Abstract
Objective
To evaluate the safety and efficacy of balloon pulmonary angioplasty (BPA) in patients with chronic thromboembolic pulmonary hypertension (CTEPH) seen at a US medical center.
Patients and Methods
Patients with inoperable or residual postendarterectomy CTEPH who underwent BPA at Mayo Clinic in Rochester, Minnesota, between August 11, 2014, and May 17, 2018, were included. Invasive hemodynamic, clinical, laboratory, and echocardiographic data were collected and analyzed retrospectively.
Results
We identified 31 patients (26 with inoperable CTEPH and 5 with residual postendarterectomy CTEPH) who underwent 75 BPA procedures performed in a staged manner to reduce complications. The median number of sessions was 2 (interquartile range [IQR], 1-3) per patient, and the number of vessels treated per session was 3 (IQR, 2-3). Of the 31 patients, 24 (77.4%) were taking pulmonary vasodilators and 22 (71.0%) were taking riociguat. The mean pulmonary arterial pressure decreased from 40 mm Hg (IQR, 29-48 mm Hg) to 29 mm Hg (IQR, 25-37 mm Hg; P<.001); pulmonary vascular resistance decreased from 5.5 Wood units (WU) (IQR, 3.0-7.6 WU) to 3.3 WU (2.2-5.2 WU; P<.001). The follow-up 6-minute walk test was performed in 13 patients and improved from 402 m (IQR, 311-439 m) to 439 m (366-510 m; P=.001). Of the 31 patients, 19 (61.3%) had improvement in New York Heart Association functional class. The mean ± SD nadir of minute ventilation/carbon dioxide production decreased by 3.4±5.5 (P=.03), reflecting improved ventilatory efficiency. Complications included hemoptysis requiring overnight intensive care unit observation (n=1) and cardiac tamponade requiring pericardiocentesis (n=1). One patient had reperfusion injury requiring intubation, recovered, and was dismissed to home but died unexpectedly within less than 30 days of the procedure. Serious complications occurred in 3 of the 75 BPA procedures (4.0%).
Conclusion
Our experience with BPA revealed that this procedure has acceptable risk and improves hemodynamics, functional class, and exercise tolerance in patients with inoperable or residual CTEPH.
Abbreviations and Acronyms: BPA, balloon pulmonary angioplasty; CTEPH, chronic thromboembolic pulmonary hypertension; ICU, intensive care unit; IQR, interquartile range; 6MWD, 6-minute walk distance; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PA, pulmonary artery; PH, pulmonary hypertension; RAP, right atrial pressure; RHC, right-sided heart catheterization; RV, right ventricular; RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion; WU, Wood units
Chronic thromboembolic pulmonary hypertension (CTEPH) is defined as pulmonary hypertension (PH) caused by unresolved pulmonary emboli that persist despite more than 3 months of anticoagulation therapy.1, 2 It is associated with high morbidity and mortality without treatment.3 Historically, CTEPH has been treated with pulmonary endarterectomy in eligible patients and medical therapy in those unable to undergo surgical treatment. Pulmonary endarterectomy is considered the approach of first choice. However, approximately one-third of patients are deemed inappropriate for this procedure because of the location of the emboli and/or comorbidities, and some patients have recurrent or residual disease after surgery.4, 5 Some patients with operable disease decline surgery and seek alternative approaches. Balloon pulmonary angioplasty (BPA) is an emerging catheter-based treatment modality that can be a useful treatment option for such patients.6 It involves balloon dilation of the lobar, segmental, or subsegmental pulmonary arteries (PAs), typically in a series of sessions.6 Limited data from small studies have shown improvement in hemodynamics with reduction in mean PA pressures and pulmonary vascular resistance, increase in cardiac index, and improvement in ventilatory efficiency with BPA.7, 8, 9, 10, 11, 12, 13 Studies predominantly performed in Japan have reported beneficial effects of BPA on systemic functions (improved dysglycemia, renal and vascular function, and nutritional status) in addition to the hemodynamic improvement.14 Complications and periprocedural mortality are low in experienced centers with high volumes.11, 12, 15, 16 The potential complications include hemoptysis, pulmonary hemorrhage, wire injury, vessel dissection or rupture, reperfusion pulmonary edema, hemorrhagic pleural effusions, and death.11 The published experience with this technique in North America is minimal and old,17 and the ultimate role, risks, and benefits of this technique need to be further assessed. This article describes the initial experience with BPA in patients with CTEPH at Mayo Clinic.
Patients and Methods
With approval from the Mayo Clinic Institutional Review Board, we retrospectively reviewed 31 consecutive patients, predominantly (26 patients) with inoperable CTEPH, who underwent BPA at Mayo Clinic, Rochester, Minnesota, between August 11, 2014, and May 17, 2018. Chronic thromboembolic pulmonary hypertension was diagnosed by standard criteria2 after thorough evaluation by a PH specialist, and options for surgical and interventional treatments were developed using a team approach with an interventional cardiologist and a surgeon. In patients with mean PA pressure over 35 mm Hg, therapy with riociguat was generally initiated in the months before the first session if the patient was not already receiving pulmonary vasodilator therapy. Patients were typically bridged with enoxaparin for the procedure, with anticoagulation resumed afterward. Any PH-targeted therapies were continued during the periprocedural period.
Details of procedures (number of sessions, number and details of target vessels), hemodynamic data (by right-sided heart catheterization [RHC]), echocardiographic data, laboratory values (N-terminal pro-B-type natriuretic peptide [NT-proBNP]), clinical data (age, sex, New York Heart Association [NYHA] functional class, 6-minute walk distance [6MWD], results of cardiopulmonary exercise testing), PH-targeted therapies, and periprocedural complications were documented and analyzed.
BPA Procedure
Before the procedure, all available imaging studies including nuclear ventilation-perfusion scan, computed tomographic angiography, invasive pulmonary angiography (either rotational DynaCT [Siemens Medical Solutions] or pulmonary digital subtraction angiography) were reviewed to guide strategy. The access for BPA was achieved through the femoral vein by advancing a balloon wedge catheter to the PA and then placing an exchange wire in order to facilitate placement of an 8F long sheath placed directly into the right or left PA. The guiding catheter was then inserted into the target pulmonary vasculature and selective angiography was performed. Target lesions of BPA were carefully delineated in lobar, segmental, or subsegmental PAs, with close attention being paid to identify target lesions with poor venous return (Figure). Chronic total occlusions were generally avoided if the distal vasculature was not visible. Pressure wire measurements were not routinely utilized. An average of 3 target lesions were dilated per session. Balloon pulmonary angioplasty was performed over multiple sessions to avoid excessive contrast medium and radiation exposure and to reduce the risk of reperfusion pulmonary edema. The goal of each balloon treatment was to adequately dilate the lesion and restore prompt pulmonary venous return (Figure). The BPA sessions were repeated in a stepwise manner until sufficient clinical improvement or a target mean PA pressure of less than 30 mm Hg was achieved. Most patients were observed in the hospital overnight and underwent chest radiography before dismissal. Withdrawal of pulmonary vasodilators was considered on an individual patient basis depending on the subsequent clinical course. The last follow-up was used for final assessment.
Figure.
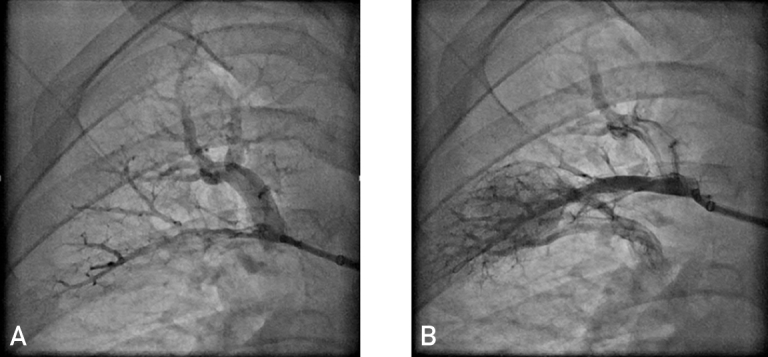
Pulmonary artery angiography before and after balloon pulmonary angioplasty (BPA). A, Preprocedure pulmonary angiogram showing an obstructive lesion with no venous flow. B, Pulmonary angiogram after BPA procedure showing arterial flow as well as good venous return.
Statistical Analyses
The primary study outcomes were a change in mean PA pressure and pulmonary vascular resistance as assessed by RHC, and the safety of the procedure was assessed by documenting the number and type of complications. The secondary study outcomes were changes in the following: NYHA functional class, NT-proBNP level, 6MWD, RHC data (mean right atrial pressure [RAP], right ventricular [RV] systolic pressure [RVSP], RV end-diastolic pressure, PA systolic pressure, PA diastolic pressure, pulmonary capillary wedge pressure, cardiac output, cardiac index, PA oxygen saturation), and transthoracic echocardiographic parameters of RV function (RAP estimate, RVSP, pulmonary valve end-diastolic velocity, RV diameters and area, RV outflow tract time velocity integral, tricuspid annular plane systolic excursion [TAPSE], tricuspid valve lateral annular systolic velocity, peak RV free wall strain, and left ventricular stroke volume index). The following markers of diastolic function were also compared before and after BPA: early mitral inflow velocity, ratio of early to late mitral inflow velocity, and medial and lateral mitral annular early filling velocity.
Data are expressed as median (interquartile range [IQR]) and number (percentage) where appropriate. A comparison of continuous variables obtained at baseline and last follow-up was made using the Wilcoxon signed rank test. Categorical variables are presented as number (percentage). P<.05 was considered statistically significant. Statistical analyses were performed using JMP statistical software, version 12.0 (SAS Institute).
Results
Thirty-one patients underwent BPA at Mayo Clinic in Rochester during the study period. The mean ± SD age of the patients was 59.7±16.7 years, and 13 (41.9%) were female. The mean ± SD duration of disease from diagnosis to the first session of BPA was 663±1072 days. Underlying conditions and comorbidities are detailed in Table 1. Five of the 31 patients (16.1%) had prior pulmonary endarterectomy. The median number of BPA sessions was 2 (IQR, 1-3), and the number of target lesions treated per session was 3 (IQR, 2-3). One patient with systemic PA pressures experienced cardiac tamponade requiring pericardiocentesis during efforts to place the guiding catheter; this patient did not have any BPA performed and is therefore not included in the current analysis. Pre-BPA, 22 patients had NYHA class III heart disease and 9 had class II. The 6MWD was 402 m (IQR, 311-439 m). Twenty-four of the 31 patients (77.4%) were receiving PH-targeted therapies—riociguat in 22 (71.0%), macitentan in 3 (9.7%), sildenafil in 1 (3.2%), tadalafil in 1 (3.2%), bosentan in 1 (3.2%), and inhaled treprostinil in 1 (3.2%). Two patients were receiving 2 PH-targeted therapies and 1 was receiving 3. In 3 of 22 patients, riociguat was discontinued after completion of revascularization. The dose was reduced in 1 patient and increased in 2. Twenty-one of the 31 patients (67.7%) were taking warfarin, and the remaining 10 (32.3%) received novel oral anticoagulants. Twenty-one of the 31 patients (67.7%) were also taking aspirin (Table 1).
Table 1.
| Variable | Value |
|---|---|
| Age (y) | 59.7±16.7 |
| Female | 13 (41.9) |
| Hypercoagulable states | |
| Myeloproliferative disorders | 5 (16.1) |
| Lupus anticoagulant positive | 3 (9.7) |
| Factor V Leiden heterozygote | 2 (6.4) |
| Klippel-Trenaunay syndrome | 1 (3.2) |
| Lupus anticoagulant and factor V Leiden heterozygote | 1 (3.2) |
| ANCA vasculitis | 1 (3.2) |
| Thoracic outlet syndrome | 1 (3.2) |
| Prior pulmonary endarterectomy | 5 (16.1) |
| 6-Minute walk distance (m) | 402 (311-439) |
| NT-proBNP (pg/mL) | 312 (71-882) |
| o2max (mL/kg/min) | 14.9 (12.6-19.9) |
| VE/co2 nadir | 38 (31-46) |
| NYHA class | |
| II | 9 (29.0) |
| III | 22 (71.0) |
| Medications | |
| PAH-targeted therapies | |
| Riociguat | 22 (71.0) |
| Sildenafil | 1 (3.2) |
| Tadalafil | 1 (3.2) |
| Bosentan | 1 (3.2) |
| Macitentan | 3 (9.7) |
| Treprostinil (inhaled) | 1 (3.2) |
| Antiplatelet/anticoagulant | |
| Aspirind | 21 (67.7) |
| Warfarin | 21 (67.7) |
| Novel oral anticoagulants | 10 (32.3) |
| Others | |
| Loop diuretic | 5 (16.1) |
| Spironolactone | 3 (9.7) |
ANCA = antineutrophil cytoplasmic antibody, NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; PAH = pulmonary arterial hypertension; VE/co2 = minute ventilation/carbon dioxide production; o2max = maximum oxygen consumption.
Data are presented as mean ± SD, No. (percentage) of patients, or median (interquartile range).
SI conversion factors: To convert NT-proBNP values to ng/L, multiply by 1.0.
One patient was taking 325 mg of acetylsalicylic acid, and the rest received 81 mg.
In terms of efficacy, there was a statistically significant improvement noted in our primary outcomes of pulmonary hemodynamics after the last BPA session. Five of the 31 patients (16.1%) still had additional sessions planned. The mean PA pressure improved from 40 mm Hg (IQR, 29-48 mm Hg) to 29 mm Hg (IQR, 25-37; P<.001), and the pulmonary vascular resistance decreased from 5.5 Wood units (WU) (IQR, 3.0-7.6 WU) to 3.3 WU (IQR, 2.2-5.2 WU; P<.001). There was also statistically significant improvement noted in other RHC variables—RVSP, 69 mm Hg (IQR, 51-82 mm Hg) to 58 mm Hg (IQR, 47-68 mm Hg; P<.001); PA systolic pressure, 70 mm Hg (IQR, 52-84 mm Hg) to 54 mm Hg (IQR, 45-64 mm Hg; P<.001); PA diastolic pressure, 26 mm Hg (IQR, 18-29 mm Hg) to 18 mm Hg (IQR, 13-22 mm Hg; P<.001); and RAP, 9 mm Hg (IQR, 6-11 mm Hg) to 7 mm Hg (IQR, 6-9 mm Hg; P=.03)—and a nonsignificant difference in RV end-diastolic pressure (13 mm Hg [IQR, 9-15 mm Hg] to 10 mm Hg [IQR, 8-13 mm Hg]; P=.11) (Table 2). The pulmonary capillary wedge pressure and cardiac output were normal at baseline, and there was no significant difference post-BPA (Table 2). Similarly, there was no significant difference in PA saturation before and after PBA (62% [IQR, 58%-68%] to 63% [IQR, 59%-70%]; P=.34).
Table 2.
Changes in the Hemodynamic Data as Assessed by Right-Sided Heart Catheterization Before and After BPAa,b
| Variable | Before BPA (N=31) | After BPA (N=23) | P valuec |
|---|---|---|---|
| Mean RA pressure (mm Hg) | 9 (6-11) | 7 (6-9) | .03 |
| RV pressure (mm Hg) | |||
| Systolic | 69 (51-82) | 58 (47-68) | <.001 |
| End-diastolic | 13 (9-15) | 10 (8-13) | .11 |
| PA pressure (mm Hg) | |||
| Systolic | 70 (52-84) | 54 (45-64) | <.001 |
| Diastolic | 26 (18-29) | 18 (13-22) | <.001 |
| Mean | 40 (29-48) | 29 (25-37) | <.001 |
| Pulmonary capillary wedge pressure (mm Hg) | 13 (9-14) | 12 (10-14) | .33 |
| Pulmonary vascular resistance (Woods unit) | 5.5 (3.0-7.6) | 3.3 (2.2-5.2) | <.001 |
| Cardiac output (L/min) | 5.1 (4.2-6.0) | 5.1 (4.3-7.0) | .23 |
| Cardiac index (L/min/m2) | 2.4 (2.1-3.1) | 2.7 (2.1-3.3) | .26 |
| Systemic arterial saturation (%) | 92 (85-96) | 95 (92-97) | .21 |
| PA saturation (%) | 62 (58-68) | 63 (59-70) | .34 |
BPA = balloon pulmonary angioplasty; PA = pulmonary artery; RA = right atrial; RV = right ventricular.
Data are presented as median (interquartile range).
Wilcoxon signed rank test.
In terms of clinical improvement, 19 patients (61.3%) had 1 class or more improvement in symptoms, 3 of whom had 2-class improvement (NYHA class III to I). Functional class did not change in 9 patients, and 3 did not have follow-up assessment at the time of this analysis. The follow-up 6-minute walk test was performed in 13 patients and improved from 402 m (IQR, 311-439 m) to 439 m (IQR, 366-510 m; P=.001). The NT-proBNP level was determined before and after BPA in 18 patients and declined from 312 pg/mL (IQR, 71-882 pg/mL) to 172 pg/mL (IQR, 32-349 pg/mL; P=.18) (to convert NT-proBNP values to ng/L, multiply by 1.0). The cardiopulmonary stress test was performed at baseline and follow-up after BPA sessions in 16 patients. The number of metabolic equivalents achieved increased from 4.3 (IQR, 3.6-7.7) to 5.5 (IQR, 3.5-7.2; P=.04). Mean ± SD maximum oxygen consumption increased by 2.2±3.9 mL/kg per minute (P=.08), and mean ± SD minute ventilation/carbon dioxide production nadir decreased by 3.4±5.5 (P=.03), reflecting improved ventilatory efficiency.
Echocardiographic data were available before PBA in 27 patients and after BPA in 15 patients, and there was significant improvement noted in peak RV strain (−21% [IQR, −12% to −26%] to −23% [IQR, −20% to −24%]; P=.02), TAPSE (18 mm [IQR, 15-23 mm] to 20 mm [IQR, 18-23 mm]; P<.001), and mid RV diameter (47 mm [IQR, 39-50 mm] to 37 mm [32-41 mm]; P=.02). However, other markers of RV function like tricuspid valve lateral annular systolic velocity were unchanged (Table 3). There were no differences in estimated RAP and parameters of left ventricular systolic and diastolic function.
Table 3.
| Variable | Before BPA (N=27) | After BPA (N=15) | P valuec |
|---|---|---|---|
| LVEF (%) | 62 (60-65) | 64 (61-66) | .03 |
| LV cardiac index (L/min/m2) | 2.8 (2.6-3.1) | 2.97 (2.8-3.7) | .35 |
| E velocity (m/s) | 0.6 (0.5-0.8) | 0.6 (0.6-0.8) | .05 |
| E/A | 0.9 (0.6-1.4) | 0.9 (0.8-1.2) | .88 |
| Medial E′ (m/s) | 0.07 (0.05-0.08) | 0.07 (0.06-0.08) | >.99 |
| Lateral E′ (m/s) | 0.1 (0.08-0.14) | 0.11 (0.08-0.12) | .66 |
| TR velocity (m/s) | 3.7 (3.4-4.0) | 3.6 (3.2-4.0) | .28 |
| Right atrial pressure estimate (mm Hg) | 5 (5-14) | 5 (5-10) | .14 |
| Pulmonary valve end-diastolic velocity (m/s) | 1.4 (1.2-2.0) | 1.3 (0.8-0.17) | .25 |
| RV basal diameter (mm) | 52 (48-57) | 48 (39-55) | .21 |
| RV mid diameter (mm) | 47 (39-50) | 37 (32-41) | .02 |
| RV base to apex length (mm) | 86 (82-95) | 87 (79-94) | .67 |
| RV diastolic area (mm2) | 35 (29-40) | 31 (23-39) | .15 |
| RV systolic area (mm2) | 26 (21-27) | 20 (13-27) | .15 |
| RVOT TVI (cm) | 11 (9-15) | 13 (11-16) | .50 |
| TV s′ (m/s) | 0.11 (0.1-0.13) | 0.12 (0.11-0.15) | .15 |
| TAPSE (mm) | 18 (15-23) | 20 (18-23) | <.01 |
| Peak RV strain (%) | −21 (−12 to −26) | −23 (−20 to −24) | .02 |
E = early diastolic mitral inflow; E′ = mitral annular early diastolic velocity; E/A = ratio of early to late diastolic mitral inflow velocity; LV = left ventricular; LVEF = LV ejection fraction; RV = right ventricular; RVOT TVI = RV outflow tract time velocity integral; TV s′ = tricuspid valve lateral annular systolic velocity; TAPSE = tricuspid annular plane systolic excursion; TR = tricuspid regurgitation.
Data are presented as median (interquartile range).
Wilcoxon signed rank test.
Overall, the procedure was safe with a low rate of complications (Table 4). Complications included self-limited scant hemoptysis in 2 patients and severe hemoptysis (∼200 mL expectorated blood) in 1 patient who required overnight intensive care unit (ICU) observation. One patient had cardiac tamponade during guide manipulation before BPA, requiring pericardiocentesis. One patient experienced pulmonary reperfusion injury requiring intubation and ICU admission, recovered, and was dismissed to home but had unexpected death within 30 days of discharge from the hospital (30-day mortality, 3.2%). Serious complications occurred in 3 of the 75 procedures (4.0%).
Table 4.
Complications of BPA Sessions in 31 Study Patients
| Complication | No. (%) of patients |
|---|---|
| Hemoptysis | 3 (9.7) |
| Reperfusion edema | 1 (3.2) |
| Cardiac tamponade | 1 (3.2) |
| Intensive care unit staya | 2 (6.5) |
| Increased oxygen requirements | 1 (3.2) |
| Hypoxemia requiring intubation | 1 (3.2) |
| Death <30 days postprocedure | 1 (3.2) |
One for hemoptysis and the other for reperfusion injury leading to intubation and mechanical ventilation.
Discussion
Our study has several important findings. First, BPA is an acceptably safe procedure with an overall low rate of complications when performed as repeated sessions. Second, BPA significantly improves mean PA pressure and pulmonary vascular resistance (both P<.001). Third, BPA is associated with significant improvement in NYHA functional class, exercise capacity (METs achieved), ventilatory efficiency (decreased minute ventilation/carbon dioxide production nadir), and 6MWD. Fourth, BPA is associated with improvement in RV function as assessed by statistically significant improvement in RV peak average free wall strain, TAPSE, and mid RV size. We observed trends toward improvement in NT-proBNP level, which did not reach statistical significance.
Patients with CTEPH are often managed with a combined medical and surgical approach. Pulmonary endarterectomy is considered the treatment of first choice for patients with CTEPH who can undergo the procedure and is associated with less than 5% perioperative mortality in expert centers.4 However, some patients are deemed inappropriate for pulmonary endarterectomy given the high surgical risk because of associated comorbidities or more distal small-vessel disease, and some patients choose not to undergo surgery. In addition, nearly 30% of patients have residual disease or recurrence after surgery,18 and BPA is an emerging treatment option for these patients. Treatment with riociguat has been found to improve hemodynamics and exercise capacity in inoperable/residual CTEPH and is the only PH-targeted therapy that is specifically approved by the US Food and Drug Administration for CTEPH; however, other pulmonary vasodilators are sometimes used as well. Recently, macitentan has been reported to improve inoperable CTEPH, although it is not approved by the US Food and Drug Administration for CTEPH.19 In our cohort, 77.4% of the patients (24) were receiving PH-targeted therapies and 71.0% (22) were treated with riociguat. Many patients who receive medical therapy for CTEPH remain severely symptomatic despite that therapy20 and may be candidates for BPA.
There is limited experience with BPA in North America. The first report was from Feinstein et al17 in 2001, who found improved hemodynamics and NYHA functional class but a high rate of reperfusion edema. The procedure was largely abandoned until it was tried again in different centers in Japan and Europe. We present the first contemporary era North American experience of successful BPA in patients with inoperable/residual CTEPH, with a low rate of complications similar to the Japanese experience.11 Reperfusion pulmonary edema was noted in only 2 of our patients; it was mild in 1 patient and led to intubation in the other, who died unexpectedly after dismissal, within 30 days of the procedure. Three patients had development of hemoptysis, which was mild in 2 patients and did not delay the discharge and severe in 1 patient who required overnight ICU observation. That patient was taking clopidogrel because of a prior coronary artery stent procedure, which prompted us to avoid BPA in patients taking such agents. Following completion of clopidogrel therapy, the patient underwent multiple additional sessions without complications. One patient with nearly systemic PA pressures and a very large RV and proximal PA experienced cardiac tamponade during initial guiding catheter placement and the procedure had to be aborted, suggesting higher risk in such patients.
Our approach differs from that reported by the Japanese centers. Individual lesions were dilated maximally as a single-step procedure, rather than a partial dilation followed by further expansion at a subsequent session. The latter approach has been recommended in an effort to reduce risk of reperfusion injury in situations of a large pressure gradient across a lesion, especially in those with a mean PA pressure above 35 mm Hg. Our incidence of reperfusion injury was nonetheless quite low, and risk of serious effects of reperfusion was minimized by limiting the number of lesions dilated per session in all patients. The vast majority of our patients were taking pulmonary vasodilators at the time of their BPA sessions. In part, this was intended to reduce the PA pressures before the BPA, thereby hopefully reducing procedural risk and incidence of reperfusion injury.
Our results are consistent with those of previous studies from Japanese and European centers. Kataoka et al8 were among the first who reported improvement in functional class, NT-proBNP levels, and hemodynamics in patients who underwent BPA for CTEPH. Shortly afterward, European centers published their experience with low rates of complications and with improvements in mean PA pressure, exercise capacity, and functional class with BPA.7, 21 Several studies from Japan have reported similar results with improvement in hemodynamics and symptoms and lower rate of complications on follow-up.11, 15, 16, 22, 23, 24 The published experience with BPA outside Japan remains limited; 2 recent studies from Germany and France reported low procedural mortality and improvement in hemodynamics similar to that found in our study.25, 26 Our study found a somewhat lower decrease in mean PA pressure compared to some of the recent studies from Japan (average decrease of 11 vs 20 mm Hg); this difference is likely due to inclusion of symptomatic patients with chronic pulmonary emboli and normal resting hemodynamics (chronic thromboembolic disease).27, 28, 29 The improvement in symptoms as assessed by NYHA functional class is similar to that in previous reports.22 The echocardiographic evaluation of RV function in our cohort revealed improvement in TAPSE and average RV strain. These results are similar to those of previous studies that evaluated the effects of BPA on RV function by echocardiography.30
Our study has several limitations. First, it is an interim analysis and not all patients had completed their revascularization and follow-up assessments at the time of this report. Consequently, follow-up echocardiographic data are unavailable in about half of the patients. In addition, there is a possibility that with longer follow-up, additional patients may be able to reduce or discontinue their pulmonary vasodilators. Third, the study design is retrospective, and the number of patients is relatively small.
Conclusion
We present the first contemporary era experience with BPA from North America. Our study found that BPA is an acceptably safe and effective treatment option for inoperable or residual CTEPH.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Kim N.H. Group 4 pulmonary hypertension: chronic thromboembolic pulmonary hypertension: epidemiology, pathophysiology, and treatment. Cardiol Clin. 2016;34(3):435–441. doi: 10.1016/j.ccl.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N., Humbert M., Vachiery J.L., et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS); Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 3.Delcroix M., Lang I., Pepke-Zaba J., et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation. 2016;133(9):859–871. doi: 10.1161/CIRCULATIONAHA.115.016522. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins D., Madani M., Fadel E., D'Armini A.M., Mayer E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):160111. doi: 10.1183/16000617.0111-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkins D. New interventions to treat chronic thromboembolic pulmonary hypertension. Heart. 2018;104(18):1480–1483. doi: 10.1136/heartjnl-2017-312110. [DOI] [PubMed] [Google Scholar]
- 6.Lang I., Meyer B.C., Ogo T., et al. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):160119. doi: 10.1183/16000617.0119-2016. [published correction appears in Eur Respir Rev. 2017;26(144):165119] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurzyna M., Darocha S., Koteja A., Pietura R., Torbicki A. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Postepy Kardiol Interwencyjnej. 2015;11(1):1–4. doi: 10.5114/pwki.2015.49176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kataoka M., Inami T., Hayashida K., et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012;5(6):756–762. doi: 10.1161/CIRCINTERVENTIONS.112.971390. [DOI] [PubMed] [Google Scholar]
- 9.Andreassen A.K., Ragnarsson A., Gude E., Geiran O., Andersen R. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart. 2013;99(19):1415–1420. doi: 10.1136/heartjnl-2012-303549. [DOI] [PubMed] [Google Scholar]
- 10.Fukui S., Ogo T., Morita Y., et al. Right ventricular reverse remodelling after balloon pulmonary angioplasty. Eur Respir J. 2014;43(5):1394–1402. doi: 10.1183/09031936.00012914. [DOI] [PubMed] [Google Scholar]
- 11.Inami T., Kataoka M., Shimura N., et al. Pressure-wire-guided percutaneous transluminal pulmonary angioplasty: a breakthrough in catheter-interventional therapy for chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol Intv. 2014;7(11):1297–1306. doi: 10.1016/j.jcin.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Kimura M., Kohno T., Kawakami T., et al. Balloon pulmonary angioplasty attenuates ongoing myocardial damage in patients with chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2016;207:387–389. doi: 10.1016/j.ijcard.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami T., Kataoka M., Arai T., Yanagisawa R., Maekawa Y., Fukuda K. Retrograde approach in balloon pulmonary angioplasty: useful novel strategy for chronic total occlusion lesions in pulmonary arteries. J Am Coll Cardiol Intv. 2016;9(2):e19–e20. doi: 10.1016/j.jcin.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Tatebe S., Sugimura K., Aoki T., et al. Multiple beneficial effects of balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Circ J. 2016;80(4):980–988. doi: 10.1253/circj.CJ-15-1212. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa A., Satoh T., Fukuda T., et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes. 2017;10(11) doi: 10.1161/CIRCOUTCOMES.117.004029. [DOI] [PubMed] [Google Scholar]
- 16.Ogo T., Fukuda T., Tsuji A., et al. Efficacy and safety of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension guided by cone-beam computed tomography and electrocardiogram-gated area detector computed tomography. Eur J Radiol. 2017;89:270–276. doi: 10.1016/j.ejrad.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Feinstein J.A., Goldhaber S.Z., Lock J.E., Ferndandes S.M., Landzberg M.J. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001;103(1):10–13. doi: 10.1161/01.cir.103.1.10. [DOI] [PubMed] [Google Scholar]
- 18.Freed D.H., Thomson B.M., Berman M., et al. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011;141(2):383–387. doi: 10.1016/j.jtcvs.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 19.Ghofrani H.A., Simonneau G., D'Armini A.M., et al. MERIT Study Investigators Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med. 2017;5(10):785–794. doi: 10.1016/S2213-2600(17)30305-3. [DOI] [PubMed] [Google Scholar]
- 20.Pepke-Zaba J., Ghofrani H.A., Hoeper M.M. Medical management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):160107. doi: 10.1183/16000617.0107-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velázquez Martin M., Albarrán González-Trevilla A., Alonso Charterina S., García Tejada J., Cortina Romero J.M., Escribano Subías P. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: preliminary experience in Spain in a series of 7 patients. Rev Esp Cardiol (Engl Ed) 2015;68(6):535–537. doi: 10.1016/j.rec.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Aoki T., Sugimura K., Nochioka K., et al. Effects of balloon pulmonary angioplasty on oxygenation in patients with chronic thromboembolic pulmonary hypertension - importance of intrapulmonary shunt. Circ J. 2016;80(10):2227–2234. doi: 10.1253/circj.CJ-16-0254. [DOI] [PubMed] [Google Scholar]
- 23.Koike H., Sueyoshi E., Sakamoto I., Uetani M., Nakata T., Maemura K. Correlation between lung perfusion blood volume and SPECT images in patients with chronic thromboembolic pulmonary hypertension by balloon pulmonary angioplasty. Clin Imaging. 2018;49:80–86. doi: 10.1016/j.clinimag.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Yamasaki Y., Nagao M., Abe K., et al. Balloon pulmonary angioplasty improves interventricular dyssynchrony in patients with inoperable chronic thromboembolic pulmonary hypertension: a cardiac MR imaging study. Int J Cardiovasc Imaging. 2017;33(2):229–239. doi: 10.1007/s10554-016-0985-y. [DOI] [PubMed] [Google Scholar]
- 25.Olsson K.M., Wiedenroth C.B., Kamp J.C., et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J. 2017;49(6):1602409. doi: 10.1183/13993003.02409-2016. [DOI] [PubMed] [Google Scholar]
- 26.Brenot P., Jais X., Taniguchi Y., et al. Balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension: the initial experience at Paris-Sud University. Am J Respir Crit Care Med. 2018;197:A7788. [Google Scholar]
- 27.Shimura N., Kataoka M., Inami T., et al. Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol. 2015;183:138–142. doi: 10.1016/j.ijcard.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 28.Sugimura K., Fukumoto Y., Satoh K., et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J. 2012;76(2):485–488. doi: 10.1253/circj.cj-11-1217. [DOI] [PubMed] [Google Scholar]
- 29.Yanagisawa R., Kataoka M., Inami T., et al. Safety and efficacy of percutaneous transluminal pulmonary angioplasty in elderly patients. Int J Cardiol. 2014;175(2):285–289. doi: 10.1016/j.ijcard.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Broch K., Murbraech K., Ragnarsson A., et al. Echocardiographic evidence of right ventricular functional improvement after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2016;35(1):80–86. doi: 10.1016/j.healun.2015.08.007. [DOI] [PubMed] [Google Scholar]


