Key Points
Question
Is a mobile technology–supported primary health care intervention associated with greater use of preventive drug treatments compared with usual care among individuals at high risk of cardiovascular disease?
Findings
In this quasi-experimental study involving 8 villages and 6579 high-risk individuals in rural Indonesia, 15.5% of individuals in the intervention villages reported appropriate use of preventive medications compared with 1.0% in the control villages. A total of 56.8% of individuals in the intervention villages used blood pressure medication vs 15.7% of individuals in the control villages.
Meaning
The primary health care intervention was associated with increased use of preventive drug therapies in people with high estimated risk of cardiovascular disease.
Abstract
Importance
Cardiovascular diseases (CVDs) are the leading cause of disease burden in Indonesia. Implementation of effective interventions for CVD prevention is limited.
Objective
To evaluate whether a mobile technology–supported primary health care intervention, compared with usual care, would improve the use of preventive drug treatment among people in rural Indonesia with a high risk of CVD.
Design, Setting, and Participants
A quasi-experimental study involving 6579 high-risk individuals in 4 intervention and 4 control villages in Malang district, Indonesia, was conducted between August 16, 2016, and March 31, 2018. Median duration of follow-up was 12.2 months. Residents 40 years or older were invited to participate. Those with high estimated 10-year risk of CVD risk (previously diagnosed CVD, systolic blood pressure [BP] >160 mm Hg or diastolic BP >100 mm Hg, 10-year estimated CVD risk of 30% or more, or 10-year estimated CVD risk of 20%-29% and a systolic BP >140 mm Hg) were followed up.
Interventions
A multifaceted mobile technology–supported intervention facilitating community-based CVD risk screening with referral, tailored clinical decision support for drug prescription, and patient follow-up.
Main Outcomes and Measures
The primary outcome was the proportion of individuals taking appropriate preventive CVD medications, defined as at least 1 BP-lowering drug and a statin for all high-risk individuals, and an antiplatelet drug for those with prior diagnosed CVD. Secondary outcomes included mean change in BP from baseline.
Results
Among 22 635 adults, 3494 of 11 647 in the intervention villages (30.0%; 2166 women and 1328 men; mean [SD] age, 58.3 [10.9] years) and 3085 of 10 988 in the control villages (28.1%; 1838 women and 1247 men; mean [SD] age, 59.0 [11.5] years) had high estimated risk of CVD. Of these, follow-up was completed in 2632 individuals (75.3%) from intervention villages and 2429 individuals (78.7%) from control villages. At follow-up, 409 high-risk individuals in intervention villages (15.5%) were taking appropriate preventive CVD medications, compared with 25 (1.0%) in control villages (adjusted risk difference, 14.1%; 95% CI, 12.7%-15.6%). This difference was driven by higher use of BP-lowering medication in those in the intervention villages (1495 [56.8%] vs 382 [15.7%]; adjusted risk difference, 39.4%; 95% CI, 37.0%-41.7%). The adjusted mean difference in change in systolic BP from baseline was −8.3 mm Hg (95% CI, −10.1 to −6.6 mm Hg).
Conclusions and Relevance
This study found that a multifaceted mobile technology–supported primary health care intervention was associated with greater use of preventive CVD medication and lower BP levels among high-risk individuals in a rural Indonesian population.
This quasi-experimental study evaluates whether a mobile technology–supported primary health care intervention, compared with usual care, would improve the use of preventive drug treatment among people in rural Indonesia with a high risk of cardiovascular disease.
Introduction
The high burden of cardiovascular diseases (CVDs) in low- and middle-income countries has increased the need for health care systems to deliver effective preventive care.1,2,3 Emphasis has been placed on strengthening primary health care systems traditionally oriented toward maternal and child health care and acute episodic care for infectious diseases.4,5 Mobile health solutions may facilitate this reorientation and strengthening of the primary health care system. However, a 2017 systematic review examining mobile health interventions for noncommunicable disease management in low- and middle-income countries found limited evidence of effectiveness, with interventions generally narrow in focus and dominated by text messaging for patients.6 This finding has led to speculation that the limited effect of mobile health innovations may be associated with a tendency to focus on single health system domains.7
In Indonesia, a lower-middle income country by World Bank classification, ischemic heart disease and cerebrovascular disease are the 2 leading causes of disability-adjusted life years lost, with CVD estimated to be the cause of one-third of all deaths in 2016.8 Existing data suggest that less than one-third of Indonesians with moderate to high risk of CVD receive any preventive care.9 Current Indonesian government policy responses articulate a strategy for preventing and managing CVD through advocacy, health promotion, and strengthening the health care system.10 As the health care system is highly decentralized, local district health agencies are pivotal in implementing these policies. The agencies are responsible for health care delivery by nurses and community health care workers at neighborhood and village health centers, and by physicians at subdistrict-level primary health care centers. To strengthen primary health care, the government is also currently implementing a comprehensive electronic health (eHealth) platform.
This policy and emerging eHealth environment provide an opportunity to develop innovative technology-enabled primary health care interventions with the potential for implementation at scale. Building on work in Australia, China, and India,11,12,13 with a common component of clinical decision support but variation in disease focus and health system integration, we adapted SMARThealth (Systematic Medical Appraisal Referral and Treatment), a mobile technology–supported, multifaceted primary health care intervention aimed at improving the provision of guideline-based assessment and management of CVD risk to the Malang district of East Java, Indonesia. We hypothesized that, compared with usual care, this intervention would be associated with greater appropriate use of preventive medication and lower blood pressure levels among individuals at high risk of CVD.
Methods
Study Design
Details of the SMARThealth intervention are outlined in eAppendix 1 in the Supplement. The study received ethics approval from the Ethical Committee, Ministry of Research, Technology, and Higher Education, Medical Faculty of Brawijaya University (330/EC/KEPK/08/2016) and was registered on the Clinical Trial Registry of India (CTRI/2017/08/009387). Written informed consent was obtained from all participants who contributed data for analysis.
In brief, the intervention enabled neighborhood-based nonphysician community health care workers (kaders in the Indonesian context), nurses at the village health centers, and physicians at the primary health care centers to assess CVD risk using basic equipment and a clinical decision support application on a mobile tablet device. The application allowed kaders to collect essential information, inform individuals of their risk status, provide lifestyle advice, and refer high-risk individuals for nurse or physician consultation. High estimated risk was defined by the presence of the following: (1) a history of CVD confirmed by a physician, (2) an extreme blood pressure elevation (systolic blood pressure >160 mm Hg or diastolic blood pressure >100 mm Hg), (3) a 10-year estimated CVD risk of 30% or more, or (4) a 10-year estimated CVD risk of 20% to 29% and a systolic blood pressure higher than 140 mm Hg. In the absence of Indonesian risk prediction charts, the 10-year risk of fatal or nonfatal major CVD event was estimated using algorithms based on the World Health Organization/International Society of Hypertension “low information” risk charts tailored to the Southeast Asian Region-B, which recommends screening individuals 40 years or older using age, sex, blood pressure, smoking and diabetes status.14 Shared electronic record functionality allowed synchronous or asynchronous capture of patient data that were securely sent to and accessed from a central server. Physicians (on monthly visits to village health centers) and nurses also used a mobile application to receive tailored decision support regarding appropriate prescription of preventive medications, using previous data collected by the kaders as well as new data collected during patient consultations. Treatment plans were immediately available to kaders, ensuring community-based follow-up. An automated system alerted high-risk individuals by text message or interactive voice response to attend follow-up visits with health care professionals and provided reminders promoting medication adherence. Community-wide health promotion; training, performance management, and activity-based remuneration of health care workers; and support of essential medication procurement underpinned this system of care. Prior to finalizing the intervention, a health systems assessment was undertaken with district health authorities. This assessment, using an adapted Rapid Assessment Protocol for Insulin Access tool,15 helped contextualize the theory-based logic model previously developed for SMARThealth and modify the components as required (eFigure 1 in the Supplement). Logic model development and subsequent intervention modifications were guided by the Behavior Change Wheel of Michie et al16 that seeks to influence capability, opportunity, and motivation to support behavior change.
From August 16, 2016, to March 31, 2018, we performed a controlled quasi-experimental study of this complex primary care intervention in 4 intervention and 4 control villages in the Malang district of East Java, Indonesia. Because the intervention was delivered through the existing health care infrastructure, close involvement of the district health authority, health care workers (including kaders), and community members in co-production and implementation was crucial. After detailed consultation, the strong preference of local partners was to identify villages for intervention in which resources could be most easily accessed and adapted for timely implementation. Consequently, random selection of villages for the intervention was deemed infeasible. The Malang District Health Agency selected 4 villages from 4 primary health care centers to maximize feasibility and geographic and socioeconomic diversity. To be eligible, each primary health care center had to have at least 1 physician, and each village health center had to have at least 1 nurse regularly providing services and willing to participate in implementation of SMARThealth.
Four control villages were subsequently chosen. Each control village was matched with an intervention village based on population size, rurality, predominant occupation of residents, distance from tobacco factories, and number of kaders. As an adequately matched control village could not be identified in the catchment area in the case of 1 primary health care center, a control village from a neighboring primary health center catchment area was selected (eFigure 2 in the Supplement).
Procedures
In all 8 villages, field researchers undertook a full census of adults 40 years or older through household visits between August 16, 2016, and April 8, 2017. This census constituted baseline data collected using identical equipment, procedures, and criteria as used by kaders in the intervention villages (eAppendix 1 in the Supplement). Independent assessors reevaluated villages between February 27 and March 31, 2018. Primary evaluation of the intervention was based on researcher-identified high-risk individuals in the intervention villages, compared with researcher-identified high-risk individuals in the control villages. Owing to anticipated discordance between researcher-identified and kader-identified high-risk individuals in the intervention villages (eTable 1 in the Supplement), prespecified sensitivity analyses were based on kader-identified high-risk individuals. To reduce the risk of ascertainment bias, field researchers were provided with lists of high-risk individuals for follow-up in all villages but were not advised of the village allocation status.
Outcomes
The primary outcome was the proportion of high-risk individuals using appropriate preventive medications at follow-up. This outcome was defined as self-reported use of at least 1 blood pressure–lowering drug and statin for people at high risk without prior physician-diagnosed CVD, or self-reported use of at least 1 blood pressure–lowering drug, statin, and an antiplatelet agent (unless there was concomitant anticoagulant use) for people with established CVD. Secondary outcomes were the proportion of high-risk individuals achieving a systolic blood pressure target of less than 140 mm Hg and the mean change in systolic and diastolic blood pressure levels from baseline to the end of follow-up among high-risk individuals. For the intervention villages, reporting of proportions of high-risk individuals referred by kaders to nurses or physicians and of high-risk individuals receiving at least 1 follow-up visit by a kader was prespecified.
Statistical Analysis
Eight villages allocated equally to intervention and control were estimated to provide 80% power with a 2-sided α = .05 to detect an absolute difference of 18% in the proportion of high-risk people taking appropriate preventive medications, assuming a baseline rate of 10%, cluster size of 144 individuals, and an intraclass correlation coefficient of 0.05.
Baseline characteristics of high-risk individuals were compared using χ2 and t tests as appropriate, with computation of standardized differences.17 The associations between the intervention and dichotomous outcomes were tested using modified Poisson models that used a robust variance estimator with generalized estimating equations to estimate the adjusted relative risk and 95% CI.18 Binomial models were used to estimate the adjusted risk difference with its 95% CI. Linear mixed models were used to report adjusted mean differences (with 95% CI) for continuous outcomes. For all outcomes, to account for correlations between participants from the same village, generalized estimating equations with an exchangeable correlation structure that assumes all pairs of observations from the same village have a common correlation were used.
All models adjusted for baseline values of the outcome as well as baseline covariates, with a between-group standardized difference of 0.1 or more (with the exception of avoiding adjusting for baseline use of individual component drug modalities for the outcome of appropriate medication use, and vice versa). For all outcomes, we performed post hoc sensitivity analyses adjusting for no covariates and for all baseline covariates.
In additional post hoc analyses, the homogeneity of associations across subgroups with the primary outcome was tested by adding interaction terms to each model. Subgroups using baseline characteristics included age (above and below the median at baseline), sex, diabetes, current smoking, educational level (primary school or less, some high school, and more than high school), high-risk group type, and systolic blood pressure (above and below the median at baseline).
All statistical significance tests were conducted using a 2-sided type I error rate of 5%. P < .05 was considered significant. For secondary outcomes, adjustment for testing multiplicity used a sequential Holm-Bonferroni method using a family size of 3, where all secondary outcomes are considered as part of the same family.19 As fewer than 2% of the primary and secondary outcome variables were missing, no imputation methods were used. Sample size was calculated using PASS 16 (NCSS LLC). All analyses were conducted using SAS Enterprise Guide, version 7.15 (SAS Institute Inc). Details for computing standardized differences, adjusting for testing multiplicity, and calculating intraclass correlation coefficients are provided in eAppendix 2 in the Supplement.
Results
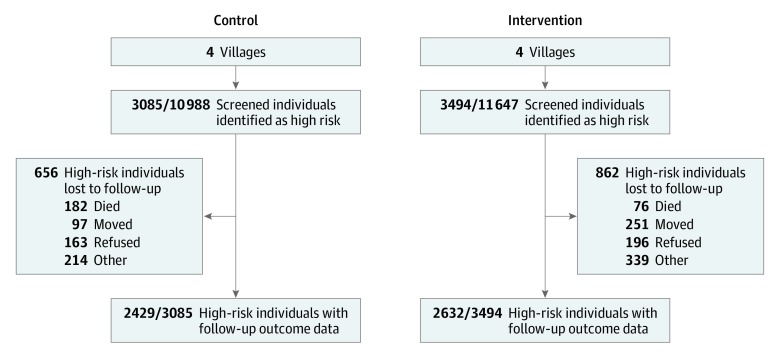
Baseline data collection began in August 2016, with follow-up data collection completed in March 2018. In total, 22 635 adults 40 years or older were identified (11 647 in the intervention villages and 10 988 in the control villages) (Figure 1; eTable 2 in the Supplement). In the intervention villages, 3494 individuals (30.0%) were identified as being at high risk of CVD, compared with 3085 individuals (28.1%) in the control villages. Of these, follow-up was completed in 2632 individuals (75.3%) from intervention villages and 2429 individuals (78.7%) from control villages. Participants who were lost to follow-up appeared to be at higher risk of CVD than those who were followed up, although baseline blood pressure and treatment rates were similar (eTable 3 in the Supplement). The median period from identification of high-risk status to follow-up assessment was 12.6 months (interquartile range [IQR], 12.2-13.1 months) for control villages. This period was shorter than the corresponding median period for intervention villages (18.0 months; IQR, 17.5-18.5 months), but similar to the median period between intervention initiation and end of follow-up (11.5; IQR, 10.9-12.2 months). This difference is explained by the need to deploy a limited number of field researchers to perform complete baseline assessments sequentially, beginning first in intervention villages, followed by control villages.
Figure 1. Flowchart of Participants Through the Study.
Several baseline characteristics of high-risk individuals were statistically significantly different between the control and intervention villages (Table 1; eTable 4 in the Supplement). Those in the intervention villages were on average younger and more educated, with a higher mean body mass index and a greater prevalence of diabetes. Preventive treatment rates with blood pressure–lowering drugs, statins, and antiplatelet medications were higher in the intervention group compared with the control group.
Table 1. Baseline Characteristics of the High-Risk Populationa.
| Characteristic | Control Villages (n = 3085) | Intervention Villages (n = 3494) | P Value | Standardized Difference |
|---|---|---|---|---|
| Age, mean (SD), y | 59.0 (11.5) | 58.3 (10.9) | .02 | .06 |
| Female, No. (%) | 1838 (59.6) | 2166 (62.0) | .07 | .03 |
| Educational level, No./total No. (%) | ||||
| Primary school or less | 2136/3085 (69.2) | 2139/3491 (61.3) | <.001 | .17 |
| Some high school | 791/3085 (25.6) | 1153/3491 (33.0) | ||
| More than high school | 158/3085 (5.1) | 199/3491 (5.7) | ||
| Diabetes, No. (%) | 247 (8.0) | 344 (9.8) | .009 | .06 |
| Current smoking, No. (%) | 595 (19.3) | 633 (18.1) | .22 | .03 |
| Systolic blood pressure, mean (SD), mm Hg | 167.3 (21.3) | 166.6 (22.2) | .20 | .03 |
| Diastolic blood pressure, mean (SD), mm Hg | 101.3 (13.1) | 101.1 (13.7) | .40 | .02 |
| Body mass index, mean (SD)b | 25.7 (4.8) | 26.0 (4.8) | .006 | .06 |
| High risk owing to known cardiovascular disease, No. (%) | 499 (16.2) | 729 (20.9) | <.001 | .12 |
| High risk owing to other reasons, No. (%) | 2586 (83.8) | 2765 (79.1) | <.001 | .12 |
| Taking appropriate preventive medications, No. (%)c | 2 (0.06) | 28 (0.8) | <.001 | .11 |
| Taking blood pressure–lowering medication(s), No. (%) | 304 (9.9) | 484 (13.9) | <.001 | .12 |
| Taking statin therapy, No. (%) | 21 (0.7) | 75 (2.1) | .001 | .12 |
| Taking antiplatelet medication(s), No./total No. (%)d | 13/499 (2.6) | 47/729 (6.4) | .002 | .18 |
Missing values: body mass index (63 control and 56 intervention villages); blood pressure (5 control and 8 intervention villages). The missing blood pressure values were due to data transmission errors from the mobile application to the central database because there were no missing values for determining high-risk status (the automatic calculation of which requires blood pressure values for those without known cardiovascular disease).
Calculated as weight in kilograms divided by height in meters squared.
Combination of blood pressure–lowering medication, statin therapy, and antiplatelet medication if high risk owing to known cardiovascular disease; combination of blood pressure–lowering medication(s) and statin therapy if high risk owing to other reasons.
Among individuals at high risk owing to known cardiovascular disease.
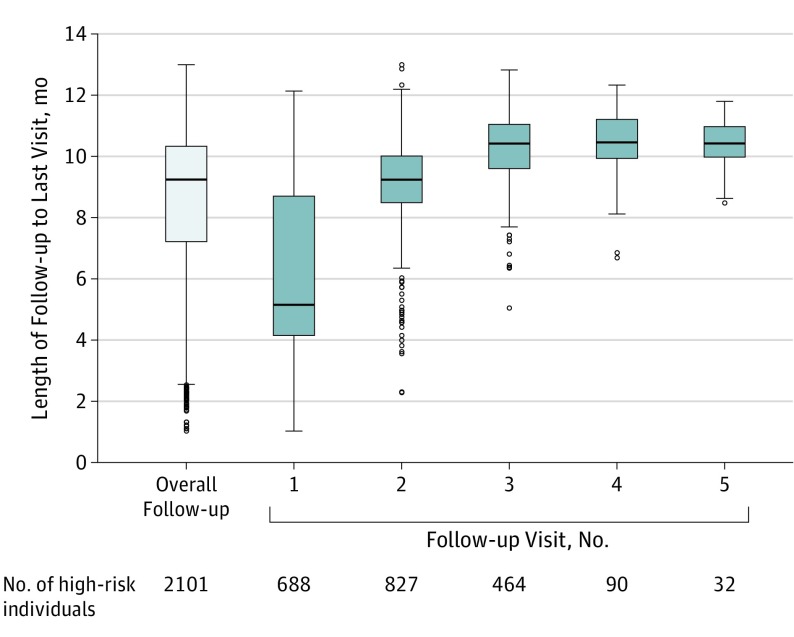
In the intervention villages, kaders screened 86.4% of the census population (10 063 of 11 647 individuals) through household visits, identifying 22.9% (2301 of 10 063 individuals) as being at high risk of CVD. There was discordance between researcher-identified and kader-identified high-risk individuals (eTable 1 in the Supplement), anticipated as a result of visit-to-visit blood pressure variability, including regression to the mean. All high-risk individuals identified by kaders were referred for further care. Of these, 1060 (46.1%) visited only a public sector nurse or a physician involved with SMARThealth, 278 (12.1%) consulted only a private sector health practitioner, and 161 (7.0%) visited both types of health care professional on at least 1 occasion. A total of 2101 high-risk individuals (91.3%) had at least 1 subsequent follow-up with a kader. The distribution of follow-up by kaders (Figure 2) indicates an overall median period of 9.2 months (IQR, 7.2-10.3) with 33% having 1 clinical interaction during this period, 39% having 2 clinical interactions during this period, and 22% having 3 clinical interactions during this period.
Figure 2. Distribution of Follow-up Visits by Kaders.
Kaders are nonphysician community health care workers in Indonesia. The horizontal line inside the box represents the median. The lower margin of the box represents the first quartile, and the upper margin of the box represents the third quartile. The upper horizontal line outside the box denotes the maximum value or the third quartile plus 1.5 times the interquartile range, whichever is smaller. The lower horizontal line outside the box denotes the minimum value or the first quartile minus 1.5 times the interquartile range, whichever is larger. The circles represent extreme observations.
At the end of follow-up, 409 researcher-identified high-risk individuals in intervention villages (15.5%) were taking appropriate preventive treatment, compared with 25 (1.0%) in control villages (adjusted relative risk, 14.8; 95% CI, 6.6-33.2; and adjusted risk difference, 14.1%; 95% CI, 12.7%-15.6%) (Table 2). This difference was particularly driven by increased use of blood pressure–lowering medication among those in the intervention villages (1495 [56.8%] vs 382 [15.7%]; adjusted relative risk, 3.6; 95% CI, 2.5-5.4; and adjusted risk difference, 39.4%; 95% CI, 37.0%-41.7%). Significant differences were observed for statin use, but antiplatelet medication use was borderline nonsignificant among those with established CVD. Similar results were obtained with no or full covariate adjustment (eTable 5 in the Supplement).
Table 2. Intervention Effects: Primary Analysis Based on Researcher-Identified High-Risk Individuals in Control and Intervention Villagesa.
| Outcome | Control Villages (n = 2429) | Intervention Villages (n = 2632) | Adjusted Risk Difference, % (95% CI) | Adjusted Relative Risk or Mean Difference (95% CI) | P Valueb | ICC |
|---|---|---|---|---|---|---|
| Appropriate treatment, No. (%)c | 25 (1.0) | 409 (15.5) | 14.1 (12.7 to 15.6) | 14.8 (6.6 to 33.2) | <.001 | .073 |
| Achieving BP target, No. (%) | 539 (22.2) | 815 (31.0) | 7.6 (5.4 to 9.9) | 1.3 (1.2 to 1.5) | <.001 | <.001 |
| Change in SBP, mean (SEM), mm Hg | −9.2 (0.4) | −17.2 (0.4) | NA | −8.3 (−10.1 to −6.6) | <.001 | .002 |
| Change in DBP, mean (SEM), mm Hg | −5.0 (0.2) | −8.3 (0.2) | NA | −3.6 (−4.5 to −2.6) | <.001 | .001 |
| BP-lowering medication, No. (%) | 382 (15.7) | 1495 (56.8) | 39.4 (37.0 to 41.7) | 3.6 (2.5 to 5.4) | <.001 | .022 |
| Lipid-lowering medication, No. (%) | 59 (2.4) | 523 (19.9) | 16.7 (15.1 to 18.3) | 9.3 (3.7 to 23.2) | <.001 | .106 |
| Antiplatelet medication, No./Total No. (%)d | 47/371 (12.7) | 128/520 (24.6) | 9.9 (5.0 to 14.8) | 1.9 (1.0 to 3.8) | .06 | .051 |
| Current smoking, No. (%) | 447 (18.4) | 420 (16.0) | Does not convergee | Does not convergee | Does not convergee | Does not convergee |
| Change in BMI, mean (SEM) | 0.0 (0.1) | −0.3 (0.1) | NA | −0.2 (−0.9 to 0.4) | .49 | .020 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; DBP, diastolic blood pressure; ICC, intraclass correlation coefficient; NA, not applicable; SBP, systolic blood pressure.
Missing values: BMI (63 control and 50 intervention villages) and BP (3 control and 9 intervention villages). The missing BP values were due to data transmission errors from the mobile application to the central database because there were no missing values for determining high-risk status (the automatic calculation of which requires BP values for those without known cardiovascular disease). For each outcome (other than the outcomes of use of individual drug modalities), the model was adjusted for baseline value of the outcome as well as baseline covariates with a between-group standardized difference of 0.1 or more (ie, baseline education, baseline appropriate medication use, and baseline high-risk category), but not baseline use of individual drug modalities (BP-lowering medication, lipid-lowering medication, or antiplatelet medication) because of the inclusion of baseline appropriate medication use. For each of the outcomes of use of individual drug modalities, the model was adjusted for the baseline value of the outcome, baseline education, and baseline high-risk category (but not baseline appropriate medication use because of the inclusion of the baseline value of the individual drug modality).
For adjusted relative risk or mean difference.
Combination of BP-lowering medication(s), statin therapy, and antiplatelet medication if high risk owing to known cardiovascular disease; combination of BP-lowering medication(s) and statin therapy if high risk of cardiovascular disease events owing to other reasons.
Among individuals at high risk owing to known cardiovascular disease at baseline.
Model does not converge with inclusion of any covariates.
A greater proportion of high-risk individuals in intervention villages achieved a systolic blood pressure target of less than 140 mm Hg at the end of follow-up, compared with those in control villages (815 [31.0%] vs 539 [22.2%]; adjusted relative risk, 1.3; 95% CI, 1.2-1.5; and adjusted risk difference, 7.6%; 95% CI, 5.4%-9.9%) (Table 2). At the end of follow-up, the mean (SEM) systolic blood pressure reduction from baseline was 17.2 (0.4) mm Hg among high-risk individuals in the intervention villages and 9.2 (0.4) mm Hg, among high-risk individuals in the control villages (adjusted mean difference, −8.3 mm Hg; 95% CI, −10.1 to −6.6 mm Hg). Similarly, diastolic blood pressure was significantly more reduced among high-risk individuals in the intervention villages compared with those in the control villages (adjusted mean difference, −3.6 mm Hg; 95% CI, −4.5 to −2.6 mm Hg). Sensitivity analyses based on kader-identified high-risk individuals in the intervention villages showed stronger associations between the intervention and treatment outcomes, compared with the primary analysis based on researcher-identified high-risk individuals (Table 3). In all analyses, there were no significant between-group differences in self-reported current smoking and measured body mass index at the end of follow-up.
Table 3. Intervention Effects: Sensitivity Analyses Based on Kader-Identified High-Risk Individuals in the Intervention Villagesa.
| Outcome | Control (n = 2429) | Intervention (n = 1894) | Adjusted Risk Difference, % (95% CI) | Adjusted Relative Risk or Mean Difference (95% CI) | P Valueb | ICC |
|---|---|---|---|---|---|---|
| Appropriate treatment, No. (%)c | 25 (1.0) | 482 (25.4) | 23.9 (21.8 to 25.9) | 24.4 (11.1 to 53.3) | <.001 | 0.068 |
| Achieving BP target, No. (%) | 539 (22.2) | 677 (35.7) | 9.8 (7.3 to 12.2) | 1.4 (1.3 to 1.6) | <.001 | <.001 |
| Change in SBP, mean (SEM), mm Hg | −9.2 (0.4) | −16.6 (0.5) | NA | −8.7 (−10.1 to −7.4) | <.001 | 0.001 |
| Change in DBP, mean (SEM), mm Hg | −5.0 (0.2) | −7.9 (0.3) | NA | −3.5 (−4.5 to −2.5) | <.001 | 0.002 |
| BP-lowering medication, No. (%) | 382 (15.7) | 1483 (78.3) | 60.9 (58.4 to 63.3) | 5.1 (3.4 to 7.5) | <.001 | 0.022 |
| Lipid-lowering medication, No. (%) | 59 (2.4) | 590 (31.2) | 28.0 (25.8 to 30.2) | 15.4 (5.9 to 39.8) | <.001 | 0.114 |
| Antiplatelet medication, No./total No. (%)d | 47/371 (12.7) | 99/301 (32.9) | 18.0 (11.4 to 24.5) | 2.6 (1.3 to 5.4) | .01 | 0.059 |
| Current smoking, No. (%)e | 447 (18.4) | 315 (16.6) | −3.0 (−5.2 to −0.8) | 0.9 (0.7 to 1.2) | .63 | 0.006 |
| Change in BMI, mean (SEM) | 0.0 (0.1) | −0.1 (0.1) | NA | −0.1 (−0.8 to 0.5) | .63 | 0.016 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; DBP, diastolic blood pressure; ICC, intraclass correlation coefficient; NA, not applicable; SBP, systolic blood pressure.
Missing values: BMI (63 control and 30 intervention villages) and BP (3 control and 26 intervention villages). The missing BP values were due to data transmission errors from the mobile application to the central database because there were no missing values for determining high-risk status (the automatic calculation of which requires BP values for those without known cardiovascular disease). For each outcome, the model was adjusted for baseline value of the outcome as well as baseline covariates with a between-group standardized difference of 0.1 or more (ie, baseline age, baseline education, baseline SBP and DBP, and baseline BMI).
For adjusted relative risk or mean difference.
Combination of BP-lowering medication(s), statin therapy, and antiplatelet medication if high risk owing to known cardiovascular disease; combination of BP-lowering medication(s) and statin therapy if high risk of cardiovascular disease events owing to other reasons.
Among individuals at high risk owing to known cardiovascular disease at baseline.
Adjusted on all baseline covariates with a standardized difference of more than 0.1, except for current smoking at baseline which had to be removed from the model owing to lack of convergence.
Post hoc subgroup analyses suggest that the associations between the intervention and the primary outcome were smaller among individuals with higher educational level, prior diagnosed CVD, and diabetes (eFigure 3 in the Supplement).
Discussion
This study showed that a mobile technology–supported, multifaceted primary health care intervention was associated with greater use of appropriate preventive CVD medications among high-risk individuals in a rural Indonesian community. The intervention was particularly associated with increased use of blood pressure–lowering medications and reductions in blood pressure levels. The more modest association with improvement in achieving target blood pressure reflects the high baseline blood pressure levels in this population.
Mobile technology–driven solutions can potentially improve the quality and efficiency of primary health care services for CVD prevention in resource-constrained environments. However, the few interventions that have undergone controlled evaluation have been shown to have modest, if any, effects.6,8,20 Much focus has been on technology, with insufficient attention on applying a multidomain health systems integration framework to development and implementation.21 The intervention evaluated in our study was developed using a theory-informed approach complemented by local health system contextualization. As a consequence, the intervention was complex, addressing barriers in multiple health system domains. Although the complex nature of intervention might be a critical contributor to improved outcomes, it inevitably leads to uncertainty about the relative contribution of each component. This will be further evaluated through a detailed process evaluation.22
Several features of the Indonesian health care system likely facilitated implementation of the intervention. First, senior district health agency officials were engaged in the context of a supportive policy environment. As a consequence of continuous data collection through the SMARThealth platform, it was recognized early that prior district-level procurement of essential CVD preventive medications, while affordable within typical procurement budgets, would be inadequate to meet demand. Although the short-term acquisition of additional medication was supported by study funding (finally supporting approximately 50% of prescribed medications), existing purchasing and supply chain processes that avoided running out of needed medication was critical.
Second, workforce characteristics in rural Indonesia enabled implementation. A core element of the theory of change was to generate community-level demand at the household level, rather than relying on promoting health care–seeking behavior among largely asymptomatic individuals. The presence of a community health care workforce already delivering care through household visits provided task-sharing opportunities through workflow modification, avoiding the need for an entirely new cadre of workers.23,24
Third, task sharing was strongly facilitated by the ability of the district health agency to authorize subsequent prescription of essential medications by nurses, with ongoing delegation where appropriate by physicians. The importance of nurse-based prescribing has been highlighted elsewhere.25,26 The positive associations between the intervention and outcomes were observed despite follow-up encompassing both public and private sector prescribers in this environment, as typically seen in many low- and middle-income countries. The latter were not using the intervention, which reinforces the important central role that community health care workers may play in ensuring integration and continuity of care.
Limitations
A key limitation of the study was the nonrandom allocation of the villages to the intervention or control scenarios, which likely introduced selection bias. Despite attempts to match villages, high-risk individuals in the intervention villages were more educated and had higher baseline treatment rates than those in the control villages. We tried to account for this in our analyses by controlling for observed differences in baseline characteristics. However, residual confounding remains a possibility, although this confounding would need to be substantial to change the overall conclusions, given the magnitude of the associations observed.27 There was anticipated discordance between researcher-identified and kader-identified high-risk individuals in the intervention villages, which was the rationale for a prespecified sensitivity analysis using data from kader-identified high-risk individuals. This discordance was driven largely by within-person differences in recorded blood pressure at levels consistent with previously reported regression to the mean and visit-to-visit blood pressure variability in people with hypertension at levels observed in this population.28,29 As a consequence, a large proportion of researcher-identified high-risk individuals would not have had an opportunity to be exposed to the intervention during the follow-up period. Thus, the primary analyses presented likely represent a more conservative assessment of associations. Conversely, the higher risk profile of participants who were not followed up, compared with those who were reassessed, may have resulted in overestimation of the true associations.
There are additional potential limitations to consider. The performance of the risk charts in this population overall and in certain subgroups is uncertain; however, this uncertainty would not introduce bias in the between-group comparisons. Participants self-reported medication use, although the prespecified secondary outcome of blood pressure provides some objective verification. Another concern may be that the study was not powered to identify associations with clinical events. However, in the context of using drugs of proven efficacy and safety, blood pressure would be considered an appropriate surrogate for CVD events.30 In addition, it is possible that community members from intervention villages may have disclosed prior exposure to the SMARThealth program to field researchers during follow-up, affecting blinded outcome assessment; we were unable to assess the extent to which this scenario may have occurred. A further potential limitation is that control villages were selected from subdistricts served by the same primary health care center as the intervention villages, providing a theoretical basis for contamination. In practice, few patients currently seek and/or receive CVD care at the primary health care center level. In addition, if there were any contamination owing to SMARThealth-exposed physicians treating control village community members, it would bias the results toward the null. Finally, the small number and selected nature of the villages included limits the generalizability of the findings.
Conclusions
Although the results are encouraging, further research is important to facilitate and demonstrate scalability and sustainability.31 Relevant data will emerge from the economic and process evaluations from this study; however, institutionalizing such interventions needs to address a range of issues for effective health care system integration. These issues include ensuring interoperability with Indonesia’s emerging eHealth strategy and infrastructure, drug and equipment supply chains, workforce and management training, and alignment with existing health care financing, social insurance, and reimbursement mechanisms. Finally, it will be necessary to broaden the disease focus to provide comprehensive primary health care services for a range of common conditions for ultimate sustainability and maximum effect.
eAppendix 1. SMARThealth Program in Indonesia
eAppendix 2. Additional Details on Statistical Methods
eFigure 1. SMARThealth Logic Model
eFigure 2. Study Villages
eFigure 3. Subgroup Analyses for Primary Outcome
eTable 1. Concordance Between Kader-Identified and Researcher-Identified High-Risk Individuals in the Intervention Villages
eTable 2. Baseline Characteristics of the Census Population
eTable 3. Baseline Characteristics of High-Risk Individuals Who Were and Were Not Followed Up
eTable 4. Additional Baseline Characteristics of High-Risk Individuals
eTable 5A. Intervention Effects – Primary Analysis Based on researcher-Identified High-Risk Individuals in Control and Intervention Villages: Without Adjustment for Baseline Covariates
eTable 5B. Intervention Effects – Primary Analysis Based on Researcher-Identified High-Risk Individuals in Control and Intervention Villages: Full Adjustment for Baseline Covariates
References
- 1.Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1-25. doi: 10.1016/j.jacc.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khatib R, McKee M, Shannon H, et al. ; PURE study investigators . Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE Study data. Lancet. 2016;387(10013):61-69. doi: 10.1016/S0140-6736(15)00469-9 [DOI] [PubMed] [Google Scholar]
- 3.Lee ES, Vedanthan R, Jeemon P, et al. Quality improvement for cardiovascular disease care in low- and middle-income countries: a systematic review. PLoS One. 2016;11(6):e0157036. doi: 10.1371/journal.pone.0157036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allotey P, Reidpath DD, Yasin S, Chan CK, de-Graft Aikins A. Rethinking health-care systems: a focus on chronicity. Lancet. 2011;377(9764):450-451. doi: 10.1016/S0140-6736(10)61856-9 [DOI] [PubMed] [Google Scholar]
- 5.Demaio AR, Kragelund Nielsen K, Pinkowski Tersbøl B, Kallestrup P, Meyrowitsch DW. Primary health care: a strategic framework for the prevention and control of chronic non-communicable disease. Glob Health Action. 2014;7:24504. doi: 10.3402/gha.v7.24504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beratarrechea A, Moyano D, Irazola V, Rubinstein A. mHealth Interventions to counter noncommunicable diseases in developing countries: still an uncertain promise. Cardiol Clin. 2017;35(1):13-30. doi: 10.1016/j.ccl.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 7.Mehl G, Labrique A. Prioritizing integrated mHealth strategies for universal health coverage. Science. 2014;345(6202):1284-1287. doi: 10.1126/science.1258926 [DOI] [PubMed] [Google Scholar]
- 8.Mboi N, Murty Surbakti I, Trihandini I, et al. On the road to universal health care in Indonesia, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10147):581-591. doi: 10.1016/S0140-6736(18)30595-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maharani A, Tampubolon G. Unmet needs for cardiovascular care in Indonesia. PLoS One. 2014;9(8):e105831. doi: 10.1371/journal.pone.0105831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kementerian Kesehtan Republik Indonesia. Rencana Strategis Kementerian Kesehatan Tahun 2015-2019. http://www.depkes.go.id/resources/download/info-publik/Renstra-2015.pdf. Accessed September 10, 2018.
- 11.Peiris D, Usherwood T, Panaretto K, et al. Effect of a computer-guided, quality improvement program for cardiovascular disease risk management in primary health care: the treatment of cardiovascular risk using electronic decision support cluster-randomized trial. Circ Cardiovasc Qual Outcomes. 2015;8(1):87-95. doi: 10.1161/CIRCOUTCOMES.114.001235 [DOI] [PubMed] [Google Scholar]
- 12.Praveen D, Patel A, Raghu A, et al. SMARThealth India: development and field evaluation of a mobile clinical decision support system for cardiovascular diseases in rural India. JMIR Mhealth Uhealth. 2014;2(4):e54. doi: 10.2196/mhealth.3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peiris D, Sun L, Patel A, et al. Systematic medical assessment, referral and treatment for diabetes care in China using lay family health promoters: protocol for the SMARTDiabetes cluster randomised controlled trial. Implement Sci. 2016;11(1):116. doi: 10.1186/s13012-016-0481-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO/ISH cardiovascular risk prediction charts. http://www.who.int/cardiovascular_diseases/guidelines/Chart_predictions/en. Accessed September 10, 2018.
- 15.Beran D, Yudkin JS, de Courten M. Assessing health systems for type 1 diabetes in sub-Saharan Africa: developing a ‘Rapid Assessment Protocol for Insulin Access’. BMC Health Serv Res. 2006;6:17. doi: 10.1186/1472-6963-6-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387-398. doi: 10.1016/S0895-4356(00)00321-8 [DOI] [PubMed] [Google Scholar]
- 18.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 19.Abdi H. Holm’s sequential Bonferroni procedure In: Salkind NJ, ed. Encyclopedia of Research Design. Thousand Oaks, CA: SAGE Publications; 2010:1-8. [Google Scholar]
- 20.Stephani V, Opoku D, Quentin W. A systematic review of randomized controlled trials of mHealth interventions against non-communicable diseases in developing countries. BMC Public Health. 2016;16:572. doi: 10.1186/s12889-016-3226-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian M, Zhang J, Luo R, et al. mHealth interventions for health system strengthening in China: a systematic review. JMIR Mhealth Uhealth. 2017;5(3):e32. doi: 10.2196/mhealth.6889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ. 2015;350:h1258. doi: 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi R, Alim M, Kengne AP, et al. Task shifting for non-communicable disease management in low and middle income countries—a systematic review. PLoS One. 2014;9(8):e103754. doi: 10.1371/journal.pone.0103754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogedegbe G, Gyamfi J, Plange-Rhule J, et al. Task shifting interventions for cardiovascular risk reduction in low-income and middle-income countries: a systematic review of randomised controlled trials. BMJ Open. 2014;4(10):e005983. doi: 10.1136/bmjopen-2014-005983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weeks G, George J, Maclure K, Stewart D. Non-medical prescribing versus medical prescribing for acute and chronic disease management in primary and secondary care. Cochrane Database Syst Rev. 2016;11:CD011227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogedegbe G, Plange-Rhule J, Gyamfi J, et al. Health insurance coverage with or without a nurse-led task shifting strategy for hypertension control: a pragmatic cluster randomized trial in Ghana. PLoS Med. 2018;15(5):e1002561. doi: 10.1371/journal.pmed.1002561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glasziou P, Chalmers I, Rawlins M, McCulloch P. When are randomised trials unnecessary? picking signal from noise. BMJ. 2007;334(7589):349-351. doi: 10.1136/bmj.39070.527986.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903-1913. doi: 10.1016/S0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 29.Mehlum MH, Liestøl K, Kjeldsen SE, et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J. 2018;39(24):2243-2251. doi: 10.1093/eurheartj/ehx760 [DOI] [PubMed] [Google Scholar]
- 30.Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527-1535. doi: 10.1016/S0140-6736(03)14739-3 [DOI] [PubMed] [Google Scholar]
- 31.Greenhalgh T, Wherton J, Papoutsi C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res. 2017;19(11):e367. doi: 10.2196/jmir.8775 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. SMARThealth Program in Indonesia
eAppendix 2. Additional Details on Statistical Methods
eFigure 1. SMARThealth Logic Model
eFigure 2. Study Villages
eFigure 3. Subgroup Analyses for Primary Outcome
eTable 1. Concordance Between Kader-Identified and Researcher-Identified High-Risk Individuals in the Intervention Villages
eTable 2. Baseline Characteristics of the Census Population
eTable 3. Baseline Characteristics of High-Risk Individuals Who Were and Were Not Followed Up
eTable 4. Additional Baseline Characteristics of High-Risk Individuals
eTable 5A. Intervention Effects – Primary Analysis Based on researcher-Identified High-Risk Individuals in Control and Intervention Villages: Without Adjustment for Baseline Covariates
eTable 5B. Intervention Effects – Primary Analysis Based on Researcher-Identified High-Risk Individuals in Control and Intervention Villages: Full Adjustment for Baseline Covariates