Abstract
The best approach to adverse-event review in solid organ transplantation is unknown. We initiated a departmental case review (DCR) method based on root-cause analysis methods in a high-volume multiorgan transplant center. We aimed to describe this process and its contributions to process improvement.
Methods
Using our prospectively maintained transplant center quality portfolio, we performed a retrospective review of a 30-month period (October 26, 2015, to May 14, 2018) after DCR-process initiation at our center. We used univariate statistics to identify counts of adverse events, DCRs, death and graft-loss events, and quality improvement action-plan items identified during case review. We evaluated variation among organ groups in action-plan items, associated phase of transplant care, and quality improvement theme.
Results
Over 30 months, we performed 1449 transplant and living donor procedures with a total of 45 deaths and 31 graft losses; 91 DCRs were performed (kidney transplant n=43; liver transplant n=24; pancreas transplant n=10; heart transplant n=6; lung transplant n=3; living donor n=5). Seventy-nine action-plan items were identified across improvement domains, including errors in clinical decision making, communication, compliance, documentation, selection, waitlist management, and administrative processes. Median time to review was 83 days and varied significantly by program. Median time to action-plan item completion was 9 weeks. Clinical decision making in the pretransplant phase was identified as an improvement opportunity in all programs.
Conclusions
DCRs provide a robust approach to transplant adverse-event review. Quality improvement targets and domains may vary based on adverse-event profiles.
Abbreviations and Acronyms: CMS, Centers for Medicare and Medicaid Services; DCR, Departmental Case Review; M&M, Morbidity and Mortality Conference; OPTN, Organ Procurement and Transplantation Network; QAPI, Quality Assurance and Performance Improvement
Transplant centers have a fiduciary duty and regulatory obligation to review adverse clinical events that occur within transplant programs. This fiduciary duty is rooted in the commitment physicians and surgeons make to transplant patients when waitlisting candidates, care of living organ donors, accepting organs, performing transplant procedures, and in conducting postoperative care. Clinicians carry the responsibility to learn from poor outcomes at all phases of transplant care and implement strategies to prevent these from recurring.1 In the United States, this fiduciary duty has transitioned from an ethical obligation to a regulatory requirement. Both the Centers for Medicare and Medicaid Services (CMS) and the Organ Procurement and Transplantation Network (OPTN) require transplant programs to maintain quality assurance and performance improvement (QAPI) programs directed toward clinical and nonclinical transplant activities.2, 3, 4 Transplant program QAPI requirements mandate review of adverse events, including patient deaths and graft losses, with the intent to change practice through the implementation of better care processes.2, 3, 4
Despite these regulatory requirements, transplant professionals lack guidelines on how best to review adverse events after transplant. Most adverse events are reviewed in a traditional surgical mortality and morbidity (M&M) conference,5 which poses a number of problems in the transplant setting. These conferences typically cover surgical decision making and may follow surgical cultural norms including assigning blame to individuals.6, 7, 8 Although it is critical to understand errors in judgment and technique at an individual provider level, traditional M&M conferences do not capture the totality of transplant decision making from the initial point of evaluation until the adverse event nor are the perspectives of the entire transplant team considered, including nursing, social work, and administrative personnel. Further, traditional M&M often fails to capture system-based factors that are critical to the occurrence of an event. This approach to adverse-event review lacks the nuance needed to facilitate improvement in multidisciplinary transplant processes and quality.9
Recently, a few transplant programs have explored new methods to review adverse events. Perkins et al describe a systematic review of all patient deaths over a 5-year period using root-cause analysis techniques in a liver transplant program, but the approach was not used to identify contributing factors in individual events.10 In our transplant program, we have initiated departmental case reviews (DCRs) to review individual adverse events systematically, based on theories applied in root-cause analysis.11 Root-cause analysis is an approach widely used across industries to evaluate failures in complex systems when an adverse event occurs.12 The goal of a DCR is to provide a comprehensive understanding of a patient's clinical course, using a timeline that outlines all phases of care and to create system-based solutions derived from multidisciplinary input. Through a facilitated discussion, DCRs aim to identify themes leading to adverse events, categorize them in domains, and create process improvement action-plan items to improve care.
In this analysis, we present our experience with the use of DCRs in a high-volume multiorgan transplant center for all adverse events (posttransplant patient deaths within 1 year, graft losses within 1 year, and other significant patient safety events) that occurred within 1 year after solid organ transplantation. This analysis, based on Standard for Quality Improvement Reporting Excellence (SQUIRE) 2.0 guidelines for describing health care improvement studies,13 may help other transplant programs develop platforms for adverse-event review that lead to process improvements, improvements in safety culture, and meet the standards of regulatory scrutiny.
Methods
Context
The goal of conducting DCRs in our transplant center was to improve how adverse events are reviewed. We theorized that a standardized approach to adverse-event review would yield consistent and meaningful action-plan items aimed at process improvement in multiple domains.
Intervention: Departmental Case Review Process
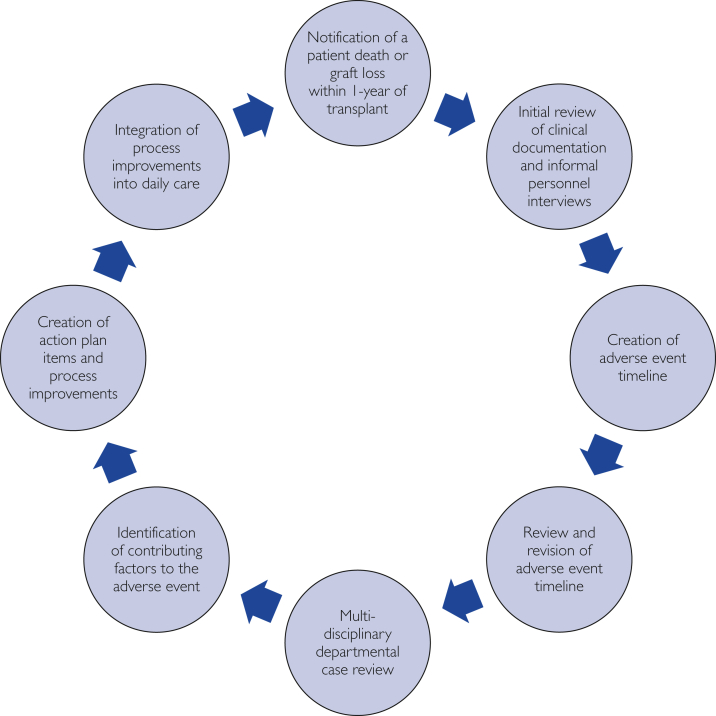
The DCR process is displayed in Figure 1. Adverse-event review is an integral part of patient safety efforts at our institution. We defined adverse events to include patient deaths, graft losses, and other significant patient-safety events. The institution has adopted a patient safety incident-review process to triage events and direct various levels of adverse-event review. DCRs in our transplant center are triggered by referral from transplant providers (physicians, surgeons, nurses, social workers) directly to transplant center QAPI personnel (2 dedicated quality and compliance nurses, 1 quality improvement advisor with expert training in quality improvement methodologies) or through the hospital-wide patient safety event-reporting system. By rule in our institutional transplant QAPI plan, all deaths and graft losses that occur within 1 year of transplant will qualify for at least a DCR or a higher level of review such as root-cause analysis, based on committee review. Other significant patient safety events, such as donor-derived transmission of disease, aborted transplant procedure, near-miss events, or others can be reviewed using DCRs. An experienced clinical risk-management officer (senior risk-management officer with a clinical nursing background) facilitates DCRs. Initial review steps include informal interviews with clinical personnel involved in the event and the creation of an event timeline, beginning at transplant evaluation. Event timelines are exhaustive: all aspects of transplant candidate or donor evaluation (clinical and nonclinical), waitlisting information for candidates, postevaluation clinical events and history, laboratory and diagnostic testing, medical documentation, deceased donor history and evaluation for applicable transplant recipients, operative procedure and/or perioperative care, posttransplant/donation management, and other information are included. The timeline is reviewed in a short small-group session (30 minutes) with a facilitator, transplant program leadership, and involved transplant clinicians. This timeline is then used in an all-inclusive multidisciplinary meeting involving all members of the transplant team to guide discussions of the event and the patient course. All members of the team are involved in the process, including transplant physicians; surgeons; pretransplant and posttransplant nurse coordinators; social workers; pharmacists; clinical dieticians; and other groups and individuals by invitation, based on the event (ie, critical care personnel for an event in the intensive care unit, anesthesia providers for intraoperative events). The DCR meeting is typically 30 to 60 minutes long and is used to identify factors contributing to the event and related themes in a systems-based context. Action-plan items aimed at process improvement are created by group consensus and assigned to small teams. Various quality improvement methodologies are applied to complete action plan items including PDSA (Plan-Do-Study-Act), DMAIC (Define, Measure, Analyze, Improve, Control), and Lean methods after DCR.14 All transplant center personnel receive formal didactic training on quality improvement methodologies through the Mayo Clinic Quality Academy and in-project training by a quality improvement advisor in our transplant center. The goal of this process is to integrate new and improved processes into daily care.
Figure 1.
The departmental case review process for adverse-event review. The review of adverse events within 1 year of transplant follows a precisely structured iterative process. The process begins with the occurrence and notification of an adverse event, including patient deaths, graft losses, and other patient-safety events. This process includes exhaustive narrative timeline review, multidisciplinary discussion, and creation of action-plan items directed to process improvement, using quality improvement methods.
Study of Intervention, Measures, and Analyses
The DCR process was initiated October 26, 2015, at our center. We retrospectively reviewed a prospectively maintained institutional database containing all transplant DCR data including date of events and review, graft type, action-plan items, and dates of resolution of action-plan items. Multiorgan transplants were classified by the leading organ; that is, simultaneous liver-kidney transplant events were counted as liver events, simultaneous pancreas-kidney events were classified under pancreas, simultaneous heart-kidney transplants were classified as heart events. This analysis was terminated after 30 months, on May 14, 2018. Action-plan items are classified in our program in domains, including improvements in clinical care, administrative, inter- and intradisciplinary communication, clinical documentation, and candidate selection processes. Clinical care improvements are process improvements aimed at supporting clinical decision making such as developing a guideline for posttransplant immunosuppression in previous cancer patients or having 2 physicians review an organ offer before decline. Communication improvements are creating processes of disseminating information among transplant team members about patients' condition, transplant logistics, or policies. Clinical documentation improvements are related to standardization of documentation for clinical ease of use and regulatory compliance. Administrative improvements are related to operational issues such as making available clinic space to accommodate multiple clinic visits for high-risk patients. Univariate statistics were used to identify trends in department case-review data, with the goal of providing a quantitative description of these activities.
This analysis was performed as a shared quality improvement activity across our transplant programs and therefore did not require institutional review board approval. Analyses were completed using Microsoft Excel (Microsoft, Redmond, WA) and JMP version 13.0 (SAS Institute, Cary, NC). The review of data and report were conducted based on SQUIRE 2.0 guidelines.13 No ethical conflicts or issues were identified in the conduct of DCRs or in the review of DCR data.
Results
Table displays the descriptive statistics for DCRs conducted over a 30-month period; 1449 solid organ transplants and living donation procedures occurred during this period (n=1292 transplants—794 kidney transplants, 306 liver transplants, 58 pancreas transplants, 129 heart transplants, 5 lung transplants—and n=157 living donor procedures). Forty-five patient deaths with functioning graft and 31 graft losses occurred across the kidney, pancreas, liver, heart, lung transplant, and living-donor programs that employed DCRs. Overall, departmental reviews were conducted in 91 cases, reflecting the use of DCRs for other patient-safety events. The majority of events were patient deaths. The kidney transplant program—the largest program by volume—had 40 adverse events, followed by the liver program (n=22) and pancreas program (n=7). The thoracic transplant programs had the lowest numbers of total events. Overall, 6.8% of all solid organ transplants had death or graft losses during this period. By ratio, the lung transplant program had the highest ratio of adverse events to transplants. Fifteen other patient safety events required DCRs. In kidney and pancreas transplant groups, 6 reviews were related to these types of patient safety events and included issues such as donor-derived transmission of infectious disease and cancellation of transplant related to transportation issues. In liver transplant patients, 2 DCRs were conducted related to these types of events, such as suspected donor-derived transmission of malignancy. Two DCRs were conducted in the heart program related to receipt of an unnecessary transjugular myocardial biopsy; the other was related to a patient fall with injury. Five DCRs were conducted for other patient-safety events in living donors, which included reviews of serious surgical complications requiring invasive intervention, nonelective conversion from laparoscopic to open nephrectomy, postoperative narcotic abuse, and pregnancy-testing practices in living donors.
Table.
Descriptive Statistics on Departmental Case Reviews Conducted From October 26, 2015 to May 14, 2018
| Kidney Trans-plant Program | Liver Transplant Programa | Pancreas Transplant Programb | Heart Transplant Programc | Lung Transplant Program | Living Donor Program | All Events | |
|---|---|---|---|---|---|---|---|
| Total procedural volume | 794 | 306 | 58 (49 KP, 9 Pancreas Transplant Alone) | 129 | 5 | 157 (150 Kidney); 6 Liver | 1449 |
| Total events | 43 | 24 | 10 | 6 | 3 | 5 | 91 |
| Departmental case reviews | 43 | 24 | 10 | 6 | 3 | 5 | 91 |
| 1-year patient deaths with functioning graft | 22 | 15 | 1 | 4 | 3 | 0 | 45 |
| 1-year graft losses | 18 | 7 | 6 | 0 | 0 | 0 | 31 |
| Other Patient Safety Events | 3 | 2 | 3 | 2 | 0 | 5 | 15 |
| Adverse-event rate (number of events/100 cases) | 5.4 | 7.8 | 17.2 | 3.8 | 60 | 3.2 | 6.8 |
| Median time from adverse event to review (days) | 91 | 62 | 140 | 54 | 20 | 13 | 83 |
| Action-plan items (total) | 38 | 19 | 2 | 12 | 1 | 7 | 79 |
| Action-plan items per review | 0.88 | 0.79 | 0.20 | 2.40 | 3.00 | 1.40 | 0.84 |
| Median time to action item completion (weeks) | 16 | 9 | 22 | 66 | 28 | 6 | 9 |
1 event occurred in a simultaneous liver-kidney (SLK) transplant.
6 events occurred in a simultaneous kidney-pancreas (SPK) transplant, 1 in pancreas transplant alone.
1 event occurred in a simultaneous heart-kidney transplant.
The median time to review of events across all organ groups was 83 days. Departmental reviews yielded 79 action plan items directed toward process improvement. The majority of these occurred as a result of kidney transplant departmental reviews (n=38). The lung transplant program had the most action plan items per review, at 3.0 items per review. Action plan items took a considerable time to complete, with a median of 9 weeks. There were significant differences between programs in the time to action plan completion.
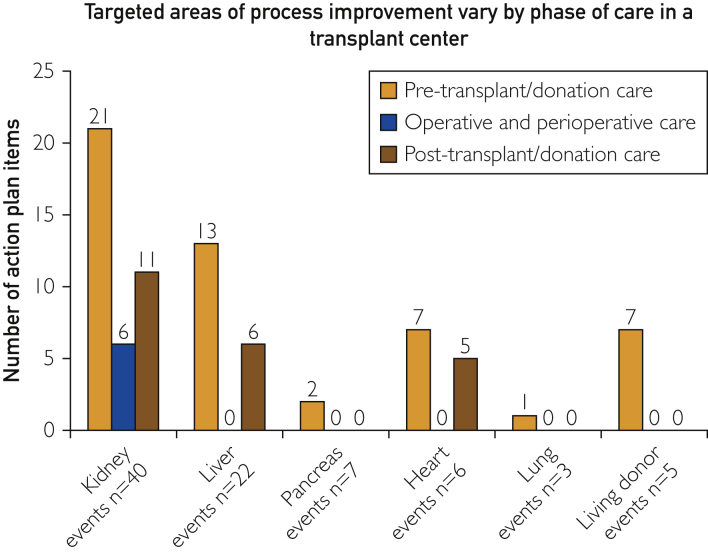
Figure 2 captures the targeted transplant/donation phase for action plan items created in DCRs. In each organ group, the most process improvement action plan items were targeted at pretransplant/donation care (n=50). Posttransplant/donation care action plan items included 22 individual items across all organ groups. Notably, there were no transplant/donation phase or perioperative action plan items in liver, pancreas, heart, lung, or living donors. Six items were targeted toward surgical processes in the kidney transplant program.
Figure 2.
Targeted areas of process improvement vary by phase of care in a transplant center. This figure demonstrates the distribution of action-plan items derived from all departmental case reviews in the study period. These data demonstrate that different phases of the transplant process were more targeted for certain organ-transplant groups than others.
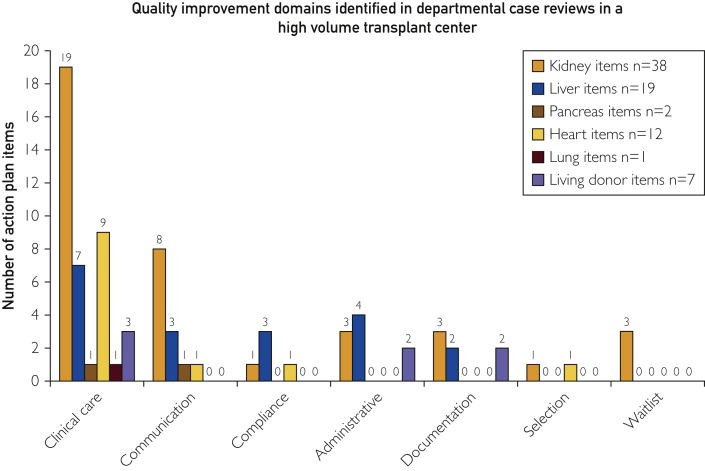
The distribution of quality improvement action item domains varied significantly by program (Figure 3); 79 action plan items were categorized. Errors in clinical care and/or clinical decision making had the highest number of attributable adverse events (n=40) and was the leading focus of action plan items in all programs. Some action plan items in this domain include examples of these include guideline development for immunosuppression in previous cancer patients and standardization of nutritional assessment to recognize extent of malnutrition. Other areas targeted for improvement included lapses in communication (create a posttransplant team huddle to reduce communication failures between providers regarding struggling patients) (n=13), administrative process failures (n=9) (standardization of workflow to schedule post-transplant annual return visits), and clinical documentation (standardization of selection conference notes to document critical findings) (n=7). Other action plan items included protocol deviations (n=5) (violation of selection guidelines in listing high cardiovascular risk patients), waitlist management errors (n=3) (workflow standardization to update waiting-list status related to a failure to inactivate a patient on the transplant waiting list), and candidate selection-process errors (n=2) (identifying patient preferences related to blood transfusion during selection process). By program, kidney transplant action-plan items were focused on clinical care and communication (n=27/38 items) but identified errors in all process domains. The liver transplant program identified errors in 5 of the 7 domains identified; 75% of the heart transplant program's action plan items were directed at clinical care, but errors were found in 4 of the 7 domains. Living donor program items were focused on clinical care and clinical documentation.
Figure 3.
Quality improvement domains identified in departmental case reviews in a high-volume transplant center. Action plan items were developed and thematically defined to help transplant center personnel understand where quality assurance and performance improvement (QAPI) efforts are targeted. In each organ group, the majority of action plan items were directed toward clinical care improvements, but a significant percentage were associated with intra- and interdisciplinary communication.
Discussion
Transplant centers must adopt a culture of continuous quality improvement to improve care. Despite improvements in outcomes in clinical transplantation over time, patient deaths and graft losses continue to occur within the first year after transplant. In this analysis, we have analyzed our experience with the use of DCRs to improve processes after adverse events. Over a 30-month period in a high-volume multiorgan transplant center, 45 deaths and 31 graft losses occurred, prompting DCRs that addressed a multitude of domains in all phases of transplant care. These DCRs yielded 79 action-plan items as targets for performance improvements. Clinical decision making has been the target of improvement in the majority of cases, but gaps in several other domains were targeted as well, including communication among personnel, problems with clinical documentation, administrative process failures, and failure to comply with internal care protocols. To date, this has led to discrete improvements in care processes across our transplant center using quality improvement methodologies including, PDSA, and Lean.
Although root-cause analysis methods have been present and shown effectiveness in health care for years,15, 16, 17 the use of DCR process to study adverse events comprehensively is a relatively novel approach in clinical transplantation.10 Adverse- event review in clinical transplantation historically takes its direction from the culture of surgical M&M conferences.5 DCRs have several advantages over traditional M&M conferences. DCRs involve a systematic review of the entirety of the transplant process; use of a linear timeline, beginning at the time of evaluation and concluding at the event of interest; and identification of systematic and human contributing factors. Quality improvement is the primary aim of this approach. M&M conferences do not follow a standardized format and instead follow arbitrary institutional norms that do not necessarily focus on system-based contributors to complications. M&M conferences are aimed to enhance trainee education.18 They are aimed at physicians and do not involve all members of the multidisciplinary transplant care team. They do not carry the expectation of a deliverable process improvement. DCRs encompass the clinical aspects of surgical M&M conference and go further; the goal is to actually improve processes across the system.
DCRs certainly have value but are resource intensive. Commitment to the use of this process for adverse-event review is predicated on having available personnel and time. Personnel are needed to help review the event and synthesize a timeline, and clinicians must be involved with reviewing this work, attendance of the review with the team, and support postreview quality and process improvement efforts. This can pose a problem for clinicians and other personnel in academic transplant centers. The commitment required for these activities is usually unfunded. Clinician time is stretched, owing to other demands and institutional-mission based activities, including education and research. Personnel to facilitate these reviews include individuals who are experienced in clinical care, risk management, and root-cause analyses in health care. Most importantly, for this process to be successful, transplant programs must create a culture committed to this level of adverse-event review and impart the significance of these activities to their hospital administrations. They must safeguard the process to ensure that the approach is honest, open, and accounts for the system-wide view as opposed to singular cause and blame; they must ensure that interventions have appropriate feedback loops and that the process is not subject to political hijacking.12 These can lead to an erosion of DCR or root-cause analysis quality. For transplant programs, CMS and OPTN have specific requirements related to quality monitoring and performance improvement. Appropriate documentation of the DCR process more than fulfills these obligations. In this context, we believe that DCRs provide a return on investment; financial and effort investment in this process can lead to process improvements and exceed the standard of regulatory review.
This analysis and root-cause analysis methods used in DCRs have limitations. When it comes to determining the optimal approach to adverse-event review, an important question is whether the approach actually improves quality as measured by a reduction in the number of graft losses or patient deaths. As clinicians understand, there are multiple factors that contribute to these events, including graft- and recipient-acceptance practices, surgical techniques and complexity, posttransplant management, and processes of care that link these together. Adverse events can occur as a result of process failures but also can occur at random. In this context, there are multiple challenges in trying to demonstrate if our approach to reviewing adverse events reduces future events. However, our approach to quality improvement is simple; we believe that the iterative approach to fine-tuning clinical and administrative process optimizes institutional conditions for a good clinical outcome. We did not include any instruments to assess patient-safety culture before and after institution of this approach, so it is difficult to assess directly what effect DCRs have had on transplant patient safety culture in our organization. Anecdotal comments from our team, however, remain positive toward this approach. Multiple members of the team have engaged in quality improvement activities and training after these reviews. Critics have opined that root-cause analysis methods in health care lack teeth, as they are typically applied by local teams that are biased in favor of their system; failure to admit human factors contributing to an event; or creating small, ineffective action plans that do not have impact on event rates.12, 19 Finally, DCRs are a resource-intense activity that may limit its generalizability, which is an important concern. We believe the DCR approach could be disseminated and used in a variety of transplant and nontransplant settings. As can be surmised from this study, DCRs require quality improvement personnel resources, clinician engagement, time, and a vibrant quality improvement culture. In the United States transplant context, regulatory bodies actually require the presence of these resources aimed at quality improvement in transplant programs. This makes application of the DCR approach inherently achievable for active transplant programs. For health care units outside of transplant, it is important to realize that DCRs do require an infrastructure and culture to execute effectively. Although there may be weaknesses in the use of root-cause analyses methods, it is only by implementation of these methods that they can be improved.
Conclusion
Adverse-event review in clinical transplantation is a critically important activity to maintaining transplant center quality. The current regulatory environment in transplantation requires the demonstration of process improvement as a result of adverse events. By using root-cause analysis methods, DCRs dissect the entire course of the transplant patient, and care decisions and broken processes can be linked to the adverse event. These reviews create an environment aimed at improving the system of transplant care. Transplant centers should consider modernizing adverse-event review by using this process, as it has several advantages over traditional M&M conferences and can lead to tangible changes in transplant care.
Footnotes
Grant Support: This study was supported by internal funds only.
Potential Competing Interests: The authors report no competing interests.
References
- 1.Institute of Medicine . National Academy Press; Washington DC: 2001. Crossing the Quality Chasm: A New Health System for the Twenty-first Century. [Google Scholar]
- 2.Medicare program; hospital conditions of participation: requirements for approval and re-approval of transplant centers to perform organ transplants: final rule. Fed Regist. 2007;72(61):15197–15280. [PubMed] [Google Scholar]
- 3.Organ Procurement and Transplantation Network Bylaws. 2017. https://optn.transplant.hrsa.gov/governance/bylaws/ [Google Scholar]
- 4.Reich D.J. Quality assessment and performance improvement in transplantation: hype or hope? Curr Opin Organ Transplant. 2013;18(2):216–221. doi: 10.1097/MOT.0b013e32835f3fcf. [DOI] [PubMed] [Google Scholar]
- 5.Gregor A., Taylor D. Morbidity and Mortality Conference: its purpose reclaimed and grounded in theory. Teach Learn Med. 2016;28(4):439–447. doi: 10.1080/10401334.2016.1189335. [DOI] [PubMed] [Google Scholar]
- 6.Harbison S.P., Regehr G. Faculty and resident opinions regarding the role of morbidity and mortality conference. Am J Surg. 1999;177(2):136–139. doi: 10.1016/s0002-9610(98)00319-5. [DOI] [PubMed] [Google Scholar]
- 7.Aboumatar H.J., Blackledge C.G., Jr., Dickson C., Heitmiller E., Freischlag J., Pronovost P.J. A descriptive study of morbidity and mortality conferences and their conformity to medical incident analysis models: results of the morbidity and mortality conference improvement study, phase 1. Am J Med Qual. 2007;22(4):232–238. doi: 10.1177/1062860607303292. [DOI] [PubMed] [Google Scholar]
- 8.Bosk C.L. 2nd ed. University of Chicago Press; Chicago, IL: 2003. Forgive and Remember: Managing Medical Failure. [Google Scholar]
- 9.Anderson J.E., Farmer D.L. A new era for the Morbidity and Mortality Conference: aligning tradition with systems-based quality improvement efforts. Acad Med. 2016;91(10):1330–1331. doi: 10.1097/ACM.0000000000001343. [DOI] [PubMed] [Google Scholar]
- 10.Perkins J.D., Levy A.E., Duncan J.B., Carithers R.L., Jr. Using root cause analysis to improve survival in a liver transplant program. J Surg Res. 2005;129(1):6–16. doi: 10.1016/j.jss.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Kellogg K.M., Hettinger Z., Shah M., et al. Our current approach to root cause analysis: is it contributing to our failure to improve patient safety? BMJ Qual Saf. 2017;26(5):381–387. doi: 10.1136/bmjqs-2016-005991. [DOI] [PubMed] [Google Scholar]
- 12.Peerally M.F., Carr S., Waring J., Dixon-Woods M. The problem with root cause analysis. BMJ Qual Saf. 2017;26(5):417–422. doi: 10.1136/bmjqs-2016-005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogrinc G., Davies L., Goodman D., Batalden P., Davidoff F., Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986–992. doi: 10.1136/bmjqs-2015-004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leaphart C.L., Gonwa T.A., Mai M.L., et al. Formal quality improvement curriculum and DMAIC method results in interdisciplinary collaboration and process improvement in renal transplant patients. J Surg Res. 2012;177(1):7–13. doi: 10.1016/j.jss.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Rex J.H., Turnbull J.E., Allen S.J., Vande Voorde K., Luther K. Systematic root cause analysis of adverse drug events in a tertiary referral hospital. Jt Comm J Qual Improv. 2000;26(10):563–575. doi: 10.1016/s1070-3241(00)26048-3. [DOI] [PubMed] [Google Scholar]
- 16.Bagian J.P., Gosbee J., Lee C.Z., Williams L., McKnight S.D., Mannos D.M. The Veterans Affairs root cause analysis system in action. Jt Comm J Qual Improv. 2002;28(10):531–545. doi: 10.1016/s1070-3241(02)28057-8. [DOI] [PubMed] [Google Scholar]
- 17.Iedema R.A., Jorm C., Braithwaite J., Travaglia J., Lum M. A root cause analysis of clinical error: confronting the disjunction between formal rules and situated clinical activity. Soc Sci Med. 2006;63(5):1201–1212. doi: 10.1016/j.socscimed.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Reines H.D., Trickey A.W., Donovan J. Morbidity and Mortality Conference is not sufficient for surgical quality control: processes and outcomes of a successful attending Physician Peer Review committee. Am J Surg. 2017;214(5):780–785. doi: 10.1016/j.amjsurg.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Trbovich P., Shojania K.G. Root-cause analysis: swatting at mosquitoes versus draining the swamp. BMJ Qual Saf. 2017;26(5):350–353. doi: 10.1136/bmjqs-2016-006229. [DOI] [PubMed] [Google Scholar]