicine9,44. Skowyra et al. used FDM printer to produce prednisolone sustained-release tablets13. Melocchi et al. explored the potential of FDM 3D printing to manufacture oral capsular devices for pulsatile release25. Chai et al. explored the potential of FDM 3D printing to prepare sustained-release drugs, which could be released in the stomach for a long time45. Goyanes et al. produced tablets with different geometrical shapes by FDM 3D printing and demonstrated that tablet shape could affect drug release profile44.
Hydroxypropyl methylcellulose (HPMC), a non-toxic and safe pharmaceutical excipient, has been employed as the forming agents and binding additives in order to obtain sustained release33,46–49. Zhang et al. explored the relationship between drug release and the geometrical shape of the 3D printed tablets with different structures printed by FDM printing50. The desktop 3D printer was used to produce bilayer tablets with a definable release profile through a hydrated HPMC gel layer12. Khaled et al. reported 3D extrusion printing of a complex multi-compartment tablet12,51. Kadry et al. employed HPMC and diltiazem to prepare both drug-free and drug-impregnated filaments and printed tablets with different infill densities and patterns49. However, the shrinkage of the gel model affected the shape of the printed tablets13. The hot melt extrusion (HME) process is indispensable in FDM 3D printing, which allows the thermally softened filaments to be extruded by a nozzle and to be deposited layer-by-layer52. Genina et al. investigated ethylene vinyl acetate (EVA) as new feedstock material for FDM 3D printing technology to print medical drug delivery devices53. Polyvinyl alcohol (PVA) is one of most widely used water-soluble synthetic polymer in various applications54–59. It has been utilized as benchmark polymer60 and drug carriers30,34,61,62 in 3D printed pharmaceuticals because of its good biocompatibility. Pluta et al. described the application of polyvinyl alcohol (PVA) in the technology of modern drug form63. Goyanes et al. used a filament extruder to obtain filaments of PVA containing paracetamol (APAP) and fabricated PVA-based caplets with specific release profiles by FDM 3D printing64. Tagami et al. produced composite tablets with a drug-loaded PVA component and a PVA or PLA filler component using FDM 3D printer with dual nozzles65. The major shortcoming of FDM 3D printing in pharmaceutical applications is the elevated extrusion and printing temperatures required in the printing process, which limits its application in 3D printing drugs41,49,66,67. So, it is not suitable to produce thermolabile drugs because the pharmaceutical excipients and active drugs may degrade at high temperature during the extrusion and printing processes30,51,60. Many investigators have attempted to address this issue. Kollamaram et al. printed ramipril printlets by reducing the FDM printing temperature to 90 °C48. Kempin et al. printed dual coated tablets by dual-extrusion-printing using three-part printing designs and utilized polycaprolactone (PCL) as the coating with a printing temperature of 58 °C68. However, the printing process is complex and PCL is nearly insoluble. Sun et al. incorporated insoluble containers and light curing materials into a tablet and obtained the release profile of the drug via controlling the area of the effective component to release in accordance with the scheduled time36. However, the challenge of preparing tablets with this method is the insoluble container and the complex manufacturing process. The main reason for limiting FDM 3D printing in pharmaceutical applications is that the 3D printing need to mix the drug to the polymeric filament and heat drug loading filament to a high temperature to extrude through a nozzel and deposit layer by layer.
We recently reported the fabrication of a tablet with a regular tetrahedron cavity printed by a 3D printer69. The support structure (shell) and the drug were fabricated separately. PVA filaments were printed as a soluble container and PVA gel containing drugs was injected into the cavity at room temperature. The tablet with a regular tetrahedron cavity can only provide one kind of drug release profile, i.e., increasing release profile.
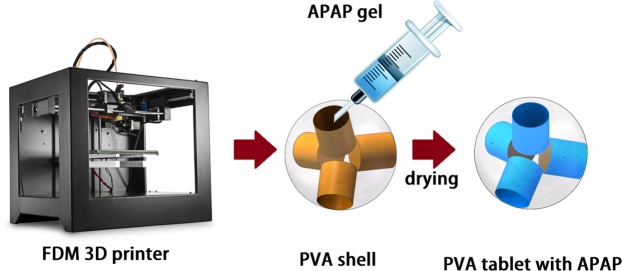
The aim of this study is to explore the use of different 3D printed internal geometries as tablet formulations to obtain multiple controlled-release profiles. PVA scaffolds with different internal architecture were printed by a commercially 3D printer and injected with drug-containing gel. Tablets produced by our method have the advantages of multiple release profiles, high temperature resistance, and no residues after dissolution.
Results and Discussion
Establishment of three tablet models
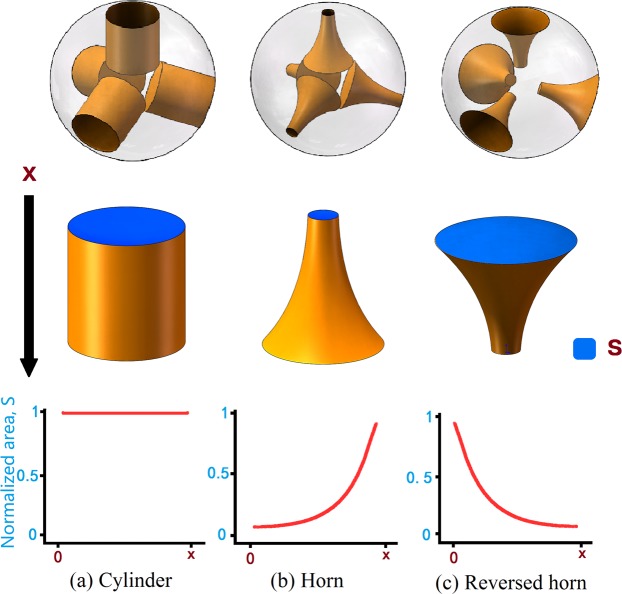
Figure 1 shows three kinds of models of tablet kernels with different shapes, i.e., Cylinder, Horn and Reversed Horn (R-Horn). Three types of cavities are embedded in the sphere for achieving different release profiles. In order to use more effective components and also make the drug release more uniformly, four symmetrically distributed cavities are designed and evenly placed in a sphere with a radius of 6 mm. X represents the height of cavities. Cavities will be filled with drug gel by injection. Tablets with different models will have distinct release attributes. The surface area S of the exposed drug will be constant, gradually increasing and gradually decreasing for Cylinder model, Horn model and R-Horn model, respectively. The inner architecture of the tablet would be gradually exposed with the decrease of the diameter of the tablet (Fig. S1) and the release rate would be different due to the change of surface area of medicine core.
Figure 1.
Three kinds of models of tablet kernels with different shapes. (a) The first model has four cylinder cavities placed in a sphere (Cylinder model). (b) The second model has four horn cavities placed in a sphere (Horn model). (c) The third model has four reversed horn cavities placed in a sphere (R-Horn model). The blue part represents the exposed area of the drug kernel. Along with the decrease of height, the surface area (S) will change in three kinds of models.
Preparation of tablets
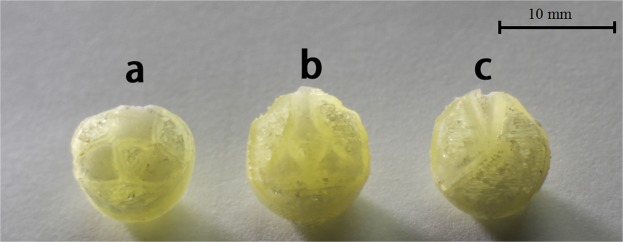
Figure 2 shows the tablets with Cylinder model, Horn model and R-Horn model printed by FDM 3D printing. The pale yellow part is PVA shell, and the milky white part is APAP-containing gel after drying. The weight and diameter of each tablet were measured. X, Stop and Sbottom represent the height of the cavity of each model, the surface area of top side and bottom side of each model in Fig. 1. The parameters of three kinds of tablets are listed in Table 1. Figure 3 shows the structure of pore and inner architecture of the tablet with Cylinder model before and after injection of the drug molecule.
Figure 2.
Photos of 3D printed tablets with the three models. (a) Cylinder model, (b) Horn model, (c) R-Horn model.
Table 1.
Parameters of the 3D printed tablets with Cylinder model, Horn model and R-Horn model.
| Model | Cavity height/mm | Stop/mm2 | Sbottom/mm2 | Weight/g | Diameter/mm |
|---|---|---|---|---|---|
| Cylinder | 4.15 ± 0.14 | 12.57 ± 0.2 | 12.57 ± 0.2 | 0.85 ± 0.026 | 9.98 ± 0.18 |
| Horn | 4.48 ± 0.21 | 0.97 ± 0.02 | 17.57 ± 0.28 | 0.92 ± 0.019 | 10.20 ± 0.15 |
| R-Horn | 4.50 ± 0.24 | 19.27 ± 0.24 | 0.79 ± 0.01 | 0.89 ± 0.016 | 10.13 ± 0.12 |
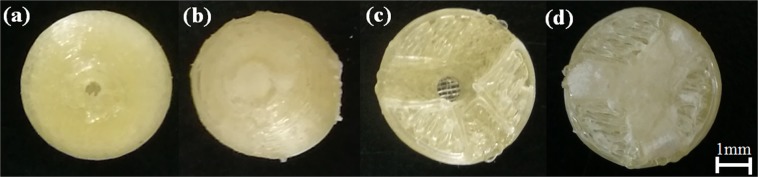
Figure 3.
The structure of the tablet with Cylinder model. (a) tablet shell with a hole, (b) tablet after injection of the drug molecule, (c) cross section of tablet shell before injection, (d) cross section of tablet shell after injection.
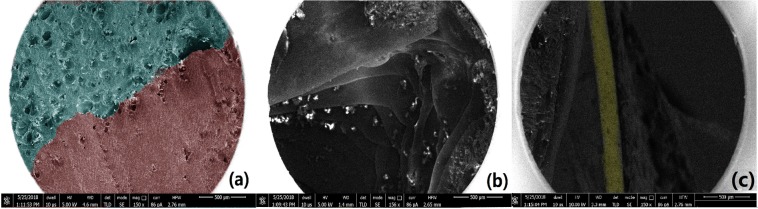
SEM images of cross-section of 3D printed PVA shell with Cylinder model and injected APAP gel are shown in Fig. 4. The fabricated tablet is sufficiently hard and does not collapse during 3D printing. Figure 4a shows that there is no gap between PVA and APAP gel after drying. Figure 4b represents the layered structure of the shell, which is the processing feature of FDM printing. Figure 4c shows a contour line that forms the perimeter of the drug.
Figure 4.
SEM visualization of cross-section of 3D printed PVA tablet with Cylinder model after injection of APAP gel. (a) Green part is the APAP gel, red part means PVA. (b) the layered structure of the shell, (c) The yellow part represents the perimeter of the drug.
In vitro drug release study
Tablets with Cylinder, Horn and R-Horn models are fabricated by printing PVA shell with FDM 3D printer and injecting APAP-containing gels into the cavity at room temperature. The phosphate buffer solution (PBS, pH = 6.8, 0.05 M, 900 mL) was used as representing the colonic environment44. In vitro drug dissolution tests were conducted by putting the tablets in phosphate buffer solution (PBS, pH = 6.8) with stirring (50 rpm) at 37 ± 0.5 °C. The concentration of active ingredient of APAP was measured by an ultraviolet spectrophotometer (UV-2601, Beijing RuiLi Analytical Instrument Co.Ltd, China). Samples were measured every 15 minutes during dissolution. The absorption peaks of APAP and PVA were detected at 243 nm and 264 nm, respectively. By the calculation, the influence of PVA could be removed, and the absorbance of the APAP could be obtained. In this way, the dissolution rate can be obtained by calculating the change of APAP concentration.
Tablets gradually melted along with the decrease of tablet diameter during dissolution. The release rate of tablets gradually varied with the change of surface area of tablets. The content of the drug was gradually exposed to PBS, which would facilitate the release rate of tablets. Tablets with three structures were dissolved according to the predetermined patterns of three models. The release was almost completed in ca. 6 hours. The release profiles of 3D printed APAP tablets are shown in Fig. 5. Tablets with three kinds of structures show constant, gradually increasing, and gradually decreasing release rate, respectively. Burst release, which is undesired for controlled-release drugs applications, is not observed from the release profiles of APAP tablets. It may be a result of using higher molecular weight PVA to decrease diffusion pathway62. In the previous work, we successfully printed a tablet with increasing release profile68. In this study, tablet of each structure has its characteristic release profile during dissolution. The Cylinder model has a constant release profile that can maintain the drug concentration within a certain range. The Horn model has an increasing release profile, which is suitable for patients with drug tolerance in the course of medication and can also be applied to hypertension which often occurs in the morning70. The R-Horn model has a decreasing release profile that can be valuable for cases where a large dose of drug is required initially to act against their targets rapidly36. The drug release data demonstrate that the internal architectures of tablets have an important effect on drug release rate.
Figure 5.
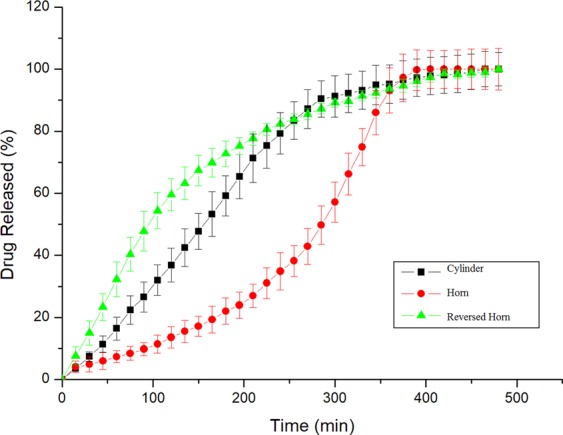
The release profiles of APAP tablets with Cylinder model, Horn model, and R-Horn model.
Figure 6 reveals the dissolution of tablets with Cylinder model every 1 hour when dissolved in PBS. Over time, tablets were gradually eroded by PBS and became smaller. Although the drug became softened during dissolution, the inner architecture of scaffolds of the tablet still existed. This is because the dissolution rate of the PVA material is lower than that of the APAP gel. This phenomenon could be explained that drug release was determined by an erosion-mediated mechanism reported by a couple of studies13,39,44. In terms of in vitro dissolution results, the release rates of the tablets with three models are positively related to the changes of surface area, which is consistent with the results reported by Goyanes44. Generally, the release profile of single-component drugs was decreasing because the surface area of active ingredients was uncontrolled. We designed different 3D printed geometries as controlled release system to govern the inner architecture of the tablets to obtain constant, gradually increasing and gradually decreasing release profiles.
Figure 6.
Tablets with Cylinder model during dissolution.
This study demonstrates that the potentials of FDM 3D printing technology to fabricate controlled release tablets with different release profiles in accordance with three kinds of models. Meanwhile, PVA can be used for different kinds of medicines, providing a positive effect for personalized medicine. Sun et al. used an insoluble container and light curing materials to produce a tablet for getting the drug release profile36. We fabricated tablets with distinguished release profiles using PVA filaments as a soluble container filled with PVA gels containing drugs. The tablets in our work were manufactured with simple fabrication process and there is no residue after dissolution because the core and shell of tablets are soluble. In our study the drug-loaded gel was injected into the cavities without high temperature and the active ingredients of the drug were not degraded. Compared to hot melt extrusion by adding APAP into PVA filament to fabricate controlled-release drugs at about 200 °C32,34, the way of injecting the drug-containing gel into the cavity at room temperature also avoids high temperature damage to the active ingredients of the tablets, and it is more suitable for the thermo-sensitive tablets. Compared to other 3D printing tablets, our work takes into account more aspects. For example, one of the typical advantages is that there is no insoluble container. Secondly, the manufacturing steps are simple so that it will be more suitable for popularizing on a large scale. Thirdly, our approach is suitable to print thermo-sensitive drugs compared to the method of adding drugs to PVA filament32. However, it still has some shortcomings such as short dissolution cycle (less than 7 hours) and lacking of the structure diversity. In the future, it is necessary to develop other gel components to fill the tablet containers so that the dissolution time would be extended.
Conclusion
We proposed a new method to fabricate customized tablets with distinguished release profiles using 3D printing technology and filling them with drug-containing gel at room temperature, which provides a new way for tailored drugs with simple fabrication process. We designed different 3D printed geometries as tablet formulations for the controlled drug release and explored the effect of inner architecture of scaffolds to obtain constant, gradually increasing or gradually decreasing release rate. The drug release rate depends on internal architectures of tablets. Tablets produced in this work could be dissolved evenly and there was no residue after dissolution. The manufacturing process did not degrade the active ingredients of drugs because drug-containing gels injected into the cavity at room temperature don’t need to be heated high temperature of over 200 °C, which could be applied to other thermolabile tablets. In addition, the simple fabrication process for customized tablets by 3D printing technologies and easily available materials also make this general method suitable for popularization on large scale, which plays an important role in the personalized medicine.
Materials and Methods
Preparation of drug gel
Water, APAP (Anta Biotechnology, China) and PVA powder (Yingjia Inc., China) were mixed at a ratio of 6:3:1, stirred evenly for 15 minutes, then put into the vacuum chamber and evacuate until the air pressure is 400 Pa to remove bubbles of the drug gel.
3D printing PVA shell
FDM 3D printer (Creator Pro, FlashForge, China) is employed to produce a soluble container of the tablet with PVA filament (MOSHU Inc., China) at the temperature of 180 °C using a nozzle diameter of 0.3 mm at an infill rate of 100% and printing speed of 60 mm/s. It has shown that infill rate can significantly affect the release profile, and the infill rate is set to 100%, excluding the interference of infill contain cavities63.
Injecting gel into PVA shell
The prepared drug gel was injected into the cavity by a 1-mL syringe with a needle (inner diameter of 0.5 mm) through a hole with a diameter of 0.7 mm, which was drilled at the thinnest part of PVA shell (Fig. 7). After injecting, the tablets were dried in a vacuum drying oven at 60 °C for 12 hours.
Figure 7.
The process of preparing tablets.
Scanning electron microscope (SEM) characterization
In order to investigate whether APAP adheres closely to PVA after injection or not, the cross-section images of the tablet were characterized using SEM (FEI Helios Nanolab 600i SEM, America). The cross section of the tablet was milled by sandpaper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Supplementary information
Acknowledgements
The authors would like to acknowledge the support provided by National Natural Science Foundation of China (No. 21105127, No. 51673214), the Fundamental Research Funds for the Central Universities of Central South University (No.2017zzts389), and the Medical Electronics Maker Space in Central South University.
Author Contributions
X.W.X. and J.L.Y. conceived the idea. J.Z.Z. and M.N.W. carried out all the experiments as well as the data collection and analysis. L.W. contributed to data analysis. All authors discussed the results and provided comments on the manuscript.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-48921-8.
References
- 1.Xiong YJ, et al. Structural broadband absorbing metamaterial based on three-dimensional printing technology. Acta Phys. Sin. 2018;67:084202. doi: 10.1007/s12110-009-9068-2. [DOI] [Google Scholar]
- 2.Feng P, et al. A Multimaterial Scaffold With Tunable Properties: Toward Bone Tissue Repair. Adv. Sci. 2018;5:1700817. doi: 10.1002/advs.201700817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melchels FPW, Feijen J, Grijpma DW. A poly(D,L-lactide) resin for the preparation of tissue engineering scaffolds by stereolithography. Biomaterials. 2009;30:3801–3809. doi: 10.1016/j.biomaterials.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Shuai CJ, et al. Fabrication optimization of nanohydroxyapatite artificial bone scaffolds. Nano. 2012;7:1250015. doi: 10.1142/s1793292012500154. [DOI] [Google Scholar]
- 5.Skoog SA, Goering PL, Narayan RJ. Stereolithography in tissue engineering. J Mater Sci Mater Med. 2014;25:845–856. doi: 10.1007/s10856-013-5107-y. [DOI] [PubMed] [Google Scholar]
- 6.Galante R, Figueiredo-Pina CG, Serro AP. Additive manufacturing of ceramics for dental applications: A review. Dent Mater. 2019;35:825–846. doi: 10.1016/j.dental.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Popov VK, et al. Laser stereolithography and supercritical fluid processing for custom-designed implant fabrication. J Mater Sci Mater Med. 2004;15:123–128. doi: 10.1023/B:JMSM.0000011812.08185.2a. [DOI] [PubMed] [Google Scholar]
- 8.Huang W, Zheng Q, Sun W, Xu H, Yang X. Levofloxacin implants with predefined microstructure fabricated by three-dimensional printing technique. Int J Pharm. 2007;339:33–38. doi: 10.1016/j.ijpharm.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Alhnan MA, et al. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm Res-Dordr. 2016;33:1817–1832. doi: 10.1007/s11095-016-1933-1. [DOI] [PubMed] [Google Scholar]
- 10.Konta AA, Garcia-Pina M, Serrano DR. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering. 2017;4:79. doi: 10.3390/bioengineering4040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyanes A, et al. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol Pharmaceut. 2015;12:4077–4084. doi: 10.1021/acs.molpharmaceut.5b00510. [DOI] [PubMed] [Google Scholar]
- 12.Khaled SA, Burley JC, Alexander MR, Roberts CJ. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int J Pharmaceut. 2014;461:105–111. doi: 10.1016/j.ijpharm.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Skowyra J, Pietrzak K, Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci. 2015;68:11–17. doi: 10.1016/j.ejps.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Huang S, Li C, Xiao Q. Yolk @ cage-Shell Hollow Mesoporous Monodispersion Nanospheres of Amorphous Calcium Phosphate for Drug Delivery with High Loading Capacity. Nanoscale Res. Lett. 2017;12:275. doi: 10.1186/s11671-017-2051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonathan G, Karim A. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int J Pharmaceut. 2016;499:376–394. doi: 10.1016/j.ijpharm.2015.12.071. [DOI] [PubMed] [Google Scholar]
- 16.Khan FA, et al. 3D Printing Technology in Customized Drug Delivery System: Current State of the Art, Prospective and the Challenges. Curr Pharm Design. 2018;24:5049–5061. doi: 10.2174/1381612825666190110153742. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Jiang W, Nam J, Moon JJ, Kim BYS. Immunomodulating Nanomedicine for Cancer Therapy. Nano Lett. 2018;18:6655–6659. doi: 10.1021/acs.nanolett.8b02340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, et al. Intercalated 2D nanoclay for emerging drug delivery in cancer therapy. Nano Res. 2017;10:2633–2643. doi: 10.1007/s12274-017-1466-x. [DOI] [Google Scholar]
- 19.Melocchi A, et al. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly(vinyl alcohol): design concept and 4D printing feasibility. Int J Pharmaceut. 2019;559:299–311. doi: 10.1016/j.ijpharm.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 20.Liang, K., Carmone, S., Brambilla, D. & Leroux, J. C. 3D printing of a wearable personalized oral delivery device: A first-in-human study. Sci Adv4, eaat2544, https://doi.org/ARTN eaat254410.1126/sciadv.aat2544 (2018). [DOI] [PMC free article] [PubMed]
- 21.Genina N, et al. Anti-tuberculosis drug combination for controlled oral delivery using 3D printed compartmental dosage forms: From drug product design to in vivo testing. J Control Release. 2017;268:40–48. doi: 10.1016/j.jconrel.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Goyanes A, Gaisford S, Basit AW. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int J Pharmaceut. 2016;503:207–212. doi: 10.1016/j.ijpharm.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Fina F, Goyanes A, Gaisford S, Basit AW. Selective laser sintering (SLS) 3D printing of medicines. Int J Pharmaceut. 2017;529:285–293. doi: 10.1016/j.ijpharm.2017.06.082. [DOI] [PubMed] [Google Scholar]
- 24.Fina F, et al. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int J Pharmaceut. 2018;547:44–52. doi: 10.1016/j.ijpharm.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 25.Melocchi A, et al. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J Drug Deliv Sci Tec. 2015;30:360–367. doi: 10.1016/j.jddst.2015.07.016. [DOI] [Google Scholar]
- 26.Sadia M, Arafat B, Ahmed W, Forbes RT, Alhnan MA. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J Control Release. 2018;269:355–363. doi: 10.1016/j.jconrel.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Cui M, et al. Exploration and Preparation of a Dose-Flexible Regulation System for Levetiracetam Tablets via Novel Semi-Solid Extrusion Three-Dimensional Printing. J Pharm Sci. 2019;108:977–986. doi: 10.1016/j.xphs.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Yu DG, et al. Novel drug delivery devices for providing linear release profiles fabricated by 3DP. Int J Pharmaceut. 2009;370:160–166. doi: 10.1016/j.ijpharm.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Katstra WE, et al. Oral dosage forms fabricated by Three Dimensional Printing (TM) J Control Release. 2000;66:1–9. doi: 10.1016/S0168-3659(99)00225-4. [DOI] [PubMed] [Google Scholar]
- 30.Goyanes A, Buanz ABM, Hatton GB, Gaisford S, Basit AW. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur J Pharm Biopharm. 2015;89:157–162. doi: 10.1016/j.ejpb.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Fu JH, Yu X, Jin YG. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int J Pharmaceut. 2018;539:75–82. doi: 10.1016/j.ijpharm.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 32.Goyanes A, et al. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int J Pharmaceut. 2015;496:414–420. doi: 10.1016/j.ijpharm.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 33.Goyanes A, et al. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int J Pharmaceut. 2017;527:21–30. doi: 10.1016/j.ijpharm.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 34.Li QJ, et al. Preparation and investigation of controlled-release glipizide novel oral device with three-dimensional printing. Int J Pharmaceut. 2017;525:5–11. doi: 10.1016/j.ijpharm.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 35.Siyawamwaya M, et al. 3D printed, controlled release, tritherapeutic tablet matrix for advanced anti-HIV-1 drug delivery. Eur J Pharm Biopharm. 2019;138:99–110. doi: 10.1016/j.ejpb.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Sun YJ, Soh S. Printing Tablets with Fully Customizable Release Profiles for Personalized Medicine. Advanced Materials. 2015;27:7847–7853. doi: 10.1002/adma.201504122. [DOI] [PubMed] [Google Scholar]
- 37.Trenfield SJ, Awad A, Goyanes A, Gaisford S, Basit AW. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol Sci. 2018;39:440–451. doi: 10.1016/j.tips.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Trenfield SJ, et al. 3D printed drug products: Non-destructive dose verification using a rapid point-and-shoot approach. Int J Pharmaceut. 2018;549:283–292. doi: 10.1016/j.ijpharm.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Goyanes A, Buanz ABM, Basit AW, Gaisford S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int J Pharmaceut. 2014;476:88–92. doi: 10.1016/j.ijpharm.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 40.Muwaffak Z, et al. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int J Pharmaceut. 2017;527:161–170. doi: 10.1016/j.ijpharm.2017.04.077. [DOI] [PubMed] [Google Scholar]
- 41.Li G, Peng B, Chai L, Wan S, Jiang L. Preparation of Antibacterial Color-Coated Steel Sheets. Int. J. Photoenergy. 2012;2012:436963. doi: 10.1155/2012/436963. [DOI] [Google Scholar]
- 42.Shu Z, Zhang Y, Yang Q, Yang H. Halloysite Nanotubes Supported Ag and ZnO Nanoparticles with Synergistically Enhanced Antibacterial Activity. Nanoscale Res. Lett. 2017;12:135. doi: 10.1186/s11671-017-1859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norman J, Madurawe RD, Moore CM, Khan MA, Khairuzzaman A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv Drug Deliv Rev. 2017;108:39–50. doi: 10.1016/j.addr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Goyanes A, Martinez PR, Buanz A, Basit AW, Gaisford S. Effect of geometry on drug release from 3D printed tablets. Int J Pharmaceut. 2015;494:657–663. doi: 10.1016/j.ijpharm.2015.04.069. [DOI] [PubMed] [Google Scholar]
- 45.Chai XY, et al. Fused Deposition Modeling (FDM) 3D Printed Tablets for Intragastric Floating Delivery of Domperidone. Sci. Rep. 2017;7:2829. doi: 10.1038/s41598-017-03097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solanki NG, Tahsin M, Shah AV, Serajuddin AM. Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J Pharm Sci-Us. 2018;107:390–401. doi: 10.1016/j.xphs.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 47.Palo M, Hollander J, Suominen J, Yliruusi J, Sandler N. 3D printed drug delivery devices: perspectives and technical challenges. Expert Rev Med Devices. 2017;14:685–696. doi: 10.1080/17434440.2017.1363647. [DOI] [PubMed] [Google Scholar]
- 48.Kollamaram G, et al. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int J Pharmaceut. 2018;545:144–152. doi: 10.1016/j.ijpharm.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 49.Kadry H, et al. Multi-purposable filaments of HPMC for 3D printing of medications with tailored drug release and timed-absorption. Int J Pharmaceut. 2018;544:285–296. doi: 10.1016/j.ijpharm.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, et al. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: Structure and drug release correlation. Carbohydr Polym. 2017;177:49–57. doi: 10.1016/j.carbpol.2017.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of tablets containing multiple drugs with defined release profiles. Int J Pharmaceut. 2015;494:643–650. doi: 10.1016/j.ijpharm.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 52.Nasereddin JM, Wellner N, Alhijjaj M, Belton P, Qi S. Development of a Simple Mechanical Screening Method for Predicting the Feedability of a Pharmaceutical FDM 3D Printing Filament. Pharm Res. 2018;35:151. doi: 10.1007/s11095-018-2432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Genina N, et al. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur J Pharm Sci. 2016;90:53–63. doi: 10.1016/j.ejps.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Guo J, Xie D, Yang B, Jiang J. Low-power logic computing realized in a single electric-double-layer MoS2 transistor gated with polymer electrolyte. Solid-State Electron. 2018;144:1–6. doi: 10.1016/j.sse.2018.02.007. [DOI] [Google Scholar]
- 55.Guo JJ, Jiang J, Zheng ZM, Yang BC. Enhanced performance of multilayer MoS2 transistor employing a polymer capping layer. Org. Electron. 2017;40:75–78. doi: 10.1016/j.orgel.2016.10.043. [DOI] [Google Scholar]
- 56.He, S. et al. Low-temperature-cured highly conductive composite of Ag nanowires & polyvinyl alcohol. Chin. Phys. B26, 078103, https://doi.org/ Artn07810310.1088/1674-1056/26/7/078103 (2017).
- 57.Liang Z, et al. Facile Synthesis of Nitrogen-Doped Microporous Carbon Spheres for High Performance Symmetric Supercapacitors. Nanoscale Res. Lett. 2018;13:314–314. doi: 10.1186/s11671-018-2713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang P, et al. Few-layered MoS2/C with expanding d-spacing as a high-performance anode for sodium-ion batteries. Nanoscale. 2017;9:12189–12195. doi: 10.1039/C7NR03690F. [DOI] [PubMed] [Google Scholar]
- 59.Zhou L, Zhou D, Gan W, Zhang Z. A ZnO/PVA/PAADDA composite electrode for rechargeable zinc-air battery. Ionics. 2017;23:3469–3477. doi: 10.1007/s11581-017-2150-6. [DOI] [Google Scholar]
- 60.Alhijjaj M, Belton P, Qi S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur J Pharm Biopharm. 2016;108:111–125. doi: 10.1016/j.ejpb.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Singh B, Sharma V. Design of psyllium-PVA-acrylic acid based novel hydrogels for use in antibiotic drug delivery. Int J Pharmaceut. 2010;389:94–106. doi: 10.1016/j.ijpharm.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 62.Rattanakit P, Moulton SE, Santiago KS, Liawruangrath S, Wallace GG. Extrusion printed polymer structures: A facile and versatile approach to tailored drug delivery platforms. Int J Pharmaceut. 2012;422:254–263. doi: 10.1016/j.ijpharm.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Pluta J, Karolewicz B. The application of polyvinyl alcohol in the technology of modern drug form. Polimery w medycynie. 2001;31:11–17. [PubMed] [Google Scholar]
- 64.Goyanes A, Kobayashi M, Martinez-Pacheco R, Gaisford S, Basit AW. Fused-filament 3D printing of drug products: Microstructure analysis and drug release characteristics of PVA-based caplets. Int J Pharm. 2016;514:290–295. doi: 10.1016/j.ijpharm.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 65.Tagami T, et al. Defined drug release from 3D-printed composite tablets consisting of drugloaded polyvinylalcohol and a water-soluble or water-insoluble polymer filler. Int J Pharmaceut. 2018;543:361–367. doi: 10.1016/j.ijpharm.2018.03.057. [DOI] [PubMed] [Google Scholar]
- 66.Awad A, Trenfield SJ, Gaisford S, Basit AW. 3D printed medicines: A new branch of digital healthcare. Int J Pharm. 2018;548:586–596. doi: 10.1016/j.ijpharm.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 67.Awad A, Trenfield SJ, Goyanes A, Gaisford S, Basit AW. Reshaping drug development using 3D printing. Drug Discov Today. 2018;23:1547–1555. doi: 10.1016/j.drudis.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 68.Kempin W, et al. Development of a dual extrusion printing technique for an acid- and thermo-labile drug. Eur J Pharm Sci. 2018;123:191–198. doi: 10.1016/j.ejps.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 69.Zhao JZ, Xu XW, Wang MN, Wang L. A New Model of a 3D-Printed Shell with Convex Drug Release Profile. Dissolut Technol. 2018;25:24–28. doi: 10.14227/DT250118P24. [DOI] [Google Scholar]
- 70.Marfella R, et al. Morning blood pressure peak, QT intervals, and sympathetic activity in hypertensive patients. Hypertension. 2003;41:237–243. doi: 10.1161/01.HYP.0000050651.96345.0E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.