Abstract
Hepatocellular carcinoma (HCC) arises in the context of cirrhosis and chronic hepatitis B virus (HBV) infections, and the diagnosis is often made at advanced stages. Because early-stage diagnosis improves survival, guidelines recommend screening patients at risk for HCC, such as patients with cirrhosis. However, adherence to screening programs is suboptimal. In this review, we discuss the value of HCC screening and provide practical guidance on patient selection and screening methods. International guidelines concordantly recommend HCC screening in patients with cirrhosis, including patients with HBV infections, hepatitis C virus infections with or without sustained virologic response, and nonalcoholic fatty liver disease. There is no consensus on screening patients without cirrhosis, although patients with advanced fibrosis, HBV infections, or nonalcoholic fatty liver disease without cirrhosis have an increased risk for development of HCC. Screening for HCC improves early tumor detection, receipt of curative treatment, and overall survival in at-risk patients. However, potential harms of HCC screening have not been well quantified. Semiannual abdominal ultrasonography is the screening modality of choice. Using ultrasonography in combination with biomarkers, such as α-fetoprotein, may increase accuracy for early HCC detection. The use of magnetic resonance imaging and computed tomography is limited by cost-effectiveness and practical considerations. Increased awareness of HCC screening will allow for earlier diagnosis and potentially curative treatment. We propose a comprehensive screening algorithm for patients at risk for development of HCC, recommending lifelong, semiannual ultrasonography combined with α-fetoprotein testing in patients with cirrhosis and selected patients without cirrhosis.
Abbreviations and Acronyms: AFP, α-fetoprotein; CT, computed tomography; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; MRI, magnetic resonance imaging; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; RCT, randomized controlled trial; SVR, sustained viral response
Article Highlights.
-
•
Early diagnosis of hepatocellular carcinoma improves survival, but adherence to screening programs is suboptimal.
-
•
International guidelines concordantly recommend screening patients with cirrhosis; consensus on screening patients without cirrhosis is lacking.
-
•
We recommend lifelong screening with semiannual ultrasonography and α-fetoprotein testing in patients with cirrhosis and selected patients without cirrhosis.
Liver cancer is the sixth most common cancer and second most common cause of cancer-related death worldwide.1 Hepatocellular carcinoma (HCC) accounts for 90% of all primary liver tumors. The prognosis of HCC is tightly linked to tumor stage, with the best survival seen in patients diagnosed at early stages. Whereas patients with early-stage HCC can undergo curative treatments and have 5-year survival rates exceeding 70%, there are no curative treatment options for advanced HCC. Survival is typically less than 1 or 2 years, depending on the underlying tumor burden, liver function, and performance status.
Hepatocellular carcinoma is most common in Asia and sub-Saharan Africa.1 However, recent reports have indicated that the incidence of HCC in East Asia is decreasing, given more widespread vaccination programs for hepatitis B virus (HBV).2 During this same time period, the incidence in developed countries, including Europe and the United States, has nearly doubled.3
Several professional societies recommend screening at-risk patients, including all patients with cirrhosis and subgroups of patients with chronic HBV infections.4, 5, 6, 7, 8, 9 Although these recommendations are largely based on expert opinion, they are supported by a large randomized controlled trial (RCT) in patients with chronic HBV, several cohort studies in patients with cirrhosis, and data showing that HCC screening fulfills the World Health Organization’s requirements for a screening program.10, 11, 12, 13
In spite of the global guidelines’ recommendations, HCC screening programs across the world are limited by low utilization rates.14, 15, 16, 17, 18, 19 Most educational efforts on the value of HCC screening to date have been aimed at subspecialists.20 However, most patients with cirrhosis seen outside tertiary care centers are followed up by primary care physicians, highlighting their central role in HCC screening. The purpose of this review is to discuss the value of HCC screening in at-risk patients with chronic liver disease. Although strictly speaking the term screening is used to describe the initial test and subsequent testing is termed surveillance, we use the colloquial term screening throughout this review.
The Value of HCC Screening
The value of cancer screening programs is based on a balance between screening benefits and harms. The best data for the benefits of HCC screening are derived from a large RCT that found that screening patients with chronic HBV improved early tumor detection (stage I, 60.5% vs 0%), receipt of curative treatment (resection, 46.5% vs 7.5%), and overall survival (37%; hazard ratio 0.63; 95% CI, 0.41-0.98) compared with not screening.10
However, level I data on screening patients with cirrhosis are lacking, largely related to perceived ethical challenges of enrolling patients with cirrhosis in an RCT for HCC screening. In a feasibility assessment for an RCT with informed consent, 99.5% of patients declined randomization and elected to participate in a screening program for HCC.21 Despite a lack of randomized data, several cohort studies have found a strong and consistent association between HCC screening and improved 3-year survival rates (odds ratio, 1.09; 95% CI, 1.67-2.17).13 The benefit persisted in the subset of studies that statistically adjusted for lead-time bias, suggesting there is likely a true benefit of screening patients with cirrhosis. A recent observational study comparing the survival of patients with HCC in Japan, where there is an intensive screening program using ultrasonography and α-fetoprotein (AFP) L3 fractions and des-gamma-carboxy prothrombin, and Hong Kong, where no program has been implemented, found higher survival in Japan (52 vs 17.8 months), mainly induced by earlier diagnosis permitting curative treatment options in more patients.11
Increasing attention is paid to the potential harms of screening, particularly in light of data suggesting harms in other cancer screening programs, such as prostate, breast, and lung cancer.22 Screening-related harms can include physical, financial, and psychological harms. A recent study suggested that up to one-third of patients with cirrhosis may experience physical harms that are related to false-positive and indeterminate screening results.23 However, most screening harms were additional diagnostic computed tomography (CT) or magnetic resonance imaging (MRI) examinations. There were few instances of severe physical harm, such as invasive procedures or procedure-related complications. Data evaluating psychological or financial harms of HCC screening are limited. Although further evaluation is needed to examine these harms, current data suggest that the benefits likely outweigh potential harms of HCC screening.
Patient Selection for Screening
Patients with Cirrhosis
Over 90% of HCCs in the Western world develop in the setting of cirrhosis, the end stage of any chronic liver injury.24 Patients with cirrhosis have an annual risk of 2% to 4% for development of HCC. Given this high risk, there is consensus among international professional society guidelines that HCC screening is recommended in all patients with cirrhosis, independent of liver disease etiology (Table).4, 5, 6, 7 The benefits of HCC screening are generally limited to patients with compensated cirrhosis (Child-Pugh class A or B). Given the lack of effective therapies, patients with Child-Pugh class C cirrhosis are typically not offered HCC screening unless they are listed for liver transplant, but an individualized screening approach is warranted. Furthermore, screening is generally not considered worthwhile in patients with a hepatic or nonhepatic disease resulting in a life expectancy of 12 months or less.
Table.
Recommended Screening Policies From International Guidelinesa
| Guideline | EASL5 | AASLD4 | JSH7 | APASL6 |
|---|---|---|---|---|
| Definition of high-risk population | • Pts with cirrhosis, Child-Pugh stage A and B | • Pts with cirrhosis, Child-Pugh stage A and B | • Extremely high-risk pts: | • Pts with cirrhosis |
| ○ Pts with cirrhosis and HBV or HCV | ||||
| • Pts with cirrhosis, Child-Pugh stage C awaiting liver transplant | • Pts with cirrhosis, Child-Pugh stage C awaiting liver transplant | • High-risk pts: | • Pts without cirrhosis with HBV: | |
| ○ Nonviral cirrhosis | ○ Asian females >50 y | |||
| • Pts without cirrhosis with HBV and an intermediate or high risk of HCC (PAGE-B score ≥10b) | • Pts without cirrhosis with HBV | ○ Pts without cirrhosis with HBV or HCV | ○ Asian males >40 y | |
| • Pts without cirrhosis with chronic HCV and bridging fibrosis | ○ Africans >20 y | |||
| ○ Family history of HCC | ||||
| Screening interval | • Every 6 mo | • Every 4-8 mo | • Every 3-4 mo in extremely high-risk pts | • Every 6 mo |
| • Every 6 mo in high-risk pts | ||||
| Imaging modality | • US (performed by experienced personnel) | • US | • US | • US |
| • CT/MRI optional every 6-12 mo in extremely high-risk pts | ||||
| Biomarkers | • Not recommended | • At discretion of physician | • AFP | • AFP (+ US) |
| • AFP-L3 fractions | ||||
| • DCP |
AASLD = American Association for the Study of Liver Diseases; AFP = α-fetoprotein; APASL = Asian Pacific Association for the Study of the Liver; CT = computed tomography; DCP = des-gamma carboxyprothrombin; EASL = European Association for the Study of the Liver; HBV = hepatitis B virus; HCC = hepatocellular carcinoma; HCV = hepatitis C virus; JSH = Japan Society of Hepatology; MRI = magnetic resonance imaging; PAGE-B = platelets, age, gender, hepatitis B; pts = patients; US = ultrasonography.
The PAGE-B score is calculated by scoring the patient’s age in years (16-29: 0 points; 30-39: 2 points; 40-49: 4 points; 50-59: 6 points; 60-69: 8 points; ≥70: 10 points), gender (female: 0 points; male: 6 points), and platelet count per mm3 (≥200,000: 0 points; 100,000-199,999: 6 points; <100,000: 9 points).25
There is regional variation in the importance of different risk factors for cirrhosis.24 Although HBV infections cause approximately 70% of HCC cases in Africa and East Asia, the majority of cases in the Western world and Japan are related to hepatitis C virus (HCV) infections. Although sustained viral response (SVR) after direct-acting antiviral treatment substantially reduces the risk of HCC in patients with cirrhosis, they remain at risk.26 In fact, HCC has even been reported a decade after SVR, despite improvement in portal hypertension and regression of fibrosis. Therefore, continued HCC screening is strongly recommended in patients with HCV cirrhosis, although the cost-effectiveness of this strategy is unclear.
Across the world, the contribution of nonalcoholic fatty liver disease (NAFLD), the hepatic manifestation of the metabolic syndrome, to the development of HCC is increasing.27, 28 In parallel with the obesity and diabetes epidemics, NAFLD-related cirrhosis is anticipated to become the most common cause of liver-related complications, including HCC, in the Western world in the near future.
Another common nonviral etiology of cirrhosis is alcohol-related cirrhosis.29 A meta-analysis found that heavy drinking (≥3 drinks per day) increased the risk of liver cancer by 16% compared with alcohol abstinence, with a linear relationship between the risk and the amount of alcohol intake.29 The Asia-Pacific guidelines also recommend screening patients with less common etiologies of cirrhosis, such as primary biliary cholangitis, hemochromatosis, and autoimmune hepatitis.6
Patients Without Cirrhosis
Advanced Fibrosis
There is debate about the value of HCC screening in patients with major fibrosis without cirrhosis. European professional society guidelines recommend screening these patients because it can be difficult to define the transition from advanced fibrosis to cirrhosis.5 This recommendation has not been included in American guidelines.4, 9
Noncirrhotic Chronic HBV
Chronic HBV infection is a well-recognized risk factor for HCC and accounts for most cases of HCC globally.24 Hepatocellular carcinoma screening has been reported to be cost-effective in patients with HBV without cirrhosis when the incidence is greater than 0.2% per year.5 Most guidelines restrict HCC screening to selected subgroups of patients without cirrhosis with HBV (Table).4, 5, 6, 7 Although antiviral treatment substantially reduces the risk of HCC in patients with chronic HBV infections, recent studies have reported persistent risk and a continued need for HCC screening.30, 31
Noncirrhotic NAFLD
Diabetes and obesity are known independent risk factors for the development of HCC and are also risk factors for the development of NAFLD.32, 33 There appears to be a common pathway via insulin resistance and the subsequent inflammatory cascade in the development of nonalcoholic steatohepatitis (NASH), the end stage of NAFLD.
Although it is well known that patients with NAFLD-related cirrhosis are at high risk of HCC, the risk for patients with noncirrhotic NAFLD is less clear. An increasing number of reports describe HCC in these patients.28, 34, 35 In a retrospective cohort study among 1500 US veterans, 13% of HCC cases developed in the absence of cirrhosis, with NAFLD being the main risk factor.36 Furthermore, it has been suggested that patients with noncirrhotic NAFLD have a higher mortality rate than patients with HCC at the background of cirrhotic NAFLD.28 However, most studies on this subject are case series or have been limited by selection bias, eg, by only including patients undergoing surgical resection, who typically have lower stages of fibrosis.28, 34, 37, 38
Despite data suggesting that noncirrhotic NAFLD may be a risk factor for the development of HCC, no guidelines recommend screening patients withNAFLD without cirrhosis (Table).4, 5, 6, 7 Currently, the incidence of HCC in patients with NAFLD without advanced fibrosis is unknown, so it is unclear if screening would be cost-effective on a population level.39
Screening Methods
Imaging
All guidelines advocate ultrasonography as the imaging modality of choice for HCC screening because it is inexpensive, noninvasive, readily available, fairly accurate, and well tolerated (Table).4, 5, 6, 7 However, the sensitivity of ultrasonography alone for detecting early-stage HCC in patients with cirrhosis is reported to be around 45%.40 The sensitivity of ultrasonography is affected by technology and operator experience. Thus, ultrasonographic screening should be performed in specialized centers by well-trained technicians and clinicians.5 Furthermore, certain areas of the liver, such as the hepatic dome, are more difficult to explore by ultrasonography.
The diagnostic accuracy of ultrasonography is also affected by patient characteristics, such as liver nodularity and the patient’s ability to momentarily stop breathing.41 The quality of the images and the sensitivity appear to be substantially worse in patients with obesity and underlying NASH.42, 43 As new antiviral therapies for HCV reduce the incidence of HCV-related HCC and the obesity epidemic continues to grow, NASH is expected to become an increasingly common risk factor for HCC. Therefore, the suboptimal sensitivity of ultrasonography is anticipated to be more problematic in the future, highlighting the need for alternative strategies to improve sensitivity for early tumor detection.
In specific patient populations in whom ultrasonographic imaging is inadequate, including obese patients or those with multinodular cirrhosis, MRI and CT may be considered potential alternatives.7, 44, 45, 46 Currently, the cost-effectiveness of these imaging modalities, as well as the nuances associated with obtaining consistent high-quality imaging, have prevented them from being included as first-line options within HCC screening guidelines.43, 45
Biomarkers
Bearing in mind the limitations of ultrasonography for HCC screening, there is a need for alternative strategies, like biomarkers, to improve sensitivity for early tumor detection. Several biomarkers, such as free cell DNA, are in development, but the best studied serum biomarker for HCC screening is AFP.47 Although inexpensive, readily available, and easy to perform, AFP testing has faced criticism given its suboptimal sensitivity and specificity when used alone. The benefit of using AFP in combination with ultrasonography is also debated, including discrepant recommendations in guidelines (Table).4, 5, 6, 7 The American Association for the Study of Liver Diseases recommends using ultrasonography with or without AFP, leaving it up to the clinician to consider the benefits and drawbacks in each individual patient.4 In contrast, European guidelines recommend ultrasonography alone.5 In attempt to increase performance, the statistical model GALAD combines AFP levels, the biomarkers AFP-L3 percentage and des-gamma-carboxy prothrombin, and the sex and age of the patient into one model.48 Although data from a case-control study seemed promising, the results of larger studies need to be awaited to determine the place of GALAD in clinical practice.
Several studies suggest that AFP can add benefit to ultrasonography by improving early tumor detection, but this improvement in sensitivity must be weighed against a decrease in specificity.49, 50, 51 A meta-analysis found that ultrasonography alone has a significantly lower sensitivity for detecting early HCC than ultrasonography combined with AFP (relative risk, 0.81; 95% CI, 0.71-0.93), although ultrasonography alone had a higher specificity (relative risk, 1.08; 95% CI, 1.05-1.09).40 The sensitivity of ultrasonography with AFP vs ultrasonography alone for detecting early HCC was 63% (95% CI, 48%-75%) vs 45% (95% CI, 30%-62%; P=.002). Of note, the specificity of AFP seems to be higher in patients with nonviral cirrhosis or post-SVR status. Therefore, as HCC epidemiology shifts from an HCV-predominant to a NASH-predominant etiology, it is anticipated that AFP accuracy will improve.52, 53, 54
Screening Interval
Most guidelines recommend ultrasonographic screening every 6 months (Table).5, 6, 7 This interval was initially recommended on the basis of tumor doubling time but has since been supported by studies reporting higher rates of early detection and improved survival with semiannual screening compared with annual screening.54 Increasing the screening frequency from every 6 months to every 3 months increases the detection of nonspecific nodules but does not improve early detection or survival.55
Underuse of Screening
Despite international recommendations, fewer than 1 in 5 high-risk patients are regularly screened.19 Guideline-adherent, biannual screening rates in the United States are even reported to be under 2%.15 In the United States and other countries with insurance-based health care systems, racial and socioeconomic factors significantly affect adherence.15 Elsewhere, factors like age, type of hepatitis, and awareness among health care professionals may play a larger role.14 Studies have suggested that the most common reason for the underuse of HCC screening is physicians failing to order screening in patients with known cirrhosis.56 Primary care physicians report several barriers to HCC screening, including lack of knowledge about the benefits of screening and clinic time constraints with competing clinical concerns.20 When health care professionals do order HCC screening, most patients are interested and adherent. However, patients who report barriers to screening, including costs or transportation, are significant less likely to complete screening.57 Studies examining interventions to improve HCC screening have suggested that simple interventions, such as electronic medical record reminders, nurse-based protocols, or mailed outreach invitations, can significantly increase HCC screening rates.58, 59, 60
Proposed Screening Algorithm
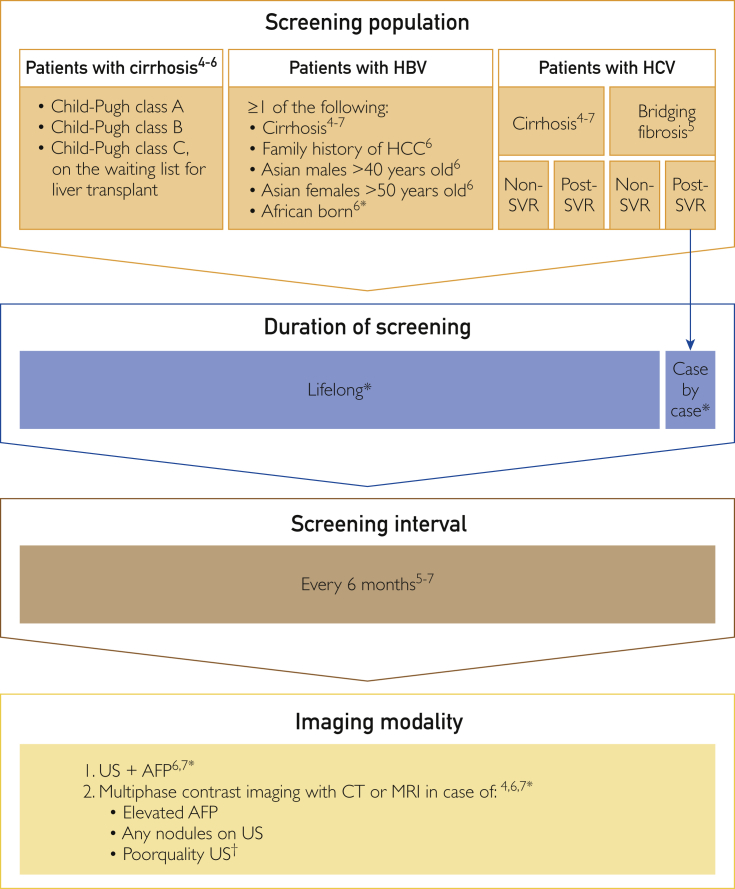
Current guidelines include diverse recommendations on the practical details of screening policies (Table).4, 5, 6, 7 This section contains a proposal for a comprehensive screening algorithm for patients at risk for HCC based on the level of liver cirrhosis or fibrosis and the underlying disease (Figure).
Figure.
Proposed screening algorithm for patients at risk for hepatocellular carcinoma (HCC), based on the American,4 European,5 Asia-Pacific,6 and Japanese7 guidelines and expert opinion (*). AFP = α-fetoprotein; CT = computed tomography; HBV = hepatitis B virus; HCV = hepatitis C virus; MRI = magnetic resonance imaging; SVR = sustained virologic response; US = ultrasonography. †Situations in which it could be worthwhile to perform cross-sectional imaging include unavailability of experienced personnel, obese patients, patients who are unable to hold their breath, and patients with an excessively nodular liver.
Patients with Child-Pugh class A or B cirrhosis and subgroups of patients without cirrhosis but with chronic HBV infections, in the absence of other medical comorbidities, should be included in an HCC screening program. Screening programs should generally consist of lifelong, semiannual abdominal ultrasonography and AFP testing. Patients with poor-quality ultrasonographic images can be further evaluated with multiphase contrast-enhanced CT or MRI.
A clear protocol for the diagnostic evaluation of patients with abnormal screening results is also key for HCC screening to be successful. The majority of lesions smaller than 1 cm are nonmalignant dysplastic or regenerative nodules, not HCCs. The optimal management of these small lesions in the setting of cirrhosis is still to be elucidated, but close follow-up with repeated short-interval ultrasonography and AFP is required to monitor these patients for malignant degeneration or growth.4, 5, 6, 7, 61 In patients with a positive screening result (mass >1 cm on ultrasonography or AFP level >20 ng/mL [to convert to μg/L, multiply by 1.0]), further evaluation with multiphase contrast imaging (CT or MRI) is required to evaluate for potential HCC.
Conclusion and Perspective
Global guidelines recommend screening high-risk populations to allow for early detection of HCC, but adherence is low. Increased awareness about the need for screening is crucial to allow more patients to qualify for curative treatment options. Lifelong, biannual screening using abdominal ultrasonography and the serum biomarker AFP is recommended for patients with cirrhosis and selected patients without cirrhosis with HBV infection.
Acknowledgments
Editorial support was provided by Kim Grootscholten, MSc, of COR2ED. Bayer AG did not have a critical role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Grant Support: This review article was written on behalf of HCC CONNECT; for more information visit www.hccconnect.info. HCC CONNECT is supported by an independent educational grant from Bayer AG.
Potential Competing Interests: Dr Frenette has served on advisory boards for Wako Diagnostics. Dr Bargellini has served on advisory boards for Bayer and Sirtex and as consultant for Bayer, Biocompatibles UK Ltd, Sirtex, GE Healthcare, and Terumo Medical Corporation. Dr Singal has served on advisory boards for Wako Diagnostics and as a consultant for Roche Diagnostics and GRAIL, Inc. Dr Saab is on the speakers bureau for Bristol-Myers Squibb Company and Bayer. Dr Isaacson reports no conflicts of interest.
References
- 1.Ervik M., Lam F., Ferlay J., et al. 2016. Cancer Today. Lyon, France: International Agency for Research on Cancer. International Agency for Research on Cancer website. http://gco.iarc.fr/today Published 2016. Accessed February 23, 2018.
- 2.Chang M.H., You S.L., Chen C.J., et al. Taiwan Hepatoma Study Group Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101(19):1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A., Ward E.M., Johnson C.J., et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9) doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heimbach J.K., Kulik L.M., Finn R., et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma [published correction appears in J Hepatol. 2019;70(4):817] J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Omata M., Cheng A.L., Kokudo N., et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kokudo N., Hasegawa K., Akahane M., et al. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: the Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines) Hepatol Res. 2015;45(2) doi: 10.1111/hepr.12464. [DOI] [PubMed] [Google Scholar]
- 8.Forner A., Reig M., Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 9.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hepatobiliary Cancers. Version 1.2018. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf [DOI] [PubMed]
- 10.Zhang B.-H., Yang B.-H., Tang Z.-Y. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130(7):417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 11.Johnson P., Berhane S., Kagebayashi C., et al. Impact of disease stage and aetiology on survival in hepatocellular carcinoma: implications for surveillance. Br J Cancer. 2017;116(4):441–447. doi: 10.1038/bjc.2016.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singal A.G., Marrero J.A. Screening for hepatocellular carcinoma. Gastroenterol Hepatol (N.Y.) 2008;4(3):201–208. [PMC free article] [PubMed] [Google Scholar]
- 13.Singal A.G., Pillai A., Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4):e1001624. doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirata A., Hirata T., Takahashi Y., Nakayama T. Surveillance rates for hepatocellular carcinoma among patients with cirrhosis, chronic hepatitis B, and chronic hepatitis C based on Japanese claims database. Hepatol Res. 2017;47(4):283–292. doi: 10.1111/hepr.12714. [DOI] [PubMed] [Google Scholar]
- 15.Singal A.G., Li X., Tiro J., et al. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am J Med. 2015;128(1):90.e1-e7. doi: 10.1016/j.amjmed.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edenvik P., Davidsdottir L., Oksanen A., Isaksson B., Hultcrantz R., Stål P. Application of hepatocellular carcinoma surveillance in a European setting: what can we learn from clinical practice? Liver Int. 2015;35(7):1862–1871. doi: 10.1111/liv.12764. [DOI] [PubMed] [Google Scholar]
- 17.Davila J.A., Henderson L., Kramer J.R., et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 18.Davila J.A., Morgan R.O., Richardson P.A., McGlynn K.A., El-Serag H.B. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52(1):132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singal A.G., Yopp A., Skinner C., Packer M., Lee W.M., Tiro J.A. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861–867. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalton-Fitzgerald E., Tiro J., Kandunoori P., Halm E.A., Yopp A., Singal A.G. Practice patterns and attitudes of primary care providers and barriers to surveillance of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(4):791–798.e1. doi: 10.1016/j.cgh.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poustchi H., Farrell G.C., Strasser S.I., Lee A.U., McCaughan G.W., George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54(6):1998–2004. doi: 10.1002/hep.24581. [DOI] [PubMed] [Google Scholar]
- 22.Kotwal A.A., Schonberg M.A. Cancer screening in the elderly: a review of breast, colorectal, lung, and prostate cancer screening. Cancer J. 2017;23(4):246–253. doi: 10.1097/PPO.0000000000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atiq O., Tiro J., Yopp A.C., et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology. 2017;65(4):1196–1205. doi: 10.1002/hep.28895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Serag H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papatheodoridis G., Dalekos G., Sypsa V., et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64(4):800–806. doi: 10.1016/j.jhep.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson I.M., Lim J.K., Fried M.W. American Gastroenterological Association Institute clinical practice update—expert review: care of patients who have achieved a sustained virologic response after antiviral therapy for chronic hepatitis C infection. Gastroenterology. 2017;152(6):1578–1587. doi: 10.1053/j.gastro.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg D., Ditah I.C., Saeian K., et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152(5):1090–1099.e1. doi: 10.1053/j.gastro.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masuzaki R., Karp S.J., Omata M. NAFLD as a risk factor for HCC: new rules of engagement [editorial]? Hepatol Int. 2016;10(4):533–534. doi: 10.1007/s12072-016-9731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turati F., Galeone C., Rota M., et al. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2014;25(8):1526–1535. doi: 10.1093/annonc/mdu020. [DOI] [PubMed] [Google Scholar]
- 30.Papatheodoridis G.V., Chan H.L., Hansen B.E., Janssen H.L., Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62(4):956–967. doi: 10.1016/j.jhep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Cho J.Y., Paik Y.H., Sohn W., et al. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut. 2014;63(12):1943–1950. doi: 10.1136/gutjnl-2013-306409. [DOI] [PubMed] [Google Scholar]
- 32.Baffy G., Brunt E.M., Caldwell S.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56(6):1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 33.Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 34.Cholankeril G., Patel R., Khurana S., Satapathy S.K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: current knowledge and implications for management. World J Hepatol. 2017;9(11):533–543. doi: 10.4254/wjh.v9.i11.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawada N., Imanaka K., Kawaguchi T., et al. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol. 2009;44(12):1190–1194. doi: 10.1007/s00535-009-0112-0. [DOI] [PubMed] [Google Scholar]
- 36.Mittal S., El-Serag H.B., Sada Y.H., et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14:124–131.e1. doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang C.M., Pu C.W., Hou Y.H., Chen Z., Alanazy M., Hebbard L. Non alcoholic steatohepatitis a precursor for hepatocellular carcinoma development. World J Gastroenterol. 2014;20(44):16464–16473. doi: 10.3748/wjg.v20.i44.16464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karagozian R., Derdák Z., Baffy G. Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism. 2014;63(5):607–617. doi: 10.1016/j.metabol.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Perumpail R.B., Wong R.J., Ahmed A., Harrison S.A. Hepatocellular carcinoma in the setting of non-cirrhotic nonalcoholic fatty liver disease and the metabolic syndrome: US experience. Dig Dis Sci. 2015;60(10):3142–3148. doi: 10.1007/s10620-015-3821-7. [DOI] [PubMed] [Google Scholar]
- 40.Tzartzeva K., Obi J., Rich N.E., et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154(6):1706–1718.e1. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Poggio P., Olmi S., Ciccarese F., et al. Italian Liver Cancer (ITA.LI.CA) Group. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12(11):1927–1933.e2. doi: 10.1016/j.cgh.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 42.Singal A.G., Nehra M., Adams-Huet B., et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol. 2013;108(3):425–432. doi: 10.1038/ajg.2012.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmons O., Fetzer D.T., Yokoo T., et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45(1):169–177. doi: 10.1111/apt.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pocha C., Dieperink E., McMaken K.A., Knott A., Thuras P., Ho S.B. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography—a randomised study. Aliment Pharmacol Ther. 2013;38(3):303–312. doi: 10.1111/apt.12370. [DOI] [PubMed] [Google Scholar]
- 45.Kim S.Y., An J., Lim Y.S., et al. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol. 2017;3(4):456–463. doi: 10.1001/jamaoncol.2016.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersson K.L., Salomon J.A., Goldie S.J., Chung R.T. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2008;6(12):1418–1424. doi: 10.1016/j.cgh.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harding J.J., Khalil D.N., Abou-Alfa G.K. Biomarkers: what role do they play (if any) for diagnosis, prognosis and tumor response prediction for hepatocellular carcinoma? Dig Dis Sci. 2019;64(4):918–927. doi: 10.1007/s10620-019-05517-6. [DOI] [PubMed] [Google Scholar]
- 48.Johnson P.J., Pirrie S.J., Cox T.F., et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23(1):144–153. doi: 10.1158/1055-9965.EPI-13-0870. [DOI] [PubMed] [Google Scholar]
- 49.Lok A.S., Sterling R.K., Everhart J.E., et al. HALT-C Trial Group Des-γ-carboxy prothrombin and α-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138(2):493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singal A., Volk M.L., Waljee A., et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singal A.G., Conjeevaram H.S., Volk M.L., et al. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol Biomarkers Prev. 2012;21(5):793–799. doi: 10.1158/1055-9965.EPI-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gopal P., Yopp A.C., Waljee A.K., et al. Factors that affect accuracy of α-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12(5):870–877. doi: 10.1016/j.cgh.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Serag H.B., Kanwal F., Davila J.A., Kramer J., Richardson P. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology. 2014;146(5):1249–1255.e1. doi: 10.1053/j.gastro.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santi V., Trevisani F., Gramenzi A., et al. Italian Liver Cancer (ITA.LI.CA) Group Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53(2):291–297. doi: 10.1016/j.jhep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 55.Trinchet J.C., Chaffaut C., Bourcier V., et al. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire (GRETCH) Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54(6):1987–1997. doi: 10.1002/hep.24545. [DOI] [PubMed] [Google Scholar]
- 56.Singal A.G., Yopp A.C., Gupta S., et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res (Phila) 2012;5(9):1124–1130. doi: 10.1158/1940-6207.CAPR-12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farvardin S., Patel J., Khambaty M., et al. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology. 2017;65(3):875–884. doi: 10.1002/hep.28770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beste L.A., Ioannou G.N., Yang Y., Chang M.F., Ross D., Dominitz J.A. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clin Gastroenterol Hepatol. 2015;13(1):172–179. doi: 10.1016/j.cgh.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 59.Singal A.G., Tiro J.A., Marrero J.A., et al. Mailed outreach program increases ultrasound screening of patients with cirrhosis for hepatocellular carcinoma. Gastroenterology. 2017;152(3):608–615.e4. doi: 10.1053/j.gastro.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goebel M., Singal A.G., Nodora J., et al. How can we boost colorectal and hepatocellular cancer screening among underserved populations? Curr Gastroenterol Rep. 2015;17(6):22. doi: 10.1007/s11894-015-0445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roskams T. Anatomic pathology of hepatocellular carcinoma: impact on prognosis and response to therapy. Clin Liver Dis. 2011;15(2):245–259, vii-x. doi: 10.1016/j.cld.2011.03.004. [DOI] [PubMed] [Google Scholar]