Abstract
The focus of this review is current knowledge about the epidemiology, clinical manifestations, diagnosis, and treatment of both pulmonary sarcoidosis and extrapulmonary sarcoidosis. Although intrathoracic involvement is the hallmark of the disease, present in over 90% of patients, sarcoidosis can affect virtually any organ. Clinical presentations of sarcoidosis are diverse, ranging from asymptomatic, incidental findings to organ failure. Diagnosis requires the presence of noncaseating granuloma and compatible presentations after exclusion of other identifiable causes. Spontaneous remission is frequent, so treatment is not always indicated unless the disease is symptomatic or causes progressive organ damage/dysfunction. Glucocorticoids are the cornerstone of treatment of sarcoidosis even though evidence from randomized controlled studies is lacking. Glucocorticoid-sparing agents and biologic agents are often used as second- and third-line therapy for patients who do not respond to glucocorticoids or experience serious adverse effects.
Abbreviations and Acronyms: ATS, American Thoracic Society; AV, atrioventricular; CMRI, cardiovascular magnetic resonance imaging; DLCO, diffusing capacity of the lung for carbon monoxide; DMARD, disease-modifying antirheumatic drugs; ECG, electrocardiographic; ERS, European Respiratory Society; FDG-PET, 18F-fluorodeoxyglucose–positron emission tomography; FVC, forced vital capacity; GI, gastrointestinal tract; LVEF, left ventricular ejection fraction; NSAID, nonsteroidal anti-inflammatory drug; PFT, pulmonary function test; TBB, transbronchial lung biopsy; TNF-α, tumor necrosis factor α; WASOG, World Association of Sarcoidosis and other Granulomatous Disorders
Article Highlights.
-
•
Sarcoidosis is a systemic disease that can affect any organ, with the lungs and intrathoracic lymph nodes being the most frequently affected sites.
-
•
The diagnosis of sarcoidosis relies on the presence of noncaseating granuloma on histopathologic examination, compatible clinical presentation, and exclusion of other causes of granulomatous inflammation.
-
•
Treatment is generally reserved for patients with disabling symptoms or with progressive organ damage/dysfunction because spontaneous remission is frequent.
-
•
Glucocorticoids are the first-line therapy, whereas glucocorticoid-sparing agents and biologic agents are used in refractory/recurrent cases.
Sarcoidosis is a multisystem disorder that can affect practically any organ of the body. The hallmark of sarcoidosis is the presence of noncaseating granuloma, a cluster of macrophages, epithelioid cells, mononuclear cells, and CD4+ T cells with a few CD8+ T cells in the peripheral zone.1, 2 The etiology of sarcoidosis is not known with certainty despite decades-long effort. It is generally thought that both genetic predisposition and environmental factors play essential roles in its pathogenesis.3, 4 Because of its heterogeneous manifestations and relative lack of data on treatment efficacy, managing patients with sarcoidosis is often a challenge for clinicians. The purpose of this review is to summarize contemporary knowledge about the epidemiology, clinical manifestations, diagnosis, and treatment of sarcoidosis.
Epidemiology of Sarcoidosis
The annual incidence of sarcoidosis among adults varies considerably across ethnic groups. The highest incidence is observed among African Americans (reported annual incidence of 17-35 per 100,000 population) followed by whites (5-12 per 100,000 population), while the lowest annual incidence is reported among Asians and Hispanics (1-3 per 100,000 population).5, 6, 7, 8, 9, 10, 11 There is a slight female predominance, with the difference being more pronounced in African Americans, in whom the female to male prevalence ratio is almost 2:1.6, 12 Sarcoidosis is a disease of middle-aged persons, with the average age at diagnosis between 35 and 50 years across different populations.5, 6, 7, 8, 9, 10, 11, 12
Clinical Manifestations
Pulmonary Sarcoidosis
The most commonly affected organs in sarcoidosis are the lungs and intrathoracic lymph nodes (over 90% of patients).5, 6, 7, 8, 9, 10, 11, 12 Pulmonary sarcoidosis can be categorized into 4 stages as described in Table 1 and Figure 1, Figure 2, Figure 3 through 4.13 Common symptoms include cough, dyspnea, and chest tightness. However, almost half of patients with pulmonary sarcoidosis are asymptomatic, especially those with stage I disease. The overall prognosis of pulmonary sarcoidosis is good, with spontaneous regression of radiographic abnormalities observed in up to 80% of patients with stage I disease and a very low rate of development of chronic respiratory impairment of less than 5% of patients over a 10-year period.14, 15, 16 However, the prognosis is less favorable among those with more advanced stage at diagnosis. For instance, spontaneous regression of radiographic abnormalities is seen in only one-third of patients with stage II and III disease, and patients with stage III and IV disease have a 5-fold increased risk of chronic respiratory impairment compared with those with stage I disease.14, 15, 16
Table 1.
Features and Frequency of Pulmonary Sarcoidosis Stages
| Stage | Radiographic features | Frequency at presentation (%)7, 10, 13 |
|---|---|---|
| I | Mediastinal and hilar adenopathy (usually bilateral) without pulmonary infiltrates | 40-50 |
| II | Mediastinal and hilar adenopathy (usually bilateral) with pulmonary infiltrates | 30-40 |
| III | Pulmonary infiltrates without adenopathy (adenopathy already regresses) | 15-20 |
| IV | Pulmonary fibrosis with volume loss. No adenopathy | 2-5 |
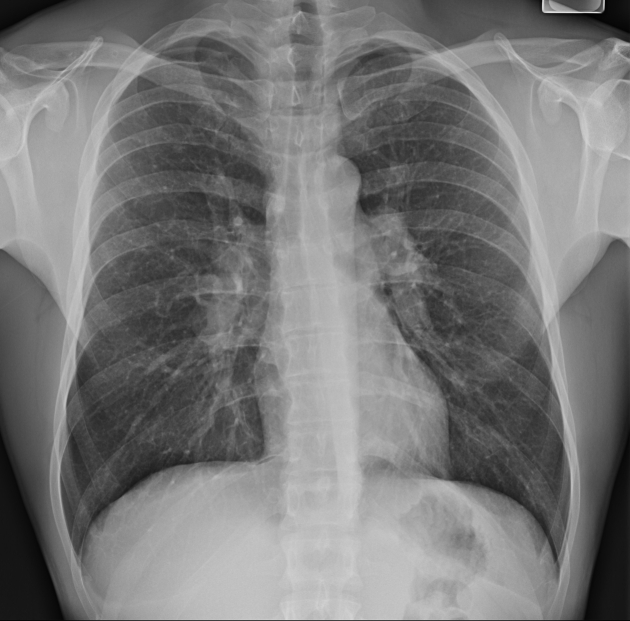
Figure 1.
Chest radiograph of a 31-year-old man with stage I pulmonary sarcoidosis, demonstrating bilateral hilar lymphadenopathy without evidence of parenchymal lung involvement.
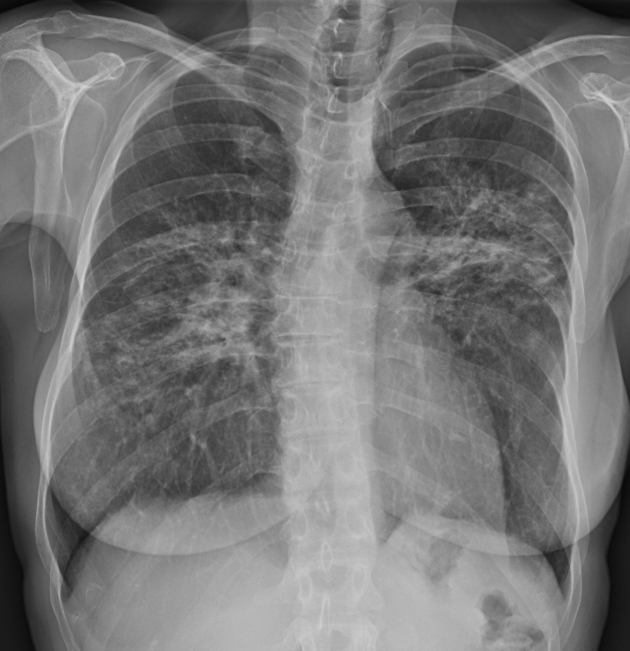
Figure 2.
Chest radiograph of a 62-year-old woman with stage II pulmonary sarcoidosis, demonstrating bilateral nodular parenchymal opacities in a perihilar and mid-lung distribution associated with bilateral hilar and aortopulmonary window lymphadenopathy.
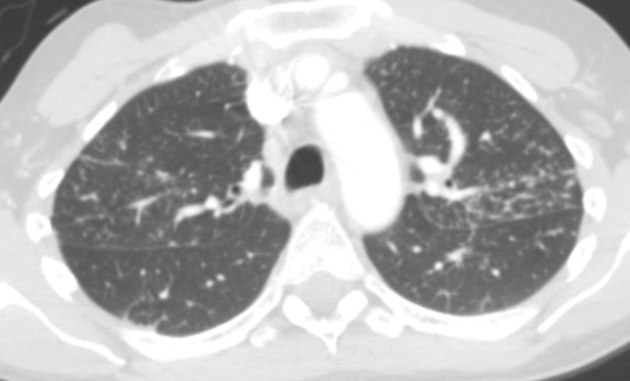
Figure 3.
Computed tomographic image of the chest of a 53-year-old woman with stage III pulmonary sarcoidosis, demonstrating numerous small pulmonary nodules that are upper-lobe predominant and perilymphatic in distribution.
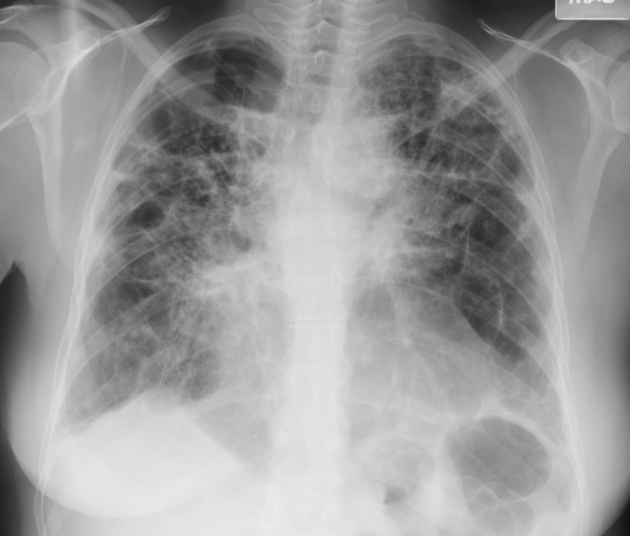
Figure 4.
Chest radiograph of a 54-year-old woman with stage IV pulmonary sarcoidosis, demonstrating extensive parenchymal scarring throughout both lungs, most marked in the upper lungs and in the perihilar regions.
Extrapulmonary Sarcoidosis
Skin
Involvement of the skin is the most common extrathoracic manifestation of sarcoidosis, present in up to one-third of patients.10, 12, 17, 18 Cutaneous sarcoidosis can be categorized into sarcoidosis-specific skin lesions (ie, skin lesions with noncaseating granuloma on histopathologic examination) and nonspecific skin lesions (ie, erythema nodosum). Both types of lesions are approximately equally common.
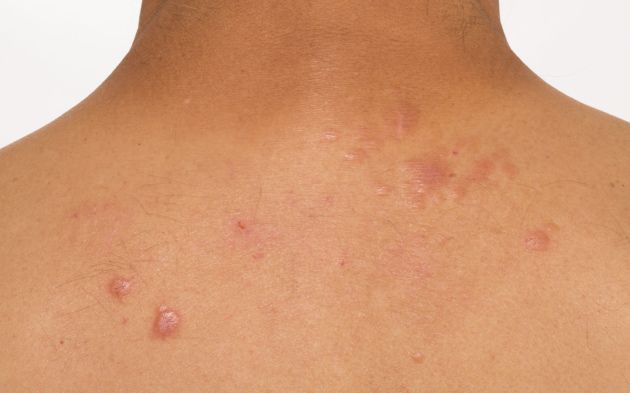
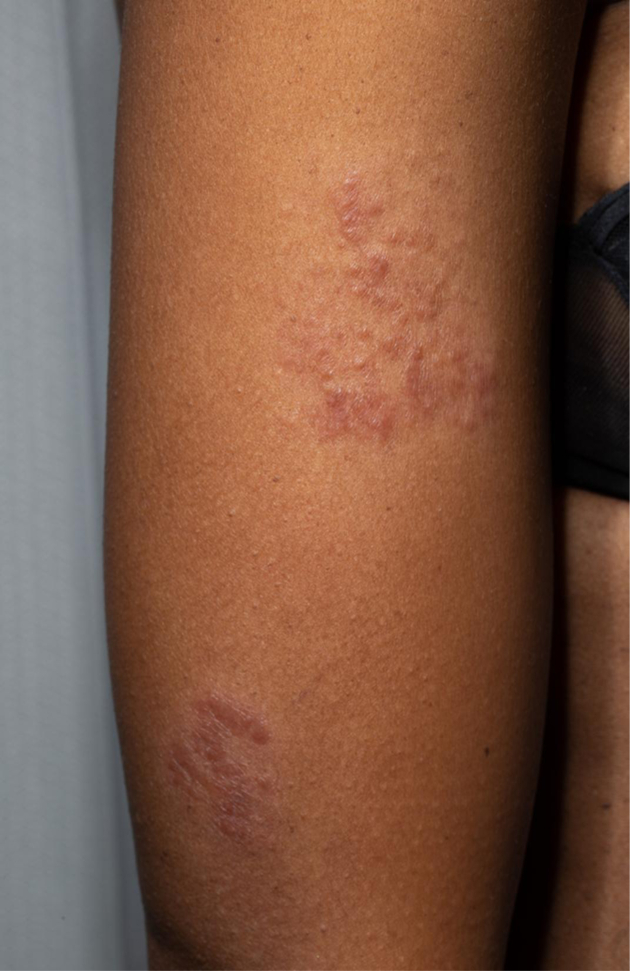
Papules/plaques and subcutaneous nodules are the most common sarcoidosis-specific skin lesions.17, 18 Papules/plaques can be skin-colored, yellow-brown, erythematous, violaceous, hyperpigmented, or hypopigmented and are more frequently found on the extremities and head and neck area than on the trunk (Figures 5 and 6). Subcutaneous nodules are caused by granulomatous inflammation of adipose tissue underneath the skin and are also commonly found on the extremities. The nodules are usually multiple and painless without overlying erythema. Less common cutaneous lesions include inflammation around tattoos or scars and lupus pernio (violaceous to erythematous papules/plaques distributed on the nose, cheeks, and ears). Skin ulcers may arise from one or the other type of lesions or develop de novo.17, 19 Skin biopsy of sarcoidosis-specific skin lesions has good sensitivity for demonstrating noncaseating granuloma (90% in one study)17 and may serve as a less invasive option for histopathologic confirmation of this disease in patients with parenchymal involvement.
Figure 5.
Papular sarcoidosis as a cutaneous manifestation seen on the upper back region. Multiple erythematous raised lesions are evident.
Figure 6.
Plaque sarcoidosis as a cutaneous manifestation seen on the upper arms. Multiple erythematous plaques are evident.
Erythema nodosum is the common nonspecific cutaneous lesion of sarcoidosis. It typically presents as painful, erythematous nodules predominantly found on the anterior surface of the lower extremities. Erythema nodosum of sarcoidosis is clinically and histologically indistinguishable from erythema nodosum from other causes.19 The presence of erythema nodosum usually indicates an acute form of sarcoidosis.17 The combination of erythema nodosum, arthritis, and bilateral hilar adenopathy on chest radiography is termed Löfgren syndrome. The onset of those symptoms is usually acute and is often accompanied by constitutional symptoms such as low-grade fever and malaise. The prognosis of Löfgren syndrome is favorable; spontaneous complete resolution within 3 to 6 months is seen in most patients.17, 20
Eyes
Ocular sarcoidosis is the second most common extrathoracic manifestation of this disease. The reported prevalence of ocular involvement ranges from 10% to 25%, with a higher prevalence observed among blacks than among whites and among females than among males (female to male ratio of about 2:1).12, 18, 21, 22, 23, 24, 25, 26 Ocular disease can be one of the first manifestations of sarcoidosis or can occur years after systemic sarcoidosis is diagnosed.21, 24, 26
Uveitis is the most common subtype of ocular sarcoidosis and can be further divided into anterior, intermediate, posterior, and diffuse uveitis (panuveitis), depending on the location of the intraocular inflammation. Patients with acute anterior uveitis usually present with visual loss, eye pain, and redness around the limbus similar to anterior uveitis from other causes. On the other hand, patients with posterior and intermediate uveitis usually present with painless visual loss and floaters. Anterior uveitis is by far the most common subtype of uveitis in whites (over 80% of cases), but posterior uveitis and panuveitis are more prevalent among blacks.21, 23, 24, 25 Sarcoid uveitis is characteristically bilateral,21, 24, 26 and features of granulomatous uveitis are usually, although not universally, seen on slit-lamp examination.25 Visual outcome of sarcoid uveitis is favorable because most patients have preserved visual acuity at diagnosis, and significant loss of visual acuity during follow-up is uncommon.21, 26, 27
Nonuveitis ocular sarcoidosis is responsible for one-third of all cases of ocular sarcoidosis. This category includes conjunctivitis/conjunctival nodules, scleritis, episcleritis, lacrimal gland involvement, orbital mass, and optic neuritis. With the exception of orbital mass and optic neuritis, nonuveitis ocular sarcoidosis tends not to affect visual acuity, and the response to treatment is generally very favorable. The conjunctiva and lacrimal gland may also serve as easier to reach targets for biopsy when they are involved.24, 26
Ocular inflammation related to sarcoidosis may have a smoldering course, and patients could remain asymptomatic for a long time. Therefore, ophthalmologic screening examination is recommended for all patients with newly diagnosed sarcoidosis, even in the absence of ocular symptoms.21
Joints
Arthropathy is another common extrathoracic manifestation of sarcoidosis that occurs in 5% to 15% of patients. True inflammatory arthritis is seen more frequently than just arthralgia.7, 10, 12, 18 Joint pain is usually one of the first symptoms that eventually leads to the diagnosis of sarcoidosis. The typical presentation is acute oligoarthritis with involvement of the large joints, especially the ankle joint (in over 90% of cases). Polyarthritis with involvement of the small joints of the hands is seen very infrequently. In these patients, other systemic arthritides such as rheumatoid arthritis, which can even co-occur with sarcoidosis, must be considered first. Erythema nodosum accompanies arthritis in approximately 25% to 30% of cases.28, 29 Chronic arthritis is extremely uncommon. Resolution of the inflammatory arthritis usually occurs within 6 weeks in most patients and within 2 years in almost all patients.20, 28, 29 Enthesitis (especially Achilles tendinitis) can also be found in patients with sarcoid arthropathy; the prevalence is about 5% to 8%.28, 30
Liver
The reported prevalence of hepatic involvement by sarcoidosis varies considerably across studies, ranging from 5% to 30%.10, 12, 18, 31 This variance is partly due to the different case definitions and reporting of hepatic sarcoidosis. Some studies required histopathologic confirmation of noncaseating granuloma in the liver, while an unexplained liver chemistry abnormality in known cases of systemic sarcoidosis was sufficient for assumed hepatic involvement in others. However, the actual prevalence is probably higher than the reports from these antemortem studies because postmortem autopsy studies have revealed a prevalence as high as 80%.32, 33 The silent nature of hepatic sarcoidosis is probably the reason behind the discrepancy between antemortem and postmortem studies.
Most patients with hepatic sarcoidosis (up to 80%) are asymptomatic, with the disease discovered incidentally by abnormal liver chemistry test results and/or imaging studies done for other reasons. Among those who are symptomatic, symptoms are usually of a general nature, such as nonspecific abdominal pain, nausea, and fatigue.31, 34, 35 Elevated alkaline phosphatase and γ-glutamyltransferase levels are the most common pattern of abnormal liver chemistry results, reflecting the infiltrative nature of sarcoidosis. Elevations of alkaline phosphatase and γ-glutamyltransferase are usually over 3 times the upper limit of normal. By contrast, elevated alanine aminotransferase and aspartate aminotransferase levels are less common and are usually of less magnitude (less than 2-3 times the upper limit of normal).31, 35, 36 Imaging studies of the liver identify abnormalities in about half of patients, with hypodense nodules being the most commonly observed finding (5%-35%), followed by hepatomegaly (8%-18%).31, 37 Magnetic resonance imaging of the liver provides the best resolution and is the most sensitive modality to detect the nodules. Computed tomography and ultrasonography are of slightly lower sensitivity but are usually more available in clinical practice.35
Definite diagnosis of hepatic sarcoidosis requires demonstration of noncaseating granuloma in the liver and exclusion of other diseases, such as infection, that can give rise to similar histopathologic changes and drug-induced liver injury that can cause transaminitis. These granulomas are commonly found in the portal and periportal areas (although can be found throughout the lobules).35, 38 Liver biopsy appears to have good sensitivity for detection of granulomas, and most studies have consistently reported sensitivity of over 90%.31, 34, 36, 38 The prognosis of hepatic sarcoidosis appears to be quite favorable because spontaneous resolution of abnormal liver chemistry results and prompt response to glucocorticoids are often observed. Nonetheless, inflammation of the liver can persist, and cirrhosis can develop in a significant minority of patients (6%-24%).31, 35, 38
Nervous System
Neurologic involvement by sarcoidosis is relatively uncommon, with a reported prevalence of 3% to 10%.10, 12, 22, 39, 40 In over 90% of cases of neurosarcoidosis, systemic manifestations of sarcoidosis are also observed, especially in the lungs and intrathoracic lymph nodes. In 70% to 80% of cases of sarcoidosis with neurologic involvement, neurologic symptoms are among the first manifestations that eventually lead to the recognition of sarcoidosis.39, 40, 41, 42, 43
Any part of the nervous system can be affected, and multiple parts can be affected in a single patient.39, 40 The most frequently affected sites are the cranial nerves, meninges, and brain parenchyma. Involvement of the pituitary gland, spinal cord, and peripheral nerves is far less common.39, 44
Any cranial nerve can be affected by neurosarcoidosis, with cranial nerves II, VII, and VIII being the most commonly involved.45 The mechanisms behind cranial neuropathy could be either epineural/perineural granulomatous inflammation of the nerve itself or granulomatous inflammation of the leptomeninges compressing the nerve.39, 45 Involvement of cranial nerve VII, which is responsible for about 10% to 25% of all cases of neurosarcoidosis, causes facial palsy that may be either unilateral or bilateral.40, 41, 44, 45 The prognosis of facial nerve neuropathy is good, with complete recovery seen in about 90% of patients when treated with glucocorticoids.39, 42
Optic neuritis is another common cranial neuropathy that is seen in 7% to 35% of cases of neurosarcoidosis.39, 42, 43 Symptoms of optic neuritis associated with sarcoidosis are similar to those of optic neuritis from other causes, including blurry vision and retrobulbar pain associated with papilledema seen on funduscopic examination. Involvement can be either unilateral or bilateral.39, 44 The prognosis of optic neuritis is not favorable, and permanent impaired visual acuity occurs in about one-third of patients. Involvement of the vestibulocochlear nerve may be present in 3% to 10% of patients with neurosarcoidosis, causing hearing loss and peripheral vertigo.43, 44, 46
Leptomeningeal abnormalities are commonly detected by imaging studies, although clinical signs and symptoms of meningeal irritation are seen in only about 10% to 20% of patients with neurosarcoidosis.39, 43, 47 Among symptomatic patients, typical disease features include subacute to chronic onset of headache and constitutional symptoms. Cerebrospinal fluid analysis usually reveals monocyte pleocytosis and a high protein level. The glucose level in cerebrospinal fluid is usually normal, but low levels are measured in about one-fifth of cases.43, 46 The overall prognosis of leptomeningeal involvement is good, although most patients will require treatment with glucocorticoids.39, 44
Intraparenchymal granulomatous lesions are less common. Lesions could be either a solitary mass or multiple nodules and may cause focal neurologic deficits, seizures, or increased intracranial pressure.39, 43, 47 Seizure from sarcoidosis is often difficult to control, and both antiepileptic medications and glucocorticoids are often required.48 The differential diagnosis for intraparenchymal lesions is quite broad, including life-threatening conditions like infection and tumor. Therefore, histopathologic confirmation is generally necessary, although biopsy of those lesions is often technically challenging, with a relatively high rate of false-negative results (up to 40% in one study49).
Small fiber neuropathy has been recognized increasingly as another manifestation of neurosarcoidosis, with a reported prevalence of up to 10% of all patients with sarcoidosis in one study.50 Patients usually present with pain, burning sensation, and paresthesia.44, 50 These symptoms can be migratory and intermittent. Dysautonomia causing orthostasis, palpitation, hyperhidrosis, gastrointestinal dysmotility, or bowel/bladder dysfunction is also observed in approximately half of patients.50 Diagnosis of small fiber neuropathy requires skin biopsy showing decreased intraepidermal nerve fiber density of lower than 5% of the population reference mean or quantitative sudomotor axonal reflex testing showing reduced sweat output.50
Kidneys
Granulomatous interstitial nephritis is the classic renal pathology of sarcoidosis, reported in up to 20% of patients in autopsy studies.51, 52 However, clinically evident interstitial nephritis is quite rare and is seen in less than 3% in antemortem studies, suggesting a nonaggressive course of renal involvement.7, 10, 12, 18 Among those diagnosed antemortem, impairment of renal function with or without abnormal urinalysis results (microscopic hematuria, aseptic pyuria, and proteinuria) is the most common presentation. The majority of these patients also have evidence of active sarcoidosis in other organs.53, 54
Up to half of renal biopsies reveal only evidence of interstitial nephritis without granuloma, which may reflect the nature of the inflammation or sampling error of the biopsy.53, 54 On the other hand, noncaseating granulomatous interstitial nephritis is not pathognomonic of renal sarcoidosis. Several diseases, such as tuberculosis, fungal infection, foreign body reaction, autoimmune diseases (granulomatosis with polyangiitis and Crohn disease), and drug allergy can give rise to the same histopathologic picture.51 Interstitial nephritis usually responds to treatment with glucocorticoids to some extent, but most patients continue to have some degree of renal impairment, particularly among those who have a high burden of fibrosis on biopsy at diagnosis.53, 54
Other renal diseases associated with sarcoidosis are nephrocalcinosis and nephrolithiasis secondary to hypercalcemia and hypercalciuria. Clinical manifestations are similar to those of nephrocalcinosis and nephrolithiasis from other causes.51
Heart
The reported prevalence of cardiac sarcoidosis varies considerably, ranging from 1% to 23%.7, 10, 12, 18, 22 The difference in methods and criteria used for detection and diagnosis of cardiac sarcoidosis is probably responsible for the disparity. For instance, a prevalence of 2% was reported by a study that used the more stringent ACCESS (A Case Control Etiologic Study of Sarcoidosis) study criteria for organ involvement,22 while the study that reported the 23% prevalence of cardiac involvement included all types of electrocardiographic (ECG) abnormalities including nonspecific ST-T changes.10 The reported prevalence of cardiac involvement is even as high as 50% to 70% in Japanese postmortem studies.32, 54 Cardiac sarcoidosis can be seen in isolation without involvement of sarcoidosis in other organs. In one study, isolated cardiac sarcoidosis accounted for two-thirds of all cases of cardiac sarcoidosis,55 although the number is generally lower in other reports.7, 56
The crucial pathology of cardiac sarcoidosis is granulomatous inflammation of the myocardium, leading to arrhythmia and cardiomyopathy.57 The most common type of arrhythmia is atrioventricular (AV) block, accounting for about half of patients, followed by ventricular tachycardia and supraventricular arrhythmia.55, 56 Patients may be asymptomatic, especially in the early stage of disease, or may present with palpitations, syncope, or even sudden cardiac death.57, 58
Heart failure as a result of cardiomyopathy is the initial manifestation of cardiac sarcoidosis in 10% to 20% of patients.55, 56 Both heart failure with reduced ejection fraction from dilated cardiomyopathy and heart failure with preserved ejection fraction from restrictive cardiomyopathy are seen.57
Because of the life-threatening nature of cardiac sarcoidosis, most expert guidelines, including the American Thoracic Society (ATS)/European Respiratory Society (ERS)/World Association of Sarcoidosis and other Granulomatous Disorders (WASOG) statement59 as well as the Heart Rhythm Society guideline,60 recommend screening for cardiac involvement for all patients diagnosed with sarcoidosis using history, physical examination, and 12-lead ECG. It should be noted that this recommendation is based on clinical experience without data from prospective studies. Further studies are still required to prove the benefit of this screening strategy.
Gastrointestinal Tract
Any part of the gastrointestinal tract (GI), from oral cavity to colon, can be affected by sarcoidosis.61 Overall, clinically evident GI sarcoidosis is rare, with a reported prevalence of less than 1%.7, 10, 12, 18, 22 The main pathologic process is granulomatous infiltration in the mucosa and muscular layer, causing mucositis, ulcer, obstruction, or stricture. The stomach is the most frequently affected hollow organ, with approximately 60 biopsy-proven cases reported in the literature. Over half of patients with gastric sarcoidosis present with epigastric pain. Other common manifestations include nausea, vomiting, diarrhea, and weight loss. About 20% of patients are asymptomatic, and sarcoid-related lesions are discovered incidentally on upper endoscopy.61, 62, 63
Diagnosis
Definitive diagnosis of sarcoidosis cannot be made on the basis of clinical and radiologic findings alone. The presence of noncaseating granuloma on biopsy is not pathognomonic of sarcoidosis because several other diseases can cause similar histopathologic changes.64, 65 The diagnosis of sarcoidosis relies on all of the following: (1) the presence of noncaseating granuloma on histopathologic examination, (2) compatible clinical presentation, and (3) exclusion of other causes of granulomatous inflammation.59 The only exceptions to the histopathologic requirement are stage I pulmonary sarcoidosis, for which the presence of bilateral hilar adenopathy alone is generally considered sufficient for diagnosis after exclusion of other possible causes, and Löfgren syndrome (ie, bilateral hilar adenopathy accompanied by erythema nodosum, fever, and arthritis).59, 64, 65
Site of Biopsy
Histopathologic confirmation is required to establish the diagnosis of sarcoidosis in most patients. However, tissue biopsy of every affected organ is not required because the presence of noncaseating granuloma in at least one organ is generally considered sufficient for diagnosis. Sarcoidal involvement of the other organs is generally assumed if signs and symptoms are compatible.64 Therefore, the easily accessible lesions, including rash, conjunctival nodules, enlarged superficial lymph nodes, and enlarged lacrimal gland, are typically the preferred sites of biopsy. If these lesions are not present or biopsy findings are nondiagnostic, intrathoracic lymph nodes and/or lung parenchyma are often the next preferred options for biopsy65 because they are involved in over 95% of patients and the lesions are usually more accessible with less risk of complications compared with other internal organs such as the liver and the kidneys.7, 22
The need for surgical lung biopsy and mediastinoscopy to obtain intrathoracic tissue has been decreasing with the availability of flexible bronchoscopy. Transbronchial lung biopsy (TBB) is widely used, but the reported diagnostic yield of TBB for sarcoidosis varies considerably from 40% to over 90%,65 with a higher yield observed among patients with higher burden of lung parenchymal disease.66 The yield is increased by approximately 20% when TBB is supplemented by endobronchial biopsy67 because endobronchial lesions are also commonly involved (about 40% of stage I disease and 70% of stages II and II disease).68 Bronchoscopists should look for areas with erythema, nodules, or a cobblestone appearance. Even when the mucosa appears normal, biopsy of tissue at the first and secondary carinas is still positive in about 30% of patients.69 Endoscopic ultrasound-guided needle aspiration to obtain samples for cytologic analysis or core needle biopsy from hilar and/or mediastinal lymph nodes is recommended for patients with stage I disease because of the significantly higher yield (over 80%) compared to TBB plus endobronchial biopsy.70 The procedure can be done by either an esophageal or endobronchial route depending on availability and the expertise of the center.
Histopathology
The characteristic histopathologic feature of sarcoidosis is the presence of multiple well-formed, noncaseating granulomas, which are compact clusters of epithelioid cells and multinucleated giant cells with minimal to no central necrosis. The granulomas are often surrounded by lymphocytes. Several types of inclusions may be seen in the granulomas, such as Schaumann bodies, asteroid bodies, Hamazaki-Wesenberg bodies, and calcium oxalate crystals.64, 65 Because sarcoidosis is a diagnosis of exclusion, careful examination of the biopsy specimen to exclude other causes of granulomatous inflammation, especially fungal and mycobacterial infection as well as foreign body reaction, must be performed. This process includes special staining and culture for mycobacteria and fungi.
Diagnosis of Neurosarcoidosis, Cardiac Sarcoidosis, and Intraocular Sarcoidosis
Diagnosis of sarcoidosis in some organs can be particularly challenging because of the inaccessibility for histopathologic confirmation. This issue includes the central nervous system, the heart, and the eyes.
Biopsy of nervous system tissue is frequently not possible because of the potential damage to the biopsied tissue itself (such as cranial nerve) or nearby structures (such as lesions in the brain stem). Therefore, the diagnosis of neurosarcoidosis often relies on histopathologic examination of tissue from an extraneural organ and indirect evidence of inflammation in the central nervous system. Proposed criteria for diagnosis of neurosarcoidosis based on this information are summarized in Table 2.47
Table 2.
Diagnosis of Neurosarcoidosis
| Diagnosis | Criteria | Additional comments |
|---|---|---|
| Definite | Suggestive clinical presentation of neurosarcoidosis | None |
| Plus | ||
| Positive histology of nervous system | Presence of sarcoid-type granulomas with epithelioid cells and macrophages without necrosis in the center, surrounded by lymphocytes, plasma cells, and mast cells in tissue biopsied from nervous system | |
| Plus | ||
| Exclusion of other possible diagnoses | None | |
| Probable | Suggestive clinical presentation of neurosarcoidosis | None |
| Plus | ||
| Evidence of inflammation in central nervous system | Elevated protein and/or cells and/or oligoclonal band in cerebrospinal fluid OR Abnormalities on brain magnetic resonance imaging compatible with neurosarcoidosis |
|
| Plus | ||
| Evidence of systemic sarcoidosis | Presence of sarcoid-type granulomas with epithelioid cells and macrophages without necrosis in the center, surrounded by lymphocytes, plasma cells, and mast cells in tissue biopsied from extraneural organs OR At least 2 indirect evidences from gallium scan, chest imaging, and elevated angiotensin-converting enzyme level |
|
| Plus | ||
| Exclusion of other possible diagnoses | None | |
| Possible | Suggestive clinical presentation of neurosarcoidosis with exclusion of other diseases when the criteria for definite and probable diagnosis are not met | None |
Data from QJM.47
Similarly, endomyocardial biopsy is an invasive procedure associated with high risk of complications. It also has low sensitivity for detection of noncaseating granulomas because of the patchy nature of cardiac sarcoidosis.71 Therefore, the diagnosis of cardiac involvement relies heavily on cardiac imaging studies. Both cardiovascular magnetic resonance imaging (CMRI) with gadolinium and 18F-fluorodeoxyglucose–positron emission tomography (FDG-PET) are useful tools to detect the presence of cardiac involvement by sarcoidosis. The characteristic CMRI pattern is multifocal areas of subepicardial and midmyocardial late gadolinium enhancement, which is an indicator of fibrosis. Late gadolinium enhancement is typically seen in the basal segments of the septum and lateral wall, although more extensive involvement, including the right ventricle, can be seen.56, 60, 72 Cardiac FDG-PET is useful for detection of myocardial inflammation, with a pattern of patchy 18F-fluorodeoxyglucose uptake, either in isolation or on a background of mild diffuse uptake, which is considered typical, although not specific, for cardiac sarcoidosis.60, 73 This abnormal uptake can also be used as a marker of disease activity to monitor response to therapy. Proper preparation is essential to enhance the accuracy of cardiac FDG-PET and may include prolonged fasting, dietary manipulation, and intravenous heparin administration to reduce the normal physiologic glucose uptake of the myocardium.73
In 2014, the Heart Rhythm Society published a guideline recommending 2 pathways to a diagnosis of cardiac sarcoidosis based on histopathology and the aforementioned imaging studies.60 The first pathway relies on the presence noncaseating granuloma in myocardial tissue. The second pathway does not require histopathologic examination of the heart but requires histopathologic diagnosis of extracardiac sarcoidosis plus at least one of the following: (1) glucocorticoid- and/or immunosuppressant-responsive cardiomyopathy and/or heart block, (2) unexplained reduced left ventricular ejection fraction (LVEF) of less than 40%, (3) unexplained sustained (spontaneous or induced) ventricular tachycardia, (4) Mobitz type II second-degree AV block or third-degree AV block, (5) patchy uptake of cardiac FDG-PET in a pattern consistent with cardiac sarcoidosis, (6) late gadolinium enhancement on CMRI in a pattern consistent with cardiac sarcoidosis, or (7) positive gallium uptake in a pattern consistent with cardiac sarcoidosis. Both pathways require exclusion of other explanations of the cardiac abnormalities.60
Diagnosis of intraocular sarcoidosis (sarcoid uveitis) is also complicated by the inaccessibility of intraocular tissue. Therefore, diagnosis of sarcoid uveitis is generally accepted in patients with unexplained uveitis who have a confirmed diagnosis of sarcoidosis in extraocular organs. In 2009, the International Workshop on Ocular Sarcoidosis proposed its first diagnostic criteria for intraocular sarcoidosis based on this principle.74 For these criteria, “definite ocular sarcoidosis” and “presumed ocular sarcoidosis” are diagnosed on the basis of the presence of compatible uveitis in patients who have biopsy-proven sarcoidosis of extraocular organ(s) and in patients who do not undergo biopsy but have classic bilateral hilar adenopathy on chest imaging, respectively. “Probable ocular sarcoidosis” is considered in patients who do not undergo biopsy and do not have classic bilateral hilar adenopathy on chest imaging but have at least 3 suggestive intraocular signs and 2 supportive laboratory investigations (Table 3). The last category is “possible ocular sarcoidosis,” which is considered in patients who have negative findings on lung biopsy but have at least 4 suggestive intraocular signs and 2 supportive laboratory investigations. All of these diagnoses are made after other causes of uveitis are excluded. It should be noted that the definition of definite ocular sarcoidosis and presumed ocular sarcoidosis does not require the presence of intraocular signs listed in Table 3 because those signs are essentially the signs of granulomatous uveitis, which are not universally seen on slit-lamp examination. The criteria are intended to allow diagnosis of sarcoid uveitis in the absence of those signs if evidence for sarcoidosis in extraocular tissue is strong.74
Table 3.
Clinical Signs and Laboratory Investigations Suggestive of Ocular Sarcoidosis According to the International Workshop on Ocular Sarcoidosis Criteria
Suggestive ocular signs
|
Laboratory signs
|
Data from Ocul Immunol Inflamm.74
Additional Tests
Serum markers have only a limited role in the diagnosis of sarcoidosis. Elevation of serum angiotensin-converting enzyme level was thought to be specific for sarcoidosis and correlate well with disease activity when it was first reported in 1975.75 However, subsequent studies revealed its sensitivity to be as low as 40% and its specificity to be poor, with false-positive rates as high as 15%.76, 77 The Kveim test, an intradermal injection of heat-sterilized splenic cells from patients with sarcoidosis to evoke granulomatous response at 4 to 6 weeks, is an old test with variable sensitivity and specificity, highly dependent on the quality of the reagent. The test is not currently used in clinical practice because of the concern about possible transmission of contagious disease and the lack of approval by the US Food and Drug Administration.64
Treatment
There are 2 fundamental facts that exert heavy influence on the management of sarcoidosis. First, sarcoidosis frequently undergoes spontaneous regression without causing any permanent damage to the affected organs.16, 78 Second, use of glucocorticoids, the cornerstone for treatment of sarcoidosis, is associated with several serious adverse effects.79, 80 Therefore, treatment is indicated only when symptoms are disabling and/or the granulomatous inflammation is relentlessly progressive, causing life- or organ-threatening disease.78
Treatment of Pulmonary Sarcoidosis
Most patients with pulmonary sarcoidosis do not require treatment. A study from the Mayo Clinic in Rochester, Minnesota, found that oral glucocorticoids were required in only about one-third of patients with pulmonary sarcoidosis.14 The ATS/ERS/WASOG statement suggests systemic therapy only for patients with progressive symptomatic disease, persistent pulmonary infiltration, and progressive decline of lung function.59 This view is shared by many experts, although conclusive evidence is lacking from randomized controlled trials that it represents optimal management and best patient outcome.78, 81, 82, 83, 84 Reduction from baseline of 10% to 15% or more for forced vital capacity (FVC) and/or of 15% to 20% or more for diffusing capacity of the lung for carbon monoxide (DLCO) are the generally accepted thresholds for significant decline of lung function.78, 81, 82 In rare situations, patients may present with disease that is severe enough to warrant treatment even without serial follow-up/pulmonary function tests (PFTs). These patients typically present with activity-limiting symptoms and have impaired PFT parameters at baseline (FVC ≤70% of predicted value and DLCO ≤60% of predicted value).81
Systemic glucocorticoids are the first-line therapy for pulmonary sarcoidosis.85 Their efficacy is summarized in a meta-analysis of 13 randomized, placebo-controlled clinical trials that found a higher rate of symptomatic, radiographic, and spirometric improvement among those who received glucocorticoids, although data on improvement of FVC and DLCO were less clear.86 The ATS/ERS/WASOG statement recommends a starting dose of 20 to 40 mg/d of prednisone (or equivalent) for 1 to 3 months before gradual taper if symptoms, radiographic changes, and PFT results are stable or improved.59 Taper should be aimed at reducing the daily dose by 5 to 10 mg every 1 to 3 months until a maintenance dose of 5 to 10 mg/d for a total duration of glucocorticoid therapy of approximately 1 year.59, 81, 84 Nonetheless, the ideal dose and duration of the therapy are not known with certainty because the regimen of glucocorticoids varies from study to study.86 Relapse is common during tapering or after discontinuation of glucocorticoids, occurring in as many as 30% of patients.86 Treatment of relapse is with dose escalation of the glucocorticoids, with tapering similar to the first episode. In addition, with relapse, most experts will also recommend disease-modifying antirheumatic drugs (DMARDs) for their corticosteroid-sparing effect because these patients tend to be corticosteroid dependent and are at a higher risk for development of glucocorticoid toxicity.83, 85
Methotrexate is the most commonly used and well-studied DMARD for pulmonary sarcoidosis.85, 86, 87, 88, 89 The usual dose is between 10 and 25 mg weekly, either orally or intramuscularly. Methotrexate has a slow onset of action, and maximal efficacy will not be observed until at least 2 to 3 months after initiation of the therapy. Less commonly used DMARDs with less evidence to support their efficacy include azathioprine, leflunomide, mycophenolate, and chloroquine/hydroxychloroquine.81, 86
Biologic agents are considered third-line therapy for patients with refractory disease that does not respond to glucocorticoids and DMARDs or for those who cannot tolerate these agents.81, 82, 83 They are reserved for later use for several reasons, including the higher cost, potential adverse effects, mode of administration, and lack of approval by the Food and Drug Administration due to the lack of unequivocal evidence of their efficacy in clinical trials. The most commonly prescribed class of biologic agents is tumor necrosis factor α (TNF-α) inhibitor; of these, infliximab is the TNF-α inhibitor with the most robust data. In a randomized controlled study of patients with chronic refractory pulmonary sarcoidosis comparing low-dose intravenous infusion of infliximab (3 mg/kg) and high-dose intravenous infusion of infliximab (5 mg/kg body weight) with intravenous placebo, a 2.5% increase in predicted FVC at 24 weeks was found in the treatment group whereas the placebo group experienced no improvement. This difference was statistically significant, although its clinical importance remained unclear.90 Another open-label prospective study reported a 6.6% increase in predicted FVC at 26 weeks among patients with refractory FDG-PET–positive pulmonary sarcoidosis given the 5 mg/kg body weight infliximab dose.91
Adalimumab is another TNF-α inhibitor with some data to support its efficacy, although the evidence was based on case series and open-label studies.92, 93, 94 Evidence does not support the use of the TNF-α inhibitor etanercept because a preliminary clinical trial of patients with progressive stage II or III pulmonary sarcoidosis reported treatment failure in 11 of 17 recruited patients.95 The difference in molecular structure of these agents might explain this discrepancy because etanercept is a soluble TNF-α receptor while infliximab and adalimumab are anti–TNF-α antibodies. Rituximab, a monoclonal antibody to CD20, is the only non–TNF-α inhibitor biologic agent for which there is some published evidence of efficacy, although the data arise from just a case series and an open-label study.96, 97
The use of H. P. Acthar Gel (Mallinckrodt Pharmaceuticals), a purified form of porcine or bovine corticotropin, has been reported in the literature. Some investigators believe that Acthar Gel can suppress the immune system through the stimulation of multiple melanocortin receptors, in addition to stimulation of endogenous glucocorticoid secretion by adrenal glands.84 In clinical practice, Acthar Gel is rarely used because of the lack of clinical data to support its efficacy. For instance, a retrospective study found that only approximately one-third of patients had objective improvement with this therapy and another one-third could not tolerate its adverse effects beyond 3 months.98 Other disadvantages of Acthar Gel include its high cost and inconvenience of parenteral route. The investigators do not recommend use of this medication for management of sarcoidosis.
Treatment of Extrapulmonary Sarcoidosis
Extrathoracic sarcoidosis tends to undergo spontaneous remission less frequently than pulmonary sarcoidosis. In addition, inflammation in some vital organs, such as the central nervous system, eyes, and heart, can lead to permanent damage and disability. Treatment for involvement of these organs with immunosuppression is required more often than for pulmonary sarcoidosis.
Skin
Erythema nodosum, the common nonspecific cutaneous lesion, is usually self-limited and often does not require any specific therapy. Short-course nonsteroidal anti-inflammatory drugs (NSAIDs) or glucocorticoids may be prescribed to alleviate pain and discomfort.17, 19
Sarcoidosis-specific cutaneous lesions can also regress spontaneously and generally do not cause significant morbidity. Treatment is not always indicated unless the lesions are disfiguring or cosmetically distressing.99 Local glucocorticoid treatment, either topical or intralesional, is first-line therapy because of its limited toxicity compared with systemic glucocorticoids and DMARDs. Ultrapotent formulations, such as clobetasol and halobetasol propionate, are generally required for topical therapy. Oral glucocorticoids are the next line of therapy after failure of local treatment but could be considered as the first option for patients with extensive or rapidly progressive cutaneous disease.19, 99 Response to glucocorticoids is generally favorable, with complete resolution seen within 2 years in most patients.17
In patients with more refractory skin disease that does not respond to systemic glucocorticoids or patients who are corticosteroid dependent, addition of DMARDs is generally considered, although data on their efficacy are quite limited. The most commonly used DMARDs for skin sarcoidosis include hydroxychloroquine, chloroquine, and methotrexate.17, 100, 101 Biologic agents are generally reserved for use when the aforementioned treatments fail to induce a meaningful clinical response. Infliximab is the most commonly used biologic agent with the most robust data, from both a case series and a randomized placebo-controlled trial.102, 103
Eyes
The presence of uveitis almost always necessitates treatment because the consequences of untreated intraocular inflammation, such as posterior synechiae, glaucoma, and neovascularization, are sight-threatening.21 Initial treatment of uveitis associated with sarcoidosis typically starts with local glucocorticoids, using eye drops for anterior uveitis and periocular/intravitreal injection or implant for posterior uveitis. Cycloplegic eye drops are often prescribed concomitantly for anterior chamber inflammation to relieve ciliary spasm, reduce pain, and prevent posterior synechiae.24, 104 Systemic glucocorticoids are required when local therapy fails to induce remission. Disease-modifying antirheumatic drugs are the next step for patients in whom glucocorticoids fail or are not tolerated, with methotrexate being the most widely used agent with the most comprehensive data.104, 105 Other DMARDs used in clinical practice include mycophenolate, azathioprine, and cyclosporine.104 Similar to pulmonary sarcoidosis, biologic agents are reserved for patients whose disease does not respond to treatment with glucocorticoids and DMARDs. Infliximab and adalimumab are the most widely used of these drugs, although data supporting their efficacy are primarily derived from studies of nonspecific noninfectious uveitis.104, 106, 107 Etanercept is not recommended for sarcoid uveitis because a randomized controlled trial of patients with chronic refractory ocular sarcoidosis found no significantly better outcome among etanercept-treated patients than a placebo group.108
External eye diseases, including scleritis, episcleritis, and conjunctivitis, generally do not cause visual impairment. Aggressive treatment of these ocular manifestations of sarcoidosis is typically not necessary.21 Nonsteroidal anti-inflammatory drugs are usually the first-line therapy, with glucocorticoids reserved for patients whose disease is not responsive to NSAIDs.104 Treatment with glucocorticoids with or without DMARDs usually works well for orbital inflammation, although sometimes orbital debulking/decompression surgery is also needed.104
Joints
Sarcoid arthropathy is usually self-limited, and treatment beyond short-course NSAIDs is generally not required.28 Short courses of low-dose glucocorticoids (equivalent to 10-20 mg of prednisone daily) may be used if NSAIDs are contraindicated or fail to improve symptoms. In rare cases in which arthritis persists, the addition of DMARDs (hydroxychloroquine and methotrexate) and, subsequently if needed, TNF-α inhibitors can be pursued similar to rheumatoid arthritis.109 Theoretically, any TNF-α inhibitors should work based on experience with other systemic arthritides. However, only infliximab has been evaluated by a randomized controlled study that found a greater percentage of resolution of joint inflammation compared with placebo, although the sample size was too small to demonstrate statistical significance.110
Liver
Data on the treatment of hepatic sarcoidosis are limited to observational studies and case reports. Most experts do not recommend treatment for patients with asymptomatic liver biochemical test abnormalities because spontaneous improvement is often seen.31 However, if the abnormal test results persist or patients are symptomatic (such as jaundice and pruritus), treatment with glucocorticoids is suggested.31, 60, 111 Disease-modifying antirheumatic drugs should be considered for cases of corticosteroid failure, intolerance, or dependence. Methotrexate, despite its potential hepatotoxicity, has been commonly used based on data from case series that reported its ability to reduce liver test abnormalities and to reduce glucocorticoid dose requirements.31, 88, 111
Nervous System
Neurosarcoidosis almost always requires treatment with glucocorticoids because spontaneous remission is uncommon, with the exception of facial nerve palsy, and damage associated with the inflammatory lesion can cause permanent neurologic deficit.39, 40 High-dose glucocorticoids (equivalent to 1 mg/kg per day of prednisone) is required for central nervous system involvement, while peripheral neuropathy and cranial neuropathy may require only moderate-intensity glucocorticoid therapy (equivalent to 0.5 mg/kg per day of prednisone).39 A short 3- to 5-day course of intravenous methylprednisolone at 1000 mg daily may be considered for patients who have severe disabling manifestations such as altered mental status, visual loss, and paralysis or whose condition deteriorates quickly.39, 44 Once the disease is controlled, gradual tapering of glucocorticoids should be undertaken in a fashion similar to pulmonary sarcoidosis,39, 44, 49 with the exception of facial nerve palsy that may require only 1 to 2 months of therapy.40
Unfortunately, neurosarcoidosis-related symptoms recur quite frequently, especially after the dose of prednisone is reduced to 20 to 25 mg daily.47 This recurrence often necessitates addition of DMARDs. Some experts suggest combination therapy of glucocorticoids and DMARDs as initial management of neurosarcoidosis.39 Methotrexate is the most commonly used DMARD with the most robust data from the literature.112, 113 Other agents with some available data on their efficacy include cyclophosphamide, chloroquine/hydroxychloroquine, mycophenolate, azathioprine, and thalidomide.39, 40 The next line of treatment is infliximab, which is the only biologic agent evaluated in a clinical trial for neurosarcoidosis. That study suggested a trend toward improvement with infliximab compared with placebo, although the sample size was too small to detect statistical significance.110 Its efficacy is also suggested by a retrospective cohort study that found a high response rate to infliximab among patients with glucocorticoid- and DMARD-refractory neurosarcoidosis.114 Successful use of adalimumab and rituximab has been described in a few case reports.39 For those with seizure, antiepileptic medications are also required in addition to immunosuppressive therapy.48
Data on treatment of sarcoidosis-associated small fiber neuropathy are very scarce. The best available data come from a Cleveland Clinic cohort of 115 patients with sarcoidosis-associated small fiber neuropathy that found that glucocorticoids and DMARDs were not effective because more than 80% of patients who received pain medications (such as gabapentin, pregabalin, and duloxetine) in combination with glucocorticoids and/or DMARDs had deterioration of their condition. Intravenous immunoglobulin and TNF-α inhibitors appear to be more effective options because about 70% of patients who received one of them or a combination of them did experience improvement within the first month of therapy.50
Kidneys
Most experts agree that treatment for renal sarcoidosis is indicated to prevent permanent damage that would ultimately lead to chronic kidney disease.51 The suggested regimen consists of initial therapy with a prolonged course of glucocorticoids followed by addition of DMARDs in refractory cases. This regimen is largely derived from experience with treatment of sarcoidosis of other organs because the data on use of these agents for renal sarcoidosis are scarce.51, 52 Hypercalcemia is also an indication for glucocorticoid therapy. Normalization of calcium levels is expected within a week after treatment with prednisone, 20 to 40 mg daily (or equivalent). A relatively quick tapering (4-6 weeks) can be attempted with frequent monitoring.51, 115
Heart
The management of cardiac sarcoidosis consists of immunosuppression and general cardiological care for arrhythmia and heart failure. Immunosuppression is prescribed to suppress ongoing myocardial inflammation with the aims of reducing damage and scarring to myocardium and preventing or reversing ventricular dysfunction. Treatment is also indicated for management of conduction tissue involvement to prevent or reverse conductive abnormalities, especially heart block. A Delphi study surveying experts in the management of cardiac sarcoidosis recommends treatment with immunosuppression for patients who have hypermetabolic activity on cardiac FDG-PET, delayed enhancement on CMRI, conduction defect, ventricular arrhythmia, left ventricular dysfunction, or right ventricular dysfunction in the absence of pulmonary hypertension.116 Glucocorticoids are the first-line therapy for these cardiac manifestations. The evidence for efficacy of glucocorticoids is more robust for conduction defects because a systematic review of 10 published studies found that among 57 patients with AV block who were treated with glucocorticoids, 27 improved whereas none of the 16 patients with AV block who were not treated got better.117
The same systematic review also evaluated the efficacy of glucocorticoids for left ventricular function and arrhythmia, but the results were more difficult to interpret because only a few controls who did not receive glucocorticoids were available.117 Another study of 43 patients with cardiac sarcoidosis found that treatment with glucocorticoids preserved LVEF in those with a pretreatment LVEF of more than 55% and improved LVEF in those with a pretreatment LVEF of 30% to 55% but did not improve LVEF in those with a baseline LVEF of less than 30%.118 The initial dose reported in the literature is prednisone at 40 to 60 mg daily with a taper regimen similar to that for pulmonary sarcoidosis.116, 117
The same diagnostic modalities used to diagnose cardiac sarcoidosis can be used to assess for response to treatment. Cardiac FDG-PET is particularly useful for this purpose, and indeed, some experts even recommend obtaining cardiac FDG-PET in every patient before initiation of immunosuppressive therapy.73 Methotrexate is the recommended DMARD after corticosteroid failure or intolerance.116, 119 Thereafter, other DMARDs including azathioprine and mycophenolate may be considered. As with other organ involvement, infliximab is the preferred biologic agent if adequate response is not achieved with the use of glucocorticoids and DMARDS.110 Nonetheless, infliximab and other TNF-α inhibitors need to be used with caution because they are known to exacerbate heart failure. A potential alternative to TNF-α inhibitors is rituximab.120
Patients with cardiac sarcoidosis who have ventricular dysfunction should also receive standard care for heart failure such as diuretics, β-blockers, and angiotensin-converting enzyme inhibitors.57 The indications for permanent pacemaker implantation are the same as those for advanced-degree heart block from other etiologies if the block cannot be permanently reversed by immunosuppression.72 Antiarrhythmic medications and catheter ablation may be useful for management of ventricular arrhythmia refractory to immunosuppression.72 The standard indications for an implantable cardioverter-defibrillator are also applicable for cardiac sarcoidosis. For those without obvious indications, electrophysiologic study may be considered to further stratify the risk of sudden cardiac death and the need for prevention with an implantable cardioverter-defibrillator.72
Gastrointestinal Tract
Because of the rarity of cases of GI sarcoidosis, the optimal treatment for GI involvement is virtually unknown. Case reports have described both cases with spontaneous remission and cases that required glucocorticoid therapy.62, 63
Recent Innovations
The most important recent innovation in the field of sarcoidosis is the development of biologic agents that has changed the landscape of treatment of refractory disease. Infliximab is the biologic agent with the most comprehensive data. More agents, such as canakinumab, roflumilast, and the nicotine patch, are currently being investigated by ongoing clinical trials.
Conclusion
Clinical presentations of sarcoidosis are diverse and may mimic several other diseases, posing diagnostic and management challenges for clinicians. The presence of noncaseating granuloma alone is not sufficient to make a definite diagnosis of sarcoidosis, and other possible causes of granuloma formation first must be excluded. Once the diagnosis is established, a systematic evaluation for the extent of disease should be conducted. This should, at minimum, include history, physical examination, measurement of calcium, liver enzyme, and creatinine levels, urinalysis, ECG, and ophthalmologic examination. Treatment is indicated for patients with disabling symptoms or with progressive organ failure. Glucocorticoids are the mainstay of therapy even though the ideal dose and duration are not known with certainty because of the lack of data from randomized controlled studies. Methotrexate is the most commonly used corticosteroid-sparing agent for corticosteroid-resistant disease or corticosteroid-intolerant patients. Recent data have supported the use of TNF-α inhibitors, especially infliximab, in refractory cases.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Thomas K.W., Hunninghake G.W. Sarcoidosis. JAMA. 2003;289(24):3300–3303. doi: 10.1001/jama.289.24.3300. [DOI] [PubMed] [Google Scholar]
- 2.Chen E.S., Moller D.R. Sarcoidosis—scientific progress and clinical challenges. Nat Rev Rheumatol. 2011;7(8):457–467. doi: 10.1038/nrrheum.2011.93. [DOI] [PubMed] [Google Scholar]
- 3.Ungprasert P., Crowson C.S., Matteson E.L. Smoking, obesity and risk of sarcoidosis: a population-based nested case-control study. Respir Med. 2016;120:87–90. doi: 10.1016/j.rmed.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen E.S., Moller D.R. Etiology of sarcoidosis. Clin Chest Med. 2008;29(3):365–377. doi: 10.1016/j.ccm.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Rybicki B.A., Major M., Popovich J., Jr., Maliarik M.J., Iannuzzi M.C. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145(3):234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 6.Baughman R.P., Field S., Costabel U., et al. Sarcoidosis in America: analysis based on health care use. Ann Am Thorac Soc. 2016;13(8):1244–1252. doi: 10.1513/AnnalsATS.201511-760OC. [DOI] [PubMed] [Google Scholar]
- 7.Ungprasert P., Carmona E.M., Utz J.P., Ryu J.H., Crowson C.S., Matteson E.L. Epidemiology of sarcoidosis 1946-2013: a population-based study. Mayo Clin Proc. 2016;91(2):183–188. doi: 10.1016/j.mayocp.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gribbin J., Hubbard R.B., Le Jeune I., Smith C.J., West J., Tata L.J. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61(11):980–985. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arkema E.V., Grunewald J., Kullberg S., Eklund A., Askling J. Sarcoidosis incidence and prevalence: a nationwide register-based assessment in Sweden. Eur Respir J. 2016;48(6):1690–1699. doi: 10.1183/13993003.00477-2016. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto T., Azuma A., Abe S., et al. Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008;31(2):372–379. doi: 10.1183/09031936.00075307. [DOI] [PubMed] [Google Scholar]
- 11.Park J.E., Kim Y.S., Kang M.J., et al. Prevalence, incidence, and mortality of sarcoidosis in Korea, 2003-2015: a nationwide population-based study. Respir Med. 2018;144S:S28–S34. doi: 10.1016/j.rmed.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Judson M.A., Boan A.D., Lackland D.T. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29(2):119–127. [PubMed] [Google Scholar]
- 13.Baughman R.P., Culver D.A., Judson M.A. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2011;183(5):573–581. doi: 10.1164/rccm.201006-0865CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ungprasert P., Crowson C.S., Carmona E.M., Matteson E.L. Outcome of pulmonary sarcoidosis: a population-based study 1976-2013. Sarcoidosis Vasc Diffuse Lung Dis. 2018;35(2):123–128. doi: 10.36141/svdld.v35i2.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillerdal G., Nöu E., Osterman K., Schmekel B. Sarcoidosis: epidemiology and prognosis; a 15-year European study. Ann Rev Respir Dis. 1984;130(1):29–32. doi: 10.1164/arrd.1984.130.1.29. [DOI] [PubMed] [Google Scholar]
- 16.Nagai S., Shigematsu M., Hamada K., Izumi T. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5(5):293–298. doi: 10.1097/00063198-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Ungprasert P., Wetter D.A., Crowson C.S., Matteson E.L. Epidemiology of cutaneous sarcoidosis, 1976-2013: a population-based study from Olmsted County, Minnesota. J Eur Acad Dermatol Venereol. 2016;30(10):1799–1804. doi: 10.1111/jdv.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cozier Y.C., Berman J.S., Palmer J.R., Boggs D.A., Serlin D.M., Rosenberg L. Sarcoidosis in black women in the United States: data from the Black Women's Health Study. Chest. 2011;139(1):144–150. doi: 10.1378/chest.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchell R.M., Judson M.A. Cutaneous sarcoidosis. Semin Respir Crit Care Med. 2010;31(4):442–451. doi: 10.1055/s-0030-1262212. [DOI] [PubMed] [Google Scholar]
- 20.Mañá J., Gómez-Vaquero C., Montero A., et al. Löfgren's syndrome revisited: a study of 186 patients. Am J Med. 1999;107(3):240–245. doi: 10.1016/s0002-9343(99)00223-5. [DOI] [PubMed] [Google Scholar]
- 21.Ungprasert P., Tooley A.A., Crowson C.S., Matteson E.L., Smith W.M. Clinical characteristics of ocular sarcoidosis: a population-based study 1976-2013. Ocul Immunol Inflamm. 2019;27(3):389–395. doi: 10.1080/09273948.2017.1386791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baughman R.P., Tierstein A.S., Judson M.A., et al. A Case Control Etiologic Study of Sarcoidosis (ACCESS) Research Group Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10, pt 1):1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 23.Birnbaum A.D., French D.D., Mirsaeidi M., Wehrli S. Sarcoidosis in the national veteran population: association of ocular inflammation and mortality. Ophthalmology. 2015;122(5):934–938. doi: 10.1016/j.ophtha.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groen F., Rothova A. Ocular involvement in sarcoidosis. Semin Respir Crit Care Med. 2017;38(4):514–522. doi: 10.1055/s-0037-1602382. [DOI] [PubMed] [Google Scholar]
- 25.Evans M., Sharma O., LaBree L., Smith R.E., Rao N.A. Differences in clinical findings between Caucasians and African Americans with biopsy-proven sarcoidosis. Ophthalmology. 2007;114(2):325–333. doi: 10.1016/j.ophtha.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 26.Rochepeau C., Jamilloux Y., Kerever S., et al. Long-term visual and systemic prognoses of 83 cases of biopsy-proven sarcoid uveitis. Br J Opthalmol. 2017;101(7):856–861. doi: 10.1136/bjophthalmol-2016-309767. [DOI] [PubMed] [Google Scholar]
- 27.Kirsch O., Frau E., Nodarian M., Labetoulle M., Offret H. Clinical course of ocular sarcoidosis in patients with histologically proven systemic sarcoidosis [in French] J Fr Ophtalmol. 2001;24(6):623–627. [PubMed] [Google Scholar]
- 28.Ungprasert P., Crowson C.S., Matteson E.L. Clinical characteristics of sarcoid arthropathy: a population-based study. Arthritis Care Res (Hoboken) 2016;68(5):695–699. doi: 10.1002/acr.22737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visser H., Vos K., Zanelli E., et al. Sarcoid arthritis: clinical characteristics, diagnostic aspects, and risk factors. Ann Rheum Dis. 2002;61(6):499–504. doi: 10.1136/ard.61.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arthritis in Sarcoidosis Group (ASG) Agarwal V., Agarwal V., Aggarwal A., et al. Arthritis in sarcoidosis: a multicentric study from India. Int J Rheum Dis. 2018;21(9):1728–1733. doi: 10.1111/1756-185X.13349. [DOI] [PubMed] [Google Scholar]
- 31.Ungprasert P., Crowson C.S., Simonetto D.A., Matteson E.L. Clinical characteristics and outcome of hepatic sarcoidosis: a population-based study 1976-2013. Am J Gastroenterol. 2017;112(10):1556–1563. doi: 10.1038/ajg.2017.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwai K., Oka H. Sarcoidosis: report of ten autopsy cases in Japan. Am Rev Respir Dis. 1964;90:612–622. doi: 10.1164/arrd.1964.90.4.612. [DOI] [PubMed] [Google Scholar]
- 33.Ricker W., Clark M. Sarcoidosis: a clinicopathologic review of three hundred cases, including twenty-two autopsies. Am J Clin Pathol. 1949;19(8):725–749. doi: 10.1093/ajcp/19.8.725. [DOI] [PubMed] [Google Scholar]
- 34.Devaney K., Goodman Z.D., Epstein M.S., Zimmerman H.J., Ishak K.G. Hepatic sarcoidosis: clinicopathologic features in 100 patients. Am J Surg Pathol. 1993;17(12):1272–1280. [PubMed] [Google Scholar]
- 35.Deutsch-Link S., Fortuna D., Weinberg E.M. A comprehensive review of hepatic sarcoid. Semin Liver Dis. 2018;38(3):284–297. doi: 10.1055/s-0038-1666853. [DOI] [PubMed] [Google Scholar]
- 36.Ennaifer R., Ayadi S., Romdhane H., et al. Hepatic sarcoidosis: a case series. Pan Afr Med J. 2016;24:209. doi: 10.11604/pamj.2016.24.209.7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warshauer D.M., Dumbleton S.A., Molina P.L., Yankaskas B.C., Parker L.A., Woosley J.T. Abdominal CT findings in sarcoidosis: radiologic and clinical correlation. Radiology. 1994;192(1):93–98. doi: 10.1148/radiology.192.1.8208972. [DOI] [PubMed] [Google Scholar]
- 38.Bihari C., Rastogi A., Kumar N., Rajesh S., Sarin S.K. Hepatic sarcoidosis: clinico-pathological characterization of symptomatic cases. Acta Gastroenterol Belg. 2015;78(3):306–313. [PubMed] [Google Scholar]
- 39.Ungprasert P., Matteson E.L. Neurosarcoidosis. Rheum Dis Clin North Am. 2017;43(4):593–606. doi: 10.1016/j.rdc.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Ungprasert P., Crowson C.S., Matteson E.L. Characteristics and long-term outcome of neurosarcoidosis: a population-based study from 1976-2013. Neuroepidemiology. 2017;48(3-4):87–94. doi: 10.1159/000477300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen R.K., Sellars R.E., Sandstrom P.A. A prospective study of 32 patients with neurosarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20(2):118–125. [PubMed] [Google Scholar]
- 42.Ferriby D., de Seze J., Stojkovic T., et al. Long-term follow-up of neurosarcoidosis. Neurology. 2001;57(5):927–929. doi: 10.1212/wnl.57.5.927. [DOI] [PubMed] [Google Scholar]
- 43.Joseph F.G., Scolding N.J. Neurosarcoidosis: a study of 30 new cases. J Neurol Neurosurg Psychiatry. 2009;80(3):297–304. doi: 10.1136/jnnp.2008.151977. [DOI] [PubMed] [Google Scholar]
- 44.Nozaki K., Judson M.A. Neurosarcoidosis: clinical manifestations, diagnosis and treatment. Presse Med. 2012;41(6, pt 2):e331–e348. doi: 10.1016/j.lpm.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Carlson M.L., White J.R., Jr., Espahbodi M., et al. Cranial base manifestations of neurosarcoidosis: a review of 305 patients. Otol Neurotol. 2014;36(1):156–166. doi: 10.1097/MAO.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 46.Pawate S., Moses H., Sriram S. Presentations and outcomes of neurosarcoidosis: a study of 54 cases. QJM. 2009;102(7):449–460. doi: 10.1093/qjmed/hcp042. [DOI] [PubMed] [Google Scholar]
- 47.Zajicek J.P., Scolding N.J., Foster O., et al. Central nervous system sarcoidosis—diagnosis and management. QJM. 1999;92(2):103–117. doi: 10.1093/qjmed/92.2.103. [DOI] [PubMed] [Google Scholar]
- 48.Krumholz A., Stern B.J., Stern E.G. Clinical implications of seizures in neurosarcoidosis. Arch Neurol. 1991;48(8):842–844. doi: 10.1001/archneur.1991.00530200084023. [DOI] [PubMed] [Google Scholar]
- 49.Stern B.J., Aksamit A., Clifford D., Scott T.F. Neurosarcoidosis Study Group. Neurologic presentations of sarcoidosis. Neurol Clin. 2010;28(1):185–198. doi: 10.1016/j.ncl.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Tavee J.O., Karwa K., Ahmed Z., Thompson N., Parambil J., Culver D.A. Sarcoidosis-associated small fiber neuropathy in a large cohort: clinical aspects and response to IVIG and anti-TNF alpha treatment. Respir Med. 2017;126:135–138. doi: 10.1016/j.rmed.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Berliner A.R., Haas M., Choi M.J. Sarcoidosis: the nephrologist's perspective. Am J Kidney Dis. 2006;48(5):856–870. doi: 10.1053/j.ajkd.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 52.Longcope W.T., Freiman D.G. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952;31(1):1–132. [PubMed] [Google Scholar]
- 53.Mahévas M., Lescure F.X., Boffa J.J., et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore) 2009;88(2):98–106. doi: 10.1097/MD.0b013e31819de50f. [DOI] [PubMed] [Google Scholar]
- 54.Kamata Y., Sato H., Joh K., et al. Clinical characteristics of biopsy-proven renal sarcoidosis in Japan. Sarcoidosis Vasc Diffuse Lung Dis. 2018;35(3):252–260. doi: 10.36141/svdld.v35i3.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kandolin R., Lehtonen J., Airaksinen J., et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131(7):624–632. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 56.Chapelon-Abric C., de Zuttere D., Duhaut P., et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore) 2004;83(6):315–334. doi: 10.1097/01.md.0000145367.17934.75. [DOI] [PubMed] [Google Scholar]
- 57.Hamzeh N., Steckman D.A., Sauer W.H., Judson M.A. Pathophysiology and clinical management of cardiac sarcoidosis. Nat Rev Cardiol. 2015;12(5):278–288. doi: 10.1038/nrcardio.2015.22. [DOI] [PubMed] [Google Scholar]
- 58.Hu X., Carmona E.M., Yi E.S., Pellikka P.A., Ryu J. Causes of death in patients with chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33(3):275–280. [PubMed] [Google Scholar]
- 59.Hunninghake G.W., Costabel U., Ando M., et al. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16(2):149–173. [PubMed] [Google Scholar]
- 60.Birnie D.H., Sauer W.H., Bogun F., et al. HRS expert consensus statement on the diagnosis and management of arrythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11(7):1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 61.Ebert E.C., Kierson M., Hagspiel K.D. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008;103(12):3184–3192. doi: 10.1111/j.1572-0241.2008.02202.x. [DOI] [PubMed] [Google Scholar]
- 62.Afshar K., BoydKing A., Sharma O.P., Shigemitsu H. Gastric sarcoidosis and review of the literature. J Natl Med Assoc. 2010;102(5):419–422. doi: 10.1016/s0027-9684(15)30577-0. [DOI] [PubMed] [Google Scholar]
- 63.Ungprasert P., Kue-A-Pai P., Srivali N., Cheungpasitporn W., Griger D.T. A rare case of symptomatic gastric sarcoidosis. QJM. 2013;106(6):569–570. doi: 10.1093/qjmed/hct067. [DOI] [PubMed] [Google Scholar]
- 64.Judson M.A. The diagnosis of sarcoidosis. Clin Chest Med. 2008;29(3):415–427. doi: 10.1016/j.ccm.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 65.Govender P., Berman J.S. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36(4):585–602. doi: 10.1016/j.ccm.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 66.de Boer S., Milne D.G., Zeng I., Wilsher M.L. Does CT scanning predict the likelihood of a positive transbronchial biopsy in sarcoidosis? Thorax. 2009;64(5):436–439. doi: 10.1136/thx.2008.105031. [DOI] [PubMed] [Google Scholar]
- 67.Shorr A.F., Torrington K.G., Hnatiuk O.W. Endobronchial biopsy for sarcoidosis: a prospective study. Chest. 2001;120(1):109–114. doi: 10.1378/chest.120.1.109. [DOI] [PubMed] [Google Scholar]
- 68.Polychronopoulos V.S., Prakash U.B.S. Airway involvement in sarcoidosis. Chest. 2009;136(5):1371–1380. doi: 10.1378/chest.08-2569. [DOI] [PubMed] [Google Scholar]
- 69.Bradley B., Branley H.M., Egan J.J., et al. British Thoracic Society Interstitial Lung Disease Guideline Group, British Thoracic Society Standards of Care Committee. Thoracic Society of Australia; New Zealand Thoracic Society. Irish Thoracic Society Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1–v58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 70.von Bartheld M.B., Dekkers O.M., Szlubowski A., et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA. 2013;309(23):2457–2464. doi: 10.1001/jama.2013.5823. [DOI] [PubMed] [Google Scholar]
- 71.Uemura A., Morimoto S., Hiramitsu S., Kato Y., Ito T., Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999;138(2, pt 1):299–302. doi: 10.1016/s0002-8703(99)70115-8. [DOI] [PubMed] [Google Scholar]
- 72.Birnie D.H., Nery P.B., Ha A.C., Beanlands R.S. Cardiac sarcoidosis. J Am Coll Cardiol. 2016;68(4):411–421. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 73.Chareonthaitawee P., Beanlands R.S., Chen W., et al. Joint SNMMI-ASNC expert consensus document on the role of 18F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Cardiol. 2017;24(5):1741–1758. doi: 10.1007/s12350-017-0978-9. [DOI] [PubMed] [Google Scholar]
- 74.Herbort C.P., Rao N.A., Mochizuki M., members of Scientific Committee of First International Workshop on Ocular Sarcoidosis International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop on Ocular Sarcoidosis (IWOS) Ocul Immunol Inflamm. 2009;17(3):160–169. doi: 10.1080/09273940902818861. [DOI] [PubMed] [Google Scholar]
- 75.Lieberman J. Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med. 1975;59(3):365–372. doi: 10.1016/0002-9343(75)90395-2. [DOI] [PubMed] [Google Scholar]
- 76.Ungprasert P., Carmona E.M., Crowson C.S., Matteson E.L. Diagnostic utility of angiotensin-converting enzyme in sarcoidosis: a population-based study. Lung. 2016;194(1):91–95. doi: 10.1007/s00408-015-9826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bunting P.S., Szalai J.P., Katic M. Diagnostic aspects of angiotensin converting enzyme in pulmonary sarcoidosis. Clin Biochem. 1987;20(3):213–219. doi: 10.1016/s0009-9120(87)80123-6. [DOI] [PubMed] [Google Scholar]
- 78.Spagnolo P., Rossi G., Trisolini R., Sverzellati N., Baughman R.P., Wells A.U. Pulmonary sarcoidosis. Lancet Respir Med. 2018;6(5):389–402. doi: 10.1016/S2213-2600(18)30064-X. [DOI] [PubMed] [Google Scholar]
- 79.Migita K., Sasaki Y., Ishizuka N., et al. Glucocorticoid therapy and the risk of infection in patients with newly diagnosed autoimmune disease. Medicine (Baltimore) 2013;92(5):285–293. doi: 10.1097/MD.0b013e3182a72299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maradit Kremers H., Reinalda M.S., Crowson C.S., Davis J.M., III, Hunder G.G., Gabriel S.E. Glucocorticoids and cardiovascular and cerebrovascular events in polymyalgia rheumatica. Arthritis Rheum. 2007;57(2):279–286. doi: 10.1002/art.22548. [DOI] [PubMed] [Google Scholar]
- 81.Judson M.A. The treatment of pulmonary sarcoidosis. Respir Med. 2012;106(10):1351–1361. doi: 10.1016/j.rmed.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 82.West S.G. Current management of sarcoidosis I: pulmonary, cardiac, and neurologic manifestations. Curr Opin Rheumatol. 2018;30(3):243–248. doi: 10.1097/BOR.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 83.Carmona E.M., Kalra S., Ryu J.H. Pulmonary sarcoidosis: diagnosis and treatment. Mayo Clin Proc. 2016;91(7):946–954. doi: 10.1016/j.mayocp.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 84.Baughman R.P., Lower E.E. Treatment of sarcoidosis. Clin Rev Allergy Immunol. 2015;49(1):79–92. doi: 10.1007/s12016-015-8492-9. [DOI] [PubMed] [Google Scholar]
- 85.Schutt A.C., Bullington W.M., Judson M.A. Pharmacotherapy for pulmonary sarcoidosis: a Delphi consensus study. Respir Med. 2010;104(5):717–723. doi: 10.1016/j.rmed.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 86.Paramothayan S., Lasserson T. Treatment for pulmonary sarcoidosis. Respir Med. 2008;102(1):1–9. doi: 10.1016/j.rmed.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 87.Baughman R.P., Winget D.B., Lower E.E. Methotrexate is steroid sparing in acute sarcoidosis: results of double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17(1):60–66. [PubMed] [Google Scholar]
- 88.Lower E.E., Baughman R.P. The use of low dose methotrexate in refractory sarcoidosis. Am J Med Sci. 1990;299(3):153–157. doi: 10.1097/00000441-199003000-00002. [DOI] [PubMed] [Google Scholar]
- 89.Vucinic V.M. What is the future of methotrexate in sarcoidosis? a study and review. Curr Opin Pulm Med. 2002;8(5):470–476. doi: 10.1097/00063198-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 90.Baughman R.P., Drent M., Kavuru M., et al. Sarcoidosis Investigators Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 91.Vorselaars A.D., Crommelin H.A., Deneer V.H., et al. Effectiveness of infliximab in refractory FDG PET-positive sarcoidosis. Eur Respir J. 2015;46(1):175–185. doi: 10.1183/09031936.00227014. [DOI] [PubMed] [Google Scholar]
- 92.Crommelin H.A., van der Burg L.M., Vorselaars A.D., et al. Efficacy of adalimumab in sarcoidosis patients who developed intolerance to infliximab. Respir Med. 2016;115:72–77. doi: 10.1016/j.rmed.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Kamphuis L.S., Lam-Tse W.K., Dik W.A., et al. Efficacy of adalimumab in chronically active and symptomatic patients with sarcoidosis. Am J Respir Crit Care Med. 2011;184(10):1214–1216. doi: 10.1164/ajrccm.184.10.1214. [DOI] [PubMed] [Google Scholar]
- 94.Sweiss N.J., Noth I., Mirsaeidi M., et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(1):46–54. [PMC free article] [PubMed] [Google Scholar]
- 95.Utz J.P., Limper A.H., Kalra S., et al. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest. 2003;124(1):177–185. doi: 10.1378/chest.124.1.177. [DOI] [PubMed] [Google Scholar]
- 96.Sweiss N.J., Lower E.E., Mirsaeidi M., et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J. 2014;43(5):1525–1528. doi: 10.1183/09031936.00224513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cinetto F., Compagno N., Scarpa R., Malipiero G., Agostini C. Rituximab in refractory sarcoidosis: a single centre experience. Clin Mol Allergy. 2015;13(1):19. doi: 10.1186/s12948-015-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baughman R.P., Barney J.B., O'Hare L., Lower E.E. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med. 2016;110:66–72. doi: 10.1016/j.rmed.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 99.Haimovic A., Sanchez M., Judson M.A., Prystowsky S. Sarcoidosis: a comprehensive review and update for the dermatologist, part I: Cutaneous disease. J Am Acad Dermatol. 2012;66(5):699.e1–699.e18. doi: 10.1016/j.jaad.2011.11.965. [DOI] [PubMed] [Google Scholar]
- 100.Zic J.A., Horowitz D.H., Arzubiaga C., King L.E., Jr. Treatment of cutaneous sarcoidosis with chloroquine: review of the literature. Arch Dermatol. 1991;127(7):1034–1040. [PubMed] [Google Scholar]
- 101.Webster G.F., Razsi L.K., Sanchez M., Shupack J.L. Weekly low-dose methotrexate therapy for cutaneous sarcoidosis. J Am Acad Dermatol. 1991;24(3):451–454. doi: 10.1016/0190-9622(91)70071-9. [DOI] [PubMed] [Google Scholar]
- 102.Heidelberger V., Ingen-Housz-Oro S., Marquet A., et al. Efficacy and tolerance of anti-tumor necrosis factor α agents in cutaneous sarcoidosis: a French study of 46 cases. JAMA Dermatol. 2017;153(7):681–685. doi: 10.1001/jamadermatol.2017.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buaghman R.P., Judson M.A., Lower E.E., et al. Sarcoidosis Investigators Infliximab for chronic cutaneous sarcoidosis: a subset analysis from a double-blind randomized clinical trial. Sarcoidosis Vasc Diffuse Lung Dis. 2016;32(4):289–295. [PubMed] [Google Scholar]
- 104.Pasadhika S., Rosenbaum J.T. Ocular sarcoidosis. Clin Chest Med. 2015;36(4):669–683. doi: 10.1016/j.ccm.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Samson C.M., Waheed N., Baltatzis S., Foster C.S. Methotrexate therapy for chronic noninfectious uveitis: analysis of a case series of 160 patients. Ophthalmology. 2001;108(6):1134–1139. doi: 10.1016/s0161-6420(01)00576-0. [DOI] [PubMed] [Google Scholar]
- 106.Suhler E.B., Smith J.R., Giles T.R., et al. Infliximab therapy for refractory uveitis: 2-year results of a prospective trial. Arch Ophthalmol. 2009;127(6):819–822. doi: 10.1001/archophthalmol.2009.141. [DOI] [PubMed] [Google Scholar]
- 107.Suhler E.B., Lowder C.Y., Goldstein D.A., et al. Adalimumab therapy for refractory uveitis: results of a multicentre, open-label, prospective trial. Br J Ophthalmol. 2013;97(4):481–486. doi: 10.1136/bjophthalmol-2012-302292. [DOI] [PubMed] [Google Scholar]
- 108.Baughman R.P., Lower E.E., Bradley D.A., Raymond L.A., Kaufman A. Etanercept for refractory ocular sarcoidosis: results of a double-blind randomized trial. Chest. 2005;128(2):1062–1067. doi: 10.1378/chest.128.2.1062. [DOI] [PubMed] [Google Scholar]
- 109.Abril A., Cohen M.D. Rheumatologic manifestations of sarcoidosis. Curr Opin Rheumatol. 2004;16(1):51–55. doi: 10.1097/00002281-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 110.Judson M.A., Baughman R.P., Costabel U., et al. Centocor T48 Sarcoidosis Investigators Efficacy of infliximab in extrapulmonary sarcoidosis: results from randomised trial. Eur Respir J. 2008;31(6):1189–1196. doi: 10.1183/09031936.00051907. [DOI] [PubMed] [Google Scholar]
- 111.Karagiannidis A., Karavalaki M., Koulaouzidis A. Hepatic sarcoidosis. Ann Hepatol. 2006;5(4):251–256. [PubMed] [Google Scholar]
- 112.Bitoun S., Bouvry D., Borie R., et al. Treatment of neurosarcoidosis: a comparative study of methotrexate and mycophenolate mofetil. Neurology. 2016;87(24):2517–2521. doi: 10.1212/WNL.0000000000003431. [DOI] [PubMed] [Google Scholar]
- 113.Soriano F.G., Caramelli P., Nitrini R., Rocha A.S. Neurosarcoidosis: therapeutic success with methotrexate. Postgrad Med J. 1990;66(772):142–143. doi: 10.1136/pgmj.66.772.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cohen Aubart F., Bouvry D., Galanaud D., et al. Long-term outcomes of refractory neurosarcoidosis treated with infliximab. J Neurol. 2017;264(5):891–897. doi: 10.1007/s00415-017-8444-9. [DOI] [PubMed] [Google Scholar]
- 115.Sharma O.P. Hypercalcemia in granulomatous disorders: a clinical review. Curr Opin Pulm Med. 2000;6(5):442–447. doi: 10.1097/00063198-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 116.Hamzeh N.Y., Wamboldt F.S., Weinberger H.D. Management of cardiac sarcoidosis in the United States: a Delphi study. Chest. 2012;141(1):154–162. doi: 10.1378/chest.11-0263. [published correction appears in Chest. 2012;142(6):1698] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sadek M.M., Yung D., Birnie D.H., Beanlands R.S., Nery P.B. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29(9):1034–1041. doi: 10.1016/j.cjca.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 118.Chiu C.Z., Nakatani S., Zhang G., et al. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95(1):143–146. doi: 10.1016/j.amjcard.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 119.Nagai S., Yokomatsu T., Tanizawa K., et al. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med. 2014;53(5):427–433. doi: 10.2169/internalmedicine.53.0794. [DOI] [PubMed] [Google Scholar]
- 120.Krause M.L., Cooper L.T., Chareonthaitawee P., Amin S. Successful use of rituximab in refractory cardiac sarcoidosis. Rheumatology (Oxford) 2016;55(1):189–191. doi: 10.1093/rheumatology/kev309. [DOI] [PubMed] [Google Scholar]