Abstract
Objective
To examine the incidence of screening, diagnosis, and treatment of hypogonadism among men treated with opioids in the United States.
Patients and Methods
Using one of the nation's largest commercial insurance databases, we identified 53,888 men aged 20 years or older who had 90 or more days of opioid prescriptions in a single 12-month period between January 1, 2010, and December 31, 2017, with no history of hypogonadism or testosterone therapy in the preceding 12 months. We matched this cohort to 53,888 men with 14 or fewer days of opioid prescriptions based on age, opioid initiation date, opioid indication, and comparable exclusion criteria. We assessed whether men, 14 or fewer days after initiation of opioid treatment, received a serum testosterone test, a diagnosis of hypogonadism, or a prescription for testosterone therapy. All men were followed up until they lost coverage from the commercial insurance plan, experienced one of the study outcomes, or the end of study (December 31, 2017).
Results
In the multivariable analyses—adjusting for age, year of opioid initiation, region, comorbid disease, glucocorticoid use, and health care utilization—the 53,888 prolonged opioid users, in comparison with 53,888 short-term users, had an increased incidence of serum testosterone screening (5991 [17.15%; 95% CI, 16.70%-17.61%] vs 3514 [11.55%; 95% CI, 11.11%-12.01%] at 5 years; hazard ratio [HR], 1.46; 95% CI, 1.38-1.55), hypogonadism diagnosis (3125 [9.44%; 95% CI, 9.09%-9.80%] vs 1421 [4.85%; 95% CI, 4.55%-5.16%; HR, 1.74; 95% CI, 1.60-1.90]), and receipt of testosterone therapy (1919 [5.76%; 95% CI, 5.49%-6.05%] vs 631 [2.21%; 95% CI, 2.04%-2.43%; HR, 2.41; 95% CI, 2.13-2.74]). Each of these findings persisted across multiple sensitivity analyses.
Conclusion
Prolonged opioid exposure was associated with increased rates of screening, diagnosis, and treatment for opioid-induced hypogonadism, but these rates were much lower than expected based on previous serum-based studies.
Abbreviations and Acronyms: CDM, Clinformatics Data Mart; HR, hazard ratio; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification
Over the past 2 decades, the use of prescription opioids in the United States has increased dramatically.1, 2 Accompanying this upsurge, opioid-related addiction, mortality, and a number of adverse outcomes have reached an epidemic level.1, 2, 3, 4 Hypogonadism—a condition in which the body produces inadequate testosterone and is associated with low libido, muscle wasting, increased adiposity, infertility, and osteoporosis5, 6, 7—has been linked with opioid use in a number of small serum-based studies, with prevalence estimates ranging from 35% to 90%.8, 9, 10, 11, 12, 13 The primary mechanism underlying opioid-induced hypogonadism is the inhibitory action of opioids on the release of gonadotropin-releasing hormone from the hypothalamus, which reduces the secretion of luteinizing hormone and follicle-stimulating hormone from the pituitary gland, leading to inadequate production of testosterone from the testis.14, 15, 16 In addition, opioids may have a direct effect on the pituitary gland and the testis.15, 17
Opioid-induced hypogonadism can have a profound impact on the patient's health and quality of life and can hinder the clinician's ability to effectively treat chronic pain and manage complex comorbidities,18 but it often goes unrecognized and untreated.14 Pain practitioners should be aware of this increasingly common condition and monitor patients accordingly. To date, the incidence of opioid-induced hypogonadism—and its related screening and treatment—has not been examined in a large, nationally representative study. Given the scale of the opioid epidemic, understanding the extent to which opioid-induced hypogonadism is screened, diagnosed, and treated among men in the United States has broad clinical and public health importance. We therefore examined this issue using one of the nation's largest commercial insurance databases.
Patients and Methods
Study Design
We conducted a retrospective cohort study using administrative health data from the Clinformatics Data Mart (CDM) database (Optum, Inc), a database of one of the nation's largest commercial health insurance programs. The CDM data have been used in numerous epidemiological and health services studies.19, 20, 21 Persons enrolled in this insurance program may be included in either a fee-for-service plan or a managed care plan, which includes health maintenance organizations, preferred provider organizations, and exclusive provider organizations. For each of these plans, providers are required to submit complete claims to receive reimbursement. We used a combination of outpatient, inpatient, and pharmacy claims data. This retrospective study was approved by the Institutional Review Board of the University of Texas Medical Branch at Galveston.
Study Population
We identified 53,888 males aged 20 years or older who had 90 or more days of opioid use within a 12-month period any time between January 1, 2010, and December 31, 2017. The cut point of 90 or more days of opioid use was chosen because it represents a widely recognized clinical threshold for prolonged opioid use, is strongly predictive of a number of adverse outcomes including opioid-related overdose, and has been used in other administrative claims–based studies.22, 23, 24 We excluded men who had a prescription for testosterone or who had any of the following diagnoses in the 12 months before their opioid initiation date: hypogonadism (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM], 257.xx; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM], E29.1), erectile dysfunction (ICD-9-CM, 607.84; ICD-10-CM, 52.xx), psychosexual dysfunction (ICD-9-CM, 302.70; ICD-10-CM, N52.xx), or infertility (ICD-9-CM, 606.xx; ICD-10-CM, N46.xx). In addition, we excluded men who did not have a minimum of 12 months of continuous enrollment before their opioid initiation date. For each patient, we identified an indication for opioid use in any of the following 12 categories: back pain, joint pain, nerve pain or neuropathy, headache, chronic pain, abdominal/chest pain, cancer, musculoskeletal pain, fracture, visceral pain, wound pain, or other pain.25 We also identified the number of days between the initiation of opioid use and the most recent indication diagnosis (same day, days 1-7, 8-30, 31-60, 61-90, 91-180, or 181-365). Overall, 16,061 of the 53,888 patients (27.8%) had more than one indication on the most recent diagnosis date; these patients were classified according to all diagnoses that occurred on that date.
For the control group, we identified a cohort of 53,888 men with short-term (≤14 days) opioid use who were matched to the prolonged use cohort based on 5-year age group, opioid indication(s), number of days from indication to opioid initiation, and the aforementioned exclusion criteria. This match resulted in 53,881 (99.9%) of the prolonged use group having a control match. The remaining 7 (0.01%) were matched on the same criteria except the 5-year age group was expanded to include a 10-year age group. For the control group, we selected a short-term opioid-exposed group rather than an unexposed group because this method allowed us to match the indication for opioid use and reduced selection bias associated with a number of clinical characteristics.
Outcomes
We assessed whether both groups of men—beginning at 14 days after initiation of opioid treatment—received any of the following: a serum testosterone screening test (Current Procedural Terminology code, 84402, 84403), a diagnosis of hypogonadism (ICD-9-CM, 257.xx; ICD-10-CM, E29.1), or a prescription for testosterone therapy (National Drug Codes for topical gel, transdermal patches, subcutaneous pellets, and oral formulations; Healthcare Common Procedure Coding System codes for injectable formulations) (Supplemental Table, available online at http://mcpiqojournal.org).
Age at opioid initiation date was obtained from the CDM database. We examined and adjusted for all conditions included in the Elixhauser Comorbidity Index.26 Each condition was examined as a separate covariate. In addition, we adjusted for diagnosis of adrenal insufficiency (ICD-9-CM, 255.4, 255.5, 255.8, 255.9; ICD-10-CM, E27.1, E27.3, E27.4) and use of glucocorticoid pharmacotherapy (therapeutic classification codes 6804010010, 6804010015, 6804010019, 6804010020, 6804010030, 6804010050, 6804010060, 6804010070, 6804010075, 6804010085) given their positive association with hypogonadism. To address the potential confounding effect of increased health care utilization—which has been linked with an increased likelihood of disease diagnosis in general—we adjusted for the total number of outpatient visits and the total number of hospitalizations in the 12 months prior to the opioid initiation date as covariates in our multivariable model. We also adjusted for the geographic region (Midwest, Northeast, South, West) of each participant.
Statistical Analyses
Unadjusted event-free survival was estimated using the Kaplan-Meier method.27 Multivariable survival analyses were performed using Cox proportional hazards regression, with the dependent variable being time to first occurrence of serum testosterone screening, hypogonadism, or testosterone therapy. Adjusted failure rates were estimated using the Cox model.27, 28 We tested the assumption of proportionality in the Cox model by determining that, for the primary exposure variable, the logarithm of the baseline cumulative hazard rates and the Schoenfield residuals were proportional with follow-up time.27, 28 Patients were censored at the date they left the commercial insurance plan or at the end of the study. To assess the robustness of these findings, we conducted several sensitivity analyses including fully adjusted models using 1- and 2-year latency periods and an analysis restricted to men who initiated opioid use in 2014-2016 (thereby ensuring more uniform follow-up periods across the 2 comparison groups). We also conducted an analysis using cataracts (ICD-9-CM, 366.x; ICD-10-CM, H25.x) as a control outcome to assess possible selection bias. All analyses were performed using SAS statistical software, version 9.4 (SAS Institute). All statistical tests were 2-sided, and P<.05 was considered significant.
Results
Comparison of the baseline characteristics in short-term vs prolonged opioid users (Table 1) shows that the 2 groups had similar distributions of the matching variables of age, and opioid indication. Prolonged opioid users, however, had a higher prevalence of prior outpatient visits, prior hospitalizations, 3 or more comorbidities, and several specific comorbidities, particularly congestive heart failure, chronic obstructive pulmonary disease, diabetes, hypertension, peripheral vascular disease, and renal failure.
Table 1.
Demographic and Clinical Characteristics of Short-term vs Prolonged Opioid Usersa
| Characteristicb | Short-term use (N=53,888) | Prolonged use (N=53,888) | P value |
|---|---|---|---|
| Age (y) at index date (mean) | |||
| Mean | 60.4±15.8 | 60.5±15.7 | .57 |
| Median | 62 (50-72) | 62 (50-73) | .61 |
| Duration of follow-up (d) | 857.8±636.6 | 1043.9±1037.9 | <.001 |
| Outpatient visits in prior year | 13.2±14.6 | 16.2±18.8 | <.001 |
| Hospitalizations in prior year | 0.25±0.73 | 0.42±1.1 | <.001 |
| Opioid initiation year | <.001 | ||
| 2010 | 9799 (18.2) | 14,304 (26.5) | |
| 2011 | 9203 (17.1) | 11,253 (20.9) | |
| 2012 | 8386 (15.6) | 8344 (15.5) | |
| 2013 | 7648 (14.2) | 6974 (12.9) | |
| 2014 | 6298 (11.7) | 5307 (9.8) | |
| 2015 | 6117 (11.4) | 4969 (9.2) | |
| 2016 | 6437 (11.9) | 2737 (5.1) | |
| Opioid indications | |||
| Back pain | 19,739 (36.6) | 19,739 (36.6) | >.99 |
| Joint pain | 18,700 (34.7) | 18,700 (34.7) | >.99 |
| Nerve pain | 3922 (7.3) | 3922 (7.3) | >.99 |
| Headache | 2266 (4.2) | 2266 (4.2) | >.99 |
| Chronic pain | 2267 (4.2) | 2267 (4.2) | >.99 |
| Chest pain | 6325 (11.7) | 6325 (11.7) | >.99 |
| Cancer | 4889 (9.1) | 4889 (9.1) | >.99 |
| Muscle pain | 9832 (18.2) | 9832 (18.2) | >.99 |
| Visceral | 2410 (4.5) | 2410 (4.5) | >.99 |
| Fracture | 1723 (3.2) | 1723 (3.2) | >.99 |
| Wound | 1303 (2.4) | 1303 (2.4) | >.99 |
| Other | 630 (1.2) | 630 (1.2) | >.99 |
| Elixhauser Comorbidity Score | >.99 | ||
| 0 | 15,484 (28.7) | 9487 (17.6) | |
| 1 | 12,126 (22.5) | 10,418 (19.3) | |
| 2 | 9306 (17.3) | 9657 (17.9) | |
| ≥3 | 16,972 (31.5) | 24,326 (45.1) | |
| Elixhauser comorbiditiesc | |||
| Alcohol abuse | 924 (1.7) | 2329 (4.3) | <.001 |
| Arrhythmia | 8413 (15.6) | 9684 (18.0) | <.001 |
| Anemia | 454 (0.8) | 717 (1.3) | <.001 |
| Congestive heart failure | 3620 (6.7) | 5767 (10.7) | <.001 |
| Chronic obstructive pulmonary disease | 7258 (13.5) | 11,667 (21.7) | <.001 |
| Coagulopathy | 1501 (2.8) | 2077 (3.9) | <.001 |
| Deficiency anemia | 1814 (3.4) | 3081 (5.7) | <.001 |
| Depression | 3657 (6.8) | 7028 (13.0) | <.001 |
| Diabetes with complications | 3706 (6.9) | 5743 (10.7) | <.001 |
| Diabetes without complications | 10,380 (19.3) | 14,561 (27.0) | <.001 |
| Drug abuse | 332 (0.6) | 1380 (2.6) | <.001 |
| Electrolyte disorders | 3637 (6.7) | 6478 (12.0) | <.001 |
| HIV | 124 (0.2) | 216 (0.4) | <.001 |
| Hypertension with complications | 3709 (6.9) | 4993 (9.3) | <.001 |
| Hypertension without complications | 27,262 (50.6) | 32,388 (60.1) | <.001 |
| Hypothyroidism | 4457 (8.3) | 4788 (8.9) | <.001 |
| Liver disease | 1949 (3.6) | 3401 (6.3) | <.001 |
| Lymphoma | 550 (1.0) | 745 (1.4) | <.001 |
| Metastatic cancer | 730 (1.4) | 2225 (4.1) | <.001 |
| Neurologic disorders | 2060 (3.8) | 3436 (6.4) | <.001 |
| Obesity | 4181 (7.8) | 5237 (9.7) | <.001 |
| Paralysis | 362 (0.7) | 1089 (2.0) | <.001 |
| Peptic ulcer disease | 451 (0.8) | 784 (1.5) | <.001 |
| Peripheral vascular disease | 4994 (9.3) | 7403 (13.7) | <.001 |
| Psychosis | 590 (1.1) | 1379 (2.6) | <.001 |
| Pulmonary circulation disorders | 1055 (2.0) | 1649 (3.1) | <.001 |
| Renal failure | 3944 (7.3) | 5731 (10.6) | <.001 |
| Tumor (no metastasis) | 6470 (12.0) | 6971 (12.9) | <.001 |
| Valvular disease | 4358 (8.1) | 4718 (8.8) | <.001 |
| Weight loss | 1353 (2.5) | 3011 (5.6) | <.001 |
| Adrenal insufficiency | 184 (0.3) | 674 (1.3) | <.001 |
| Glucocorticoid use | 11,185 (20.8) | 12,163 (22.6) | <.001 |
Data are presented as mean ± SD, median (interquartile range), or No. (percentage) of patients.
Cohorts were matched for age and index/diagnosis date.
Comorbid conditions were measured using the factors that comprise the Elixhauser Comorbidity Index.
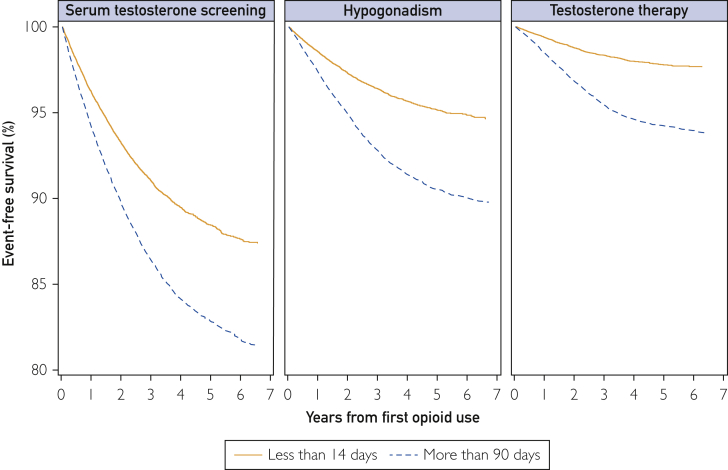
The Figure presents the Kaplan-Meier curves showing event-free survival for short-term vs prolonged opioid users over 7 years of follow-up with each of the 3 outcomes at 1, 3, and 5 years of follow-up as follows. The incidence of serum testosterone screening (Table 2) was 3.73% (95% CI, 3.57%-3.91%), 8.94% (95% CI, 8.64%-9.27%), and 11.55% (95% CI, 11.11%-12.01%) among short-term users and 5.76% (95% CI, 5.56%-5.97%), 13.60% (95% CI, 13.25%-13.96%), and 17.15% (95% CI, 16.70%-17.61%) among long-term users. The incidence of hypogonadism was 1.42% (95% CI, 1.32%-1.53%), 3.59% (95% CI, 3.39%-3.80%), and 4.85% (95% CI, 4.55%-5.16%) among short-term users and 2.62% (95% CI, 2.49%-2.77%), 7.18% (95% CI, 6.92%-7.46%), and 9.44% (95% CI, 9.09%-9.80%) among prolonged users. The incidence of filling a testosterone prescription was 0.61% (95% CI, 0.54%-0.68%), 1.65% (95% CI, 1.52%-1.80%), and 2.21% (95% CI, 2.04%-2.43%) among short-term users and 1.50% (95% CI, 1.40%-1.62%), 4.55% (95% CI, 4.34%-4.77%), and 5.76% (95% CI, 5.49%-6.05%) among prolonged users.
Figure.
Kaplan-Meier curves showing event-free survival for short-term vs prolonged opioid users over 7 years of follow-up with each of the 3 outcomes at 1, 3, and 5 years of follow-up.
Table 2.
Incidencea of Opioid-Induced Hypogonadism Outcomes in Short-term vs Prolonged Opioid Users
| Brief opioid exposure | Short-term opioid use, absolute risk, % (95% CI) | Prolonged opioid use, absolute risk, % (95% CI) |
|---|---|---|
| Serum testosterone test | ||
| 1 year | 3.73 (3.57-3.91) | 5.76 (5.56-5.97) |
| 3 years | 8.94 (8.64-9.27) | 13.60 (13.25-13.96) |
| 5 years | 11.55 (11.11-12.01) | 17.15 (16.70-17.61) |
| Hypogonadism diagnosis | ||
| 1 year | 1.42 (1.32-1.53) | 2.62 (2.49-2.77) |
| 3 years | 3.59 (3.39-3.80) | 7.18 (6.92-7.46) |
| 5 years | 4.85 (4.55-5.16) | 9.44 (9.09-9.80) |
| Testosterone therapy | ||
| 1 year | 0.61 (0.54-0.68) | 1.50 (1.40-1.62) |
| 3 years | 1.65 (1.52-1.80) | 4.55 (4.34-4.77) |
| 5 years | 2.21 (2.04-2.43) | 5.76 (5.49-6.05) |
Based on Kaplan-Meier estimates.
Table 3 presents the results of the Cox regression model. In these multivariable analyses— adjusting for age, region, year, comorbidity, number of outpatient visits, and number of hospitalizations—prolonged opioid users, compared with short-term users, had an increased incidence of serum testosterone screening (hazard ratio [HR], 1.46; 95% CI, 1.38-1.55), hypogonadism diagnosis (HR, 1.74; 95% CI, 1.60-1.90), and receipt of testosterone therapy (HR, 2.41; 95% CI, 2.13-2.74). These general findings persisted across multiple sensitivity analyses, including fully adjusted models using 1- and 2-year latency periods, restricted to men who initiated opioid use in 2014-2016. Prolonged opioid use was associated with a decreased risk of the control outcome (cataracts) (HR, 0.86; 95% CI, 0.82-0.89).
Table 3.
Hazard Ratios Assessing the Association of Prolonged vs Short-term Opioid Use With Hypogonadism Outcomes, Analyzed by Various Approachesa,b,c
| Variable | Serum testosterone test, HR (95% CI) | Hypogonadism diagnosis, HR (95% CI) | Testosterone therapy, HR (95% CI) |
|---|---|---|---|
| Model | |||
| Unadjusted | 1.56 (1.48-1.63) | 1.87 (1.73-2.01) | 2.54 (2.29-2.83) |
| Adjusted for age, region, and year | 1.48 (1.4-1.56) | 1.78 (1.65-1.93) | 2.37 (2.11-2.66) |
| Adjusted for age, region, year, and comorbidity | 1.47 (1.39-1.56) | 1.77 (1.62-1.92) | 2.42 (2.13-2.74) |
| Adjusted for age, region, year, comorbidity, adrenal insufficiency, glucocorticoid use, prior outpatient visits, and prior hospitalizations | 1.46 (1.38-1.55) | 1.74 (1.60-1.90) | 2.41 (2.13-2.74) |
| Sensitivity analyses | |||
| Adjusted for all covariates; included only matched pairs who initiated opioid use from 2014-2016 | 1.83 (1.42-2.37) | 1.62 (1.03-2.54) | 16.21 (1.71-154.09) |
| Adjusted for all covariates, 1-year latency period | 1.56 (1.44-1.69) | 1.81 (1.6-2.04) | 2.5 (2.11-2.96) |
| Adjusted for all covariates, 2-year latency period | 1.69 (1.53-1.86) | 2.03 (1.67-2.45) | 2.7 (2.05-3.54) |
HR = hazard ratio.
Total outpatient visits in the year before diagnosis/index date.
Comorbid conditions were measured using the factors that comprise the Elixhauser Comorbidity Index.21
Discussion
In this study of 107,776 commercially insured men, we found that prolonged opioid exposure was associated with increased rates of screening, diagnosis, and treatment of opioid-induced hypogonadism but that these rates were much lower than expected based on prior serum-based studies.8, 9, 10, 11, 12, 13 To our knowledge, this is the first large-scale, nationally representative, population-based study to examine this issue. Our findings persisted across multiple sensitivity analyses, including analyses with latency periods of 1 and 2 years and alternative follow-up periods. In addition, prolonged opioid exposure was associated with a decreased risk of the control outcome, cataracts.
Opioid-induced hypogonadism is characterized by opioid-associated reductions in gonadotropin-releasing hormone by the hypothalamus resulting in decreased secretion of luteinizing hormone and follicle-stimulating hormone by the pituitary gland, which leads to an inadequate production of testosterone by the testis.14, 15, 16 Hypogonadism is associated with a number of adverse effects including sexual dysfunction, muscle wasting, adiposity, metabolic syndrome, infertility, and osteoporosis.5, 6, 7 Given the dramatic increase in opioid use over the past 20 years,14 it is likely that opioid-induced hypogonadism has become increasingly common. Our finding that only 3125 men (9.44%) with prolonged opioid use were diagnosed with hypogonadism over a 5-year period is substantially lower than estimates reported in previous serum-based studies, which range from 35% to 90%.8, 9, 10, 11, 12, 13 This large disparity may be attributable, in part, to our assessment of incident rather than prevalent hypogonadism and our reliance on administrative claims data, which often underestimate chronic health conditions.
In their study of 20 male cancer survivors who received long-term opioid treatment vs 20 nonopioid matched controls, Rajagopal et al13 reported that 90% of the long-term opioid users exhibited hypogonadism—defined as a serum total testosterone level of less than 345 ng/dL—compared with 40% of opioid nonusers. The extent to which hypogonadism was attributable to underlying cancer diagnosis or chemotherapy in either group, however, is not clear. Second, in their study of 12 men treated with oral opioids for at least 1 year, Fraser et al11 reported that 75% were diagnosed as having hypogonadism, defined as a total testosterone level below 9.1 nmol/L for men aged 20 to 49 years and 6.3 nmol/L for men 50 years or older. Third, in their retrospective study of 1585 men enrolled in a large regional health system, Rubenstein and Carpenter8 reported that 57% of men receiving long-acting opioids were diagnosed as having hypogonadism, defined as a serum total testosterone level of less than 345 ng/dL. Because this study was restricted to men who were referred by a clinician to undergo a serum testosterone test—presumably selected on the basis of observed or reported hypogonadal symptoms—the reported incidence of hypogonadism is likely to be increased as a result of selection bias. Despite the methodological issues addressed previously, the magnitude of the difference in opioid-associated hypogonadism incidence between our study and the prior, more restrictive, serum-based investigations suggests that this condition has been substantially underdiagnosed in the United States.
Screening for hypogonadism was surprisingly low among prolonged opioid users in our study—2920 (5.76%; 95% CI, 5.56%-5.97%) at 1 year and 5991 (17.15%; 95% CI, 16.70%-17.61%) at 5 years—given prior studies' estimates of opioid-induced hypogonadism, ranging as high as 90%.8, 9, 10, 11, 12, 13 Our estimates of Current Procedural Terminology code–based testosterone screening should be reasonably accurate given that clinics must submit these claims to the insurance company to receive payment. This finding suggests a widespread underscreening of opioid-induced hypogonadism during critical years of the opioid epidemic in the United States. It is not clear what factors drove this exceedingly low rate of serum testosterone screening. It may reflect a lack of awareness by some clinicians of the association between long-term opioid use and hypogonadism. Additionally, many clinicians—when treating patients with multimorbid disease and complex drug regimens—may be reluctant to screen for conditions that would require additional pharmacotherapy, especially in view of concerns related to comorbid psychiatric disorders and substance use disorders, both of which are strongly associated with long-term opioid use.29, 30, 31 From a patient's perspective, it is possible that men who are struggling with chronic pain and associated conditions are less concerned than their peers about early hypogonadal symptoms, such as low libido, sexual dysfunction, increased adiposity, and decreased muscle mass. They may, therefore, be less likely to bring these symptoms to the attention of their physician or to request a hypogonadism screening test. Our finding that prolonged opioid users had a reduced incidence of cataracts (our control outcome) is consistent with this possibility.
Finally, our observation that only 5.76% (95% CI, 5.49%-6.05%) of prolonged opioid users received testosterone therapy at 5 years and only 1.50% (95% CI, 1.40%-1.62%) at 1 year suggests that this condition is undertreated in the United States. Our previous study of testosterone prescribing among all men aged 30 years or older, using the same database, found an annual incidence ranging from 0.5% (95% CI, 0.5%-0.6%) to 1.3% (95% CI, 1.2%-1.3%) per year during the same time period.32 These rates are only slightly lower than those observed in the present study. It is possible that this finding reflects some clinicians' concerns about conflicting reports regarding adverse cardiovascular effects associated with testosterone therapy.32, 33, 34, 35, 36
The results of our study may have been influenced by the several limitations. First, information on outcomes and risk factors are based on diagnosis codes included in charges for outpatient and hospitalization services. Such diagnoses are not always accurate or complete.26 For example, we were unable to determine whether diagnoses of hypogonadism met the established serum and symptom criteria for this condition. Second, given the retrospective nature of this study, it is possible that undetected selection bias may have affected the findings. For example, men who were prescribed prolonged opioid therapy may have been more likely than their counterparts to have had subsequent diagnoses. However, we attempted to address this potential bias by either matching or adjusting for a broad range of demographic and clinical factors, including indication for opioid use. Third, our database lacked information on several important health behaviors such as smoking status, exercise, and diet. Fourth, prescription claims data do not capture information on drugs obtained outside the plan. Given the various stigmas and restrictions associated with both opioid and testosterone therapy, some men may have accessed these drugs outside their health care setting.
Despite these limitations, we believe that this study has important strengths including a large sample size, a long follow-up period, representation of all US geographic regions, and inclusion of a clinically diverse cohort. In view of the widespread underrecognition of opioid-induced hypogonadism observed in this study, public education on this condition will be an important step in helping men and their physicians make complex decisions about managing chronic pain, treating comorbid conditions, and understanding the short- and long-term risks, such as osteoporosis. Future research should continue to examine opioid-induced hypogonadism, with particular attention to the factors that have driven its underscreening and underdiagnosis during the opioid epidemic of the past 20 years.
Conclusion
Prolonged opioid users—in comparison to short-term users—had an increased incidence of serum testosterone screening, hypogonadism diagnosis, and receipt of testosterone therapy, but these rates were much lower than expected based on prior serum-based studies. These findings persisted across multiple sensitivity analyses. Given the scale of the opioid epidemic in the United States, clinicians should be aware of this condition and monitor patients accordingly.
Acknowledgments
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Grant Support: This study was supported by grants UL1TR000071 from the National Center for Advancing Translational Sciences, R24HS022134 from the Agency for Healthcare Research and Quality, P30AG024832 from the National Institute on Aging, and R01DA039192 from the National Institutes of Health.
Potential Competing Interests: Dr Baillargeon has received consulting fees from AbbVie Inc, Auxillium Pharmaceuticals, Inc, GlaxoSmithKline plc, and Endo Pharmaceuticals Inc. The other authors report no conflicts of interest.
Supplemental Online Material
Supplemental material can be found online at http://mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Dart R.C., Surratt H.L., Cicero T.J., et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 2.Rudd R.A., Seth P., David F., Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 3.Seth P., Scholl L., Rudd R.A., Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349–358. doi: 10.15585/mmwr.mm6712a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedegaard H., Warner M., Miniño A.M. Drug overdose deaths in the United States, 1999-2016. NCHS Data Brief. 2017;(294):1–8. [PubMed] [Google Scholar]
- 5.Bhasin S., Cunningham G.R., Hayes F.J., et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–2559. doi: 10.1210/jc.2009-2354. [published correction appears in J Clin Endocrinol Metab. 2010;95(8):4101] [DOI] [PubMed] [Google Scholar]
- 6.Nieschlag E. Current topics in testosterone replacement of hypogonadal men. Best Pract Res Clin Endocrinol Metab. 2015;29(1):77–90. doi: 10.1016/j.beem.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Orwoll E., Lambert L.C., Marshall L.M., et al. Osteoporotic Fractures in Men Study Group Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med. 2006;166(19):2124–2131. doi: 10.1001/archinte.166.19.2124. [DOI] [PubMed] [Google Scholar]
- 8.Rubinstein A., Carpenter D.M. Elucidating risk factors for androgen deficiency associated with daily opioid use. Am J Med. 2014;127(12):1195–1201. doi: 10.1016/j.amjmed.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Rubinstein A.L., Carpenter D.M. Association between commonly prescribed opioids and androgen deficiency in men: a retrospective cohort analysis. Pain Med. 2017;18(4):637–644. doi: 10.1093/pm/pnw182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abs R., Verhelst J., Maeyaert J., et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85(6):2215–2222. doi: 10.1210/jcem.85.6.6615. [DOI] [PubMed] [Google Scholar]
- 11.Fraser L.A., Morrison D., Morley-Forster P., et al. Oral opioids for chronic non-cancer pain: higher prevalence of hypogonadism in men than in women. Exp Clin Endocrinol Diabetes. 2009;117(1):38–43. doi: 10.1055/s-2008-1076715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajagopal A., Vassilopoulou-Sellin R., Palmer J.L., Kaur G., Bruera E. Hypogonadism and sexual dysfunction in male cancer survivors receiving chronic opioid therapy. J Pain Symptom Manage. 2003;26(5):1055–1061. doi: 10.1016/s0885-3924(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopal A., Vassilopoulou-Sellin R., Palmer J.L., Kaur G., Bruera E. Symptomatic hypogonadism in male survivors of cancer with chronic exposure to opioids. Cancer. 2004;100(4):851–858. doi: 10.1002/cncr.20028. [DOI] [PubMed] [Google Scholar]
- 14.Smith H.S., Elliott J.A. Opioid-induced androgen deficiency (OPIAD) Pain Physician. 2012;15(3, suppl):ES145–ES156. [PubMed] [Google Scholar]
- 15.De Maddalena C., Bellini M., Berra M., Meriggiola M.C., Aloisi A.M. Opioid-induced hypogonadism: why and how to treat it. Pain Physician. 2012;15(3, suppl):ES111–ES118. [PubMed] [Google Scholar]
- 16.Vuong C., Van Uum S.H., O'Dell L.E., Lutfy K., Friedman T.C. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31(1):98–132. doi: 10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams M.L., Sewing B., Forman J.B., Meyer E.R., Cicero T.J. Opioid-induced suppression of rat testicular function. J Pharmacol Exp Ther. 1993;266(1):323–328. [PubMed] [Google Scholar]
- 18.Rubinstein A.L., Carpenter D.M., Minkoff J.R. Hypogonadism in men with chronic pain linked to the use of long-acting rather than short-acting opioids. Clin J Pain. 2013;29(10):840–845. doi: 10.1097/AJP.0b013e31827c7b5d. [DOI] [PubMed] [Google Scholar]
- 19.Loughlin J., Seeger J.D., Eng P.M., et al. Risk of hyperkalemia in women taking ethinylestradiol/drospirenone and other oral contraceptives. Contraception. 2008;78(5):377–383. doi: 10.1016/j.contraception.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Loughlin J., Quinn S., Rivero E., et al. Tegaserod and the risk of cardiovascular ischemic events: an observational cohort study. J Cardiovasc Pharmcol Ther. 2010;15(2):151–157. doi: 10.1177/1074248409360357. [DOI] [PubMed] [Google Scholar]
- 21.Ziyadeh N., Fife D., Walker A.M., Wilkinson G.S., Seeger J.D. A matched cohort study of the risk of cancer in users of becaplermin. Adv Skin Wound Care. 2011;24(1):31–39. doi: 10.1097/01.ASW.0000392922.30229.b3. [DOI] [PubMed] [Google Scholar]
- 22.Kuo Y.F., Raji M.A., Chen N.W., Hasan H., Goodwin J.S. Trends in opioid prescriptions among Part D Medicare recipients from 2007 to 2012. Am J Med. 2016;129(2):221.e21–221.e30. doi: 10.1016/j.amjmed.2015.10.002. [published correction appears in Am J Med. 2017;130(5):615-616] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke H., Soneji N., Ko D.T., Yun L., Wijeysundera D.N. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;348:g1251. doi: 10.1136/bmj.g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunn K.M., Saunders K.W., Rutter C.M., et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seal K.H., Shi Y., Cohen G., et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940–947. doi: 10.1001/jama.2012.234. [published correction appears in JAMA. 2012;307(23):2489] [DOI] [PubMed] [Google Scholar]
- 26.Klabunde C.N., Warren J.L., Legler J.M. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(8, suppl):IV-26–IV-35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 27.Klein J.P., Moeschberger M.L. Springer-Verlag; New York, NY: 1997. Survival Analysis: Techniques for Censored and Truncated Data. [Google Scholar]
- 28.Allison P.D. SAS Institute; Cary, NC: 1995. Survival Analysis Using the SAS System: A Practical Guide. [Google Scholar]
- 29.Brummett C.M., Waljee J.F., Goesling J., et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6) doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun E.C., Darnall B.D., Baker L.C., Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286–1293. doi: 10.1001/jamainternmed.2016.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connolly J., III, Javed Z., Raji M.A., Chan W., Kuo Y.F., Baillargeon J. Predictors of long-term opioid use following lumbar fusion surgery. Spine (Phila Pa 1976) 2017;42(18):1405–1411. doi: 10.1097/BRS.0000000000002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baillargeon J., Kuo Y.F., Westra J.R., Urban R.J., Goodwin J.S. Testosterone prescribing in the United States, 2002-2016. JAMA. 2018;320(2):200–202. doi: 10.1001/jama.2018.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigen R., O'Donnell C.I., Barón A.E., et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829–1836. doi: 10.1001/jama.2013.280386. [published correction appears in JAMA. 2014;311(9):967] [DOI] [PubMed] [Google Scholar]
- 34.Finkle W.D., Greenland S., Ridgeway G.K., et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma R., Oni O.A., Gupta K., et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36(40):2706–2715. doi: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
- 36.Baillargeon J., Urban R.J., Kuo Y.F., et al. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother. 2014;48(9):1138–1144. doi: 10.1177/1060028014539918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.