Key Points
Question
Is delay discounting a transdiagnostic process in psychiatric disorders?
Findings
In this meta-analysis of 57 effect sizes from 43 studies across 8 diagnostic categories, robust differences in delay discounting were observed between people with psychiatric disorders and controls. Most individuals with disorders (including depression, bipolar disorder, schizophrenia, borderline personality disorder, bulimia nervosa, and binge-eating disorder) exhibited steeper discounting compared with controls, whereas those with anorexia nervosa exhibited shallower discounting compared with controls.
Meaning
Evidence from this study suggests that delay discounting decision-making is a robust transdiagnostic process across a range of psychiatric disorders and may be a viable target for treatment interventions.
This meta-analysis reviews articles from PubMed, MEDLINE, PsycInfo, Embase, and Web of Science to examine the role of delay discounting in individuals with psychiatric illness.
Abstract
Importance
Delay discounting is a behavioral economic index of impulsive preferences for smaller-immediate or larger-delayed rewards that is argued to be a transdiagnostic process across health conditions. Studies suggest some psychiatric disorders are associated with differences in discounting compared with controls, but null findings have also been reported.
Objective
To conduct a meta-analysis of the published literature on delay discounting in people with psychiatric disorders.
Data Sources
PubMed, MEDLINE, PsycInfo, Embase, and Web of Science databases were searched through December 10, 2018. The psychiatric keywords used were based on DSM-IV or DSM-5 diagnostic categories. Collected data were analyzed from December 10, 2018, through June 1, 2019.
Study Selection
Following a preregistered Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) protocol, 2 independent raters reviewed titles, abstracts, and full-text articles. English-language articles comparing monetary delay discounting between participants with psychiatric disorders and controls were included.
Data Extraction and Synthesis
Hedges g effect sizes were computed and random-effects models were used for all analyses. Heterogeneity statistics, one-study-removed analyses, and publication bias indices were also examined.
Main Outcomes and Measures
Categorical comparisons of delay discounting between a psychiatric group and a control group.
Results
The sample included 57 effect sizes from 43 studies across 8 diagnostic categories. Significantly steeper discounting for individuals with a psychiatric disorder compared with controls was observed for major depressive disorder (Hedges g = 0.37; P = .002; k = 7), schizophrenia (Hedges g = 0.46; P = .004; k = 12), borderline personality disorder (Hedges g = 0.60; P < .001; k = 8), bipolar disorder (Hedges g = 0.68; P < .001; k = 4), bulimia nervosa (Hedges g = 0.41; P = .001; k = 4), and binge-eating disorder (Hedges g = 0.34; P = .001; k = 7). In contrast, anorexia nervosa exhibited statistically significantly shallower discounting (Hedges g = –0.30; P < .001; k = 10). Modest evidence of publication bias was indicated by a statistically significant Egger test for schizophrenia and at the aggregate level across studies.
Conclusions and Relevance
Results of this study appear to provide empirical support for delay discounting as a transdiagnostic process across most of the psychiatric disorders examined; the literature search also revealed limited studies in some disorders, notably posttraumatic stress disorder, which is a priority area for research.
Introduction
Examination of underlying neurocognitive processes that transcend multiple diagnostic categories is a long-standing priority in psychiatry. Consistent with this focus is the Research Domain Criteria (RDoC) framework from the US National Institute of Mental Health,1,2 which seeks to characterize the fundamental domains of cognitive, perceptual, and social processing with the aim of identifying novel targets for the treatment of mental health disorders. Within the RDoC framework, the behavioral economic index of delay discounting, which captures the extent to which rewards lose value over a temporal delay, has emerged as a promising paradigm.3 Delay discounting is commonly assessed through intertemporal choice tasks involving choices between immediate and delayed rewards (eg, money) to estimate a person’s discounting rate (k) or other quantitative indices (eg, area under the curve, impulsive choice ratio). Steeper delay discounting and, subsequently, smaller area under the discounting curve is frequently interpreted as reflecting an impulsive preference for immediate rewards over delayed gratification.4,5
A growing body of research has solidified the relevance of delay discounting in the context of psychiatric disorders. This relevance has led to the proposal that excessive discounting of delayed rewards is a transdiagnostic process (ie, a behavior exhibited across multiple disorders that may provide novel insights into the common underlying features of those disorders).6,7 Furthermore, Levin et al8 proposed that investigating delay discounting across disorders may help inform transdiagnostic treatments by identifying target behavioral processes and providing markers of change in existing treatments.
Previous narrative reviews by Bickel et al6 and Lempert et al3 have summarized evidence of steep discounting associated with numerous health conditions, with addictive disorders, attention-deficit/hyperactivity disorder (ADHD), and obesity being among the most extensively studied domains to date. Several meta-analyses have reported consistent evidence of impulsive discounting associated with each of these disorders.9,10,11,12 In addition, Bickel et al6 and Lempert et al3 also summarized evidence of steep delay discounting in several other psychiatric disorders, including schizophrenia,13,14 bipolar disorder,13,15 major depressive disorder,16,17 and borderline personality disorder.18,19 In contrast, disorders such as anorexia nervosa20,21 and obsessive-compulsive personality disorder22 are associated with shallower discounting compared with healthy controls. Therefore, the existing literature suggests that delay discounting lies on a continuum (Figure 2 in Lempert et al3). Indexing the location of different disorders along this continuum may elucidate the degree to which delay discounting should be considered as a viable and necessary treatment target in the pursuit of ameliorating transdiagnostic symptoms.
Narrative reviews are valuable for summarizing findings and stimulating new research on the role of delay discounting in the broad field of psychiatry, but to our knowledge, a quantitative synthesis of the research on psychiatric disorders (apart from addictive disorders and ADHD) has yet to be published. A quantitative meta-analysis is necessary for several reasons. First, although a preponderance of individual studies have reported statistically significant differences between individuals with psychiatric disorders and healthy controls, a notable number of studies have not found these differences,23,24,25,26 suggesting a need to clarify the nature and relative weight of the collective evidence to date. Second, a meta-analytic approach involves a systematic literature search that may identify additional studies or disorder categories otherwise excluded from narrative reviews. Third, a meta-analysis provides important quantitative data, including estimates of aggregate effect sizes across studies, indices of between-study heterogeneity, and evaluation of publication bias.
The goal of the current study was to conduct a meta-analysis of studies comparing delay discounting between individuals with psychiatric disorders and nonclinical comparison groups. Based on the hypothesis that delay discounting is a transdiagnostic process, we hypothesized the existence of robust differences across studies between individuals with psychiatric disorders and healthy controls.
Methods
Search Strategy
The meta-analysis protocol was preregistered on PROSPERO (The International Prospective Register of Systematic Reviews) (CRD42018105385). Candidate studies were identified through searches of PubMed, MEDLINE, PsycInfo, Embase, and Web of Science through December 10, 2018. Discounting keywords were combined using Boolean logic with psychiatric keywords based on DSM-IV and DSM-5 diagnostic categories (the complete list of search terms is presented in eTable 1 in the Supplement, and a licensed clinical psychologist [R.E.M.] reviewed the psychiatric keyword list). Addiction or ADHD-associated keywords were not included to avoid redundancy with published meta-analyses.9,11,12 Keywords associated with other neurodevelopmental disorders (eg, autism spectrum disorders) were excluded. In addition, the reference lists of recent reviews were manually searched for additional studies.
Inclusion Criteria and Study Selection
For inclusion, studies had to meet the following criteria: (1) published in a peer-reviewed journal, (2) available in the English language, (3) involved human participants, (4) included a monetary delay discounting measure, (5) performed a categorical comparison between individuals with a psychiatric diagnosis based on a validated diagnostic instrument (eg, SCID [Structured Clinical Interview for DSM]27,28) and controls, and (6) assessed monetary delay discounting under neutral conditions (eg, no experimental stress or affect manipulations). Studies with multiple discounting assessments (eg, accelerated vs delayed versions21,22,29) were included as these were not considered to be manipulations designed to alter mood or emotional state; however, these studies were collapsed into a single effect size in a follow-up analysis. Although a limited number of studies have assessed nonmonetary commodities (eg, food, effort), we focused on money as the most commonly and consistently assessed reward. Studies focused on comorbid substance use disorders and psychiatric disorders30,31 were not included because disentangling the associations between substance use disorder and psychiatric illness was not possible. A minimum of 4 effect sizes was required for a diagnostic category to be included in the meta-analysis.
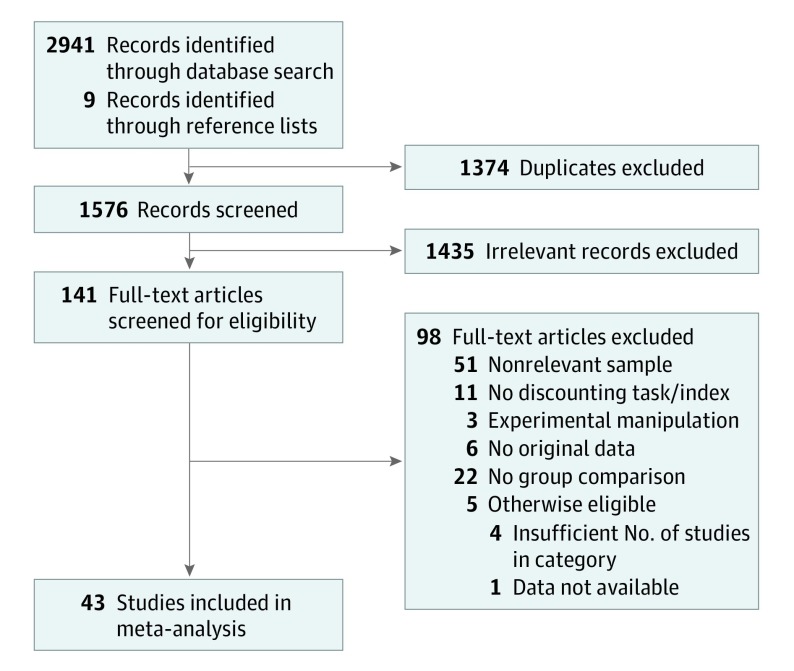
Study selection was completed in Covidence (Veritas Health Innovation Inc). The selection procedure is depicted in Figure 1 and followed Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) standards.32 Two of us (M.A. and E.M.) independently screened titles and abstracts for clearly eligible or ineligible studies. Studies with conflicting ratings were discussed, and a consensus decision was reached between M.A. and E.M., with additional clarification from R.M as needed. The same process was repeated for full-text articles.
Figure 1. Diagram of Study Selection and Inclusion.
Sample Characteristics
Characteristics of the included studies13,14,15,16,17,18,19,20,21,22,23,24,25,26,29,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60are presented in Table 1; a list of excluded full-text articles and reasons for exclusion is provided in eTable 4 in the Supplement. Disorder categories reflected the primary diagnosis for the clinical group; inclusion or exclusion of other comorbidities varied across studies but was not considered in the present analyses. Notable details of the participant characteristics were that only 1 study on bipolar disorder15 specified whether participants were in a manic or depressive state at testing, 1 study on binge-eating disorder33 included controls who were overweight or obese, and Wierenga et al34 included individuals with remitted anorexia nervosa. Although studies that explicitly focused on concurrent addictive and psychiatric disorders were excluded, a number of studies did include participants with varying levels of substance use. However, levels of substance use were not consistently reported or controlled for across studies, so we were unable to examine this factor in the present analyses. Readers are encouraged to consult the original articles for information on psychiatric comorbidities and substance use.
Table 1. Characteristics of Included Studies.
| Source | Groups (No. of Participants) | Diagnostic Tool | DD Measure | Delayed Amount | DD Index |
|---|---|---|---|---|---|
| Major Depressive Disorder | |||||
| Brown et al,39 2018 | MDD (32); HC (61) | DSM-IV | DDT | $10 | k |
| Cáceda et al,17 2014 | MDD (20); HC (20) | DSM-IV | MCQ | $55 (mean) | k |
| Dombrovski et al,40 2011 | MDD (42); HC (31) | DSM-IV | MCQ | $55 (mean) | k |
| Engelmann et al,38 2013 | MDD (11); HC (15) | DSM-IV | DDT | $10 000 | β |
| Imhoff et al,16 2014 | MDD (20); HC (20) | BDI | DDQ | $10 | AUC |
| Pulcu et al,41 2014 | MDD (24); HC (29) | DSM-IV | MCQ | $75-$85 | k |
| Weidberg et al,23 2015 | MDD (30); HC (65) | BDI | DDT | €1000 | k |
| Schizophrenia | |||||
| Ahn et al,13 2011 | SZ/SZA (21); HC (30) | DSM-IV | DDT | $800 | k |
| Avsar et al,36 2013 | SZ/SZA (14); HC (14) | DSM-IV | DDT | $28-86 | k |
| Brown et al,39 2018 | SZ/SZA (31); HC (61) | DSM-IV | DDT | $10 | k |
| Heerey et al,14 2007 | SZ/SZA (42); HC (29) | DSM-IV | MCQ | $55 (mean) | k |
| Heerey et al,37 2011 | SZ (37); HC (24) | DSM-IV | DDT | $75-$85 | k |
| Horan et al,42 2017 | SZ (131); HC (70) | DSM-IV | DDT | $1000 | AUC |
| MacKillop and Tidey,43 2011 | SZ/SZA (23); HC (24) | DSM-IV | MCQ | $25-$85 | k |
| Wang et al,44 2018 | SZ (25); HC (30) | DSM-IV | DDT | $45 | ICR |
| Wing et al,24 2012 | SZ/SZA (34); HC (37) | DSM-IV | MCQ | $55 (mean) | k |
| Yu et al,45 2017 | SZ/SZA (47); HC (42) | DSM-IV | DDT | $25-$35 | k |
| Borderline Personality Disorder | |||||
| Barker et al,18 2015 | BPD (19); HC (21) | DSM-IV | DDT | £100 | k |
| Berenson et al,46 2016 | BPD (35); HC (45) | DSM-IV | DDT | $55 (mean) | k |
| Coffey et al,47 2011 | BPD (19); HC (28) | DSM-IV | DDT | $1000 | k |
| Dougherty et al,48 1999 | BPD (13); HC (17) | DSM-III | DDT | $.15 | ICR |
| Krause-Utz et al,49 2016 | BPD (25); HC (24) | DSM-IV | DDT | €100 | k |
| Lawrence et al,19 2010 | BPD (30); HC (28) | DSM-IV | DDT | $1000 | k |
| Maraz et al,25 2016 | BPD (36); HC (111) | ICD-10 | DDT | 50 000 HUF | k |
| Bipolar Disorder | |||||
| Ahn et al,13 2011 | BP (22); HC (30); manic-depressive state not specified | DSM-IV | DDT | $800 | k |
| Brown et al,39 2018 | BP (16); HC (61); manic-depressive state not specified | DSM-IV | DDT | $10 | k |
| Strakowski et al,15 2010 | BP (108); HC (48); acute manic/mixed-episode state | DSM-IV | eDDT | $.15 | ICR |
| Urošević et al,50 2016 | BP (32); HC (32); manic-depressive state not specified | DSM-IV | DDT | $10 | AUC |
| Obsessive-Compulsive Disorder | |||||
| Norman et al,51 2017 | OCD (20); HC (20) | ICD-10 | DDT | £100 | k |
| Pinto et al,22 2014 | OCD (25); HC (25) | DSM-IV | DDT | $80-$100 | AUC |
| Sohn et al,35 2014 | OCD (80); HC (76) | DSM-IV | DDT | $100 | k |
| Steinglass et al,52 2017 | OCD (50); HC (75) | DSM-IV | DDT | $47-$78 | k |
| Bulimia Nervosa | |||||
| Bartholdy et al,53 2017 | BN (27); HC (28); all female | DSM-5 | DDT | £100 | AUC |
| Kekic et al,29 2016 | BN (39); HC (53) | DSM-5 | DDT | £100-£130 | DF |
| Neveu et al,54 2014 | BN (18); HC (18); all female | DSM-IV | DDT | €10 | k |
| Binge-Eating Disorder | |||||
| Bartholdy et al,53 2017 | BED (11); HC (28); all female | DSM-5 | DDT | £100 | AUC |
| Davis et al,55 2010 | BED (65); HC (71) | DSM-IV | DDT | $100 | IDP |
| Manasse et al,33 2015 | BED (31); obese/overweight controls (43); all female | EDE | DDT | $1000 | AUC |
| Manwaring et al,56 2011 | BED (27); HC (30); all female | DSM-IV | DDT | $100 | AUC |
| Mole et al,57 2015 | BED (30); HC (30) | DSM-IV | MCQ | $55 (mean) | k |
| Steward et al,58 2017 | BED (24); HC (80); all female | DSM-IV | MCQ | $55 (mean) | k |
| Yan et al,59 2018 | BE (85); HC (928) | BES | DDT | ¥10 000 | k |
| Anorexia Nervosa | |||||
| Bartholdy et al,53 2017 | AN (28); HC (28); all female | DSM-5 | DDT | £100 | AUC |
| Decker et al,20 2015 | AN (54); HC (39) | DSM-5 | DDT | $5-$40 | k |
| King et al,60 2016 | AN (31); HC (31); all female | DSM-IV | DDT | €30 | k |
| Neveu et al,54 2014 | AN-R (16); HC (18); all female | DSM-IV | DDT | €10 | k |
| Ritschel et al,26 2015 | AN (34); HC (53); all female | DSM-IV | DDT | €20-€789 | k |
| Steinglass et al,21 2012 | AN (36); HC (28) | DSM-IV | DDT | $80-100 | DF |
| Steinglass et al,52 2017 | AN (27); HC (75) | DSM-IV | DDT | $47-$78 | k |
| Steward et al,58 2017 | AN-R (37); HC (80); all female | DSM-IV | MCQ | $55 (mean) | k |
| Wierenga et al,34 2015 | Remitted AN (23); HC (17) | DSM-IV | DDT | $47-$78 | ICR |
Abbreviations: AN, anorexia; AN-R, anorexia-restrictive subtype; AUC, area under the curve; BDI, Beck Depression Inventory; BED, binge-eating disorder; BES, Binge Eating Scale; BN, bulimia nervosa; BP, bipolar disorder; BPD, borderline personality disorder; DD, delay discounting; DDT, delay discounting task; DF, discount factor; eDDT, experiential-type delay discounting task; EDE, Eating Disorder Examination; HC, healthy controls; ICR, impulsive choice ratio; IDP, indifference point; k, hyperbolic discounting rate; MCQ, monetary choice task; MDD, major depressive disorder; OCD, obsessive-compulsive disorder; SZ, schizophrenia; SZA, schizoaffective disorder; €, Euro; HUF, Hungarian Forint; ¥, Japanese yen; £, UK pound; $, US dollar.
Studies also varied in the methods used to measure and quantify delay discounting (Table 1). Although all studies examined money, the reward magnitudes varied from small (ie, $0.15 to approximately $30) to large (ie, $500-$1000). Most studies used either a delay discounting task (ie, adjusting amount or delay) or the monetary choice questionnaire,61 with 1 study using an experiential discounting task.15 Hyperbolic discounting rate and area under the curve were the most common indices of discounting, with impulsive choice ratio and other indices also used.
Meta-analytic Approach
Comprehensive Meta-Analysis, version 3.0 (Biostat), was used for all analyses. The primary effect size was Hedges g, which is ideal when aggregating studies with small sample sizes owing to the statistic’s correction for small study bias.62 Two of us (M.A. and E.M.) independently extracted and checked quantitative values (raw data values are provided in eTable 2 in the Supplement). When the required data were not reported in the published article, we contacted the corresponding authors (9 authors provided data, and data remained unavailable for 1 study). Effect sizes from studies using area under the curve or indifference points were reversed prior to analysis.
Separate meta-analyses were conducted for each diagnosis category using a random-effects model. Several indices of effect size heterogeneity were calculated. Cochran Q test reflects the sum of squared differences between individual weighted study effects and the overall mean. I2 statistic captures the proportion of variation within study effect sizes explained by heterogeneity. The tau (τ) reflects the SD of the mean effect. Borenstein et al62 emphasized that Q is less reliable with small sample sizes, whereas I2 and τ are not affected by sample size; thus, all 3 statistics were reported to be comprehensive. A one-study removed (OSR) analysis quantified the association of individual studies with the aggregate results.63 Furthermore, to evaluate the overrepresentation by studies adding multiple effect sizes, we repeated the primary analysis after consolidation into a single effect size per study.
Publication bias was evaluated using multiple indices, including examination of the funnel plots using the 2-tailed Begg-Mazumdar test64 and the 1-tailed Egger test.65 Owing to low statistical power for the funnel plot indices with small sample sizes,66 statistical significance of the funnel plot indices was considered only in categories with 10 or more effect sizes. Adjusted estimates of effect size were also generated according to imputed unpublished studies using the Duval and Tweedie trim-and-fill approach.67 A 2-tailed significance value of P < .05 was used for all aggregate tests.
Results
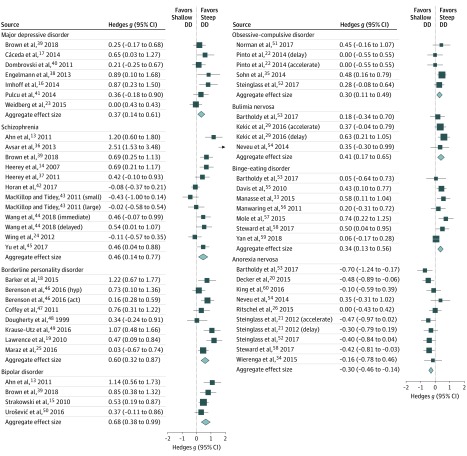
The results of the meta-analyses by disorder category are presented in Table 2, and forest plots by category are provided in Figure 2. Complete statistical results for individual studies are provided in eTable 3 in the Supplement. A total of 43 studies met the inclusion criteria, yielding 57 effect sizes. Eight disorder categories had sufficient effect sizes (ie, k≥4) to be included (Table 2). All disorder categories, except anorexia nervosa, exhibited statistically significantly steeper (more impulsive) delay discounting compared with controls: major depressive disorder (Hedges g = 0.37; P = .002; k = 7), schizophrenia (Hedges g = 0.46; P = .004; k = 12), borderline personality disorder (Hedges g = 0.60; P < .001; k = 8), bipolar disorder (Hedges g = 0.68; P < .001; k = 4), bulimia nervosa (Hedges g = 0.41; P = .001; k = 4), binge-eating disorder (Hedges g = 0.34; P = .001; k = 7), and obsessive-compulsive disorder (Hedges g = 0.30; P = .002; k = 5). Studies of anorexia nervosa revealed the opposite pattern, with the clinical group exhibiting shallower (less impulsive) discounting compared with controls (Hedges g = –0.30; P < .001; k = 10). The largest aggregate effect sizes were observed for bipolar disorder and borderline personality disorder, with each of these reflecting medium-sized effects based on conventional interpretation. Small to medium effect sizes (Hedges g = 0.30-0.46) were observed for the other categories.
Table 2. Meta-analytic Results by Disorder Category.
| Disorder | No. of Effect Sizes | Total No. of Unique Individuals | Hedges g Effect Size | P Value | 95% CI | OSR | Cochran Q Test of Homogeneity | Cochran Q Test P Value | I2 Statistic | SD of Aggregate Effect Size, τ |
|---|---|---|---|---|---|---|---|---|---|---|
| Major depressive disorder | 7 | 420 | 0.37 | .002 | 0.14 to 0.61 | 0.29 to 0.44 | 8.31 | .22 | 27.82 | 0.17 |
| Schizophrenia | 12 | 766 | 0.46 | .004 | 0.14 to 0.77 | 0.34 to 0.53 | 52.97 | <.001 | 79.23 | 0.48 |
| Borderline personality disorder | 8 | 451 | 0.60 | <.001 | 0.32 to 0.87 | 0.51 to 0.67 | 15.74 | .028 | 55.53 | 0.29 |
| Bipolar disorder | 4 | 349 | 0.68 | <.001 | 0.37 to 0.98 | 0.55 to 0.78 | 4.91 | .18 | 38.853 | 0.20 |
| Obsessive-compulsive disorder | 5 | 371 | 0.30 | .002 | 0.11 to 0.49 | 0.20 to 0.34 | 3.77 | .44 | 0.00 | 0.00 |
| Bulimia nervosa | 4 | 183 | 0.41 | .001 | 0.17 to 0.65 | 0.31 to 0.47 | 1.85 | .60 | 0.00 | 0.00 |
| Binge-eating disorder | 7 | 1483 | 0.34 | .001 | 0.13 to 0.56 | 0.29 to 0.45 | 10.63 | .10 | 43.53 | 0.18 |
| Anorexia nervosa | 10 | 655 | −0.30 | <.001 | −0.46 to −0.14 | −0.27 to −0.35 | 10.31 | .33 | 12.73 | 0.09 |
Abbreviations: I2, proportion of variability from heterogeneity; OSR, one-study removed, or range of effect sizes obtained from OSR jackknife analysis.
Figure 2. Forest Plots of Primary Meta-analytic Results by Disorder Category.
Act indicates actual rewards; DD, delay discounting; and Hyp, hypothetical rewards. Square data points reflect effect size (Hedges g) for each study, with whiskers reflecting 95% CIs. Diamonds reflect aggregate effect sizes (Hedges g) for each category, with width of diamond indicating 95% CI.
Statistically significant evidence of heterogeneity based on the Cochran Q statistic was found for 3 of the disorder categories (major depressive disorder, schizophrenia, and borderline personality disorder). However, the I2 and τ statistics suggested that heterogeneity was also present for bipolar disorder and binge-eating disorder. A nonsignificant Cochran Q may result from low power from the small number of studies in these disorder categories.
The OSR analysis showed that the results for all disorder categories except obsessive-compulsive disorder were generally stable (eTable 3 in the Supplement). For obsessive-compulsive disorder, omitting the study by Sohn et al35 resulted in a nonsignificant aggregate effect size (Hedges g = 0.20; P = .11). Although the remaining OSR analyses yielded statistically significant aggregate effect sizes, a small number of studies with larger effect sizes tended to have a disproportionate association with the aggregate effect sizes for major depressive disorder23 (OSR Hedges g = 0.45; P < .001) and schizophrenia36 (OSR Hedges g = 0.34; P = .01).
For the 5 disorder categories that included multiple effect sizes from individual studies, we recalculated the aggregate effect sizes after consolidating to a single effect size per study. This recalculation yielded generally similar estimates of aggregate effect size for schizophrenia (Hedges g = 0.52), borderline personality disorder (Hedges g = 0.64), obsessive-compulsive disorder (Hedges g = 0.30), anorexia nervosa (Hedges g = –0.30), and bulimia nervosa (Hedges g = 42).
An exploratory analysis examined the association between reward magnitude and the effect sizes obtained, irrespective of diagnosis. Magnitudes were coded into 3 categories: small (<$100; k = 33), medium ($100-$499; k = 14) or large (≥$500; k = 10). Modest differences in effect sizes were observed for small (Hedges g = 0.41; 95% CI, 0.29-0.52; P < .001), medium (Hedges g = 0.38; 95% CI, 0.23-0.53; P < .001), and large (Hedges g = 0.53; 95% CI, 0.21-0.85; P = .001) rewards, but the test of heterogeneity was nonsignificant (Cochran Q = 0.71; P = .70).
Publication Bias
Publication bias indices are reported in Table 3. Two disorder categories (schizophrenia and anorexia nervosa) had more than the recommended minimum of 10 studies for the Begg-Mazumdar test or Egger test. The Egger intercept was statistically significant for schizophrenia. Major depressive disorder and bipolar disorder were determined to have missing effect sizes, using the trim-and-fill method (see Table 3 for imputed effect sizes). To explore publication bias more broadly, we aggregated the 57 effect sizes into a single analysis. The Kendal tau was nonsignificant (P = .24), but the Egger test intercept was significant (Egger intercept = 2.2; P = .001). The trim-and-fill method indicated no missing studies. In sum, minimal to modest evidence for publication bias was found, but these indices should be considered with caution given the relatively small number of studies for most disorder categories.66
Table 3. Publication Bias Indices by Disorder Category.
| Disorder | Kendall τ | P Value | Egger Intercept | SE | P Value | Trim and Fill | Imputed Hedges g |
|---|---|---|---|---|---|---|---|
| Major depressive disorder | 0.71 | .02a | 4.93 | 0.96 | .002a | 2 | 0.26 |
| Schizophrenia | 0.24 | .27 | 4.97 | 2.02 | .02a | 0 | NA |
| Borderline personality disorder | 0.00 | >.99a | 1.21 | 2.87 | .34a | 0 | NA |
| Bipolar disorder | 0.33 | .497a | 3.55 | 3.22 | .19a | 1 | 0.58 |
| Obsessive-compulsive disorder | −0.33 | .46a | −1.98 | 1.54 | .14a | 0 | NA |
| Bulimia nervosa | 0.00 | >.99a | −2.04 | 2.43 | .25a | 0 | NA |
| Binge-eating disorder | 0.14 | .65a | 1.98 | 1.26 | .09a | 0 | NA |
| Anorexia nervosa | −0.22 | .42 | 2.64 | 2.15 | .13 | 0 | NA |
| Aggregate, All studies | 0.11 | .22 | 2.22 | 0.69 | .001 | 0 | NA |
Abbreviation: NA, not applicable.
Caution is warranted when interpreting P values for categories comprising fewer than 10 studies because of low statistical power.
Discussion
This meta-analysis evaluated the evidence supporting delay discounting as a transdiagnostic process in psychiatric disorders. Consistent with our hypotheses, statistically significant aggregate effect sizes were observed for all disorder categories included in the meta-analysis, although OSR sensitivity analyses indicated that the aggregate effect size was not reliable for obsessive-compulsive disorder. Although the relatively small number of studies in many of the disorder categories precluded thorough consideration of publication bias, the tests examined suggested modest evidence of small study bias.
The primary findings are consistent with the view that delay discounting exists on a continuum.3 Most of the disorder categories examined were characterized by steep discounting in those who had the disorder compared with controls, whereas individuals with anorexia nervosa exhibited the opposite pattern. Taken together, the results support the transdiagnostic nature of delay discounting, although the overall magnitude of differences is not uniform across psychiatric disorders. Bipolar disorder and borderline personality disorder had the largest effect sizes, with differences between groups being in the medium magnitude range. The effect sizes for these disorders are comparable to the effect sizes reported in addiction studies (d = 0.67 in MacKillop et al9). The other disorder categories had somewhat smaller effect sizes that were generally comparable to that for ADHD in meta-analytic findings (d = 0.43 in Jackson and MacKillop12). From the standpoint of the RDoC framework, these findings appear to highlight the need to continue looking into different ways to classify presenting difficulties using a continuum rather than general categories based on DSM diagnoses.
These results raise intriguing questions about the shared underlying mechanisms that might explain the consistent association among disorder categories. One neurocognitive mechanism that is commonly discussed in the context of addiction is impaired self-control, which is associated with dysfunction in competing neurobehavioral decision systems68,69 The competing neurobehavioral decision systems model posits that delay discounting may be associated with 2 competing neural systems: a frontal cortical system that exerts executive control and a limbic-subcortical system that drives immediate reward seeking. According to this model, addiction is characterized by excessive activation of the limbic circuit and dysfunction in the frontal circuit. Disruption in these neural systems has theoretical relevance to many of the other psychiatric disorders we examined.70,71,72,73,74,75,76 For example, the various eating disorder diagnoses illustrate both ends of the competing neurobehavioral decision systems balance. Excessive self-control over food intake in anorexia has been associated with exaggerated activity in dorsal cognitive circuits,77 whereas reduced self-control in bulimia nervosa and binge-eating disorder is partially associated with disruption in the similar frontal circuits.77,78
Other psychological mechanisms may explain the observed results. First, future-oriented cognitive processes, such as episodic future thinking,79 are important for prospectively considering larger delayed rewards in the context of delay discounting. Numerous psychiatric disorders are characterized by deficits in episodic future thinking, including major depressive disorder, bipolar disorder, schizophrenia, borderline personality disorder, eating disorders, and addictive disorders.37,80,81,82,83 Moreover, shifting a person’s focus toward the future through experimental episodic future thinking training has been shown to decrease impulsive delay discounting in healthy samples or individuals with addiction,84,85,86,87 but this shift had not been examined in the other psychiatric disorders included in this study. A second psychological mechanism relates to intolerance of uncertainty, or the tendency to react negatively to uncertain situations.88 Because delayed rewards may be interpreted as uncertain, increased preferences for immediate rewards on discounting tasks could also be explained by heightened intolerance of uncertainty. Consistent with this hypothesis, a positive correlation between steeper discounting and higher scores on an intolerance-of-uncertainty scale was found in a study of healthy participants.89 Although numerous psychiatric disorders are characterized by heightened intolerance of uncertainty,88,90,91 we are not aware of any studies in psychiatric samples that have examined the intersection between this construct and discounting.
Further clarifying the clinical significance of differences in delay discounting appears to be a priority for psychiatric research. In particular, examining whether delay discounting is associated with specific symptoms or symptom clusters may provide greater clinical precision. The studies included in this meta-analysis focused on broad diagnostic categories and not specific subtypes or symptoms within disorders. This focus is an important consideration given that a limited number of previous studies have reported symptom-level associations. For example, the presence of anhedonia (a symptom of major depressive disorder) is associated with decreased discounting,92 and discounting is associated with specific symptoms of schizophrenia (eg, apathy93). Unfortunately, insufficient research is currently available on symptom-level associations to permit meta-analyses.
Another priority is determining whether discounting prospectively estimates treatment outcomes, in which the motivation and willingness to take active steps toward therapeutic goals may be more challenging for individuals who struggle to reliably weigh the advantages of short-term against long-term rewards. This research would dovetail with previous studies on discounting and substance use treatment outcomes.31,94 Future research should also investigate whether discounting rates can be normalized via treatment interventions.95 Various interventions such as episodic future thinking training have been shown to reduce impulsive discounting in individuals with addictive disorders.85,86 Most of these techniques have focused on reducing discounting, which makes them less applicable to disorders with shallow discounting such as anorexia nervosa. How discounting rates can be modified in both directions is an especially novel area of research.
Limitations
This study has a number of limitations. First, despite its comprehensive literature search strategy, the study identified a relatively small number of studies for some disorder categories. This small number may reduce confidence in the accuracy of the aggregate effect sizes observed and constrained power for heterogeneity and publication bias tests. Also notable was the insufficient number of articles on several key disorders, including posttraumatic stress disorder, generalized anxiety disorder, and other personality disorders. Although a few studies examined delay discounting in the context of trauma or posttraumatic stress disorder,38,96 the study designs and samples varied considerably. Characterizing delay discounting in posttraumatic stress disorder seems to be a priority.
Second, studies used a range of criteria and scales to establish clinical diagnoses, which may have exaggerated heterogeneity between studies. Third, although we excluded studies that explicitly examined comorbid substance use and psychiatric disorders, a few of the remaining studies included participants who endorsed use of alcohol or tobacco, whereas others did not report substance use data. Reporting of this information was highly inconsistent across articles; therefore, we were unable to identify the extent to which concurrent substance use may have been a factor in the effect sizes obtained. Fourth, this analysis focused exclusively on monetary discounting. Effect sizes were still in the small-to-medium range for the eating disorder categories despite the use of monetary rewards, but it is possible that other commodities (eg, food rewards or effort discounting97,98) may be more sensitive in specific disorders.
Conclusions
To our knowledge, this meta-analysis is the first quantitative synthesis of delay discounting findings in psychiatric disorders, except ADHD and addictive disorders. This meta-analysis provides relatively strong evidence that delay discounting is a transdiagnostic process in psychiatric disorders. The findings suggest that discounting is not universally increased in all psychiatric disorders but is more appropriately conceptualized as falling on a continuum. Together, the findings generally support the inclusion of delay discounting in the RDoC framework and suggest that discounting is a robust marker of psychiatric illness that may have clinical utility as a target for novel interventions.
eTable 1. Complete List of Search Terms
eTable 2. Raw Data Used to Calculate Effect Size (Hedges g) for Each Study
eTable 3. Complete Meta-analytic Results by Disorder Category
eTable 4. Studies Excluded at Full-Text Stage with Reasons for Exclusion
References
- 1.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751. doi: 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- 2.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lempert KM, Steinglass JE, Pinto A, Kable JW, Simpson HB. Can delay discounting deliver on the promise of RDoC? Psychol Med. 2019;49(2):190-199. doi: 10.1017/S0033291718001770 [DOI] [PubMed] [Google Scholar]
- 4.de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14(1):22-31. doi: 10.1111/j.1369-1600.2008.00129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madden GJ, Bickel WK. Impulsivity: The Behavioral and Neurological Science of Discounting. Washington, DC: American Psychological Association; 2009. [Google Scholar]
- 6.Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012;134(3):287-297. doi: 10.1016/j.pharmthera.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bickel WK, Mueller ET. Toward the study of trans-disease processes: a novel approach with special reference to the study of co-morbidity. J Dual Diagn. 2009;5(2):131-138. doi: 10.1080/15504260902869147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin ME, Haeger J, Ong CW, Twohig MP. An examination of the transdiagnostic role of delay discounting in psychological inflexibility and mental health problems. Psychol Rec. 2018;68(2):201-210. doi: 10.1007/s40732-018-0281-4 [DOI] [Google Scholar]
- 9.MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl). 2011;216(3):305-321. doi: 10.1007/s00213-011-2229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amlung M, Petker T, Jackson J, Balodis I, MacKillop J. Steep discounting of delayed monetary and food rewards in obesity: a meta-analysis. Psychol Med. 2016;46(11):2423-2434. doi: 10.1017/S0033291716000866 [DOI] [PubMed] [Google Scholar]
- 11.Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J. Steep delay discounting and addictive behavior: a meta-analysis of continuous associations. Addiction. 2017;112(1):51-62. doi: 10.1111/add.13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson JN, MacKillop J. Attention-deficit/hyperactivity disorder and monetary delay discounting: a meta-analysis of case-control studies. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(4):316-325. doi: 10.1016/j.bpsc.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn W-Y, Rass O, Fridberg DJ, et al. Temporal discounting of rewards in patients with bipolar disorder and schizophrenia. J Abnorm Psychol. 2011;120(4):911-921. doi: 10.1037/a0023333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cogn Neuropsychiatry. 2007;12(3):213-221. doi: 10.1080/13546800601005900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strakowski SM, Fleck DE, DelBello MP, et al. Impulsivity across the course of bipolar disorder. Bipolar Disord. 2010;12(3):285-297. doi: 10.1111/j.1399-5618.2010.00806.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imhoff S, Harris M, Weiser J, Reynolds B. Delay discounting by depressed and non-depressed adolescent smokers and non-smokers. Drug Alcohol Depend. 2014;135(1):152-155. doi: 10.1016/j.drugalcdep.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 17.Cáceda R, Durand D, Cortes E, et al. Impulsive choice and psychological pain in acutely suicidal depressed patients. Psychosom Med. 2014;76(6):445-451. doi: 10.1097/PSY.0000000000000075 [DOI] [PubMed] [Google Scholar]
- 18.Barker V, Romaniuk L, Cardinal RN, Pope M, Nicol K, Hall J. Impulsivity in borderline personality disorder. Psychol Med. 2015;45(9):1955-1964. doi: 10.1017/S0033291714003079 [DOI] [PubMed] [Google Scholar]
- 19.Lawrence KA, Allen JS, Chanen AM. Impulsivity in borderline personality disorder: reward-based decision-making and its relationship to emotional distress. J Pers Disord. 2010;24(6):786-799. doi: 10.1521/pedi.2010.24.6.785 [DOI] [PubMed] [Google Scholar]
- 20.Decker JH, Figner B, Steinglass JE. On weight and waiting: delay discounting in anorexia nervosa pretreatment and posttreatment. Biol Psychiatry. 2015;78(9):606-614. doi: 10.1016/j.biopsych.2014.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinglass JE, Figner B, Berkowitz S, Simpson HB, Weber EU, Walsh BT. Increased capacity to delay reward in anorexia nervosa. J Int Neuropsychol Soc. 2012;18(4):773-780. doi: 10.1017/S1355617712000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinto A, Steinglass JE, Greene AL, Weber EU, Simpson HB. Capacity to delay reward differentiates obsessive-compulsive disorder and obsessive-compulsive personality disorder. Biol Psychiatry. 2014;75(8):653-659. doi: 10.1016/j.biopsych.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weidberg S, García-Rodríguez O, Yoon JH, Secades-Villa R. Interaction of depressive symptoms and smoking abstinence on delay discounting rates. Psychol Addict Behav. 2015;29(4):1041-1047. doi: 10.1037/adb0000073 [DOI] [PubMed] [Google Scholar]
- 24.Wing VC, Moss TG, Rabin RA, George TP. Effects of cigarette smoking status on delay discounting in schizophrenia and healthy controls. Addict Behav. 2012;37(1):67-72. doi: 10.1016/j.addbeh.2011.08.012 [DOI] [PubMed] [Google Scholar]
- 25.Maraz A, Andó B, Rigó P, et al. The two-faceted nature of impulsivity in patients with borderline personality disorder and substance use disorder. Drug Alcohol Depend. 2016;163:48-54. doi: 10.1016/j.drugalcdep.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 26.Ritschel F, King JA, Geisler D, et al. Temporal delay discounting in acutely ill and weight-recovered patients with anorexia nervosa. Psychol Med. 2015;45(6):1229-1239. doi: 10.1017/S0033291714002311 [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association Structured Clinical Interview for DSM-5: Research Version. Washington, DC: American Psychiatric Association Publishing; 2015. [Google Scholar]
- 28.First MB, Gibbon M. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I, Version 2.0.). New York, NY: New York State Psychiatric Institute, Biometrics Research Department; 1995. [Google Scholar]
- 29.Kekic M, Bartholdy S, Cheng J, et al. Increased temporal discounting in bulimia nervosa. Int J Eat Disord. 2016;49(12):1077-1081. doi: 10.1002/eat.22571 [DOI] [PubMed] [Google Scholar]
- 30.Moody L, Franck C, Bickel WK. Comorbid depression, antisocial personality, and substance dependence: relationship with delay discounting. Drug Alcohol Depend. 2016;160:190-196. doi: 10.1016/j.drugalcdep.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Exp Clin Psychopharmacol. 2007;15(2):176-186. doi: 10.1037/1064-1297.15.2.186 [DOI] [PubMed] [Google Scholar]
- 32.Stewart LA, Clarke M, Rovers M, et al. ; PRISMA-IPD Development Group . Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313(16):1657-1665. doi: 10.1001/jama.2015.3656 [DOI] [PubMed] [Google Scholar]
- 33.Manasse SM, Forman EM, Ruocco AC, Butryn ML, Juarascio AS, Fitzpatrick KK. Do executive functioning deficits underpin binge eating disorder? A comparison of overweight women with and without binge eating pathology. Int J Eat Disord. 2015;48(6):677-683. doi: 10.1002/eat.22383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wierenga CE, Bischoff-Grethe A, Melrose AJ, et al. Hunger does not motivate reward in women remitted from anorexia nervosa. Biol Psychiatry. 2015;77(7):642-652. doi: 10.1016/j.biopsych.2014.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sohn SY, Kang JI, Namkoong K, Kim SJ. Multidimensional measures of impulsivity in obsessive-compulsive disorder: cannot wait and stop. PLoS One. 2014;9(11):e111739. doi: 10.1371/journal.pone.0111739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avsar KB, Weller RE, Cox JE, Reid MA, White DM, Lahti AC. An fMRI investigation of delay discounting in patients with schizophrenia. Brain Behav. 2013;3(4):384-401. doi: 10.1002/brb3.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heerey EA, Matveeva TM, Gold JM. Imagining the future: degraded representations of future rewards and events in schizophrenia. J Abnorm Psychol. 2011;120(2):483-489. doi: 10.1037/a0021810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engelmann JB, Maciuba B, Vaughan C, Paulus MP, Dunlop BW. Posttraumatic stress disorder increases sensitivity to long term losses among patients with major depressive disorder. PLoS One. 2013;8(10):e78292. doi: 10.1371/journal.pone.0078292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown HE, Hart KL, Snapper LA, Roffman JL, Perlis RH. Impairment in delay discounting in schizophrenia and schizoaffective disorder but not primary mood disorders. NPJ Schizophr. 2018;4(1):9. doi: 10.1038/s41537-018-0050-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dombrovski AY, Szanto K, Siegle GJ, et al. Lethal forethought: delayed reward discounting differentiates high- and low-lethality suicide attempts in old age. Biol Psychiatry. 2011;70(2):138-144. doi: 10.1016/j.biopsych.2010.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pulcu E, Trotter PD, Thomas EJ, et al. Temporal discounting in major depressive disorder. Psychol Med. 2014;44(9):1825-1834. doi: 10.1017/S0033291713002584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horan WP, Johnson MW, Green MF. Altered experiential, but not hypothetical, delay discounting in schizophrenia. J Abnorm Psychol. 2017;126(3):301-311. doi: 10.1037/abn0000249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacKillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology (Berl). 2011;216(1):91-99. doi: 10.1007/s00213-011-2185-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Jin S, He K, et al. Increased delayed reward during intertemporal decision-making in schizophrenic patients and their unaffected siblings. Psychiatry Res. 2018;262(262):246-253. doi: 10.1016/j.psychres.2017.12.040 [DOI] [PubMed] [Google Scholar]
- 45.Yu LQ, Lee S, Katchmar N, Satterthwaite TD, Kable JW, Wolf DH. Steeper discounting of delayed rewards in schizophrenia but not first-degree relatives. Psychiatry Res. 2017;252(252):303-309. doi: 10.1016/j.psychres.2017.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berenson KR, Gregory WE, Glaser E, et al. Impulsivity, rejection sensitivity, and reactions to stressors in borderline personality disorder. Cognit Ther Res. 2016;40(4):510-521. doi: 10.1007/s10608-015-9752-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coffey SF, Schumacher JA, Baschnagel JS, Hawk LW, Holloman G. Impulsivity and risk-taking in borderline personality disorder with and without substance use disorders. Personal Disord. 2011;2(2):128-141. doi: 10.1037/a0020574 [DOI] [PubMed] [Google Scholar]
- 48.Dougherty DM, Bjork JM, Huckabee HCG, Moeller FG, Swann AC. Laboratory measures of aggression and impulsivity in women with borderline personality disorder. Psychiatry Res. 1999;85(3):315-326. doi: 10.1016/S0165-1781(99)00011-6 [DOI] [PubMed] [Google Scholar]
- 49.Krause-Utz A, Cackowski S, Daffner S, et al. Delay discounting and response disinhibition under acute experimental stress in women with borderline personality disorder and adult attention deficit hyperactivity disorder. Psychol Med. 2016;46(15):3137-3149. doi: 10.1017/S0033291716001677 [DOI] [PubMed] [Google Scholar]
- 50.Urošević S, Youngstrom EA, Collins P, Jensen JB, Luciana M. Associations of age with reward delay discounting and response inhibition in adolescents with bipolar disorders. J Affect Disord. 2016;190:649-656. doi: 10.1016/j.jad.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norman LJ, Carlisi CO, Christakou A, et al. Neural dysfunction during temporal discounting in paediatric attention-deficit/hyperactivity disorder and obsessive-compulsive disorder. Psychiatry Res Neuroimaging. 2017;269(September):97-105. doi: 10.1016/j.pscychresns.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steinglass JE, Lempert KM, Choo T-H, et al. Temporal discounting across three psychiatric disorders: anorexia nervosa, obsessive compulsive disorder, and social anxiety disorder. Depress Anxiety. 2017;34(5):463-470. doi: 10.1002/da.22586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartholdy S, Rennalls S, Danby H, et al. Temporal discounting and the tendency to delay gratification across the eating disorder spectrum. Eur Eat Disord Rev. 2017;25(5):344-350. doi: 10.1002/erv.2513 [DOI] [PubMed] [Google Scholar]
- 54.Neveu R, Neveu D, Barsumian F, et al. Improved planning abilities in binge eating. PLoS One. 2014;9(8):e105657. doi: 10.1371/journal.pone.0105657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis C, Patte K, Curtis C, Reid C. Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite. 2010;54(1):208-213. doi: 10.1016/j.appet.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 56.Manwaring JL, Green L, Myerson J, Strube MJ, Wilfley DE. Discounting of various types of rewards by women with and without binge eating disorder: evidence for general rather than specific differences. Psychol Rec. 2011;61(4):561-582. doi: 10.1007/BF03395777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mole TB, Irvine MA, Worbe Y, et al. Impulsivity in disorders of food and drug misuse. Psychol Med. 2015;45(4):771-782. doi: 10.1017/S0033291714001834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steward T, Mestre-Bach G, Vintró-Alcaraz C, et al. Delay discounting of reward and impulsivity in eating disorders: from anorexia nervosa to binge eating disorder. Eur Eat Disord Rev. 2017;25(6):601-606. doi: 10.1002/erv.2543 [DOI] [PubMed] [Google Scholar]
- 59.Yan W-S, Zhang RR, Lan Y, Li Z-M, Li Y-H. Questionnaire-based maladaptive decision-coping patterns involved in binge eating among 1013 college students. Front Psychol. 2018;9:609. doi: 10.3389/fpsyg.2018.00609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.King JA, Geisler D, Bernardoni F, et al. Altered neural efficiency of decision making during temporal reward discounting in anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 2016;55(11):972-979. doi: 10.1016/j.jaac.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 61.Kirby KN, Maraković NN. Delay-discounting probabilistic rewards: rates decrease as amounts increase. Psychon Bull Rev. 1996;3(1):100-104. doi: 10.3758/BF03210748 [DOI] [PubMed] [Google Scholar]
- 62.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. West Sussex, UK: John Wiley & Sons, Ltd; 2009. doi: 10.1002/9780470743386 [DOI] [Google Scholar]
- 63.Tukey JW. Bias and confidence in not quite large samples. Ann Math Stat. 1958;29:614. doi: 10.1214/aoms/1177706647 [DOI] [Google Scholar]
- 64.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 65.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 67.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 68.Bickel WK, Yi R. Temporal discounting as a measure of executive function: insights from the competing neuro-behavioral decision system hypothesis of addiction. Adv Health Econ Health Serv Res. 2008;20:289-309. doi: 10.1016/S0731-2199(08)20012-9 [DOI] [PubMed] [Google Scholar]
- 69.Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90(Suppl 1)(suppl 1):S85-S91. doi: 10.1016/j.drugalcdep.2006.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anticevic A, Hu S, Zhang S, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 2014;75(8):595-605. doi: 10.1016/j.biopsych.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin NY, Lee TY, Kim E, Kwon JS. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2014;44(6):1121-1130. doi: 10.1017/S0033291713001803 [DOI] [PubMed] [Google Scholar]
- 72.Mak ADP, Lam LCW. Neurocognitive profiles of people with borderline personality disorder. Curr Opin Psychiatry. 2013;26(1):90-96. doi: 10.1097/YCO.0b013e32835b57a9 [DOI] [PubMed] [Google Scholar]
- 73.Sebastian A, Jung P, Krause-Utz A, Lieb K, Schmahl C, Tüscher O. Frontal dysfunctions of impulse control - a systematic review in borderline personality disorder and attention-deficit/hyperactivity disorder. Front Hum Neurosci. 2014;8:698. doi: 10.3389/fnhum.2014.00698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen C-H, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13(1):1-15. doi: 10.1111/j.1399-5618.2011.00893.x [DOI] [PubMed] [Google Scholar]
- 75.Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117(1-2):1-17. doi: 10.1016/j.jad.2008.11.021 [DOI] [PubMed] [Google Scholar]
- 76.Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117(1):1-12. doi: 10.1016/j.schres.2009.12.022 [DOI] [PubMed] [Google Scholar]
- 77.Kaye WH, Wagner A, Fudge JL, Paulus M. Neurocircuity of eating disorders. Curr Top Behav Neurosci. 2011;6:37-57. doi: 10.1007/7854_2010_85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Friederich H-C, Wu M, Simon JJ, Herzog W. Neurocircuit function in eating disorders. Int J Eat Disord. 2013;46(5):425-432. doi: 10.1002/eat.22099 [DOI] [PubMed] [Google Scholar]
- 79.Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: concepts, data, and applications. Ann N Y Acad Sci. 2008;1124:39-60. doi: 10.1196/annals.1440.001 [DOI] [PubMed] [Google Scholar]
- 80.Hallford DJ, Austin DW, Takano K, Raes F. Psychopathology and episodic future thinking: a systematic review and meta-analysis of specificity and episodic detail. Behav Res Ther. 2018;102:42-51. doi: 10.1016/j.brat.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 81.D’Argembeau A, Raffard S, Van der Linden M. Remembering the past and imagining the future in schizophrenia. J Abnorm Psychol. 2008;117(1):247-251. doi: 10.1037/0021-843X.117.1.247 [DOI] [PubMed] [Google Scholar]
- 82.Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93(5):729-738. doi: 10.1046/j.1360-0443.1998.9357298.x [DOI] [PubMed] [Google Scholar]
- 83.Rasmussen AS, Jørgensen CR, O’Connor M, et al. The structure of past and future events in borderline personality disorder, eating disorder, and obsessive–compulsive disorder. Psychol Conscious Theory. 2017;4(2):190-210. doi: 10.1037/cns0000109 [DOI] [Google Scholar]
- 84.Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66(1):138-148. doi: 10.1016/j.neuron.2010.03.026 [DOI] [PubMed] [Google Scholar]
- 85.Snider SE, LaConte SM, Bickel WK. Episodic future thinking: expansion of the temporal window in individuals with alcohol dependence. Alcohol Clin Exp Res. 2016;40(7):1558-1566. doi: 10.1111/acer.13112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stein JS, Wilson AG, Koffarnus MN, Daniel TO, Epstein LH, Bickel WK. Unstuck in time: episodic future thinking reduces delay discounting and cigarette smoking. Psychopharmacology (Berl). 2016;233(21-22):3771-3778. doi: 10.1007/s00213-016-4410-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Daniel TO, Stanton CM, Epstein LH. The future is now: reducing impulsivity and energy intake using episodic future thinking. Psychol Sci. 2013;24(11):2339-2342. doi: 10.1177/0956797613488780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Einstein DA. Extension of the transdiagnostic model to focus on intolerance of uncertainty: a review of the literature and implications for treatment. Clin Psychol (New York). 2014;21(3):280-300. doi: 10.1111/cpsp.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luhmann CC, Ishida K, Hajcak G. Intolerance of uncertainty and decisions about delayed, probabilistic rewards. Behav Ther. 2011;42(3):378-386. doi: 10.1016/j.beth.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 90.Brown M, Robinson L, Campione GC, Wuensch K, Hildebrandt T, Micali N. Intolerance of uncertainty in eating disorders: a systematic review and meta-analysis. Eur Eat Disord Rev. 2017;25(5):329-343. doi: 10.1002/erv.2523 [DOI] [PubMed] [Google Scholar]
- 91.Tolin DF, Abramowitz JS, Brigidi BD, Foa EB. Intolerance of uncertainty in obsessive-compulsive disorder. J Anxiety Disord. 2003;17(2):233-242. doi: 10.1016/S0887-6185(02)00182-2 [DOI] [PubMed] [Google Scholar]
- 92.Lempert KM, Pizzagalli DA. Delay discounting and future-directed thinking in anhedonic individuals. J Behav Ther Exp Psychiatry. 2010;41(3):258-264. doi: 10.1016/j.jbtep.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hartmann MN, Hager OM, Reimann AV, et al. Apathy but not diminished expression in schizophrenia is associated with discounting of monetary rewards by physical effort. Schizophr Bull. 2015;41(2):503-512. doi: 10.1093/schbul/sbu102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sheffer C, Mackillop J, McGeary J, et al. Delay discounting, locus of control, and cognitive impulsiveness independently predict tobacco dependence treatment outcomes in a highly dependent, lower socioeconomic group of smokers. Am J Addict. 2012;21(3):221-232. doi: 10.1111/j.1521-0391.2012.00224.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koffarnus MN, Jarmolowicz DP, Mueller ET, Bickel WK. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: a review. J Exp Anal Behav. 2013;99(1):32-57. doi: 10.1002/jeab.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li J-Z, Li S, Liu H. How has the Wenchuan earthquake influenced people’s intertemporal choices?. J Appl Soc Psychol. 2011;41(11):2739-2752. doi: 10.1111/j.1559-1816.2011.00847.x [DOI] [Google Scholar]
- 97.Docx L, de la Asuncion J, Sabbe B, et al. Effort discounting and its association with negative symptoms in schizophrenia. Cogn Neuropsychiatry. 2015;20(2):172-185. doi: 10.1080/13546805.2014.993463 [DOI] [PubMed] [Google Scholar]
- 98.Robertson SH, Rasmussen EB. Comparison of potentially real versus hypothetical food outcomes in delay and probability discounting tasks. Behav Processes. 2018;149:8-15. doi: 10.1016/j.beproc.2018.01.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Complete List of Search Terms
eTable 2. Raw Data Used to Calculate Effect Size (Hedges g) for Each Study
eTable 3. Complete Meta-analytic Results by Disorder Category
eTable 4. Studies Excluded at Full-Text Stage with Reasons for Exclusion