Abstract
Celiac Disease (CeD) is defined as a chronic small intestinal immune-mediated enteropathy that is precipitated by exposure to dietary gluten in genetically predisposed individuals. CeD is one of the most common autoimmune disorders affecting around 1% of the population worldwide. Currently, the only acceptable treatment for CeD is strict, lifelong adherence to a gluten-free diet (GFD) which can often present a challenging task. A GFD alone is not sufficient to control symptoms and prevent mucosal damage that can result from unintentional gluten exposure. Moreover, long-term complications can occur in many patients. Consequently, there is an unmet need for non-dietary therapies for the management of CeD. Such therapies could serve as an adjunct to the GFD but eventually may replace it. This review will focus on and discuss non-dietary therapies currently in clinical development for the management of CeD.
Methodology
We searched clinicaltrials.gov and PubMed to extract articles about celiac disease. We used keywords including, but not limited to, “celiac disease,” “non-dietary,” “therapeutics,” “pathophysiology,” “Endopeptidases,” “tight junction modulators,” “vaccine,” and “Nexvax2”. We focused mainly on articles that conducted pathophysiologic and therapeutic research in human trials.
Keywords: Celiac Disease, Drug Therapy, Latiglutenase, Larazotide Acetate, Nexvax2, Necator americanus
Abbreviations used in this paper: AN-PEP, Aspergillus niger endopeptidase; CeD, celiac disease; GFD, gluten-free diet; IEL, intraepithelial lymphocyte; IFN-γ, interferon gamma; IL, interleukin; PEP, prolyl endopeptidase; RCD, refractory celiac disease; TG2, transglutaminase 2; TNF-α, tumor necrosis factor alpha
Summary.
The review discusses the latest non-dietary therapies currently in clinical development for the management of celiac disease.
Celiac disease (CeD) is defined as a chronic small intestinal immune-mediated enteropathy that is precipitated by exposure to dietary gluten in genetically predisposed individuals.1 CeD is one of the most common autoimmune disorders, affecting around 1% of the population worldwide.2 There has been a notable rise in the prevalence of CeD in the last 50 years and a rise in the rate of diagnosis in the last 10 years.3 According to the Corazza-Villanacci classification, histopathology of duodenal biopsy tissue in CeD is divided into nonatrophic lesions (grade A) and atrophic lesions (grade B), grade B lesions are further divided into grade B1, in which the villous to crypt ratio is less than 3:1, with detectable villi, and grade B2, in which the villi are no longer detectable.4
Currently, the only acceptable treatment for CeD is a strict, lifelong adherence to a gluten-free diet (GFD), which often presents a challenging task.3 Despite the rigid nature of the GFD, strict adherence is highly encouraged. Untreated and partially treated CeD is associated with an increased risk for poor outcomes such as infertility, osteoporosis, neuropathies, and lymphomas. Adhering to a GFD is associated with a reduction of these outcomes.5
However, the GFD alone is frequently not sufficient to control symptoms and prevent mucosal damage that can result from unintentional gluten exposure. Adherence to the diet can pose a challenge and lead to frustration, and many CeD patients are exposed to gluten inadvertently via contamination of food, medications, and supplements.6 Owing to the substantial difficulties associated with adherence to the GFD, most patients with CeD are highly interested in nondietary therapies for the management of their condition, as shown by patient survey data on this topic.7
Pathogenesis of Celiac Disease
The pathogenesis of CeD is summarized in Figure 1. The pathways that are targeted for nondietary therapies of celiac disease discussed below are summarized with the respective therapy in Figure 2. Other therapies are discussed in this section. The pathogenesis of refractory celiac disease (RCD) is not elaborated here, as it is beyond the scope of this review.
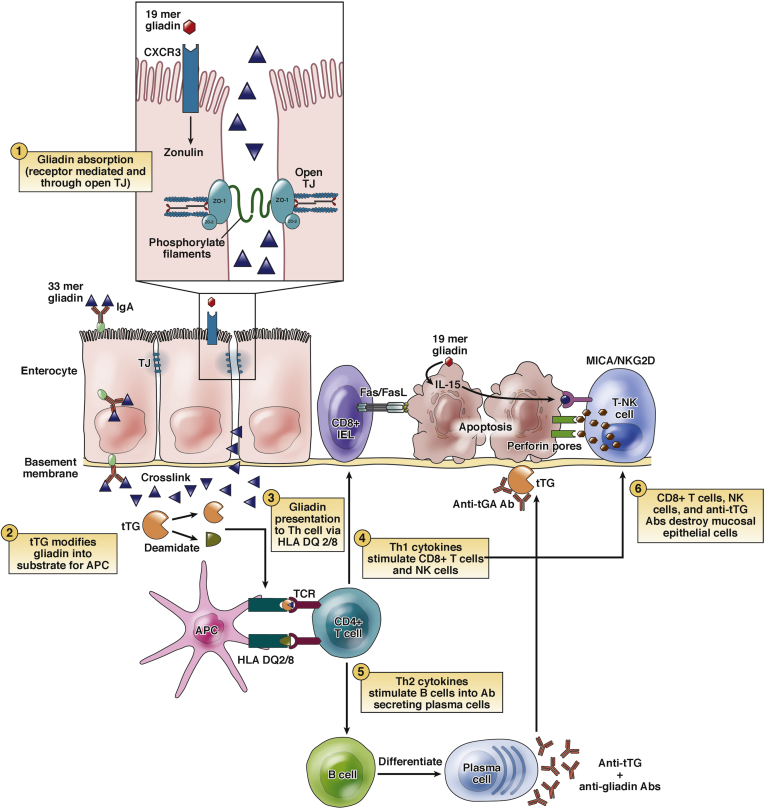
Figure 1.
Pathophysiology of celiac disease. Gliadin resists digestion in the duodenal lumen and may be directly toxic to the enterocytes of CeD patients. Undigested gliadin peptides cross into the intestinal submucosa via paracellular and transcellular passage. Tissue transglutaminase (tTG) deaminates gliadin peptides in the lamina propria. Deaminated gliadin is then recognized by HLA-DQ2 or HLA-DQ8 molecules on antigen-presenting cells (APCs), stimulating an immune reaction. This leads to the activation of Th1 and Th2 inflammatory pathways. Th1 cells stimulate CD8+ and natural killer (NK) cells, which causes apoptosis of the enterocytes via the Fas/FasL system. Th2 cells stimulate B cells to differentiate into plasma cells that produce antibodies (anti-tTG and antigliadin). The interaction between extracellular tTG and anti-tTG may cause further epithelial damage. Modified with permission from Di Sabatino and Corazza.58 NK, natural killer; TJ, tight junction.
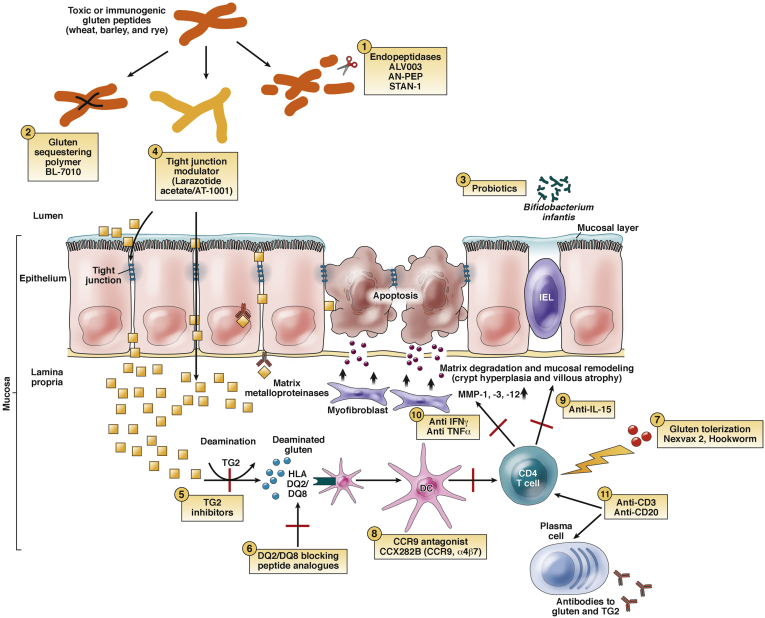
Figure 2.
Novel therapies for celiac disease. (1) Endopeptidases: latiglutenase (formerly ALV003), AN-PEP, and STAN-1 degrade gluten into nonimmunogenic particles, thereby alleviating mucosal injury. (2) Gluten-sequestering polymer: BL-7010 binds to intraluminal gliadin and prevents its release and breakdown into immunogenic peptides. (3) Probiotics: Bifidobacterium infantis protects epithelial cells from damage caused by gliadin by downregulating the proinflammatory immune response. (4) Tight junction modulator: larazotide acetate/AT-1001 works as a tight junction modulator to prevent gliadin-induced epithelial permeability. (5) TG2 inhibitor: blocks the transformation of native gliadin peptides to the far more antigenically potent deamidated gliadin peptides. (6) DQ2/DQ8 blocking peptide analogues prevent presentation of gliadin from activating T cells. (7) Gluten tolerization: Nexvax2 and hookworm (Necator americanus) inoculation aim to downregulate the immune response to gluten. (8) CCR9 antagonist: CCX282B blocks this chemokine receptor to block lymphocyte homing. (9) Anti-IL-15 is a monoclonal antibody that may prevent immune-mediated tissue destruction. (10) Anti IFN-γ may prevent inflammation. (11) Anti-CD3 antibodies suppress gluten activated T cells; anti-CD20 antibodies suppress B cells. Modified with permission from Castillo et al.59 MMP, matrix metalloproteinase.
A critical step in CeD pathogenesis is the ingestion of gluten which encompasses the insoluble prolamin polypeptides found in wheat (gliadins and glutenins), rye (secalin), barley (hordein), and other closely related grains.8 Gluten is resistant to proteolytic breakdown by gastric, pancreatic, and intestinal digestive proteases due to its high proline content. These nondigested gliadin polypeptides cross into the intestinal submucosa by mechanisms that are not well understood but are considered to involve both paracellular and transcellular passage.9 The gluten peptides are deamidated in the lamina propria by transglutaminase 2 (TG2) enzyme.10
In vitro studies have shown that Th1 and Th2 cytokines such as interferon gamma (IFN-γ), IL-4 and IL-10 are elevated in the serum of untreated celiac subjects. They decrease significantly in treated celiac subjects on a GFD.11 Similarly, activated intraepithelial lymphocytes in CeD have been shown to express tumor necrosis factor alpha (TNF-α) in the mucosa.12 A study has shown that RNA transcripts of TNF- α were significantly upregulated in patients with RCD when compared with patients with active uncomplicated CeD. Thus, anti-TNF-α agents such as infliximab do not have a therapeutic role in uncomplicated CeD.13
The interleukin-15 (IL-15) and IL-21 cytokines appear to play an important role in CeD pathogenesis. High numbers of IL-21-producing lamina propria T cells were observed in pediatric CeD lesions including early/mild Marsh 1-2 lesions.14 Furthermore, IL-15 upregulates IL-21 production in CeD.15 Increased expression of IL-15 in enterocytes, lamina propria mononuclear cells, and the mucosa of untreated CeD and RCD results in overexpression of IL-15 receptor α on intraepithelial lymphocytes (IELs) causing increased cell proliferation and production of inflammatory cytokines. Thus, in turn, results in cytotoxicity and decreased apoptosis of IELs.12, 16 Antiapoptotic signals produced by IL-15 have been found to be due to Bcl-2 and/or Bcl-xL, IL-15Rβ, Jak3, and STAT5. Murine models have shown that treatment with this antibody induces IEL apoptosis and abolishes the massive IEL accumulation in mice overexpressing human IL-15 in their gut epithelium.17
During antigenic stimulation of T cells via their T cell receptor, CD28 and CTLA-4 molecules (expressed by T lymphocytes) interact with their respective ligands, B7-1 (CD80) and B7-2 (CD86). As part of this interaction, CD28 provides a costimulatory signal to T cell activation, while CTLA-4 acts as a negative regulator of T cell activation. As such, these molecules may also have a role in the pathogenesis of CeD and, therefore, a potential role in CeD therapeutics.18
NonDietary Therapies for CeD
Endopeptidase
Latiglutenase (formerly ALV003) is a combination of ALV001, an EP-B2 cysteine endopeptidase that is derived from the endosperm of germinating barley, and ALV002, a Sphingomonas capsulata prolyl endopeptidase (PEP).19 ALV001 degrades gluten proteins and reduces the immunogenic potential of gluten.20, 21 Similarly, ALV002 catalyzes the postproline hydrolysis of proteins and peptides, similarly reducing the immunogenic potential of gluten.21, 22 A phase 0 study found that ALV003 pretreatment can abolish peripheral blood T cell IFN-γ ELISpot responses to an immunogenic gliadin-derived 33mer induced by gluten (16 g daily for 3 days) in patients with CeD.23 The results of 2 phase I, single, escalating-dose clinical trials showed that ALV003 appears to be stable in the fed stomach and degrades dietary gluten in this compartment.24 All escalating dose levels of ALV003 (100, 300, 900, and 1800 mg) were well tolerated with no serious adverse events or allergic reactions.24 In phase II clinical trial, ALV003 was shown to attenuate gluten-induced small intestinal mucosal injury in patients with CeD who underwent a 6-week gluten challenge. Histologic changes in duodenal biopsies in the placebo group showed evidence of mucosal injury after gluten challenge. However, no significant mucosal deterioration was observed in duodenal biopsies in the ALV003 group, which is a promising finding. Interestingly, there were no statistically significant differences in symptoms between CeD patients who received ALV003 and the CeD control group.25 Another phase II clinical trial in CeD patients with persisting symptoms showed no improvement of histologic findings and symptom scores in patients who received latiglutenase when compared with placebo.26 In a post hoc subgroup analysis of seropositive CeD patients, those receiving the highest dose of latiglutenase while on a GFD had some improvement in bloating and tiredness. There was no improvement in abdominal pain and constipation, which is a major concern.27 While additional studies are required to fully evaluate the therapeutic potential of endopeptidase therapy in celiac patients on a GFD, the previous studies suggest they could represent a viable therapeutic option given improvement in both mucosal inflammation and clinical symptoms, at least as seen in the post hoc subgroup analysis. See Table 1 for a summary of phase II clinical trials.
Table 1.
Summary of Phase II Clinical Trials
| Treatment Class | Agent | Mechanism of Action | ClinicalTrials.gov | Summary of Clinical Trial Results |
|---|---|---|---|---|
| Endopeptidases | Latiglutenase (formerly ALV003) | Enzymatic degradation of gluten | NCT00959114/NCT01255696 | Attenuated gluten challenge–induced small intestinal mucosal injury and increases in intraepithelial lymphocyte counts. |
| NCT01917630 | Showed no improvement of histologic findings and symptom scores when compared with placebo. In a post hoc subgroup analysis of seropositive CeD patients, symptomatic improvement was observed in those received the highest dose of latiglutenase while on a GFD. | |||
| AN-PEP | PEP derived from the fungus Aspergillus niger | NCT00810654 | It was not effective in preventing mucosal damage induced by 7 g of gluten per day for 2 weeks | |
| Gluten-sequestering polymer | BL-7010 | Sequesters intraluminal gliadin and prevents its breakdown into immunogenic peptides | NCT01990885 | Not available |
| Probiotics | Bifidobacterium infantis | Protect epithelial cells from damage caused by gliadin | NCT01257620 | Significantly improved CeD symptoms. Final vs baseline IgA tTG and IgA DGP antibody concentration ratios were lower than the control group. Intestinal permeability, measured by LAMA fractional excretion ratio, was nonsignificant. |
| Tight junction modulators | Larazotide acetate (AT-1001) | Tight junction modulator preventing gliadin-induced permeability | NCT00362856 | Limited gluten-challenge induced gastrointestinal symptom severity. |
| NCT00492960 | Reduced gluten-challenge induced symptoms and serum tTG increase. No significant difference in the designated primary outcome measure of intestinal permeability (LAMA ratios). | |||
| NCT01396213 | In patients with NRCD on a GFD a dose of 0.5 mg reduced CeD symptoms better than placebo. | |||
| TG2 inhibitors | ZED 1227 | Direct and specific inhibitor of TG2 | 2017-002241-30 (Clinical Trials Register [European Union]) | Not available |
| Gluten tolerization and immunomodulation | Hookworm infection: Necator americanus | Regulate the immune system by inhibiting Th1 immune response | NCT01661933 | Hookworm infection group: experienced less lethargy than control, showed unchanged intraepithelial lymphocyte counts and Marsh scores following gluten challenge. Intestinal T cells expressing IFN-γ were reduced following hookworm infection with an increase in CD4(+) Foxp3(+) regulatory T cells. |
| NCT00671138 | Hookworm infection imposed no obvious benefit on CeD pathology. | |||
| Immune cell-targeted therapies | AMG 714 | Anti-IL-15 monoclonal antibody | NCT02637141/NCT02633020/NCT03439475 | Not available |
| CCR9 antagonist (CCX282-B) | Block the chemokine receptor CCR9 to prevent intestinal T-cell homing | NCT00540657 | Not available |
AN-PEP, Aspergillus niger endopeptidase; CeD, celiac disease; DGP, deamidated gliadin peptide; GFD, gluten-free diet; IFN-γ, interferon gamma; IL, interleukin; LAMA, lactulose-to-mannitol; NRCD, nonresponsive celiac disease; PEP, prolyl endopeptidase; TG2, transglutaminase 2; TNF-α, tumor necrosis factor alpha; tTG, tissue transglutaminase.
An additional endopeptidase that has been explored is STAN-1, a cocktail of microbial enzymes designed to degrade gluten before absorption in the gastrointestinal tract. It was hypothesized that treatment with STAN-1 would lead to a clinical decrease in tTG-IgA levels in CeD patients. In a randomized, double-blinded, placebo-controlled trial, 35 patients with CeD on a GFD for at least 1 year with persistently elevated tTG-IgA were randomized to STAN-1 or placebo for 12 weeks in conjunction with a daily load of 1 g of gluten. The primary endpoint of the study was the tTG-IgA titer. The authors found no significant difference between the 2 groups, suggesting that a therapeutic role for this agent remains unclear.28
Aspergillus niger endopeptidase (AN-PEP) is a second PEP derived from the fungus Aspergillus niger. In a randomized, double-blinded, placebo-controlled study of 16 adult patients with CeD on a GFD, AN-PEP was well tolerated. The CeD quality of subjects consuming gluten with placebo or gluten with AN-PEP did not deteriorate significantly and no difference was observed between the 2 groups. This can be attributed to small sample size and the short duration of the gluten challenge; as no significant deterioration was observed regarding immunohistological and flow cytometric evaluation between the groups. It remains to be seen whether AN-PEP can be effective in preventing mucosal damage induced by gluten.
Gluten-Sequestering Polymers (BL-7010)
BL-7010 is a synthetic, nonabsorbable copolymer of styrene sulfate with hydroxyethyl methacrylate. This polymer is reported to have high affinity to α-gliadin peptides. Its proposed mechanism of action is to sequester intraluminal gliadin and prevent its breakdown into immunogenic peptides.29 A preclinical study in a NOD-DQ8 mouse model showed that BL-7010 was effective at preventing gluten-induced reduction in villous-to-crypt ratios, intraepithelial lymphocytosis, and alterations in paracellular permeability.29 The study also showed that BL-7010 interacted with high affinity with gliadin and had no interaction with tested vitamins and digestive enzymes.29 A phase I/II, randomized, double-blind, placebo-controlled study was conducted to evaluate the safety and systemic exposure of single escalating administrations and repeated administration of BL-7010 in well-controlled CeD. This clinical trial has been completed, and the results are yet to be published (NCT01990885). While the concept of sequestering gluten before it reaches the small intestine is an attractive one, more data are needed regarding the safety profile of such an agent given the potential that it could bind nonspecifically to other important medications.
Probiotics
The intestinal microbiota plays a significant role in maintaining a healthy status. As we learn more about the microbiome and its relationship to health and disease, it appears that imbalance in the microbiome might be implicated in various disease states. In particular, dysbiosis has been associated with chronic inflammatory disorders, including CeD. A condition called small intestinal bacterial overgrowth has been well described in the literature, and associated with diseases such as celiac and irritable bowel syndrome. Probiotics work by downregulating the proinflammatory immune response in CeD patients.30, 31 A recently published study compared fecal bifidobacterial concentrations among celiac patients and healthy individuals before and after the daily intake of 100 g of yogurt containing probiotic for 30 days.32 Fecal bifidobacterial concentration was markedly increased in celiac patients after the probiotic supplementation, but it did not reach the concentration found in healthy individuals prior to probiotic consumption.32 Another recently published study confirmed that probiotic Lactobacillus strains have enzymatic abilities for hydrolyzing gluten peptides.33 The results suggested that probiotic formulas may be used as an adjunctive dietary treatment for CeD patients.33 The use of Bifidobacterium natrum life start strain was assessed in the treatment of CeD. The testing period was approximately 3 weeks between the results of the serological testing and before the intestinal biopsy procedure during which time participants were consuming a gluten-containing diet. B. infantis ingestion was associated with improved gastrointestinal symptoms based on the Gastrointestinal Symptom Rating Scale compared with placebo. The final: baseline IgA tTG and IgA DGP antibody concentration ratios were lower in the probiotic group. Intestinal permeability, measured by the urinary lactulose-to-mannitol fractional excretion ratio, was not significantly affected by treatment.34 Currrently, there are no firm guidelines to recommend probiotic use indiscriminantly in all patients with CeD. However, the data suggest a strong adjunctive role in symptom management and management of conditions like small intestinal bacterial overgrowth.
Tight Junction Modulator: Larazotide Acetate
Larazotide acetate (or AT-1001) is a synthetic octapeptide whose sequence was derived from similarity to the zonula occludens toxin secreted by Vibrio cholerae. Larazotide acetate acts to improve tight junction integrity, preventing gliadin-induced permeability and thereby reducing small intestinal inflammation. Several phase I and phase II clinical trials involving larazotide acetate have been completed. In phase I, double-blind, randomized placebo-controlled safety study, larazotide acetate was found to be safe, well tolerated and diminished diarrhea after gluten exposure. However, there was no statistical difference in permeability and proinflammatory cytokine production.35 A phase II, placebo-controlled study showed that larazotide acetate was well tolerated and prevented the increase in gastrointestinal symptom severity induced by gluten challenge.36 A recently completed phase II clinical trial showed that larazotide acetate 0.5 mg taken 3 times daily reduced the symptoms in CeD patients on a GFD better than a GFD alone.37 The study represented a successful trial of larazotide as a novel therapeutic agent in patients with CeD who are symptomatic despite a GFD.37 larazotide acetate was also found to reduce gluten-induced immune reactivity (ie, tTG response) and symptoms in patients with CeD undergoing gluten challenge and was generally well tolerated.38 While these data are promising with regard to symptom improvement, lack of information on histologic improvement despite symptom improvement is a drawback in these studies; the implications of that are unknown.
HLA-DQ2 or HLA-DQ8 Blockers
Nearly all CeD patients carry either human leukocyte antigen DQ2 or DQ8.39 In susceptible individuals, the deamidated gliadin peptides bind with high affinity to DQ2 or DQ8 expressed on antigen-presenting cells which, in turn, stimulate gliadin-specific T cells.40 The activation of gliadin-specific Th1 and Th2 inflammatory pathways may have a self-perpetual role in ensuring the entry of additional gliadin peptides to the lamina propria through increased intestinal permeability and/or a CD71- sIgA-gliadin complex transport mechanisms.41, 42 HLA-DQ2 and HLA-DQ8 lead to the preferential presentation of gliadin peptides to CD4+ helper T cells in the lamina propria, leading to induction of secretion of IFN-γ dominated inflammatory cytokines.3, 10 DQ-2/DQ-8 blocking peptide analogs act by preventing immune activation. This novel therapy remains in the preclinical phase. Modified gluten peptide has been shown to have a higher binding affinity for HLA-DQ2 compared with natural gluten peptides, but it is considered insufficient to markedly suppress the activation of gluten-reactive T cells in the intestine in CeD patients. Moreover, some of these peptides were found to be nonimmunogenic and block gluten-induced immune responses.43, 44 Overall, it remains unclear whether this therapeutic strategy can be successful in the management of CeD as there is no available data on in vivo efficacy and safety of DQ-2/DQ-8 blockers.
TG2 Inhibitors
TG2 plays a major role in CeD pathogenesis via deamidation and transamidation of gluten peptides leading to an immune-based response characterized by inflammation in the small intestinal mucosa. Thus, inhibition of TG2 may prevent the presentation of gluten peptides by HLA-DQ2 and HLA-DQ8.45 A proof-of-concept study was conducted to investigate whether 2 TG2 inhibitors, cell-impermeable R281, and cell-permeable R283, can prevent the toxic effects of gliadin in vitro (using intestinal Caco cells) and ex vivo (using organ culture of celiac patients’ derived small intestinal biopsies). TG2 inhibitors proved to be protective against gliadin induced toxic effects, an increase in CD25 and IL15 positive cells, upregulation of T regulatory cells, and crypt cell proliferation.46 A new generation of TG2 inhibitors was developed in 2009 based on a high-affinity thiol-binding group. This generation of TG2 inhibitors showed 70- to 225-fold specificity for intestinal TG2 over other TGs on in vitro testing. ZED1227 is a new drug that acts as a direct and specific inhibitor to TG2 in patients with CeD. The drug was already tested in phase I clinical trials and have been shown to be safe in healthy volunteers. A proof-of-concept study will enroll patients with CeD in a phase II clinical trial aiming to show the protective effect of ZED1227 during a 6-week gluten challenge (Clinical Register: 2017-002241-30). As TG2 plays a pivotal role in inflammation and wound healing in the intestines, the safety and efficacy of these drugs remain unclear.
Gluten Tolerization and Immunomodulation
Nexvax2
Nexvax2 is a peptide-based, epitope-specific, gluten tolerizing agent utilizing the principles of immunotherapy in a similar fashion to the desensitization strategies used for allergic conditions. A randomized, double-blind, placebo-controlled phase I study showed that antigenic peptides recognized by CD4+ T cells in CeD can be safely administered to patients at high maintenance dose levels without immune activation if preceded by gradual dose escalation.47 Two randomized, double-blind, placebo-controlled phase I studies showed that the maximum tolerated dose of Nexvax2 (150 μg) given intradermally, twice weekly over 8 weeks had modified the immune responsiveness to Nexvax2 peptides without deterioration in the duodenal histology.48 The gastrointestinal symptoms that followed the first intradermal administration of the vaccine resembled those symptoms associated with oral gluten challenge.48 We believe that gastrointestinal symptoms induced by Nexvax2 administration indicate intestinal immune activation similar to that of oral gluten ingestion. Overall, Nexvax2’s safety profile was considered to be acceptable. Further data are required to determine its role in CeD management.
Hookworm Infection (Necator americanus)
It is hypothesized that intestinal parasitic infections help regulate the immune system and prevent autoimmune and allergic diseases. According to the “hygiene hypothesis,” the lack of early childhood exposure to intestinal parasites has created a predisposition to develop autoimmune diseases in developed countries.49, 50 A randomized, double-blind, placebo-controlled trial was conducted to study the effect of hookworm infection on gluten challenge in CeD.51 There was no statistical difference in the histological damage and induction of IFN-γ producing cells in the peripheral blood after a 5-day gluten challenge in patients inoculated with the hookworm Necator americanus and the placebo group.51 The study was continued for another 12 weeks of gluten challenge. The study results have not been published yet (NCT01661933). Another study examined the influence of experimental hookworm infection in preventing intestinal damage and symptoms using escalating gluten challenges in CeD patients. Twelve CeD patients on a GFD were inoculated with N. americanus larvae within a 52-week duration and were given escalating doses of gluten. The median villous height-to crypt depth ratios did not decrease as predicted after the gluten challenge, quality of life indices improved and the mean tTG-IgA titers declined despite the escalating doses of gluten.52 Based on these findings, we believe that induction of infection with N. americanus can promote immune regulation with tolerance to gluten in CeD. Given the nature of this novel therapy, it is difficult to imagine the routine clinical use of N. americanus in the management of CeD.
Nanoparticle
TIMP-GLIA (Cour Pharmaceuticals, Northbrook, IL) is a nanoparticle-based investigational product, which proposes to develop immune tolerance to gluten exposure through noninflammatory antigen presentation. The Food and Drug Administration has granted Fast Track Designation for the product, and a phase I study is underway to characterize its safety and tolerability when either 1 or 2 intravenous doses are given to subjects with CeD (NCT03486990).
Cathepsin S Inhibitor
Cathepsin is a lysosomal cysteine protease that has a role in antigen presentation to MHC class II. RO5459072 is an orally administered competitive inhibitor of cathepsin S.53 A phase 1 randomized, double-blind, placebo-controlled, multiple dose, parallel study to investigate the pharmacokinetics, pharmacodynamics, safety, and tolerability of RO5459072 in subjects who have well-controlled CeD. The primary outcome of this study was measured by the numbers of participants who responded to a gluten challenge. The study has been completed, and we await the publication of results (NCT02679014).
Immune Cell–Targeted Therapies
IL-15 Antagonists
IL-15 is a cytokine that regulates the activation and proliferation of T lymphocytes and natural killer cells. IL-15 overexpression leads to increased numbers of IELs, a characteristic finding in CeD. IL-15 has antiapoptotic properties. Many antiapoptotic pathways of IL-15 have been identified, including Bcl-2 or Bcl-XL, JAK 1, and JAK 3. Tofacitinib is a pan-JAK inhibitor that blocks 1L-15 signaling. It has been approved by the Food and Drug Administration for the treatment of rheumatoid arthritis. Tofacitinib has been effective in a transgenic mouse model of CeD in which there is overexpression of human IL-15. Tofacitinib therapy led to a lasting reversal of pathologic manifestations.54 Currently, tofacitinib is being considered for use in celiac disease patients with RCD in the future. Another potential agent is AMG 714, a human monoclonal antibody to IL-15. Three phase II clinical trials investigating this agent are underway in patients with RCD and nonresponsive CeD (NCT02637141, NCT02633020, and NCT03439475). Moreover, a phase I clinical trial is underway in RCD patients using Hu-Mik-β-1, a monoclonal antibody that blocks IL-2 and IL-15 (NCT01893775).
IL-10 Agonists
Although this review does not focus on the management and treatment of RCD, it is worth mentioning an exciting possible therapeutic, the IL-10 agonist. IL-10 is an immune-modulating interleukin secreted by T regulatory cells and it may have a protective role in CeD when exposed to low doses of gluten. So far, it has only been studied in the RCD patient population. Its role in the treatment of CeD has not yet been defined.
Anti-CD3 and Anti-CD20
CD3 antigen is part of the T cell receptor complex that helps activate both T helper cells and cytotoxic T cells. Theoretically, therapy with anti-CD3 antibody may suppress gluten-activated T cells and therefore reduce inflammation in CeD. Studies have identified that T regulatory cells can be induced in vitro by anti-CeD3 antibodies and may promote tolerance to gluten in CeD.55 However, published data are lacking to support the use of anti-CD3 in CeD.
Also, B cells play a major role in the pathogenesis of CeD via the production of various antibodies. CD20 is a B-cell marker that is targeted by various monoclonal antibodies. However, it is known that the generation of IgA plasmablasts in the gut mucosa is not abolished by CD20 antagonists.56 Therefore, CD20 antagonists may not be effective in the treatment of CeD. Although several monoclonal antibodies against CD20 have already been approved for clinical use, their role in the treatment of CeD has not yet been established.
CCR9 Antagonist
CCR9 is a chemokine receptor known to be essential for lymphocyte migration into the intestine. CCX282-B is a CCR9 receptor antagonist that was studied as a therapeutic option for CeD and inflammatory bowel disease. A phase II clinical trial was performed in patients with CeD who followed a strict GFD for at least 24 months to evaluate the effect of CCX282-B on villous height /crypt depth ratio of small intestinal biopsy specimens before and after gluten challenge. This study has been completed, but the results have not been published yet (NCT00540657). Given that the lymphocyte homing mechanism involving CCR9 is not antigen-specific, concerns have been raised that this sort of agent could increase the risk of infection, particularly, gastrointestinal infections. The safety of these drugs remains unclear.
Expert Commentary
The GFD is the only available treatment for CeD and will remain the mainstay of treatment until safe adjuncts and alternatives are available. While the majority of CeD patients respond to the GFD, a significant proportion continues to have symptoms and/or laboratory abnormalities consistent with CeD. The ubiquitous nature of gluten makes adhering to a GFD difficult and requires patient education, continuous motivation, and follow-up, all of which add to the disease burden. Hence, there is an unmet need for potential nondietary therapies for CeD. Currently, the aim of these nondietary therapies is to serve as adjunctive agents in conjunction with the GFD to control symptoms not controlled by the GFD alone and eventually to protect the mucosa from the action of gliadin peptides. While many trials are ongoing or underway to explore nondietary treatments for celiac disease, there is no consensus on outcome measures in CeD trials. This issue was explored by expert-based recommendations in an article published earlier this year. To increase transparency and comparability of CeD therapeutic trials, careful evaluation and reporting of outcomes are needed.57
Footnotes
Conflicts of interest This author discloses the following: Ciaran P. Kelly has acted as a scientific consultant for Amgen, Cellimmune, Cour, Glutenostics, Immunogenx, Innovate, and Takeda. The remaining authors disclose no conflicts.
References
- 1.Ludvigsson J.F., Leffler D.A., Bai J.C., Biagi F., Fasano A., Green P.H., Hadjivassiliou M., Kaukinen K., Kelly C., Leonard J.N., Lundin K.E., Murray J.A., Sanders D.S., Walker M.M., Zingone F., Ciacci C. The Oslo definitions for coeliac disease and related terms. Gut. 2012;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh P., Arora A., Strand T.A., Leffler D.A., Catassi C., Green P.H., Kelly C.P., Ahuja V., Makharia G.K. Global prevalence of celiac disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018:823–836. doi: 10.1016/j.cgh.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 3.Rubio-Tapia A., Hill I.D., Kelly C.P., Calderwood A.H., Murray J.A. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656–676. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corazza G.R., Villanacci V. Coeliac disease. J Clin Pathol. 2005;58:573–574. doi: 10.1136/jcp.2004.023978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunn B., Murphy K.E., Greenblatt E.M. Unexplained infertility and undiagnosed celiac disease: study of a multiethnic canadian population. J Obstet Gynaecol Canada. 2018;40:293–298. doi: 10.1016/j.jogc.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Sansotta N., Amirikian K., Guandalini S., Jericho H. Celiac disease symptom resolution: Effectiveness of the gluten-free diet. J Pediatr Gastroenterol Nutr. 2018;66:48–52. doi: 10.1097/MPG.0000000000001634. [DOI] [PubMed] [Google Scholar]
- 7.Tennyson C.A., Simpson S., Lebwohl B., Lewis S., Green P.H.R. Interest in medical therapy for celiac disease. Therap Adv Gastroenterol. 2013;6:358–364. doi: 10.1177/1756283X13492580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rostom A., Murray J.A., Kagnoff M.F. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Ménard S., Lebreton C., Schumann M., Matysiak-Budnik T., Dugave C., Bouhnik Y., Malamut G., Cellier C., Allez M., Crenn P., Schulzke J.D., Cerf-Bensussan N., Heyman M. Paracellular versus transcellular intestinal permeability to gliadin peptides in active celiac disease. Am J Pathol. 2012;180:608–615. doi: 10.1016/j.ajpath.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Sollid L.M. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–655. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 11.Manavalan J.S., Hernandez L., Shah J.G., Konikkara J., Naiyer A.J., Lee A.R., Ciaccio E., Minaya M.T., Green P.H., Bhagat G. Serum cytokine elevations in celiac disease: Association with disease presentation. Hum Immunol. 2010;71:50–57. doi: 10.1016/j.humimm.2009.09.351. [DOI] [PubMed] [Google Scholar]
- 12.Di Sabatino A., Ciccocioppo R., Cupelli F., Cinque B., Millimaggi D., Clarkson M.M., Paulli M., Cifone M.G., Corazza G.R. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut. 2006;55:469–477. doi: 10.1136/gut.2005.068684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caruso R., Marafini I., Sedda S., Del Vecchio Blanco G., Giuffrida P., MacDonald T.T., Corazza G.R., Pallone F., Di Sabatino A., Monteleone G. Analysis of the cytokine profile in the duodenal mucosa of refractory coeliac disease patients. Clin Sci (Lond) 2014;458:451–458. doi: 10.1042/CS20130478. [DOI] [PubMed] [Google Scholar]
- 14.van Leeuwen M.A., Lindenbergh-Kortleve D.J., Raatgeep H.C., de Ruiter L.F., de Krijger R.R., Groeneweg M., Escher J.C., Samsom J.N. Increased production of interleukin-21, but not interleukin-17A, in the small intestine characterizes pediatric celiac disease. Mucosal Immunol. 2013;6:1202–1213. doi: 10.1038/mi.2013.19. [DOI] [PubMed] [Google Scholar]
- 15.Sarra M., Cupi M.L., Monteleone I., Franzè E., Ronchetti G., Di Sabatino A., Gentileschi P., Franceschilli L., Sileri P., Sica G., Del Vecchio Blanco G., Cretella M., Paoluzi O.A., Corazza G.R., Pallone F., Monteleone G. IL-15 positively regulates IL-21 production in celiac disease mucosa. Mucosal Immunol. 2013;6:244–255. doi: 10.1038/mi.2012.65. [DOI] [PubMed] [Google Scholar]
- 16.Mention J.-J., Ben Ahmed M., Bègue B., Barbe U., Verkarre V., Asnafi V., Colombel J.F., Cugnenc P.H., Ruemmele F.M., McIntyre E., Brousse N., Cellier C., Cerf-Bensussan N. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–745. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 17.Malamut G., El Machhour R., Montcuquet N., Martin-Lannerée S., Dusanter-Fourt I., Verkarre V., Mention J.J., Rahmi G., Kiyono H., Butz E.A., Brousse N., Cellier C., Cerf-Bensussan N., Meresse B. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J Clin Invest. 2010;120:2131–2143. doi: 10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King A.L., Moodie S.J., Fraser J.S., Curtis D., Reid E., Dearlove A.M., Ellis H.J., Ciclitira P.J. CTLA-4/CD28 gene region is associated with genetic susceptibility to coeliac disease in UK families. J Med Genet. 2002;39:51–54. doi: 10.1136/jmg.39.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gass J., Bethune M.T., Siegel M., Spencer A., Khosla C. Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology. 2007;133:472–480. doi: 10.1053/j.gastro.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Bethune M.T., Strop P., Tang Y., Sollid L.M., Khosla C. Heterologous expression, purification, refolding, and structural-functional characterization of EP-B2, a self-activating barley cysteine endoprotease. Chem Biol. 2006;13:637–647. doi: 10.1016/j.chembiol.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Siegel M., Bethune M.T., Gass J., Ehren J., Xia J., Johannsen A., Stuge T.B., Gray G.M., Lee P.P., Khosla C. Rational design of combination enzyme therapy for celiac sprue. Chem Biol. 2006;13:649–658. doi: 10.1016/j.chembiol.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Marti T. Prolyl endopeptidase-mediated destruction of T cell epitopes in whole gluten: chemical and immunological characterization. J Pharmacol Exp Ther. 2004;312:19–26. doi: 10.1124/jpet.104.073312. [DOI] [PubMed] [Google Scholar]
- 23.Tye-Din J.A., Anderson R.P., Ffrench R.A., Brown G.J., Hodsman P., Siegel M., Botwick W., Shreeniwas R. The effects of ALV003 pre-digestion of gluten on immune response and symptoms in celiac disease in vivo. Clin Immunol. 2010;134:289–295. doi: 10.1016/j.clim.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Siegel M., Garber M.E., Spencer A.G., Botwick W., Kumar P., Williams R.N., Kozuka K., Shreeniwas R., Pratha V., Adelman D.C. Safety, tolerability, and activity of ALV003: results from two phase 1 single, escalating-dose clinical trials. Dig Dis Sci. 2012;57:440–450. doi: 10.1007/s10620-011-1906-5. [DOI] [PubMed] [Google Scholar]
- 25.Lähdeaho M.-L., Kaukinen K., Laurila K., Vuotikka P., Koivurova O.P., Kärjä-Lahdensuu T., Marcantonio A., Adelman D.C., Mäki M. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology. 2014;146:1649–1658. doi: 10.1053/j.gastro.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Murray J.A., Kelly C.P., Green P.H.R., Marcantonio A., Wu T.T., Mäki M., Adelman D.C. No difference between latiglutenase and placebo in reducing villous atrophy or improving symptoms in patients with symptomatic celiac disease. Gastroenterology. 2017;152:787–798.e2. doi: 10.1053/j.gastro.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Syage J.A., Murray J.A., Green P.H.R., Khosla C. Latiglutenase improves symptoms in seropositive celiac disease patients while on a gluten-free diet. Dig Dis Sci. 2017;62:2428–2432. doi: 10.1007/s10620-017-4687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehren J., Moron B., Martin E., Bethune M.T., Gray G.M., Chaitan A food-grade enzyme preparation with modest gluten detoxification properties. PLoS One. 2009;4:e0006313. doi: 10.1371/journal.pone.0006313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mccarville J.L., Nisemblat Y., Galipeau H.J., Jury J., Tabakman R., Cohen A., Naftali E., Neiman B., Halbfinger E., Murray J.A., Anbazhagan A.N., Dudeja P.K., Varvak A., Leroux J.C., Verdu E.F. BL-7010 demonstrates specific binding to gliadin and reduces gluten-associated pathology in a chronic mouse model of gliadin sensitivity. PLoS One. 2014;9:0109972. doi: 10.1371/journal.pone.0109972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindfors K., Blomqvist T., Juuti-Uusitalo K., Stenman S., Venäläinen J., Mäki M., Kaukinen K. Live probiotic Bifidobacterium lactis bacteria inhibit the toxic effects induced by wheat gliadin in epithelial cell culture. Clin Exp Immunol. 2008;152:552–558. doi: 10.1111/j.1365-2249.2008.03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Palma G., Cinova J., Stepankova R., Tuckova L., Sanz Y. Pivotal Advance: Bifidobacteria and Gram-negative bacteria differentially influence immune responses in the proinflammatory milieu of celiac disease. J Leukoc Biol. 2010;87:765–778. doi: 10.1189/jlb.0709471. [DOI] [PubMed] [Google Scholar]
- 32.Martinello F., Roman C.F., Souza PA De. Effects of probiotic intake on intestinal bifidobacteria of celiac patients. Arg Gastroenterol. 2017:85–90. doi: 10.1590/S0004-2803.201700000-07. [DOI] [PubMed] [Google Scholar]
- 33.Francavilla R., De Angelis M., Rizzello C.G., Cavallo N., Dal Bello F., Gobbetti M. Selected probiotic lactobacilli have the capacity to hydrolyze gluten peptides during simulated gastrointestinal digestion. Appl Environ Microbiol. 2017;83 doi: 10.1128/AEM.00376-17. e00376-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smecuol E., Hwang H.J., Sugai E., Corso L., Cherñavsky A.C., Bellavite F.P., González A., Vodánovich F., Moreno M.L., Vázquez H., Lozano G., Niveloni S., Mazure R., Meddings J., Mauriño E., Bai J.C. Exploratory, randomized, double-blind, placebo-controlled study on the effects of bifidobacterium infantis natren life start strain super strain in active celiac disease. J Clin Gastroenterol. 2013;47:139–147. doi: 10.1097/MCG.0b013e31827759ac. [DOI] [PubMed] [Google Scholar]
- 35.Paterson B.M., Lammers K.M., Arrieta M.C., Fasano A., Meddings J.B. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–766. doi: 10.1111/j.1365-2036.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- 36.Leffler D.A., Kelly C.P., Abdallah H.Z., Colatrella A.M., Harris L.A., Leon F., Arterburn L.A., Paterson B.M., Lan Z.H., Murray J.A. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am J Gastroenterol. 2012;107:1554–1562. doi: 10.1038/ajg.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leffler D.A., Kelly C.P., Green P.H.R., Fedorak R.N., DiMarino A., Perrow W., Rasmussen H., Wang C., Bercik P., Bachir N.M., Murray J.A. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology. 2015;148:1311–1319.e6. doi: 10.1053/j.gastro.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly C.P., Green P.H.R., Murray J.A., Dimarino A., Colatrella A., Leffler D.A., Alexander T., Arsenescu R., Leon F., Jiang J.G., Arterburn L.A., Paterson B.M., Fedorak R.N. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther. 2013;37:252–262. doi: 10.1111/apt.12147. [DOI] [PubMed] [Google Scholar]
- 39.Pallav K., Kabbani T., Tariq S., Vanga R., Kelly C.P., Leffler D.A. Clinical utility of celiac disease-associated HLA testing. Dig Dis Sci. 2014;59:2199–2206. doi: 10.1007/s10620-014-3143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiao S.-W., Bergseng E., Molberg O., Jung G., Fleckenstein B., Sollid L.M. Refining the rules of gliadin T cell epitope binding to the disease-associated DQ2 molecule in celiac disease: importance of proline spacing and glutamine deamidation. J Immunol. 2005;175:254–261. doi: 10.4049/jimmunol.175.1.254. [DOI] [PubMed] [Google Scholar]
- 41.Rauhavirta T., Qiao S.-W., Jiang Z., Myrsky E., Loponen J., Korponay-Szabó I.R., Salovaara H., Garcia-Horsman J.A., Venäläinen J., Männistö P.T., Collighan R., Mongeot A., Griffin M., Mäki M., Kaukinen K., Lindfors K. Epithelial transport and deamidation of gliadin peptides: a role for coeliac disease patient immunoglobulin A. Clin Exp Immunol. 2011;164:127–136. doi: 10.1111/j.1365-2249.2010.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matysiak-Budnik T., Moura I.C., Arcos-Fajardo M., Lebreton C., Ménard S., Candalh C., Ben-Khalifa K., Dugave C., Tamouza H., van Niel G., Bouhnik Y., Lamarque D., Chaussade S., Malamut G., Cellier C., Cerf-Bensussan N., Monteiro R.C., Heyman M. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med. 2008;205:143–154. doi: 10.1084/jem.20071204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kapoerchan V.V., Wiesner M., Overhand M., van der Marel G.A., Koning F., Overkleeft H.S. Design of azidoproline containing gluten peptides to suppress CD4+ T-cell responses associated with celiac disease. Bioorg Med Chem. 2008;16:2053–2062. doi: 10.1016/j.bmc.2007.10.091. [DOI] [PubMed] [Google Scholar]
- 44.Kapoerchan V.V., Wiesner M., Hillaert U., Drijfhout J.W., Overhand M., Alard P., van der Marel G.A., Overkleeft H.S., Koning F. Design, synthesis and evaluation of high-affinity binders for the celiac disease associated HLA-DQ2 molecule. Mol Immunol. 2010;47:1091–1097. doi: 10.1016/j.molimm.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 45.Xia J., Siegel M., Bergseng E., Sollid L.M., Khosla C. Inhibition of HLA-DQ2-mediated antigen presentation by analogues of a high affinity 33-residue peptide from α2-gliadin. J Am Chem Soc. 2006;128:1859–1867. doi: 10.1021/ja056423o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rauhavirta T., Oittinen M., Kivistö R., Männistö P.T., Garcia-Horsman J.A., Wang Z., Griffin M., Mäki M., Kaukinen K., Lindfors K. Are Transglutaminase 2 inhibitors able to reduce gliadin-induced toxicity related to celiac disease? A proof-of-concept study. J Clin Immunol. 2013;33:134–142. doi: 10.1007/s10875-012-9745-5. [DOI] [PubMed] [Google Scholar]
- 47.Daveson A.J.M., Ee H.C., Andrews J.M., King T., Goldstein K.E., Dzuris J.L., MacDougall J.A., Williams L.J., Treohan A., Cooreman M.P., Anderson R.P. Epitope-specific immunotherapy targeting CD4-positive T cells in celiac disease: safety, pharmacokinetics, and effects on intestinal histology and plasma cytokines with escalating dose regimens of Nexvax2 in a randomized, double-blind, placebo-controlled phase 1 study. EBioMedicine. 2017;26:78–90. doi: 10.1016/j.ebiom.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goel G., King T., Daveson A.J., Andrews J.M., Krishnarajah J., Krause R., Brown G.J.E., Fogel R., Barish C.F., Epstein R., Kinney T.P., Miner P.B., Jr., Tye-Din J.A., Girardin A., Taavela J., Popp A., Sidney J., Mäki M., Goldstein K.E., Griffin P.H., Wang S., Dzuris J.L., Williams L.J., Sette A., Xavier R.J., Sollid L.M., Jabri B., Anderson R.P. Epitope-specific immunotherapy targeting CD4-positive T cells in coeliac disease: two randomised, double-blind, placebo-controlled phase 1 studies. Lancet Gastroenterol Hepatol. 2017;2:479–493. doi: 10.1016/S2468-1253(17)30110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strachan D.P. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bach J.-F. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 51.Daveson A.J., Jones D.M., Gaze S., McSorley H., Clouston A., Pascoe A., Cooke S., Speare R., Macdonald G.A., Anderson R., McCarthy J.S., Loukas A., Croese J. Effect of hookworm infection on wheat challenge in celiac disease – a randomised double-blinded placebo controlled trial. PLoS One. 2011;6:e0017366. doi: 10.1371/journal.pone.0017366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Croese J., Giacomin P., Navarro S., Clouston A., McCann L., Dougall A., Ferreira I., Susianto A., O'Rourke P., Howlett M., McCarthy J., Engwerda C., Jones D., Loukas A. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J Allergy Clin Immunol. 2015;135:508–516. doi: 10.1016/j.jaci.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 53.Theron M., Bentley D., Nagel S., Manchester M., Gerg M., Schindler T., Silva A., Ecabert B., Teixeira P., Perret C., Reis B. Pharmacodynamic monitoring of Ro5459072, a small molecule inhibitor of cathepsin S. Front Immunol. 2017;8:806. doi: 10.3389/fimmu.2017.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yokoyama S., Perera P.-Y., Waldmann T.A., Hiroi T., Perera L.P. Tofacitinib, a janus kinase inhibitor demonstrates efficacy in an IL–15 transgenic mouse model that recapitulates pathologic manifestations of celiac disease. J Clin Immunol. 2013;33:586–594. doi: 10.1007/s10875-012-9849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abraham M., Karni A., Dembinsky A., Miller A., Gandhi R., Anderson D., Weiner H.L. In vitro induction of regulatory T cells by anti-CD3 antibody in humans. J Autoimmun. 2008;30:21–28. doi: 10.1016/j.jaut.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mei H.E., Frölich D., Giesecke C., Loddenkemper C., Reiter K., Schmidt S., Feist E., Daridon C., Tony H.P., Radbruch A., Dörner T. Steady-state generation of mucosal IgA+ plasmablasts is not abrogated by B-cell depletion therapy with rituximab. Blood. 2010;116:5181–5190. doi: 10.1182/blood-2010-01-266536. [DOI] [PubMed] [Google Scholar]
- 57.Ludvigsson J.F., Ciacci C., Green P.H., Kaukinen K., Korponay-Szabo I.R., Kurppa K., Murray J.A., Lundin K.E.A., Maki M.J., Popp A., Reilly N.R., Rodriguez-Herrera A., Sanders D.S., Schuppan D., Sleet S., Taavela J., Voorhees K., Walker M.M., Leffler D.A. Outcome measures in coeliac disease trials: the Tampere recommendations. Gut. 2018:1410–1424. doi: 10.1136/gutjnl-2017-314853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Sabatino A., Corazza G.R. Coeliac disease. Lancet. 2017;373:1480–1493. doi: 10.1016/S0140-6736(09)60254-3. [DOI] [PubMed] [Google Scholar]
- 59.Castillo N.E., Theethira T.G., Leffler D.A. The present and the future in the diagnosis and management of celiac disease. Gastroenterol Rep. 2015;3:3–11. doi: 10.1093/gastro/gou065. [DOI] [PMC free article] [PubMed] [Google Scholar]