Abstract
Glioblastoma (GBM) remains one of the most malignant primary tumors in adults, with a 5-year survival rate less than 10% because of lacking effective treatment. Here, we aimed to explore whether B7-H3 could serve as a novel therapeutic target for GBM in chimeric antigen receptor (CAR) T cell therapy. In this study, a CAR targeting B7-H3 was constructed and transduced into T cells by lentivirus. Antitumor effects of B7-H3-specific CAR-T cells were assessed with primary and GBM cell lines both in vitro and in vivo. Our results indicated that B7-H3 was positively stained in most of the clinical glioma samples, and its expression levels were correlated to the malignancy grade and poor survival in both low-grade glioma (LGG) and GBM patients. Specific antitumor functions of CAR-T cells were confirmed by cytotoxic and ELISA assay both in primary glioblastoma cells and GBM cell lines. In the orthotropic GBM models, the median survival of the CAR-T-cell-treated group was significantly longer than that of the control group. In conclusion, B7-H3 is frequently overexpressed in GBM patients and may serve as a therapeutic target in CAR-T therapy.
Keywords: B7-H3, chimeric antigen receptor, glioblastoma, low grade glioma, immunotherapy
Introduction
Glioblastoma (GBM) is still the most common and lethal form of brain cancer. Despite multiple treatments available including surgery, radiotherapy, and chemotherapy, GBM still exhibits a poor, with 5-year survival rate less than 10%, because of low cure rate, high recurrence, and mortality.1 New strategies that could prolong the overall survival and reduce toxic side effects are yet to be developed. In recent years, adoptive transfer of chimeric antigen receptor T cell (CAR-T) therapy come to be one of the most effective and promising approaches for treating hematologic tumor.2, 3 More recently, CAR-T cells also showed impressive antitumor efficiency in preclinical and clinical treatment studies of GBM.4, 5 However, the progress and popularization of the CAR-T therapy for GBM was limited by the lack of an applicable therapeutic target.6, 7 Although several targets applied for CAR-T therapy, including interleukin-13 receptor alpha 2 (IL-13Rα2), human epidermal growth factor receptor 2 (HER2), and epidermal growth factor receptor variant III (EGFRvIII) have been proved to induce antitumor response,8, 9, 10 these immunotherapy targets were not expressed highly and ubiquitously within tumor tissues and could not meet the standard of an ideal tumor immunotherapeutic target. Thus, novel suitable therapeutic targets for CAR application await discovery.
B7-H3, a type I transmembrane protein, is encoded by chromosome 15 in human beings. The extracellular domain of B7-H3 has two kinds of isoforms named 4IgB7-H3 and 2IgB7-H3.11 Previous studies suggest B7-H3 has both co-stimulatory function and co-inhibitory function in the regulation of different T cell subsets.12, 13, 14 So far, the receptor of the B7-H3 is unknown, and its biological functions remain largely undefined. Recently, it has been reported that B7-H3 is broadly overexpressed by multiple tumor types on cancer cells, tumor-infiltrating dendritic cells, macrophages, and blood vessels, suggesting it may serve as a valuable target for immunotherapy against cancer.15 Some studies show that B7-H3 is highly expressed on medulloblastoma and that children diffuse intrinsic pontine glioma, and it has been recommended to be a promising candidate target for immunotherapy.16, 17 More recent studies demonstrated the potent antitumor effects of B7-H3 CAR-T cells in several solid tumor preclinical models, including pancreatic ductal adenocarcinoma, ovarian cancer, neuroblastoma, and various pediatric cancers.18, 19 Given the wider expression of B7-H3 in various solid tumors, the present study was to explore whether B7-H3 can serve as an immunotherapy target for GBM.
Our study provided the evidence that B7-H3 was expressed on glioma of different WHO grades, but not on the tumor-adjacent normal tissues or normal brain tissue. We also found that B7-H3 expression level was correlated to tumor malignancy grade and poor survival. Furthermore, we constructed a B7-H3-targeting CAR vector using a B7-H3 single-chain antibody fragment (scFv) and evaluated its efficacy against primary GBM cells and several GBM cell lines both in vitro and in vivo. Our results suggested that B7-H3 may serve as a promising therapeutic target for CAR-T therapy against GBM.
Result
B7-H3 Expression Level among Glioma and the GBM Cell Lines
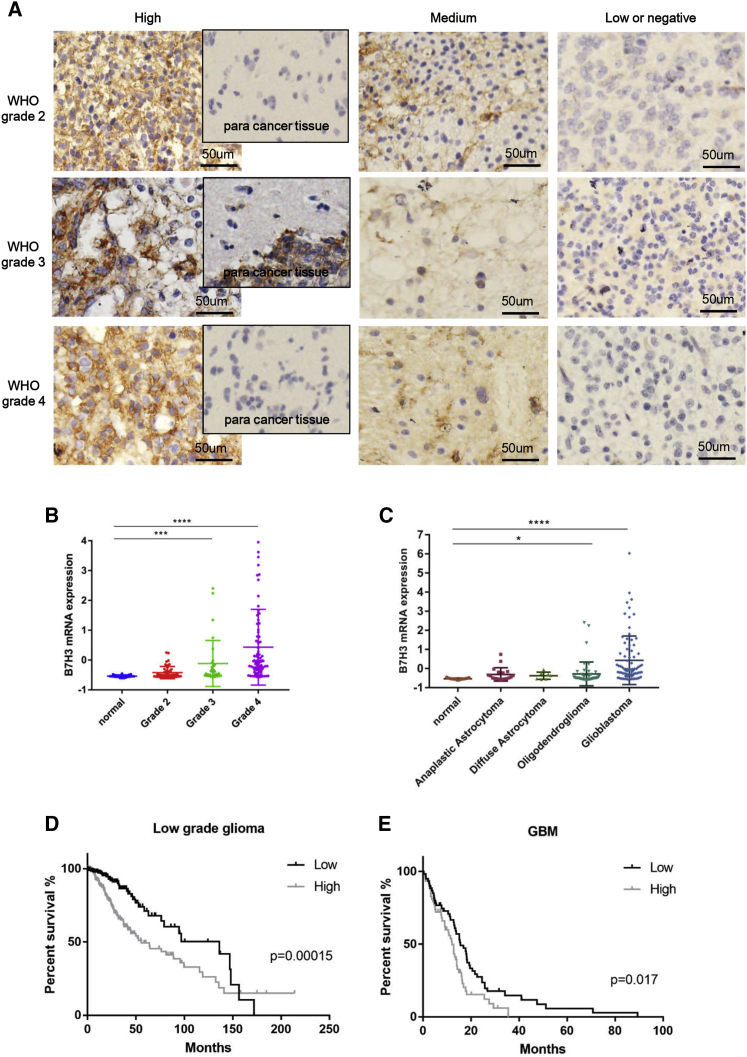
GBM tumor samples of WHO grade 2 and above were collected from neurosurgical resections and analyzed for expression of B7-H3 by immunohistochemistry staining. The result showed that 8/34 of samples were strongly stained and 11/34 of samples stained moderately, while there was no detectable B7-H3 expression in tumor-adjacent tissues and normal cerebral tissue. The details of the tumor specimens were shown in Table 1. Representative cases including para cancer tissues from each grade were shown in Figure 1A, and other cases with strongly stained and normal brain tissue samples were presented in Figure S1. We utilized the data from Oncomine to analyze the association between B7-H3 expression level and the malignancy grade of glioma. As shown in Figure 1B, significantly higher expression of B7-H3 was found in high-grade glioma, while negative and lower expression levels were observed in normal cerebral tissues and low-grade glioma, respectively. For different types of glioma, B7-H3 was significantly overexpressed in oligodendrogliaoma and especially in GBM compared with normal brain tissues, anaplastic astrocytoma and diffuse astrocytoma (Figure 1C). We also analyzed the survival of low-grade glioma (LGG) and GBM patients based on the data from The Cancer Genome Atlas (TCGA) (Figures 1D and 1E). As shown, higher expression of B7-H3 was significantly correlated to decreased survival in both GBM (p < 0.017) and LGG (p < 0.00015). In LGG, the medium overall survival of patients with higher B7-H3 expression was 55.5 months, while it was 136.1 months in lower-expression group.
Table 1.
B7H3 Positivity Rate in Resected Tumor Samples
| WHO Grade | Total Patients | B7-H3 Expression |
Positive Rate (%) | ||
|---|---|---|---|---|---|
| High Positive (Sum: 8) | Medium Positive (Sum: 8) | Low and Positive (Sum: 8) | |||
| 2 | 12 | 2 | 4 | 6 | 50 |
| 3 | 10 | 4 | 2 | 4 | 60 |
| 4 | 12 | 2 | 5 | 5 | 64 |
Figure 1.
Analysis of the Expression Level of B7-H3 in Glioma Sample
(A) Representative cases of 34 diagnosed glioma samples including para cancer tissues from each grade. (B and C) Differential expression of B7-H3 in normal brain tissue and glioma with different grade (B) or type (C) was analyzed with RNA-seq datasets from Oncomine. (D and E) Survival analysis of LGG (D) and GBM (E) patient was shown (*p < 0.05, **p < 0.01, ***p < 0.001).
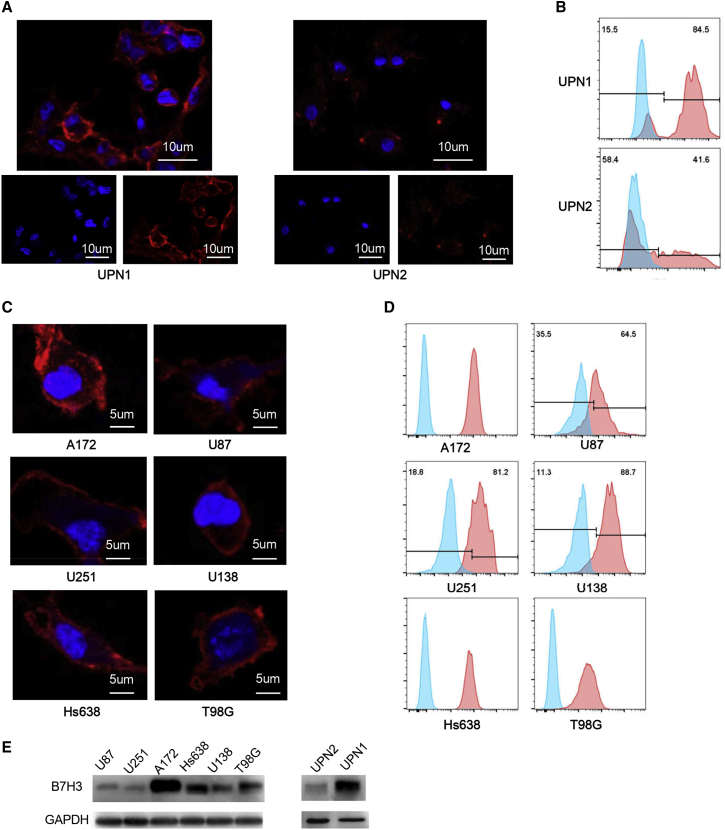
Next, we examined the expression of B7-H3 in primary GBM cells isolated from clinical samples and several GBM cell lines (U87, U138, U251, T98G, Hs638, and A172) using immunofluorescence, flow cytometry, and western blot. As shown, B7-H3 was positively stained in primary tumor cells from GBM samples (UPN1, unique patient number 1; Figures 2A and 2B) and GBM cell lines A172, Hs638, and T98G, while GBM primary cells from UPN2, as well as U87, U251, and U138 cells were weakly stained (Figure 2C). Likewise, western blot results indicated that B7-H3 was highly expressed in the sample of UPN1, A172, Hs638, and T98G cell lines (Figure 2E).
Figure 2.
B7-H3 Expression Level in Primary GBM Cells and GBM Cell Lines
(A and C) Immunofluorescence staining indicated the expression of B7-H3 (red) on cell surface in GBM primary cells (A) and glioma cell lines (C). (B and D) Consistent with the immunofluorescence result, the flow cytometry analysis shows that high-level expression of B7-H3 was observed in UPN1 among the primary GBM cells (B) and in A172 cells among the GBM cell lines (D).
Construction of B7-H3-Specific CAR-T Cells
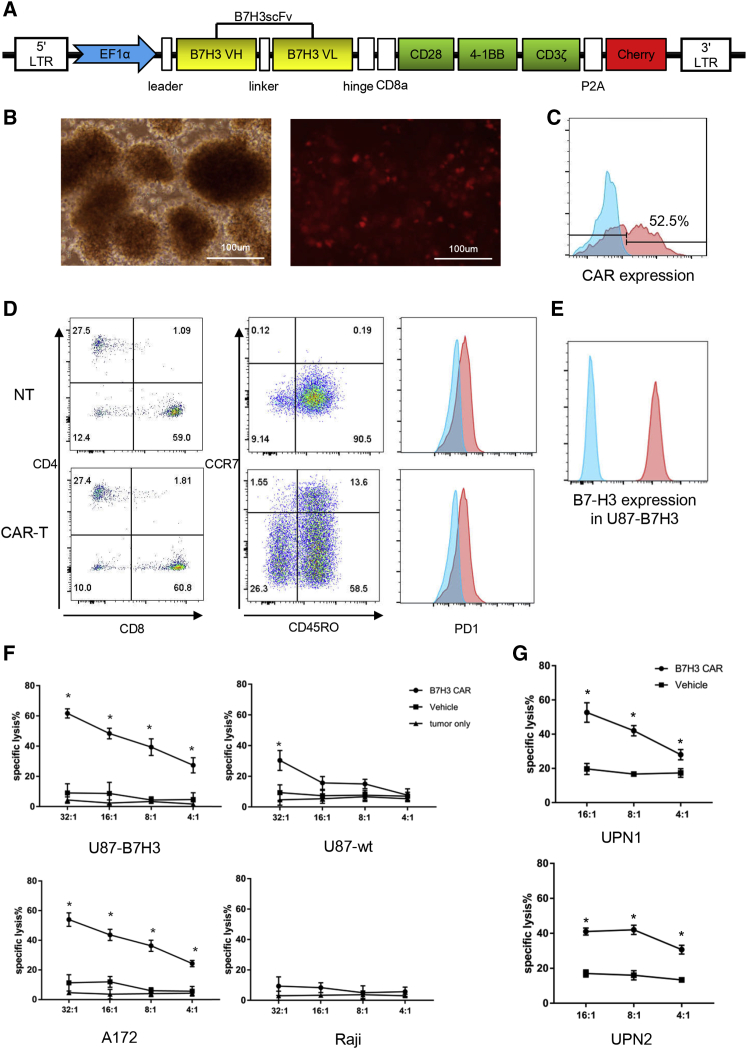
After confirming the B7-H3 expression in GBM specimens and cell lines, we generated a B7-H3 targeted third-generation CAR with 4-1BB and CD28 costimulatory domain to explore whether B7-H3 could be used as a novel CAR-T immunotherapy target in glioma (Figure 3A). The expression of the CARs on T cells after lentivirus transduction was confirmed by detection of the co-expressed mCherry spaced by a P2A self-cleaving peptide with fluorescence microscope (red fluorescence in Figure 3B) and flow cytometry (PE Texas red in Figure 3C), respectively.
Figure 3.
Construction of B7-H3-Specific CAR-T Cells and Cytotoxic Assays
(A) Lentiviral vector construct with the EF1α promoter followed by the leader sequence, anti-B7-H3 scFv, hinge, CD8 transmembrance domain, CD28, 4-1BB, CD3ζ, P2A, and mCherry. (B and C) The transduction efficiency of CAR was measured using fluorescence microscope (B) and flow cytometry analysis of mCherry (C). (D) NT and CAR-T cell subsets and phenotype were analyzed by flow cytometry at 10 days after the transduction of CAR lentivirus. (E) The B7-H3 expression level of lenti-transduced U87 cells was assessed by flow cytometry, and the positive rate was 100%. (F and G) 51Cr-release assays of CAR-T cells against B7-H3+/− cell lines (F) and primary GBM cells (G) in different E:T ratios (*p < 0.05).
Functional Test of B7-H3 CAR-T Cells In Vitro
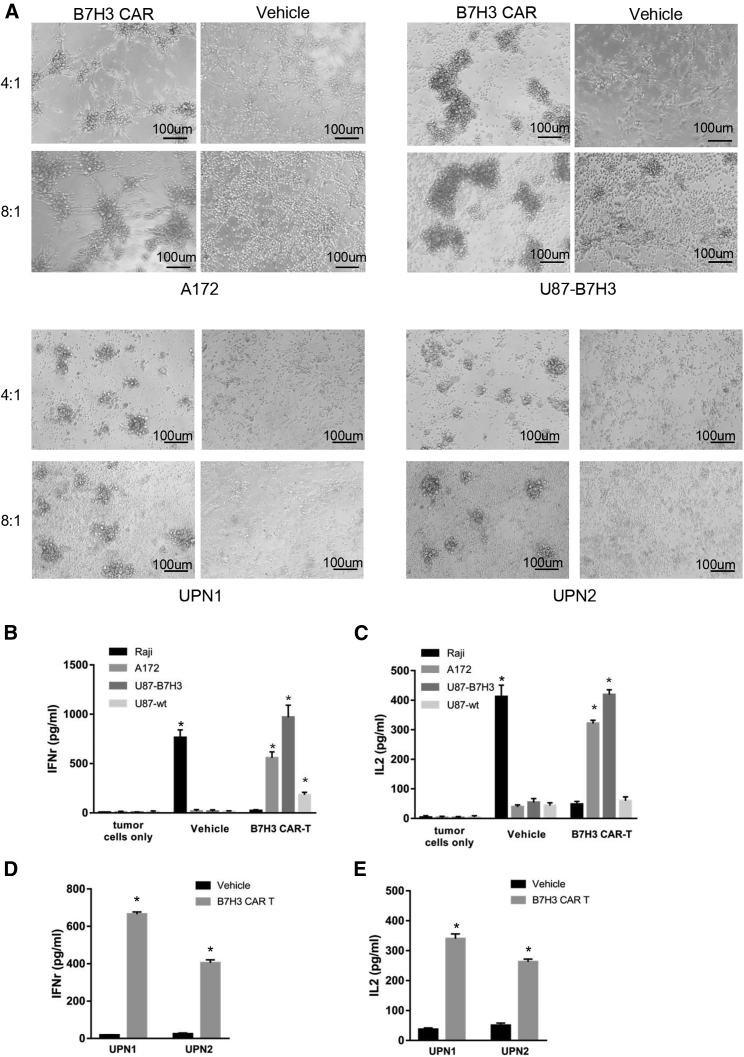
Before the in vitro and in vivo antitumor assay, the phenotype of CAR-T cells was analyzed by flow cytometry (Figure 3D). 10 days after transduction, the ratio of CD4+/CD8+ T cells was about 1:2, and there was no significant difference between CAR-T cells and non-transduced T cell groups (NT). Most CAR-T cells exhibited a phenotype of memory cells (CD45RO+/PD-1−). The specific and potent cytotoxicity of B7-H3 CAR-T cells was evaluated by a co-culture assay with primary GBM cells and B7-H3high/low GBM cell lines. Because of the non-tumorigenic characteristic of B7-H3 highly expressed A172, Hs638, and T98G cell lines in vivo and the low expression of B7-H3 in tumorigenic U87, the U87 cells were stably transduced via lentivirus encoding B7-H3. The flow cytometry result showed the ratio of B7-H3-positive cells of U87 B7-H3 overexpression strain (U87-B7-H3) (Figure 3E). To evaluate the specific antitumor effect of B7-H3 CAR-T cells, Cr51-release cytotoxic assays were carried out to test the cytotoxic effects of B7-H3 CAR-T cells (Figures 3F and 3G). As shown, strong specific lysis was observed in B7-H3 highly expressed A172, U87-B7-H3, and UPN1 cells, moderate cytotoxicity in UPN2, while no significant response was observed in B7-H3 negative or low cells, such as Raji and U87 wild-type cells. Representative bright-field images were shown in Figure 4A. To determine the relative cytokine secretion level, primary glioma cells and GBM cell lines were co-cultured with CAR or vehicle control T cells at an E:T (effect cells: target cells) ratio of 4:1. Supernatants were collected and detected by an ELISA kit. Increased release of interferon γ (IFNγ) and IL-2 can be detected in the supernatants of A172, U87-B7-H3, UPN1, and UPN2 cells co-cultured with CAR-T cells, while not in Raji cells (Figures 4B–4E).
Figure 4.
Tumor Recognition and Cytokine Production of B7-H3-Specific CAR-T Cells In Vitro
(A) Images of co-culture of vehicle and CAR-T cells with autologous GBM cells or GBM cell lines in E:T ratios of 4:1 and 8:1 at 8 h. (B–E) The IFNγ (B: GBM cell lines; C: primary GBM cells) and IL-2 (D: GBM cell lines; E, primary GBM cells) secretion levels of vehicle and CAR-T cells co-cultured with target cells (60,000 T cells to 15,000 tumor cells) for 12 h were measured by ELISA kit (*p < 0.05).
Antitumor Effect of B7-H3-Specific CAR-T Cells In Vivo
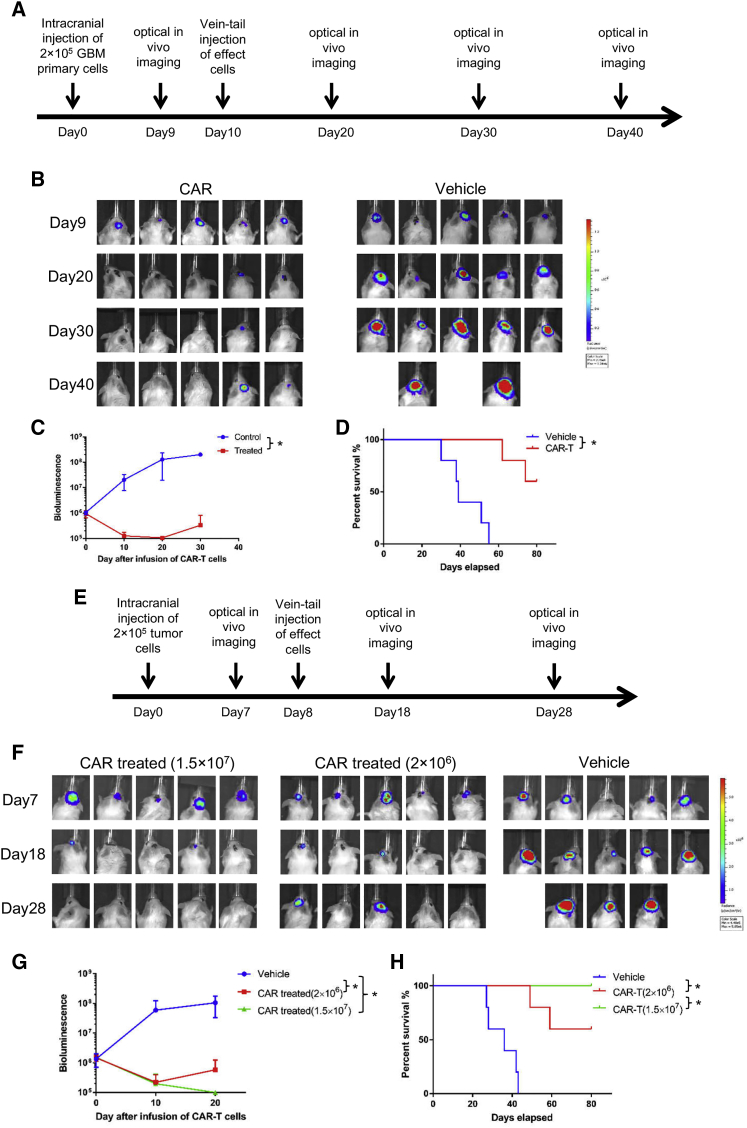
A xenograft orthotropic GBM PDX model was established to evaluate the antitumor effect of B7-H3-specific CAR-T cells in vivo. General protocol schema is illustrated in Figure 5A. As shown in Figure 5B, mouse models were established successfully, which was confirmed by detecting bioluminescence signal 9 days after intracranial injection of engineered GBM primary cells expressing luciferase. A significant tumor growth repression was observed after tail-vein injection of 2 × 106 B7-H3 CAR-T cells compared with vehicle-transduced T cells as measured by bioluminescent imaging (Figure 5C). Prolonged survival was observed in CAR- T cell-treated group compared with the vehicle control group, but tumor recurrence was observed in two mice brain of CAR-T cells treated group (Figure 5D). In this in vivo study, we used a cell line stably expressing B7-H3, so the antigen loss resulting from tumor heterogeneity may not occur. To further investigate whether tumor recurrence was due to the insufficient dose of CAR-T cells in the GBM PDX model, we further designed high (1.5 × 107/mouse) and low (2 × 106/mouse) doses of B7-H3 CAR-T cells against U87-B7-H3 cell xenografts. The treatment program was shown in Figure 5E. Normally, a wider range of doses were used in preclinical animal-model research compared with the clinical trial, facilitating the evaluation of the safety and feasibility. As shown, a complete regression of tumor was observed in high-dose CAR-treated group, and the complete remission of tumor was maintained for about 2 months. In low-dose CAR-treated group, obvious tumor suppression effects were also observed, while no effect was detected in the vehicle group by bioluminescent imaging and survival analysis (Figures 5F and 5H).
Figure 5.
Antitumor Efficacy of B7-H3-Specific CAR-T Cells In Vivo
(A and E) The treatment scheme of orthotropic GBM PDX (A) and U87-B7-H3 xenograft model (E) including intracranial injection of tumor cells, vein-tail injection of vehicle and CAR-T cells, and the timing of in vivo imaging. (B and F) Images of bioluminescence signal in mouse brain before and after injection of vehicle and CAR-T cells in orthotropic GBM PDX (B) and U87-B7-H3 xenograft model (F). (C and G) Tumor total flux (p/s) was calculated by using Living Image software after tail-vein injection of vehicle and CAR-T cells in orthotropic GBM PDX (C) and U87-B7-H3 xenograft model (G). (D and H) Log rank test was used to analyze the overall survival of tumor-bearing mice (*p < 0.05) in orthotropic GBM PDX (D) and U87-B7-H3 xenograft model (H).
Discussion
For CAR-T cell therapy, the selection of therapeutic target is important for the safety and feasibility. Recent studies have demonstrated that B7-H3 is overexpressed by multiple tumor types in cancer cells, tumor-infiltrating dendritic cells, macrophages, and blood vessels.20, 21 It has been reported that B7-H3 is broadly overexpressed by multiple tumor types on both cancer cells and tumor-infiltrating blood vessels and anti-B7-H3 drug conjugates display potent antitumor and antimetastatic activity, suggesting it is a potentially ideal dual-compartment therapeutic target.15 In murine systems, B7-H3 was reported to possess both co-stimulatory and co-inhibitory function in regulation of different T cell subsets.14 With human T cells, it is reported that B7-H3 potently and consistently down-modulated human T cell responses in the presence of strong activating signals. More recently, Lee et al.13 reported that expression of B7-H3 was found in multiple tumor lines, tumor-infiltrating dendritic cells, and macrophages, and B7-H3-deficient mice or mice treated with an antagonistic antibody to B7-H3 showed reduced growth of multiple tumors, which depended on NK and CD8+ T cells. Together, these studies suggested that B7-H3 is a potentially ideal multi-compartment therapeutic target. In the present study, B7-H3 was positively stained in 58% clinical glioma samples as well as in primary GBM cells and GBM cell lines, suggesting it may serve as a potential target for glioma therapy.
Although adoptive transfer of CAR-T cells in treating hematologic cancer has achieved remarkable success, the use of CAR-T cells toward solid tumor faced various barriers, including target antigen heterogeneity, trafficking, and hostile tumor microenvironment.22 For glioma, IL-13Rα2 and EGFRvIII are two most famous CAR-T targets, with which many clinical trials are carried out throughout the world. Earlier studies reported that IL-13Rα2 is expressed by a vast majority of glioblastoma multiforme explants.23 Recent research indicated that the percentage of increased expression of IL-13Rα2 in GBM specimens relative to non-neoplastic brain was less than 50%, and its expression was highly variable both within and across specimens.24 Similarly, although EGFRvIII was reported to be expressed in 10%–30% of patients with GBM, significant regional heterogeneity was observed within tumor specimens for both EGFRvIII and EGFR wild-type (WT),25 and researchers even concluded that the major barrier to targeting EGFRvIII as a single antigen is the heterogeneity of its expression.26 On the other hand, CAR-T or chemotherapies commonly result in the loss of drug targets, which is the most important cause of treatment failure. In IL-13Rα2-targeted CAR-T clinical trial, decreased expression of IL-13Rα2 was observed after treatment, and tumor eventually recurred.27 In a preclinical study, Krenciute et al.28 reported that transgenic expression of IL-15 improves T cell persistence and antiglioma activity of IL-13Rα2 CAR-T cells but results in antigen loss variants. Gliomas with downregulated IL-13Rα2 expression recurred 40 days after T cell injection. Similarly, O’Rourke et al.26 found that peripheral infusion of EGFRvIII-directed CAR-T cells could mediate antigen loss and induce adaptive resistance in patients with recurrent glioblastoma. In the PDX model of our study, target antigen heterogeneity also might be one of the reasons tumors recurred. Therefore, combinatorial targeting of two or three antigens was recommended by some researchers to offset interpatient variability and antigen escape. Very recently, CD70 and CSPG4 were also suggested to be promising targets for CAR-T therapy of GBM.29, 30 In the present study, we found that B7-H3 was highly expressed in GBM and may serve as a novel promising target for CAR-T therapy. With more and more targets identified, more choices are available for molecularly targeted therapy.
In conclusion, our results indicated that B7-H3 was expressed in 58% of clinical glioma samples, and its expression level was correlated to the malignancy grade of glioma and the poor survival of both LGG and GBM patients. Overexpression of B7-H3 was also observed both in primary glioblastoma cells and in GBM cell lines. B7-H3-specific CAR-T cells presented obvious cytotoxic effects on GBM cells in vitro and significantly induced tumor regression in orthotropic GBM models. Our data indicated that B7-H3 may serve as a promising therapeutic target for CAR-T therapy of GBM.
Materials and Methods
Patient Sample and Immunohistochemistry Analysis
All glioma samples were collected from neurosurgery department, West China Hospital. The para cancer tissue was obtained from the extended excision domain of GBM neurosurgical resections, and the normal brain tissue displayed in Figure S1 was from the neurosurgical resections of epilepsy patients. All of samples were dealt with 10% formalin and embedded by paraffin. Paraffin slices were dried for 90 min at 65°C and blocked with 3% H2O2 in distilled water for 20 min, then blocked with PBS containing 10% normal goat serum (Boster) for 30 min at room temperature. Slices were stained with anti-B7-H3 mono-antibody (1:200, Santa Cruz, F11) at 4°C overnight. The next day, slides were developed using DAB chromogen (ZSGB-Bio), counterstained with hematoxylin (BioSharp), dehydrated in ethanol, and cleared in xylene.
The positive rate of each sample was counted by two independent pathologists at five randomized high-magnification fields. More than 60% and 20%–60% of tumor cell surface stained were set as high and moderate positive, respectively. For RNA-sequencing (RNA-seq) and survival analysis, the data were downloaded from Oncomine (http://www.oncomine.org) and TCGA (http://www.oncolnc.org).
Primary Tumor Cells and GBM Cell Lines
GBM primary cells obtained from neurosurgical resections were grown in RPMI (HyClone), and GBM cell lines including U87, A172, U251, and U138 (purchased from ATCC) were cultured with DMEM (HyClone). All the media were supplemented with 10% fetal calf serum (HyClone) with 2 mmol/L glutamine and 1.0 mmol/L penicillin streptomycin combination (HyClone). The B7-H3/luciferase (Luc) double-positive U87 cell line was established by virus transduction and puromycin selection using a lentivirus vector with double promoters (EF1α for B7-H3 and PGK1 for Luc-P2A-puromycin).
Expression and Purification of B7-H3-Specific scFv-Mouse Fragment Crystallizable (mFC) Recombinant Protein
cDNA coding human B7-H3-specific scFv were synthesized by Genewizz according to a previously published amino acid sequence.31 The B7-H3-specific scFv does not have cross-reaction with mouse B7-H3. The cDNA was subcloned into a eukaryotic expression vector with murine Fc and His tags at the C-terminal. Construct was transiently transfected into HEK293FT cells in Freestyle serum-free medium (Thermo Fisher Scientific), and the supernatant was harvested 1 week post-transfection. Recombinant protein was initially isolated by protein A and nickel-nitrilotriacetic acid (Ni-NTA) affinity columns and subsequently subjected to size-exclusion chromatography.
Immunofluorescent Staining and Western Blot
GBM cells were incubated in 24-well plates with poly-L-lysine-treated coverslips at 37°C. After 24 h, specimens were stained with primary antibody or scFv-Fc for 1 h at 4°C and fixed in 4% paraformaldehyde in PBS for 15 min. The slides were stained in turn by a Cy3-conjugated secondary antibody (Proteintech) and DAPI (Beyotime). The immunofluorescent staining was imaged by confocal microscopy.
Western blots were used to probe for B7-H3 expression in isolated glioma primary cells and GBM cell lines. Cells were lysed in radioimmunoprecipitation (RIPA) buffer supplemented with protease inhibitor cocktail (Sigma) for 15 min and then centrifuged at 10,000 × g for 15 min at 4°C. Protein concentrations were determined with a BCA protein assay kit (Thermo Fisher Scientific). Equal amounts of protein were loaded on 10% SDS-PAGE gel and subsequently transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore). After blocking in 5% skimmed milk, the membranes were incubated with anti-B7-H3 (1:500, Santa Cruz, F-11) or anti-GAPDH (1:1,000; Beyotime, AF0006) primary antibody overnight. Horseradish peroxidase (HRP)-conjugated secondary antibody (1:2,000; Beyotime, A0216) was used at a dilution of 1:2,000. Western blot results were visualized using ChemiScope 6000 Touch (Clinx).
Flow Cytometry Analysis
Cells were filtered through a 100-μM strainer filter and washed twice with PBS containing 0.5% BSA before incubation with antibodies for 30 min at 4°C in the dark. A FACSCalibur instrument was used to analyze the cells, and percentages of positive cells were calculated via FlowJo-V10 software for analysis. In addition, CAR-T cells expressing the mCherry protein were detected in PE Texas red channel, and T cell phenotype was assessed by CD4, CD8 (BD Biosciences, RPA-T4; RPA-T8), CCR7, CD45RO, and PD1 (BioLegend, G043H7; UCHL1; EH12.2H7). B7-H3 expression in GBM primary cells and cell lines was evaluated by using a B7-H3-specific APC-conjugated antibody (BioLegend, MIH42).
Construction of CAR Vector
A CAR sequence consisting of CD8α signal peptide, B7-H3-specific scFv, hinge, CD8 transmembrane, the cytoplasmic domain of human CD28, 4-1BB and CD3ζ, P2A, and mCherry were synthesized by a vendor (Genecreate, China). The CAR sequences were subcloned into a lentivirus vector. Vehicle control was constructed by substituting B7-H3 scFv sequence with CD19 scFv.
Manufacture of Lentivirus
HEK293T cells were co-transfected with the CAR vector, pMDLg/pRRE (Addgene plasmid#12251), pRSV-Rev (Addgene plasmid #12253), and pMD2.G (Addgene plasmid #12259) by using polyetherimide (PEI; Roche Applied Science). The supernatant was collected at 36 h and 60 h and then centrifuged at 15,000 rpm for 2 h at 4°C. The pellet was resuspended in cold RPMI medium and stored at −80°C.
Primary Human Lymphocytes and Transduction of T Cells
On day 1, peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation (800 × g for 15 min at room temperature) using Lymphoprep (Greiner Bio-One). Peripheral blood mononuclear cells cultured in X-vivo medium (Lonza) were stimulated with CD3 monoclonal antibody (mAb) (OKT3, 200 ng/mL, BioLegend), CD28 mAb (CD28.2, 100 ng/mL, BioLegend), and recombinant human IL-2 (100 units/mL, Life Science) at 37°C in a humidified atmosphere with 5% CO2. On day 3, anti-CD3 (OKT3)/anti-CD28 activated T cells were transduced with lentivirus (MOI 3–10) over the recombinant fibronectin fragment (Novoprotein) in the presence of IL-2. After 12 h, T cells were collected and cultured in the presence of 100 U/mL IL-2 for 8–10 days before use. Vehicle control T cells were established in the same conditions, and NT cells were stimulated with CD3 and CD28 mAb but not transduced with lentivirus.
Cytotoxicity Assays
Cytotoxicity of T cells was examined by using 51Cr assay as previously described elsewhere.32 In brief, 1 × 106 cells/mL target cells (tumor cells) labeled with sodium chromate (molecular formula, Na251CrO4) were incubated with effector cells (T cells) at an E:T ratio of 32:1, 16:1, 8:1, and 4:1. After 4 h, the supernatants were collected, and the radioactivity was examined by a gamma counter. The percentage of specific lysis was calculated by the following formula: (test release − spontaneous release)/(maximal release − spontaneous release) × 100.
Analysis of Cytokine Secretion
1.5 × 104 target cells were co-cultured with 6 × 104 effect cells (E:T ratio, 4:1) in a 96-well plate at 37°C without the addition of exogenous cytokines. Vehicle control T cells served as the negative control. After 12 h, the supernatant was collected to analyze the IFNγ and IL-2 secretion from the effector cells using ELISA kits (Thermo Fisher Scientific).
Animal Studies
For the orthotropic GBM model, NOD-SCID mice 8 to 10 weeks old were purchased from Beijing Vital River Laboratory Animal Technology Company. Each mouse received an intracranial injection of 2 × 105 (GBM primary cells or U87-B7-H3) tumor cells in a volume of 5 μL at 1 μL/min using the stereotactic tumor establishment apparatus. 6–9 days after injection, progressively growing xenografts were confirmed by bioluminescence signal. Mice with established tumors were divided into different groups randomly (five mice/group). The group included vehicle and B7-H3-specific CAR-T cell treatment groups. Mice were injected with B7-H3 CAR-T cells or equivalent number of vehicle control T cells on day 10 (as indicated in Figure 5) after tumor injection. For bioluminescence imaging, mice were intraperitoneally injected with 150 mg/kg D-luciferin (Beyotime) and imaged using the IVIS system (Caliper Life Sciences) 10 min after injection. The IVIS data was analyzed using Living Image software (Caliper Life Sciences). A “survival analysis” experiment was used to compare B7-H3-specific CAR with vehicle T cells on their antitumor effect. Mouse death served as the endpoint of the experiment.
Statistical Analysis
Statistical tests were calculated and conducted by GraphPad software. For RNA-seq analysis of B7-H3 expression in glioma, unpaired t tests were used (*p < 0.05, **p < 0.01, ***p < 0.001). For survival analysis of LGG and GBM patients and orthotropic GBM models, the survival curve was obtained by Kaplan-Meier plot, and a log rank test was used. p < 0.05 was served as a level to designate significant differences. For cytokine and cytolysis in vitro experiments (n = 3 wells), the experiments were repeated twice.
Study Approval
The animal experiments were approved by the West China Hospital of Sichuan University Biomedical Ethics Committee, and all experiments conformed to all relevant regulatory standards. Primary tumor samples obtained from patients with GBM and blood samples from GBM patients and healthy donors were also approved by West China Hospital of Sichuan University Biomedical Ethics Committee (ethical approval document 2018-061). Written informed consent was obtained from patients with GBM and healthy donors.
Author Contributions
X.T. and S.Z. carried out experiments, acquisition of data, and the draft the manuscript. Y.W. and Y.Z. participated in sample collection and animal experiments. Z.Z., M.Y., Y.Z., and G.Z. participated in acquisition of data and study supervision. G.G. participated in critical revision of the manuscript. A.T. and L.Z. conceived and designed the experiment.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31471286 and 81772693), the National Major Scientific and Technological Special Project for Significant New Drugs Development (2015ZX09102010), and the Postdoctoral Research Fund of Sichuan University (2018SCU12035).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omto.2019.07.002.
Contributor Information
Aiping Tong, Email: aipingtong@scu.edu.cn.
Liangxue Zhou, Email: liangxue_zhou@126.com.
Supplemental Information
References
- 1.Oike T., Suzuki Y., Sugawara K., Shirai K., Noda S.E., Tamaki T., Nagaishi M., Yokoo H., Nakazato Y., Nakano T. Radiotherapy plus concomitant adjuvant temozolomide for glioblastoma: Japanese mono-institutional results. PLoS ONE. 2013;8:e78943. doi: 10.1371/journal.pone.0078943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster S.J., Svoboda J., Chong E.A., Nasta S.D., Mato A.R., Anak Ö., Brogdon J.L., Pruteanu-Malinici I., Bhoj V., Landsburg D. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow K.K., Naik S., Kakarla S., Brawley V.S., Shaffer D.R., Yi Z., Rainusso N., Wu M.F., Liu H., Kew Y. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol. Ther. 2013;21:629–637. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hegde M., Mukherjee M., Grada Z., Pignata A., Landi D., Navai S.A., Wakefield A., Fousek K., Bielamowicz K., Chow K.K. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Invest. 2016;126:3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Migliorini D., Dietrich P.Y., Stupp R., Linette G.P., Posey A.D., Jr., June C.H. CAR T-Cell Therapies in Glioblastoma: A First Look. Clin. Cancer Res. 2018;24:535–540. doi: 10.1158/1078-0432.CCR-17-2871. [DOI] [PubMed] [Google Scholar]
- 7.Bagley S.J., Desai A.S., Linette G.P., June C.H., O’Rourke D.M. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro-oncol. 2018;20:1429–1438. doi: 10.1093/neuonc/noy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohno M., Natsume A., Ichiro Iwami K., Iwamizu H., Noritake K., Ito D., Toi Y., Ito M., Motomura K., Yoshida J. Retrovirally engineered T-cell-based immunotherapy targeting type III variant epidermal growth factor receptor, a glioma-associated antigen. Cancer Sci. 2010;101:2518–2524. doi: 10.1111/j.1349-7006.2010.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed N., Salsman V.S., Kew Y., Shaffer D., Powell S., Zhang Y.J., Grossman R.G., Heslop H.E., Gottschalk S. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin. Cancer Res. 2010;16:474–485. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krebs S., Chow K.K., Yi Z., Rodriguez-Cruz T., Hegde M., Gerken C., Ahmed N., Gottschalk S. T cells redirected to interleukin-13Rα2 with interleukin-13 mutein--chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Rα1. Cytotherapy. 2014;16:1121–1131. doi: 10.1016/j.jcyt.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberger P., Majdic O., Derdak S.V., Pfistershammer K., Kirchberger S., Klauser C., Zlabinger G., Pickl W.F., Stöckl J., Knapp W. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J. Immunol. 2004;172:2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 12.Castellanos J.R., Purvis I.J., Labak C.M., Guda M.R., Tsung A.J., Velpula K.K., Asuthkar S. B7-H3 role in the immune landscape of cancer. Am. J. Clin. Exp. Immunol. 2017;6:66–75. [PMC free article] [PubMed] [Google Scholar]
- 13.Lee Y.H., Martin-Orozco N., Zheng P., Li J., Zhang P., Tan H., Park H.J., Jeong M., Chang S.H., Kim B.S. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27:1034–1045. doi: 10.1038/cr.2017.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo L., Zhu G., Xu H., Yao S., Zhou G., Zhu Y., Tamada K., Huang L., Flies A.D., Broadwater M. B7-H3 Promotes Pathogenesis of Autoimmune Disease and Inflammation by Regulating the Activity of Different T Cell Subsets. PLoS ONE. 2015;10:e0130126. doi: 10.1371/journal.pone.0130126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seaman S., Zhu Z., Saha S., Zhang X.M., Yang M.Y., Hilton M.B., Morris K., Szot C., Morris H., Swing D.A. Eradication of Tumors through Simultaneous Ablation of CD276/B7-H3-Positive Tumor Cells and Tumor Vasculature. Cancer Cell. 2017;31:501–515.e8. doi: 10.1016/j.ccell.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theruvath J., Heitzeneder S., Majzner R., Graef C.M., Cui K., Nellan A., Cheshier S.H., Mackall C., Mitra S. Checkpoint Molecule B7-H3 Is Highly Expressed on Medulloblastoma and Proves to Be a Promising Candidate for Car T Cell Immunotherapy. Neuro Oncol. 2017;19(Suppl 4):iv28–iv29. [Google Scholar]
- 17.Souweidane M.M., Kramer K., Pandit-Taskar N., Zhou Z., Haque S., Zanzonico P., Carrasquillo J.A., Lyashchenko S.K., Thakur S.B., Donzelli M. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018;19:1040–1050. doi: 10.1016/S1470-2045(18)30322-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majzner R.G., Theruvath J.L., Nellan A., Heitzeneder S., Cui Y., Mount C.W., Rietberg S.P., Linde M.H., Xu P., Rota C. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin. Cancer Res. 2019;25:2560–2574. doi: 10.1158/1078-0432.CCR-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du H., Hirabayashi K., Ahn S., Kren N.P., Montgomery S.A., Wang X., Tiruthani K., Mirlekar B., Michaud D., Greene K. Antitumor Responses in the Absence of Toxicity in Solid Tumors by Targeting B7-H3 via Chimeric Antigen Receptor T Cells. Cancer Cell. 2019;35:221–237.e8. doi: 10.1016/j.ccell.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nygren M.K., Tekle C., Ingebrigtsen V.A., Mäkelä R., Krohn M., Aure M.R., Nunes-Xavier C.E., Perälä M., Tramm T., Alsner J. Identifying microRNAs regulating B7-H3 in breast cancer: the clinical impact of microRNA-29c. Br. J. Cancer. 2014;110:2072–2080. doi: 10.1038/bjc.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crispen P.L., Sheinin Y., Roth T.J., Lohse C.M., Kuntz S.M., Frigola X., Thompson R.H., Boorjian S.A., Dong H., Leibovich B.C. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin. Cancer Res. 2008;14:5150–5157. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newick K., O’Brien S., Moon E., Albelda S.M. CAR T Cell Therapy for Solid Tumors. Annu. Rev. Med. 2017;68:139–152. doi: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 23.Debinski W., Gibo D.M., Hulet S.W., Connor J.R., Gillespie G.Y. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin. Cancer Res. 1999;5:985–990. [PubMed] [Google Scholar]
- 24.Jarboe J.S., Johnson K.R., Choi Y., Lonser R.R., Park J.K. Expression of interleukin-13 receptor alpha2 in glioblastoma multiforme: implications for targeted therapies. Cancer Res. 2007;67:7983–7986. doi: 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- 25.Harms U., Li N., Rouault M., Wilkens K., Monroe R., Ma X.J., Park E. In-situ detection and characterization of EGFR and EGFRvIII expression heterogeneity in glioblastoma FFPE tissues. Ann. Oncol. 2017;28(Suppl 7) vii17. [Google Scholar]
- 26.O’Rourke D.M., Nasrallah M.P., Desai A., Melenhorst J.J., Mansfield K., Morrissette J.J.D., Martinez-Lage M., Brem S., Maloney E., Shen A. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017;9:eaaa0984. doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown C.E., Alizadeh D., Starr R., Weng L., Wagner J.R., Naranjo A., Ostberg J.R., Blanchard M.S., Kilpatrick J., Simpson J. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krenciute G., Prinzing B.L., Yi Z., Wu M.F., Liu H., Dotti G., Balyasnikova I.V., Gottschalk S. Transgenic Expression of IL15 Improves Antiglioma Activity of IL13Rα2-CAR T Cells but Results in Antigen Loss Variants. Cancer Immunol. Res. 2017;5:571–581. doi: 10.1158/2326-6066.CIR-16-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin L., Ge H., Long Y., Yang C., Chang Y.E., Mu L., Sayour E.J., De Leon G., Wang Q.J., Yang J.C. CD70, a novel target of CAR T-cell therapy for gliomas. Neuro-oncol. 2018;20:55–65. doi: 10.1093/neuonc/nox116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellegatta S., Savoldo B., Su C., Ferrone S., Finocchiaro G., Dotti G. Chondroitin sulfate proteoglycan 4 (CSPG4)-redirected T Cells eliminate glioblastoma-derived neurospheres. Mol. Ther. 2016;24(Suppl 1) S202. [Google Scholar]
- 31.Ahmed M., Cheng M., Zhao Q., Goldgur Y., Cheal S.M., Guo H.F., Larson S.M., Cheung N.K. Humanized Affinity-matured Monoclonal Antibody 8H9 Has Potent Antitumor Activity and Binds to FG Loop of Tumor Antigen B7-H3. J. Biol. Chem. 2015;290:30018–30029. doi: 10.1074/jbc.M115.679852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottschalk S., Edwards O.L., Sili U., Huls M.H., Goltsova T., Davis A.R., Heslop H.E., Rooney C.M. Generating CTLs against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive immunotherapy of EBV-associated malignancies. Blood. 2003;101:1905–1912. doi: 10.1182/blood-2002-05-1514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.