Abstract
Purpose
Photodynamic therapy (PDT) is an emerging focal treatment modality for prostate cancer. However, the efficacy, safety, and functional outcomes of PDT are not clear. We performed a meta-analysis of available single-arm studies and control trials which used PDT for prostate cancer.
Materials and methods
We searched Pubmed, Embase, Ovid and the Cochrane library (until March,2018) for studies about PDT in patients with prostate cancer. The negative biopsy rate after PDT, PSA decreasing rate, pooled rate of functional outcome (IPSS or IIEF-5), and adverse events were analyzed.
Results
14 studies containing 654 patients were included. Nine of the 14 included studies had evaluated a negative biopsy rate after PDT. The pooled rate was 55.0% (95.0% CI: 0.44–0.66, I2 = 85.7%). Twelve of the 14 included studies which evaluated PSA decreasing rate with the pooled rate of 35.0% (95.0% CI: 0.24–0.47, I2 = 88.7%). Six of the included studies evaluated IPSS with decreasing rate of 29.1% (95.0 % CI: 2.7%–55.5%, I2 = 96.9%). Five of the included studies evaluated IIEF-5 with decreasing rate of 14.9% (95.0% CI: 6.8%–23.0%, I2 = 44.2%). The most common adverse events were haematuria (28.1%, 95.0% CI: 17.1%–39.2%, I2 = 79.8%), erectile dysfunction (23.1%, 95.0% CI: 9.7%–36.5%, I2 = 87.7%), and dysuria (18.6%, 95.0% CI: 12.1%–25.0 %, I2 = 53.4 %).
Conclusions
The meta-analysis results shows that PDT for patients with prostate cancer can be considered as effective based on single-arm clinical trials. Meanwhile, this study reveals that there are not only low levels of side effect rates but also insignificant effect on both urinary and erectile function. However, more high-quality RCTs are needed to evaluate the comparative efficacy, safety, and functional outcomes of PDT for patients with prostate cancer.
Keywords: Meta-analysis, Photochemotherapy, Prostatic neoplasms
1. Introduction
Prostate cancer (PC) is the one of the most common cancer in males. According to the United States' National Center for Health Statistics in 2017, there were 161,360 new cases of PC and 26,730 deaths from it in the USA.1 Current treatment options for men with localized PC include active surveillance and radical therapy. The ideal treatment would provide cancer control with few side effects.2 Radical prostatectomy is the first-line therapy for patients with PC. However, considering the morbidity and prognosis, the risks and efficacy of radical therapy were frequently not identified.3 Focal therapy (FT) is an emerging treatment modality for localized PC which aims to reduce the morbidity seen with radical therapy, while maintaining oncological control.
Photodynamic therapy (PDT) is one of the FTs for cancer. It is a treatment modality that uses laser of a specific wavelength in the presence of oxygen to activate a photosensitizing drug. This then causes localized cell death or tissue necrosis.4 PDT has been used for tumors including cancers of lung, head and neck, pancreas, esophagus, and bladder.5 Since the 1990s, studies of PDT for localized PC have been reported.6 As an FT for the localized PC, transrectal ultrasound (TRUS) and abdominal ultrasound-guided procedures were frequently used in vascular-targeted PDT. Several phase II and III clinical studies investigated the efficacy of TRUS and abdominal ultrasound-guided PDT for localized PC, yet they had variable outcomes and results of active surveillance in follow-up duration. Low mortality and morbidity rates of the PDT were reported; as far as we knew, the effect on urinary and erectile function was not clear.
Therefore, we performed a meta-analysis of available single-arm studies and control trials which used PDT for PC to evaluate the efficacy, safety, and functional outcomes postoperatively in patients.
2. Methods
2.1. Literature searching and study selection
We searched PubMed, Embase, Ovid, and Cochrane library sites (until March, 2018) for studies about PDT in patients with PC. Our search strategy included the following terms: “photodynamic therapy”, “photochemotherapy”, “prostate cancer”, “prostate carcinoma”, and “photosensitizer”. They were combined with the Boolean search terms of “AND” or “OR” for searching relevant articles restricted to human clinical studies published in English. Studies were chosen when they fulfilled the following criteria: (1) enrolled patients with PC who underwent PDT; (2) a diagnosis of PC was confirmed by biopsy; (3) the study design was a randomized controlled trial (RCT), case–control trial, or single-arm trial; (4) at least five patients were enrolled; and (5) at least one follow-up outcome which included a biopsy after PDT, prostate-specific antigen (PSA) before and after PDT, functional outcomes [International Prostate Symptom Score (IPSS) or Five Question International Index of Erectile Function (IIEF-5)], or adverse events. All the enrolled patients must be confirmed as having localized PC using at least an image examination technique, and they should not have had radical surgical treatment or other FT (such as cryotherapy and brachytherapy) before PDT. Both unilateral and bilateral lobes of PC can be taken into account. However, PDT must be performed in the lobes of positive biopsy. Studies were excluded if they met the following criteria: (1) case reports, reviews, or comments; (2) study had irrelevant or unclear data; or (3) studies were duplicate reports where two authors conducted literature searching and study selection independently with divergences resolved by consensus.
2.2. Data extraction and quality assessment
Two investigators separately extracted data and assessed the risk of bias. The following data were extracted: the year of publication, study design, numbers of patients, average age, photosensitizer type and dose, Gleason score, energy and wave of light, guidance, negative biopsy after PDT (of patients, not lobes), PSA before and after PDT, follow-up duration, functional outcome (IPSS or IIEF-5), and adverse events. Literature studies that met all inclusion criteria but in which specific data were unclear were marked by a slash. The methodological quality of the studies was assessed by the Agency for Healthcare Research and Quality score.7 The quality of literature was assessed and ranked as follows: low quality (0–3), moderate quality (4–7), and high quality (8–11). Divergences were resolved by consensus.
2.3. Statistical analysis
All analyses were performed using STATA, version 13.0 (Statacorp, USA). Pooled rates included negative biopsy rate after PDT, and PSA decreasing rate was expressed as effect size (ES) with 95% confidence interval (CI). For those comparative studies with two arms, preplanned analyses using the individual data were needed to avoid unfairness and to have a consistent conclusion. Categorical variables such as biopsy result were expressed as odds ratio with 95% CI, and continuous variables such as change of PSA were expressed as standardized mean difference (SMD) with 95% CI. We also calculated the pooled rate of functional outcome (IPSS or IIEF-5) and adverse events. Statistical heterogeneity of the pooled rates among the studies was assessed by I2 test. Heterogeneity was considered as low (25.0–50.0%), moderate (50.0–75.0%), or high (>75.0%).8 A random effects model was used when I2 > 75.0%; otherwise, a fixed effect model was used. Preplanned meta-regression was conducted to determine factors related to negative biopsy, PSA decreasing, and functional outcome after PDT. The possibility of publication bias was assessed by using Egger's test and funnel plots.9 A P-value < 0.05 was considered statistically significant.
3. Results
3.1. Searching results
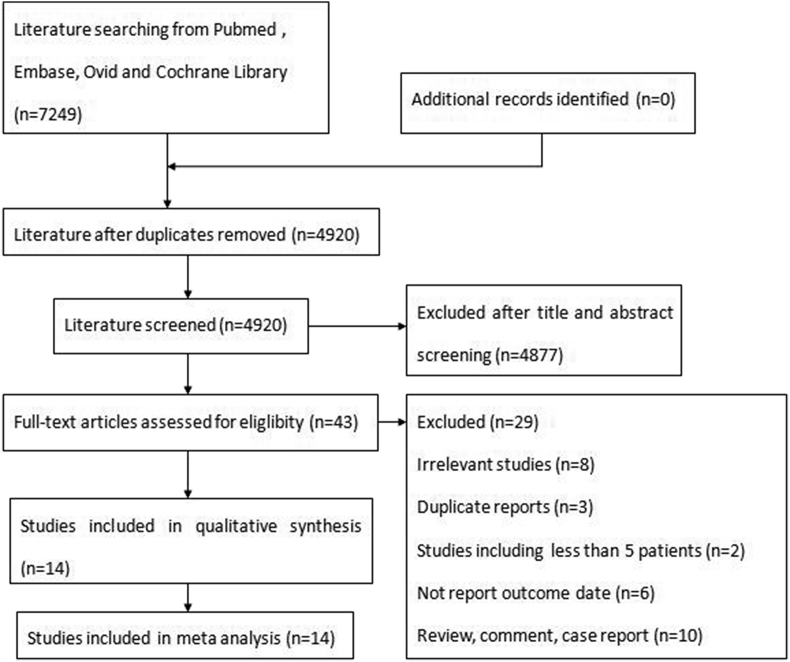
The search and study selection process is shown in Fig. 1. The initial searching identified 7249 articles, of which 4877 articles were excluded in the first screening. Of these, 43 full-text, potentially relevant articles were screened. Ultimately, 14 studies (including one RCT,10 one case–control trial,11 and 12 single-arm trials12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23), containing 654 patients, were eligible for the meta-analysis.
Fig. 1.
Flow diagram of article screening and selection process.
3.2. Study characteristics
Characteristics of the 14 studies are shown in Table 1. All studies were published from 2002 to 2017 and had a follow-up duration of 6–24 months. The types of photosensitizers used were temoporfin, 5-aminolevulinic acid (5-ALA), motexafin lutetium, padeliporfin, and padoporfin. The energy and wave of light and photosensitizer dose were associated with the types of photosensitizer. The ways of guidance were abdominal ultrasound and TRUS. The Gleason score of the PC ranged from 6 (3 + 3) to 9 (4 + 5). The PSA before PDT ranged from 3.3 ng/mL to 22.4 ng/mL. For quality assessment, two of these studies were assessed to be of high quality, 11 were of moderate quality, and one was of low quality.
Table 1.
Characteristics of included studies.
| Study | Year | Design | Number | Age | Photosensitizer | Gleason | Energy (mW/cm) | Wave (nm) |
|---|---|---|---|---|---|---|---|---|
| Nathan TR12 | 2002 | Single arm | 14 | 70 | Temoporfin | <4 + 4 | 100–150 | 652 |
| Zaak D13 | 2003 | Single arm | 6 | / | 5-ALA | <4 + 4 | / | 633 |
| Verigos K14 | 2006 | Single arm | 15 | 69 | Motexafin lutetium | <4 + 5 | 150 | 732 |
| Du KL15 | 2006 | Single arm | 17 | 69 | Motexafin lutetium | <3 + 3 | 150 | 732 |
| Moore CM16 | 2006 | Single arm | 6 | 66 | Temoporfin | <3 + 3 | 150 | 652 |
| Trachteberg J17 | 2008 | Single arm | 28 | / | Padoporfin | / | / | 763 |
| Eymerit-Morin C18 | 2013 | Single arm | 56 | 63 | Padeliporfin | <3 + 3 | 150 | 753 |
| Barret E11 | 2013 | Case control | 23 | 66.5 | Padeliporfin | <3 + 3 | / | / |
| Moore CM19 | 2014 | Single arm | 38 | 63.5 | Padeliporfin | <3 + 3 | 150 | 753 |
| Azzouzi AR20 | 2015 | Single arm | 114 | 62.2 | Padeliporfin | <3 + 4 | 150 | 753 |
| Lebdai S21 | 2015 | Single arm | 19 | 64 | Padeliporfin | <4 + 5 | / | / |
| Taneja SS22 | 2016 | Single arm | 30 | / | Padeliporfin | <3 + 3 | / | 753 |
| Azzouzi AR10 | 2017 | RCT | 206 | 64.2 | Padeliporfin | <3 + 3 | 150 | 753 |
| Lebdai S23 | 2017 | Single arm | 82 | 63 | Padeliporfin | <3 + 3 | 150 | 753 |
| Study | Year | Dose (mg/kg) | Guidance | Biopsy negative | Pre-PSA (ng/mL) | Post-PSA (ng/mL) | Follow-up (month) | AHRQ |
|---|---|---|---|---|---|---|---|---|
| Nathan TR12 | 2002 | 0.15 | TRUS | 4 | 22.4 | 12.9 | 10 | 6 |
| Zaak D13 | 2003 | 20 | TRUS | / | 7.4 | 4.5 | 6 | 4 |
| Verigos K14 | 2006 | 0.5–2 | TRUS | / | / | / | 12 | 4 |
| Du KL15 | 2006 | 0.5–2 | TRUS | / | 7.4 | 7.1 | 24 | 5 |
| Moore CM16 | 2006 | 0.15 | TRUS | 0 | 7.3 | 4.1 | 6 | 3 |
| Trachteberg J17 | 2008 | 2 | Abdominal ultrasound | 8 | 5.1 | 4.7 | 6 | 5 |
| Eymerit-Morin C18 | 2013 | / | Abdominal ultrasound | 29 | 6.2 | 3.7 | 6 | 6 |
| Barret E11 | 2013 | / | Abdominal ultrasound | / | 5.7 | 2.8 | 6 | 5 |
| Moore CM19 | 2014 | 2–6 | TRUS | 20 | / | / | 6 | 4 |
| Azzouzi AR20 | 2015 | 4 | TRUS | 78 | 5.6 | 3.5 | 6 | 8 |
| Lebdai S21 | 2015 | / | TRUS | / | 6.3 | 3.7 | 10 | 5 |
| Taneja SS22 | 2016 | 2–6 | TRUS | 22 | 3.3 | 2.3 | 12 | 7 |
| Azzouzi AR10 | 2017 | 4 | TRUS | 101 | 6.2 | 3.2 | 24 | 8 |
| Lebdai S23 | 2017 | 4 | TRUS | 62 | 6.1 | 3 | 6 | 6 |
AHRQ, Agency for Healthcare Research and Quality; PSA, prostate-specific antigen; RCT, randomized controlled trial; TRUS, transrectal ultrasound.
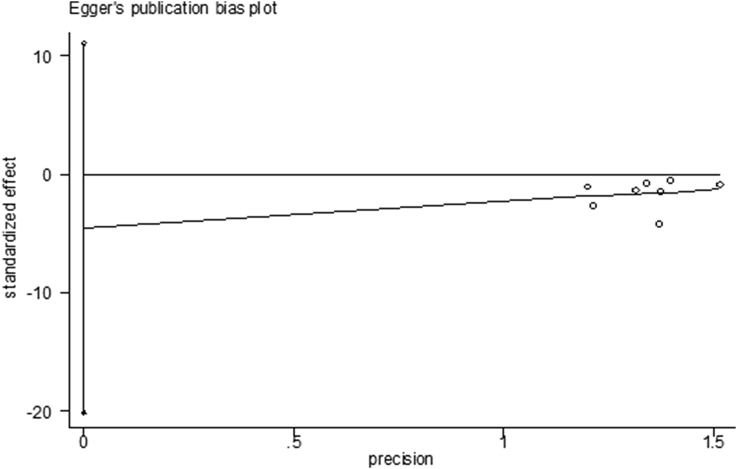
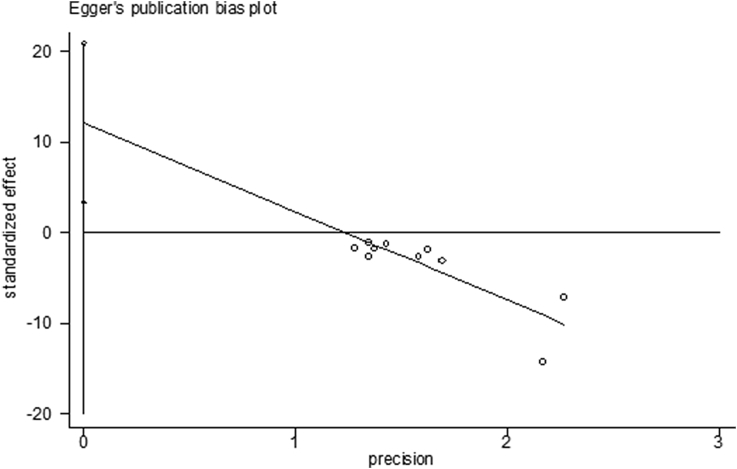
3.3. Evaluation of publication bias
Funnel plots and Egger's tests of the pooled rates for treatment outcomes, including negative biopsy rates after PDT and PSA decreasing rates, were conducted to evaluate the publication bias (Fig. 2, Fig. 3). No publication bias was visually shown for negative biopsy rates after PDT (Egger's test P = 0.504), whereas there was publication bias in PSA decreasing rates (Egger's test P = 0.013). The small sample size may be the reason.
Fig. 2.
Egger's publication bias plot of negative biopsy after PDT for prostate cancer. PDT, photodynamic therapy.
Fig. 3.
Egger's publication bias plot of PSA after PDT for prostate cancer. PDT, photodynamic therapy.
3.4. Main funding
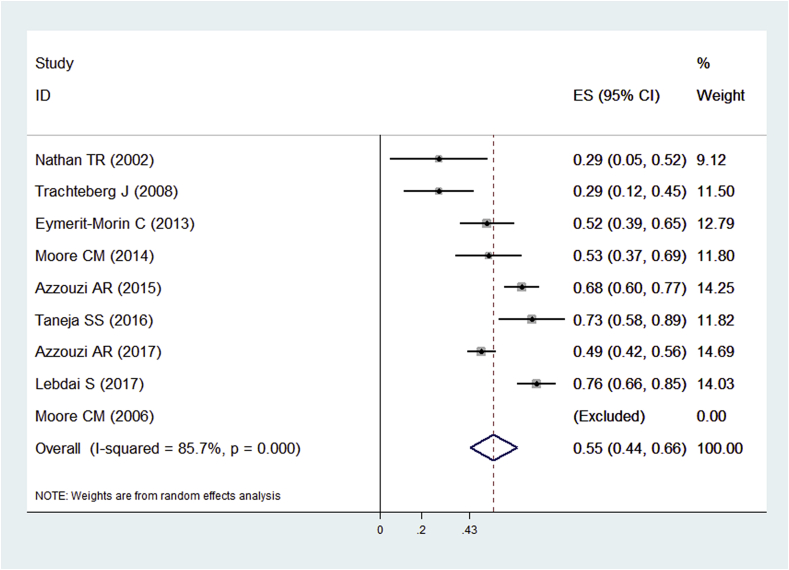
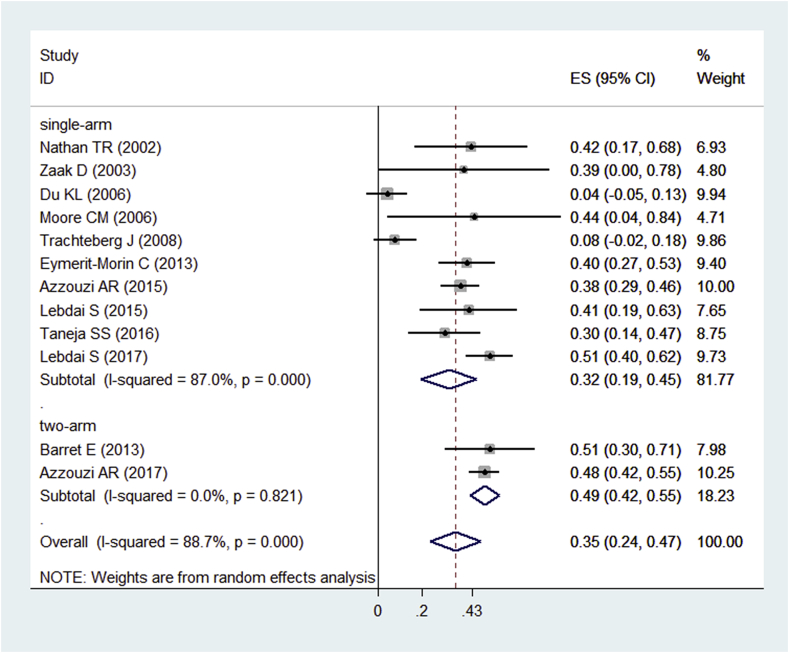
Nine of the 14 included studies10, 12, 16, 17, 18, 19, 20, 22, 23 evaluated the negative biopsy rates after PDT. The pooled rate was 55.0% (95.0% CI: 0.44–0.66, I2 = 85.7%) (Fig. 4). One study had no negative biopsy after PDT.16 Twelve of the 14 included studies10, 11, 12, 13, 15, 16, 17, 18, 20, 21, 22, 23 evaluated PSA decreasing rates. The pooled rate was 35.0% (95.0% CI: 0.24–0.47, I2 = 88.7%) (Fig. 5). We tried to perform a meta-analysis using the individual data for the comparative studies with two arms10, 11 to evaluate biopsy outcome and PSA changes of both the PDT group and control group. However, Barret11 et al. did not evaluate biopsy results after treatment, so we only performed a pooled analysis of PSA changes for them. Unfortunately, the heterogeneity was too high (I2 = 95.6%) to make a pooled analysis. Then, we performed a subgroup analysis for the included single-arm and two-arm studies (Fig. 5). Functional outcomes were summarized in Table 2. Six of included studies12, 16, 18, 19, 20, 22 evaluated the IPSS before and after PDT. The preoperative IPSS ranged from 4.0 to 10.1. The decreasing rate was 29.1% (95.0% CI: 2.7–55.5%, I2 = 96.9%). Five of the included studies12, 16, 18, 19, 22 evaluated the IIEF-5 before and after PDT. The preoperative IIEF-5 ranged from 17.7 to 23.0. The decreasing rate was 14.9% (95.0% CI: 6.8–23.0%, I2 = 44.2%). The I2 of the IIEF-5 was less than 50.0%, so a fixed-effects model was used.
Fig. 4.
Forest plots of biopsy-negative rate after PDT for prostate cancer. Note: Weights are from random effects analysis. CI, confidence interval; PDT, photodynamic therapy.
Fig. 5.
Forest plots of PSA decreasing rate after PDT for prostate cancer. Note: Weights are from random effects analysis. CI, confidence interval; PDT, photodynamic therapy.
Table 2.
Pooled decreasing rate of IPSS and IIEF-5 in patients with prostate cancer who underwent PDT.
| Study | IPSS |
IIEF-5 |
||
|---|---|---|---|---|
| Preoperative IPSS | Postoperative IPSS | Preoperative IIEF-5 | Postoperative IIEF-5 | |
| Nathan TR (2002)12 | 10.1 | 9.9 | 17.9 | 14.5 |
| Moore CM (2006)16 | 6 | 6 | 23 | 13 |
| Eymerit-Morin C (2013)18 | 6 | 4.7 | 19.4 | 15.3 |
| Moore CM (2014)19 | 4 | 3 | 23 | 20 |
| Azzouzi AR (2015)20 | 6 | 2 | / | / |
| Taneja SS (2016)22 | 7.3 | 5.1 | 17.7 | 16.6 |
| Pooled decreasing rate (95% CI) | 29.1% (2.7–55.5%) I2 = 96.9% |
14.9% (6.8–23.0% I2 = 44.2% |
||
CI, confidence interval; IIEF-5, Five Question International Index of Erectile Function; IPSS, International Prostate Symptom Score; PDT, photodynamic therapy.
To evaluate safety of PDT, pooled rates of incidence of adverse events were calculated. Six of the 14 included studies10, 12, 14, 19, 22, 23 reported adverse event outcomes such as dysuria (18.6%, 95.0% CI: 12.1–25.0%, I2 = 53.4%), hematuria (28.1%, 95.0% CI: 17.1–39.2%, I2 = 79.8%), micturition urgency (12.5%, 95.0% CI: 5.9–19.1%, I2 = 58.6%), erectile dysfunction (23.1%, 95.0% CI: 9.7–36.5%, I2 = 87.7%), perineal pain (12.1%, 95.0% CI: 8.7–15.4%, I2 = 0.0), and prostatic pain (13.4%, 95.0% CI: 9.7–36.6%, I2 = 88.2%). More details are summarized in Table 3.
Table 3.
Incidence of adverse events.
| Adverse events | Pooled rate (%) | 95% CI (%) | I2 (%) |
|---|---|---|---|
| Dysuria | 18.6 | 12.1–25.0 | 53.4 |
| Hematuria | 28.1 | 17.1–39.2 | 79.8 |
| Ejaculation failure | 8.5 | 5.3–11.7 | 0 |
| Micturition urgency | 12.5 | 5.9–19.1 | 58.6 |
| Pollakiuria | 7.6 | 2.4–12.9 | 62.7 |
| Erectile dysfunction | 23.1 | 9.7–36.5 | 87.7 |
| Urinary incontinence | 9.3 | 6.3–12.4 | 0 |
| Urinary retention | 11.4 | 5.5–17.3 | 63.9 |
| Perineal pain | 12.1 | 8.7–15.4 | 0 |
| Prostatic pain | 13.4 | 9.7–36.6 | 88.2 |
| Prostatitis | 3.9 | 1.7–6.1 | 0 |
| Hematospermia | 9.6 | 2.1–17.2 | 55.8 |
| Urinary tract infection | 11.5 | 2.5–20.5 | 56.2 |
CI, confidence interval.
3.5. Meta-regression analysis
We conducted meta-regression analysis to explore the source of heterogeneity and determine factors related to negative biopsies, PSA decreasing, and IPSS according to the heterogeneity (Table 4). The covariates included guidance, photosensitizer, and follow-up duration. The results shown that the photosensitizer was related to negative biopsy (P = 0.003, Coef = −3.869, 95.0% CI: −6.404 to −1.334), PSA decreasing (P = 0.020, Coef = −0.755, 95.0% CI: −1.389 to −0.121), and IPSS (P = 0.003, Coef = 1.034, 95.0% CI: 0.360–1.707). Follow-up time was only related to PSA decreasing (P = 0.017, Coef = 0.161, 95.0% CI: 0.029–0.293). Guidance was not effective for the PDT and functional outcome.
Table 4.
Meta-regression of negative biopsy rate, PSA decreasing rate, and IPSS decreasing rate.
| Covariates | Biopsy negative | PSA | IPSS | |
|---|---|---|---|---|
| Guidance | Coef | 1.891 | 1.250 | 0.277 |
| 95% CI | −3.368, 7.151 | −0.817, 3.318 | −0.650, 1.205 | |
| P | 0.481 | 0.236 | 0.558 | |
| Photosensitizer | Coef | −3.869 | −0.755 | 1.034 |
| 95% CI | −6.404, −1.334 | −1.389, −0.121 | 0.360, 1.707 | |
| P | 0.003 | 0.020 | 0.003 | |
| Follow-up (months) | Coef | 0.175 | 0.161 | −0.096 |
| 95% CI | −0.229, 0.580 | 0.029, 0.293 | −0.285, 0.094 | |
| P | 0.395 | 0.017 | 0.322 | |
CI, confidence interval; IPSS, International Prostate Symptom Score; PSA, prostate-specific antigen.
4. Discussion
As the most common treatment alternatives for localized PC, radical surgery and radiotherapy are used with a considerable morbidity. Patients with low risk and localized PC do not benefit from radical prostatectomy.24 A number of FTs such as high-intensity focused ultrasound, cryotherapy, and radiofrequency have been used.5 Although cancerous cells are destroyed, traditional FT frequently leaves the tumor vessels intact, which can lead to recurrence of the tumor while treatment is insufficient, leaving not only the tumor parenchyma but also tumor vessels. PDT is specialized in target ablating and can prevents recurrence by reactive oxygen species such as singlet oxygen and free radicals.3, 25 However, there is little known about the efficacy, safety, and functional outcomes of PDT in patients with localized PC. To our knowledge, this is the first systematic review to assess those three aspects.
Percent negative biopsy, Gleason score, clinical stages, and PSA were tools for risk estimation for PC. Unlike radical prostatectomy and radiotherapy, it is suitable for a biopsy-based outcome after PDT.10, 24 The most important finding of our studies was that the pooled rate of negative biopsy after PDT and decreased PSA were 55.0% and 35.0%, respectively. Because of the high heterogeneity, a pooled analysis of PSA changes for the two-arm studies was failed to be performed; we think that the difference of control groups was the source of heterogeneity. The control group in the studies by Azzouzi et al.10 and Barret et al.11 was active surveillance and several other FT (such as cryotherapy and brachytherapy), respectively. As compared to the single-arm group, heterogeneity of the two-arm group was lower and PSA decreasing rate was higher according to the subgroup analysis. It was revealed that comparative studies with two arms, especially the RCT, were methodologically stronger than the single-arm studies. According to the result of the RCT,10 negative biopsy rate of active surveillance was less than one-third of the rate in the PDT group. Not only was absent clinically significant cancer for those patients who underwent PDT in the single-arm trails, but also the RCT comparing active surveillance versus PDT with strong results of both negative biopsy and PSA decreasing showed feasibility and efficacy. All the patients of the studies in this systematic review were considered having low-risk, localized PC which was well or moderately differentiated (most biopsy Gleason score was less than 6). The PSA after PDT was less than 4.0 ng/mL in the follow-up duration. In the study of high-risk PC,12 although the PSA decreasing rate was 42.0%, the PSA after PDT was still higher than 10.0 ng/mL. This suggested that PDT was not suitable for the patients with high-risk, poorly differentiated PC. On the other hand, PDT can play an important role in patients who have recurrence after radical prostatectomy or who have failed previous definitive radiotherapy.15
The present meta-analysis shows that pooled rates of adverse events were variable but at a low level. By comparing PDT with cryotherapy, brachytherapy, and high-intensity focused ultrasound, PDT appears to have a reasonably low rate of side effects.11 The most common adverse events were hematuria, erectile dysfunction, and dysuria. Owing to the vascular target toxicity, hematuria always emerges in the duration of early posttreatment in about seven days. Sometimes, hemorrhagic suffusions can be detected in both treated and untreated lobes on magnetic resonance imaging.26 Another notable complication was retention. We found that retention was eighth in the adverse events included in the analysis, with a pooled rate of 11.4%. Instead, Azzouzi et al.10 found that retention was the most common serious adverse event in patients who underwent PDT. They thought it was associated with timing of withdrawal of the urinary catheter. Other rare adverse events such as rectourethral fistulae and injury of seminal vesicle were almost asymptomatic and with a self-healing process, probably for extraprostatic sliding of an optical fiber.17, 26 It is worth noting that photo toxicity is an inherent risk when using a photosensitizer.3 Unfortunately, evaluation of photo toxicity was not available in these studies. To avoid this phototoxic skin reaction, inhibition of intense light exposure is needed.
The expected survival benefit of treatment for PC must be balanced against the related side effects such as erectile dysfunction and dysuria.27 We found that the pooled decreasing rates of the IPSS and IIEF-5 after PDT were 29.1% and 14.9%, respectively. There is an asymmetric prostate after PDT due to unilateral scar tissue; however, the relevance of attracting of the urethra and functional outcome is not clear. The IPSS and IIEF-5 showed transient deterioration and then smooth rising.10, 22 Marien et al.28 found that occurrence of erectile dysfunction and incontinence during high-intensity focused ultrasound and cryotherapy was not only variable but also high (20.0–55.0%, 0.0–10.0%, 15.0–40.0%, and 1.0–10.0%, respectively). In comparison to high-intensity focused ultrasound and cryotherapy, PDT has less effect on urinary and erectile function.29
To explore the source of heterogeneity, the present meta-regression analysis showed that negative biopsy, decreasing PSA, and IPSS after PDT might be associated with types of photosensitizer. Efficacy and functional outcomes of PDT were variable when using different photosensitizers. Padeliporfin and motexafin lutetium were usually used in PC, whereas temoporfin was usually used in lung and head and neck malignancies.30 Moore et al.16 conducted PDT for six patients with PC using temoporfin, with no negative biopsies after treatment. The selection of photosensitizer is important for patients if PDT is planned. Decreasing PSA was also associated with the follow-up duration. It indicated that a long-time follow-up is necessary when evaluating the changing PSA. We did not conduct a meta-regression for the IIEF-5 because of the low I2. A multiple meta-regression analysis was not performed because of the insufficient number of studies.
This analysis has several limitations and should be interpreted with caution. First, only nine of the 14 studies evaluated biopsy after PDT, six of the 14 studies evaluated the side effects, and six evaluated the functional outcomes. Second, only one RCT was included in this meta-analysis, and most trials involved were phase II, single-arm trials. Some confounding factors such as patient population, severity, and treated prostate lobes were uncontrollable. Third, there was significant heterogeneity in these studies, and substantial efforts led the possible causes. The research details in these studies (Gleason score, mean age, energy of laser, and photosensitizer dose) were shown to be insufficient to interpret the results. Fourth, small sample size of some enrolled studies may reduce the power of statistical analysis.
5. Conclusion
The present results show that PDT for patients with PC can be considered effective based on both single-arm clinical trials and RCT included. Meanwhile, this study reveals not only low levels of side effect rates but also insignificant effect on both urinary and erectile function. However, owing to the limitation of current available evidence, more high-quality RCTs are urgently needed to evaluate the comparative efficacy, safety, and functional outcomes of PDT for patients with PC.
Conflicts of interest
All authors have no conflict of interest to declare.
Acknowledgments
The authors would like to thank Dr. Morgan A Mc Clure for language editing.
Contributor Information
Lang Wang, Email: 857344243@qq.com.
Hanfeng Yang, Email: yhf_nsmc@163.com.
Bing Li, Email: 39865701@qq.com.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017 Jan;67(1):7–30. doi: 10.3322/caac.21387. PMID: 28055103. [DOI] [PubMed] [Google Scholar]
- 2.Kasivisvanathan V., Emberton M., Ahmed H.U. Focal therapy for prostate cancer: rationale and treatment opportunities. Clin Oncol (R Coll Radiol) 2013 Aug;25(8):461–473. doi: 10.1016/j.clon.2013.05.002. PMID: 23759249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawczyk-Krupka A., Wawrzyniec K., Musiol S.K., Potempa M., Bugaj A.M., Sieroń A. Treatment of localized prostate cancer using WST-09 and WST-11 mediated vascular targeted photodynamic therapy – a review. Photodiagnosis Photodyn Ther. 2015 Dec;12(4):567–574. doi: 10.1016/j.pdpdt.2015.10.001. PMID: 26467273. [DOI] [PubMed] [Google Scholar]
- 4.Zhu T.C., Finlay J.C., Hahn S.M. Determination of the distribution of light, optical properties, drug concentration, and tissue oxygenation in-vivo in human prostate during motexafin lutetium-mediated photodynamic therapy. J Photochem Photobiol B. 2005 Jun 1;79(3):231–241. doi: 10.1016/j.jphotobiol.2004.09.013. PMID: 15896650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gheewala T., Skwor T., Munirathinam G. Photosensitizers in prostate cancer therapy. Oncotarget. 2017 May 2;8(18):30524–30538. doi: 10.18632/oncotarget.15496. PMID: 28430624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Windahl T., Andersson S.O., Lofgren L. Photodynamic therapy of localized prostatic cancer. Lancet. 1990 Nov 3;336(8723):1139. doi: 10.1016/0140-6736(90)92626-s. PMID: 1978022. [DOI] [PubMed] [Google Scholar]
- 7.Hu J., Dong Y., Chen X., Liu Y., Ma D., Liu X. Prevalence of suicide attempts among Chinese adolescents: a meta-analysis of cross-sectional studies. Compr Psychiatry. 2015 Aug;61:78–89. doi: 10.1016/j.comppsych.2015.05.001. PMID: 26005111. [DOI] [PubMed] [Google Scholar]
- 8.Turner R.M., Davey J., Clarke M.J., Thompson S.G., Higgins J.P. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol. 2012;41:818–827. doi: 10.1093/ije/dys041. PMID: 22461129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. PMID: 9310563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azzouzi A.R., Vincendeau S., Barret E., Cicco A., Kleinclauss F., van der Poel H.G. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomized controlled trial. Lancet Oncol. 2017 Feb;18(2):181–191. doi: 10.1016/S1470-2045(16)30661-1. PMID: 28007457. [DOI] [PubMed] [Google Scholar]
- 11.Barret E., Ahallal Y., Sanchez-Salas R., Galiano M., Cosset J.M., Validire P. Morbidity of focal therapy in the treatment of localized prostate cancer. Eur Urol. 2013 Apr;63(4):618–622. doi: 10.1016/j.eururo.2012.11.057. PMID: 23265382. [DOI] [PubMed] [Google Scholar]
- 12.Nathan T.R., Whitelaw D.E., Chang S.C., Lees W.R., Ripley P.M., Payne H. Photodynamic therapy for prostate cancer recurrence after radiotherapy: a phase I study. J Urol. 2002 Oct;168(4 Pt 1):1427–1432. doi: 10.1016/S0022-5347(05)64466-7. PMID: 12352410. [DOI] [PubMed] [Google Scholar]
- 13.Zaak D., Sroka R., Hoppner M., Khoder W., Reich O., Tritschler S. Photodynamic therapy by means of 5-ALA induced PPIX in human prostate cancer – preliminary results. Med Laser Appl. 2003;18:91–95. [Google Scholar]
- 14.Verigos K., Stripp D.C., Mick R., Zhu T.C., Whittington R., Smith D. Updated results of a phase I trial of motexafin lutetium-mediated interstitial photodynamic therapy in patients with locally recurrent prostate cancer. J Environ Pathol Toxicol Oncol. 2006;25(1–2):373–387. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.230. PMID: 16566729. [DOI] [PubMed] [Google Scholar]
- 15.Du K.L., Mick R., Busch T.M., Zhu T.C., Finlay J.C., Yu G. Preliminary results of interstitial motexafin lutetium-mediated PDT for prostate cancer. Lasers Surg Med. 2006 Jun;38(5):427–434. doi: 10.1002/lsm.20341. PMID: 16788929. [DOI] [PubMed] [Google Scholar]
- 16.Moore C.M., Nathan T.R., Lees W.R., Mosse C.A., Freeman A., Emberton M. Photodynamic therapy using meso tetra hydroxy phenyl chlorin (mTHPC) in early prostate cancer. Lasers Surg Med. 2006 Jun;38(5):356–363. doi: 10.1002/lsm.20275. PMID: 16392142. [DOI] [PubMed] [Google Scholar]
- 17.Trachtenberg J., Weersink R.A., Davidson S.R., Haider M.A., Bogaards A., Gertner M.R. Vascular-targeted photodynamic therapy (padoporfin, WST09) for recurrent prostate cancer after failure of external beam radiotherapy: a study of escalating light doses. BJU Int. 2008 Aug 5;102(5):556–562. doi: 10.1111/j.1464-410X.2008.07753.x. PMID: 18494829. [DOI] [PubMed] [Google Scholar]
- 18.Eymerit-Morin C., Zidane M., Lebdai S., Triau S., Azzouzi A.R., Rousselet M.C. Histopathology of prostate tissue after vascular-targeted photodynamic therapy for localized prostate cancer. Virchows Arch. 2013 Oct;463(4):547–552. doi: 10.1007/s00428-013-1454-9. PMID: 23948957. [DOI] [PubMed] [Google Scholar]
- 19.Moore C.M., Azzouzi A.R., Barret E., Villers A., Muir G.H., Barber N.J. Determination of optimal drug dose and light dose index to achieve minimally invasive focal ablation of localised prostate cancer using WST11-vascular-targeted photodynamic (VTP) therapy. BJU Int. 2014;116(6):888–896. doi: 10.1111/bju.12816. PMID: 24841929. [DOI] [PubMed] [Google Scholar]
- 20.Azzouzi A.R., Barret E., Bennet J., Moore C., Taneja S., Muir G. TOOKAD® Soluble focal therapy: pooled analysis of three phase II studies assessing the minimally invasive ablation of localized prostate cancer. World J Urol. 2015 Jul;33(7):945–953. doi: 10.1007/s00345-015-1505-8. PMID: 25712310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebdai S., Villers A., Barret E., Nedelcu C., Bigot P., Azzouzi A.R. Feasibility, safety, and efficacy of salvage radical prostatectomy after Tookad® Soluble focal treatment for localized prostate cancer. World J Urol. 2015 Jul;33(7):965–971. doi: 10.1007/s00345-015-1493-8. PMID: 25614256. [DOI] [PubMed] [Google Scholar]
- 22.Taneja S.S., Bennett J., Coleman J., Grubb R., Andriole G., Reiter R.E. Final results of a phase I/II multicenter trial of WST11 vascular targeted photodynamic therapy for hemi-ablation of the prostate in men with unilateral low risk prostate cancer performed in the United States. J Urol. 2016 Oct;196(4):1096–1104. doi: 10.1016/j.juro.2016.05.113. PMID: 27291652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebdai S., Bigot P., Leroux P.A., Berthelot L.P., Maulaz P., Azzouzi A.R. Vascular targeted photodynamic therapy with padeliporfin for low risk prostate cancer treatment: midterm oncologic outcomes. J Urol. 2017 Aug;198(2):335–344. doi: 10.1016/j.juro.2017.03.119. PMID: 28322857. [DOI] [PubMed] [Google Scholar]
- 24.Nelson J.B. Perspectives on the clinical management of localized prostate cancer. Asian J Androl. 2014 Jul–Aug;16(4):511–514. doi: 10.4103/1008-682X.123672. PMID: 24589461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolmans D.E., Fukumura D., Jain R.K. Photodynamic therapy for cancer. Nat Rev Cancer. 2003 May;3(5):380–387. doi: 10.1038/nrc1071. PMID: 12724736. [DOI] [PubMed] [Google Scholar]
- 26.Kulik M., Nedelcu C., Martin F., Lebdai S., Rousselet M.C., Azzouzi A.R. Post-treatment MRI aspects of photodynamic therapy for prostate cancer. Insights Imaging. 2014 Dec;5(6):697–713. doi: 10.1007/s13244-014-0359-8. PMID: 25288529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachdeva A., van der Meulen J.H., Emberton M., Cathcart P.J. Evaluating variation in use of definitive therapy and risk-adjusted prostate cancer mortality in England and the USA. BMJ Open. 2015 Feb 24;5(2):e006805. doi: 10.1136/bmjopen-2014-006805. PMID: 25712821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marien A., Gill I., Ukimura O., Betrouni N., Villers A. Target ablation – image-guided therapy in prostate cancer. Urol Oncol. 2014 Aug;32(6):912–923. doi: 10.1016/j.urolonc.2013.10.014. PMID: 24411788. [DOI] [PubMed] [Google Scholar]
- 29.Gómez-Veiga F., Martínez-Breijo S., Solsona-Narbón E., Hernández C., Ciudin A., Ribal M.J. Focal therapy for prostate cancer. Alternative treatment. Actas Urol Esp. 2014 Sep;38(7):465–475. doi: 10.1016/j.acuro.2013.12.006. PMID: 24612733. [DOI] [PubMed] [Google Scholar]
- 30.Dąbrowski J.M., Arnaut L.G. Photodynamic therapy (PDT) of cancer: from local to systemic treatment. Photochem Photobiol Sci. 2015 Oct;14(10):1765–1780. doi: 10.1039/c5pp00132c. PMID: 26219737. [DOI] [PubMed] [Google Scholar]