Abstract
Thrombospondin 1 (TSP1) is a matricellular extracellular matrix protein that has diverse roles in regulating cellular processes important for the pathogenesis of fibrotic diseases. We will present evidence for the importance of TSP1 control of latent transforming growth factor beta activation in renal fibrosis with an emphasis on diabetic nephropathy. Other functions of TSP1 that affect renal fibrosis, including regulation of inflammation and capillary density, will be addressed. Emerging roles for TSP1 N-terminal domain regulation of collagen matrix assembly, direct effects of TSP1–collagen binding, and intracellular functions of TSP1 in mediating endoplasmic reticulum stress responses in extracellular matrix remodeling and fibrosis, which could potentially affect renal fibrogenesis, will also be discussed. Finally, we will address possible strategies for targeting TSP1 functions to treat fibrotic renal disease.
Keywords: calreticulin, chronic kidney disease, collagen, diabetic nephropathy, endoplasmic reticulum, extracellular matrix, fibrosis, kidney, thrombospondin, transforming growth factor beta
Introduction
Matricellular proteins are components of the extracellular matrix (ECM) that interact with cells and other ECM components: Their functions are to regulate cell behavior and ECM organization rather than acting as primary structural elements of the ECM.1–3 Thrombospondin 1 (TSP1) is a prototypic matricellular ECM protein with diverse and significant roles in tissue remodeling and fibrosis. TSP1 is a disulfide-bonded, homotrimeric secreted glycoprotein and a member of the group A subfamily of five TSP family members.4 TSP1 is composed of multiple structural domains with distinct intermolecular interactions, cellular receptors, and functional roles.1,5,6 First studied as a component of platelet α-granules for its role in platelet aggregation, TSP1 is now known to be widely expressed by diverse cells in a highly regulated manner.7 TSP1 regulates an array of cellular processes, primarily through its interactions with cellular receptors and also through interactions with other extracellular molecules, including ECM proteins and glycosaminoglycans, matrix-regulating enzymes, and growth factors.1,4 TSP1 has roles in regulating cell adhesion and deadhesion, cell migration, apoptosis, ECM organization, activity of matrix-degrading enzymes such as matrix metalloproteinases and lysyl oxidases, and growth factor activity.
Given these roles in regulating cellular functions, it is not surprising that TSP1 is important in disease processes involving tissue remodeling—both fibrotic and normal reparative states—and in inflammation.5,8 Several functions of TSP1 have been shown to be involved in fibrotic disease. The most prominent role for TSP1 in fibrosis centers on its ability to activate latent transforming growth factor beta (TGF-β), particularly in diabetic nephropathy.9 Other studies suggest that TSP1 regulates metabolic inflammation and fibrosis.10,11 In addition, there are roles for TSP1 in regulating collagen matrix assembly and crosslinking.5,12,13 Moreover, there is an emerging appreciation that intracellular TSP1 might have roles in endoplasmic reticulum (ER) stress and calcium regulation.14,15
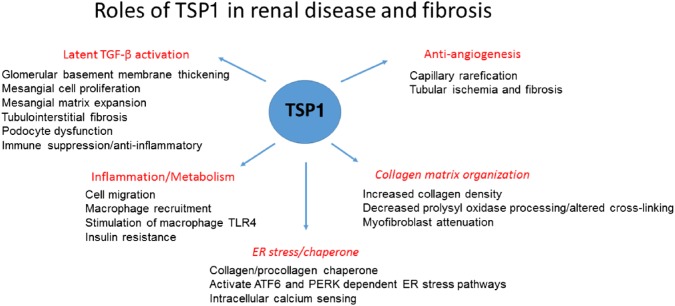
In this review, we will first focus on known roles for TSP1 in fibrotic renal diseases, with an emphasis on its role in controlling latent TGF-β activation (Fig. 1). We will also consider some emerging functions of TSP1 in ECM remodeling and discuss how these TSP1 functions could also contribute to dysregulation of ECM in fibrosis. Finally, we will discuss how these TSP1 functions might be regulated for therapeutic interventions.
Figure 1.
TSP1 has diverse functions in renal disease and fibrosis. Major functions are described in red, with specific implications for renal cells and disease pathogenesis listed below the headings. TSP1’s functions in collagen organization and as an ER stress regulator (headings are italicized) have not yet been investigated in the context of renal disease, although these functions might indeed be relevant to the pathogenesis of some renal diseases. Abbreviations: TSP1, thrombospondin 1; ER, endoplasmic reticulum; TGF-β, transforming growth factor beta.
TSP1 as an Activator of Latent TGF-β
Our lab discovered that human platelet TSP1 purified from releasates of thrombin-stimulated platelets was associated with biologically active TGF-β, also a major component of platelet α-granules.16 Further studies by our group identified that TSP1 binds to both the small and large forms of latent TGF-β and that this binding interaction can convert the inactive precursor to a biologically active form of TGF-β in a protease-independent manner.17,18 We identified key sequences in both TSP1 and the latent TGF-β complex necessary for latency and for TSP1-dependent activation of the latent complex.19–22 Small peptides of the TSP1-activating sequence (KRFK) or of the TGF-β latency-associated peptide (LSKL) were used as probes to either mimic or inhibit TSP1-dependent TGF-β activation as a means of assessing the relevance of the TSP1–TGF-β pathway in vitro and in multiple animal models of disease.5,23–26 This function is specific to the TSP1 family member as thrombospondin-2 (TSP2) can bind to the latent complex but lacks the KRFK activation sequence, and the thrombospondin (TSP) type B subfamily members lack the type 1 repeat region that contains the KRFK and WxxW TGF-β binding sequences.19 A detailed discussion of the mechanism and cellular variations by which TSP1 activates latent TGF-β was recently published.26
TSP1 as an Activator of Latent TGF-β in Renal Fibrosis
TGF-β is a key mediator of renal fibrosis in multiple animal models and in diverse human renal diseases.27–29 With the discovery by our lab that TSP1 can activate latent TGF-β, multiple groups investigated whether TSP1 plays a role in renal fibrosis through its ability to control latent TGF-β activation.30 TSP1 is widely expressed at low levels under quiescent conditions by most renal cell types, including podocytes, mesangial cells, tubular epithelial cells, and macrophages.31–34 As detailed in the specific studies described in the following sections, TSP1 is upregulated in human renal fibrotic diseases, in various animal models of renal fibrosis, and in renal cell culture systems exposed to fibrotic or inflammatory stimuli.35–37
Using multiple different models of renal injury, Hugo et al. showed that TSP1 expression was upregulated in tubular cells and myofibroblasts and by some macrophages and that TSP1 expression predicted the severity of tubulointerstitial fibrosis.36,38 Daniel et al. used antisense oligonucleotides to TSP1 in the anti-Thy-1 antibody rat model of mesangioproliferative glomerulonephritis: Thy-1 is a protein expressed on mesangial cells, and the anti-Thy-1 antibody model is a rapidly developing model of glomerulonephritis which mimics human mesangial cell proliferative glomerulonephritis characterized by mesangial cell lysis with later repopulation by proliferating myofibroblast-like mesangial cells, inflammatory cell infiltration, and glomerular fibrosis with eventual resolution.39,40 Treatment with antisense oligonucleotides to TSP1 decreased TGF-β activity and Smad signaling as well as reduced ED-A fibronectin and collagen I deposition, but there was no effect on macrophage infiltration or glomerular mesangial or endothelial cell proliferation.40 Further studies from this group used two different peptides that block TSP1 binding to latent TGF-β (LSKL, AAWSHW) to prevent TSP1-dependent activation and observed reduced TGF-β activation, glomerular ECM deposition, and proteinuria in the anti-Thy-1 antibody rat model.9 The blocking LSKL peptide also reduced renal interstitial fibrosis in rats subjected to unilateral ureteral obstruction (UUO): Myofibroblast accumulation, phosphorylated Smad 2, tubular injury, and collagen deposition were reduced in rats treated with LSKL peptide.41 In models of chronic kidney disease, mice lacking both Col4α3 and Thbs1 had delayed disease progression with reduced TGF-β activity, fibroblast proliferation, and fibrosis compared with animals lacking only Col4α3, but expressing TSP1.42 In the absence of TSP1-activated TGF-β, there was a shift to a Th1 type of inflammation with decreased Th17 and FOXP3+ T-regulatory cells, likely due to loss of the anti-inflammatory activity of TGF-β, but also potentially due to loss of direct TSP1 actions.42
Diabetes is the most common cause of end-stage renal failure in the United States.29,43 TGF-β is well documented as a major factor in the pathogenesis of diabetic nephropathy, with involvement in the various processes leading to renal failure, including glomerular hypertrophy with mesangial matrix expansion, glomerular basement membrane thickening, renal tubular injury and interstitial fibrosis, and alterations in podocyte slit barrier function, leading to proteinuria.29,44–46 A myriad of factors in the diabetic milieu, including increased levels of glucose and advanced glycation end products, increased activity of the renin–angiotensin system and hypertension, and increased oxidative stress, are linked to increased TGF-β in diabetic renal disease.47–53 Evidence from multiple labs has established TSP1 as a major regulator of the inappropriate TGF-β activity in diabetic nephropathy. Renal biopsy samples from patients with both type 1 and type 2 diabetic nephropathy showed corresponding increases in glomerular TSP1 and latent and active TGF-β expression during progression of diabetic nephropathy, and correlation of TSP1 expression, Smad signaling, and proteinuria was seen in patients with type 2 diabetic nephropathy.37,54 TSP1 is also induced by both glucose and angiotensin II, two factors directly linked to increased TGF-β activity in diabetic nephropathy.55–58 Our lab demonstrated that high glucose or angiotensin II treatment induced TSP1 protein by rat mesangial cells.56,59 Glucose also induces TSP1 mRNA and protein by podocytes.33 Glycated albumin stimulates increased USF2 expression and tubular TSP1 protein.60,61 Under normal glucose conditions, nitric oxide–dependent activation of cGMP-dependent protein kinase (protein kinase G [PKG]) normally suppresses TSP1 transcription.62,63 In contrast, increased oxidative stress under high glucose conditions decreases PKG activity, resulting in attenuation of transcriptional repression of TSP1 and upregulation of the transcription factor USF2 to mediate increased TSP1 transcription.64 The importance of USF2 stimulation of TSP1 expression was shown in studies demonstrating that streptozotocin-treated USF2-overexpressing mice had accelerated development of diabetic nephropathy, with increased TSP1, collagen I, and TGF-β protein in glomeruli isolated from these mice.65 The hexosamine pathway was also shown to induce TSP1 transcription and mRNA levels by high-glucose-treated vascular smooth muscle cells.66 More direct evidence for the importance of TSP1 in regulating TGF-β activity in diabetic nephropathy comes from studies in two different models of type 1 diabetic nephropathy. The first model showed that streptozotocin-treated Thbs1 null mice are protected from glomerulosclerosis and podocyte loss,67 and in the second model, treatment of uninephrectomized type 1 diabetic Akita mice with the TSP1 antagonist peptide LSKL reduced proteinuria and tubulointerstitial fibrosis and decreased glomerular TGF-β signaling as measured by phosphorylated Smad 2.25 The Akita mouse has a mutated cysteine in the insulin gene, which prevents proper protein folding and is in effect an insulin-deficient, non-obese model of type 1 diabetes.68
In addition to these models of type 1 diabetic nephropathy, Thbs1 null mice are protected from renal dysfunction and had reduced glomerular phosphorylated Smad 2 and 3 in a high-fat diet model of obesity.11 Recent data showed that the TSP1 blocking peptide LSKL reduced active TGF-β in macrophages exposed to hypoxia.69 Furthermore, in studies with myeloid cell–specific deletion of Thbs1, but not adipocyte deletion, mice had reduced adipose tissue macrophage infiltration and inflammation, reduced adipose tissue TGF-β signaling and fibrosis, and improved glucose tolerance and insulin sensitivity.69 Together, these data suggest that TSP1-dependent TGF-β activation is important in obesity-related renal and metabolic diseases, such as hypertension and type 2 diabetes. Moreover, these findings suggest that specific cellular sources of TSP1 can variably affect regulation of TGF-β activation in disease.
Renin–angiotensin antagonists such as angiotensin-converting enzyme inhibitors or angiotensin receptor blockers are the current standard of care for diabetic kidney disease, and these drugs reduce proteinuria due to normalization of glomerular hyperfiltration, but do not reverse or halt progression to end-stage renal disease.70,71 Nearly 20% of treated patients progress to end-stage renal disease.72 Interestingly, we showed that the angiotensin II type 1 receptor antagonist valsartan reduces angiotensin II stimulation of TSP1 expression by rat mesangial cells.59 Synergy between renin–angiotensin system blockers and TGF-β antagonists has been demonstrated in animal models of diabetic nephropathy despite some overlap in their targets.73–75 It remains to be determined whether TSP1-TGF-β antagonists might have synergy with renin–angiotensin blockers.
Interestingly, drugs that tightly regulate glucose have unexpectedly shown cardiovascular and renal benefit in clinical trials and in rodent models of nephropathy in obese and type 2 diabetic animals: Those drugs that target glucose transporters, such as the sodium glucose cotransporter 2 (SGLT2) inhibitors, empagliflozin and canagliflozin, slow renal decline, independent of their effects on glycemia.76–79 A recent study investigating possible mechanisms of renoprotection showed that SGLT2 inhibitors decrease TSP1 expression in two human proximal tubular cell lines.80
TGF-β-Independent Functions of TSP1 in Renal Fibrosis
It should be noted that other important functions of TSP1 potentially contribute to renal fibrosis, independent of its role in activating latent TGF-β. TSP1 binding to its receptor CD36 is involved in podocyte apoptosis.81 TSP1 has a pro-inflammatory effect on macrophages by stimulating increased toll-like receptor 4 expression, which in turn induces tumor necrosis factor-α production in a manner blocked by peptides that prevent TSP1–CD36 binding.82
TSP1 is a known inhibitor of angiogenesis through its ability to downregulate nitric oxide signaling downstream of binding to CD47, and the TSP1–CD47 pathway is well documented to exacerbate ischemia–reperfusion injury.83,84 TSP1 signaling through CD47 has been shown to inhibit tubular epithelial repair, potentially predisposing to later development of chronic kidney disease.84 TSP1 can also antagonize FGF2, also known as basic fibroblast growth factor (bFGF), and vascular endothelial grown factor (VEGF) binding to cellular receptors to impede angiogenesis while promoting platelet-derived growth factor (PDGF) binding to mesenchymal stromal cells.85,86 A decrease in peritubular capillary density, termed capillary rarefaction, promotes hypoxia and progression of chronic kidney disease with fibrosis and tubular atrophy.87 In the UUO model, TSP1 short hairpin RNA increased VEGF protein levels and peritubular capillary density and reduced tubulointerstitial fibrosis.88 A peptide (peptide 545) from the type 1 repeat of TSP containing the WSXW sequence, but lacking the TGF-β-activating KRFK sequence, blocked glomerular endothelial cell proliferation stimulated by bFGF or PDGF in vitro and in vivo in the anti-Thy1 model.89 Increased TSP1 in the aging kidney has been associated with decreased peritubular and glomerular capillary density and concomitant tubulointerstitial fibrosis and glomerulosclerosis.90 It is possible that under these conditions, both the TGF-β-activating and the antiangiogenic functions of TSP1 drive fibrogenic progression.87,91
TSP2 shares many structural and functional similarities with TSP1.8 However, TSP2 lacks the TGF-β-activating sequence of TSP1 and can actually antagonize latent TGF-β activation by TSP1.19 Daniel et al. explored the role of TSP2 in the anti-Thy-1 model of glomerulonephritis and showed that Thbs2 null mice had increased cell proliferation, inflammation, and matrix metalloproteinase 2 activity.92 Forced overexpression of TSP2 in this mouse model reduced endothelial cell proliferation, inflammation, TGF-β activation, and ECM deposition, although prolonged TSP2 overexpression resulted in capillary rarefication in a model of chronic allograft nephropathy.93,94
TSP1 Actions in Repair and Fibrosis in Non-renal Systems
In the following sections, we will discuss additional functions of TSP1 in regulating ECM remodeling in repair and fibrosis. Many of these actions have been studied in non-renal models. However, given the complexity and context dependence of TSP1 actions, it is useful to have a broad perspective on the diverse abilities of this molecule. Hopefully, a more comprehensive view of the multiplicity of its actions will lead to new insights into its possible roles in renal fibrosis.
TSP1 as a Regulator of Collagen Matrix Organization
A key characteristic of matricellular proteins is that in vitro surfaces coated with these ECM proteins do not support full cell adhesion with focal adhesion and stress fiber formation.1,95 We identified a heparin-binding sequence in the N-terminal domain of TSP1 and TSP2 (aa 17–35) that stimulates the disassembly of focal adhesions in fibroblasts and endothelial cells.96,97 TSP and peptide mimetics of this sequence bind to a cell-surface form of the ER stress and calcium regulatory protein, calreticulin, to signal focal adhesion disassembly in a phosphoinositide 3-kinase–dependent manner.98–100 This peptide (hep I) also triggered both random and directed cell migration of fibroblasts and endothelial cells.101 As calreticulin lacks a transmembrane domain, TSP1 or hep I peptide bound to calreticulin triggers signaling by inducing calreticulin association with LDL receptor–related protein 1 (LRP1) to activate G protein signaling and transiently downregulate Rho activity.100,102,103 In further studies, we showed that the N-terminal TSP1 domain or the hep I peptide prevented anoikis of non-adherent fibroblasts through binding to calreticulin/LRP1.104
Increased cell migration and persistence of fibroblasts are frequently observed in fibrosis.105,106 To address the in vivo effects of TSP1 signaling through the calreticulin/LRP1 pathway, we developed a model of the foreign body response using sponges with forced local expression of the secreted TSP1 calreticulin-binding sequence.13 In animals implanted with collagen-loaded sponges overexpressing this secreted calreticulin-binding sequence, we observed the formation of a dense and highly organized collagen capsule surrounding the foreign body in comparison with animals implanted with control sponges expressing an inactive, mutated TSP1 sequence, despite no observed differences in the number or types of cells infiltrating into the foreign body sponge. In vitro studies confirmed the ability of the TSP1 calreticulin-binding sequence to stimulate increased fibrillar collagen protein expression in conditioned media and incorporation of collagen I into a deoxycholate-insoluble ECM. N-terminal domain regulation of collagen matrix occurred independent of TSP1’s TGF-β-activating ability and was dependent on signaling through Akt.13 The increased collagen expression and ECM deposition induced by the TSP1 calreticulin-binding sequence does not involve changes in collagen I transcript or altered N- and C-terminal collagen propeptide processing.13 The specific mechanism by which TSP1 binding to the calreticulin/LRP1 complex increases collagen expression and matrix organization remains unclear, but could possibly involve regulation of posttranscriptional secretory processes, as well as of factors involved in collagen fibril assembly.
The N-terminal domain of TSP1 can be released by multiple proteases involved in tissue remodeling, including ADAMTS1, cathepsin G, and neutrophil elastase, as well as by a collagenase used clinically for wound debridement (Santyl), suggesting roles in tissue repair responses.107–111 The protein receptors, calreticulin and LRP1, for this sequence of TSP1 have variable roles in fibrosis. Calreticulin is upregulated in the UUO model of renal fibrosis and in bleomycin-induced lung fibrosis, and downregulation of calreticulin attenuates fibrosis.112,113 Furthermore, we showed that calreticulin in the ER regulates collagen transcription, processing, and assembly into the ECM, as well as TGF-β-mediated ECM transcription through calcium-dependent activation of nuclear factor of activated T cells (NFAT).114,115 However, these studies focused on intracellular ER calreticulin rather than on calreticulin at the cell surface, where it would be a ligand for TSP1 and a co-receptor with LRP1. In fact, exogenous extracellular calreticulin promotes scarless wound healing.116,117 However, there is a recent report that cell surface calreticulin on HK-2 proximal tubule cells binds to surfactant protein-A to induce oxidative stress, type 1 collagen expression, and cellular apoptosis and to downregulate matrix metalloproteinases, suggesting a possible role in renal fibrosis.118 In addition, both profibrotic and antifibrotic actions have been reported for LRP1 through both its signaling and endocytic functions. Cellular responses appear to be cell type specific and possibly vary depending on which ligand engages LRP1: For example, tissue plasminogen activator binding to LRP1 engages signaling through integrins that has profibrotic effects on kidney interstitial fibroblasts.119–123 Ultimately, direct examination of TSP1 N-terminal domain binding to calreticulin–LRP1 on collagen organization in fibrotic remodeling remains to be addressed using TSP-binding calreticulin peptides that block TSP1–calreticulin interactions in animal models.13,99
The TSP family has been long known to play a role in the regulation of collagenous ECM fibril organization. Thbs2–/– mice have irregular collagen fibril formation, and it was recently shown that TSP2-regulated miR-29 is decreased in these mice, leading to reduced lysyl oxidase and defective collagen crosslinking.124,125 Cartilage oligomeric matrix protein (COMP), also known as thrombospondin-5 (TSP5), is an important regulator of collagen fibrillogenesis and acts to increase the rate of fibrillogenesis.126 TSP1 is well known to bind to various fibrillar collagens, and altered collagen matrix has been noted in Thbs1–/– mice.127,128 New data provide interesting evidence for direct TSP1–collagen interactions in regulating collagen fibril formation.12 In this work, Rosini et al.12 identified TSP1 binding sites in the C-propeptide domain of intracellular pools of collagen and in a KGHR containing conserved sequence in crosslinking sites in the triple helical region of mature, secreted collagen. Interestingly, both COMP and thrombospondin-4 (TSP4) were shown to bind to the GXKGHR motif in collagen type II, suggesting a conservation of this interaction with fibrillar collagens among these TSP family members.129 Thbs1–/– dermal fibroblasts have altered collagen crosslinking, a finding that was recently confirmed in both Thbs1- and Thbs2-deficient female bone diphyses.12,130 TSP1 binds prolysyl oxidase and prevents prolysyl oxidase activation by BMP-1. The TSP1–KGHR interaction is important in fibroblast homeostasis as peptides that disrupt this interaction increase alpha-smooth muscle actin (α-SMA)–positive myofibroblasts in a manner dependent on signaling through TGF-β receptor I.12 These findings are intriguing and suggest that TSP1–procollagen/collagen interactions potentially regulate collagen trafficking, processing, and fibril assembly. These data also suggest that TSP1 bound to collagen in the ECM potentially “normalizes” collagen matrix and prevents myofibroblast differentiation, thus indicating a possible antifibrotic role for collagen-bound TSP1. This would be distinct from various profibrotic activities of secreted TSP1 (which is presumably soluble or receptor bound) in promoting TGF-β activation, cell migration, or dense collagenous capsule formation. It is not known whether these TSP1–collagen interactions are functionally related to the observations of increased collagen organization in response to extracellular stimulation with the calreticulin binding N-terminal domain sequence. These multiple collagen regulatory functions of TSP1 suggest that we do not yet fully understand the breadth of TSP1 functional complexity in the regulation of the collagenous ECM. Studies that potentially address alterations in collagen crosslinking and myofibroblast induction in Thbs1-deficient mice in models of wound healing and renal fibrosis would be useful in confirming these functions in pathology.
Intracellular TSP1 as an ER Stress Regulator of ECM
Given the diversity of the TSP1 interactome,6 it seems almost intuitive that TSP1 might play intracellular roles in regulating protein trafficking in the ER. However, until recently, possible intracellular TSP1 functions have been largely unexplored.1,131
The unfolded protein response (UPR) is an adaptive cellular response, called ER stress, to ensure proper proteostasis under challenging conditions such as increased oxidative stress, exposure to high glucose levels as in diabetes, or altered nutrient conditions.132 Under acute stress conditions, mechanisms are activated to attenuate protein translation, to increase chaperone expression to assure proper protein folding, and to degrade misfolded proteins. However, under prolonged stress, these responses become detrimental and lead to apoptotic cell death and activation of fibrosis pathways.133–135 This is analogous to acute tissue repair responses that are active in fibrotic processes due to continued inappropriate stimuli. There is now a significant body of literature that links the ER stress response with fibrosis and enhanced TGF-β activity in various organ systems.113,135–141 It is well established that ER stress pathways are activated in fibrotic renal disease, including in diabetic nephropathy and in ischemia, and that chemical chaperones such as 4-phenylbutyrate and tauroursodeoxycholic acid have been shown to reduce ER stress and result in fibrosis in animal models of renal disease.142–149
It is unclear how TSP1 and other TSP family members might be participating in the UPR and other branches of the ER stress response and whether intracellular TSP1 functions are involved in fibrogenic processes. Studies on mutant COMP (TSP5) in models of pseudoachondroplasia showed a more severe phenotype in animals in which the mutant protein was expressed than in knockout mice.150,151 It was also observed that mutant COMP associated with other ECM proteins and chaperones such as calreticulin in the ER, suggesting the formation of intracellular ECM vesicles.152 Indeed, Rosini et al.12 reported intracellular vesicles of TSP1 and procollagen. Moreover, TSP1 and TSP4 bind to the ER luminal domain of activating transcription factor 6 (ATF6), a branch of the UPR that increases chaperone expression and downregulates protein translation.15 TSP–ATF6 binding occurs through the type 3 repeats of TSP as demonstrated in studies with recombinant TSP4 fragments.15 TSP1, similar to TSP4, binds to ATF6 specifically in the Golgi compartment and increases nuclear shuttling of ATF6 and chaperone expression to activate ER stress protective mechanisms, particularly in cardiac myocytes and in skeletal muscle cells.15 Further studies showed that TSP1 prevents pancreatic β-cell death from lipotoxic palmitate by activating the ER stress pathway PKR-like ER kinase (PERK)–NRF2 antioxidant signaling.153 It is interesting that this cell-protective effect was only induced by intracellular TSP1 engineered with an ER retention KDEL sequence and was not observed with conditioned medium TSP1.153,154 In this pancreatic β-cell system, ER TSP1 colocalized with protein disulfide isomerase. Further studies showed that TSP1 was also cytoprotective to β cells acutely exposed to the inflammatory cytokines interleukin-1β or interferon-γ or to thapsigargin-induced ER stress, although prolonged exposure (4–24 hr) resulted in downregulation of TSP1 expression and proteosomal degradation, leading to cell death.154 Under these conditions, TSP1’s cytoprotective effect was dependent on the expression of mesencephalic astrocyte-derived neutrotrophic factor rather than ATF6 or modulation of oxidative stress.154 These studies suggest that in acute or under limited stress or injury, TSP1 might have cytoprotective effects that could be lost upon chronic stimulation. The conditions that determine ER retention of TSP1 are not entirely clear, although low calcium levels have been shown to retain TSP1 in the ER of renal carcinoma cells.155 In addition, given TSP1’s complex disulfide bond arrangement and its glycosylation, it is reasonable that TSP1 could be improperly folded under conditions of ER stress and thus retained in the ER or targeted for degradation through the endoplasmic reticulum–associated degradation pathway. Proper O-glycosylation of TSP1 type 1 repeats stabilizes proper folding and promotes transit from the ER.156 This suggests a scenario in which TSP1 is initially a cytoprotective protein in cellular homeostasis. However, TSP1 message and protein levels are increased in many fibrotic processes: One scenario might be that during prolonged ER stress, an increasing fraction of TSP1 with altered glycosylation or defective protein folding could escape the ER, which could potentially enhance extracellular interactions with profibrotic receptors or factors such as latent TGF-β.
It has also been suggested that TSP1 might regulate calcium signaling through binding of the C-terminal signature domain of TSP1 to the N-terminal ER luminal domain of the ER membrane calcium sensor, STIM1 (stromal interaction molecule 1).14 STIM1 senses low calcium levels and activates store operated calcium entry (SOCE) mechanisms to restore ER calcium levels.157 Although it is unknown whether TSP1 affects STIM1 function, overexpression of COMP attenuates ER calcium release–activated calcium channel activity, whereas it increases arachidonate calcium channels at the plasma membrane and results in increased thapsigargin-induced cytosolic calcium levels.14 Interestingly, extracellular TSP1 binds to plasma membrane STIM1 on platelets.158 Currently, there are no data to directly link ER TSP1 to fibrosis through regulation of SOCE mechanisms, although there are data from cardiac fibrosis models showing that SOCE and STIM1 can induce fibrosis through downstream activation of NFAT and TGF-β signaling.159 In renal models, TGF-β signals transcription of fibronectin and collagen type 1 in mesangial cells in an NFAT-dependent manner,160–162 and an NFAT inhibitor reduces renal fibrosis in mouse models of diabetic nephropathy.163 Forced TSP1 overexpression in cardiomyocytes increases expression of the ER chaperone and calcium regulatory protein, calreticulin, which our lab showed is critical for TGF-β-mediated transcription of fibronectin and collagen in fibrosis models through stimulating calcium-dependent activation of NFAT.15,114,115,164,165 Moreover, the NoC1 calreticulin-binding fragment of TSP1 increases calreticulin expression in fibroblasts (unpublished data). TSP1 is also a transcriptional target of NFAT,166,167 representing a possible feedforward mechanism. However, indirect TSP1 activation of NFAT might be cell type or cell context specific, because TSP1 binding to CD36 activates both JNK2 and NFκB, which block NFAT activation and inhibit angiogenesis.168 Nonetheless, possible intracellular TSP regulation of cytosolic calcium levels in modulation of transcriptional pathways such as NFAT downstream of TGF-β in fibrosis warrants further exploration.
TSP1 as a Therapeutic Target
This review highlights some of the diverse functions of TSP1 in the pathogenesis of fibrosis in the kidney (Table 1). Given the multiple roles of TSP1 in the pathogenesis of fibrosis, it will be important to identify which specific interactions and functions of TSP1 are important in a particular disease entity and target only those specific actions. This focused approach could avoid side effects from general blockade of all TSP1 functions. For example, studies that induced renal overexpression of TSP2 to competitively antagonize TSP1-dependent TGF-β activation in a model of chronic allograft nephropathy not only decreased TGF-β activity as predicted, but also induced glomerular and peritubular capillary rarefication due to excessive antiangiogenic activity of TSP2.94
Table 1.
Diverse Interactions and Roles of Thrombospondin 1 in Renal Disease.
| TSP1 Domain | TSP Tools | Cellular Location | Receptor or Ligand | Function | Renal Disease Relevance | References |
|---|---|---|---|---|---|---|
| Type 1 repeat (RFK in second type 1 repeat) | Purified protein, proteolytic fragment, bacterial and insect cell domain, peptides | Secreted/cell surface | Latent TGF-β (LAP, mature TGF-β) | Activation of TGF-β, fibrosis | UUO model, anti-Thy-1 antibody membranous glomerulonephritis, type 1 and type 2 diabetic nephropathy, obesity-related nephropathy | 9, 11, 25, 40, 41, 67, 69 |
| CSVTCG in second and third type 1 repeat | Purified protein, recombinant fragments, peptide | Cell surface bound | CD36 | Podocyte apoptosis, macrophage TLR4 activation | Renal inflammation and podocyte injury | 81, 82 |
| TSP1 type 1 repeats (bFGF, binding domain) | Short hairpin TSP1 RNA D-reverse type 1 domain peptide 545 |
Secreted/cell surface | VEGF and bFGF antagonism | Antiangiogenic activity | Capillary rarefication and fibrosis in UUO model and age-related nephropathy, blockade of mesangial and glomerular endothelial proliferation in anti-Thy1 model | 88–90 |
| TSP1 signature domain (E123CaG) FIRVVMYEGKK |
Purified protein, insect cell fragment, peptide | Cell surface bound | CD47 | Inhibition of nitric oxide–mediated vasodilation, inhibition of tubular epithelial repair | Ischemia–reperfusion injury | 83, 84, 169 |
This table summarizes evidence for different TSP1 functions in renal disease discussed in this review. The domain or sequence of TSP1 and its corresponding ligand or receptor are indicated. Abbreviations: TSP1, thrombospondin 1; TGF-β, transforming growth factor beta; UUO, unilateral ureteral obstruction; bFGR, basic fibroblast growth factor; VEGF, vascular endothelial grown factor.
As described above, genetic and biochemical approaches to blocking TSP1-dependent TGF-β activation have been effective in reducing TGF-β activity, fibrosis, and renal dysfunction in a number of preclinical animal models of renal fibrosis, particularly in models of diabetic nephropathy. To date, the biochemical approaches have involved use of peptide antagonists or antisense oligonucleotides. Because of the short half-life of peptides and the need for injectable delivery routes, longer lived, orally available small molecules would represent more useful drugs for treating patients with chronic kidney disease. Such oral small molecule antagonists are currently under development.
TGF-β has long been a target for the treatment of fibrosis, and in particular, diabetic renal disease. However, results from clinical studies with anti-TGF-β neutralizing antibodies have been disappointing.170–173 In addition, there are cardiotoxicity concerns with general TGF-β ligand or signaling antagonists.174 It is becoming more widely appreciated that interventions that block disease-specific mechanisms of latent TGF-β activation, such as blocking TSP1 or integrin-dependent activation, are likely to have a greater therapeutic index than more broadly targeted antagonists.26,173,175 Interestingly, some reports suggest that certain integrins can regulate TSP1 expression. In a corneal wound model that deals with fibrosis, knockout of the β6 integrin delayed initial healing and fibrosis and reduced TSP1 levels; however, TSP1 expression increased later in healing and was associated with α-SMA-positive cells.176 Furthermore, an antibody to the αvβ3 integrin that reduces proteinuria also decreases TSP1 and TGF-β expression in diabetic rats.177 These intriguing observations suggest that there might be synergy between integrin and TSP-dependent modes of latent TGF-β activation.
Although in vitro studies show that TSP1, TGF-β, and TGF-β activation are regulated by angiotensin II and glucose and that the renin–angiotensin system and sodium glucose cotransporter antagonists downregulate TSP1 and TGF-β expression in renal cells,59,80 data from animal studies using combinations of TSP1-TGF-β antagonists with antagonists of these pathways are lacking. It will be interesting to determine whether there is synergy in using drugs that inhibit multiple related, but distinct, pathways to treat chronic kidney disease in diabetes and hypertension.
Approaches that target more than one action of TSP1, such as blocking both TGF-β activation and angiogenesis inhibition or growth factor binding through combining function-specific inhibitory molecules, could be of benefit. Anti-CD47 antibodies and small molecules that mimic the TSP binding site for bFGF are being tested in clinical trials, in preclinical models, or are under development for various diseases.169,178–182 Antagonists of these antiangiogenic TSP1 functions have not been tested with antagonists of TSP1’s TGF-β activating function. Combined use of a variety of TSP1 functional antagonists could represent a multipronged approach to treating the complex pathogenesis of fibrosis.
Acknowledgments
The author acknowledges all of the outstanding trainees and collaborators who contributed to the original studies described in this review.
Footnotes
Competing Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Murphy-Ullrich is a coinventor on several patents to develop antagonists of Thrombospondin 1-dependent transforming growth factor beta activation. There are no other conflicts to report.
Author Contribution: JEM-U wrote the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Murphy-Ullrich’s lab is currently supported by National Institutes of Health grants 1R01 CA175012 and 1R01 EY027924 and by funds from the Alabama Drug Discovery Alliance.
Literature Cited
- 1. Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130(3):503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest. 2001;107(8):929–34. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3(10):a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sweetwyne MT, Murphy-Ullrich JE. Thrombos-pondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31(3):178–86. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Resovi A, Pinessi D, Chiorino G, Taraboletti G. Current understanding of the thrombospondin 1 interactome. Matrix Biol. 2014;37:83–91. doi: 10.1016/j.matbio.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 7. Stenina-Adognravi O. Invoking the power of thrombospondins: regulation of thrombospondins expression. Matrix Biol. 2014;37:69–82. doi: 10.1016/j.matbio.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36(6):1115–25. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 9. Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, Hugo C. Thrombospondin 1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int. 2004;65(2):459–68. doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 10. Li Y, Tong X, Rumala C, Clemons K, Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS ONE. 2011;6(10):e26656. doi: 10.1371/journal.pone.0026656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cui W, Maimaitiyiming H, Qi X, Norman H, Wang S. Thrombospondin 1 mediates renal dysfunction in a mouse model of high-fat diet-induced obesity. Am J Physiol Renal Physiol. 2013;305(6):F871–80. doi: 10.1152/ajprenal.00209.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosini S, Pugh N, Bonna AM, Hulmes DJS, Farndale RW, Adams JC. Thrombospondin 1 promotes matrix homeostasis by interacting with collagen and lysyl oxidase precursors and collagen cross-linking sites. Sci Signal. 2018;11(532). doi: 10.1126/scisignal.aar2566. [DOI] [PubMed] [Google Scholar]
- 13. Sweetwyne MT, Pallero MA, Lu A, Van Duyn Graham L, Murphy-Ullrich JE. The calreticulin-binding sequence of thrombospondin 1 regulates collagen expression and organization during tissue remodeling. Am J Pathol. 2010;177(4):1710–24. doi: 10.2353/ajpath.2010.090903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duquette M, Nadler M, Okuhara D, Thompson J, Shuttleworth T, Lawler J. Members of the thrombospondin gene family bind stromal interaction molecule 1 and regulate calcium channel activity. Matrix Biol. 2014;37:15–24. doi: 10.1016/j.matbio.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lynch JM, Maillet M, Vanhoutte D, Schloemer A, Sargent MA, Blair NS, Lynch KA, Okada T, Aronow BJ, Osinska H, Prywes R, Lorenz JN, Mori K, Lawler J, Robbins J, Molkentin JD. A thrombospondin-dependent pathway for a protective ER stress response. Cell. 2012;149(6):1257–68. doi: 10.1016/j.cell.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy-Ullrich JE, Schultz-Cherry S, Höök M. Transforming growth factor-beta complexes with thrombospondin. Mol Biol Cell. 1992;3(2):181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122(4):923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schultz-Cherry S, Ribeiro S, Gentry L, Murphy-Ullrich JE. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-beta in a chemically defined system. J Biol Chem. 1994;269(43):26775–82. [PubMed] [Google Scholar]
- 19. Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270(13):7304–10. [DOI] [PubMed] [Google Scholar]
- 20. Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin 1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem. 1999;274(19):13586–93. [DOI] [PubMed] [Google Scholar]
- 21. Young GD, Murphy-Ullrich JE. Molecular interactions that confer latency to transforming growth factor-beta. J Biol Chem 2004;279(36):38032–9. doi: 10.1074/jbc.M405658200. [DOI] [PubMed] [Google Scholar]
- 22. Young GD, Murphy-Ullrich JE. The tryptophan-rich motifs of the thrombospondin type 1 repeats bind VLAL motifs in the latent transforming growth factor-beta complex. J Biol Chem. 2004;279(46):47633–42. doi: 10.1074/jbc.M404918200. [DOI] [PubMed] [Google Scholar]
- 23. Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin 1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93(7):1159–70. [DOI] [PubMed] [Google Scholar]
- 24. Belmadani S, Bernal J, Wei CC, Pallero MA, Dell’italia L, Murphy-Ullrich JE, Berecek KH. A thrombospondin 1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol. 2007;171(3):777–89. doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu A, Miao M, Schoeb TR, Agarwal A, Murphy-Ullrich JE. Blockade of TSP1-dependent TGF-beta activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am J Pathol. 2011;178(6):2573–86. doi: 10.1016/j.ajpath.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murphy-Ullrich JE, Suto MJ. Thrombospondin 1 regulation of latent TGF-beta activation: A therapeutic target for fibrotic disease. Matrix Biol. 2018;68–9:28–43. doi: 10.1016/j.matbio.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang AS, Hathaway CK, Smithies O, Kakoki M. Transforming growth factor-beta1 and diabetic nephropathy. Am J Physiol Renal Physiol. 2016;310(8):F68-99. doi: 10.1152/ajprenal.00502.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sureshbabu A, Muhsin SA, Choi ME. TGF-β signaling in the kidney: pro-fibrotic and protective effects. Am J Physiol Renal Physiol. 2016;310:F596–606. doi: 10.1152/ajprenal.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ziyadeh FN. Mediators of diabetic renal disease: the case for TGF-β as the major mediator. J Am Soc Nephrol. 2004;15Suppl1:S55–7. [DOI] [PubMed] [Google Scholar]
- 30. Hugo C, Daniel C. Thrombospondin in renal disease. Nephron Exp Nephrol. 2009;111(3):e61–6. doi: 10.1159/000198235. [DOI] [PubMed] [Google Scholar]
- 31. Raugi GJ, Lovett DH. Thrombospondin secretion by cultured human glomerular mesangial cells. Am J Pathol. 1987;129(2):364–72. [PMC free article] [PubMed] [Google Scholar]
- 32. Sarkozi R, Hauser C, Noppert SJ, Kronbichler A, Pirklbauer M, Haller VM, Grillari J, Grillari-Voglauer R, Mayer G, Schramek H. Oncostatin M is a novel inhibitor of TGF-β1-induced matricellular protein expression. Am J Physiol Renal Physiol. 2011;301(5):F1014–25. doi: 10.1152/ajprenal.00123.2011. [DOI] [PubMed] [Google Scholar]
- 33. Han SH, Yang S, Jung DS, Li JJ, Kim JJ, Kwak SJ, Kim DK, Moon SJ, Lee JE, Han DS, Kang SW. Gene expression patterns in glucose-stimulated podocytes. Biochem Biophys Res Commun. 2008;370(3):514–8. doi: 10.1016/j.bbrc.2008.03.121. [DOI] [PubMed] [Google Scholar]
- 34. Barnes JL, Mitchell RJ, Kanalas JJ, Barnes VL. Differential expression of thrombospondin and cellular fibronectin during remodeling in proliferative glomerulonephritis. J Histochem Cytochem. 1999;47(4):533–44. doi: 10.1177/002215549904700412. [DOI] [PubMed] [Google Scholar]
- 35. McGregor B, Colon S, Mutin M, Chignier E, Zech P, McGregor J. Thrombospondin in human glomerulopathies. A marker of inflammation and early fibrosis. Am J Pathol. 1994;144(6):1281–7. [PMC free article] [PubMed] [Google Scholar]
- 36. Hugo C, Shankland SJ, Pichler RH, Couser WG, Johnson RJ. Thrombospondin 1 precedes and predicts the development of tubulointerstitial fibrosis in glomerular disease in the rat. Kidney Int. 1998;53(2):302–11. doi: 10.1046/j.1523-1755.1998.00774.x. [DOI] [PubMed] [Google Scholar]
- 37. Wahab NA, Schaefer L, Weston BS, Yiannikouris O, Wright A, Babelova A, Schaefer R, Mason RM. Glomerular expression of thrombospondin 1, transforming growth factor beta and connective tissue growth factor at different stages of diabetic nephropathy and their interdependent roles in mesangial response to diabetic stimuli. Diabetologia. 2005;48(12):2650–60. doi: 10.1007/s00125-005-0006-5. [DOI] [PubMed] [Google Scholar]
- 38. Hugo C, Kang DH, Johnson RJ. Sustained expression of thrombospondin 1 is associated with the development of glomerular and tubulointerstitial fibrosis in the remnant kidney model. Nephron. 2002;90(4):460–70. doi: 10.1159/000054735. [DOI] [PubMed] [Google Scholar]
- 39. Johnson RJ. The glomerular response to injury: progression or resolution? Kidney Int. 1994;45(6):1769–82. [DOI] [PubMed] [Google Scholar]
- 40. Daniel C, Takabatake Y, Mizui M, Isaka Y, Kawashi H, Rupprecht H, Imai E, Hugo C. Antisense oligonucleotides against thrombospondin 1 inhibit activation of TGF-β in fibrotic renal disease in the rat in vivo. Am J Pathol. 2003;163(3):1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xie XS, Li FY, Liu HC, Deng Y, Li Z, Fan JM. LSKL, a peptide antagonist of thrombospondin 1, attenuates renal interstitial fibrosis in rats with unilateral ureteral obstruction. Arch Pharm Res. 2010;33(2):275–84. doi: 10.1007/s12272-010-0213-6. [DOI] [PubMed] [Google Scholar]
- 42. Zeisberg M, Tampe B, LeBleu V, Tampe D, Zeisberg EM, Kalluri R. Thrombospondin 1 deficiency causes a shift from fibroproliferative to inflammatory kidney disease and delays onset of renal failure. Am J Pathol. 2014;184(10):2687–98. doi: 10.1016/j.ajpath.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ritz E, Rychlik I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34(5):795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 44. Tsuchida K, Cronin B, Sharma K. Novel aspects of transforming growth factor-Beta in diabetic kidney disease. Nephron. 2002;92(1):7–21. doi: 10.1159/000064486. [DOI] [PubMed] [Google Scholar]
- 45. Wolf G. Growth factors and the development of diabetic nephropathy. Curr Diab Rep. 2003;3(6):485–90. [DOI] [PubMed] [Google Scholar]
- 46. Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diab Rev. 2008;4(1):39–45. [DOI] [PubMed] [Google Scholar]
- 47. Wolf G, Ziyadeh FN. The role of angiotensin II in diabetic nephropathy: emphasis on nonhemodynamic mechanisms. Am J Kidney Dis. 1997;29(1):153–63. [DOI] [PubMed] [Google Scholar]
- 48. Sharma K, Ziyadeh FN. Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes. 1995;44(10):1139–46. [DOI] [PubMed] [Google Scholar]
- 49. Chen S, Cohen MP, Lautenslager GT, Shearman CW, Ziyadeh FN. Glycated albumin stimulates TGF-β 1 production and protein kinase C activity in glomerular endothelial cells. Kidney Int. 2001;59(2):673–81. doi: 10.1046/j.1523-1755.2001.059002673.x. [DOI] [PubMed] [Google Scholar]
- 50. Jandeleit-Dahm K, Cooper ME. Hypertension and diabetes: role of the renin-angiotensin system. Endocrinol Metab Clin North Am. 2006;35(3):469–90, vii. doi: 10.1016/j.ecl.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 51. Lassila M, Cooper ME, Jandeleit-Dahm K. Antiproteinuric effect of RAS blockade: new mechanisms. Curr Hypertens Rep. 2004;6(5):383–92. [DOI] [PubMed] [Google Scholar]
- 52. Park SH, Choi HJ, Lee JH, Woo CH, Kim JH, Han HJ. High glucose inhibits renal proximal tubule cell proliferation and involves PKC, oxidative stress, and TGF-β 1. Kidney Int. 2001;59(5):1695–705. doi: 10.1046/j.1523-1755.2001.0590051695.x. [DOI] [PubMed] [Google Scholar]
- 53. Kandhare AD, Mukherjee A, Bodhankar SL. Antioxidant for treatment of diabetic nephropathy: A systematic review and meta-analysis. Chem Biol Interact. 2017;278:212–21. doi: 10.1016/j.cbi.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 54. Hohenstein B, Daniel C, Hausknecht B, Boehmer K, Riess R, Amann KU, Hugo CP. Correlation of enhanced thrombospondin 1 expression, TGF-β signalling and proteinuria in human type-2 diabetic nephropathy. Nephrol Dial Transplant. 2008;23(12):3880–7. doi: 10.1093/ndt/gfn399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yevdokimova N, Wahab NA, Mason RM. Thrombospondin 1 is the key activator of TGF-β1 in human mesangial cells exposed to high glucose. J Am Soc Nephrol. 2001;12(4):703–12. [DOI] [PubMed] [Google Scholar]
- 56. Poczatek MH, Hugo C, Darley-Usmar V, Murphy-Ullrich JE. Glucose stimulation of transforming growth factor-beta bioactivity in mesangial cells is mediated by thrombospondin 1. Am J Pathol. 2000;157(4):1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scott-Burden T, Resink TJ, Hahn AW, Buhler FR. Induction of thrombospondin expression in vascular smooth muscle cells by angiotensin II. J Cardiovasc Pharmacol. 1990;16(Suppl7):S17–20. [PubMed] [Google Scholar]
- 58. Naito T, Masaki T, Nikolic-Paterson DJ, Tanji C, Yorioka N, Kohno N. Angiotensin II induces thrombospondin 1 production in human mesangial cells via p38 MAPK and JNK: a mechanism for activation of latent TGF-β1. Am J Physiol Renal Physiol. 2004;286(2):F27-88. doi: 10.1152/ajprenal.00139.2003. [DOI] [PubMed] [Google Scholar]
- 59. Zhou Y, Poczatek MH, Berecek KH, Murphy-Ullrich JE. Thrombospondin 1 mediates angiotensin II induction of TGF-beta activation by cardiac and renal cells under both high and low glucose conditions. Biochem Biophys Res Commun. 2006;339(2):633–41. doi: 10.1016/j.bbrc.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 60. Yang YL, Chuang LY, Guh JY, Liu SF, Hung MY, Liao TN, Huang YL. Thrombospondin 1 mediates distal tubule hypertrophy induced by glycated albumin. Biochem J. 2004;379(Pt1):89–97. doi: 10.1042/BJ20031730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li Y, Wang S. Glycated albumin upregulates upstream stimulatory factor 2 gene transcription in mesangial cells. Am J Physiol Renal Physiol. 2010;299(1):F121–7. doi: 10.1152/ajprenal.00074.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang S, Wu X, Lincoln TM, Murphy-Ullrich JE. Expression of constitutively active cGMP-dependent protein kinase prevents glucose stimulation of thrombospondin 1 expression and TGF-beta activity. Diabetes. 2003;52(8):2144–50. [DOI] [PubMed] [Google Scholar]
- 63. Wang S, Shiva S, Poczatek MH, Darley-Usmar V, Murphy-Ullrich JE. Nitric oxide and cGMP-dependent protein kinase regulation of glucose-mediated thrombospondin 1-dependent transforming growth factor-beta activation in mesangial cells. J Biol Chem. 2002;277(12):9880–8. doi: 10.1074/jbc.M108360200. [DOI] [PubMed] [Google Scholar]
- 64. Wang S, Skorczewski J, Feng X, Mei L, Murphy-Ullrich JE. Glucose up-regulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J Biol Chem. 2004;279(33):34311–22. doi: 10.1074/jbc.M401629200. [DOI] [PubMed] [Google Scholar]
- 65. Liu S, Shi L, Wang S. Overexpression of upstream stimulatory factor 2 accelerates diabetic kidney injury. Am J Physiol Renal Physiol. 2007;293(5):F1727–35. doi: 10.1152/ajprenal.00316.2007. [DOI] [PubMed] [Google Scholar]
- 66. Raman P, Krukovets I, Marinic TE, Bornstein P, Stenina OI. Glycosylation mediates up-regulation of a potent antiangiogenic and proatherogenic protein, thrombospondin 1, by glucose in vascular smooth muscle cells. J Biol Chem. 2007;282(8):5704–14. doi: 10.1074/jbc.M610965200. [DOI] [PubMed] [Google Scholar]
- 67. Daniel C, Schaub K, Amann K, Lawler J, Hugo C. Thrombospondin 1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes. 2007;56(12):2982–9. doi: 10.2337/db07-0551. [DOI] [PubMed] [Google Scholar]
- 68. Gurley SB, Mach CL, Stegbauer J, Yang J, Snow KP, Hu A, Meyer TW, Coffman TM. Influence of genetic background on albuminuria and kidney injury in Ins2(+/C96Y) (Akita) mice. Am J Physiol Renal Physiol. 2010;298(3):F78-89. doi: 10.1152/ajprenal.90515.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Memetimin H, Li D, Tan K, Zhou C, Liang Y, Wu Y, Wang S. Myeloid specific deletion of thrombospondin 1 protects against inflammation and insulin resistance in long-term diet-induced obese male mice. Am J Physiol Endocrinol Metab. 2018; 315(6). doi: 10.1152/ajpendo.00273.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ruggenenti P, Cravedi P, Remuzzi G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol. 2010;6:319. doi: 10.1038/nrneph.2010.58. [DOI] [PubMed] [Google Scholar]
- 71. Breyer MD, Susztak K. Developing treatments for chronic kidney disease in the 21st century. Semin Nephrol. 2016;36(6):436–47. doi: 10.1016/j.semnephrol.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Johnson SA, Spurney RF. Twenty years after ACEIs and ARBs: emerging treatment strategies for diabetic nephropathy. Am J Physiol Renal Physiol. 2015;309(10):F80-72. doi: 10.1152/ajprenal.00266.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sugaru E, Nakagawa T, Ono-Kishino M, Nagamine J, Tokunaga T, Kitoh M, Hume WE, Nagata R, Taiji M. Enhanced effect of combined treatment with SMP-534 (antifibrotic agent) and losartan in diabetic nephropathy. Am J Nephrol. 2006;26(1):50–8. doi: 10.1159/000091786. [DOI] [PubMed] [Google Scholar]
- 74. Sugaru E, Nakagawa T, Ono-Kishino M, Nagamine J, Tokunaga T, Kitoh M, Hume WE, Nagata R, Taiji M. Amelioration of established diabetic nephropathy by combined treatment with SMP-534 (antifibrotic agent) and losartan in db/db mice. Nephron Exp Nephrol. 2007;105(2):e45–52. doi: 10.1159/000097603. [DOI] [PubMed] [Google Scholar]
- 75. Benigni A, Zoja C, Corna D, Zatelli C, Conti S, Campana M, Gagliardini E, Rottoli D, Zanchi C, Abbate M, Ledbetter S, Remuzzi G. Add-on anti-TGF-beta antibody to ACE inhibitor arrests progressive diabetic nephropathy in the rat. J Am Soc Nephrol. 2003;14(7):1816–24. [DOI] [PubMed] [Google Scholar]
- 76. Alicic RZ, Johnson EJ, Tuttle KR. SGLT2 inhibition for the prevention and treatment of diabetic kidney disease: a review. Am J Kidney Dis. 2018;72(2):267–77. doi: 10.1053/j.ajkd.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 77. Smyth B, Perkovic V. New hypoglycemic agents and the kidney: what do the major trials tell us? F1000res. 2018;7. doi: 10.12688/f1000research.16135.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gembardt F, Bartaun C, Jarzebska N, Mayoux E, Todorov VT, Hohenstein B, Hugo C. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol. 2014;307(3):F31-72. doi: 10.1152/ajprenal.00145.2014. [DOI] [PubMed] [Google Scholar]
- 79. Ishibashi Y, Matsui T, Yamagishi SI. Tofogliflozin, a selective inhibitor of sodium-glucose cotransporter 2, suppresses renal damage in KKAy/Ta mice, obese and type 2 diabetic animals. Diab Vasc Dis Res. 2016;13(6):438–41. doi: 10.1177/1479164116657304. [DOI] [PubMed] [Google Scholar]
- 80. Pirklbauer M, Schupart R, Fuchs LC, Staudinger P, Corazza U, Sallaberger S, Leierer J, Mayer G, Schramek H. Unravelling reno-protective effects of SGLT2 inhibition in human proximal tubular cells. Am J Physiol Renal Physiol. 2019;316(3):F44-96. doi: 10.1152/ajprenal.00431.2018. [DOI] [PubMed] [Google Scholar]
- 81. Cui W, Maimaitiyiming H, Zhou Q, Norman H, Zhou C, Wang S. Interaction of thrombospondin1 and CD36 contributes to obesity-associated podocytopathy. Biochim Biophys Acta. 2015;1852(7):1323–33. doi: 10.1016/j.bbadis.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li Y, Qi X, Tong X, Wang S. Thrombospondin 1 activates the macrophage Toll-like receptor 4 pathway. Cell Mol Immunol. 2013;10(6):506–12. doi: 10.1038/cmi.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Isenberg JS, Roberts DD. The role of CD47 in pathogenesis and treatment of renal ischemia reperfusion injury. Pediatr Nephrol. Epub 2018 Nov 3. doi: 10.1007/s00467-018-4123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rogers NM, Zhang ZJ, Wang JJ, Thomson AW, Isenberg JS. CD47 regulates renal tubular epithelial cell self-renewal and proliferation following renal ischemia reperfusion. Kidney Int. 2016;90(2):334–47. doi: 10.1016/j.kint.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 85. Margosio B, Rusnati M, Bonezzi K, Cordes BL, Annis DS, Urbinati C, Giavazzi R, Presta M, Ribatti D, Mosher DF, Taraboletti G. Fibroblast growth factor-2 binding to the thrombospondin 1 type III repeats, a novel antiangiogenic domain. Int J Biochem Cell Biol. 2008;40(4):700–9. doi: 10.1016/j.biocel.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Belotti D, Capelli C, Resovi A, Introna M, Taraboletti G. Thrombospondin 1 promotes mesenchymal stromal cell functions via TGFbeta and in cooperation with PDGF. Matrix Biol. 2016;55:106–16. doi: 10.1016/j.matbio.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 87. Afsar B, Afsar RE, Dagel T, Kaya E, Erus S, Ortiz A, Covic A, Kanbay M. Capillary rarefaction from the kidney point of view. Clin Kidney J. 2018;11(3):295–301. doi: 10.1093/ckj/sfx133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sun D, Ma Y, Han H, Yin Z, Liu C, Feng J, Zhou X, Li X, Xiao A, Yu R. Thrombospondin 1 short hairpin RNA suppresses tubulointerstitial fibrosis in the kidney of ureteral obstruction by ameliorating peritubular capillary injury. Kidney Blood Press Res. 2012;35(1):35–47. doi: 10.1159/000330718. [DOI] [PubMed] [Google Scholar]
- 89. Hugo CP, Pichler RP, Schulze-Lohoff E, Prols F, Adler S, Krutsch HC, Murphy-Ullrich JE, Couser WG, Roberts DD, Johnson RJ. Thrombospondin peptides are potent inhibitors of mesangial and glomerular endothelial cell proliferation in vitro and in vivo. Kidney Int. 1999;55(6):2236–49. doi: 10.1046/j.1523-1755.1999.00459.x. [DOI] [PubMed] [Google Scholar]
- 90. Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, Gordon KL, Oyama TT, Hughes J, Hugo C, Kerjaschki D, Schreiner GF, Johnson RJ. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin 1 in renal disease. Am J Kidney Dis. 2001;37(3):601–11. [DOI] [PubMed] [Google Scholar]
- 91. Gewin L, Zent R, Pozzi A. Progression of chronic kidney disease: too much cellular talk causes damage. Kidney Int. 2017;91(3):552–60. doi: 10.1016/j.kint.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Daniel C, Amann K, Hohenstein B, Bornstein P, Hugo C. Thrombospondin 2 functions as an endogenous regulator of angiogenesis and inflammation in experimental glomerulonephritis in mice. J Am Soc Nephrol. 2007;18(3):788–98. doi: 10.1681/ASN.2006080873. [DOI] [PubMed] [Google Scholar]
- 93. Daniel C, Wagner A, Hohenstein B, Hugo C. Thrombospondin-2 therapy ameliorates experimental glomerulonephritis via inhibition of cell proliferation, inflammation, and TGF-beta activation. Am J Physiol Renal Physiol. 2009;297(5):F1299–309. doi: 10.1152/ajprenal.00254.2009. [DOI] [PubMed] [Google Scholar]
- 94. Daniel C, Vogelbacher R, Stief A, Grigo C, Hugo C. Long-term gene therapy with thrombospondin 2 inhibits TGF-beta activation, inflammation and angiogenesis in chronic allograft nephropathy. PLoS ONE. 2013;8(12):e83846. doi: 10.1371/journal.pone.0083846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107(7):785–90. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Murphy-Ullrich JE, Höök M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989;109(3):1309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Murphy-Ullrich JE, Gurusiddappa S, Frazier WA, Höök M. Heparin-binding peptides from thrombospondins 1 and 2 contain focal adhesion-labilizing activity. J Biol Chem. 1993;268(35):26784–9. [PubMed] [Google Scholar]
- 98. Greenwood JA, Pallero MA, Theibert AB, Murphy-Ullrich JE. Thrombospondin signaling of focal adhesion disassembly requires activation of phosphoinositide 3-kinase. J Biol Chem. 1998;273(3):1755–63. [DOI] [PubMed] [Google Scholar]
- 99. Goicoechea S, Pallero MA, Eggleton P, Michalak M, Murphy-Ullrich JE. The anti-adhesive activity of thrombospondin is mediated by the N-terminal domain of cell surface calreticulin. J Biol Chem. 2002;277(40):37219–28. doi: 10.1074/jbc.M202200200. [DOI] [PubMed] [Google Scholar]
- 100. Orr AW, Pallero MA, Murphy-Ullrich JE. Thrombospondin stimulates focal adhesion disassembly through Gi- and phosphoinositide 3-kinase-dependent ERK activation. J Biol Chem. 2002;277(23):20453–60. doi: 10.1074/jbc.M112091200. [DOI] [PubMed] [Google Scholar]
- 101. Orr AW, Elzie CA, Kucik DF, Murphy-Ullrich JE. Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J Cell Sci. 2003;116(Pt14):2917–27. doi: 10.1242/jcs.00600. [DOI] [PubMed] [Google Scholar]
- 102. Orr AW, Pedraza CE, Pallero MA, Elzie CA, Goicoechea S, Strickland DK, Murphy-Ullrich JE. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J Cell Biol. 2003;161(6):1179–89. doi: 10.1083/jcb.200302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Orr AW, Pallero MA, Xiong WC, Murphy-Ullrich JE. Thrombospondin induces RhoA inactivation through FAK-dependent signaling to stimulate focal adhesion disassembly. J Biol Chem. 2004;279(47):48983–92. doi: 10.1074/jbc.M404881200. [DOI] [PubMed] [Google Scholar]
- 104. Pallero MA, Elzie CA, Chen J, Mosher DF, Murphy-Ullrich JE. Thrombospondin 1 binding to calreticulin-LRP1 signals resistance to anoikis. FASEB J. 2008;22(11):3968–79. doi: 10.1096/fj.07-104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pakshir P, Hinz B. The big five in fibrosis: Macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018;68–69:81–93. doi: 10.1016/j.matbio.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 107. Sheets AR, Demidova-Rice TN, Shi L, Ronfard V, Grover KV, Herman IM. Identification and characterization of novel matrix-derived bioactive peptides: a role for collagenase from Santyl® ointment in post-debridement wound healing? PLoS ONE. 2016;11(7):e0159598. doi: 10.1371/journal.pone.0159598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lawler JW, Slayter HS. The release of heparin binding peptides from platelet thrombospondin by proteolytic action of thrombin, plasmin and trypsin. Thromb Res. 1981;22(3):267–79.0 [DOI] [PubMed] [Google Scholar]
- 109. Rabhi-Sabile S, Pidard D, Lawler J, Renesto P, Chignard M, Legrand C. Proteolysis of thrombospondin during cathepsin-G-induced platelet aggregation: functional role of the 165-kDa carboxy-terminal fragment. FEBS Lett. 1996;386(1):82–6. [DOI] [PubMed] [Google Scholar]
- 110. Lee NV, Sato M, Annis DS, Loo JA, Wu L, Mosher DF, Iruela-Arispe ML. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J. 2006;25(22):5270–83. doi: 10.1038/sj.emboj.7601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Elzie CA, Murphy-Ullrich JE. The N-terminus of thrombospondin: the domain stands apart. Int J Biochem Cell Biol. 2004;36(6):1090–101. doi: 10.1016/j.biocel.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 112. Kypreou KP, Kavvadas P, Karamessinis P, Peroulis M, Alberti A, Sideras P, Psarras S, Capetanaki Y, Politis PK, Charonis AS. Altered expression of calreticulin during the development of fibrosis. Proteomics. 2008;8(12):2407–19. doi: 10.1002/pmic.200700831. [DOI] [PubMed] [Google Scholar]
- 113. Prakoura N, Politis PK, Ihara Y, Michalak M, Charonis AS. Epithelial calreticulin up-regulation promotes profibrotic responses and tubulointerstitial fibrosis development. Am J Pathol. 2013;183(5):1474–87. doi: 10.1016/j.ajpath.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 114. Van Duyn Graham L, Sweetwyne MT, Pallero MA, Murphy-Ullrich JE. Intracellular calreticulin regulates multiple steps in fibrillar collagen expression, trafficking, and processing into the extracellular matrix. J Biol Chem. 2010;285(10):7067–78. doi: 10.1074/jbc.M109.006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zimmerman KA, Graham LV, Pallero MA, Murphy-Ullrich JE. Calreticulin regulates transforming growth factor-beta-stimulated extracellular matrix production. J Biol Chem. 2013;288(20):14584–98. doi: 10.1074/jbc.M112.447243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Greives MR, Samra F, Pavlides SC, Blechman KM, Naylor SM, Woodrell CD, Cadacio C, Levine JP, Bancroft TA, Michalak M, Warren SM, Gold LI. Exogenous calreticulin improves diabetic wound healing. Wound Repair Regen. 2012;20(5):715–30. doi: 10.1111/j.1524-475X.2012.00822.x. [DOI] [PubMed] [Google Scholar]
- 117. Nanney LB, Woodrell CD, Greives MR, Cardwell NL, Pollins AC, Bancroft TA, Chesser A, Michalak M, Rahman M, Siebert JW, Gold LI. Calreticulin enhances porcine wound repair by diverse biological effects. Am J Pathol. 2008;173(3):610–30. doi: 10.2353/ajpath.2008.071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hao J, Zhao X, Yu W, Huang X, Huang Y. Surfactant protein A induces the pathogenesis of renal fibrosis through binding to calreticulin. Exp Ther Med. 2019;17(1):459–64. doi: 10.3892/etm.2018.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hu K, Wu C, Mars WM, Liu Y. Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J Clin Invest. 2007;117(12):3821–32. doi: 10.1172/JCI32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kang LI, Isse K, Koral K, Bowen WC, Muratoglu S, Strickland DK, Michalopoulos GK, Mars WM. Tissue-type plasminogen activator suppresses activated stellate cells through low-density lipoprotein receptor-related protein 1. Lab Invest. 2015;95(10):1117–29. doi: 10.1038/labinvest.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bellaye PS, Shimbori C, Yanagihara T, Carlson DA, Hughes P, Upagupta C, Sato S, Wheildon N, Haystead T, Ask K, Kolb M. Synergistic role of HSP90alpha and HSP90beta to promote myofibroblast persistence in lung fibrosis. Eur Respir J. 2018;51(2). doi: 10.1183/13993003.00386-2017. [DOI] [PubMed] [Google Scholar]
- 122. Wujak L, Schnieder J, Schaefer L, Wygrecka M. LRP1: a chameleon receptor of lung inflammation and repair. Matrix Biol. 2018;68–9:366–81. doi: 10.1016/j.matbio.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 123. Gerritsen KG, Bovenschen N, Nguyen TQ, Sprengers D, Koeners MP, van Koppen AN, Joles JA, Goldschmeding R, Kok RJ. Rapid hepatic clearance of full length CCN-2/CTGF: a putative role for LRP1-mediated endocytosis. J Cell Commun Signal. 2016;10(4):295–303. doi: 10.1007/s12079-016-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Calabro NE, Barrett A, Chamorro-Jorganes A, Tam S, Kristofik NJ, Xing H, Loye AM, Sessa WC, Hansen K, Kyriakides TR. Thrombospondin-2 regulates extracellular matrix production, LOX levels, and cross-linking via downregulation of miR-29. Matrix Biol. Epub 2019 Mar 13. doi: 10.1016/j.matbio.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140(2):419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Halasz K, Kassner A, Morgelin M, Heinegard D. COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem. 2007;282(43):31166–73. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- 127. Posey KL, Hankenson K, Veerisetty AC, Bornstein P, Lawler J, Hecht JT. Skeletal abnormalities in mice lacking extracellular matrix proteins, thrombospondin 1, thrombospondin-3, thrombospondin-5, and type IX collagen. Am J Pathol. 2008;172(6):1664–74. doi: 10.2353/ajpath.2008.071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Galvin NJ, Vance PM, Dixit VM, Fink B, Frazier WA. Interaction of human thrombospondin with types I-V collagen: direct binding and electron microscopy. J Cell Biol. 1987;104(5):1413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gebauer JM, Kohler A, Dietmar H, Gompert M, Neundorf I, Zaucke F, Koch M, Baumann U. COMP and TSP-4 interact specifically with the novel GXKGHR motif only found in fibrillar collagens. Sci Rep. 2018;8(1):17187. doi: 10.1038/s41598-018-35447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shearer D, Mervis MO, Manley E, Jr, Reddy AB, Alford AI. TSP1 and TSP2 deficiencies affect LOX protein distribution in the femoral diaphysis and pro-peptide removal in marrow-derived mesenchymal stem cells in vitro. Connect Tissue Res. Epub 2019 Apr 2. doi: 10.1080/03008207.2019.1593391. [DOI] [PubMed] [Google Scholar]
- 131. Hellewell AL, Adams JC. Insider trading: extracellular matrix proteins and their non-canonical intracellular roles. Bioessays. 2016;38(1):77–88. doi: 10.1002/bies.201500103. [DOI] [PubMed] [Google Scholar]
- 132. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 133. Inagi R, Ishimoto Y, Nangaku M. Proteostasis in endoplasmic reticulum —new mechanisms in kidney disease. Nat Rev Nephrol. 2014;10(7):369–78. doi: 10.1038/nrneph.2014.67. [DOI] [PubMed] [Google Scholar]
- 134. Lindenmeyer MT, Rastaldi MP, Ikehata M, Neusser MA, Kretzler M, Cohen CD, Schlondorff D. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol. 2008;19(11):2225–36. doi: 10.1681/ASN.2007121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Tanjore H, Lawson WE, Blackwell TS. Endoplasmic reticulum stress as a pro-fibrotic stimulus. Biochim Biophys Acta. 2013;1832(7):940–7. doi: 10.1016/j.bbadis.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cybulsky AV. Endoplasmic reticulum stress in proteinuric kidney disease. Kidney Int. 2010;77(3):187–93. doi: 10.1038/ki.2009.389. [DOI] [PubMed] [Google Scholar]
- 137. Baek HA, Kim DS, Park HS, Jang KY, Kang MJ, Lee DG, Moon WS, Chae HJ, Chung MJ. Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am J Respir Cell Mol Biol. 2012;46(6):731–9. doi: 10.1165/rcmb.2011-0121OC. [DOI] [PubMed] [Google Scholar]
- 138. Chiang CK, Hsu SP, Wu CT, Huang JW, Cheng HT, Chang YW, Hung KY, Wu KD, Liu SH. Endoplasmic reticulum stress implicated in the development of renal fibrosis. Mol Med. 2011;17(11–2):1295–305. doi: 10.2119/molmed.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ayala P, Montenegro J, Vivar R, Letelier A, Urroz PA, Copaja M, Pivet D, Humeres C, Troncoso R, Vicencio JM, Lavandero S, Diaz-Araya G. Attenuation of endoplasmic reticulum stress using the chemical chaperone 4-phenylbutyric acid prevents cardiac fibrosis induced by isoproterenol. Exp Mol Pathol. 2012;92(1):97–104. doi: 10.1016/j.yexmp.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 140. Groenendyk J, Lee D, Jung J, Dyck JR, Lopaschuk GD, Agellon LB, Michalak M. Inhibition of the Unfolded Protein Response Mechanism Prevents Cardiac Fibrosis. Plos One. 2016;11(7):e0159682. doi: 10.1371/journal.pone.0159682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Burman A, Tanjore H, Blackwell TS. Endoplasmic reticulum stress in pulmonary fibrosis. Matrix Biol. 2018;68–9:355–65. doi: 10.1016/j.matbio.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Liu H, Bowes RC, 3rd, van de Water B, Sillence C, Nagelkerke JF, Stevens JL. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J Biol Chem. 1997;272(35):21751–9. [DOI] [PubMed] [Google Scholar]
- 143. Shu S, Zhu J, Liu Z, Tang C, Cai J, Dong Z. Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. Ebiomedicine. 2018;37:269–80. doi: 10.1016/j.ebiom.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Noh MR, Woo CH, Park MJ, In Kim J, Park KM. Ablation of C/EBP homologous protein attenuates renal fibrosis after ureteral obstruction by reducing autophagy and microtubule disruption. Biochim Biophys Acta Mol Basis Dis. 2018;1864(5PtA):1634–41. doi: 10.1016/j.bbadis.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 145. Lei J, Zhao L, Zhang Y, Wu Y, Liu Y. High glucose-induced podocyte injury involves activation of mammalian target of rapamycin (mTOR)-induced endoplasmic reticulum (ER) stress. Cell Physiol Biochem. 2018;45(6):2431–43. doi: 10.1159/000488231. [DOI] [PubMed] [Google Scholar]
- 146. Gallazzini M, Pallet N. Endoplasmic reticulum stress and kidney dysfunction. Biol Cell. 2018;110(9):205–16. doi: 10.1111/boc.201800019. [DOI] [PubMed] [Google Scholar]
- 147. Mohammed-Ali Z, Lu C, Marway MK, Carlisle RE, Ask K, Lukic D, Krepinsky JC, Dickhout JG. Endoplasmic reticulum stress inhibition attenuates hypertensive chronic kidney disease through reduction in proteinuria. Sci Rep. 2017;7:41572. doi: 10.1038/srep41572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Maekawa H, Inagi R. Stress signal network between hypoxia and ER stress in chronic kidney disease. Front Physiol. 2017;8:74. doi: 10.3389/fphys.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Liu SH, Yang CC, Chan DC, Wu CT, Chen LP, Huang JW, Hung KY, Chiang CK. Chemical chaperon 4-phenylbutyrate protects against the endoplasmic reticulum stress-mediated renal fibrosis in vivo and in vitro. Oncotarget. 2016;7(16):22116–27. doi: 10.18632/oncotarget.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Coustry F, Posey KL, Maerz T, Baker K, Abraham AM, Ambrose CG, Nobakhti S, Shefelbine SJ, Bi X, Newton M, Gawronski K, Remer L, Veerisetty AC, Hossain MG, Chiu F, Hecht JT. Mutant cartilage oligomeric matrix protein (COMP) compromises bone integrity, joint function and the balance between adipogenesis and osteogenesis. Matrix Biol. 2018;67:75–89. doi: 10.1016/j.matbio.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hecht JT, Hayes E, Haynes R, Cole WG. COMP mutations, chondrocyte function and cartilage matrix. Matrix Biol. 2005;23(8):525–33. doi: 10.1016/j.matbio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 152. Hecht JT, Hayes E, Snuggs M, Decker G, Montufar-Solis D, Doege K, Mwalle F, Poole R, Stevens J, Duke PJ. Calreticulin, PDI, Grp94 and BiP chaperone proteins are associated with retained COMP in pseudoachondroplasia chondrocytes. Matrix Biol. 2001;20(4):251–62. [DOI] [PubMed] [Google Scholar]
- 153. Cunha DA, Cito M, Carlsson PO, Vanderwinden JM, Molkentin JD, Bugliani M, Marchetti P, Eizirik DL, Cnop M. Thrombospondin 1 protects pancreatic beta-cells from lipotoxicity via the PERK-NRF2 pathway. Cell Death Differ. 2016;23(12):1995–2006. doi: 10.1038/cdd.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Cunha DA, Cito M, Grieco FA, Cosentino C, Danilova T, Ladriere L, Lindahl M, Domanskyi A, Bugliani M, Marchetti P, Eizirik DL, Cnop M. Pancreatic beta-cell protection from inflammatory stress by the endoplasmic reticulum proteins thrombospondin 1 and mesencephalic astrocyte-derived neutrotrophic factor (MANF). J Biol Chem. 2017;292(36):14977–88. doi: 10.1074/jbc.M116.769877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Veliceasa D, Ivanovic M, Hoepfner FT, Thumbikat P, Volpert OV, Smith ND. Transient potential receptor channel 4 controls thrombospondin 1 secretion and angiogenesis in renal cell carcinoma. FEBS J. 2007;274(24):6365–77. doi: 10.1111/j.1742-4658.2007.06159.x. [DOI] [PubMed] [Google Scholar]
- 156. Vasudevan D, Takeuchi H, Johar SS, Majerus E, Haltiwanger RS. Peters plus syndrome mutations disrupt a noncanonical ER quality-control mechanism. Curr Biol. 2015;25(3):286–95. doi: 10.1016/j.cub.2014.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Putney JW. The physiological function of store-operated calcium entry. Neurochem Res. 2011;36(7):1157–65. doi: 10.1007/s11064-010-0383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ambily A, Kaiser WJ, Pierro C, Chamberlain EV, Li Z, Jones CI, Kassouf N, Gibbins JM, Authi KS. The role of plasma membrane STIM1 and Ca(2+)entry in platelet aggregation. STIM1 binds to novel proteins in human platelets. Cell Signal. 2014;26(3):502–11. doi: 10.1016/j.cellsig.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhang B, Jiang J, Yue Z, Liu S, Ma Y, Yu N, Gao Y, Sun S, Chen S, Liu P. Store-operated Ca(2+) entry (SOCE) contributes to angiotensin II-induced cardiac fibrosis in cardiac fibroblasts. J Pharmacol Sci. 2016;132(3):171–80. doi: 10.1016/j.jphs.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 160. Gooch JL, Gorin Y, Zhang BX, Abboud HE. Involvement of calcineurin in transforming growth factor-beta-mediated regulation of extracellular matrix accumulation. J Biol Chem. 2004;279(15):15561–70. doi: 10.1074/jbc.M308759200. [DOI] [PubMed] [Google Scholar]
- 161. Cobbs SL, Gooch JL. NFATc is required for TGF-beta-mediated transcriptional regulation of fibronectin. Biochem Biophys Res Commun. 2007;362(2):288–94. doi: 10.1016/j.bbrc.2007.07.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Gooch JL, Barnes JL, Garcia S, Abboud HE. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol. 2003;284(1):F14-45. doi: 10.1152/ajprenal.00158.2002. [DOI] [PubMed] [Google Scholar]
- 163. Zhang L, Li R, Shi W, Liang X, Liu S, Ye Z, Yu C, Chen Y, Zhang B, Wang W, Lai Y, Ma J, Li Z, Tan X. NFAT2 inhibitor ameliorates diabetic nephropathy and podocyte injury in db/db mice. Br J Pharmacol. 2013;170(2):426–39. doi: 10.1111/bph.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zimmerman KA, Xing D, Pallero MA, Lu A, Ikawa M, Black L, Hoyt KL, Kabarowski JH, Michalak M, Murphy-Ullrich JE. Calreticulin regulates neointima formation and collagen deposition following carotid artery ligation. J Vasc Res. 2015;52(5):306–20. doi: 10.1159/000443884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Owusu BY, Zimmerman KA, Murphy-Ullrich JE. The role of the endoplasmic reticulum protein calreticulin in mediating TGF-beta-stimulated extracellular matrix production in fibrotic disease. J Cell Commun Signal. 2018;12(1):289–99. doi: 10.1007/s12079-017-0426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Schadler KL, Crosby EJ, Zhou AY, Bhang DH, Braunstein L, Baek KH, Crawford D, Crawford A, Angelosanto J, Wherry EJ, Ryeom S. Immunosurveillance by antiangiogenesis: tumor growth arrest by T cell-derived thrombospondin 1. Cancer Res. 2014;74(8):2171–81. doi: 10.1158/0008-5472.CAN-13-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin 1 axis. Cell. 2014;156(3):440–55. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Mirochnik Y, Kwiatek A, Volpert OV. Thrombospondin and apoptosis: molecular mechanisms and use for design of complementation treatments. Curr Drug Targets. 2008;9(10):851–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Xu M, Wang X, Banan B, Chirumbole DL, Garcia-Aroz S, Balakrishnan A, Nayak DK, Zhang Z, Jia J, Upadhya GA, Gaut JP, Hiebsch R, Manning PT, Wu N, Lin Y, Chapman WC. Anti-CD47 monoclonal antibody therapy reduces ischemia-reperfusion injury of renal allografts in a porcine model of donation after cardiac death. Am J Transplant. 2018;18(4):855–67. doi: 10.1111/ajt.14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Voelker J, Berg PH, Sheetz M, Duffin K, Shen T, Moser B, Greene T, Blumenthal SS, Rychlik I, Yagil Y, Zaoui P, Lewis JB. Anti-TGF-β1 antibody therapy in patients with diabetic nephropathy. J Am Soc Nephrol. 2017;28(3):953–62. doi: 10.1681/ASN.2015111230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Francois H, Chatziantoniou C. Renal fibrosis: recent translational aspects. Matrix Biol. 2018;68–9:318–32. doi: 10.1016/j.matbio.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 172. Allinovi M, De Chiara L, Angelotti ML, Becherucci F, Romagnani P. Anti-fibrotic treatments: a review of clinical evidence. Matrix Biol. 2018;68–9:333–54. doi: 10.1016/j.matbio.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 173. Gyorfi AH, Matei AE, Distler JHW. Targeting TGF-beta signaling for the treatment of fibrosis. Matrix Biol. 2018;68–9:8–27. doi: 10.1016/j.matbio.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 174. Herbertz S, Sawyer JS, Stauber AJ, Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, Desaiah D, Guba SC, Benhadji KA, Slapak CA, Lahn MM. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther. 2015;9:4479–99. doi: 10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Lachapelle P, Li M, Douglass J, Stewart A. Safer approaches to therapeutic modulation of TGF-beta signaling for respiratory disease. Pharmacol Ther. 2018;187:98–113. doi: 10.1016/j.pharmthera.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 176. Wu W, Hutcheon AEK, Sriram S, Tran JA, Zieske JD. Initiation of fibrosis in the integrin αvβ6 knockout mice. Exp Eye Res. 2018;180:23–8. doi: 10.1016/j.exer.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Maile LA, Gollahon K, Wai C, Dunbar P, Busby W, Clemmons D. Blocking αvβ3 integrin ligand occupancy inhibits the progression of albuminuria in diabetic rats. J Diabetes Res. 2014;2014:421827. doi: 10.1155/2014/421827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wernig G, Chen SY, Cui L, Van Neste C, Tsai JM, Kambham N, Vogel H, Natkunam Y, Gilliland DG, Nolan G, Weissman IL. Unifying mechanism for different fibrotic diseases. Proc Natl Acad Sci USA. 2017;114(18):4757–62. doi: 10.1073/pnas.1621375114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Huang Y, Ma Y, Gao P, Yao Z. Targeting CD47: the achievements and concerns of current studies on cancer immunotherapy. J Thorac Dis. 2017;9(2):E16-87. doi: 10.21037/jtd.2017.02.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Pinessi D, Foglieni C, Bugatti A, Moroni E, Resovi A, Ribatti D, Rusnati M, Giavazzi R, Colombo G, Taraboletti G. PO-15—Antiangiogenic small molecule ligands of FGF2 derived from the endogenous inhibitor thrombospondin 1. Thromb Res. 2016;140(Suppl1):S182. doi: 10.1016/S0049-3848(16)30148-7. [DOI] [PubMed] [Google Scholar]
- 181. Foglieni C, Pagano K, Lessi M, Bugatti A, Moroni E, Pinessi D, Resovi A, Ribatti D, Bertini S, Ragona L, Bellina F, Rusnati M, Colombo G, Taraboletti G. Integrating computational and chemical biology tools in the discovery of antiangiogenic small molecule ligands of FGF2 derived from endogenous inhibitors. Sci Rep. 2016;6:23432. doi: 10.1038/srep23432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Rusnati M, Borsotti P, Moroni E, Foglieni C, Chiodelli P, Carminati L, Pinessi D, Annis DS, Paiardi G, Bugatti A, Gori A, Longhi R, Belotti D, Mosher DF, Colombo G, Taraboletti G. The calcium-binding type III repeats domain of thrombospondin-2 binds to fibroblast growth factor 2 (FGF2). Angiogenesis. 2019;22:133–44. doi: 10.1007/s10456-018-9644-3. [DOI] [PubMed] [Google Scholar]