This cross-sectional study of young Finnish men examines the associations of aerobic fitness and muscular strength with metabolome measures that are associated with cardiometabolic risks.
Key Points
Question
Are aerobic fitness and maximal muscular strength associated with metabolites that are associated with cardiometabolic disease risk?
Findings
In this cross-sectional study of 580 young Finnish men, after adjusting for covariates, aerobic fitness accounted for more than an additional 5% of the variation of 25 serum metabolome measures that are associated with a reduction in cardiometabolic risk. There were fewer beneficial associations of maximal muscular strength with the studied metabolic risk factors.
Meaning
Aerobic fitness was associated with beneficial levels of metabolites associated with reduced vascular and metabolic disease risk.
Abstract
Importance
High physical fitness is associated with a reduction in risk of cardiovascular diseases and death, but the underlying mechanisms are insufficiently understood.
Objective
To determine how aerobic fitness and muscular strength are associated with serum metabolome measures.
Design, Setting, and Participants
This cross-sectional study included Finnish men receiving military refresher training from May 5, 2015, to November 28, 2015, representing partly overlapping groups of individuals with the lowest vs highest aerobic fitness and the lowest vs highest muscular strength. Data analyses were conducted from January 1, 2018, to May 31, 2019.
Main Outcomes and Measures
The associations of aerobic fitness (determined with maximum oxygen consumption in milliliters per minute per kilogram, measured with maximal cycle ergometer test) and muscular strength (determined with a maximal strength test for lower extremities in kilograms) with 66 metabolome measures from fasting serum samples (nuclear magnetic resonance–based metabolomics) were analyzed.
Results
Participants included 580 Finnish men (mean [SD] age, 26.1 [6.5] years). Including overlap between groups, there were 196 men in the lowest aerobic fitness group and 197 men in the highest aerobic fitness group as well as 196 men in the lowest muscular strength group and 197 men in the highest muscular strength group. Of 66 studied metabolome measures, 48 differed between high vs low aerobic fitness groups, including small very low-density lipoprotein (standardized median difference, −0.67; 95% CI, −0.83 to −0.49), large high-density lipoprotein (standardized median difference, 0.89; 95% CI, 0.69-1.15), total triglyceride levels (standardized median difference, −0.52; 95% CI, −0.65 to −0.34), isoleucine (standardized median difference, −0.37; 95% CI, −0.55 to −0.16), leucine (standardized median difference, −0.55; 95% CI, −0.72 to −0.34), phenylalanine (standardized median difference, −0.54; 95% CI, −0.71 to −0.32), glycerol (standardized median difference, −0.64; 95% CI, −0.81 to −0.48), and glycoprotein (standardized median difference, −0.78; 95% CI, −0.95 to −0.62) concentration, a high unsaturation degree of fatty acids (standardized median difference, 0.59; 95% CI, 0.42-0.81), and apolipoprotein B to apolipoprotein A1 ratio (standardized median difference, −0.88; 95% CI, −1.08 to −0.67). Adding aerobic fitness into the regression model after age, education, smoking, use of alcohol, and dietary factors accounted for more than an additional 5% of variation for 25 metabolome measures (R2 range, 5.01%-15.90% by measure). With these 2 criteria, maximal muscular strength was not associated with any metabolome measures. Aerobic fitness was associated with high large high-density lipoprotein particle concentration (R2, 14.97%; 95% CI, 10.65%-20.85%), low apolipoprotein B to apolipoprotein A1 ratio (R2, 14.49%; 95% CI, 10.58%-19.51%), and low glycoprotein concentration (R2, 15.90%; 95% CI, 11.22%-21.51%). Aerobic fitness was also associated with low very low-density lipoprotein, triglyceride, isoleucine, leucine, phenylalanine, glycerol, and glycoprotein concentrations and with a high unsaturation degree of fatty acids. Adjusting for recent physical activity influenced the results minimally. Adjusting for body fat percentage showed that some of the associations were mechanistically associated with body fat percentage.
Conclusions and Relevance
This study provides data on the association of high aerobic fitness with underlying oxidative lipid metabolism associated with a reduction in cardiometabolic risk. High maximal muscular strength is not similarly associated with these benefits.
Introduction
Aerobic fitness, also called cardiorespiratory fitness in clinical medicine, is a measure of the body’s ability to take oxygen from the atmosphere and use it for energy production in the cells. Maximum oxygen consumption (V̇o2max), measured in milliliters per minute per kilogram, is a commonly used measure of aerobic fitness. Many factors influence aerobic fitness, including sex, age, genetic factors, body composition, diseases, physical training background, pulmonary and cardiac functions, neural factors, and skeletal muscle properties. Maximal muscular strength is the ability of a muscle or muscle group to generate maximal force. One repetition maximum, measured in kilograms, is a commonly used measure of muscular strength. Aside from the properties of skeletal muscle, maximal muscular strength is mainly determined by muscle mass as well as the number of active motor units and their firing rate. At the muscular level, high aerobic fitness is associated with high capacity for oxidative lipid metabolism, or muscular endurance. Muscular endurance is defined as the muscle’s ability to exert successive submaximal force for a certain time and is also influenced by aerobic fitness.
According to several observational studies,1,2,3,4,5,6,7,8,9,10 aerobic fitness, muscular strength, and participation in physical activity measured in different ways are associated with better cardiometabolic health and lower risk of death. According to epidemiological evidence,1,2,3,7 aerobic fitness in particular is an indicator of good health and reduced risk of premature death. Aerobic fitness is a stronger predictor of reduced risk of death than physical activity level both in human and in animal studies.4,11,12,13 In a 2003 study14 of former top-level Finnish male athletes and matched control participants, endurance sports athletes with proven high aerobic capacity had lower occurrence of type 2 diabetes and cardiovascular diseases and reduced risk of death compared with power athletes or control participants. However, genetic pleiotropy may explain part of these associations.13
Aerobic fitness is associated with cardiometabolic risk factors.15,16 Some studies,17,18,19 but not all,20 have reported an association of cardiovascular risk factors with muscular strength, but these associations attenuated after adjusting for aerobic fitness.17,18,21 In addition to traditional cardiometabolic risk factors measured from the blood, novel metabolomics platforms provide a way to understand the mechanisms associated with the development of diseases. Recently, it has been shown how differing physical activity levels are associated with serum metabolome measures.22,23,24
To our knowledge, little research has been conducted on the associations of physical fitness with serum metabolome measures using valid measures of aerobic fitness and muscular strength among humans without diseases or predisease conditions.25,26 The skeletal muscle system is the largest and metabolically active organ system in the human body and contributes to the serum metabolome measures associated with aerobic and muscular fitness.22 The purpose of this study was to investigate how measured aerobic fitness and maximal muscular strength are associated with serum metabolome among 580 young Finnish men to better understand the mechanisms mediating the association of high physical fitness with low morbidity and mortality.
Methods
Participants
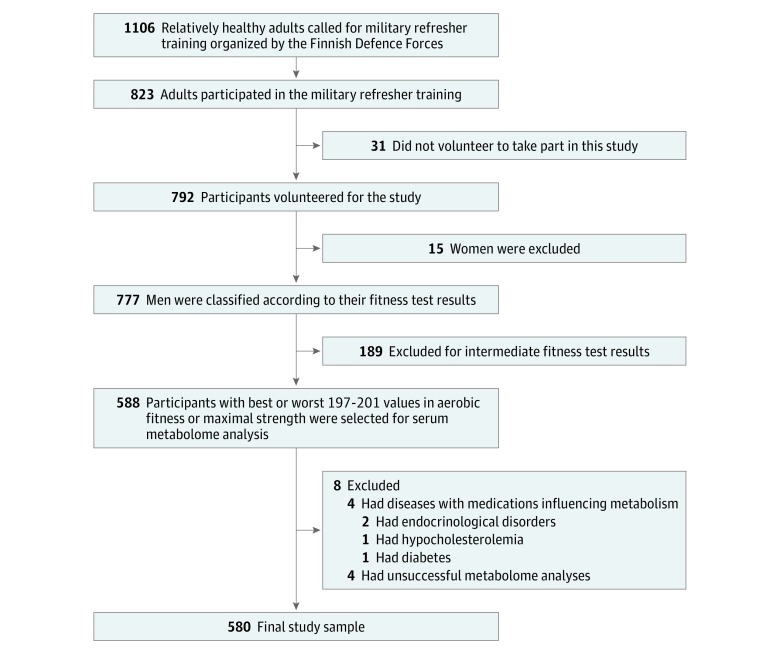
Study participants were identified from the participants of a previous clinical study of 776 participants in military refresher training from May 5, 2015, to November 28, 2015, during which aerobic fitness and muscular strength were measured and blood samples collected (Figure 1; eAppendix 1 in the Supplement). We identified a group of men with the highest aerobic fitness (approximately 200 participants with the highest fitness test results) and a group with the lowest aerobic fitness (approximately 200 participants with the lowest fitness test results) as well as a corresponding group with the highest muscular strength (approximately 200 participants with the highest strength test results) and a group with the lowest muscular strength (approximately 200 participants with the lowest strength test results). Before fitness tests, all participants underwent exercise eligibility screening, and each included participant was able to exercise to subjective maximum without disease-related symptoms interrupting the test. Individuals with (nonsevere) hypertension, nonoptimal lipid levels, occasional symptoms of asthma or allergy, diabetes, or musculoskeletal symptoms not interfering with maximal exercise were included. Seven participants were taking medication for hypertension, but none of them were taking β-blocker medication. Participants who had diseases or took medications that influenced metabolism or whose metabolome analyses were unsuccessful were excluded. The study protocol was explained in detail to the participants before they provided written informed consent.
Figure 1. Flowchart of Study Cohort.
The study was approved by the ethics committees of the University of Jyväskylä and the Central Finland Health Care District as well as the Headquarters of the Finnish Defense Forces. Data analyses were conducted from January 1, 2018, to May 31, 2019. This study was reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Demographic, Mediating, and Confounding Variables
The demographic and confounding variables assessed using structured questions included age, education, working status, use of alcohol, smoking, dietary habits (including food frequency questions on vegetable, fruit, fish, chicken, and meat consumption), and physical activity (Table 1; eAppendix 2 in the Supplement). Body mass and height were measured on a commercial scale to the closest 0.1 kg and 0.1 cm, respectively. Body mass index was calculated as weight in kilograms divided by height in meters squared, and waist circumference was measured using a cloth tape measure at the level of the iliac crest after exhaling. Body composition was determined after an overnight fast using bioelectrical impedance analysis (InBody 720; InBody) to determine fat mass, body fat percentage, and lean mass. The bioelectrical impedance analysis estimates of body composition have been shown to strongly correlate with the dual-energy radiography absorptiometry method (r range, 0.82-0.95).27
Table 1. Participant Characteristics.
| Characteristic | Aerobic Fitness | Muscular Strength | ||||
|---|---|---|---|---|---|---|
| Low (n = 196)a | High (n = 197)a | P Valueb | Low (n = 196)c | High (n = 197)c | P Valueb | |
| Age, y, mean (SD) | 26.8 (6.9) | 25.0 (5.8) | .007 | 25.4 (6.4) | 27.0 (6.4) | .02 |
| V̇O2max, mL/min/kg, mean (SD) | 31.8 (3.8) | 50.7 (4.2) | <.001 | 40.2 (7.6) | 41.7 (7.9) | .05 |
| Muscular strength, kg, mean (SD) | 334 (85) | 347 (94) | .15 | 240 (20) | 460 (69) | <.001 |
| Muscular strength/body mass, kg/kg, mean (SD) | 3.7 (0.8)d | 4.8 (1.2)e | <.001 | 3.3 (0.6)f | 5.4 (1.2)g | <.001 |
| Height, cm, mean (SD) | 179.8 (6.6) | 179.2 (5.8) | .33 | 178.6 (6.0) | 180.5 (6.5) | .003 |
| Body mass, kg, mean (SD) | 92.3 (17.9)d | 73.1 (9.0)e | <.001 | 73.5 (12.7)f | 88.3 (15.1)g | <.001 |
| BMI, mean (SD) | 28.5 (4.9)d | 22.7 (2.3)e | <.001 | 23.0 (3.7)f | 27.1 (4.2)g | <.001 |
| Waist circumference, cm, mean (SD) | 96.8 (12.4) | 80.3 (5.9) | <.001 | 83.0 (10.4) | 90.9 (11.1) | <.001 |
| Lean mass, kg, mean (SD) | 39.0 (5.9)d | 36.6 (4.4)e | <.001 | 34.2 (4.4)f | 41.1 (4.7)g | <.001 |
| Fat mass, kg, mean (SD) | 23.8 (11.6)h | 9.0 (4.2)e | <.001 | 13.1 (8.3)f | 16.6 (10.7)g | <.001 |
| Body fat, %, mean (SD) | 24.8 (8.1)d | 12.4 (5.6)e | <.001 | 16.9 (7.9)f | 17.9 (8.0)g | .23 |
| Obese (BMI ≥30), No./total No. (%) | 71/192 (37.0) | 0/194 | <.001 | 10/194 (5.2) | 36/196 (18.4) | <.001 |
| Waist circumference >102 cm, No./total No. (%) | 67/196 (34.2) | 0/197 | <.001 | 8/196 (4.1) | 31/197 (15.7) | <.001 |
| Education, No./total No. (%) | ||||||
| ≤Vocational school | 116/194 (59.8) | 86/196 (43.9) | .002 | 114/194 (58.8) | 95/195 (48.7) | .05 |
| ≥Upper secondary school | 78/194 (40.2) | 110/196 (56.1) | 80/194 (41.2) | 100/195 (51.3) | ||
| Employment status, No./total No. (%) | ||||||
| Working | 129/194 (66.5) | 128/196 (65.3) | .008 | 116/194 (59.8) | 152/196 (77.6) | <.001 |
| Student | 44/194 (22.7) | 61/196 (31.1) | 58/194 (29.9) | 40/196 (20.4) | ||
| Unemployed or other | 21/194 (10.8) | 7/196 (3.6) | 20/194 (10.3) | 4/196 (2.0) | ||
| Smoking, No./total No. (%) | ||||||
| Never | 79/194 (40.7) | 116/196 (59.2) | <.001 | 97/194 (50.0) | 97/196 (49.5) | <.001 |
| Quit | 46/194 (23.7) | 47/196 (24.0) | 31/194 (16.0) | 61/196 (31.1) | ||
| Regular | 69/194 (35.6) | 33/196 (16.8) | 66/194 (34.0) | 38/196 (19.4) | ||
| Use of alcohol, No./total No. (%) | ||||||
| Never | 11/194 (5.7) | 31/196 (15.8) | .29 | 14/194 (7.2) | 14/196 (7.1) | .65 |
| ≤1-2 Times/wk | 143/194 (73.7) | 138/196 (70.4) | 142/194 (73.2) | 150/196 (76.5) | ||
| ≥3 Times/wk | 40/194 (20.6) | 27/196 (13.8) | 38/194 (19.6) | 32/196 (16.3) | ||
| ≥6 Drinks on 1 occasion, ≥1 time/wk | 52/194 (26.8) | 48/196 (24.5) | .64 | 54/194 (27.8) | 44/196 (22.4) | .24 |
| Diet ≥3 d/wk, No./total No. (%) | ||||||
| Fresh vegetables | 102/194 (52.6) | 125/196 (63.8) | .03 | 99/194 (51.0) | 138/196 (70.4) | <.001 |
| Cooked vegetables | 41/194 (21.1) | 53/196 (27.0) | .19 | 42/194 (21.6) | 65/196 (33.2) | .01 |
| Fruits or berries | 56/194 (28.9) | 102/196 (52.0) | <.001 | 53/194 (27.3) | 94/196 (48.0) | <.001 |
| Chicken | 47/194 (24.2) | 64/196 (32.7) | .07 | 36/194 (18.6) | 69/196 (35.2) | <.001 |
| Fish | 12/194 (6.2) | 12/196 (5.9) | .10 | 12/194 (6.2) | 13/196 (6.6) | .10 |
| Meat | 137/194 (70.6) | 150/196 (76.5) | .21 | 134/194 (69.1) | 143/196 (73.0) | .44 |
| Processed | 35/194 (18.0) | 44/196 (22.4) | .31 | 40/194 (20.6) | 25/196 (12.8) | .04 |
| Total amount of physical activity/wk, h, mean (SD) | ||||||
| Light aerobic | 4.3 (9.2)g | 6.8 (13.0) | .03 | 4.2 (8.5)g | 5.6 (11.4) | .14 |
| Moderate aerobic | 2.4 (5.4)f | 3.1 (5.6) | .20 | 2.6 (6.1)f | 2.7 (6.1) | .90 |
| Vigorous aerobic | 0.9 (1.9)f | 2.6 (2.8) | <.001 | 1.1 (1.8)f | 1.8 (2.3) | <.001 |
| Strength training | 1.1 (3.0)f | 2.0 (2.6) | .001 | 0.7 (1.5)f | 2.4 (2.9) | <.001 |
| Sum of moderate to vigorous aerobic training | 3.3 (6.2)f | 5.7 (6.5) | <.001 | 3.7 (6.3)f | 4.5 (6.5) | .20 |
| Work-related loading, No./total No. (%) | ||||||
| Sedentary work | 53/192 (27.6) | 41/193 (21.2) | .42 | 52/193 (26.9) | 53/188 (28.2) | .88 |
| Walking and other light activity | 28/192 (14.6) | 35/193 (18.1) | 35/193 (18.1) | 29/188 (15.4) | ||
| Walking and lifting material | 64/192 (33.3) | 63/193 (32.6) | 56/193 (29.0) | 59/188 (31.4) | ||
| Heavy manual work | 47/192 (24.5) | 54/193 (28.0) | 50/193 (25.9) | 47/188 (25.0) | ||
| Blood pressure, mm Hg, mean (SD) | ||||||
| Systolic | 127.2 (11.7) | 121.8 (11.4) | <.001 | 119.9 (10.5) | 126.5 (12.5) | <.001 |
| Diastolic | 78.0 (8.8) | 71.7 (8.3) | <.001 | 73.9 (8.7) | 74.6 (10.3) | .47 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); V̇O2max, maximum oxygen consumption.
Ranges: low, 15.5-36.6 mL/min/kg; high, 45.4-67.1 mL/min/kg.
P values for the difference between high fitness vs low fitness groups for categorical variables by χ2 tests and for continuous variables by permutation median test.
Ranges: low, 115-279 kg; high, 382-738 kg.
Data were missing for 4 participants.
Data were missing for 3 participants.
Data were missing for 2 participants.
Data were missing for 1 participant.
Data were missing for 5 participants.
Assessment of Physical Fitness
Maximal aerobic fitness, as measured by V̇o2max, was determined using an indirect graded cycle ergometer (Ergoline 800S, Ergoselect 100K, and Ergoselect 200K; Ergoline) test until exhaustion. A progressive protocol was used that started at a power output of 50 W and increased 25 W every 2 minutes until exhaustion. Heart rate was continuously recorded during the test using Polar Vantage NV or Polar S610, S710, or S810 heart rate monitors (Polar). Predicted V̇o2max was estimated from heart rate and maximal power (W) using Fitware fitness testing software (Fitware) using the equation V̇o2max = 12 × 35 × Wattsmax/kg + 3.5, in which Wattsmax indicates the maximal power and kg refers to the unit of body mass. This protocol is accurate (SD, 3%) and reliable to estimate V̇O2max values in healthy men, with the reported intraclass correlation being high (r range, 0.82-0.94).28
The maximal isometric leg extension was measured using a dynamometer as a test of maximal strength.29 The repeatability has been reported to be high in maximal isometric strength tests (r, 0.98; coefficient of variation, 4.1%).30 Additional details are presented in eAppendix 1 in the Supplement.
Serum Metabolome Measures
Participants’ circulating metabolomes, including lipids, lipoproteins, and metabolites, were assessed using a high-throughput proton nuclear magnetic resonance spectroscopy metabolomics platform.31,32 Collectively, the 153 metabolic traits measured by the platform represent a broad molecular signature of systemic metabolism.31,32 The platform quantifies various measures of lipoprotein metabolism, certain lipids, ketone bodies, and amino acids, as well as glycolysis and gluconeogenesis-related metabolites in absolute concentration units. Based on our 2013 study22 on physical activity and metabolomics, 66 metabolites or their ratios were selected for this study (eTable 2 in the Supplement).
Statistical Analysis
The primary aim of our study was to examine whether V̇o2max per kilogram of body mass and maximal muscular strength without adjusting for body mass or body composition are associated with metabolome. First, we analyzed the unadjusted results of metabolome measures between high vs low aerobic fitness and high vs low muscular strength groups. Second, all of the participants were included in linear regression analyses in which we investigated separately how aerobic fitness and muscular strength (independent continuous variables) were associated with different metabolome measures (dependent continuous variables). The models were first adjusted for age (continuous variable), and education level, smoking, use of alcohol, and indicators of dietary factors (dichotomous variables) (eAppendix 2 in the Supplement). Next, we investigated how adjusting for concomitants of fitness, such as reported physical activity habits (aerobic activities and strength training volumes; continuous variables), and body fat percentage (continuous variable) affected the associations.
As it may be physiologically more relevant to express muscular strength per mass, a comparison of how the results differed when calculated using maximal strength vs maximal strength per body mass was also reported. Maximal strength per body mass was expected to correlate more strongly with maximal aerobic fitness. Finally, we also included aerobic fitness and muscular strength in a regression model to determine which had a stronger correlation with the variation in the metabolome measures.
P values were 2-tailed, calculated using χ2 tests or permutation median tests, and were not corrected for multiple testing. Our previous estimation of the multiple test correction can be used for the interpretation of statistical significance in all the 66 studied metabolome measures. We used principal component analysis to determine the minimum number of orthogonal linear components from a similar full metabolomics measures panel that explained 99% of the observed variance in a large data set. The minimum number of orthogonal linear components was analyzed, and the highest number observed in the previously studied cohort22 was 26 components, which we used as a consistent conservative estimate in all multiple testing interpretations using Bonferroni method. Bonferroni-corrected P value of .002 or less was set as the level for statistical significance, which corresponds to a P value of less than .05.
The data were analyzed with R statistical software version 3.4.1 (R Project for Statistical Computing) and SPSS statistical software version 25.0 (IBM). Pretreatment of the variables included imputation of the minimum values in instances in which the level of the specific metabolome measure was too small to detect (eTable 2 in the Supplement). Descriptive statistics, such as frequencies, means, and SDs, as well as medians and nonparametric bias-corrected and accelerated bootstrapped 95% CIs for the variance of medians, were calculated. Natural log transformations were used for all of the regression analyses for metabolome measures that were not normally distributed (eTable 2 in the Supplement). Linear regression analyses were conducted to define R2 as a measure of variance accounted for, as well as change in R2 after the addition of the variable of interest. For the change in coefficients of determination, bias-corrected and accelerated 95% CIs were computed with boot and boot.ci from the library boot in R (Table 2).
Table 2. Variation in Selected Metabolome Measures Accounted for by the Covariates and Physical Fitness.
| Metabolome Measure | % | ||||
|---|---|---|---|---|---|
| Model 1 R2a | Model 2 R2b,c | Model 2−Model 1 R2 Difference (95% CI)d | Model 3 R2c,e | Model 3−Model 1 R2 Difference (95% CI) | |
| Lipoprotein particle concentration | |||||
| Large VLDL | 5.86 | 12.13 (−) | 6.28 (3.27-11.02) | 5.91 (+) | 0.06 (0-0.69) |
| Medium VLDL | 8.36 | 17.14 (−) | 8.78 (5.08-13.40) | 8.83 (+) | 0.47 (0-2.16) |
| Small VLDL | 13.27 | 22.27 (−) | 9.00 (5.47-13.46) | 14.01 (+) | 0.74 (0.01-2.51) |
| Very small VLDL | 18.24 | 24.37 (−) | 6.13 (3.49-9.54) | 19.13 (+) | 0.89 (0.02-2.62) |
| Very large HDL | 5.53 | 13.97 (+) | 8.43 (5.19-13.11) | 5.76 (−) | 0.23 (0-1.47) |
| Large HDL | 8.21 | 23.18 (+) | 14.97 (10.65-20.85) | 8.93 (−) | 0.72 (0.02-2.78) |
| Lipoprotein particle size | |||||
| VLDL diameter | 4.68 | 10.94 (−) | 6.27 (3.11-10.48) | 4.81 (+) | 0.13 (0-1.28) |
| HDL diameter | 8.90 | 23.35 (+) | 14.45 (10.21-20.00) | 9.40 (−) | 0.50 (0-2.22) |
| APOs | |||||
| APOB | 14.65 | 22.91 (−) | 8.26 (5.10-12.38) | 15.55 (+) | 0.90 (0.03-2.95) |
| APOB:APOA1 ratio | 15.20 | 29.69 (−) | 14.49 (10.58-19.51) | 16.47 (+) | 1.27 (0.12-3.75) |
| TGs | |||||
| Total TG | 10.34 | 20.07 (−) | 9.73 (5.95-14.55) | 10.86 (+) | 0.52 (0-2.31) |
| VLDL TG | 8.60 | 18.17 (−) | 9.57 (5.82-14.22) | 9.02 (+) | 0.42 (0-2.09) |
| IDL TG | 15.38 | 21.60 (−) | 6.22 (3.21-10.17) | 15.72 (+) | 0.34 (0-1.65) |
| Cholesterol | |||||
| VLDL | 13.60 | 21.30 (−) | 7.70 (4.49-11.85) | 14.40 (+) | 0.80 (0.01-2.77) |
| HDL | 5.72 | 17.19 (+) | 11.47 (7.81-16.90) | 6.61 (−) | 0.89 (0.04-3.20) |
| HDL subfraction 2 | 7.07 | 19.34 (+) | 12.27 (8.34-17.80) | 7.96 (−) | 0.89 (0.06-3.12) |
| FAs | |||||
| Unsaturation degree | 9.05 | 17.27 (+) | 8.22 (4.64-13.07) | 9.17 (+) | 0.13 (0-1.26) |
| ω-6 FA ratio | 8.15 | 16.64 (+) | 8.49 (4.78-13.83) | 8.66 (−) | 0.51 (0-2.38) |
| Saturated FA | 13.78 | 18.79 (−) | 5.01 (2.32-8.82) | 14.14 (+) | 0.36 (0-1.65) |
| Monounsaturated FA | 13.56 | 19.97 (−) | 6.41 (3.38-10.55) | 13.78 (+) | 0.22 (0-1.34) |
| Metabolic substrates, amino acids, and other | |||||
| Glycerol | 4.03 | 20.19 (−) | 16.17 (10.81-22.79) | 4.52 (−) | 0.50 (0-2.17) |
| Isoleucine (BCAA) | 0.90 | 7.85 (−) | 6.95 (3.77-11.22) | 1.42 (+) | 0.52 (0-2.53) |
| Leucine (BCAA) | 2.04 | 8.91 (−) | 6.88 (3.58-10.79) | 2.82 (+) | 0.78 (0.01-3.25) |
| Phenylalanine | 4.06 | 10.52 (−) | 6.46 (3.10-11.21) | 6.08 (+) | 2.02 (0.36-4.91) |
| Glycoproteins | 6.21 | 22.11 (−) | 15.90 (11.22-21.51) | 6.38 (+) | 0.17 (0-1.54) |
Abbreviations: APO, apolipoprotein; BCAA, branched-chain amino acid; FA, fatty acid; HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; TG, triglyceride; VLDL, very low-density lipoprotein.
Model 1 R2 from linear regression analysis shows how much age, education, smoking, use of alcohol, and dietary factors (consumption of vegetables, fruits, fish, chicken, and meat) account for the variation in each of the metabolome measures.
Model 2 R2 shows how much age, education, smoking, use of alcohol, dietary factors (consumption of vegetables, fruits, fish, chicken, and meat), and aerobic fitness account for the variation in each of the metabolome measures.
+ and – indicate the direction of the association of the fitness variable with each metabolome measure.
Statistically significant additional R2 value with P ≤ .002.
Model 3 R2 shows how much age, education, smoking, use of alcohol, dietary factors (consumption of vegetables, fruits, fish, chicken, and meat), and muscular strength account for the variation in each of the metabolome measures.
Results
From 588 individuals selected for having the best or worst values in aerobic fitness or maximal strength, we excluded 4 participants having a disease or using medication that influenced metabolism and 4 participants owing to unsuccessful metabolome analyses (Figure 1). As there was overlap between the aerobic fitness and muscular strength groups (eTable 1 in the Supplement), the total cohort included 580 adult white Finnish men (mean [SD] age, 26.1 [6.5] years). Our final groups included 196 participants with low aerobic fitness (V̇o2max range, 15.5-36.6 mL/min/kg), 197 participants with high aerobic fitness (V̇o2max range, 45.4-67.1 mL/min/kg), 196 participants with low muscular strength (maximal muscular strength range, 115-279 kg), and 197 participants with high muscular strength (maximal muscular strength range, 382-738 kg) (Table 1). Compared with participants with high aerobic fitness, participants with low aerobic fitness, had a higher mean (SD) body mass index (28.5 [4.9] vs 22.7 [2.3]) and a larger mean (SD) waist circumference (96.8 [12.4] cm vs 80.3 [5.9] cm). Compared with participants with low muscular strength, participants with high muscular strength had a higher mean (SD) body mass index (27.1 [4.2] vs 23.0 [3.7]) and a larger mean (SD) waist circumference (90.9 [11.1] cm vs 83.0 [10.4] cm). Participants with high aerobic fitness had the highest rate of upper secondary school educations or higher (110 participants [56.1%]). Participants with high muscular strength had the highest rate of working at the time of study participation (152 participants [77.6%]). Both types of fitness were associated with more mean (SD) moderate to vigorous aerobic training (high aerobic fitness, 5.7 (6.2) h/wk; low aerobic fitness, 3.3 [6.2] h/wk; high muscular strength, 4.5 [6.5] h/wk; low muscular strength, 3.7 [6.3] h/wk), more mean (SD) strength training (high aerobic fitness, 2.0 (2.6) h/wk; low aerobic fitness, 1.1 [3.0] h/wk; high muscular strength, 2.4 [2.9] h/wk; low muscular strength, 0.7 [1.5] h/wk), and lower incidence of regular smoking (high aerobic fitness, 33 participants [16.8%]; low aerobic fitness, 69 participants [35.6%]; high muscular strength, 38 participants [19.4%]; low muscular strength, 66 participants [34.0%]) (Table 1).
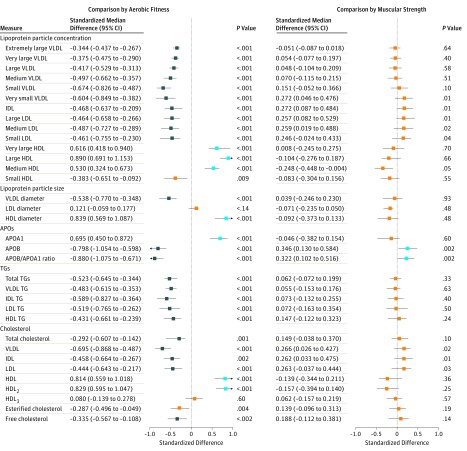
Nonadjusted differences in serum metabolome measures by aerobic fitness and muscular strength groups are shown in Figure 2 and Figure 3 and in eTable 3 and eTable 4 in the Supplement. There were many differences between the participants with high aerobic fitness vs low aerobic fitness but fewer differences between those with high muscular strength vs low muscular strength. The largest median differences between the high aerobic fitness vs low aerobic fitness groups relative to pooled SDs were found for large high-density lipoprotein (HDL) particles (median standardized difference, 0.89; 95% CI, 0.69-1.15; P < .001), the ratio of apolipoproteins (APOs) APOB to APOA1 (median standardized difference, −0.88; 95% CI, −1.08 to −0.67; P < .001), and glycoproteins (median standardized difference, −0.78; 95% CI, −0.95 to −0.62; P < .001). According to age-adjusted regression analysis among the 580 participants, the findings were in accordance with the group comparisons (eTable 5 and eTable 6 in the Supplement).
Figure 2. Serum Metabolome Measures in Individuals With High Fitness Compared With Individuals With Low Fitness According to Aerobic Fitness and Muscular Strength.
The points and lines indicate the differences in individuals with high fitness compared with individuals with low fitness in medians and their bootstrapped 95% CIs scaled in SDs of the pooled data of all 580 participants. Blue indicates serum levels that were statistically significantly higher among individuals with high fitness compared with those with low fitness; navy, serum levels that were statistically significantly lower among individuals with high fitness compared with those with low fitness; and orange, differences were not statistically significant. APO indicates apolipoprotein; HDL, high-density lipoprotein; HDL2, HDL subfraction 2; HDL3, HDL subfraction 3; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; VLDL, very low-density lipoprotein.
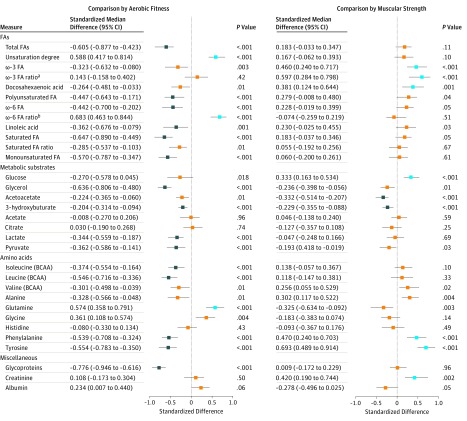
Figure 3. Serum Metabolome Measures in Individuals With High Fitness Compared With Individuals With Low Fitness According to Aerobic Fitness and Muscular Strength.
The points and lines indicate the differences in individuals with high fitness compared with individuals with low fitness in medians and their bootstrapped 95% CIs scaled in SDs of the pooled data of all 580 participants. Blue indicates serum levels that were statistically significantly higher among individuals with high fitness compared with those with low fitness; navy, serum levels that were statistically significantly lower among individuals with high fitness compared with those with low fitness; and orange, differences were not statistically significant. BCAA indicates branched-chain amino acid; FA, fatty acid.
aω-3 FA ratio is the ratio of ω-3 FAs to total FAs.
bω-6 FA ratio is the ratio of ω-6 FAs to total FAs.
The associations of fitness characteristics with metabolome measures were similar in most cases after adjusting for age, education level, smoking, use of alcohol, and dietary factors (Table 2; eTable 7 and eTable 8 in the Supplement). Twenty-five of the 66 studied metabolome measures differed between high vs low aerobic fitness groups (Figure 2 and Figure 3; eTable 3 and eTable 4 in the Supplement), and among the 580 men, adding aerobic fitness into the regression model after age, education, smoking, use of alcohol, and dietary factors accounted for more than an additional 5% of their variation (R2 range, 5.01%-15.90%) (Table 2; eTable 7 in the Supplement). Within these 2 criteria, maximal muscular strength was not associated with any metabolome measures. After adjusting for covariates, aerobic fitness was associated with high large HDL particle concentration (R2, 14.97%; 95% CI, 10.65-20.85), with low APOB/APOA1 ratio (R2, 14.49%; 95% CI, 10.58-19.51), and with low glycoprotein concentration (R2, 15.90%; 95% CI, 11.22-21.51). The eFigure in the Supplement shows a correlation heat map of fitness characteristics with metabolome measures, of which variation was accounted for by at least 5% by aerobic fitness after adjusting for the main covariates. All of the variables are linked to fat metabolism.
Most associations of physical fitness with metabolome measures remained statistically significant with only small changes in the explanation rates after adjusting for physical activity (eTable 9 and eTable 10 in the Supplement). After adjusting for age and body fat percentage, aerobic fitness was statistically significantly associated with 19 of the included metabolome measures, including higher levels of large HDL particles (R2, 4.15%; 95% CI, 1.75%-7.33%) and APOA1 (R2, 3.25%; 95% CI, 1.05%-6.39%); lower levels of medium very low-density lipoprotein (R2, 1.36%; 95% CI, 0.25%-3.76%), very low-density lipoprotein triglycerides (R2, 1.72%; 95% CI, 0.33%-4.12%), and total triglycerides (R2, 1.49%; 95% CI, 0.24%-3.77%); unsaturation degree of fatty acids (FAs) (R2, 2.62%; 95% CI, 0.98%-4.84%); and lower levels of glycerol (R2, 3.17%; 95% CI, 0.88%-8.18%), acetoacetate (R2, 3.76%; 95% CI, 1.26%-7.54%), 3-hydroxybuturate (R2, 2.63%; 95% CI, 0.56%-6.25%), and glycoproteins (R2, 2.76%; 95% CI, 0.80%-5.88%) (eTable 11 in the Supplement). After adjusting for age and body fat percentage, muscular strength was associated with 8 of the measures, including high levels of ω-3 FAs (R2, 1.58%; 95% CI, 0.26%-3.58%), phenylalanine (R2, 1.72%; 95% CI, 0.32%-4.00%), tyrosine (R2, 4.74%; 95% CI, 2.08%-8.18%), and creatinine (R2, 5.90%; 95% CI, 2.39%-10.41%) and low levels of glycerol (R2, 1.74%; 95% CI, 0.29%-4.02%), acetoacetate (R2, 3.55%; 95% CI, 1.14%-7.10%), and 3-hydroxybuturate (R2, 3.53%; 95% CI, 0.96%-6.58%) (eTable 12 in the Supplement). A summary of the changes in the explanation rates when adding different covariates to the models is shown in eTable 13 in the Supplement.
The correlation between maximal aerobic fitness and maximal muscular strength was stronger when muscular strength was expressed per body mass (r = 0.43; 95% CI, 0.37-0.49; P < .001) compared with when muscular strength was expressed as an absolute value (r = 0.09; 95% CI, 0.01-0.15; P = .04). The unfavorable associations of metabolome measures with muscle strength were usually attenuated when we used muscular strength expressed per body mass (eTable 14 in the Supplement). After adjusting for age, body fat percentage, aerobic fitness, and maximal muscular strength per body mass, we found that the associations with aerobic fitness largely persisted, but after these adjustments, muscular strength was only associated with high creatinine concentration (eTable 15 in the Supplement).
Discussion
This study found many associations of high aerobic fitness with metabolome measures, indicating reduced cardiometabolic risk in relatively healthy young men, while maximal muscular strength had fewer such associations with metabolome measures. Many of the associations were mechanistically associated with body fat percentage. To our knowledge, there are no previous similar studies with similarly targeted metabolomics platforms.
The associations of lower very low-density lipoprotein, intermediate-density lipoprotein, and low-density lipoprotein particle concentrations as well as lower triglyceride levels with high aerobic fitness are similar to the findings in 2 previous studies22,33 of physically active individuals that suggested decreased risk of coronary heart disease. Body fat percentage explained or mediated much of these associations. After adjusting for body fat percentage, aerobic fitness explained an additional 4.2% of the variation in the content of large HDL particles. Although drugs that increase HDL cholesterol levels are not effective in reducing cardiovascular disease risk, high HDL cholesterol levels were associated with low risk of coronary heart disease in a review by Natarajan et al,34 and several studies35,36,37 have shown that HDL cholesterol levels increase with exercise. In particular, the functionally beneficial larger HDL particle concentration is increased in physically active individuals.22 Our findings are in agreement with studies by Kujala et al22 and Blazek et al37 that reported that high aerobic fitness or high physical activity was associated with the improved function of HDL particles. In addition to previously known mechanisms,37 the function of HDL particles may be associated with high oxygen uptake and associated muscle metabolism.22,38 Further research is warranted to explain these mechanisms, as there is evidence that suggests HDL modulates skeletal muscle cellular respiration.38
Aerobic fitness was associated with lower levels of most types of FA concentrations, including total and saturated FA, monounsaturated FA, and ω-6 FA, unlike maximum strength, which was associated with higher docosahexaenoic acid and ω-3 FA concentrations. Interestingly, after adjusting for body fat percentage, aerobic fitness correlated more closely with the degree of unsaturation than with any other FA parameter. High aerobic fitness was associated with a free FA profile, which is a characteristic of reduced cardiovascular event risk.39 However, substituting ω-6, ω-3, or total polyunsaturated FAs for saturated FAs in the diet is not very efficient in reducing cardiovascular disease risk.40,41,42,43 Our results suggest that fitness characteristics themselves at least partly define the favorable serum FA profile but also that some FAs may influence fitness characteristics.44
Lower serum lactate and pyruvate concentrations in the participants with high aerobic fitness compared with the participants with low aerobic fitness are most likely owing to several interacting factors. First, more oxidative muscle fibers can exist more frequently in skeletal muscles of aerobically fit people owing to heredity45 or training,46 thus using more lactate for energy production than glycolytic fibers.47 Skeletal muscle mitochondria use lactate for oxidation more efficiently than they use pyruvate, suggesting that glucose is first largely metabolized to lactate, secreted out of muscle cells, and only then transported back to the same or other cells.48 This supports the idea that type IIB fibers produce lactate that is consumed by type I and IIA fibers for energy production and that this cycle is more efficient in people who are aerobically fit. Second, in participants who were aerobically fit, skeletal muscle glycogenesis for restoring glycogen stores at rest may have been more efficient than in those who were less fit. Third, hepatic gluconeogenesis from lactate is likely higher in people who are aerobically fit than in those who are less fit.49
Interestingly, high aerobic fitness and high muscle strength were associated with lower levels of glycerol and ketone bodies acetoacetate and 3-hydroxybutyrate, suggesting increased fat catabolism. A 2015 study50 found that lipolysis-derived serum glycerol levels at rest can be lower in aerobically trained individuals than in untrained individuals,50 similar to this study, but 2 older studies51,52 reported no observed difference. Differences in the results can be explained by variations in glycerol kinetics, as glycerol appearance and disappearance rates are considerably higher in people who have undergone endurance training compared with those who have not.52 Lower glycerol levels at rest may be owing to enhanced hepatic gluconeogenesis from glycerol in individuals who are physically fit. Ketone bodies are mainly produced in the liver from FAs released from adipose tissue and used for energy production (eg, in the skeletal muscles, heart, and brain). Skeletal muscle accounts for the highest amount of the use of ketone bodies.53 In individuals who were aerobically fit, lower serum ketone body concentrations may be associated with increased activity of ketolytic enzymes in the skeletal muscles, as seen in rodents.54 In people with high muscle strength, the lower ketone body levels may be explained by higher muscle mass capable of using ketone bodies for energy.
Increased serum levels of branched-chain amino acids have been shown to predict the occurrence of type 2 diabetes55 and are associated with physical inactivity and unfavorable lipid metabolism.22 This is most likely because branched-chain amino acids degradation mechanistically connects to tricarboxylic acid cycle, intramyocellular lipid storage, and oxidation, thus allowing more efficient mitochondrial energy production from lipids as well as providing better metabolic health.56
In our study and in a 2013 study57 examining the association of aerobic fitness with metabolic profiles, serum phenylalanine concentration was negatively associated with high aerobic fitness, supporting the finding of Würtz et al,39 in which a high serum phenylalanine level was associated with the increased risk of cardiovascular events. Acute phenylalanine supplementation combined with exercise is associated with increased glucagon concentration and increased whole body fat oxidation.58 This implies that lower phenylalanine concentration in individuals who are aerobically fit may be due to more efficient removal of phenylalanine from the bloodstream to enhance fat oxidation. To our knowledge, the details of the mechanism for this effect of phenylalanine remain to be shown. Glycoprotein acetyls, in particular α1-acid glycoprotein, are indicators of glycosylation modification of secreted inflammatory proteins and have been reported to predict mortality.59,60 In this study, the creatinine levels were higher among those with high muscular strength and high skeletal muscle mass.
Limitations
This study used carefully validated methods to measure the fitness characteristics, but the maximal aerobic fitness test method was an indirect way to estimate V̇o2max. Furthermore, the participants were relatively healthy white Finnish men, which led to little confounding due to diseases. It is unclear how our results could be generalized to individuals from other races, women, and older people with noncommunicable diseases. Estrogens, aging, development of diseases, and development of nonalcoholic fatty liver disease may influence the associations of physical fitness with metabolomics.61,62
Conclusions
This study shows confirmatory and novel associations of aerobic fitness with vascular and metabolic disease risk factors already present in young, relatively healthy men. Many of the mechanisms may be associated with skeletal muscle metabolism, body fat content, and metabolism. Although the most beneficial associations were mostly seen for aerobic fitness, muscular endurance training also increases aerobic fitness. In addition, muscular training is an important component in maintaining or improving function among patients with long-term diseases and in the later years of life.63 Further interventional research is needed on whether the observed associations are more genetically determined or whether improving fitness by physical training has beneficial influences on health.
eAppendix 1. Study Plan
eAppendix 2. Questionnaire Items on Covariates Used in the Regression Analyses
eFigure. Heat Map of Correlations Between Fitness Characteristics and Metabolome Measures
eTable 1. Number of Participants in the Overlapping High Fitness vs Low Aerobic Fitness and High Muscular Strength vs Low Muscular Strength Groups
eTable 2. List of Analyzed Metabolome Measures, Their Abbreviations, and Number of Imputed Values
eTable 3. Serum Metabolome Measures by Aerobic Fitness and Muscular Strength Groups in SI Units
eTable 4. Differences in the Medians of Serum Metabolome Measures Between Aerobic Fitness Groups and Between the Muscular Strength Groups Relative to 1 SD of Each Variable
eTable 5. Linear Regression Analysis of the Age-Adjusted Association of Aerobic Fitness With Serum Metabolome Measures
eTable 6. Linear Regression Analysis of the Age-Adjusted Association of Maximal Muscular Strength With Serum Metabolome Measures
eTable 7. Linear Regression Analysis of the Age-, Education-, Smoking-, Use of Alcohol-, and Diet-Adjusted Association of Aerobic Fitness With Serum Metabolome Measures
eTable 8. Linear Regression Analysis of the Age-, Education-, Smoking-, Use of Alcohol-, and Diet-Adjusted Association of Maximal Muscular Strength With Serum Metabolome Measures
eTable 9. Linear Regression Analysis of the Age-Adjusted and Physical Activity–Adjusted Association of Aerobic Fitness With Serum Metabolome Measures
eTable 10. Linear Regression Analysis of the Age-Adjusted and Physical Activity–Adjusted Association of Maximal Muscular Strength With Serum Metabolome Measures
eTable 11. Linear Regression Analysis of the Age-Adjusted and Body Fat Percentage–Adjusted Association of Aerobic Fitness With Serum Metabolome Measures
eTable 12. Linear Regression Analysis of the Age-Adjusted and Body Fat Percentage–Adjusted Association of Maximal Muscular Strength With Serum Metabolome Measures
eTable 13. Summary of the ΔR2 Values From Regression Models Indicating the Additional Variation Accounted for by Each Fitness Variable
eTable 14. Additional Explanation Rate for Absolute and Relative-to-Body-Weight Maximal Muscular Strength After Adjusting for Age and After Adjusting for Age and Body Fat Percentage
eTable 15. Regression Models Including Age, Body Fat Percentage, Aerobic Fitness, and Relative Muscular Strength
References
- 1.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):-. doi: 10.1056/NEJMoa011858 [DOI] [PubMed] [Google Scholar]
- 2.Kodama S, Saito K, Tanaka S, et al. . Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024-2035. doi: 10.1001/jama.2009.681 [DOI] [PubMed] [Google Scholar]
- 3.Lee DC, Sui X, Church TS, Lavie CJ, Jackson AS, Blair SN. Changes in fitness and fatness on the development of cardiovascular disease risk factors hypertension, metabolic syndrome, and hypercholesterolemia. J Am Coll Cardiol. 2012;59(7):665-672. doi: 10.1016/j.jacc.2011.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross R, Blair SN, Arena R, et al. ; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653-e699. doi: 10.1161/CIR.0000000000000461 [DOI] [PubMed] [Google Scholar]
- 5.Sui X, Sarzynski MA, Lee DC, Kokkinos PF. Impact of changes in cardiorespiratory fitness on hypertension, dyslipidemia and survival: an overview of the epidemiological evidence. Prog Cardiovasc Dis. 2017;60(1):56-66. doi: 10.1016/j.pcad.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 6.Farrell SW, Finley CE, Barlow CE, et al. . Moderate to high levels of cardiorespiratory fitness attenuate the effects of triglyceride to high-density lipoprotein cholesterol ratio on coronary heart disease mortality in men. Mayo Clin Proc. 2017;92(12):1763-1771. doi: 10.1016/j.mayocp.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 7.Mandsager K, Harb S, Cremer P, Phelan D, Nissen SE, Jaber W. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open. 2018;1(6):e183605. doi: 10.1001/jamanetworkopen.2018.3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozemek C, Laddu DR, Lavie CJ, et al. . An update on the role of cardiorespiratory fitness, structured exercise and lifestyle physical activity in preventing cardiovascular disease and health risk. Prog Cardiovasc Dis. 2018;61(5-6):484-490. doi: 10.1016/j.pcad.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC health promotion series. J Am Coll Cardiol. 2018;72(14):1622-1639. doi: 10.1016/j.jacc.2018.08.2141 [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Lee DC, Li Y, et al. . Associations of resistance exercise with cardiovascular disease morbidity and mortality. Med Sci Sports Exerc. 2019;51(3):499-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DC, Sui X, Ortega FB, et al. . Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br J Sports Med. 2011;45(6):504-510. doi: 10.1136/bjsm.2009.066209 [DOI] [PubMed] [Google Scholar]
- 12.Karvinen S, Waller K, Silvennoinen M, et al. . Physical activity in adulthood: genes and mortality. Sci Rep. 2015;5:18259. doi: 10.1038/srep18259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kujala UM. Is physical activity a cause of longevity: it is not as straightforward as some would believe: a critical analysis. Br J Sports Med. 2018;52(14):914-918. doi: 10.1136/bjsports-2017-098639 [DOI] [PubMed] [Google Scholar]
- 14.Kujala UM, Marti P, Kaprio J, Hernelahti M, Tikkanen H, Sarna S. Occurrence of chronic disease in former top-level athletes: predominance of benefits, risks or selection effects? Sports Med. 2003;33(8):553-561. doi: 10.2165/00007256-200333080-00001 [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Kuk JL, Katzmarzyk PT, Blair SN, Church TS, Ross R. Cardiorespiratory fitness attenuates metabolic risk independent of abdominal subcutaneous and visceral fat in men. Diabetes Care. 2005;28(4):895-901. doi: 10.2337/diacare.28.4.895 [DOI] [PubMed] [Google Scholar]
- 16.Rhéaume C, Arsenault BJ, Dumas M-P, et al. . Contributions of cardiorespiratory fitness and visceral adiposity to six-year changes in cardiometabolic risk markers in apparently healthy men and women. J Clin Endocrinol Metab. 2011;96(5):1462-1468. doi: 10.1210/jc.2010-2432 [DOI] [PubMed] [Google Scholar]
- 17.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37(11):1849-1855. doi: 10.1249/01.mss.0000175865.17614.74 [DOI] [PubMed] [Google Scholar]
- 18.Wijndaele K, Duvigneaud N, Matton L, et al. . Muscular strength, aerobic fitness, and metabolic syndrome risk in Flemish adults. Med Sci Sports Exerc. 2007;39(2):233-240. doi: 10.1249/01.mss.0000247003.32589.a6 [DOI] [PubMed] [Google Scholar]
- 19.Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA; Members of the Florey Adelaide Male Ageing Study . Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism. 2009;58(7):1013-1022. doi: 10.1016/j.metabol.2009.02.027 [DOI] [PubMed] [Google Scholar]
- 20.Vaara JP, Fogelholm M, Vasankari T, Santtila M, Häkkinen K, Kyröläinen H. Associations of maximal strength and muscular endurance with cardiovascular risk factors. Int J Sports Med. 2014;35(4):356-360. [DOI] [PubMed] [Google Scholar]
- 21.Artero EG, Lee DC, Lavie CJ, et al. . Effects of muscular strength on cardiovascular risk factors and prognosis. J Cardiopulm Rehabil Prev. 2012;32(6):351-358. doi: 10.1097/HCR.0b013e3182642688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kujala UM, Mäkinen V-P, Heinonen I, et al. . Long-term leisure-time physical activity and serum metabolome. Circulation. 2013;127(3):340-348. doi: 10.1161/CIRCULATIONAHA.112.105551 [DOI] [PubMed] [Google Scholar]
- 23.Xiao Q, Moore SC, Keadle SK, et al. . Objectively measured physical activity and plasma metabolomics in the Shanghai Physical Activity Study. Int J Epidemiol. 2016;45(5):1433-1444. doi: 10.1093/ije/dyw033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukai K, Harada S, Iida M, et al. . Metabolic profiling of total physical activity and sedentary behavior in community-dwelling men. PLoS One. 2016;11(10):e0164877. doi: 10.1371/journal.pone.0164877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lustgarten MS, Price LL, Logvinenko T, et al. . Identification of serum analytes and metabolites associated with aerobic capacity. Eur J Appl Physiol. 2013;113(5):1311-1320. doi: 10.1007/s00421-012-2555-x [DOI] [PubMed] [Google Scholar]
- 26.Lustgarten MS, Price LL, Fielding RA. Analytes and metabolites associated with muscle quality in young, healthy adults. Med Sci Sports Exerc. 2015;47(8):1659-1664. doi: 10.1249/MSS.0000000000000578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boneva-Asiova Z, Boyanov MA. Body composition analysis by leg-to-leg bioelectrical impedance and dual-energy x-ray absorptiometry in non-obese and obese individuals. Diabetes Obes Metab. 2008;10(11):1012-1018. doi: 10.1111/j.1463-1326.2008.00851.x [DOI] [PubMed] [Google Scholar]
- 28.Santtila M, Häkkinen K, Pihlainen K, Kyröläinen H. Comparison between direct and predicted maximal oxygen uptake measurement during cycling. Mil Med. 2013;178(2):234-238. doi: 10.7205/MILMED-D-12-00276 [DOI] [PubMed] [Google Scholar]
- 29.Häkkinen K, Häkkinen A. Neuromuscular adaptations during intensive strength training in middle-aged and elderly males and females. Electromyogr Clin Neurophysiol. 1995;35(3):137-147. [PubMed] [Google Scholar]
- 30.Viitasalo JT, Saukkonen S, Komi PV. Reproducibility of measurements of selected neuromuscular performance variables in man. Electromyogr Clin Neurophysiol. 1980;20(6):487-501. [PubMed] [Google Scholar]
- 31.Soininen P, Kangas AJ, Würtz P, et al. . High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst. 2009;134(9):1781-1785. doi: 10.1039/b910205a [DOI] [PubMed] [Google Scholar]
- 32.Soininen P, Kangas AJ, Würtz P, Suna T, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet. 2015;8(1):192-206. doi: 10.1161/CIRCGENETICS.114.000216 [DOI] [PubMed] [Google Scholar]
- 33.Kraus WE, Houmard JA, Duscha BD, et al. . Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347(19):1483-1492. doi: 10.1056/NEJMoa020194 [DOI] [PubMed] [Google Scholar]
- 34.Natarajan P, Ray KK, Cannon CP. High-density lipoprotein and coronary heart disease: current and future therapies. J Am Coll Cardiol. 2010;55(13):1283-1299. doi: 10.1016/j.jacc.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 35.Huttunen JK, Länsimies E, Voutilainen E, et al. . Effect of moderate physical exercise on serum lipoproteins: a controlled clinical trial with special reference to serum high-density lipoproteins. Circulation. 1979;60(6):1220-1229. doi: 10.1161/01.CIR.60.6.1220 [DOI] [PubMed] [Google Scholar]
- 36.Kodama S, Tanaka S, Saito K, et al. . Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med. 2007;167(10):999-1008. doi: 10.1001/archinte.167.10.999 [DOI] [PubMed] [Google Scholar]
- 37.Blazek A, Rutsky J, Osei K, Maiseyeu A, Rajagopalan S. Exercise-mediated changes in high-density lipoprotein: impact on form and function. Am Heart J. 2013;166(3):392-400. doi: 10.1016/j.ahj.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 38.Lehti M, Donelan E, Abplanalp W, et al. . High-density lipoprotein maintains skeletal muscle function by modulating cellular respiration in mice. Circulation. 2013;128(22):2364-2371. doi: 10.1161/CIRCULATIONAHA.113.001551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Würtz P, Havulinna AS, Soininen P, et al. . Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation. 2015;131(9):774-785. doi: 10.1161/CIRCULATIONAHA.114.013116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aung T, Halsey J, Kromhout D, et al. ; Omega-3 Treatment Trialists’ Collaboration . Associations of omega-3 fatty acid supplement use with cardiovascular disease risk: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3(3):225-234. doi: 10.1001/jamacardio.2017.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdelhamid AS, Martin N, Bridges C, et al. . Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;11:CD012345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hooper L, Al-Khudairy L, Abdelhamid AS, et al. . Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;11:CD011094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maki KC, Eren F, Cassens ME, Dicklin MR, Davidson MH. ω-6 polyunsaturated fatty acids and cardiometabolic health: current evidence, controversies, and research gaps. Adv Nutr. 2018;9(6):688-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Da Boit M, Hunter AM, Gray SR. Fit with good fat: the role of n-3 polyunsaturated fatty acids on exercise performance. Metabolism. 2017;66:45-54. doi: 10.1016/j.metabol.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kivelä R, Silvennoinen M, Lehti M, et al. . Gene expression centroids that link with low intrinsic aerobic exercise capacity and complex disease risk. FASEB J. 2010;24(11):4565-4574. doi: 10.1096/fj.10-157313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch. 1985;403(4):369-376. doi: 10.1007/BF00589248 [DOI] [PubMed] [Google Scholar]
- 47.Donovan CM, Pagliassotti MJ. Quantitative assessment of pathways for lactate disposal in skeletal muscle fiber types. Med Sci Sports Exerc. 2000;32(4):772-777. doi: 10.1097/00005768-200004000-00009 [DOI] [PubMed] [Google Scholar]
- 48.Hui S, Ghergurovich JM, Morscher RJ, et al. . Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551(7678):115-118. doi: 10.1038/nature24057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shephard RJ, Johnson N. Effects of physical activity upon the liver. Eur J Appl Physiol. 2015;115(1):1-46. doi: 10.1007/s00421-014-3031-6 [DOI] [PubMed] [Google Scholar]
- 50.Nørregaard J, Gram M, Vigelsø A, et al. . The effect of reduced physical activity and retraining on blood lipids and body composition in young and older adult men. J Aging Phys Act. 2015;23(4):489-495. doi: 10.1123/japa.2014-0079 [DOI] [PubMed] [Google Scholar]
- 51.Coggan AR, Raguso CA, Gastaldelli A, Sidossis LS, Yeckel CW. Fat metabolism during high-intensity exercise in endurance-trained and untrained men. Metabolism. 2000;49(1):122-128. doi: 10.1016/S0026-0495(00)90963-6 [DOI] [PubMed] [Google Scholar]
- 52.Klein S, Weber JM, Coyle EF, Wolfe RR. Effect of endurance training on glycerol kinetics during strenuous exercise in humans. Metabolism. 1996;45(3):357-361. doi: 10.1016/S0026-0495(96)90291-7 [DOI] [PubMed] [Google Scholar]
- 53.Balasse EO, Féry F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev. 1989;5(3):247-270. doi: 10.1002/dmr.5610050304 [DOI] [PubMed] [Google Scholar]
- 54.Winder WW, Baldwin KM, Holloszy JO. Enzymes involved in ketone utilization in different types of muscle: adaptation to exercise. Eur J Biochem. 1974;47(3):461-467. doi: 10.1111/j.1432-1033.1974.tb03713.x [DOI] [PubMed] [Google Scholar]
- 55.Wang TJ, Larson MG, Vasan RS, et al. . Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448-453. doi: 10.1038/nm.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kainulainen H, Hulmi JJ, Kujala UM. Potential role of branched-chain amino acid catabolism in regulating fat oxidation. Exerc Sport Sci Rev. 2013;41(4):194-200. doi: 10.1097/JES.0b013e3182a4e6b6 [DOI] [PubMed] [Google Scholar]
- 57.Morris C, Grada CO, Ryan M, et al. . The relationship between aerobic fitness level and metabolic profiles in healthy adults. Mol Nutr Food Res. 2013;57(7):1246-1254. doi: 10.1002/mnfr.201200629 [DOI] [PubMed] [Google Scholar]
- 58.Ueda K, Sanbongi C, Yamaguchi M, Ikegami S, Hamaoka T, Fujita S. The effects of phenylalanine on exercise-induced fat oxidation: a preliminary, double-blind, placebo-controlled, crossover trial. J Int Soc Sports Nutr. 2017;14:34. doi: 10.1186/s12970-017-0191-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henry OF, Blacher J, Verdavaine J, Duviquet M, Safar ME. Alpha 1-acid glycoprotein is an independent predictor of in-hospital death in the elderly. Age Ageing. 2003;32(1):37-42. doi: 10.1093/ageing/32.1.37 [DOI] [PubMed] [Google Scholar]
- 60.Lawler PR, Akinkuolie AO, Chandler PD, et al. . Circulating N-linked glycoprotein acetyls and longitudinal mortality risk. Circ Res. 2016;118(7):1106-1115. doi: 10.1161/CIRCRESAHA.115.308078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Auro K, Joensuu A, Fischer K, et al. . A metabolic view on menopause and ageing. Nat Commun. 2014;5:4708. doi: 10.1038/ncomms5708 [DOI] [PubMed] [Google Scholar]
- 62.Pirola CJ, Sookoian S. Multiomics biomarkers for the prediction of nonalcoholic fatty liver disease severity. World J Gastroenterol. 2018;24(15):1601-1615. doi: 10.3748/wjg.v24.i15.1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pasanen T, Tolvanen S, Heinonen A, Kujala UM. Exercise therapy for functional capacity in chronic diseases: an overview of meta-analyses of randomised controlled trials. Br J Sports Med. 2017;51(20):1459-1465. doi: 10.1136/bjsports-2016-097132 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Study Plan
eAppendix 2. Questionnaire Items on Covariates Used in the Regression Analyses
eFigure. Heat Map of Correlations Between Fitness Characteristics and Metabolome Measures
eTable 1. Number of Participants in the Overlapping High Fitness vs Low Aerobic Fitness and High Muscular Strength vs Low Muscular Strength Groups
eTable 2. List of Analyzed Metabolome Measures, Their Abbreviations, and Number of Imputed Values
eTable 3. Serum Metabolome Measures by Aerobic Fitness and Muscular Strength Groups in SI Units
eTable 4. Differences in the Medians of Serum Metabolome Measures Between Aerobic Fitness Groups and Between the Muscular Strength Groups Relative to 1 SD of Each Variable
eTable 5. Linear Regression Analysis of the Age-Adjusted Association of Aerobic Fitness With Serum Metabolome Measures
eTable 6. Linear Regression Analysis of the Age-Adjusted Association of Maximal Muscular Strength With Serum Metabolome Measures
eTable 7. Linear Regression Analysis of the Age-, Education-, Smoking-, Use of Alcohol-, and Diet-Adjusted Association of Aerobic Fitness With Serum Metabolome Measures
eTable 8. Linear Regression Analysis of the Age-, Education-, Smoking-, Use of Alcohol-, and Diet-Adjusted Association of Maximal Muscular Strength With Serum Metabolome Measures
eTable 9. Linear Regression Analysis of the Age-Adjusted and Physical Activity–Adjusted Association of Aerobic Fitness With Serum Metabolome Measures
eTable 10. Linear Regression Analysis of the Age-Adjusted and Physical Activity–Adjusted Association of Maximal Muscular Strength With Serum Metabolome Measures
eTable 11. Linear Regression Analysis of the Age-Adjusted and Body Fat Percentage–Adjusted Association of Aerobic Fitness With Serum Metabolome Measures
eTable 12. Linear Regression Analysis of the Age-Adjusted and Body Fat Percentage–Adjusted Association of Maximal Muscular Strength With Serum Metabolome Measures
eTable 13. Summary of the ΔR2 Values From Regression Models Indicating the Additional Variation Accounted for by Each Fitness Variable
eTable 14. Additional Explanation Rate for Absolute and Relative-to-Body-Weight Maximal Muscular Strength After Adjusting for Age and After Adjusting for Age and Body Fat Percentage
eTable 15. Regression Models Including Age, Body Fat Percentage, Aerobic Fitness, and Relative Muscular Strength