Abstract
Kidney fibrosis is the common histological end-point of progressive, chronic kidney diseases (CKDs) regardless of the underlying etiology. The hallmark of renal fibrosis, similar to all other organs, is pathological deposition of extracellular matrix (ECM). Renal ECM is a complex network of collagens, elastin, and several glycoproteins and proteoglycans forming basal membranes and interstitial space. Several ECM functions beyond providing a scaffold and organ stability are being increasingly recognized, for example, in inflammation. ECM composition is determined by the function of each of the histological compartments of the kidney, that is, glomeruli, tubulo-interstitium, and vessels. Renal ECM is a dynamic structure undergoing remodeling, particularly during fibrosis. From a clinical perspective, ECM proteins are directly involved in several rare renal diseases and indirectly in CKD progression during renal fibrosis. ECM proteins could serve as specific non-invasive biomarkers of fibrosis and scaffolds in regenerative medicine. The gold standard and currently only specific means to measure renal fibrosis is renal biopsy, but new diagnostic approaches are appearing. Here, we discuss the localization, function, and remodeling of major renal ECM components in healthy and diseased, fibrotic kidneys and the potential use of ECM in diagnostics of renal fibrosis and in tissue engineering.
Keywords: biomarker, bioprinting, chronic kidney disease, decellularization, extracellular matrix, fibrosis, tissue engineering, transforming growth factor beta
Introduction
Prevalence of chronic kidney disease (CKD) has reached epidemic proportions, with estimates of prevalence ranging between 11% and 13% of world population.1 CKD is associated with a significantly higher mortality compared to the general population.2 The common histological correlate and end-point of CKD is renal fibrosis, which is defined as an excessive, pathological accumulation of extracellular matrix (ECM). The kidney has a complex structure, with three histologically distinct compartments: glomeruli, tubulo-interstitium, and vasculature, each possessing different types of basal-membrane and non-basal membrane (interstitial) ECMs, all of which can be affected by fibrosis (Figs. 1–3).
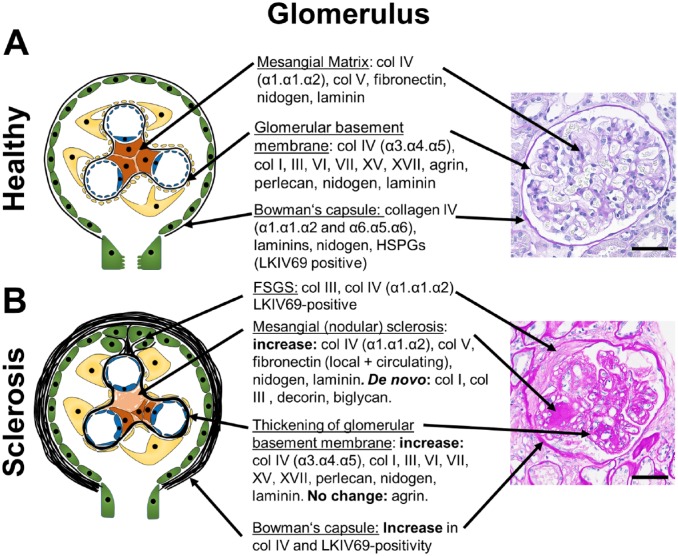
Figure 1.
Glomerular ECM components and changes during glomerulosclerosis. A schematic of a healthy glomerulus (A) and a glomerulus with nodular or focal segmental glomerulosclerosis (B) with description of the major ECM-components and their alterations in fibrosis in the mesangial ECM, the glomerular basement membrane and the Bowman’s capsule are shown. Apart from increased Collagen IV- and LKIV69-positivity, little is known on the exact composition of the Bowman’s capsule and the ECM forming FSGS lesions, albeit the ECM of FSGS is likely similar to the ECM of Bowman’s capsule, as it is mainly produced by the parietal epithelial cells. The right panel shows representative PAS stained human sections. (B): PAS-Stain was provided by Dr. Cannata-Ortiz. Scale bars indicate 50 µm. Abbreviations: BC, Bowman’s capsule; PAS, periodic acid-Schiff; FSGS, focal segmental glomerulosclerosis; ECM, extracellular matrix; HSPGs, heparan sulfate proteoglycans.
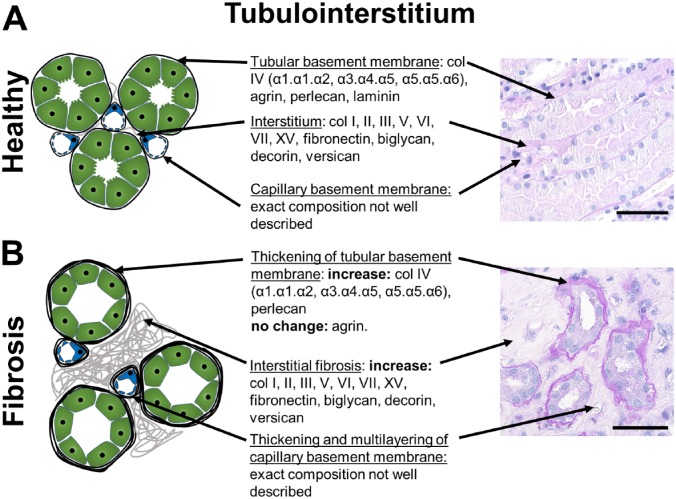
Figure 2.
Tubulo-interstitial ECM components and changes during fibrosis. A schematic of a healthy (A) and fibrotic tubulo-interstitium (B) with description of the major ECM-components and their alterations in fibrosis in the interstitial ECM, the tubular and the peritubular capillary basement membranes are shown. The exact composition of the basement membrane of peritubular capillaries has not been investigated. Alterations of the basement membrane of peritubular capillaries are best appreciated by electron microscopy. The right panel shows representative PAS stained human sections. Scale bars indicate 50µm. Abbreviation: ECM, extracellular matrix; PAS, periodic acid-Schiff.
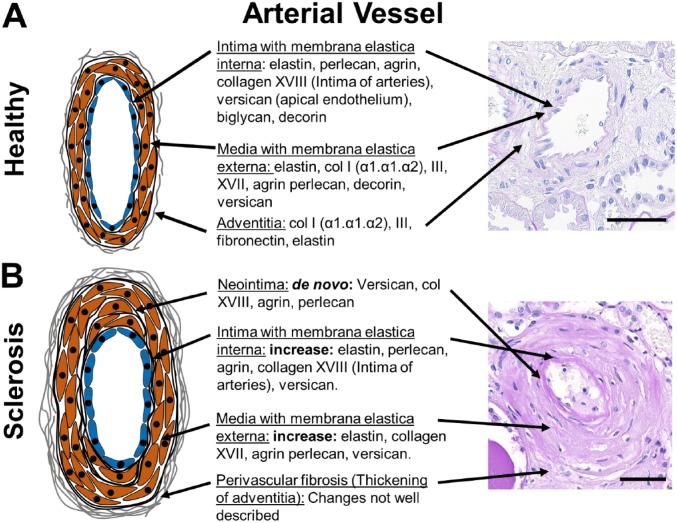
Figure 3.
Vascular ECM components and changes during arteriosclerosis. A schematic of a healthy (A) and sclerotic renal arterial vessels (B) with description of the major ECM-components and their alterations in arteriosclerosis in the intima/neointima, media and adventitia are shown. Little is known on specific changes of adventitia in renal fibrosis. The right panel shows representative PAS stained human sections. Scale bars indicate 50µm. Abbreviation: ECM, extracellular matrix; PAS, periodic acid-Schiff.
ECM in the glomerular basement membrane (GBM) mainly consists of collagen type IV (α3.α4.α5 heterotrimers), nidogen, laminins (mainly α5β2γ1), and heparan sulfate proteoglycans (HSPGs) (agrin, perlecan). Glomerular mesangial ECM mainly consists of collagen type IV (α1.α1.α2 heterotrimers), collagen type V, fibronectin, laminins, and HSPGs. The basement membrane of the Bowman’s capsule is composed of collagen IV (α1.α1.α2 and α5.α5.α6 heterotrimers), laminins, nidogen, and HSPGs3 (Fig. 1). A proteomic study of glomerular ECM, that is, of both mesangium and GBM, revealed 144 structural components.4 The most abundantly detected proteins were collagen IV with α1.α1.α2- and α3.α4.α5-heterotrimers, collagen I, laminin α5β2γ1, and HSPGs.
The ECM of the tubulo-interstitial compartment consists of basal membranes of peritubular capillaries and tubules, and of the interstitial space (Fig. 2). The latter is most commonly, and often exclusively, analyzed in studies of renal fibrosis. Interstitial ECM mainly consists of different collagens (types I, III, IV, V, VI, VII, and VIII), glycosaminoglycans (e.g., hyaluronan), polysaccharides, and glycoproteins (e.g., fibronectin, versican, biglycan, decorin). Renal medulla physiologically has a higher content of interstitial ECM compared to cortex. It is not yet clear whether there are major differences in the composition of cortical versus medullary interstitial ECM. Very little is known about the composition of the basement membrane of the peritubular capillaries.
Renal arteries consist of three compartments, the intima with subendothelial basal membrane and subendothelial interstitial ECM, media with smooth muscle cells and predominantly elastic fibers, and adventitia with interstitial type of collagen rich ECM. A comprehensive analysis of vascular ECM in the kidney is largely missing, although it is likely similar to vessels in other organs, reviewed elsewhere5 (Fig. 3).
In renal pathology diagnostics, specific terms are used to describe fibrotic changes in each of the compartments, that is, glomerulosclerosis, which can be either nodular or focal segmental, in the glomeruli, interstitial fibrosis in the tubulo-interstitium and arteriosclerosis (and perivascular fibrosis) in the vessels. No specific terms exist for thickening and fibrosis of basement membranes, although they can be observed as an isolated change, for example, in diabetic nephropathy, where thickening of the GBM is one the earliest pathological signs of the disease. In the tubules, thickened basement membranes are linked with the term tubular atrophy rather than interstitial fibrosis, albeit both processes most often go hand-in-hand. Thickening and multi-layering of peritubular capillary basement membranes is less well described, most likely due to methodological issues requiring high-resolution or electron microscopy for its assessment. Such changes were recognized in chronic antibody-mediated rejection and in a lesser extent seem to be also a common finding in renal fibrosis in animals and humans.6 Arteriosclerosis, that is, thickening of the intima is a common finding in renal fibrosis and is especially linked to hypertension.7 However, it can also be found in healthy individuals without hypertension and is linked to aging and systemic atherosclerosis.8 Little is known about perivascular (adventitial) fibrosis, although it is a relatively common morphological finding in renal fibrosis.
The main cells producing mesangial and interstitial ECM are mesangial cells in the glomeruli, and fibroblasts, myofibroblasts, and pericytes in the tubulo-interstitium. On the other hand, ECM of the basement membranes is mainly produced by the cells lining them, that is, podocytes and endothelial cells in the GBM, parietal epithelial cells in the Bowman’s capsule, tubular epithelial cells and endothelial cells in the tubular and peritubular capillary basement membranes, respectively. These respective cells are also the most important contributors to fibrosis of the respective ECM. For example, in focal segmental sclerosis (FSGS), parietal epithelial cells are the major ECM producing cells, and ECM of FSGS-lesions is similar to Bowman’s capsule.9–11 A detailed discussion of cellular and molecular mechanisms of renal fibrosis can be found elsewhere.12–14
Components of the Renal ECM
Collagens
Collagens are the most abundant components of the ECM, constituting approximately 30% of total body protein.15 There are 54 collagen-genes in humans.16 Each gene encodes a monomer collagen chain, termed alpha-chain,17 which assemble into homo- or heterotrimers, forming 28 collagen types. For example, collagen type IV has six alpha-chains forming three different trimer combinations, that is, α1.α1.α2, α3.α4.α5 and α5.α5.α6.
The GBM is formed by the collagen type IV α1.α1.α2-heterotrimer during embryonic development, followed by a shift to the α3.α4.α5-heterotrimer in adult kidneys.18 Collagen IV α3.α4.α5 heterotrimer is essential for glomerular function. This is well documented by rare diseases, such as Alport syndrome, a hereditary disorder characterized by mutations in the COL4A3, COL4A4 or COL4A5 genes19,20 and anti-GBM glomerulonephritis or Goodpasture Syndrome, caused by autoantibodies against collagen IV (Table 1).21,22 Proteomic analysis of the renal GBM identified the presence of collagens I, III, VI, XII, and XV.4 Another collagen expressed in the GBM is collagen XVII, a transmembrane protein, which is considered important for glomerular maturation.23
Table 1.
Renal Diseases With Primary Involvement of ECM Components.
| Disease | Cause/Clinical Presentation | Main Pathological Findings | References |
|---|---|---|---|
| Alport-Syndrome | • Mutation in COL4A3, COL4A4, COL4A5
• Hearing loss, proteinuria, hematuria, progressive renal functional decline • Prevalence 1:50.000 |
• Marked thickening and lamellation (“basket weave” pattern) of GBM • FSGS • In thin basement membrane nephropathy: GBM thinner than 250 nm |
20, 24–34 |
| Anti-GBM-GN or Goodpasture-Syndrome | • Autoantibodies against collagen IV (α3) • Hematuria, proteinuria, often flu-like illness before onset • Incidence 1:1.000.000 |
• Glomerular fibrinoid necrosis, GBM ruptures and crescent formation • Breaks in Bowman’s capsule • Breaks in tubular basement membrane |
21, 22, 35–37 |
| Nail-Patella-Syndrome | • Heterozygous LMX1b-Mutations • Nail hypo-/dysplasia, bone abnormalities. Renal involvement: proteinuria, hematuria • Estimated prevalence 1:50.000 |
• Initially non-specific. • Advanced: FSGS or globally sclerotic glomeruli. Increase in GBM-thickness and mesangial matrix expansion • Collagen III- deposits in mesangial matrix |
38–42 |
| Col III- Glomerulopathy | • Cause unknown • Early and late onset, proteinuria, hypertension, progression to ESRD • <100 described cases |
• Mesangial and peripheral capillary loop expansion and thickening due to deposits of collagen III | 43–45 |
| Pierson-Syndrome | • LAMB2 mutations (over 50 described) • Congenital nephrotic syndrome, rapid progression to ESRD. Ocular anomalies (microcoria). • <30 described cases |
• Diffuse mesangial sclerosis • Irregular thickening of the GBM • Dilated tubules with protein casts, epithelial flattening • Fibrous and epithelial crescents |
46–51 |
| Fibronectin-Glomerulopathy | • Associated to mutations in FN1-Gene (40% of cases, rest: unknown) • Proteinuria, hematuria, hypertension, tubular acidosis, progression to ESRD • <20 described families |
• Enlargement and lobular appearance of glomeruli • Increased mesangial extracellular material and capillary wall thickening due to fibronectin deposition |
52–54 |
Different rare renal diseases primarily involve ECM components. If described in the literature the cause is given, as well as clinical presentation and main pathologic findings. Abbreviations: LM, light microscopy; GBM, glomerular basement membrane; ESRD, end stage renal disease; ECM, extracellular matrix; FSGS, focal segmental glomerulosclerosis.
Pathological deposition of collagen III is found in both mesangial nodular and focal segmental glomerulosclerosis,55,56 but also in specific rare diseases, that is, collagen type III glomerulopathy and Nail-Patella Syndrome38,57 (Table 1).
Collagens I, II, III, V, VI, VII, and XV are expressed in the renal interstitium and increased deposition was found during renal fibrosis.58 Upregulation of collagen I and III is considered an early event in renal fibrosis.55,58,59 Collagen I is widely accepted as a major component of fibrotic tissues. A study using a cell-type specific knock-out of collagen type I provided first evidence for direct functional involvement of collagen in kidney diseases. The effects were dependent on disease context, that is, during reversion of early obstructive nephropathy, collagen I deficiency resulted in reduced renal functional recovery, whereas in a model of crystal-induced CKD and fibrosis, collagen I deposition was harmful and disease aggravating.60
Glycoproteins and Proteoglycans
Glycoproteins are a large group of proteins with covalently attached oligosaccharide chains, and proteoglycans are glycosaminoglycans that are covalently bound to a protein core.61
Fibronectin is an adhesive high molecular weight glycoprotein that plays a crucial role in wound-healing and ECM formation.62 Although only one fibronectin-gene exists, there are multiple different fibronectin proteins generated through alternative splicing.63 Fibronectin is found in the glomerular mesangium, Bowman’s capsule and tubulo-interstitium and its expression increased after injury during fibrosis64–66 (Figs. 1, 2 and 4). In addition to locally produced fibronectin, it was shown that circulating fibronectin can be incorporated into mesangial ECM during diabetes, thereby contributing to mesangial expansion.67 Fibronectin is a major component of glomerular extracapillary proliferates (crescents).68 Approximately 40% of patients with mutations in the FN1-gene develop fibronectin glomerulopathy, a very rare disease caused by pathological deposition of fibronectin in the mesangium and capillary walls of the glomeruli.52,53 Little is known about fibronectin in renal vessels: an increased expression in acute diffuse proliferative glomerulonephritis and malignant hypertension65 and in renal cell carcinomas was described.69
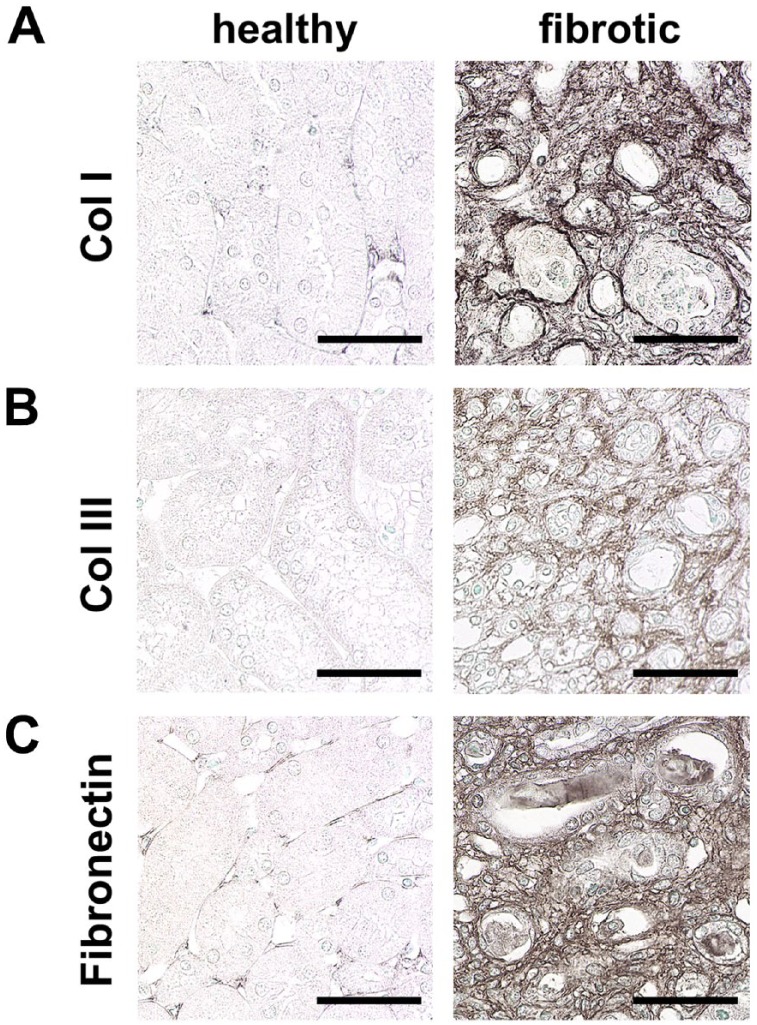
Figure 4.
Hallmarks of renal interstitial fibrosis. Immunohistochemical staining of ECM components shows prominent upregulation of collagen I (A), collagen III (B), and fibronectin (C) in interstitial fibrosis compared to healthy mice. Scale bars indicate 50 µm. Abbreviation: ECM, extracellular matrix.
HSPGs are glycoproteins that contain one or more covalently attached heparan sulfate chains.70 They can be divided into three different groups: membrane HSPG (e.g., glypicans), secreted ECM HSPGs (e.g., agrin, perlecan), and the secretory vesicle proteoglycan serglycin. In the healthy kidney, agrin is the major HSPG of the GBM, while perlecan is less abundant. In the mesangial matrix of healthy kidneys, both agrin and perlecan are expressed.71 Both agrin and perlecan contribute to the electric charge of the GBM, but seem not to be important for glomerular filtration.72 In a rat model of chronic transplant dysfunction, perlecan was markedly induced in the GBM and the mesangial matrix.71 In IgA-nephropathy, perlecan was significantly upregulated and high expression correlated with a better outcome of patients.73 Both perlecan and agrin are expressed in tubular basement membranes, and perlecan is also expressed in basement membranes of peritubular capillaries. In healthy and diseased kidneys, perlecan and agrin are not expressed in interstitium, but in vessel walls and neointima in arteriosclerosis.71
Laminins, which are a group of heterotrimeric glycoproteins, are one of the main constituents of basement membranes and are involved in cell and tissue differentiation, homeostasis, and survival. Laminins consist of an alpha, a beta, and a gamma chain.74 There are five alpha, four beta, and three gamma chains.75 In total, 16 different combinations of these chains have been identified in mammals, for example, laminin α1β1γ1.76 Laminins are a major constituent of the GBM,77 mesangial ECM58 and tubular basement membranes.78 Similar to the change in the collagen IV network, there is a shift in laminin isoform expression during renal development in the GBM. Laminin α5β2γ1 is expressed in the mature GBM, while Laminin α1β1γ1 is expressed during development.79 Pierson syndrome, a rare genetic disorder is caused by a mutation in the LAMB2 gene encoding the laminin β2-chain46 (Table 1). A podocyte-specific knockout of laminin β2 led to severe proteinuria in mice.80 Introduction of a Lamb2 gene with C321R missense mutation leading to Pierson Syndrome in these Lamb2-knockout mice initially reduced proteinuria, but eventually led to progressive disease with development of end-stage renal disease (ESRD).80 Increased deposition of laminins in the mesangial matrix and GBM, but not in the interstitium, has been shown in diabetic nephropathy.81 There is no comprehensive description of laminins in tubular basement membranes in renal fibrosis.
Nidogens are monomeric glycoproteins expressed in basal membranes, important for normal development of some organs like heart or lung.82 In the kidney, they are expressed in the GBM and tubular basement membranes but also in the mesangial ECM.58,83 The functional role of nidogen is not yet clear as global knockouts of nidogen 1 or nidogen 2 showed no kidney phenotype with normally developed GBM.84,85 Upregulation of nidogens in the mesangial matrix and the GBM was described in patients with glomerular diseases, that is, diabetic nephropathy, IgA-nephropathy, and lupus nephritis.86
Versican is a large chondroitin sulfate proteoglycan considered an anti-adhesion molecule with multiple binding partners, for example, hyaluronan, collagen I, and fibronectin.87–89 In the healthy kidney, versican is expressed in the tubulo-interstitium and in intima and media of vessels,90 but not in glomeruli.71 In kidney diseases, both in animal models and patients, versican was found to be upregulated in interstitium during fibrosis91 and it was found to be expressed de novo in glomerular crescents.92 During arteriosclerosis, it is induced in the neointima.92 High versican-expression is associated with worsening of CKD.91
Decorin and biglycan belong to the large group of small leucine-rich proteoglycans that are either bound to the ECM or released as soluble mediators.93–96 In healthy kidneys, they are mainly expressed in the interstitium97 but not in glomeruli.98 Both biglycan and decorin showed de novo expression in glomeruli and elevated expression in the interstitium in various renal diseases and fibrosis in animal models and patients.92,98 In healthy renal arterial vessels, decorin and biglycan are expressed throughout all layers.97 Both ECM-bound decorin and biglycan interact with collagens and regulate ECM-assembly.95 After proteolytic cleavage, both biglycan and decorin can act as soluble mediators,99 interact with toll-like-receptors and are considered ECM-derived promotors of inflammatory response.100 Decorin was shown to exert antifibrotic functions in the kidney by blocking transforming growth factor β (TGF-β), a key protein in pro-fibrotic signaling.101 Biglycan can also interact with TGF-β,102 however, no effects of biglycan-treatment were found in pulmonary fibrosis.103 Biglycan was recently found to induce autophagy in macrophages via TLR4 and CD44, thereby limiting inflammation and ischemia-reperfusion induced kidney injury in mice.104
ECM Remodelling
All types of ECM undergo constant remodeling, with an equilibrium between degradation and production in healthy state.105 In the kidney, the exact analyses of the half-life of the various ECM components is largely missing. In fibrosis, the equilibrium between ECM synthesis and breakdown is disturbed with increased production and reduced degradation leading to excessive ECM deposition. Degradation of ECM is mainly done by matrix-metalloproteinases (MMPs), which are counteracted by the tissue-inhibitors of metalloproteinases (TIMPs), both of which can be dysregulated in fibrosis.
MMPs are a group of zinc-dependent endopeptidases that can degrade various ECM components.105 They are considered as one of the main enzymes responsible for ECM remodeling.106,107 Of the 28 MMPs described in vertebrae, 23 are expressed in humans.108 At least 10 MMPs are expressed in the kidney (MMP-1, -2, -3, -9, -13, -14, -24, -25, -27, -28).58,109 MMPs can be divided into different groups based on their substrates and structure: the collagenases (MMP-1, -8, -13, -18),108,110 the gelatinases (MMP-2, -9), the stromelysins (MMP-3, -10, -11), the matrilysins (MMP-7, -26), the membrane-type MMPs (MMP-14, -15, -16, -17, -24, -25), and other MMPs (MMP-12, -19-23, -27, -28).108 Almost all of them cleave both collagen- and non-collagen-substrates.108 Their ability to degrade ECM suggested a beneficial role in renal fibrosis. However, effects of MMPs are far more complex and different MMPs were shown to play divergent roles in fibrogenesis. For example, high MMP-2-expression in mesangial cells led to increased collagen production.111 MMP-9 produced collagen fragments that are chemotactic for neutrophils and stimulate MMP9 production in these cells.112 Conversely, MMP-7 knockout mice showed reduced fibrosis in unilateral ureteral obstruction (UUO).113
Three of the four TIMPs, that is, TIMP-1, TIMP-2 and most strongly TIMP-3, are expressed in the kidney.109,114 Deficiency of TIMP-1 in mice undergoing UUO did not reduce tubulointerstitial fibrosis.115 A knockout of TIMP-2 did not show increased collagen I and III deposition following renal injury, and these mice had reduced MMP-2 activity.116 However, TIMP-3 knockout mice showed increased renal fibrosis and more collagen I and III deposition compared to wildtype controls in UUO.114 MMP-2 activation was also elevated in TIMP-3 knockout mice.116
Other proteases involved in ECM remodeling are the a-disintegrin-and-metalloproteinases with thrombospondin motifs (ADAMTS). ADAMTS have a variety of substrates including versican and fibrillar procollagens.117 Little is known on the potential role of ADAMTS in renal fibrosis, despite their ability to degrade a variety of matrix proteins. Interestingly, ADAMTS-1-knockout mice developed dilated calyces and renal interstitial fibrosis after birth.118 Recently, it was shown, that ADAMTS-1, -12 and -15 are increasingly expressed in the Adriamycin model of renal fibrosis.119
During fibrosis, ECM is not only changed in a quantitative but also qualitative manner. For example, tissue-transglutaminase 2 can cross-link various proteins, including collagens and fibronectin.120 This cross-linking of ECM-proteins leads to increased stability of ECM and resistance to proteolytic degradation. Tissue-transglutaminase 2 is upregulated in experimental models of renal fibrosis121 and tissue-transglutaminase 2 knockout mice develop less interstitial fibrosis.122 In diabetes, advanced glycation end-products were shown to increase stiffness of collagens by crosslinking, which also reduces collagen-digestibility and masks integrin-binding sites.123,124
Cell-ECM Interactions
Interactions between ECM and cells are mediated by ECM-binding transmembrane receptors, such as integrins. Integrins provide a mechanical link between the ECM and intracellular actin- and intermediate filaments of the cytoskeleton.125 They were shown to modulate cell survival, migration, proliferation, and tissue homeostasis, providing a communication hub between the ECM and cells.126 Integrins are transmembrane glycoproteins with 18 α- and 8 β-subunits, forming 24 heterodimers127 and bind to a wide variety of ligands including ECM components like collagens, laminins, and fibronectin. Binding of extracellular ligands to integrins induces intracellular signaling via intracellular adaptor proteins, enabling cell-matrix signal transduction.128 In the glomerulus, integrin α3β1, a laminin receptor, was shown to be expressed on podocytes128 and integrin α1β1, a collagen receptor, on the mesangial cells.129 In early stage diabetic nephropathy, α3β1-integrin was upregulated in podocytes, but was downregulated in later stages.130 Mice with a podocyte-specific knockout of the integrin α3 subunit showed podocyte foot process effacement.131 Knock-out of the α1β1-integrin in Alport mice led to reduced proteinuria and significantly prolonged life-span of these mice.132
α6β1-integrin is expressed in renal tubular cells.133–135 Deletion of the α6-subunit in collecting ducts lead to a higher vulnerability of epithelial cells to obstructive nephropathy.78 In a mouse model of polycystic kidney disease, knock-out of the β1-subunit reduced cyst formation.136 Several integrin subunits were found in renal vascular endothelial cells, for example, α1, β2, β3.137 A knockout of the α8-subunit, which is expressed in vascular smooth muscle cells and mesangial cells in the kidney, lead to a widening of the lumen of peritubular capillaries.138 Integrins containing the αv-subunit, which act as receptors for fibronectin and laminin, were found to be key modulators in organ fibrosis.139,140 A knockout of the αv-integrin-subunit in myofibroblasts led to less fibrosis in lung, liver, and kidney and a specific inhibitor of αv-integrins effectively reduced fibrosis in liver and lung.141 In a model of lipopolysaccharide-induced proteinuria in mice activation of the αvβ3-integrin by the urokinase receptor (uPAR) aggravated proteinuria.142 Activation of β3-integrin via periostin was proposed as an important mechanism driving crescentic glomerulonephritis in mice via influencing podocyte foot process effacement, increased cell motility, and survival.143
Syndecans are transmembrane HSPGs, attached to the actin cytoskeleton.144 Both heparan sulfate and chondroitin sulfate chains can be bound to syndecans. As receptors, they can bind several ECM-proteins, including collagens and fibronectin.145 In the kidney, syndecan-1, -2, and -4 were shown to be expressed.146 A knockout of syndecan-4 reduced renal fibrosis by reducing transglutaminase 2 mediated ECM cross-linking.147 Discoidin domain receptors (DDRs) are receptor tyrosine kinases that mainly bind to collagens and are generally considered profibrotic in glomerular and interstitial kidney diseases. In healthy kidneys, they are expressed mainly in the tubules. DDR1 is expressed de novo in glomerular injury in rats,148 and DDR1-knockout mice showed less fibrosis and less proteinuria in a model of hypertension149 and in Alport mice.150 Dystroglycan is another transmembrane receptor connecting extracellular glycoproteins, for example, laminin and agrin, to the cytoskeleton. This complex is called the dystroglycan glycoprotein complex151 and is expressed in podocytes and tubular epithelial cells in the kidney.152,153 However, both ubiquitous and a kidney cell-specific knockout of dystroglycan had no significant impact neither in healthy nor diseased kidneys.154
Renal ECM Components—Biomarker Potential?
The extent of renal fibrosis is currently the best predictor of progressive functional decline of kidney diseases.12 Increased deposition of ECM is the core hallmark and definition of fibrosis, and therefore ECM components can be viewed as the only truly specific fibrosis markers. Histological assessment of renal fibrosis on kidney biopsy is currently the only specific method for fibrosis quantification.
Renal interstitial fibrosis can appear in various morphological patterns. In diseases which affect renal parenchyma in a diffuse manner, a diffuse interstitial fibrosis is observed. In diseases with focal injury, for example, in many glomerular diseases, fibrosis develops in a patchy manner. A striped appearance of fibrosis was associated to calcineurin-inhibitor use155 and broad fibrotic scars of interstitial fibrosis are observed after pyelonephritis or anemic infarction, similar to scars after myocardial infarction.156 Given the focal nature of fibrosis, sampling bias could be a confounder when measuring fibrosis in biopsies. However, there are no studies that would systematically and comprehensively analyze such a bias. On the contrary, one study comparing two biopsy cores from various kidney locations showed no significant sampling error, suggesting that such a bias might not be as relevant as is often anticipated.157
In renal biopsy diagnostic, the degree of interstitial fibrosis is assessed on routinely performed stains, preferentially on special stains for ECM, such as the Sirius red or Masson’s trichrome stain. Immunohistochemistry or immunofluorescence can be used for a more specific assessment of particular ECM components (Fig. 4), but this is currently not used in routine diagnostics. Given the predictive value of interstitial fibrosis, the degree of interstitial fibrosis is included as an integral part in the renal biopsy pathology report. The extent of interstitial fibrosis is most common assessed by renal pathologists in a semiquantitative manner using scoring systems or an estimate of the fibrotic area, which might have low reproducibility.158–160 Digitalization of pathological slides, that is, virtual microscopy, opens the possibility of automated, more exact and more reproducible quantification of fibrosis.161–163 Various alternative methods were shown to be useful for analysis of interstitial fibrosis. For example, a combination of Fluorescence Lifetime Imaging (FLIM) with Second Harmonic Generation (SHG) allowing a label-free, deep tissue imaging of a biopsy reproducibly detected fibrosis.164 However, this technique requires sophisticated microscopy equipment, which is not freely available in routine pathology labs. Combining such novel microscopy techniques with tissue clearing,165 opens exciting possibilities of 3D assessment of kidney morphology and fibrosis in research,166 but is currently not useful or applicable for routine pathology diagnostics.
However, the invasive nature and the potential sampling bias are some of the disadvantages rendering renal biopsies not perfectly suitable for fibrosis monitoring. An ideal biomarker of renal fibrosis should be non-invasive and closely and specifically reflect kidney fibrosis. Several studies analyzed collagens and their production and degradation fragments as non-invasive biomarkers of renal fibrosis. N-terminal propeptide of type III collagen (PIIINP) is a fragment generated during production of collagen III. It was elevated in urine and serum of patients with different renal diseases and correlated with increased collagen III in biopsies.55 The ratio of urinary PIIINP to creatinine showed a positive correlation with renal function parameters, increased with CKD stage and correlated with the extent of interstitial fibrosis.167 Elevated urinary levels of PIIINP were associated with CKD-progression in elderly patients.168
MMP-mediated collagen I and III degradation fragments, C1M and C3M, were shown to be a potential biomarker in animal models of fibrosis169,170 and acute kidney injury.171 In two cohorts of IgA-nephropathy patients, collagen III-turnover suggested a profibrotic ECM remodeling with increasing CKD stage, with steadily increasing Pro-C3 as a marker of collagen III production and progressively decreasing C3M as a marker of collagen III degradation.172 Similarly, renal transplant recipients with high CKD stage exhibited low urinary levels of C3M.173 In a large study analyzing the urinary proteome of patients with and without CKD, fragments of collagen I and III were shown to have a strong positive correlation to progression in CKD.174
High urinary collagen type IV was associated with lower renal function in a cohort of Japanese patients with type 2 diabetes.175 In a long-term follow-up of patients with type 1 diabetes, it was possible to predict microalbuminuria by higher urinary collagen type IV.176 In a Japanese non-diabetic population, a high urinary collagen IV to creatinine ratio was associated with an increased risk of 10% decline of eGFR per year.177 Although collagen IV is increased in renal fibrosis, urinary and circulating collagen IV did not correlate with fibrosis in kidney biopsies in a cohort of patients with different diseases, that is, membranous glomerulonephritis, mesangioproliferative glomerulonephritis, tubulointerstitial nephritis, and ESRD.55 However, high serum amounts of a fragment of collagen IV termed P4NP_7S were shown to be associated with worse survival in hemodialysis patients.178
Endotrophin is a C-terminal fragment of collagen VI that is generated during production of collagen VI.179 High serum endotrophin was associated with increased risk for developing ESRD and a higher mortality in a group of CKD patients.180 Endotrophin was expressed de novo in interstitial fibrosis in patients and high urinary endotrophin was associated with a higher rate of one-year-progression to ESRD in CKD patients.181
Increased serum and urinary LG1M, a specific neoepitope generated by cleavage of the laminin γ1 chain by MMP-9, was associated with a higher rate of development of end stage renal disease and with mortality.182
Given the excretory function of the kidney and the fact that most, if not all ECM components are not kidney-specific, the specificity of urinary and circulating biomarkers in detecting and monitoring kidney fibrosis is limited. Non-invasive imaging of ECM could circumvent this problem and showed some promising results in other organs, particularly the liver.183 A number of imaging techniques, in particular using magnetic resonance imaging (MRI), ultrasonography, and elastography, were evaluated in assessment of renal fibrosis with rather contradictory and not convincing results.14,183–185 Molecular imaging of ECM components might represent a more specific approach to image fibrosis. First promising data showed the feasibility of molecular fibrosis imaging using elastin-specific molecular probes.186 The above discussed approaches to monitor fibrosis are summarized in Table 2.
Table 2.
Summary of Different Ways to Monitor Renal Fibrosis.
| Component/Fragment | Fluid | Regulation | |
|---|---|---|---|
| ECM-components in body fluids | • PIIINP | • Serum and urine | • ↑ urinary correlated with col III in biopsies • ↑ PIIINP/creatinine correlated with interstitial fibrosis • ↑ PIIINP/creatinine in ↑ CKD-stage |
| • C1M, C3M | • Urine | • ↑ ProC3 and ↓ C3M in IgA-Nephropathy • ↓ C3M in transplant recipients with ↑ CKD-stage |
|
| • Col IV | • Serum and urine | • ↑ col IV in urine in ↓ renal function • ↑ col IV in urine predicted microalbuminuria • ↑ col IV/creatinine ↑ risk of renal functional decline • No correlation between serum and urine col IV with fibrosis score in biopsies |
|
| • P4NP_7S | • Serum | • ↑ P4NP_7S ↓ survival in hemodialysis patients | |
| • Endotrophin | • Serum and urine | • ↑ serum endotrophin ↑ risk of developing ESRD • ↑ urinary endotrophin ↑ one year progression to ESRD |
|
| • LG1M | • Serum and urine | • ↑ LG1M ↑ development of ESRD and ↑ mortality | |
| Method | |||
| Non-invasive Imaging | • MRI | • Detects changes in microarchitecture and function | |
| • Ultrasonography | • Detects anatomical variances | ||
| • Elastography | • Measures tissue stiffness | ||
| • Molecular imaging | • Detects amount of a specific ECM components, such as elastin | ||
| Method | |||
| Renal Tissue | • Semiquantitative assessment by pathologist (microscope) | • Easy, fast, low reproducibility | |
| • Computer assisted analysis using whole slide imaging | • Requires digital pathology (Whole Slide Imaging), reproducible, precise | ||
| • FLIM & SHG | • Requires special microscopy equipment | ||
Fibrosis can be monitored in different ways with renal biopsy being the gold standard right now. Additionally, different non-invasive biomarkers are being developed trying to monitor fibrosis via sampling of biological fluids. Several different imaging techniques have been developed, the most promising being molecular imaging. Abbreviations: ESRD, end stage renal disease; MRI, magnetic resonance imaging; FLIM, fluorescence lifetime imaging; SHG, second harmonic generation; ECM, extracellular matrix; PIIINP, N-terminal propeptide of type III collagen; CKD, chronic kidney disease.
Renal ECM in Tissue Engineering
Tissue engineering provides a potential alternative for transplantation. Building up kidneys from patient-own cells is an exciting future perspective. As a first step in this direction, kidney organoids, that is, organ-like 3D structures of organ-specific cells,187 were successfully generated in vitro using human embryonic stem cells or human-induced pluripotent stem cells (iPSCs).188,189 To date, it is only possible to recapitulate early embryonic development of the kidney, which is still far from generating organs suitable for transplantation. A possible alternative approach is to repopulate a kidney scaffold with iPSCs or more differentiated, kidney-committed precursors.190 For this, renal ECM itself might be the best suitable scaffold, providing an exact functional and structural environment for repopulating cells.191,192 Decellularization, that is, organ perfusion with compounds destroying cell membranes and leading to cell death (e.g., Triton X-100 or sodium dodecyl sulfate), generates a cell-free ECM-scaffold that can be used for tissue reconstruction of a whole kidney.190,193 This is followed by repopulation of the ECM scaffolds with cells, potentially leading to generation of functional kidneys.193,194 Currently, also this approach is yet far from clinical applicability.
Another concept of tissue engineering is bioprinting, that is, 3D-organ printing from tissue components.195 In this technique, cellular and non-cellular components are printed in parallel to form a replica of a tissue. Most biomaterials currently used for bioprinting do not replicate the complexity of the ECM.196 However, decellularized ECM can be solubilized to be used as bio-ink. This solubilized ECM behaves as a pre-gel, being soluble at low temperatures allowing 3D-printing followed by incubation at 37C, generating a gel (a process termed gelation).196 First examples of 3D-bioprinting of proximal tubules were shown to be suitable for drug testing.197 However, the kidney is a very complex structure which might pose a substantial hurdle for whole organ 3D bioprinting.
In conclusion, the ECM of the kidney is a complex, dynamic structure essential for normal renal function. This is well documented by several rare diseases leading to progressive and currently non-treatable renal diseases. In addition, pathological ECM deposition is a hallmark of renal fibrosis and thereby involved in virtually all CKDs. Components of the ECM could be exploited as specific biomarkers of renal fibrosis, being potentially powerful progression markers. ECM scaffolds from decellularized kidneys might be crucial in tissue engineering of kidneys.
Acknowledgments
We thank Dr. Pablo Cannata-Ortiz from Fundación Jiménez Díaz, Madrid, for providing a picture of mesangial sclerosis with a sclerotic adhesion in Fig. 1.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: RDB drafted the manuscript and arranged figures and table, PB critically reviewed the manuscript, tables, and figures both for intellectual content and form. Both RDB and PB have read and approved the final version of the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financed by the German Research Foundation (DFG: SFB/TRR57 and SFB/TRR219, BO3755/3-1 and BO3755/6-1), the German Ministry of Education and Research (BMBF: STOP-FSGS-01GM1901A), and the RWTH Interdisciplinary Center for Clinical Research (IZKF: O3-7).
Contributor Information
Roman David Bülow, Institute of Pathology, RWTH Aachen University Hospital, Aachen, Germany.
Peter Boor, Institute of Pathology, RWTH Aachen University Hospital, Aachen, Germany; Department of Nephrology and Immunology, RWTH Aachen University Hospital, Aachen, Germany.
Literature Cited
- 1. Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FD. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS ONE. 2016;11(7):e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neovius M, Jacobson SH, Eriksson JK, Elinder CG, Hylander B. Mortality in chronic kidney disease and renal replacement therapy: a population-based cohort study. BMJ Open. 2014;4(2):e004251. doi: 10.1136/bmjopen-2013-004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Michael AF. The glomerular mesangium. Contrib Nephrol. 1984;40:7–16. [PubMed] [Google Scholar]
- 4. Lennon R, Byron A, Humphries JD, Randles MJ, Carisey A, Murphy S, Knight D, Brenchley PE, Zent R, Humphries MJ. Global analysis reveals the complexity of the human glomerular extracellular matrix. J Am Soc Nephrol. 2014;25(5):939–51. doi: 10.1681/ASN.2013030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu J, Shi GP. Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta. 2014;1842(11):2106–119. doi: 10.1016/j.bbadis.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Babickova J, Klinkhammer BM, Buhl EM, Djudjaj S, Hoss M, Heymann F, Tacke F, Floege J, Becker JU, Boor P. Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int. 2017;91(1):70–85. doi: 10.1016/j.kint.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 7. Hughson MD, Puelles VG, Hoy WE, Douglas-Denton RN, Mott SA, Bertram JF. Hypertension, glomerular hypertrophy and nephrosclerosis: the effect of race. Nephrol Dial Transpl. 2014;29(7):1399–409. doi: 10.1093/ndt/gft480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hommos MS, Glassock RJ, Rule AD. Structural and functional changes in human kidneys with healthy aging. J Am Soc Nephrol. 2017;28(10):2838–44. doi: 10.1681/ASN.2017040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J, Moeller MJ. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol. 2011;22(7):1262–74. doi: 10.1681/ASN.2010090970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smeets B, Stucker F, Wetzels J, Brocheriou I, Ronco P, Grone HJ, D’Agati V, Fogo AB, van Kuppevelt TH, Fischer HP, Boor P, Floege J, Ostendorf T, Moeller MJ. Detection of activated parietal epithelial cells on the glomerular tuft distinguishes early focal segmental glomerulosclerosis from minimal change disease. Am J Pathol. 2014;184(12):3239–48. doi: 10.1016/j.ajpath.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smeets B, Te Loeke NA, Dijkman HB, Steenbergen ML, Lensen JF, Begieneman MP, van Kuppevelt TH, Wetzels JF, Steenbergen EJ. The parietal epithelial cell: a key player in the pathogenesis of focal segmental glomerulosclerosis in Thy-1.1 transgenic mice. J Am Soc Nephrol. 2004;15(4):928–39. [DOI] [PubMed] [Google Scholar]
- 12. Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med. 2019;65:16–36. doi: 10.1016/j.mam.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 13. Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124(6):2299–306. doi: 10.1172/Jci72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klinkhammer BM, Goldschmeding R, Floege J, Boor P. Treatment of renal fibrosis-turning challenges into opportunities. Adv Chronic Kidney D. 2017;24(2):117–29. doi: 10.1053/j.ackd.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 15. Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem. 2002;277(6):4223–31. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 16. Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 2010;339(1):247–57. doi: 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kadler KE. Fell Muir lecture: collagen fibril formation in vitro and in vivo. Int J Exp Pathol. 2017;98(1):4–16. doi: 10.1111/iep.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chew C, Lennon R. Basement membrane defects in genetic kidney diseases. Front Pediatr. 2018;6:11. doi: 10.3389/fped.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kruegel J, Rubel D, Gross O. Alport syndrome-insights from basic and clinical research. Nat Rev Nephrol. 2013;9(3):170–8. doi: 10.1038/nrneph.2012.259. [DOI] [PubMed] [Google Scholar]
- 20. Cosgrove D, Liu SG. Collagen IV diseases: a focus on the glomerular basement membrane in Alport syndrome. Matrix Biol. 2017;57–58:45–54. doi: 10.1016/j.matbio.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodpasture EW. The significance of certain pulmonary lesions in relation to the etiology of influenza. AM J Med Sci. 1919;158:863. [DOI] [PubMed] [Google Scholar]
- 22. Kalluri R, Wilson CB, Weber M, Gunwar S, Chonko AM, Neilson EG, Hudson BG. Identification of the alpha 3 chain of type IV collagen as the common autoantigen in antibasement membrane disease and Goodpasture syndrome. J Am Soc Nephrol. 1995;6(4):1178–85. [DOI] [PubMed] [Google Scholar]
- 23. Hurskainen T, Moilanen J, Sormunen R, Franzke CW, Soininen R, Loeffek S, Huilaja L, Nuutinen M, Bruckner-Tuderman L, Autio-Harmainen H, Tasanen K. Transmembrane collagen XVII is a novel component of the glomerular filtration barrier. Cell and Tissue Research. 2012;348(3):579–88. doi: 10.1007/s00441-012-1368-x. [DOI] [PubMed] [Google Scholar]
- 24. Funk SD, Lin MH, Miner JH. Alport syndrome and Pierson syndrome: diseases of the glomerular basement membrane. Matrix Biol. 2018;71–72:250–61. doi: 10.1016/j.matbio.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gubler M, Levy M, Broyer M, Naizot C, Gonzales G, Perrin D, Habib R. Alport’s syndrome. A report of 58 cases and a review of the literature. Am J Med. 1981;70(3):493–505. [DOI] [PubMed] [Google Scholar]
- 26. Heidet L, Gubler MC. The renal lesions of Alport syndrome. J Am Soc Nephrol. 2009;20(6):1210–15. doi: 10.1681/ASN.2008090984. [DOI] [PubMed] [Google Scholar]
- 27. Kashtan CE, Gubler MC, Sisson-Ross S, Mauer M. Chronology of renal scarring in males with Alport syndrome. Pediatr Nephrol. 1998;12(4):269–74. [DOI] [PubMed] [Google Scholar]
- 28. White RH, Raafat F, Milford DV, Komianou F, Moghal NE. The Alport nephropathy: clinicopathological correlations. Pediatr Nephrol. 2005;20(7):897–903. doi: 10.1007/s00467-005-1955-0. [DOI] [PubMed] [Google Scholar]
- 29. Badenas C, Praga M, Tazon B, Heidet L, Arrondel C, Armengol A, Andrés A, Morales E, Camacho JA, Lens X, Dávila S, Milà M, Antignac C, Darnell A, Torra R. Mutations in the COL4A4 and COL4A3 genes cause familial benign hematuria. J Am Soc Nephrol. 2002;13(5):1248–54. [DOI] [PubMed] [Google Scholar]
- 30. Foster K, Markowitz GS, D’Agati D. Pathology of thin basement membrane nephropathy. Semin Nephrol. 2005;25(3):149–58. doi: 10.1016/j.semnephrol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 31. Schroder CH, Bontemps CM, Assmann KJM, Stekhoven JHS, Foidart JM, Monnens LA, Veerkamp JH. Renal biopsy and family studies in 65 children with isolated hematuria. Acta Paediatr Scand. 1990;79(6-7):630–6. doi: 10.1111/j.1651-2227.1990.tb11527.x. [DOI] [PubMed] [Google Scholar]
- 32. Tina L, Jenis E, Jose P, Medani C, Papadopoulou Z, Calcagno P. The glomerular basement membrane in benign familial hematuria. Clin Nephrol. 1982;17(1):1–4. [PubMed] [Google Scholar]
- 33. Wang YY, Rana K, Tonna S, Lin T, Sin L, Savige J. COL4A3 mutations and their clinical consequences in thin basement membrane nephropathy (TBMN). Kidney Int. 2004;65(3):786–90. doi: 10.1111/j.1523-1755.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- 34. Yoshikawa N, Hashimoto H, Katayama Y, Yamada Y, Matsuo T, Okada S. The thin glomerular basement-membrane in children with hematuria. J Pathol. 1984;142(4):253–7. doi: 10.1002/path.1711420403. [DOI] [PubMed] [Google Scholar]
- 35. Martinez JS, Kohler PF. Variant “goodpasture’s syndrome”? The need for immunologic criteria in rapidly progressive glomerulonephritis and hemorrhagic pneumonitis. Ann Intern Med. 1971;75(1):67–76. [DOI] [PubMed] [Google Scholar]
- 36. Andres G, Brentjens J, Kohli R, Anthone R, Anthone S, Baliah T, Montes M, Mookerjee BK, Prezyna A, Sepulveda M, Venuto R, Elwood C. Histology of human tubulo-interstitial nephritis associated with antibodies to renal basement membranes. Kidney Int. 1978;13(6):480–91. [DOI] [PubMed] [Google Scholar]
- 37. McAdoo SP, Pusey CD. Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol. 2017;12(7):1162–72. doi: 10.2215/CJN.01380217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cohen AH. Collagen type III glomerulopathies. Adv Chronic Kidney D. 2012;19(2):101–6. doi: 10.1053/j.ackd.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 39. Morello R, Zhou G, Dreyer SD, Harvey SJ, Ninomiya Y, Thorner PS, Miner JH, Cole W, Winterpacht A, Zabel B, Oberg KC, Lee B. Regulation of glomerular basement membrane collagen expression by LMX1B contributes to renal disease in nail patella syndrome. Nat Genet. 2001;27(2):205–8. doi: 10.1038/84853. [DOI] [PubMed] [Google Scholar]
- 40. Del Pozo E, Lapp H. Ultrastructure of the kidney in the nephropathy of the nail–patella syndrome. Am J Clin Pathol. 1970;54(6):845–51. [DOI] [PubMed] [Google Scholar]
- 41. Ben-Bassat M, Cohen L, Rosenfeld J. The glomerular basement membrane in the nail-patella syndrome. Arch Pathol. 1971;92(5):350–5. [PubMed] [Google Scholar]
- 42. Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469(7–8):927–36. doi: 10.1007/s00424-017-2013-z. [DOI] [PubMed] [Google Scholar]
- 43. Fogo AB, Lusco MA, Najafian B, Alpers CE. AJKD atlas of renal pathology: type III collagen glomerulopathy. Am J Kidney Dis. 2017;69(6):e25–6. doi: 10.1053/j.ajkd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 44. Chen X, Wang H, Xu W, Zhu J. Collagen type III glomerulopathy: case report and review of the literature. Clin Nephrol. 2017;87(2017):39–46. doi: 10.5414/CN108907. [DOI] [PubMed] [Google Scholar]
- 45. Ikeda K, Yokoyama H, Tomosugi N, Kida H, Ooshima A, Kobayashi K. Primary glomerular fibrosis: a new nephropathy caused by diffuse intra-glomerular increase in atypical type III collagen fibers. Clin Nephrol. 1990;33(4):155–9. [PubMed] [Google Scholar]
- 46. Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wühl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dötsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin beta 2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13(21):2625–32. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- 47. Zenker M, Tralau T, Lennert T, Pitz S, Mark K, Madlon H, Dötsch J, Reis A, Müntefering H, Neumann LM. Congenital nephrosis, mesangial sclerosis, and distinct eye abnormalities with microcoria: an autosomal recessive syndrome. Am J Med Genet A. 2004;130A(2):138–45. doi: 10.1002/ajmg.a.30310. [DOI] [PubMed] [Google Scholar]
- 48. Matejas V, Hinkes B, Alkandari F, Al-Gazali L, Annexstad E, Aytac MB, Barrow M, Bláhová K, Bockenhauer D, Cheong HI, Maruniak-Chudek I, Cochat P, Dötsch J, Gajjar P, Hennekam RC, Janssen F, Kagan M, Kariminejad A, Kemper MJ, Koenig J, Kogan J, Kroes HY, Kuwertz-Bröking E, Lewanda AF, Medeira A, Muscheites J, Niaudet P, Pierson M, Saggar A, Seaver L, Suri M, Tsygin A, Wühl E, Zurowska A, Uebe S, Hildebrandt F, Antignac C, Zenker M. Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat. 2010;31(9):992–1002. doi: 10.1002/humu.21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kagan M, Cohen AH, Matejas V, Vlangos C, Zenker M. A milder variant of Pierson syndrome. Pediatr Nephrol. 2008;23(2):323–7. doi: 10.1007/s00467-007-0624-x. [DOI] [PubMed] [Google Scholar]
- 50. Choi HJ, Lee BH, Kang JH, Jeong HJ, Moon KC, Ha IS, Yu YS, Matejas V, Zenker M, Choi Y, Cheong HI. Variable phenotype of Pierson syndrome. Pediatr Nephrol. 2008;23(6):995–1000. doi: 10.1007/s00467-008-0748-7. [DOI] [PubMed] [Google Scholar]
- 51. Glastre C, Cochat P, Bouvier R, Colon S, Cottin X, Giffon D, Wright C, Dijoud F, David L. Familial infantile nephrotic syndrome with ocular abnormalities. Pediatr Nephrol. 1990;4(4):340–2. [DOI] [PubMed] [Google Scholar]
- 52. Strom EH, Banfi G, Krapf R, Abt AB, Mazzucco G, Monga G, Gloor F, Neuweiler J, Riess R, Stosiek P, Hebert LA, Sedmak DD, Gudat F, Mitsch MJ. Glomerulopathy associated with predominant fibronectin deposits: a newly recognized hereditary disease. Kidney Int. 1995;48(1):163–70. [DOI] [PubMed] [Google Scholar]
- 53. Ishimoto I, Sohara E, Ito E, Okado T, Rai T, Uchida S. Fibronectin glomerulopathy. Clin Kidney J. 2013;6(5):513–5. doi: 10.1093/ckj/sft097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Takii M, Suehiro T, Shima A, Yotsueda H, Hisano S, Katafuchi R. Fibronectin glomerulopathy complicated with persistent cloaca and congenital esophageal atresia: a case report and literature review. Bmc Nephrol. 2017;18(1):288. doi: 10.1186/s12882-017-0704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Soylemezoglu O, Wild G, Dalley AJ, MacNeil S, MilfordWard A, Brown CB, el Nahas AM. Urinary and serum type III collagen: markers of renal fibrosis. Nephrol Dial Transpl. 1997;12(9):1883–9. doi: 10.1093/ndt/12.9.1883. [DOI] [PubMed] [Google Scholar]
- 56. Yoshioka K, Takemura T, Tohda M, Akano N, Miyamoto H, Ooshima A, Maki S. Glomerular localization of type-III collagen in human-kidney disease. Kidney Int. 1989;35(5):1203–11. doi: 10.1038/ki.1989.111. [DOI] [PubMed] [Google Scholar]
- 57. Zuppan CW, Weeks DA, Cutler D. Nail-patella glomerulopathy without associated constitutional abnormalities. Ultrastruct Pathol. 2003;27(5):357–61. doi: 10.1080/01913120390239971. [DOI] [PubMed] [Google Scholar]
- 58. Genovese F, Manresa AA, Leeming DJ, Karsdal MA, Boor P. The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair. 2014;7(1):4. doi: 10.1186/1755-1536-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sharma AK, Mauer SM, Kim Y, Michael AF. Interstitial fibrosis in obstructive nephropathy. Kidney Int. 1993;44(4):774–88. doi: 10.1038/ki.1993.312. [DOI] [PubMed] [Google Scholar]
- 60. Buchtler S, Grill A, Hofmarksrichter S, Stockert P, Schiechl-Brachner G, Rodriguez Gomez M, Neumayer S, Schmidbauer K, Talke Y, Klinkhammer BM, Boor P, Medvinsky A, Renner K, Castrop H, Mack M. Cellular origin and functional relevance of collagen I production in the kidney. J Am Soc Nephrol. 2018;29(7):1859–73. doi: 10.1681/ASN.2018020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(Pt 24):4195–200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lenselink EA. Role of fibronectin in normal wound healing. Int Wound J. 2015;12(3):313–6. doi: 10.1111/iwj.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115(20):3861–3. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 64. Assad L, Schwartz MM, Virtanen I, Gould VE. Immunolocalization of tenascin and cellular fibronectins in diverse glomerulopathies. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63(5):307–16. [DOI] [PubMed] [Google Scholar]
- 65. Dixon AJ, Burns J, Dunnill MS, McGee JO. Distribution of fibronectin in normal and diseased human kidneys. J Clin Pathol. 1980;33(11):1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van Vliet AI, Baelde HJ, Vleming LJ, de Heer E, Bruijn JA. Distribution of fibronectin isoforms in human renal disease. J Pathol. 2001;193(2):256–62. [DOI] [PubMed] [Google Scholar]
- 67. Klemis V, Ghura H, Federico G, Wurfel C, Bentmann A, Gretz N, Miyazaki T, Gröne HJ, Nakchbandi IA. Circulating fibronectin contributes to mesangial expansion in a murine model of type 1 diabetes. Kidney Int. 2017;91(6):1374–85. doi: 10.1016/j.kint.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 68. Yoshioka K, Takemura T, Akano N, Miyamoto H, Iseki T, Maki S. Cellular and non-cellular compositions of crescents in human glomerulonephritis. Kidney Int. 1987;32(2):284–91. [DOI] [PubMed] [Google Scholar]
- 69. Galler K, Junker K, Franz M, Hentschel J, Richter P, Gajda M, Göhlert A, von Eggeling F, Heller R, Giavazzi R, Neri D, Kosmehl H, Wunderlich H, Berndt A. Differential vascular expression and regulation of oncofetal tenascin-C and fibronectin variants in renal cell carcinoma (RCC): implications for an individualized angiogenesis-related targeted drug delivery. Histochem Cell Biol. 2012;137(2):195–204. doi: 10.1007/s00418-011-0886-z. [DOI] [PubMed] [Google Scholar]
- 70. Esko JD, Kimata K, Lindahl U. Proteoglycans and sulfated glycosaminoglycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. p. 207–222. [PubMed] [Google Scholar]
- 71. Rienstra H, Katta K, Celie JW, van Goor H, Navis G, van den Born J, Hillebrands JL. Differential expression of proteoglycans in tissue remodeling and lymphangiogenesis after experimental renal transplantation in rats. PLoS ONE. 2010;5(2):e9095. doi: 10.1371/journal.pone.0009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Harvey SJ, Miner JH. Revisiting the glomerular charge barrier in the molecular era. Curr Opin Nephrol Hypertens. 2008;17(4):393–8. doi: 10.1097/MNH.0b013e32830464de. [DOI] [PubMed] [Google Scholar]
- 73. Ebefors K, Granqvist A, Ingelsten M, Molne J, Haraldsson B, Nystrom J. Role of glomerular proteoglycans in IgA nephropathy. PLoS ONE. 2011;6(4):e18575. doi: 10.1371/journal.pone.0018575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24(5):326–32. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 75. Aumailley M. The laminin family. Cell Adh Migr. 2013;7(1):48–55. doi: 10.4161/cam.22826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yao Y. Laminin: loss-of-function studies. Cell Mol Life Sci. 2017;74(6):1095–115. doi: 10.1007/s00018-016-2381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Borza DB. Glomerular basement membrane heparan sulfate in health and disease: a regulator of local complement activation. Matrix Biol. 2017;57–58:299–310. doi: 10.1016/j.matbio.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Viquez OM, Yazlovitskaya EM, Tu T, Mernaugh G, Secades P, McKee KK, Georges-Labouesse E, De Arcangelis A, Quaranta V, Yurchenco P, Gewin LC, Sonnenberg A, Pozzi A, Zent R. Integrin alpha6 maintains the structural integrity of the kidney collecting system. Matrix Biol. 2017;57–58:244–57. doi: 10.1016/j.matbio.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Abrahamson DR, St John PL, Stroganova L, Zelenchuk A, Steenhard BM. Laminin and type IV collagen isoform substitutions occur in temporally and spatially distinct patterns in developing kidney glomerular basement membranes. J Histochem Cytochem. 2013;61(10):706–18. doi: 10.1369/0022155413501677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen YM, Zhou YF, Go G, Marmerstein JT, Kikkawa Y, Miner JH. Laminin 2 gene missense mutation produces endoplasmic reticulum stress in podocytes. J Am Soc Nephrol. 2013;24(8):1223–33. doi: 10.1681/Asn.2012121149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hu C, Sun L, Xiao L, Han Y, Fu X, Xiong X, Xu X, Liu Y, Yang S, Liu F, Kanwar YS. Insights into the mechanisms involved in the expression and regulation of extracellular matrix proteins in diabetic nephropathy. Curr Med Chem. 2015;22(24):2858–70. doi: 10.2174/0929867322666150625095407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pozzi A, Yurchenco PD, Iozzo RV. The nature and biology of basement membranes. Matrix Biol. 2017;57–58:1–11. doi: 10.1016/j.matbio.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gersdorff N, Otto S, Roediger M, Kruegel J, Miosge N. The absence of one or both nidogens does not alter basement membrane composition in adult murine kidney. Histol Histopathol. 2007;22(10):1077–84. doi: 10.14670/HH-22.1077. [DOI] [PubMed] [Google Scholar]
- 84. Murshed M, Smyth N, Miosge N, Karolat J, Krieg T, Paulsson M, Nischt R. The absence of nidogen 1 does not affect murine basement membrane formation. Mol Cell Biol. 2000;20(18):7007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schymeinsky J, Nedbal S, Miosge N, Poschl E, Rao C, Beier DR, Skarnes WC, Timpl R, Bader BL. Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol Cell Biol. 2002;22(19):6820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Katz A, Fish AJ, Kleppel MM, Hagen SG, Michael AF, Butkowski RJ. Renal entactin (nidogen): isolation, characterization and tissue distribution. Kidney Int. 1991;40(4):643–52. [DOI] [PubMed] [Google Scholar]
- 87. Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14(5):617–23. [DOI] [PubMed] [Google Scholar]
- 88. Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989;8(10):2975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15(7):483–94. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 90. Bode-Lesniewska B, Dours-Zimmermann MT, Odermatt BF, Briner J, Heitz PU, Zimmermann DR. Distribution of the large aggregating proteoglycan versican in adult human tissues. J Histochem Cytochem. 1996;44(4):303–12. [DOI] [PubMed] [Google Scholar]
- 91. Rudnicki M, Perco P, Neuwirt H, Noppert SJ, Leierer J, Sunzenauer J, Eder S, Zoja C, Eller K, Rosenkranz AR, Müller GA, Mayer B, Mayer G. Increased renal Versican expression is associated with progression of chronic kidney disease. PLoS ONE. 2012;7(9):e44891. doi: 10.1371/journal.pone.0044891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stokes MB, Hudkins KL, Zaharia V, Taneda S, Alpers CE. Up-regulation of extracellular matrix proteoglycans and collagen type I in human crescentic glomerulonephritis. Kidney Int. 2001;59(2):532–42. doi: 10.1046/j.1523-1755.2001.059002532.x. [DOI] [PubMed] [Google Scholar]
- 93. Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2010;339(1):237–46. doi: 10.1007/s00441-009-0821-y. [DOI] [PubMed] [Google Scholar]
- 94. Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360(6402):361–4. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 95. Chen S, Birk DE. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013;280(10):2120–37. doi: 10.1111/febs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283(31):21305–9. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schaefer L, Grone HJ, Raslik I, Robenek H, Ugorcakova J, Budny S, Schaefer RM, Kresse H. Small proteoglycans of normal adult human kidney: distinct expression patterns of decorin, biglycan, fibromodulin, and lumican. Kidney Int. 2000;58(4):1557–68. doi: 10.1046/j.1523-1755.2000.00317.x. [DOI] [PubMed] [Google Scholar]
- 98. Stokes MB, Holler S, Cui Y, Hudkins KL, Eitner F, Fogo A, Alpers CE. Expression of decorin, biglycan, and collagen type I in human renal fibrosing disease. Kidney Int. 2000;57(2):487–98. doi: 10.1046/j.1523-1755.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 99. Nastase MV, Janicova A, Roedig H, Hsieh LT, Wygrecka M, Schaefer L. Small leucine-rich proteoglycans in renal inflammation: two sides of the coin. J Histochem Cytochem. 2018;66(4):261–72. doi: 10.1369/0022155417738752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289(51):35237–45. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schaefer L, Mihalik D, Babelova A, Krzyzankova M, Grone HJ, Iozzo RV, Young MF, Seidler DG, Lin G, Reinhardt DP, Schaefer RM. Regulation of fibrillin-1 by biglycan and decorin is important for tissue preservation in the kidney during pressure-induced injury. Am J Pathol. 2004;165(2):383–96. doi: 10.1016/S0002-9440(10)63305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schaefer L. Small leucine-rich proteoglycans in kidney disease. J Am Soc Nephrol. 2011;22(7):1200–7. doi: 10.1681/ASN.2010050570. [DOI] [PubMed] [Google Scholar]
- 104. Poluzzi C, Nastase MV, Zeng-Brouwers J, Roedig H, Hsieh LT, Michaelis JB, Buhl EM, Rezende F, Manavski Y, Bleich A, Boor P, Brandes RP, Pfeilschifter J, Stelzer EHK, Münch C, Dikic I, Brandts C, Iozzo RV, Wygrecka M, Schaefer L. Biglycan evokes autophagy in macrophages via a novel CD44/Toll-like receptor 4 signaling axis in ischemia/reperfusion injury. Kidney Int. 2019;95:540–62. doi: 10.1016/j.kint.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 105. Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol. 2012;302(11):F1351–61. doi: 10.1152/ajprenal.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ahmed AK, Haylor JL, El Nahas AM, Johnson TS. Localization of matrix metalloproteinases and their inhibitors in experimental progressive kidney scarring. Kidney Int. 2007;71(8):755–63. doi: 10.1038/sj.ki.5002108. [DOI] [PubMed] [Google Scholar]
- 107. Kato T, Hagiyama M, Ito A. Renal ADAM10 and 17: their physiological and medical meanings. Front Cell Dev Biol. 2018;6:153. doi: 10.3389/fcell.2018.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl. 2017;147:1–73. doi: 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Catania JM, Chen G, Parrish AR. Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol-Renal. 2007;292(3):F905–11. doi: 10.1152/ajprenal.00421.2006. [DOI] [PubMed] [Google Scholar]
- 110. Inanc S, Keles D, Oktay G. An improved collagen zymography approach for evaluating the collagenases MMP-1, MMP-8, and MMP-13. Biotechniques. 2017;63(4):174–80. doi: 10.2144/000114597. [DOI] [PubMed] [Google Scholar]
- 111. Turck J, Pollock AS, Lee LK, Marti HP, Lovett DH. Matrix metalloproteinase 2 (gelatinase A) regulates glomerular mesangial cell proliferation and differentiation. J Biol Chem. 1996;271(25):15074–83. [DOI] [PubMed] [Google Scholar]
- 112. Xu X, Jackson PL, Tanner S, Hardison MT, Abdul Roda M, Blalock JE, Gaggar A. A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation. PLoS ONE. 2011;6(1):e15781. doi: 10.1371/journal.pone.0015781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhou D, Tian Y, Sun L, Zhou LL, Xiao LX, Tan RJ, Tian J, Fu H, Hou FF, Liu Y. Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J Am Soc Nephrol. 2017;28(2):598–611. doi: 10.1681/Asn.2016030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kassiri Z, Oudit GY, Kandalam V, Awad A, Wang XH, Ziou XH, Maeda N, Herzenberg AM, Scholey JW. Loss of TIMP3 enhances interstitial nephritis and fibrosis. J Am Soc Nephrol. 2009;20(6):1223–35. doi: 10.1681/Asn.2008050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kim H, Oda T, Lopez-Guisa J, Wing D, Edwards DR, Soloway PD, Eddy AA. TIMP-1 deficiency does not attenuate interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2001;12(4):736–48. [DOI] [PubMed] [Google Scholar]
- 116. Wang ZC, Famulski K, Lee J, Das SK, Wang XH, Halloran P, Oudit GY, Kassiri Z. TIMP2 and TIMP3 have divergent roles in early renal tubulointerstitial injury. Kidney Int. 2014;85(1):82–93. doi: 10.1038/ki.2013.225. [DOI] [PubMed] [Google Scholar]
- 117. Kelwick R, Desanlis I, Wheeler GN, Edwards DR. The ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family. Genome Biol. 2015;16:113. doi: 10.1186/s13059-015-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mittaz L, Ricardo S, Martinez G, Kola I, Kelly DJ, Little MH, Hertzog PJ, Pritchard MA. Neonatal calyceal dilation and renal fibrosis resulting from loss of Adamts-1 in mouse kidney is due to a developmental dysgenesis. Nephrol Dial Transpl. 2005;20(2):419–23. doi: 10.1093/ndt/gfh603. [DOI] [PubMed] [Google Scholar]
- 119. Armutcu F, Demircan K, Yildirim U, Namuslu M, Yagmurca M, Celik H. Hypoxia causes important changes of extracellular matrix biomarkers and ADAMTS proteinases in the adriamycin-induced renal fibrosis model. Nephrology (Carlton). Epub 2019 Feb 4. doi: 10.1111/nep.13572. [DOI] [PubMed] [Google Scholar]
- 120. Lin CH, Chen J, Zhang Z, Johnson GV, Cooper AJ, Feola J, Bank A, Shein J, Ruotsalainen HJ, Pihlajaniemi TA, Goligorsky MS. Endostatin and transglutaminase 2 are involved in fibrosis of the aging kidney. Kidney Int. 2016;89(6):1281–92. doi: 10.1016/j.kint.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Johnson TS, Griffin M, Thomas GL, Skill J, Cox A, Yang B, Nicholas B, Birckbichler PJ, Muchaneta-Kubara C, Meguid El, Nahas A. The role of transglutaminase in the rat subtotal nephrectomy model of renal fibrosis. J Clin Invest. 1997;99(12):2950–60. doi: 10.1172/JCI119490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Shweke N, Boulos N, Jouanneau C, Vandermeersch S, Melino G, Dussaule JC, Chatziantoniou C, Ronco P, Boffa JJ. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-beta activation and cell infiltration. Am J Pathol. 2008;173(3):631–42. doi: 10.2353/ajpath.2008.080025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gautieri A, Redaelli A, Buehler MJ, Vesentini S. Age- and diabetes-related nonenzymatic crosslinks in collagen fibrils: candidate amino acids involved in advanced glycation end-products. Matrix Biol. 2014;34:89–95. doi: 10.1016/j.matbio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 124. Brings S, Zhang S, Choong YS, Hogl S, Middleditch M, Kamalov M, Brimble MA, Gong D, Cooper GJ. Diabetes-induced alterations in tissue collagen and carboxymethyllysine in rat kidneys: association with increased collagen-degrading proteinases and amelioration by Cu(II)-selective chelation. Biochim Biophys Acta. 2015;1852(8):1610–8. doi: 10.1016/j.bbadis.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 125. Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Bio. 2009;10(1):21–33. doi:10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 126. Borza CM, Chen X, Zent R, Pozzi A. Cell receptor-basement membrane interactions in health and disease: A kidney-centric view. Curr Top Membr. 2015;76:231–53. doi:10.1016/bs.ctm.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8(5):215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pozzi A, Zent R. Integrins in kidney disease. J Am Soc Nephrol. 2013;24(7):1034–9. doi: 10.1681/ASN.2013010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kreidberg JA, Symons JM. Integrins in kidney development, function, and disease. Am J Physiol Renal Physiol. 2000;279(2):F233–42. doi: 10.1152/ajprenal.2000.279.2.F233. [DOI] [PubMed] [Google Scholar]
- 130. Sawada K, Toyoda M, Kaneyama N, Shiraiwa S, Moriya H, Miyatake H, Tanaka E, Yamamoto N, Miyauchi M, Kimura M, Wada T, Fukagawa M. Upregulation of α3β1-integrin in podocytes in early-stage diabetic nephropathy. J Diabetes Res. 2016;2016:9265074. doi: 10.1155/2016/9265074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol. 2006;175(1):33–9. doi: 10.1083/jcb.200603073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cosgrove D, Rodgers K, Meehan D, Miller C, Bovard K, Gilroy A, Gardner H, Kotelianski V, Gotwals P, Amatucci A, Kalluri R. Integrin alpha1beta1 and transforming growth factor-beta1 play distinct roles in alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am J Pathol. 2000;157(5):1649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Korhonen M, Ylanne J, Laitinen L, Virtanen I. The alpha 1-alpha 6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J Cell Biol. 1990;111(3):1245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Patey N, Halbwachs-Mecarelli L, Droz D, Lesavre P, Noel LH. Distribution of integrin subunits in normal human kidney. Cell Adhes Commun. 1994;2(2):159–67. [DOI] [PubMed] [Google Scholar]
- 135. Rahilly MA, Fleming S. Differential expression of integrin alpha chains by renal epithelial cells. J Pathol. 1992;167(3):327–34. doi: 10.1002/path.1711670311. [DOI] [PubMed] [Google Scholar]
- 136. Lee K, Boctor S, Barisoni LM, Gusella GL. Inactivation of integrin-beta1 prevents the development of polycystic kidney disease after the loss of polycystin-1. J Am Soc Nephrol. 2015;26(4):888–95. doi: 10.1681/ASN.2013111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Liu Z, Xu B, Nameta M, Zhang Y, Magdeldin S, Yoshida Y, Yamamoto K, Fujinaka H, Yaoita E, Tasaki M, Nakagawa Y, Saito K, Takahashi K, Yamamoto T. Profiling of kidney vascular endothelial cell plasma membrane proteins by liquid chromatography-tandem mass spectrometry. Clin Exp Nephrol. 2013;17(3):327–37. doi: 10.1007/s10157-012-0708-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Haas CS, Amann K, Schittny J, Blaser B, Muller U, Hartner A. Glomerular and renal vascular structural changes in alpha8 integrin-deficient mice. J Am Soc Nephrol. 2003;14(9):2288–96. [DOI] [PubMed] [Google Scholar]
- 139. Munger JS, Huang XZ, Kawakatsu H, Griffiths MJD, Dalton SL, Wu JF, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–28. doi: 10.1016/S0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 140. Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CF, 3rd, Sheppard D, Gardner HA, Violette SM. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol. 2007;170(1):110–25. doi: 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, Griggs DW, Prinsen MJ, Maher JJ, Iredale JP, Lacy-Hulbert A, Adams RH, Sheppard D. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19(12):1617–24. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wei C, Moller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14(1):55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 143. Prakoura N, Kavvadas P, Kormann R, Dussaule JC, Chadjichristos CE, Chatziantoniou C. NFkappaB-induced periostin activates integrin-beta3 signaling to promote renal injury in GN. J Am Soc Nephrol. 2017;28(5):1475–90. doi: 10.1681/ASN.2016070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Afratis NA, Nikitovic D, Multhaupt HA, Theocharis AD, Couchman JR, Karamanos NK. Syndecans—key regulators of cell signaling and biological functions. FEBS J. 2017;284(1):27–41. doi: 10.1111/febs.13940. [DOI] [PubMed] [Google Scholar]
- 145. Chung H, Multhaupt HAB, Oh ES, Couchman JR. Minireview: syndecans and their crucial roles during tissue regeneration. Febs Lett. 2016;590(15):2408–17. doi: 10.1002/1873-3468.12280. [DOI] [PubMed] [Google Scholar]
- 146. Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell. 1994;5(7):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Scarpellini A, Huang L, Burhan I, Schroeder N, Funck M, Johnson TS, Verderio EA. Syndecan-4 knockout leads to reduced extracellular transglutaminase-2 and protects against tubulointerstitial fibrosis. J Am Soc Nephrol. 2014;25(5):1013–27. doi: 10.1681/ASN.2013050563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Borza CM, Pozzi A. The role of cell-extracellular matrix interactions in glomerular injury. Exp Cell Res. 2012;318(9):1001–10. doi: 10.1016/j.yexcr.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Flamant M, Placier S, Rodenas A, Curat CA, Vogel WF, Chatziantoniou C, Dussaule JC. Discoidin domain receptor 1 null mice are protected against hypertension-induced renal disease. J Am Soc Nephrol. 2006;17(12):3374–81. doi: 10.1681/ASN.2006060677. [DOI] [PubMed] [Google Scholar]
- 150. Gross O, Girgert R, Beirowski B, Kretzler M, Kang HG, Kruegel J, Miosge N, Busse AC, Segerer S, Vogel WF, Müller GA, Weber M. Loss of collagen-receptor DDR1 delays renal fibrosis in hereditary type IV collagen disease. Matrix Biol. 2010;29(5):346–56. doi: 10.1016/j.matbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 151. Kopp JB. Dystroglycan in the molecular diagnosis of the podocytopathies. Clin J Am Soc Nephrol. 2009;4(11):1696–8. doi: 10.2215/CJN.06910909. [DOI] [PubMed] [Google Scholar]
- 152. Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P. Distribution of dystroglycan in normal adult mouse tissues. J Histochem Cytochem. 1998;46(4):449–57. doi: 10.1177/002215549804600404. [DOI] [PubMed] [Google Scholar]
- 153. Haenggi T, Schaub MC, Fritschy JM. Molecular heterogeneity of the dystrophin-associated protein complex in the mouse kidney nephron: differential alterations in the absence of utrophin and dystrophin. Cell Tissue Res. 2005;319(2):299–313. doi: 10.1007/s00441-004-0999-y. [DOI] [PubMed] [Google Scholar]
- 154. Jarad G, Pippin JW, Shankland SJ, Kreidberg JA, Miner JH. Dystroglycan does not contribute significantly to kidney development or function, in health or after injury. Am J Physiol-Renal. 2011;300(3):F811–20. doi: 10.1152/ajprenal.00725.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Liptak P, Ivanyi B. Primer: histopathology of calcineurin-inhibitor toxicity in renal allografts. Nat Clin Pract Nephrol. 2006;2(7):398–404; quiz following 404. doi: 10.1038/ncpneph0225. [DOI] [PubMed] [Google Scholar]
- 156. Farris AB, Colvin RB. Renal interstitial fibrosis: mechanisms and evaluation. Curr Opin Nephrol Hypertens. 2012;21(3):289–300. doi: 10.1097/MNH.0b013e3283521cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gerth J, Busch M, Illner N, Traut M, Grone HJ, Wolf G. Are tissue samples from two different anatomical areas of the kidney necessary for adequate diagnosis? Clin Nephrol. 2010;74(4):258–65. [DOI] [PubMed] [Google Scholar]
- 158. Azancot MA, Moreso F, Salcedo M, Cantarell C, Perello M, Torres IB, Montero A, Trilla E, Sellarés J, Morote J, Seron D. The reproducibility and predictive value on outcome of renal biopsies from expanded criteria donors. Kidney Int. 2014;85(5):1161–8. doi: 10.1038/ki.2013.461. [DOI] [PubMed] [Google Scholar]
- 159. Furness PN, Taub N; Convergence of European Renal Transplant Pathology Assessment Procedures Project. International variation in the interpretation of renal transplant biopsies: report of the CERTPAP Project. Kidney Int. 2001;60(5):1998–2012. doi: 10.1046/j.1523-1755.2001.00030.x. [DOI] [PubMed] [Google Scholar]
- 160. Furness PN, Taub N, Assmann KJM, Banfi G, Cosyns JP, Dorman AM, Hill CM, Kapper SK, Waldherr R, Laurinavicius A, Marcussen N, Martins AP, Nogueira M, Regele H, Seron D, Carrera M, Sund S, Taskinen EI, Paavonen T, Tihomirova T, Rosenthal R. International variation in histologic grading is large, and persistent feedback does not improve reproducibility. Am J Surg Pathol. 2003;27(6):805–10. doi: 10.1097/00000478-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 161. Barisoni L, Gimpel C, Kain R, Laurinavicius A, Bueno G, Zeng CH, Liu Z, Schaefer F, Kretzler M, Holzman LB, Hewitt SM. Digital pathology imaging as a novel platform for standardization and globalization of quantitative nephropathology. Clin Kidney J. 2017;10(2):176–87. doi: 10.1093/ckj/sfw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Barisoni L, Hodgin JB. Digital pathology in nephrology clinical trials, research, and pathology practice. Curr Opin Nephrol Hypertens. 2017;26(6):450–9. doi: 10.1097/MNH.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Tey WK, Kuang YC, Ooi MP, Khoo JJ. Automated quantification of renal interstitial fibrosis for computer-aided diagnosis: a comprehensive tissue structure segmentation method. Comput Methods Programs Biomed. 2018;155:109–20. doi: 10.1016/j.cmpb.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 164. Ranjit S, Dobrinskikh E, Montford J, Dvornikov A, Lehman A, Orlicky DJ, Nemenoff R, Gratton E, Levi M, Furgeson S. Label-free fluorescence lifetime and second harmonic generation imaging microscopy improves quantification of experimental renal fibrosis. Kidney Int. 2016;90(5):1123–8. doi: 10.1016/j.kint.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Torres R, Velazquez H, Chang JJ, Levene MJ, Moeckel G, Desir GV, Safirstein R. Three-dimensional morphology by multiphoton microscopy with clearing in a model of cisplatin-induced CKD. J Am Soc Nephrol. 2016;27(4):1102–12. doi: 10.1681/ASN.2015010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Peti-Peterdi J. A practical new way to measure kidney fibrosis. Kidney Int. 2016;90(5):941–2. doi: 10.1016/j.kint.2016.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ghoul BE, Squalli T, Servais A, Elie C, Meas-Yedid V, Trivint C, Vanmassenhove J, Grünfeld JP, Olivo-Marin JC, Thervet E, Noël LH, Prié D, Fakhouri F. Urinary procollagen III aminoterminal propeptide (PIIINP): a fibrotest for the nephrologist. Clin J Am Soc Nephrol. 2010;5(2):205–10. doi: 10.2215/CJN.06610909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ix JH, Biggs ML, Mukamal K, Djousse L, Siscovick D, Tracy R, Katz R, Delaney JA, Chaves P, Rifkin DE, Hughes-Austin JM, Garimella PS, Sarnak MJ, Shlipak MG, Kizer JR. Urine collagen fragments and CKD progression-the cardiovascular health study. J Am Soc Nephrol. 2015;26(10):2494–503. doi: 10.1681/ASN.2014070696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Papasotiriou M, Genovese F, Klinkhammer BM, Kunter U, Nielsen SH, Karsdal MA, Floege J, Boor P. Serum and urine markers of collagen degradation reflect renal fibrosis in experimental kidney diseases. Nephrol Dial Transpl. 2015;30(7):1112–21. doi: 10.1093/ndt/gfv063. [DOI] [PubMed] [Google Scholar]
- 170. Hijmans RS, Rasmussen DGK, Yazdani S, Navis G, van Goor H, Karsdal MA, Genovese F, van den Born J. Urinary collagen degradation products as early markers of progressive renal fibrosis. J Transl Med. 2017;15:63. doi: 10.1186/s12967-017-1163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Rasmussen DGK, Nielsen PM, Kasab-Oglo OY, Nielsen SH, Kierulf-Lassen C, Karsdal MA, Genovese F, Nørregaard R. A non-invasive biomarker of type III collagen degradation reflects ischaemia reperfusion injury in rats. Nephrol Dial Transpl. Epub 2018 Dec 7. doi: 10.1093/ndt/gfy345. [DOI] [PubMed] [Google Scholar]
- 172. Genovese F, Boor P, Papasotiriou M, Leeming DJ, Karsdal MA, Floege J. Turnover of type III collagen reflects disease severity and is associated with progression and microinflammation in patients with IgA nephropathy. Nephrol Dial Transpl. 2016;31(3):472–9. doi: 10.1093/ndt/gfv301. [DOI] [PubMed] [Google Scholar]
- 173. Stribos EGD, Nielsen SH, Brix S, Karsdal MA, Seelen MA, van Goor H, Bakker SJL, Olinga P, Mutsaers HAM, Genovese F. Non-invasive quantification of collagen turnover in renal transplant recipients. PLoS ONE. 2017;12(4):e0175898. doi: 10.1371/journal.pone.0175898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Schanstra JP, Zurbig P, Alkhalaf A, Argiles A, Bakker SJ, Beige J, Bilo HJ, Chatzikyrkou C, Dakna M, Dawson J, Delles C, Haller H, Haubitz M, Husi H, Jankowski J, Jerums G, Kleefstra N, Kuznetsova T, Maahs DM, Menne J, Mullen W, Ortiz A, Persson F, Rossing P, Ruggenenti P, Rychlik I, Serra AL, Siwy J, Snell-Bergeon J, Spasovski G, Staessen JA, Vlahou A, Mischak H, Vanholder R. Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol. 2015;26(8):1999–2010. doi: 10.1681/ASN.2014050423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Araki S, Haneda M, Koya D, Isshiki K, Kume S, Sugimoto T, Kawai H, Nishio Y, Kashiwagi A, Uzu T, Maegawa H. Association between urinary type IV collagen level and deterioration of renal function in type 2 diabetic patients without overt proteinuria. Diabetes Care. 2010;33(8):1805–10. doi: 10.2337/dc10-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Morita M, Hanai K, Uchigata Y. Urinary type IV collagen as a predictor for the incidence of microalbuminuria in young patients with Type 1 diabetes. Diabet Med. 2014;31(2):213–18. doi: 10.1111/dme.12317. [DOI] [PubMed] [Google Scholar]
- 177. Kishi F, Nagai K, Takamatsu N, Tominaga T, Tamaki M, Shibata E, Murakami T, Kishi S, Abe H, Koezuka Y, Minagawa N, Ichien G, Doi T. Urinary type IV collagen excretion is involved in the decline in estimated glomerular filtration rate in the Japanese general population without diabetes: a 5-year observational study. PLoS ONE. 2018;13(4):e0195523. doi: 10.1371/journal.pone.0195523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Leeming DJ, Karsdal MA, Rasmussen LM, Scholze A, Tepel M. Association of systemic collagen type IV formation with survival among patients undergoing hemodialysis. PLoS ONE. 2013;8(8):e71050. doi: 10.1371/journal.pone.0071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Sun S, Henriksen K, Karsdal MA, Byrjalsen I, Rittweger J, Armbrecht G, Belavy DL, Felsenberg D, Nedergaard AF. Collagen type III and VI turnover in response to long-term immobilization. PLoS ONE. 2015;10(12):e0144525. doi: 10.1371/journal.pone.0144525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Fenton A, Jesky MD, Ferro CJ, Sorensen J, Karsdal MA, Cockwell P, Genovese F. Serum endotrophin, a type VI collagen cleavage product, is associated with increased mortality in chronic kidney disease. PLoS ONE. 2017;12(4):e0175200. doi: 10.1371/journal.pone.0175200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Rasmussen DGK, Fenton A, Jesky M, Ferro C, Boor P, Tepel M, Karsdal MA, Genovese F, Cockwell P. Urinary endotrophin predicts disease progression in patients with chronic kidney disease. Sci Rep. 2017;7(1):17328. doi: 10.1038/s41598-017-17470-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Nielsen SH, Rasmussen DGK, Brix S, Fenton A, Jesky M, Ferro CJ, Karsdal M, Genovese F, Cockwell P. A novel biomarker of laminin turnover is associated with disease progression and mortality in chronic kidney disease. PLoS ONE. 2018;13(10):e0204239. doi: 10.1371/journal.pone.0204239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Baues M, Dasgupta A, Ehling J, Prakash J, Boor P, Tacke F, Kiessling F, Lammers T. Fibrosis imaging: current concepts and future directions. Adv Drug Deliv Rev. 2017;121:9–26. doi: 10.1016/j.addr.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Correas JM, Anglicheau D, Joly D, Gennisson JL, Tanter M, Helenon O. Ultrasound-based imaging methods of the kidney-recent developments. Kidney Int. 2016;90(6):1199–210. doi: 10.1016/j.kint.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 185. Boor P, Floege J. Renal allograft fibrosis: biology and therapeutic targets. Am J Transplant. 2015;15(4):863–86. doi: 10.1111/ajt.13180. [DOI] [PubMed] [Google Scholar]
- 186. Sun Q, Baues M, Klinkhammer BM, Ehling J, Djudjaj S, Drude NI, Daniel C, Amann K, Kramann R, Kim H, Saez-Rodriguez J, Weiskirchen R, Onthank DC, Botnar RM, Kiessling F, Floege J, Lammers T, Boor P. Elastin imaging enables non-invasive staging and treatment monitoring of kidney fibrosis. Sci Transl Med. 2019;11:eaat4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 188. Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Lopes SM, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526(7574):564–8. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 189. Takasato M, Er PX, Chiu HS, Little MH. Generation of kidney organoids from human pluripotent stem cells. Nat Protoc. 2016;11(9):1681–92. doi: 10.1038/nprot.2016.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Destefani AC, Sirtoli GM, Nogueira BV. Advances in the knowledge about kidney decellularization and repopulation. Front Bioeng Biotechnol. 2017;5:34. doi: 10.3389/fbioe.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. He M, Callanan A, Lagaras K, Steele JAM, Stevens MM. Optimization of SDS exposure on preservation of ECM characteristics in whole organ decellularization of rat kidneys. J Biomed Mater Res B Appl Biomater. 2017;105(6):1352–60. doi: 10.1002/jbm.b.33668. [DOI] [PubMed] [Google Scholar]
- 194. Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19(5):646–51. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Huang Y, Zhang XF, Gao G, Yonezawa T, Cui X. 3D bioprinting and the current applications in tissue engineering. Biotechnol J. 2017;12(8):1600734. doi: 10.1002/biot.201600734. [DOI] [PubMed] [Google Scholar]
- 196. Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, Kim DH, Cho DW. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, Lewis JA. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep. 2016;6:34845. doi: 10.1038/srep34845. [DOI] [PMC free article] [PubMed] [Google Scholar]