Abstract
The midbrain dopamine system via the dorsal and ventral striatum regulates a wide range of behaviors. To dissect the role of dopaminergic projections to the dorsal striatum (nigrostriatal projection) and ventral striatum (mesolimbic projection) in sleep-wake behavior, we selectively chemogenetically stimulated nigrostriatal or mesolimbic projections and examined the resulting effects on sleep in rats. Stimulation of nigrostriatal pathways increased sleep and EEG delta power, while stimulation of mesolimbic pathways decreased sleep and reduced cortical EEG power. These results indicate that midbrain dopamine signaling in the dorsal or ventral striatum promotes sleep or wake respectively.
Keywords: nigrostriatal pathway, mesolimbic pathway, dopamine, sleep, wake, EEG
INTRODUCTION
Midbrain dopaminergic neurons have massive nigrostriatal and mesolimbic projections to the dorsal striatum (caudoputamen, CPu) and ventral striatum (nucleus accumbens, NAc), where dopamine tunes cortical inputs and regulates cognition, motor behavior, and sleep-wake states (Bjorklund and Dunnett 2007; Malenka et al. 2009; Nambu 2008; Schultz 2007). Dopamine and its D1 and D2 receptors are required for maintaining arousal (Feenstra et al. 2000; Isaac and Berridge 2003; Lu et al. 2006; Ongini et al. 1993; Qu et al. 2008; Smith et al. 1992). However, nigrostriatal dopamine has been shown to promote sleep in rats via activation of the globus pallidus externa (GPe) (Qiu et al. 2016c). Lesions of the ventral tegmental area (VTA) in rats and cats do not significantly affect sleep-wake behavior, while lesions of the substantia nigra pars compacta (SNc) increase wakefulness in rats, cats and monkeys (Belaid et al. 2014; Gerashchenko et al. 2006; Lai et al. 2008; Lai et al. 1999). Consistent with these findings, chemogenetic inhibition of VTA dopamine neurons has no effect on sleep (Oishi et al. 2017). These results suggest that under baseline conditions, VTA dopamine neurons are not sufficient for wakefulness, while SNc dopamine neurons are necessary for sleep.
Anterograde and retrograde tracing, together with optogenetic stimulation, has revealed a direct GABAergic projection from GPe to the GABAergic interneurons in the frontal promotor cortex (Chen et al. 2015), strongly supporting the hypothesis that SNc DA promotes sleep via the GPe as an extra-thalamocortical pathway (Qiu et al. 2016c). The projections of NAc are much more diverse than that of the CPu, targeting multiple sites in the forebrain, diencephalon and brainstem (Zhang et al. 2013). Accordingly, the mesolimbic pathway may have a multifaceted influence on cortical activity. Jones and colleagues revealed that cats exhibited decreased behavioral arousal but no significant changes in electrocortical wakefulness after electrolytic lesion of the VTA (Jones et al. 1973). Similarly, lesions to the VTA dopamine cells in rats with the neurotoxin hypocretin2-saporin produced no significant changes in arousal behavior as assessed by electroencephalographic recording (Gerashchenko et al. 2006), suggesting that the VTA is not essential for electrocortical activation.
With the help of genetic-based tools and approaches, several recent mouse studies have shown that optogenetic and chemogenetic stimulation of dopamine neurons in the VTA promotes wakefulness. Contrary to optogenetic stimulation (Qiu et al. 2016c), chemogenetic stimulation of SNc dopaminergic neurons has no effect on sleep (Eban-Rothschild et al. 2016; Oishi et al. 2017). The differences in the findings may stem from the different dopamine neuron populations activated by the different methods. In consideration of the complexity of the neural circuits relating to the basal ganglia, SNc dopamine neurons not only innervate the CPu, but also innervate the STN, SNr/GPi and even brainstem nuclei, such as the mesencephalic locomotor command regions (Grillner and Robertson 2016). Though Oishi et al. used dopamine transporter (DAT)-Cre mice to ensure that their findings were specific to SNc dopamine neurons, however, multiple SNc dopaminergic pathways may have a combined effect on arousal. In order to elucidate the role of specific dopamine pathways inside the BG (nigrostriatal pathway and mesolimbic pathway) in sleep, we used a combinatorial viral-based retrograde-activation approach. Using this approach, we demonstrated that chemogenetic activation of nigrostriatal pathway increased sleep and cortical EEG delta power, while chemogenetic stimulation of mesolimbic pathway had the opposite effect. Together, these results suggest that midbrain dopamine pathways targeting the basal ganglia have opposite roles in sleep-wake regulation.
RESULTS
Chemogenetic stimulation of dopamine nigrostriatal pathway promotes sleep.
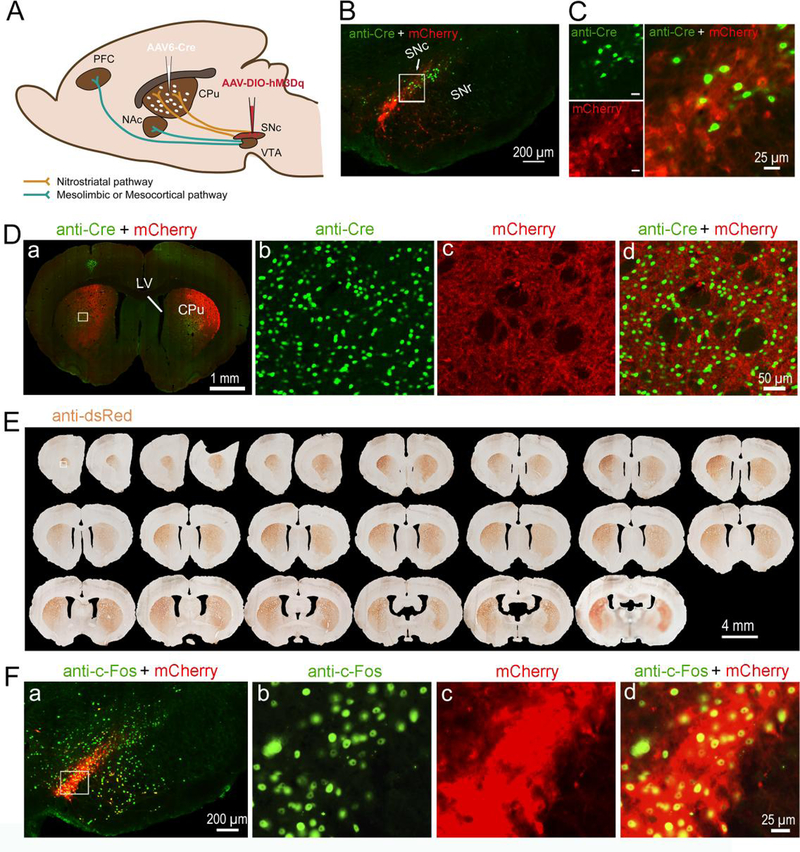
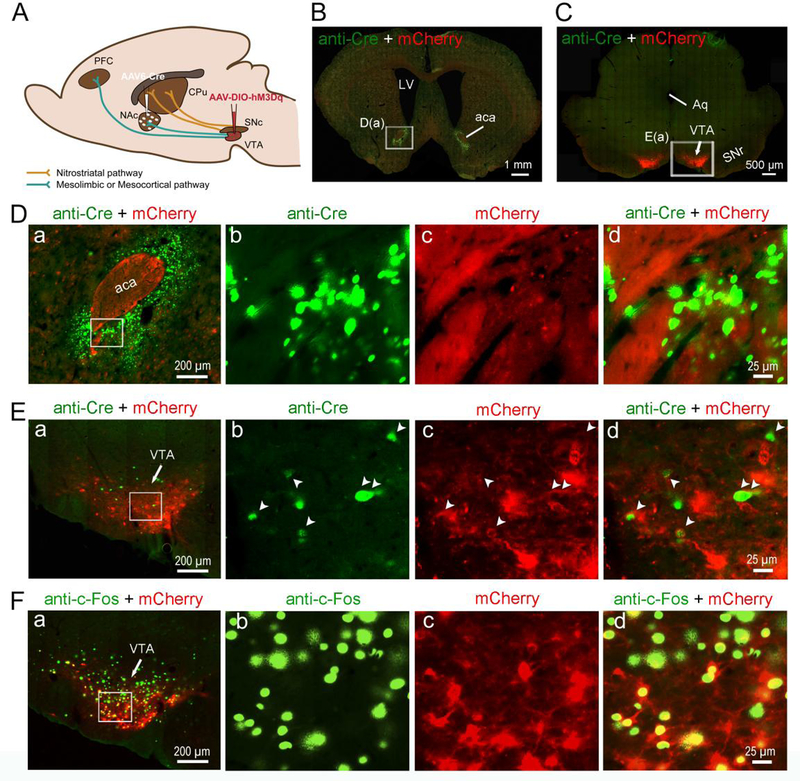
To determine the role of nigrostriatal dopamine neurons in sleep-wake regulation, we injected a retrograde viral vector AAV6-cre (Towne et al. 2008; Towne et al. 2010) into the CPu and cre-dependent AAV8-hM3Dq (AAV8-DIO-hM3Dq:mCherry) into the SNc to selectively insert hM3Dq receptors in SNc dopamine neurons that project to the CPu (Figure. 1A). The hM3Dq receptors expression in SNc dopamine neurons was verified by immunohistology (anti-dsRed) or directly visualized by the fusion reporter mCherry (red color) under the fluorescent microscope (Figure 1, B–F). Two animals were excluded from the group analysis, as few expression of hM3Dq-mCherry was observed in one side of the SNc. The specific insertion of hM3Dq receptors in SNc dopamine neurons were further confirmed by anti-tyrosine hydroxylase (TH) immunohistochemistry staining (supplementary figure 1), such that mCherry (red fluorescence) was expressed in SNc TH-positive neurons (green color). The AAV6-cre injections in the CPu and retrograde AAV6-cre in the SNc were verified by cre immunostaining (Figure 1, B–D). In the CPu, robust mCherry labeled terminals exclusively from the SNc were clearly seen (Figure 1, D and E). Activation of SNc dopamine (nigrostriatal) neurons by clozapine-N-oxide (CNO; 0.2mg/kg) was confirmed by intense nuclear c-Fos immunofluorescence (green fluorescence) in mCherry expressed SNc dopamine neurons (red fluorescence) (Figure 1F).
Figure 1. Chemogenetic stimulation of nigrostriatal dopamine neurons.
A. Experimental design: injections of AAV6-Cre into the CPu and AAV8-DIO-hM3Dq-mCherry into the SNc lead insertion of hM3Dq in the nigrostriatal neurons in the SNc.
B. Cre is shown by anti-Cre immunofluorescence staining (green) and Cre-dependent expression of hM3Dq receptors is shown by native fluorescent mCherry (red) excited at 594 nm in SNc.
C. High-magnification photomicrographs of the white rectangular area in “B”.
D. a: Cre is shown by anti-Cre immunofluorescence staining (green) and Cre-dependent expression of hM3Dq receptors is shown by native fluorenscent mCherry (red) on nigrostriatal dopamine terminals in CPu. “b”, “c” and “d”: High-magnification photomicrographs of the white rectangular area in “a”.
E. Anti-dsRed-immunostaining (brown) labeling nigrostriatal terminals from hM3Dq receptors containing neurons in the SNc.
F. a: c-Fos are shown by anti c-Fos immunofluorescence staining (green) and Cre-dependent expression of hM3Dq receptors is shown by native fluorescent mCherry (red) excited at 594 nm in SNc. “b”, “c” and “d”: High-magnification photomicrographs of the white rectangular area in “a”.
CPu, caudate putamen; LV, lateral ventricle; NAc, nucleus accumbens; PFC, prefrontal cortex; SNc, substantia nigra, compacta part; SNr, substantia nigra, reticular part; VTA, ventral tegmental area.
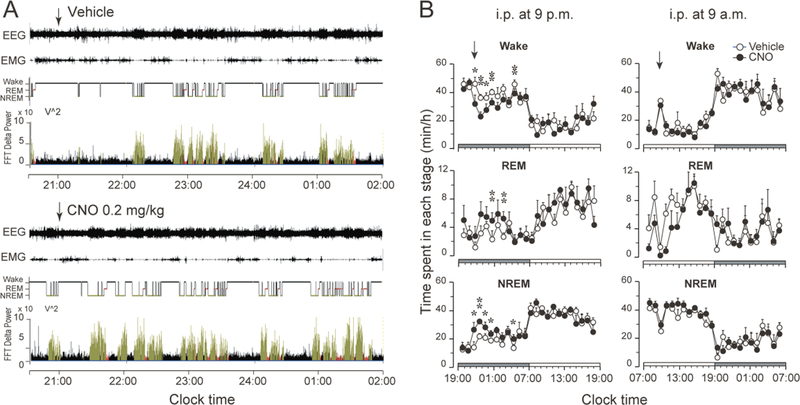
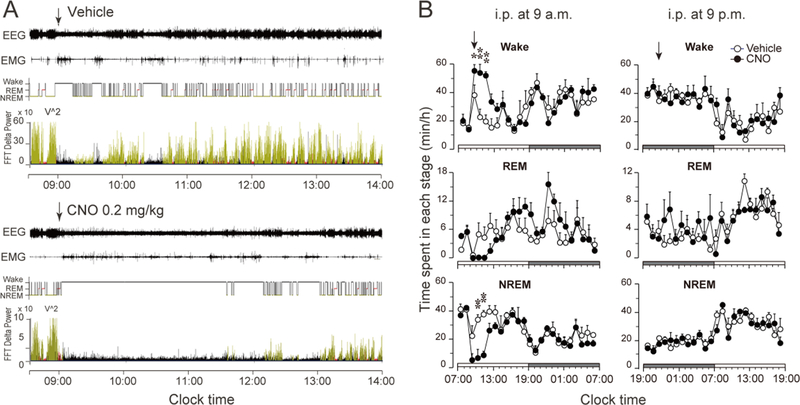
To examine sleep-wake effects of chemogenetic activation of nigrostriatal dopamine neurons, we injected saline vehicle (first day) and CNO (second day) at 9 a.m. or 9 p.m., respectively. Compared with vehicle injections, CNO injection at 9 p.m. in the rats expressing the hM3Dq receptors in SNc-CPu projecting neurons significantly decreased the amount of wakefulness during the 4 hr post-injection period (CNO: 110.7 ± 6.1 min vs. vehicle: 154.1 ± 6.1 min, P = 0.0004, n=5); and increased the amount of NREM (CNO: 110.8 ± 5.3 min vs. vehicle: 75.8 ± 5.5 min, P = 0.0008, n=5) and REM sleep (CNO: 18.4 ± 1.9 min vs. vehicle: 10.1 ± 1.8 min, P = 0.0183, n=5) correspondingly (Figure 2A, Figure 2B, left column, Figure 3A, left column, upper panel).
Figure 2. Chemogenetic stimulation of the nigrostriatal pathway promotes sleep.
A. Typical examples of polygraphic recordings and corresponding hypnograms and FFT delta power following vehicle or CNO (0.2 mg/kg, i.p. at 9 p.m.) administration in a rat with hM3Dq receptors in the SNc. (black, wakefulness; red, REM sleep; yellow, NREM sleep).
B. Time course changes of wake, REM and NREM sleep produced by saline or CNO (0.2 mg/kg) injection at 9 p.m. or at 9 a.m. Data are represented by hourly mean ± SEM of wake, REM and NREM sleep. *p < 0.05; **p < 0.01.
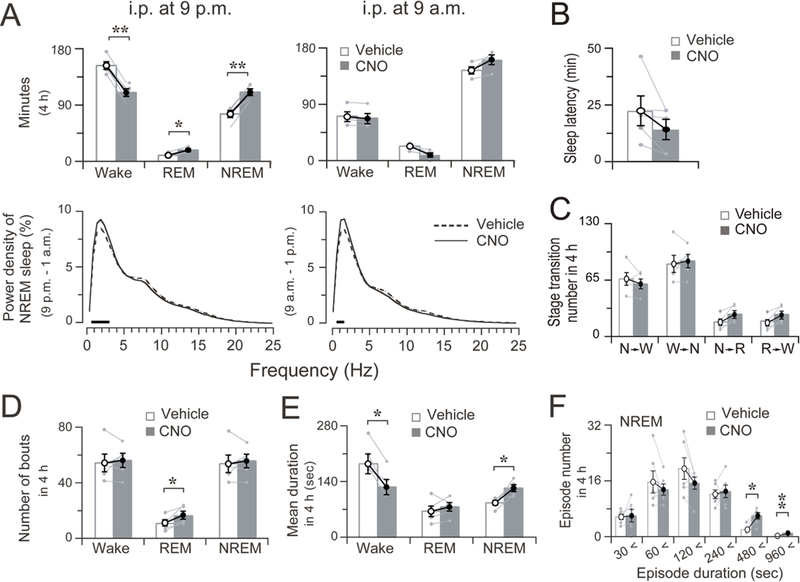
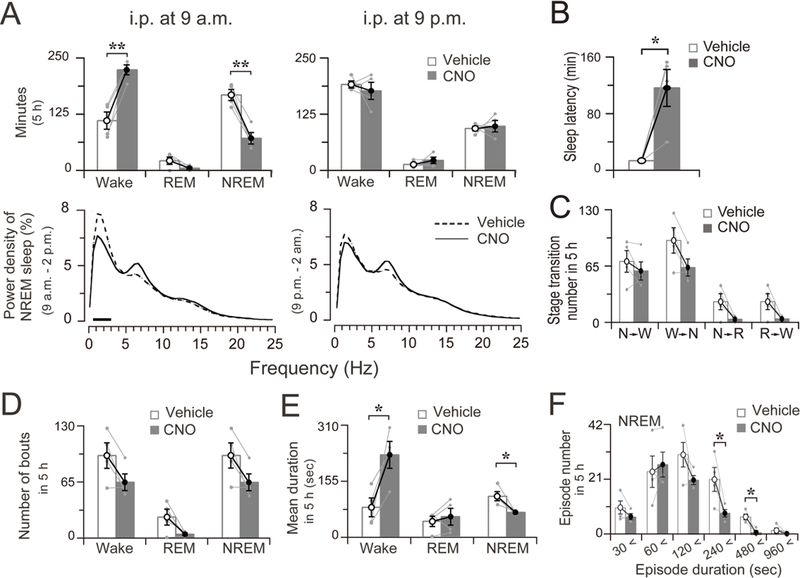
Figure 3. Four-hour sleep-wake profile after chemogenetic stimulation of the nigrostriatal neurons.
A. Total time spent in wakefulness, REM and NREM sleep and EEG power density of NREM sleep during four hours after vehicle and CNO treatment. The bars indicate statistical difference (P < 0.05) between CNO and vehicle control.
B. Sleep latency after vehicle and CNO treatment at 9 p.m. C. Sleep-wake state transitions during four hours after vehicle and CNO treatment at 9 p.m.
D. Total number of wake, REM, and NREM bouts during 4 h after vehicle and CNO treatment at 9 p.m.
E. Mean duration of wake, NREM, and REM bouts during 4 h after vehicle and CNO treatment at 9 p.m.
F. Changes in number of NREM sleep bouts at different ranges of episode duration in 4 h after the administration of vehicle or CNO at 9 p.m.
Data are represented by mean ± SEM. *p < 0.05, **p < 0.01. Each pair of grey dots indicates data from one rat.
Nigrostriatal pathway activation resulted in a moderate increase in 0.5–3.0 Hz EEG power density of NREM sleep (Figure 3A, left column, lower panel). Between baseline saline control and CNO stimulation, there were no significant differences in NREM sleep latency (Figure 3B, p = 0.1312, n=5), sleep-wake transitions (Figure 3C), or bout numbers (Figure 3D, Wake: p = 0.6677, REM: p = 0.0477, NREM: P = 0.6126, n=5). The NREM sleep increase was mainly due to a decrease in duration of wake bouts (p = 0.0106, n=5) and an increase in duration of NREM bouts (p = 0.0220, n=5) (Figure 3E). The number of NREM sleep bouts with duration ranges of 240–480 (p = 0.0103, n=6) and 480–960 (p = 0.0041, n=6) were increased, while the numbers of shorter-duration bouts were unaffected (Figure 3F). Chemogenetic stimulation of SNc dopamine neurons during the day period (i.p. at 9 a.m.) had no effect on sleep amount (Figure 2B, right column and Figure 3A, right column, upper panel), and did not affect either the bout number or the mean duration of NREM sleep or wakefulness (data not shown) although the EEG power density of NREM sleep was slightly increased in the 0–1.5 Hz frequency range (Figure 3A, right column, lower panel). No abnormal motor behaviors were seen.
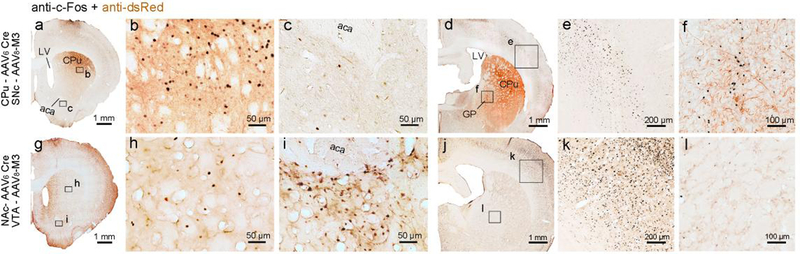
Chemogenetic activation of the nigrostriatal pathway also resulted in a robust increase in c-Fos expression in its downstream target, CPu, while a lesser expression of c-Fos was observed in the cerebral cortex and NAc, though the animal were in activate period. Surprisingly, expression of c-Fos was upregulated in the GPe (Figure 4, a–f).
Figure 4. c-Fos expressions after chemogenetic activation of nigrostriatal and mesolimbic pathways.
Representative photomicrographs of c-Fos (black color) and dsRed (brown color) immunostaining in the CPu, NAc, cerebral cortex and GP after activation of nigrostriatal pathway (CNO administrated at 9 p.m., a-f) and mesolimbic pathway (CNO administrated at 9 a.m., g-k). b and c, e and f, h and i, k and l are magnified views of the rectangular boxes marked in a, d, g and j, respectively.
Chemogenetic stimulation of mesolimbic pathway promotes wakefulness.
To determine if selective stimulation of mesolimbic pathway promotes wakefulness, we injected AAV6-cre into the NAc and cre-dependent AAV-hM3Dq into the VTA (Figure 5A). This approach selectively inserted hM3Dq into the VTA dopamine neurons projecting to the NAc (Figure 5B–F, supplementary figure 2). Five animals were excluded from the group analysis, as the expressions of hM3Dq-mCherry were also found in the medial part of the SNc. Anti-cre immunostaining was adopted to verify the AAV6-cre injections in the NAc and the retrograde AAV6-cre in the VTA (Figure 5, D and E). Activation of VTA dopamine neurons by CNO (0.2mg/kg) was verified by intense nuclear c-Fos immunofluorescence labeling (green fluorescence) within mCherry+ VTA dopamine neurons (red fluorescence) (Figure 5F).
Figure 5. Chemogenetic stimulation of mesolimbic dopamine neurons.
A. Experiment design: injections of AAV6-Cre into the NAc and AAV8-DIO-hM3Dq-mCherry into the VTA lead insertion of hM3Dq in the VTA mesolimbic neurons.
B. Cre in NAc is shown by anti-Cre immunofluorescence staining (green).
C. Cre is shown by anti-Cre immunofluorescence staining (green) and Cre-dependent expression of hM3Dq receptors is shown by native fluorescent mCherry (red) excited at 594 nm in VTA.
D. a: High-magnification photomicrographs of the white rectangular area in “B”. “b”, “c” and “d”: High-magnification photomicrographs of the white rectangular area in “D a”.
E. a: High-magnification photomicrographs of the white rectangular area in “C”. “b”, “c” and “d”: High-magnification photomicrographs of the white rectangular area in “E a”.
F. a: CNO-induced c-Fos (green) and Cre-dependent expression of hM3Dq receptors (red) in mesolimbic dopamine neurons. “b”, “c” and “d”: High-magnification photomicrographs of the white rectangular area in “F a”. aca, anterior commissure, anterior part; Aq, aqueduct.
In contrast to the sleep-promoting effects of activating the nigrostriatal pathway, activation of the mesolimbic pathway at 9 a.m. produced a robust increase in wakefulness, compared to vehicle injection in the same rats (Figure 6A, Figure 6B, left column and Figure 7A, left column, upper panel). The increase in wakefulness lasted about 5 h after injection (Figure 6A, lower panel, and Figure 6B, left column, upper panel). There was no further disruption of sleep-wake architecture during the subsequent period (Figure 6B, left column), i.e., no sleep rebound was seen following the wakefulness. The average NREM sleep latency after the stimulation was 117.5 ± 21.6 min, which was significantly prolonged compared to that after vehicle control injection 13.5 ± 1.0 min (Figure 7B, p=0.029, n=4). We calculated the total time spent in wake, REM and NREM sleep for 5 h after activation. VTA dopaminergic activation significantly increased the amount of wakefulness by 100.5% (CNO: 222.9 ± 11.0 min vs. vehicle: 111.2 ± 19.0 min, P = 0.0099, n=4), and decreased the amount of NREM sleep by 57.0% (CNO: 167.1 ± 12.1 min vs. vehicle: 71.8 ± 12.6 min, P = 0.0078, n=4), respectively, during that 5-h period compared to the vehicle control. There was no significant change in REM sleep amount after injection (Figure 7A, left column, upper panel).
Figure 6. Chemogenetic stimulation of mesolimbic pathway increases wakefulness.
A. Typical examples of polygraphic recordings and corresponding hypnograms and FFT delta power following vehicle or CNO (0.2 mg/kg, i.p. at 9 a.m.) administration in a rat with hM3Dq receptors in the mesolimbic dopamine neurons (black, wakefulness; red, REM sleep; yellow, NREM sleep).
B. Time course of wake, REM and NREM sleep produced by saline or CNO (0.2 mg/kg) injection at 9 a.m. or at 9 p.m. Data is shown by hourly mean ± SEM of wake, REM and NREM sleep. *p < 0.05; **p < 0.01.
Figure 7. Five-hour sleep-wake profile after chemogenetic stimulation of the mesolimbic neurons.
A. Total time spent in wakefulness, REM and NREM sleep and EEG power density of NREM sleep during five hours after vehicle and CNO treatment at 9 a.m. or at 9 p.m. The horizontal bars indicate a statistical difference (P < 0.05) between CNO and vehicle control.
B. Sleep latency after vehicle and CNO treatment at 9 a.m.
C. Sleep-wake state transitions during five hours after vehicle and CNO treatment at 9 a.m.
D. Total number of wake, NREM, and REM bouts during five hours after vehicle and CNO treatment at 9 a.m.
E. Mean duration of wake, NREM, and REM bouts during five hours after vehicle and CNO treatment at 9 a.m.
F. Number of NREM sleep bouts at different ranges of episode duration in five hours after the administration of vehicle or CNO at 9 a.m.
Data are represented by mean ± SEM. *p < 0.05. Each pair of grey dots indicates data from one rat.
The EEG power density of NREM sleep in the 0.5–3.0 Hz frequency range was decreased during the first 5 hours after CNO administration (Fig 7A, left column, lower panel). Sleep-wake transitions (Figure 7C) and bout numbers (Fig 7D, Wake: p = 0.0829, REM: p = 0.1075, NREM: P = 0.0822, n=4) were not significantly affected. Wake increase after CNO administration was mainly due to an increase in wake bout duration and a decrease in NREM sleep bout duration (Figure 7E, Wake: p = 0.0387, NREM: P = 0.0479, n=4). The number of NREM sleep bouts with duration ranges of 120–240 (p = 0.0359, n=4) and 240–480 (p = 0.0321, n=4) were decreased (Figure 7F). Chemogenetic stimulation of VTA dopamine neurons at 9 p.m. (night) showed no effects on sleep amount (Figure 6, B right column and Figure 7A, right column, upper panel). No abnormal motor behaviors were observed.
Chemogenetic activation of the mesolimbic pathway resulted in a robust increase of c-Fos expression in its downstream NAc. High expression of c-Fos was seen in the CPu and cortex, though the animals were sacrificed during their resting phase. In contrast to the results of activating the nigrostriatal pathway, the GPe was in a quiet state as indicated by sparse c-Fos expression (Figure 4, g–k).
We statistically compare the sleep-wake profiles under vehicle condition (Vehicle i.p. at 9 pm or 9 am) between the two different dopamine pathways. No obvious differences were observed in the time course of the hourly amounts of or the total time spent in wake, REM and NREM sleep of each group (supplementary figure 3).
DISCUSSION
The nigrostriatal and mesolimbic dopamine neurons play key roles in regulating sleep and behavior via their projections to the basal ganglia. We demonstrate that chemogenetic stimulation of dopamine nigrostriatal and mesolimbic pathways promote sleep and wake respectively, accompanied by increased or decreased NREM sleep delta power, respectively in rats. These results suggest that the midbrain dopaminergic system regulates both sleep-wake state and cortical activity via the basal ganglia.
SNc dopamine neurons project to the dorsal striatum (or caudoputamen, CPu), via the nigrostriatal pathway. In the CPu, dopamine acts on D2 receptors at the presynaptic sites of GABAergic striatopallidal axons in the GPe, disinhibiting GPe neurons and promoting sleep and regulating cortical activity (Cooper and Stanford 2001; Qiu et al. 2016c; Querejeta et al. 2001). Consistent with this, optogenetic stimulation and deep brain stimulation of GPe neurons, as well as optogenetic stimulation of nigrostriatal terminals, promotes sleep in rats (Qiu et al. 2016b; Qiu et al. 2016c). And in this study, chemogenetic activation of the nigrostriatal pathway induces c-Fos expression in GP and cerebral cortex resembles sleep state, despite animals are perfused at the night (Figure 4). Because thalamic lesions do not alter sleep-wake behavior (Fuller et al. 2011), we propose a non-thalamic circuit for sleep regulation via direct pallidocortical neurons (Chen et al. 2015; Guo et al. 2016; Qiu et al. 2016c). We hypothesize that SNc dopamine regulates sleep by regulating pallidocortical tuning of cortical activity, which also explains the changes in EEG delta power after stimulation of SNc dopamine neurons.
SNc dopaminergic lesions reduce sleep in rats, cats, primates, and humans (e.g. Parkinson’s disease) (Belaid et al. 2014; Fifel et al. 2014; Gerashchenko et al. 2006; Lai et al. 2008; Lai et al. 1999; Videnovic et al. 2014). In humans, dopamine D2 agonists are known to trigger sleep attacks (Hirayama et al. 2008; Lipford and Silber 2012; Paus et al. 2003), and low dose D2 agonists induce sleep in rats and mice (Dimpfel 2008; Laloux et al. 2008a). However, MPTP induced SNc lesions in mice result in marginal reductions in sleep (Laloux et al. 2008b), and chemogenetic activation of SNc dopamine neurons in mice does not affect sleep (Oishi et al. 2017). The inconsistency between mice experiments and our results from rats may due to the different methodology applied in the experiments and species difference. It is known that MPTP induced DA lesions are partial and temporary in mice, and chemogenetic stimulation of the SNc may include some VTA neurons, lacking of specificity in neural pathway modulation.
VTA mesolimbic projections terminate in the NAc, where dopamine acts on D2 receptors to mediate arousal. Systemic D2 antagonists block the arousal effects of stimulating VTA dopamine neurons in mice (Oishi et al. 2017). Consistent with this, lesions of the NAc increase wakefulness by 27% in rats (Qiu et al. 2010) and chemogenetic and optogenetic stimulation of the VTA dopamine neurons in mice and chemogenetic stimulation of mesolimbic dopamine neurons in rats induce wakefulness. NAc D2-containing neurons may promote wakefulness via the ventral pallidum and lateral hypothalamus (Zhang et al. 2013). There are also GABAergic neurons and possibly glutamatergic neurons in the VTA projecting to the NAc. However, compared to the dopaminergic projections, other projections are relatively sparse (Taylor et al. 2014).
Due to large area of the dorsal striatum, it was impossible to fill the dorsal striatum by AAV6-cre, thus our approach most probably activated the partial SNc (supplementary figure 1). Even so, mCherry (hM3Dq) was robustly and extensively expressed in CPu. This suggests that each dopamine neuron from the SNc can innervate a large target area and act on many neurons. If a large proportion of the SNc-CPu dopamine projecting neurons were activated, a much stronger sleep promoting effect could be evoked. Compared to the dorsal striatum, the ventral striatum is much smaller. Our approach likely activated most of the VTA dopamine neurons (supplementary figure 2).
The sleep-wake effects of the midbrain dopamine system appeared to be circadian dependent: sleep promotion occurred at night and wake promotion occurred during daytime. We speculate that midbrain dopamine system may rely on other sleep-wake systems that are tightly regulated by the circadian clock for sleep-wake regulation.
In conclusion, midbrain dopamine released by nigrostriatal and mesolimbic terminals in the basal ganglia promotes sleep and wake respectively in rats. The nigrostriatal and mesolimbic circuits share anatomic and circuit-based similarities but have very different functions in not only sleep-wake but also highly evolved cortical functions such as motor, mental and cognition. The interaction of sleep-wake regulation and other mental and motor behaviors by midbrain dopamine and basal ganglia remains to be explored.
METHODS
Animals
Pathogen-free, adult, male Sprague-Dawley rats (275–325 grams, Harlan) were individually housed and had ad libitum access to food and water. All animals were housed under controlled conditions (12 hr light starting at 7 am, 100 lux) in an isolated ventilated chamber maintained at 20–22 °C. All protocols were approved by Committee on the Ethics of Animal Experiments of School of Basic Medical Science, Shanghai Medical College, Fudan University(Permit Number: 20110307049) and the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center, and all experiments were performed in accordance with relevant guidelines and regulations. Every effort was made to minimize the number of animals used and any pain and discomfort experienced by the subjects.
Surgery
Rat were anesthetized with ketamine/xylazine (100 mg/kg and 10 mg/kg, respectively, i.p.) and then placed on a stereotaxic apparatus. To express the hM3Dq receptors specifically in dopamine nigrostriatal projection neurons, we injected AAV6-CAG-Cre (AAV6-Cre, 150 nl, titers at 1–2 × 1012, AAV6 = AAV2/6: AAV serotype 2 genome packaged in the serotype 6 capsid, from the F.M. Kirby Neurobiology Center Viral Core), which is taken up by the axonal terminals and retrograde transported to the neuronal bodies (Qiu et al. 2016a; Towne et al. 2008; Towne et al. 2010), into caudate putamen (CPu, AP = + 0.24 mm, ML= ± 2.9 mm, DV = − 5 mm). And then we injected recombinant AAV8 carrying hM3Dq-mCherry (AAV8-hSyn-DIO-hM3Dq-mCherry, 150 nl, titers at 1–2 × 1012, from University of North Carolina Gene Therapy Center Vector Core) into the substantia nigra pars compacta (SNc, AP = − 5 mm, ML= ± 1.8 mm, DV = − 7.8 mm), which expression of hM3Dq receptor is dependent on the presence of Cre recombinase. The viruses were given into the target areas as following procedures: a burr hole was made and a fine glass pipette (1 mm glass stock, tapering slowly to a 10–20 micron tip) containing recombinant AAV was lowered to the CPu or SNc bilaterally, as per the atlas of Paxinos and Watson (Paxinos and Watson 2009). A total of 150 nl virus was delivered over a 5 min period per hemisphere via nitrogen gas pulses of 20–40 psi using an air compression system previously described (Lu et al. 2000). After 2 additional minutes, the pipette was slowly withdrawn. For testing the roles of the dopamine mesolimbic projection neurons in sleep-wake regulation, weinjected AAV6-Cre (90 nl) into nucleus accumbens (NAc, AP= + 1.2 mm, ML= ± 1.8 mm, DV = −7 mm). And then we injected recombinant AAV8-hM3Dq (90 nl) into the ventral tegmental area (VTA, AP= − 5 mm, ML= ± 0.6 mm, DV = − 8 mm). Following the injections, rats were implanted with 4 screw electrodes for recording EEG (2 on the frontal bone and 2 on the parietal bone) and 2 wire electrodes on to the nuchal muscles for recording EMG. The other ends of the electrodes were connected to a 6-pin pedestal (Plastics One, USA) that was then secured on to the skull using dental cement. For postoperative care, rats were injected intraperitoneally with meloxicam (0.5 mg per kg). All injection sites were verified by immunohistochemistry. The ‘misses’ or ‘partial hits’ ones were excluded from data analyses.
Sleep-wake recording and analysis
After surgical procedures, rats were allowed to recover in individual housing for at least three weeks. The animals were then transferred to the recording room and habituated to the recording cables and conditions for 2 days. Following this habituation period, EEG/EMG activity from the beginning of the light period (7 a.m.) or the dark period (7 p.m.) was recorded (AM systems, USA) from all the rats. The cortical EEG and EMG signal were amplified, digitized at a sampling rate of 256 Hz, and recorded using VitalRecorder (Kissei Comtec, Nagano, Japan). The behavior of the animals was recorded simultaneously with time-locked video recordings. EEG/EMG were filtered (EEG, 0.5–40 Hz band-pass; EMG 10 Hz high-pass) and automatically scored offline in 10-sec epochs as wake, non-rapid eye movement (NREM) sleep, or rapid eye movement (REM) sleep in SleepSign (Kissei Comtec, Nagano, Japan) using established criteria (Lu et al. 2000; Lu et al. 2001). After automatic scoring, sleep-wake stages were examined and manually corrected. The amount of time spent in wake, NREM and REM sleep was determined from the scored EEG/EMG data. EEG power spectra for each epoch were analyzed offline using Fast Fourier Transformation (512 point, Hanning window, 0–24.5 Hz with 0.5 Hz resolution using SleepSign).
CNO injections
Clozapine N-oxide (CNO, 0.2 mg/kg, final concentration 0.1 mg/ml, C0832, Sigma) in saline was injected by i.p. at 9 a.m. or at 9 p.m. For baseline data, rats were injected i.p, with saline vehicle (2ml/kg) at 9 a.m., or at 9 p.m., respectively.
Perfusion and immunohistochemistry
After sleep-wake recording, the rats were returned to their normal housing room for another week. Then the animals received CNO (0.2 mg/kg, i.p., 9 a.m. or 9 p.m.) and 2 h later, they were deeply anesthetized with 7% chloral hydrate, and were perfused transcardially with saline followed by 10% neutral phosphate buffered formalin (Fisher Scientific Co.) at a flow rate of 5 ml/min. The saline and the formalin were precooled in a refrigerator (4°C) and the procedure was processed at room temperature. The brains were harvested, post-fixed, and cryoprotected in 20% sucrose in PBS overnight, then sectioned in the coronal plane on a freezing microtome into 4 series of 40 µm sections.
Immunohistochemistry was performed in accordance with the free floating method described previously (Qiu et al. 2010). For immunofluorescence labeling, sections were blocked with 10% normal donkey serum/0.25% Triton-X-100 in PBS for 1 h at room temperature and then incubated overnight at room temperature with the rabbit anti-c-Fos (1:5000, Ab5, Cat# PC38, Oncogene Research Products) or rabbit anti-Cre (1:5000, Cat# 69050–3, Novagen) diluted in blocking buffer, after washed three times with PBS, the sections were then incubated for 2 h at room temperature with Alexa Fluor-488 conjugated donkey anti rabbit secondary antibody (1:500, Cat# A21206, Molecular Probes, Invitrogen), following additional washes in PBS, sections were mounted to glass slides and cover slipped with VECTASHIELD mounting medium (Cat# H-1000, Vector Laboratories, CA, USA). Fluorescence images were captured with Olympus VS120 slide scanner microscope.
Statistical Analysis
The quantitative data were presented as the mean ± standard error of mean. Statistical significance was assessed with the Student’s paired t-test, or two-way repeated measures analysis of variance with Student-Newman-Keuls post-hoc when appropriate, with P < 0.05 taken as the threshold of significance. All statistical tests were two-sided.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by National Natural Science Foundation of China 31171049 and 81771430 (M.H.Q.), the Scientific Research Foundation for Returned Overseas Chinese Scholars, State Education Ministry (M.H.Q.), and NIH NS061841 and NS095986 (J.L)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
COMPETING FINANCIAL INTERESTS STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Belaid H, Adrien J, Laffrat E, Tande D, Karachi C, Grabli D, Arnulf I, Clark SD, Drouot X, Hirsch EC, Francois C (2014) Sleep disorders in Parkinsonian macaques: effects of L-dopa treatment and pedunculopontine nucleus lesion. J Neurosci 34 (27):9124–9133. doi: 10.1523/JNEUROSCI.0181-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci 30 (5):194–202. doi: 10.1016/j.tins.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Chen MC, Ferrari L, Sacchet MD, Foland-Ross LC, Qiu MH, Gotlib IH, Fuller PM, Arrigoni E, Lu J (2015) Identification of a direct GABAergic pallidocortical pathway in rodents. Eur J Neurosci 41 (6):748–759. doi: 10.1111/ejn.12822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJ, Stanford IM (2001) Dopamine D2 receptor mediated presynaptic inhibition of striatopallidal GABA(A) IPSCs in vitro. Neuropharmacology 41 (1):62–71 [DOI] [PubMed] [Google Scholar]
- Dimpfel W (2008) Pharmacological modulation of dopaminergic brain activity and its reflection in spectral frequencies of the rat electropharmacogram. Neuropsychobiology 58 (3–4):178–186. doi: 10.1159/000191124 [DOI] [PubMed] [Google Scholar]
- Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L (2016) VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci 19 (10):1356–1366. doi: 10.1038/nn.4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH, Mastenbroek S (2000) Dopamine and noradrenaline efflux in the prefrontal cortex in the light and dark period: effects of novelty and handling and comparison to the nucleus accumbens. Neuroscience 100 (4):741–748 [DOI] [PubMed] [Google Scholar]
- Fifel K, Vezoli J, Dzahini K, Claustrat B, Leviel V, Kennedy H, Procyk E, Dkhissi-Benyahya O, Gronfier C, Cooper HM (2014) Alteration of Daily and Circadian Rhythms following Dopamine Depletion in MPTP Treated Non-Human Primates. PloS one 9 (1):e86240. doi: 10.1371/journal.pone.0086240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J (2011) Reassessment of the structural basis of the ascending arousal system. The Journal of comparative neurology 519 (5):933–956. doi: 10.1002/cne.22559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Blanco-Centurion CA, Miller JD, Shiromani PJ (2006) Insomnia following hypocretin2-saporin lesions of the substantia nigra. Neuroscience 137 (1):29–36. doi: 10.1016/j.neuroscience.2005.08.088 [DOI] [PubMed] [Google Scholar]
- Grillner S, Robertson B (2016) The Basal Ganglia Over 500 Million Years. Curr Biol 26 (20):R1088–R1100. doi: 10.1016/j.cub.2016.06.041 [DOI] [PubMed] [Google Scholar]
- Guo CN, Machado NL, Zhan SQ, Yang XF, Yang WJ, Lu J (2016) Identification of Cholinergic Pallidocortical Neurons. CNS Neurosci Ther 22 (10):863–865. doi: 10.1111/cns.12602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama M, Nakamura T, Hori N, Koike Y, Sobue G (2008) The video images of sleep attacks in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 23 (2):288–290. doi: 10.1002/mds.21830 [DOI] [PubMed] [Google Scholar]
- Isaac SO, Berridge CW (2003) Wake-promoting actions of dopamine D1 and D2 receptor stimulation. J Pharmacol Exp Ther 307 (1):386–394. doi: 10.1124/jpet.103.053918 [DOI] [PubMed] [Google Scholar]
- Jones BE, Bobillier P, Pin C, Jouvet M (1973) The effect of lesions of catecholamine-containing neurons upon monoamine content of the brain and EEG and behavioral waking in the cat. Brain Res 58 (1):157–177. doi: 10.1016/0006-8993(73)90830-5 [DOI] [PubMed] [Google Scholar]
- Lai YY, Hsieh KC, Nguyen D, Peever J, Siegel JM (2008) Neurotoxic lesions at the ventral mesopontine junction change sleep time and muscle activity during sleep: an animal model of motor disorders in sleep. Neuroscience 154 (2):431–443. doi: 10.1016/j.neuroscience.2008.03.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Shalita T, Hajnik T, Wu JP, Kuo JS, Chia LG, Siegel JM (1999) Neurotoxic N-methyl-D-aspartate lesion of the ventral midbrain and mesopontine junction alters sleep-wake organization. Neuroscience 90 (2):469–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloux C, Derambure P, Houdayer E, Jacquesson JM, Bordet R, Destee A, Monaca C (2008a) Effect of dopaminergic substances on sleep/wakefulness in saline- and MPTP-treated mice. Journal of sleep research 17 (1):101–110. doi: 10.1111/j.1365-2869.2008.00625.x [DOI] [PubMed] [Google Scholar]
- Laloux C, Derambure P, Kreisler A, Houdayer E, Brueziere S, Bordet R, Destee A, Monaca C (2008b) MPTP-treated mice: long-lasting loss of nigral TH-ir neurons but not paradoxical sleep alterations. Experimental brain research 186 (4):635–642. doi: 10.1007/s00221-008-1268-1 [DOI] [PubMed] [Google Scholar]
- Lipford MC, Silber MH (2012) Long-term use of pramipexole in the management of restless legs syndrome. Sleep medicine 13 (10):1280–1285. doi: 10.1016/j.sleep.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB (2000) Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci 20 (10):3830–3842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Jhou TC, Saper CB (2006) Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci 26 (1):193–202. doi: 10.1523/JNEUROSCI.2244-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB (2001) Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci 21 (13):4864–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Neslter EJ, E. HS (2009) Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin. In: Sydor A, Brown RY (eds) Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd edn. New York: McGraw-Hill Medical, pp 147–148, 154–157 [Google Scholar]
- Nambu A (2008) Seven problems on the basal ganglia. Curr Opin Neurobiol 18 (6):595–604. doi: 10.1016/j.conb.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Oishi Y, Suzuki Y, Takahashi K, Yonezawa T, Kanda T, Takata Y, Cherasse Y, Lazarus M (2017) Activation of ventral tegmental area dopamine neurons produces wakefulness through dopamine D2-like receptors in mice. Brain Struct Funct doi: 10.1007/s00429-017-1365-7 [DOI] [PubMed]
- Ongini E, Bonizzoni E, Ferri N, Milani S, Trampus M (1993) Differential effects of dopamine D-1 and D-2 receptor antagonist antipsychotics on sleep-wake patterns in the rat. J Pharmacol Exp Ther 266 (2):726–731 [PubMed] [Google Scholar]
- Paus S, Brecht HM, Koster J, Seeger G, Klockgether T, Wullner U (2003) Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 18 (6):659–667. doi: 10.1002/mds.10417 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2009) The rat brain. In stereotaxic coordinates Compace sixth edition edn. Elsevier Inc., [Google Scholar]
- Qiu MH, Chen MC, Fuller PM, Lu J (2016a) Stimulation of the Pontine Parabrachial Nucleus Promotes Wakefulness via Extra-thalamic Forebrain Circuit Nodes. Curr Biol 26 (17):2301–2312. doi: 10.1016/j.cub.2016.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu MH, Chen MC, Wu J, Nelson D, Lu J (2016b) Deep brain stimulation in the globus pallidus externa promotes sleep. Neuroscience 322:115–120. doi: 10.1016/j.neuroscience.2016.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu MH, Vetrivelan R, Fuller PM, Lu J (2010) Basal ganglia control of sleep-wake behavior and cortical activation. Eur J Neurosci 31 (3):499–507. doi: 10.1111/j.1460-9568.2009.07062.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu MH, Yao QL, Vetrivelan R, Chen MC, Lu J (2016c) Nigrostriatal Dopamine Acting on Globus Pallidus Regulates Sleep. Cereb Cortex 26 (4):1430–1439. doi: 10.1093/cercor/bhu241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y (2008) Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci 28 (34):8462–8469. doi: 10.1523/JNEUROSCI.1819-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querejeta E, Delgado A, Valdiosera R, Erlij D, Aceves J (2001) Intrapallidal D2 dopamine receptors control globus pallidus neuron activity in the rat. Neuroscience letters 300 (2):79–82 [DOI] [PubMed] [Google Scholar]
- Schultz W (2007) Multiple dopamine functions at different time courses. Annu Rev Neurosci 30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722 [DOI] [PubMed] [Google Scholar]
- Smith AD, Olson RJ, Justice JB Jr. (1992) Quantitative microdialysis of dopamine in the striatum: effect of circadian variation. J Neurosci Methods 44 (1):33–41 [DOI] [PubMed] [Google Scholar]
- Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, Picciotto MR (2014) GABAergic and glutamatergic efferents of the mouse ventral tegmental area. The Journal of comparative neurology 522 (14):3308–3334. doi: 10.1002/cne.23603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne C, Raoul C, Schneider BL, Aebischer P (2008) Systemic AAV6 delivery mediating RNA interference against SOD1: neuromuscular transduction does not alter disease progression in fALS mice. Mol Ther 16 (6):1018–1025. doi: 10.1038/mt.2008.73 [DOI] [PubMed] [Google Scholar]
- Towne C, Schneider BL, Kieran D, Redmond DE Jr., Aebischer P (2010) Efficient transduction of non-human primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene Ther 17 (1):141–146. doi: 10.1038/gt.2009.119 [DOI] [PubMed] [Google Scholar]
- Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, Rademaker AW, Simuni T, Zadikoff C, Zee PC (2014) Circadian Melatonin Rhythm and Excessive Daytime Sleepiness in Parkinson Disease. JAMA neurology doi: 10.1001/jamaneurol.2013.6239 [DOI] [PMC free article] [PubMed]
- Zhang JP, Xu Q, Yuan XS, Cherasse Y, Schiffmann SN, de Kerchove d’Exaerde A, Qu WM, Urade Y, Lazarus M, Huang ZL, Li RX (2013) Projections of nucleus accumbens adenosine A2A receptor neurons in the mouse brain and their implications in mediating sleep-wake regulation. Front Neuroanat 7:43. doi: 10.3389/fnana.2013.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.