This cohort study assesses the incidence of and risk factors and outcomes associated with breast cancer recurrence of the nipple-areola complex among patients with invasive breast cancer who underwent nipple-sparing mastectomy and immediate breast reconstruction.
Key Points
Question
What are the incidence of and risk factors and long-term outcomes associated with breast cancer recurrence at the nipple-areola complex after nipple-sparing mastectomy for invasive breast cancer?
Findings
In this cohort study of 962 breasts from 944 patients who underwent nipple-sparing mastectomy and immediate breast reconstruction for invasive breast cancer, 39 cases of cancer recurrence at the nipple-areola complex were identified as the first event; multifocality or multicentricity, negative hormone receptor and ERBB2 (formerly HER2 or HER2/neu)–positive subtype, high histologic grade, and extensive intraductal component were independently associated with recurrence. Patients with and without recurrence of cancer at the nipple-areola complex as the first event did not differ significantly with regard to distant metastasis-free or overall survival.
Meaning
The findings suggest that biological features of the breast cancer tumor should be considered when planning nipple-sparing mastectomy.
Abstract
Importance
The main concern associated with nipple-sparing mastectomy (NSM) is the risk of local breast cancer recurrence at the retained nipple-areola complex (NAC) consequent to occult nipple involvement. Long-term follow-up data regarding the oncologic safety of modern therapeutic NSM in terms of cancer recurrence at the NAC and survival are limited.
Objective
To assess the incidence, risk factors, treatment, and long-term outcomes associated with cancer recurrence at the NAC in a large series of patients with invasive breast cancer who underwent NSM and immediate breast reconstruction.
Design, Setting, and Participants
In this retrospective cohort study at a single institution (Asan Medical Center) in Seoul, Republic of Korea, 962 breasts from 944 patients who underwent NSM and immediate breast reconstruction for invasive breast cancer were analyzed between March 3, 2003, and December 31, 2015. Patients who underwent neoadjuvant systemic therapy or palliative surgery were excluded. Data analysis was performed from June 4, 2018, to August 31, 2018.
Main Outcomes and Measures
Univariate and multivariate Cox proportional hazards regression models were used to analyze the association between clinicopathologic variables and cancer recurrence at the NAC. To evaluate the association between cancer recurrence at the NAC and prognosis, distant metastasis-free survival, and overall survival were estimated using the Kaplan-Meier method and compared using the log-rank test.
Results
Among the 944 study patients (median age at diagnosis, 43 years [range, 23-67 years]) during a median follow-up of 85 months (range, 14–185 months), 39 cases (4.1%) of cancer recurrence at the NAC were identified as the first event after NSM. The 5-year cumulative incidence of cancer recurrence at the NAC was 3.5% (n = 34). In multivariate analyses, multifocality or multicentricity (hazard ratio [HR], 3.309; 95% CI, 1.501-7.294; P = .003), negative hormone receptor or ERBB2 (formerly HER2 or HER2/neu)–positive subtype (HR, 3.051; 95% CI, 1.194-7.796; P = .02), high histologic grade (HR, 2.641; 95% CI, 1.132-6.160; P = .03), and extensive intraductal component (HR, 3.338; 95% CI, 1.262-8.824; P = .02) were independently associated with cancer recurrence at the NAC after NSM. All 39 recurrent cases involved wide local excision. Patients with and without cancer recurrence at the NAC as the first event did not differ significantly with regard to distant metastasis-free survival (P = .95) or overall survival (P = .21). The 10-year distant metastasis-free survival rates were 89.3% among patients with cancer recurrence at the NAC and 94.3% among patients without recurrence. The 10-year overall survival rates were 100% among patients with cancer recurrence at the NAC and 94.5% among those without recurrence.
Conclusions and Relevance
Patients had a low incidence of cancer recurrence at the NAC after NSM and immediate breast reconstruction in this study. The findings suggest that multifocal or multicentric disease, hormone receptor–negative/ERBB2–positive subtype, high histologic grade, and positive extensive intraductal component should be considered before determining the NSM procedure.
Introduction
Breast-conserving surgery is the most frequently performed surgical procedure for breast cancer treatment. However, 34.0% of patients with breast cancer in the Republic of Korea require mastectomy.1 Nipple-sparing mastectomy (NSM), which evolved from skin-sparing mastectomy, is characterized by the preservation of the entire nipple-areola complex (NAC) and breast skin envelope despite removal of the mammary tissue.
Multiple prospective and retrospective studies2,3,4 on NSM have demonstrated the oncologic and surgical safety of this technique as well as the superior aesthetic outcomes and improved quality of life achieved when NSM is combined with immediate breast reconstruction. Present National Comprehensive Cancer Network guidelines state that NSM is an acceptable surgical option for carefully selected patients with breast cancer.5 Nevertheless, the application of NSM for breast cancer remains controversial because of the limited available data, including long-term follow-up data, from accurate evaluations of modern therapeutic NSM. Currently, the main concern associated with NSM is the risk of local cancer recurrence at the retained NAC consequent to occult nipple involvement. Because increasing numbers of patients with breast cancer are selecting NSM,6,7 it is important to identify the incidence of cancer recurrence at the NAC after NSM, describe the associated factors, and determine its association with prognosis. Previous studies8,9,10,11,12 have reported cancer recurrence incidence rates at the NAC from 0% to 3.7% after NSM. However, most of those series included heterogeneous populations with both invasive and noninvasive breast cancer as well as short follow-up durations. Furthermore, the factors associated with cancer recurrence at the NAC and its association with distant metastasis and survival are not yet well defined to our knowledge.
The present study, which included long-term follow-up, assessed the incidence and treatment of cancer recurrence at the NAC as well as risk factors and outcomes associated with cancer recurrence at the NAC in a large series of patients with invasive breast cancer who underwent NSM and immediate breast reconstruction at a single institution in the Republic of Korea.
Methods
Study Population
This cohort study included 19 964 patients with breast cancer who underwent surgical treatment at the Asan Medical Center in Seoul, Korea, from March 3, 2003, through December 31, 2015. We retrospectively analyzed 962 breasts from 944 of these patients who underwent NSM and immediate breast reconstruction for invasive breast cancer. Patients who underwent neoadjuvant systemic therapy or palliative surgery were excluded from this study. The study was approved by the institutional review board of Asan Medical Center, Seoul, Korea. Because of the retrospective nature of the study, the requirement for informed consent was waived. Data analysis was performed from June 4, 2018, to August 31, 2018.
Indications and Surgical Technique for NSM
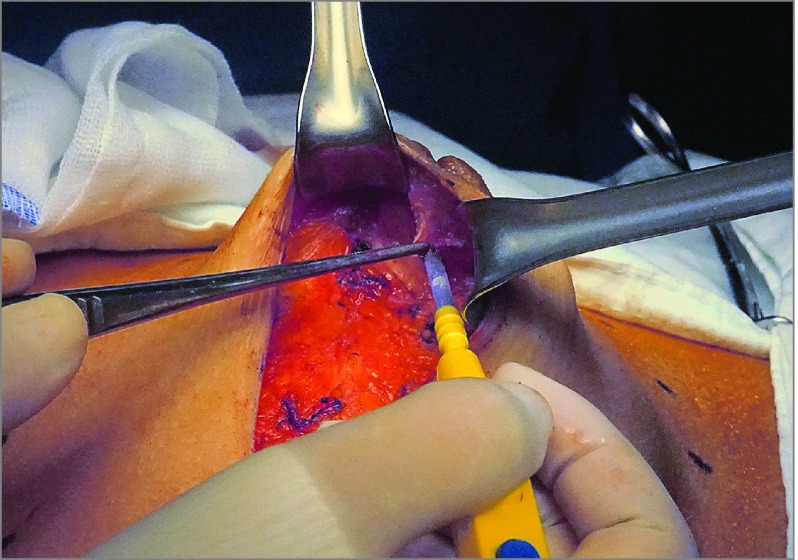
The indications for NSM were any stage, tumor size, and tumor-areola distance with indications for mastectomy. Patients with a clinically normal NAC and no skin involvement were offered the option of NSM. The NSM was performed as previously described.2 The most commonly used incision type in this cohort was lateral radial incision (85.9%), followed by periareolar incision with lateral extension (11.1%). In addition, lateral inframammary fold, periareolar, reduction or mastopexy, and previous scar incisions accounted for 3.0% of the total cases. In all cases, a retroareolar frozen section was collected and examined intraoperatively. The subdermal glandular tissue was undermined in the retroareolar area, leaving 1 to 2 mm of intact dermis. Next, a thin layer of glandular tissue was collected under the areola for review after a frozen section was obtained (Figure 1). The NAC was preserved if the shape, color, and palpated features of the nipple were normal and when the NAC ducts were confirmed to be tumor free in intraoperatively collected frozen biopsy samples. Sentinel lymph node biopsy and/or axillary lymph node dissection were performed. All patients underwent immediate breast reconstruction through autologous or prosthetic methods by plastic surgeons (J.-S.E.). Adjuvant systemic treatment was performed according to the contemporary recommendations of the St Gallen Consensus Conference13 and National Comprehensive Cancer Network5 guidelines.
Figure 1. Intraoperative Frozen Section Examination of the Retroareolar Margin.
Characteristics
Clinicopathologic and survival data were obtained from the Asan Medical Center–Breast Cancer Center database. The age at diagnosis, type of surgery, type of adjuvant systemic treatment, tumor stage, node stage, histologic grade, multifocality or multicentricity, lymphovascular invasion, subtype, tumor-nipple distance, extensive intraductal component, and tumor histologic type were carefully reviewed. The extensive intraductal component was considered to be positive when it comprised more than 20% of the tumor size. Tumor and node staging were conducted according to the 7th edition of the American Joint Committee on Cancer Cancer Staging Manual.14
Follow-up
The patients were regularly followed up postoperatively every 3 to 6 months for the first 5 years and annually thereafter. Recurrence and metastasis were identified based on the results of clinical examination, mammography, chest radiography, and tumor marker (cancer antigen 15-3 [CA 15–3]) measurements, which were performed during every follow-up visit. Abnormal clinical findings may have been evaluated through further studies, including computed tomography of the chest, bone scan, and liver ultrasonography. Punch needle or incisional biopsy was performed to evaluate suspected lesions in the NAC, and cancer recurrence at the NAC was defined as a biopsy-proven cancer found in the tissue of the NAC. Patients who failed to present for examination were contacted via telephone to confirm that they were alive.
Statistical Analysis
The primary end point was cancer recurrence at the NAC as the first event. Patients with initial recurrences at other sites were excluded from the recurrence group. The time to recurrence or metastasis was measured from the date of surgery until occurrence of the event. Distant metastasis-free survival was defined as the interval from the date of surgery to the first occurrence of distant metastasis, whereas overall survival was defined as the interval from the date of surgery to death. Univariate and multivariate Cox proportional hazards regression models were used to analyze the association between clinicopathologic variables and cancer recurrence at the NAC and to identify the potential risk factors associated with cancer recurrence at the NAC after NSM. To evaluate the association of cancer recurrence at the NAC with prognosis, distant metastasis-free survival and overall survival were estimated using the Kaplan-Meier method and compared using the log-rank test. All statistical analyses were performed using SPSS, version 24.0 for Windows (IBM Corporation). A 2-sided P < .05 indicated statistical significance.
Results
Patient and Treatment Characteristics
The clinicopathologic characteristics of the 944 study patients are shown in Table 1. The median age of patients at diagnosis was 43 years (range, 23-67 years). Most of the reconstruction procedures used pedicle or free transverse rectus abdominis myocutaneous flaps (628 of 962 breasts [65.3%]). Of the 944 patients, 498 (52.8%) received adjuvant chemotherapy; 750 (79.5%), adjuvant hormonal therapy, and 97 (10.1%), adjuvant radiotherapy.
Table 1. Clinicopathologic Characteristics of the Patients and Univariate Analyses of Factors Associated With Cancer Recurrence at the NAC.
| Variable | Patients, No. (%) (n = 962) | Cancer Recurrence at the NAC, No. (%) | 5-y Cancer Recurrence at the NAC, No. | 5-y Cumulative Incidence, % | P Value |
|---|---|---|---|---|---|
| Age, y | |||||
| <50 | 774 (80.5) | 36 (4.7) | 32 | 4.1 | .07 |
| ≥50 | 188 (19.5) | 3 (1.6) | 2 | 1.1 | |
| Multifocality or multicentricity | |||||
| Yes | 509 (52.9) | 30 (5.9) | 27 | 5.3 | .003 |
| No | 453 (47.1) | 9 (2.0) | 7 | 1.6 | |
| No. of positive lymph nodes | |||||
| 0 | 657 (68.3) | 35 (5.3) | 30 | 4.6 | .09 |
| 1-3 | 234 (24.3) | 4 (1.7) | 4 | 1.7 | |
| ≥4 | 71 (7.4) | 0 | 0 | 0 | |
| Tumor-nipple distance, cm | |||||
| >1 | 584 (60.7) | 18 (3.1) | 15 | 2.6 | .06 |
| ≤1 | 364 (37.8) | 21 (5.8) | 19 | 5.2 | |
| Unknown | 14 (1.5) | NA | NA | NA | |
| Histologic type | |||||
| Ductal | 841 (87.4) | 38 (4.5) | 33 | 3.9 | .54 |
| Lobular | 65 (6.8) | 0 | 0 | 0 | |
| Mixed | 25 (2.6) | 0 | 0 | 0 | |
| Other | 31 (3.2) | 1 (3.2) | 1 | 3.2 | |
| Histologic grade | |||||
| 1-2 | 690 (71.7) | 14 (2.0) | 12 | 1.7 | <.001 |
| 3 | 258 (26.8) | 21 (8.1) | 18 | 7.0 | |
| Unknown | 14 (1.5) | 4 (28.6) | 4 | NA | |
| Lymphovascular invasion | |||||
| Yes | 231 (24.0) | 4 (1.7) | 4 | 1.7 | .20 |
| No | 720 (74.8) | 34 (4.7) | 29 | 4.0 | |
| Unknown | 11 (1.1) | 1 (9.1) | 1 | NA | |
| Subtype | |||||
| HR-positive/ERBB2-negative | 606 (63.0) | 12 (2.0) | 11 | 1.8 | <.001 |
| HR-positive /ERBB-positive | 123 (12.8) | 3 (2.4) | 2 | 1.6 | |
| HR–negative/ERBB2-positive | 152 (15.8) | 20 (13.2) | 17 | 11.2 | |
| Triple negative | 81 (8.4) | 4 (4.9) | 4 | 4.9 | |
| Tumor stage | |||||
| T1 | 598 (62.2) | 32 (5.4) | 28 | 4.7 | .09 |
| T2 | 331 (34.4) | 7 (2.1) | 6 | 1.8 | |
| T3 | 33 (3.4) | 0 | 0 | 0 | |
| Extensive intraductal component | |||||
| Positive | 579 (60.2) | 32 (5.5) | 27 | 4.7 | .008 |
| Negative | 383 (39.8) | 7 (1.8) | 7 | 1.8 |
Abbreviations: HR, hormone receptor; NA, not applicable; NAC, nipple-areola complex.
Cancer Recurrence at the NAC
In a median follow-up duration of 85 months (range, 14-185 months), 39 cases (4.1%) involved cancer recurrence at the NAC as the first event after NSM; these excluded cancer recurrences at the NAC diagnosed after a locoregional recurrence or distant metastases. In addition, 42 cases (4.4%) involved local recurrence in the skin or chest wall outside the NAC as the first event. The 5-year cumulative incidence of cancer recurrence at the NAC was 3.5% (n = 34), and the 5-year cumulative incidence of local recurrence other than at the NAC was 3.4% (n = 33). No association was found between the incision type and local recurrence. The median time from surgery to cancer recurrence at the NAC was 35 months (range, 7-135 months). In all cases of cancer recurrence at the NAC, both frozen and permanent biopsy sections revealed tumor-negative retroareolar resection margins.
The characteristics and outcomes of the 39 patients (4.1%) who developed cancer recurrence at the NAC are shown in Table 2. These patients had a median age of 37 years (range, 26-54 years). The median primary tumor size was 4.7 cm (range, 0.5-12.0 cm). The primary tumor histologic feature was invasive ductal carcinoma in 38 cases (97%) and invasive papillary carcinoma in 1 case (3%). Recurrent tumor histologic features were invasive ductal carcinoma in 14 patients (36%), Paget disease with or without ductal carcinoma in situ in 18 patients (46%), ductal carcinoma in situ in 6 patients (15%), and Paget disease with microinvasive ductal carcinoma in 1 patient (3%).
Table 2. Characteristics of Patients and Outcomes of Cancer Recurrence at the Nipple-Areola Complexa.
| Patient No./Age, y | Primary Tumor | TTR, mo | Recurrent Tumor | Treatment Type | Follow-up After Recurrence, mo | |||
|---|---|---|---|---|---|---|---|---|
| Stage | ER/PR/ERBB2 | Histotype | Distant Metastasis | ER/PR/ERBB2 | ||||
| 1/30s | 1 | N/N/N | 42 | IDC | NA | N/N/N | Excision | 121 |
| 2/40s | 1 | N/N/P | 135 | Paget | NA | N/N/P | Excision | 27 |
| 3/30s | 1 | N/N/N | 57 | DCIS | NA | P/P/N | Excision and HT | 94 |
| 4/30s | 1 | N/N/P | 30 | Paget | NA | NA | Excision | 120 |
| 5/20s | 1 | N/N/P | 35 | Paget | NA | N/N/P | Excision and RT | 109 |
| 6/40s | 1 | P/P/P | 77 | Paget | NA | P/N/P | Excision | 65 |
| 7/20s | 1 | P/P/N | 54 | IDC | NA | P/P/N | Excision, RT, and HT | 82 |
| 8/40s | 1 | P/P/N | 84 | DCIS | Liver, bone | P/P/N | Excision, RT, and HT | 48 |
| 9/30s | 1 | N/N/P | 29 | DCIS and Paget | NA | N/N/P | Excision | 95 |
| 10/30s | 2 | P/P/N | 44 | IDC | NA | P/P/N | Excision, CTx, and HT | 71 |
| 11/30s | 1 | P/P/N | 11 | IDC | NA | N/P/N | Excision and HT | 101 |
| 12/30s | 2 | N/N/N | 7 | IDC | NA | N/N/N | Excision and CTx | 100 |
| 13/30s | 2 | P/P/N | 58 | IDC | NA | P/P/N | Excision and HT | 45 |
| 14/30s | 1 | N/N/P | 43 | DCIS and Paget | NA | NA | Excision | 55 |
| 15/30s | 1 | P/P/N | 29 | IDC | NA | P/P/N | Excision and HT | 66 |
| 16/40s | 1 | N/N/P | 61 | DCIS and Paget | NA | N/N/P | Excision | 32 |
| 17/30s | 2 | P/P/N | 59 | IDC | NA | P/P/N | Excision, RT, and HT | 34 |
| 18/40s | 1 | N/N/P | 23 | DCIS and Paget | NA | N/N/P | Excision | 70 |
| 19/40s | 1 | P/P/N | 48 | IDC | NA | P/P/N | Excision, RT, and HT | 38 |
| 20/40s | 1 | P/N/N | 25 | DCIS and Paget | NA | NA | Excision and HT | 60 |
| 21/30s | 1 | N/N/P | 44 | DCIS and Paget | NA | N/N/P | Excision | 40 |
| 22/30s | 1 | N/N/P | 42 | DCIS | NA | NA | Excision and RT | 42 |
| 23/30s | 1 | N/N/P | 24 | DCIS and Paget | NA | N/N/P | Excision | 57 |
| 24/30s | 1 | N/N/P | 25 | DCIS and Paget | NA | N/N/P | Excision | 55 |
| 25/50s | 2 | P/P/N | 45 | IDC | NA | P/N/N | Excision and HT | 33 |
| 26/30s | 1 | N/N/P | 23 | DCIS and Paget | NA | N/N/P | Excision | 52 |
| 27/40s | 1 | P/P/N | 13 | IDC | NA | P/P/N | Excision, RT, and HT | 59 |
| 28/30s | 1 | N/N/P | 14 | IDC | Contralateral ALN | N/N/P | Excision, RT, CTx, and Herceptin | 55 |
| 29/50s | 2 | N/N/N | 28 | IDC | NA | P/N/N | Excision, ALN dissection, RT, CTx, and HT | 39 |
| 30/50s | 2 | N/N/P | 62 | IDC | NA | N/N/P | Excision and RT | 5 |
| 31/40s | 1 | N/N/P | 51 | DCIS and Paget | NA | N/N/P | Excision and RT | 12 |
| 32/30s | 1 | P/P/P | 54 | microIDC and Paget | NA | N/N/P | Excision and HT | 8 |
| 33/30s | 1 | N/N/P | 36 | DCIS and Paget | NA | N/N/P | Excision, RT, CTx, and Herceptin | 25 |
| 34/30s | 1 | N/N/P | 18 | DCIS and Paget | NA | NA | Excision | 33 |
| 35/20s | 1 | N/N/P | 27 | DCIS | NA | N/N/P | Excision and RT | 22 |
| 36/40s | 1 | P/P/N | 8 | DCIS | NA | NA | Excision and HT | 40 |
| 37/40s | 1 | P/P/P | 35 | DCIS and Paget | NA | P/N/P | Excision and HT | 11 |
| 38/40s | 1 | N/N/P | 17 | DCIS | NA | N/N/P | Excision and RT | 25 |
| 39/30s | 1 | N/N/P | 15 | Paget | NA | N/N/P | Excision | 18 |
Abbreviations: ALN, axillary lymph node; CTx, chemotherapy; DCIS, ductal carcinoma in situ; ER, estrogen receptor; HT, hormonal therapy; IDC, invasive ductal carcinoma; N, negative; NA, not applicable; P, positive; PR, progesterone receptor; RT, radiation therapy; TTR, time to recurrence.
All patients were alive at the end of follow-up.
Risk Factors for Cancer Recurrence at the NAC
In univariate analysis, multifocality or multicentricity, subtype, histologic grade, and extensive intraductal component were associated with cancer recurrence at the NAC (Table 1). Of these, multifocality or multicentricity (hazard ratio [HR], 3.309; 95% CI, 1.501-7.294; P = .003), hormone receptor–negative/ERBB2 (formerly HER2 or HER2/neu)–positive subtype (HR, 3.051; 95% CI, 1.194–7.796; P = .02), high histologic grade (HR, 2.641; 95% CI, 1.132–6.160; P = .03), and presence of extensive intraductal component (HR, 3.338; 95% CI, 1.262–8.824; P = .02) were independently associated with cancer recurrence at the NAC in a multivariate analysis (Table 3).
Table 3. Multivariate Analyses of the Factors Associated With Cancer Recurrence at the Nipple-Areola Complex.
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Multifocality or multicentricity | 3.309 (1.501-7.294) | .003 |
| Histologic grade | 2.641 (1.132-6.160) | .03 |
| Extensive intraductal component | 3.338 (1.262-8.824) | .02 |
| Subtype | ||
| HR-positive/ERBB2-negative | 1 [Reference] | NA |
| HR-positive/ERBB2-positive | 0.972 (0.261-3.620) | .97 |
| HR-negative/ERBB2-positive | 3.051 (1.194-7.796) | .02 |
| Triple negative | 1.511 (0.376-6.066) | .56 |
Abbreviations: HR, hormone receptor; NA, not applicable.
Treatment and Prognosis of Cancer Recurrence at the NAC
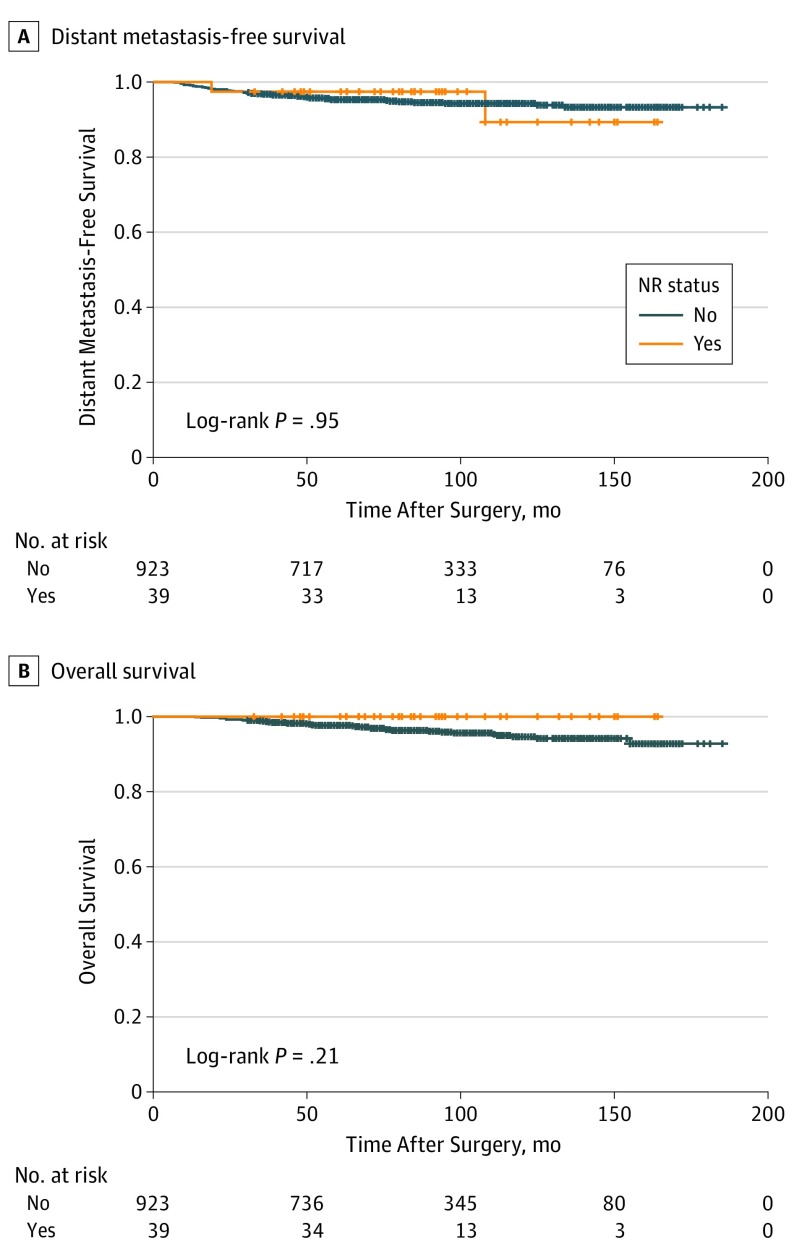
All 39 patients with cancer recurrence at the NAC underwent wide local excision. Of these, 25 (64%) patients received a combination of systemic hormone therapy, chemotherapy, and radiation therapy. Patients with cancer recurrence at the NAC had a mean follow-up duration of 51 months (range, 5–121 months) after NAC removal, during which 2 patients (5.2%) developed distant metastasis (1 patient within 24 months and the other within 5 months). All patients who had cancer recurrence at the NAC were confirmed to be alive at the last follow-up. The 10-year distant metastasis-free survival rates were 89.3% among patients with cancer recurrence at the NAC and 94.3% among patients without recurrence. The 10-year overall survival rates were 100% among patients with cancer recurrence at the NAC and 94.5% among those without recurrence. A Kaplan-Meier survival analysis revealed no statistically significant differences in distant metastasis-free survival (log-rank test, P = .95) and overall survival (log-rank test, P = .21) between patients with and without cancer recurrence at the NAC as the first event (Figure 2).
Figure 2. Kaplan-Meier Curves of Distant Metastasis-Free and Overall Survival According to Status of Cancer Recurrence at the Nipple-Areola Complex.
NR indicates cancer recurrence at the nipple-areola complex.
Discussion
Nipple-sparing mastectomy followed by immediate breast reconstruction for the surgical treatment of breast cancer has gained increased acceptance, with a growing emphasis on the achievement of excellent aesthetic results and improved quality of life without compromising oncologic safety. However, limited long-term follow-up data are available regarding the oncologic safety of modern NSM in terms of cancer recurrence at the NAC and survival. To our knowledge, the current study was the first to focus specifically on the incidence of and risk factors associated with cancer recurrence at the NAC as the first event after NSM in a large series of patients with invasive breast cancer in a long-term follow-up period (>5 years). We also examined treatments and outcomes of cancer recurrence at the NAC after NSM.
Although published studies8,9,10,11,12,15,16,17,18,19 have shown low rates of cancer recurrence at the NAC (0%-3.7%) after NSM, these findings were observed in a heterogeneous group of patients, including those with invasive and noninvasive disease, and reported variable follow-up durations. In a series by Jensen et al,16 no case of cancer recurrence at the NAC was reported among 149 patients who underwent NSM during a mean follow-up of 60.2 months; however, 57% of these cases did not involve invasive cancer. In a study by Wang et al,17 no case of cancer recurrence at the NAC was reported among 981 patients who underwent NSM; however, the follow-up evaluation was limited to 29 months, and 52% of the surgeries were performed prophylactically or for in situ disease.17 During a median follow-up duration of 78 months, Sakurai et al10 reported a cancer recurrence rate at the NAC of 3.7% among 788 patients who underwent NSM without radiotherapy between 1985 and 2004. A few other series on NSM20,21,22 that reported follow-up durations of at least 5 years involved patients treated in the 1980s and/or 1990s, when the adjuvant systemic therapy and radiation therapy regimens currently used in clinics had not yet been established as the criterion standard. In our study, we included only patients with invasive breast cancer who underwent NSM and immediate breast reconstruction between 2003 and 2015 and identified a low 5-year cumulative incidence of cancer recurrence at the NAC of 3.5%, which we believe was acceptable given the study population in the current series and the relatively long follow-up period.
Previously, only 3 studies15,18,19 to our knowledge had analyzed the risk factors associated with cancer recurrence at the NAC after NSM; of these, 2 involved only univariate analyses because of the small number of events. Only 1 study investigated variables through a multivariate analysis. In a study by Petit et al18 of 934 NSMs for invasive and intraepithelial breast cancer with a follow-up duration of 50 months that included 11 cases of cancer recurrence at the NAC, tumor size, receptor status, ERBB2 status, grade, and Ki-67 proliferation index were associated with the risk of recurrence in a multivariate analysis. Regarding the operative technique for NSM, Petit et al18 described leaving a 5-mm–thick layer of glandular tissue beneath the NAC to avoid flap necrosis and for the intraoperative delivery of electron-beam radiotherapy exclusively to the NAC to minimize the risk of local recurrence. This technique differed substantially from the protocol used at our institution; therefore, the outcomes may not be applicable to our series. In another study from the same institution of Petit et al,18 Lohsiriwat et al19 reported 7 cases involving local recurrence of Paget disease after NSM. In that study, univariate analyses identified primary carcinoma with ductal intraepithelial neoplasia or invasive ductal carcinoma with extensive intraductal component, negative hormonal receptor status, high pathological grade, ERBB2 overexpression, and ERBB2 positivity as risk factors associated with local recurrence of Paget disease. Shimo et al15 found that young age, estrogen receptor negativity, ERBB2 overexpression, and ERBB2-enriched subtype were significantly associated with a higher rate of cancer recurrence at the NAC in a univariate analysis.15 In our study, in multivariate analysis, multifocality or multicentricity, hormone receptor–negative/ERBB2–positive subtype, high histologic grade, and presence of extensive intraductal component were associated with an increased risk of cancer recurrence at the NAC after NSM. Although some of these risk factors were similar between the studies, our study showed that multifocality or multicentricity and presence of extensive intraductal component has the strongest associations with cancer recurrence at the NAC. Although we analyzed the tumor size and tumor-nipple distance, we did not find significant associations between these factors and cancer recurrence at the NAC.
Although the criteria used to select NSM for breast cancer treatment have broadened over time,23 consensus has not yet been reached regarding this issue. The traditional guidelines were based on studies recommending the selection of patients with the lowest risk of NAC involvement. The variables reported to be associated with occult NAC involvement include tumor size, tumor-nipple distance, ERBB2 amplification, lymphovascular invasion, extensive intraductal component, multifocal disease, and axillary lymph nodal metastasis.24,25,26 Our indications for NSM were any stage, tumor size, and tumor-areola distance. Furthermore, we routinely performed intraoperative frozen biopsy examinations of the retroareolar resection margins to determine occult nipple involvement. The NAC was preserved if the palpation findings, shape, and color of the nipple were normal and the NAC ducts were confirmed to be tumor free in frozen biopsy specimens collected intraoperatively. Our results support the validity of our indications for NSM and an associated low incidence of cancer recurrence at the NAC.
The current study describes our experience with the treatment and outcomes of cancer recurrence at the NAC after NSM. All patients with cancer recurrence at the NAC underwent wide local excision, and 64% of these patients received multimodal adjuvant treatment according to the biologic disease features, including hormonal therapy, chemotherapy, and/or radiotherapy. Of the 39 patients with cancer recurrence at the NAC as the first event in the present series, 2 developed distant metastasis during the follow-up period; however, all 39 patients survived, with a mean follow-up duration of 51 months after NAC removal. We observed no statistically significant differences in distant metastasis-free survival and overall survival between patients with and without cancer recurrence at the NAC. These results suggest that cancer recurrence at the NAC had no statistically significant association with prognosis.
Limitations
The main limitation of present study was that it involved retrospective analysis, although from a prospectively maintained database, and may include bias. However, our study included one of the largest NSM series for invasive breast cancer to date with long-term follow-up from a single institution.
Conclusions
Our study revealed a low incidence of cancer recurrence at the NAC after NSM and immediate breast reconstruction. Patients with multifocal or multicentric disease, hormone receptor–negative/ ERBB2–positive subtype, high histologic grade, and positive extensive intraductal component had a significantly increased risk of cancer recurrence at the NAC. These findings suggest that factors should be considered when planning for NSM. Most patients with cancer recurrence at the NAC had a favorable prognosis after receiving appropriate comprehensive treatment. As more patients with breast cancer undergo NSM, these data may be useful in estimating a prognosis for cancer recurrence at the NAC and guiding management decisions.
References
- 1.Park EH, Min SY, Kim Z, et al. ; Korean Breast Cancer Society . Basic facts of breast cancer in Korea in 2014: the 10-year overall survival progress. J Breast Cancer. 2017;20(1):1-11. doi: 10.4048/jbc.2017.20.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HJ, Park EH, Lim WS, et al. . Nipple areola skin-sparing mastectomy with immediate transverse rectus abdominis musculocutaneous flap reconstruction is an oncologically safe procedure: a single center study. Ann Surg. 2010;251(3):493-498. doi: 10.1097/SLA.0b013e3181c5dc4e [DOI] [PubMed] [Google Scholar]
- 3.Moo TA, Pinchinat T, Mays S, et al. . Oncologic outcomes after nipple-sparing mastectomy. Ann Surg Oncol. 2016;23(10):3221-3225. doi: 10.1245/s10434-016-5366-1 [DOI] [PubMed] [Google Scholar]
- 4.Bailey CR, Ogbuagu O, Baltodano PA, et al. . Quality-of-life outcomes improve with nipple-sparing mastectomy and breast reconstruction. Plast Reconstr Surg. 2017;140(2):219-226. doi: 10.1097/PRS.0000000000003505 [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 4. 2017. https://www.nccn.org/professionals/physician_gls/default/aspx. Accessed March 1, 2018. [DOI] [PubMed]
- 6.Agarwal S, Agarwal S, Neumayer L, Agarwal JP. Therapeutic nipple-sparing mastectomy: trends based on a national cancer database. Am J Surg. 2014;208(1):93-98. doi: 10.1016/j.amjsurg.2013.09.030 [DOI] [PubMed] [Google Scholar]
- 7.Frey JD, Salibian AA, Karp NS, Choi M. Comparing therapeutic versus prophylactic nipple-sparing mastectomy: does indication inform oncologic and reconstructive outcomes? Plast Reconstr Surg. 2018;142(2):306-315. doi: 10.1097/PRS.0000000000004548 [DOI] [PubMed] [Google Scholar]
- 8.Smith BL, Tang R, Rai U, et al. . Oncologic safety of nipple-sparing mastectomy in women with breast cancer. J Am Coll Surg. 2017;225(3):361-365. doi: 10.1016/j.jamcollsurg.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 9.Krajewski AC, Boughey JC, Degnim AC, et al. . Expanded indications and improved outcomes for nipple-sparing mastectomy over time. Ann Surg Oncol. 2015;22(10):3317-3323. doi: 10.1245/s10434-015-4737-3 [DOI] [PubMed] [Google Scholar]
- 10.Sakurai T, Zhang N, Suzuma T, et al. . Long-term follow-up of nipple-sparing mastectomy without radiotherapy: a single center study at a Japanese institution. Med Oncol. 2013;30(1):481. doi: 10.1007/s12032-013-0481-3 [DOI] [PubMed] [Google Scholar]
- 11.Orzalesi L, Casella D, Santi C, et al. . Nipple sparing mastectomy: surgical and oncological outcomes from a national multicentric registry with 913 patients (1006 cases) over a six year period. Breast. 2016;25:75-81. doi: 10.1016/j.breast.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 12.De La Cruz L, Moody AM, Tappy EE, Blankenship SA, Hecht EM. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systematic review. Ann Surg Oncol. 2015;22(10):3241-3249. doi: 10.1245/s10434-015-4739-1 [DOI] [PubMed] [Google Scholar]
- 13.Curigliano G, Burstein HJ, Winer EP, et al. ; St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2017 . De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28(8):1700-1712. doi: 10.1093/annonc/mdx308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edge S, Byrd DR, Compton CC, et al. . AJCC Cancer Staging Manual. 7th ed New York, NY: Springer; 2010. [Google Scholar]
- 15.Shimo A, Tsugawa K, Tsuchiya S, et al. . Oncologic outcomes and technical considerations of nipple-sparing mastectomies in breast cancer: experience of 425 cases from a single institution. Breast Cancer. 2016;23(6):851-860. doi: 10.1007/s12282-015-0651-6 [DOI] [PubMed] [Google Scholar]
- 16.Jensen JA, Orringer JS, Giuliano AE. Nipple-sparing mastectomy in 99 patients with a mean follow-up of 5 years. Ann Surg Oncol. 2011;18(6):1665-1670. doi: 10.1245/s10434-010-1475-4 [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Peled AW, Garwood E, et al. . Total skin-sparing mastectomy and immediate breast reconstruction: an evolution of technique and assessment of outcomes. Ann Surg Oncol. 2014;21(10):3223-3230. doi: 10.1245/s10434-014-3915-z [DOI] [PubMed] [Google Scholar]
- 18.Petit JY, Veronesi U, Orecchia R, et al. . Risk factors associated with recurrence after nipple-sparing mastectomy for invasive and intraepithelial neoplasia. Ann Oncol. 2012;23(8):2053-2058. doi: 10.1093/annonc/mdr566 [DOI] [PubMed] [Google Scholar]
- 19.Lohsiriwat V, Martella S, Rietjens M, et al. . Paget’s disease as a local recurrence after nipple-sparing mastectomy: clinical presentation, treatment, outcome, and risk factor analysis. Ann Surg Oncol. 2012;19(6):1850-1855. doi: 10.1245/s10434-012-2226-5 [DOI] [PubMed] [Google Scholar]
- 20.Gerber B, Krause A, Dieterich M, Kundt G, Reimer T. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg. 2009;249(3):461-468. doi: 10.1097/SLA.0b013e31819a044f [DOI] [PubMed] [Google Scholar]
- 21.Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol. 2008;34(2):143-148. doi: 10.1016/j.ejso.2007.06.010 [DOI] [PubMed] [Google Scholar]
- 22.Horiguchi J, Takei H, et al. . A comparative study of subcutaneous mastectomy with radical mastectomy. Anticancer Res. 2001;21(4B):2963-2967. [PubMed] [Google Scholar]
- 23.Peled AW, Wang F, Foster RD, et al. . Expanding the indications for total skin-sparing mastectomy: is it safe for patients with locally advanced disease? Ann Surg Oncol. 2016;23(1):87-91. doi: 10.1245/s10434-015-4734-6 [DOI] [PubMed] [Google Scholar]
- 24.Byon W, Kim E, Kwon J, Park YL, Park C. Magnetic resonance imaging and clinicopathological factors for the detection of occult nipple involvement in breast cancer patients. J Breast Cancer. 2014;17(4):386-392. doi: 10.4048/jbc.2014.17.4.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laronga C, Kemp B, Johnston D, Robb GL, Singletary SE. The incidence of occult nipple-areola complex involvement in breast cancer patients receiving a skin-sparing mastectomy. Ann Surg Oncol. 1999;6(6):609-613. doi: 10.1007/s10434-999-0609-z [DOI] [PubMed] [Google Scholar]
- 26.Brachtel EF, Rusby JE, Michaelson JS, et al. . Occult nipple involvement in breast cancer: clinicopathologic findings in 316 consecutive mastectomy specimens. J Clin Oncol. 2009;27(30):4948-4954. doi: 10.1200/JCO.2008.20.8785 [DOI] [PubMed] [Google Scholar]