Tamoxifen (TX) is widely used to induce Cre recombinase-estrogen receptor fusion proteins. However, gastric toxicity resulting from parietal cell death limits the utility of Cre recombinase-estrogen receptor drivers.1 Here, we compared dose and method of TX administration in wild-type and gastrin knockout (GKO)2 mice to define the toxic response and role of gastrin in injury and repair.
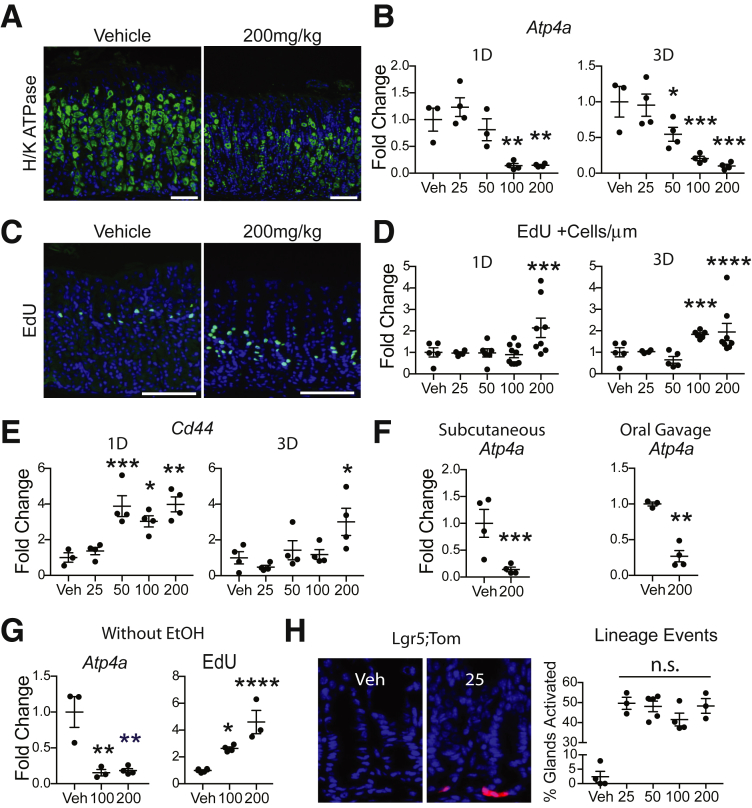
Adult mice were injected once intraperitoneally with TX (25, 50, 100, or 200 mg/kg) or vehicle (Veh) and corpus tissue was analyzed 1 or 3 days later for parietal cells and cellular proliferation. TX-induced gastric injury was apparent in hematoxylin and eosin–stained sections, with parietal cell loss shown by H/K-ATPase-α immunostaining (Supplementary Figure 1 and Figure 1A). Measurement of parietal cell Atp4a mRNA revealed a graded response (Figure 1B). The lowest dose, 25 mg/kg, showed no parietal cell loss, with extensive damage observed with 100–200 mg/kg, and intermediate loss at 50 mg/kg. The proliferative response, measured by EdU incorporation, was also graded (Supplementary Figure 2A–J and Figure 1C and D). Increased proliferation was observed at the 2 highest doses, suggesting that a threshold of cellular damage is required to induce proliferation. In addition, the proliferative zone expanded downward in the damaged corpus glands. There was no change in antral proliferation at any TX dose (Supplementary Figure 2K–S).
Figure 1.
TX-induced gastric corpus damage is dose- and time-dependent, and route of administration and ethanol do not affect the response. (A) Mice treated intraperitoneally with Veh or 200-mg/kg TX were H/K-ATPase-α–immunostained (green) with DAPI (blue) at 3 days. (B) Reverse-transcription quantitative polymerase chain reaction (RT-qPCR) measurement of Atp4a messenger RNA. (C) EdU staining (green) with DAPI (blue). (A, C) Scale bar = 100 μm. (D) EdU+ cells. (E) RT-qPCR measurement of Cd44 mRNA. (F) RT-qPCR measurement of Atp4a messenger RNA 3 days after 200-mg/kg TX via subcutaneous injection or oral gavage. (G) IP TX without EtOH at 3 days for Atp4a mRNA and EdU+ cells. (H) Lgr5-eGFP-IRES-CreERT2;ROSA-CAG-LSL-tdTomato-WPRE mice treated with TX and examined for tdTomato reporter activation (red) 1 day later. 1D, 1 day; 3D, 3 days. Quantitative data are presented as mean ± SEM. n = 3–9 mice/treatment; *P < .05, **P < .01, ***P < .001, ****P < .0001 vs Veh by 1-way analysis of variance with Dunnett’s post-test or Student’s t test.
Similar to other modes of gastric injury, TX has been reported to induce expression of the stem cell marker Cd44.3 We observed rapid increases in Cd44 messenger RNA for all but the 25-mg/kg dose (Figure 1E).
To determine if the mode of TX administration affects damage, mice were treated by subcutaneous injection or oral gavage, and examined for parietal cell marker expression (Supplementary Figure 3 and Figure 1F). As observed with intraperitoneal injection, subcutaneous injection or oral gavage induced parietal cell loss, with similar damage induced by all modes of TX administration.
To test whether the EtOH used to dissolve the TX into solution is a potential cause of the gastric toxicity, TX was made without EtOH and tissue was examined 3 days after intraperitoneal administration. This showed gastric damage, with fewer H/K-ATPase-α expressing cells (Supplementary Figure 4A–C), reduced Atp4a mRNA abundance, and increased proliferation (Figure 1G and Supplementary Figure 4D–F). The response was similar to TX with EtOH, showing that it is TX, and not EtOH, that causes gastric injury.
With the observation of a dose-dependent damage response, 25-mg/kg TX was the only dose without damage. To confirm that this dose was sufficient to induce recombination we examined lineage tracing in Lgr5-eGFP-IRES-CreERT2;ROSA-CAG-LSL-tdTomato-WPRE mice, comparing the extent of tdTomato-marked cells after TX activation. This analysis showed that all doses, including 25 mg/kg, induced a similar amount of labeling (Figure 1H). Thus, 25 mg/kg is sufficient to induce Cre recombination, and furthermore, increasing the TX dose does not increase the extent of recombination.
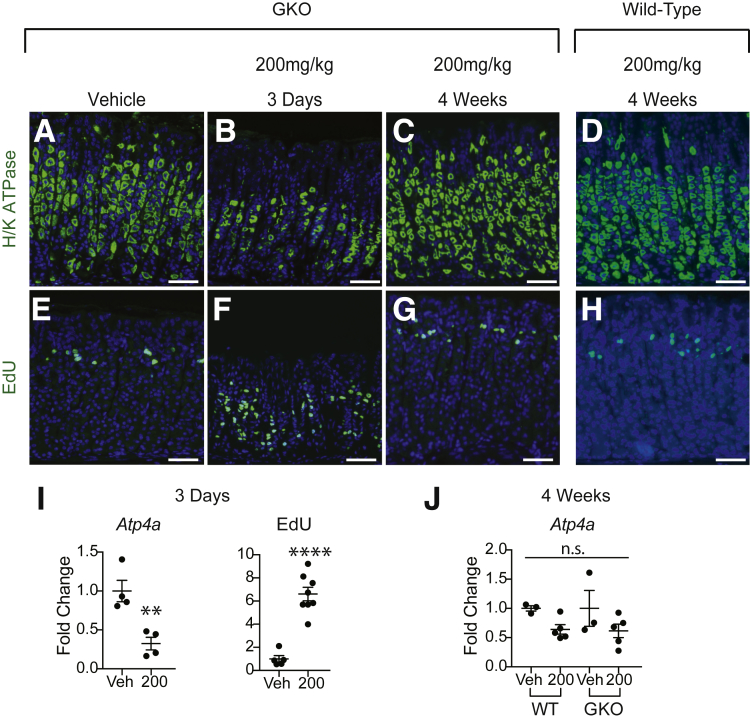
The gastric hormone gastrin induces corpus glandular hypertrophy and hyperproliferation in mouse genetic models with parietal cell loss or dysfunction.4, 5, 6 To determine if gastrin is responsible for the increased epithelial proliferation observed in the corpus after TX treatment and to test the possible role of gastrin in tissue repair, GKO mice were treated with either vehicle or 200-mg/kg TX and the response was measured at 3 days to assess damage, and 4 weeks to assess restitution (Figure 2). This showed that GKO mice had a similar damage response as wild-type mice, with parietal cell loss and, importantly, markedly increased proliferation at 3 days post-TX (Figure 2B, F, and I). Further, gastric damage was similarly repaired in wild-type and GKO mice 4 weeks post-TX (Figure 2C, D, G, H, and J). Together, these data show that gastrin is not required for the TX gastric injury response or for subsequent tissue repair.
Figure 2.
Gastrin is dispensable for TX damage and recovery. GKO or wild-type (WT) mice were injected intraperitoneally with 200-mg/kg TX or Veh and examined (B, F) 3 days or (C, D, G, H) 4 weeks later. Sections were stained for (A–D) H/K-ATPase-α (green) or (E–H) EdU (green), with DAPI (blue). (I) Atp4a messenger RNA abundance or EdU+ cells in GKO mice at 3 days and (J) Atp4a messenger RNA abundance in GKO and WT mice 4 weeks post-TX. Quantitative data are presented as mean ± SEM. n = 3–8 mice/treatment; **P < .01, **** P < .0001 vs vehicle by Student’s t test. Scale bar = 100 μm.
Together our findings confirm work from the Mills laboratory, which originally reported TX-induced parietal cell loss.1, 7, 8 We elucidated variables that have been suggested to affect TX toxicity by systematically analyzing dose, presence or absence of EtOH, and route of administration. Parietal cell loss and increased proliferation is a consistent feature of TX administration regardless of mode of administration or method of TX preparation. Unfortunately, many publications that use high doses of TX do not take gastric toxicity into account. As the mechanism of TX damage has not yet been uncovered, all studies that utilize TX, including both past and future studies, should consider parietal cell injury and tissue regeneration as confounding factors for any conclusions. The dynamic gastric response to TX, with rapid, yet transient changes, underscores the importance of including controls at appropriate short time points after TX administration to detect injury. Further, our studies suggest that Cd44 messenger RNA measured 1-day post-TX would be a sensitive test of TX injury.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding The research was funded by an National Institutes of Health project award to Linda C. Samuelson (grant no. P01-DK06041) and core support from the Michigan Gastrointestinal Research Center National Institutes of Health grant no. P30-DK34933.
Supplementary Material
References
- 1.Huh W.J. Gastroenterology. 2012;142:21–24. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friis-Hansen L. Am J Physiol Gastroint Liver Physiol. 1998;274:G561–568. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- 3.Khurana S.S. J Biol Chem. 2013;288:16085–16897. doi: 10.1074/jbc.M112.445551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franic T.V. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1502–1511. doi: 10.1152/ajpgi.2001.281.6.G1502. [DOI] [PubMed] [Google Scholar]
- 5.Jain R.N. J Clin Invest. 2008;118:2459–2470. doi: 10.1172/JCI33569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todisco A. Physiol Rep. 2015;3 doi: 10.14814/phy2.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burclaff J. Gastroenterology. 2017;152:762–766. doi: 10.1053/j.gastro.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saenz J.B. Methods Mol Biol. 2016;1422:329–339. doi: 10.1007/978-1-4939-3603-8_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.